Indication of Long-Term Changes of Algae Communities in a Hydrologically Transformed Estuary Sasyk, Black Sea, Ukraine
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Environmental Data Collection
2.3. Algae Floristic Data Collection
2.4. Bioindicators and Statistical Analysis
3. Results
3.1. Environmental Variables in Long-Term Changes
3.2. Algae Composition Characteristics of the Stages of the Sasyk’s Existence
3.3. Ecological Assessment Based on Bioindicators for the Different Stages of the Sasyk’s Existence
3.4. Species–Environment Relationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Taxa | Estuary-Lake | Forming a Reservoir | Reservoir | Hab | T | Oxy | pH | Sal | Wat | Sapro | Index S | Tro | Aut-Het |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacillariophyta | |||||||||||||
| Achnanthes armillaris (O.F.Müller) Guiry | 1 | 0 | 0 | B | - | - | - | hl | - | - | - | - | - |
| Achnanthes brevipes C.Agardh | 1 | 1 | 0 | B | - | - | alf | hl | - | b | 2.0 | me | - |
| Achnanthes minima J.R.Carter | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Achnanthes sp. | 1 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Achnanthidium minutissimum (Kützing) Czarn. | 0 | 0 | 1 | P-B | eterm | st-str | ind | i | es | x-b | 0.95 | o-e | ate |
| Amphiprora gigantea Grunow | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Amphiprora paludosa var. subsalina Cleve | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Amphora libyca Ehrenberg | 0 | 0 | 1 | B | temp | st | alf | i | es | o-b | 1.5 | o-m | - |
| Amphora ovalis (Kützing) Kützing | 1 | 1 | 1 | B | temp | st-str | alf | i | sx | o-b | 1.5 | me | ate |
| Amphora pediculus (Kützing) Grunow ex A.Schmidt | 0 | 1 | 1 | B | temp | st | alf | i | es | b-o | 1.7 | o-m | ate |
| Amphora sp. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Amphora copulata (Kützing) Schoeman et R.E.M.Archibald | 0 | 0 | 1 | B | temp | st | alf | i | es | o-b | 1.5 | e | ate |
| Amphora inariensis Krammer | 0 | 0 | 1 | B | - | - | alf | oh | - | o-x | 0.7 | o-m | - |
| Amphora recens Levkov et Nakov | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Aneumastus tuscula (Ehrenberg) D.G.Mann et A.J.Stickle | 1 | 0 | 0 | P-B | - | - | alf | i | - | x-b | 0.9 | o-e | - |
| Asterionella formosa Hassal | 1 | 1 | 0 | P | - | st-str | alf | i | sx | o | 1.35 | me | ate |
| Asterionella formosa var. gracillima (Hanztsch) Grunow | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Aulacoseira granulata (Ehrenberg) Simonsen | 1 | 1 | 1 | P-B | temp | st-str | ind | i | es | b | 2.0 | me | ate |
| Aulacoseira granulata var. angustissima (O.F.Müller) Simonsen | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Aulacoseira italica (Ehrenberg) Simonsen | 1 | 1 | 0 | P-B | cool | st-str | ind | i | es | o-b | 1.45 | me | - |
| Aulacoseira italica var. tenuissima (Grunow) Simonsen | 1 | 1 | 0 | P | cool | st-str | ind | i | es | o | 1.3 | me | ate |
| Azpeitia nodulifera (A.W.F.Schmidt) G.A.Fryxell et P.A.Sims | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Bacillaria paxillifera (O.F.Müller) T.Marsson | 1 | 1 | 1 | P-B | - | - | ind | hl | es | b | 2.3 | me | ate |
| Bacillaria socialis var. baltica Grunow | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Belonastrum cf. berolinense (Lemmermann) Round & Maidana | 0 | 0 | 1 | P-B | - | st-str | alf | hl | - | b | 2.2 | he | ate |
| Biddulphia subaequa (Kützing) J.Ralfs | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Brachysira exilis (Kützing) Round et D.G.Mann | 1 | 0 | 0 | P-B | - | - | - | - | - | o | - | - | - |
| Brebissonia lanceolata (C.Agardh) R.K.Mahoney et Reimer | 0 | 1 | 0 | B | - | - | alf | hl | - | b | 2.0 | - | - |
| Caloneis amphisbaena (Bory) Cleve | 0 | 1 | 0 | B | - | st-str | alf | i | - | b | 2.3 | me | ate |
| Caloneis sp. | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Campylodiscus clypeus (Ehrenberg) Ehrenberg ex Kützing | 1 | 0 | 0 | B | temp | - | alb | mh | - | b | 2.0 | e | - |
| Campylodiscus echensis Ehrenberg | 0 | 1 | 0 | P | - | st | - | hl | - | - | - | - | - |
| Campylodiscus fastuosus Ehrenberg | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Cerataulina pelagica (Cleve) Hendey | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chaetoceros compressus Lauder | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chaetoceros curvisetus Cleve | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chaetoceros dubius Proshkina-Lavrenko | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chaetoceros laciniosus F.Schütt | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chaetoceros lauderi Ralfs | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chaetoceros lorenzianus f. subsalinus Proshkina-Lavrenko | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chaetoceros lorenzianus Grunow | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chaetoceros simplex Ostenfeld | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chaetoceros socialis H.S.Lauder | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chaetoceros wighamii Brightwell | 1 | 0 | 0 | P-B | - | - | alb | mh | - | - | - | - | - |
| Chaetoceros willei Gran | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Cocconeis lineata Ehrenberg | 0 | 0 | 1 | P-B | - | st-str | alf | i | sx | o | 1.2 | o-m | ate |
| Cocconeis neodiminuta Krammer | 0 | 0 | 1 | P-B | temp | st-str | alf | i | sx | x-b | 0.9 | me | - |
| Cocconeis pediculus Ehrenberg | 1 | 1 | 1 | B | - | st-str | alf | i | sx | o-a | 1.8 | me | ate |
| Cocconeis placentula Ehrenberg | 1 | 1 | 1 | P-B | temp | st-str | alf | i | es | o | 1.35 | me | ate |
| Cocconeis placentula var. euglypta (Ehrenberg) Grunow | 0 | 1 | 0 | P-B | temp | st-str | alf | i | sx | o | 1.3 | o-m | ate |
| Cocconeis pseudolineata (Geitler) Lange-Bertalot | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Cocconeis scutellum Ehrenberg | 1 | 1 | 0 | B | - | - | alf | hl | - | b | 2.0 | me | - |
| Cocconeis sp. | 0 | 1 | 1 | - | - | - | - | - | - | - | - | - | - |
| Coronia daemeliana (Grunow) Ruck et Guiry | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Coscinodiscopsis commutata (Grunow) E.A.Sar et I.Sunesen | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Coscinodiscopsis jonesiana (Greville) E.A.Sar et I.Sunesen | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Coscinodiscus granii Gough | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Coscinodiscus granii var. aralensis (Ostenfeld) Hustedt | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Coscinodiscus janischii A.Schmidt | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Coscinodiscus sp. | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Craticula halophila (Grunow) D.G.Mann | 1 | 0 | 1 | B | - | st-str | alf | mh | es | a | 3.0 | e | ate |
| Craticula subminuscula (Manguin) C.E.Wetzel et Ector | 0 | 0 | 1 | B | - | - | alf | i | sp | a-o | 2.6 | e | hce |
| Ctenophora pulchella (Ralfs ex Kützing) D.M.Williams et Round | 0 | 0 | 1 | P-B | - | st-str | alf | i | - | b | 2.3 | o-m | ate |
| Cyclostephanos dubius (Fricke) Round | 0 | 1 | 1 | P-B | - | st-str | alf | i | es | b | 2.0 | o-m | ate |
| Cyclostephanos invisitatus (M.H.Hohn et Hellermann) E.C.Theriot, Stoermer et Håkasson | 0 | 0 | 1 | P | - | - | alf | - | es | o-a | 1.9 | o-m | - |
| Cyclotella choctawhatcheeana Prasad | 1 | 0 | 0 | P | - | - | - | hl | - | - | - | - | - |
| Cyclotella meneghiniana Kützing | 1 | 1 | 1 | P-B | temp | st | alf | hl | sp | a-o | 2.8 | e | hne |
| Cyclotella radiosa (Grunow) Lemmermann | 0 | 1 | 0 | P | - | st-str | alb | i | sx | o | 1.2 | o-m | ats |
| Cyclotella sp. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Cyclotella atomus Hustedt | 0 | 0 | 1 | P-B | - | st-str | alf | i | sp | b-a | 2.5 | me | ate |
| Cylindrotheca closterium (Ehrenberg) Reimann et J.C.Lewin | 1 | 1 | 0 | B | - | - | alf | i | - | b | 2.0 | - | - |
| Cylindrotheca gracilis (Brébisson ex Kützing) Grunow | 1 | 0 | 0 | B | - | st | - | hl | - | a-o | 2.8 | e | - |
| Cymatopleura elliptica (Brébisson) W.Smith | 0 | 0 | 1 | P-B | - | st-str | alf | i | - | b-o | 1.7 | e | ate |
| Cymatopleura librile (Ehrenberg) Pant. | 0 | 1 | 0 | P-B | - | st-str | alf | i | - | o | 1.0 | - | - |
| Cymatopleura solea var. apiculata (W.Smith) Ralfs | 1 | 1 | 0 | B | - | - | alf | i | - | x-o | 0.5 | - | - |
| Cymatopleura solea var. gracilis Grunow | 0 | 1 | 0 | B | - | - | alf | i | - | - | - | - | - |
| Cymatopleura sp. | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Cymbella affinis Kützing | 0 | 0 | 1 | B | temp | st-str | alf | i | sx | o | 1.1 | ot | ats |
| Cymbella cistula (Ehrenberg) O.Kirchner | 0 | 0 | 1 | B | - | st-str | alf | i | sx | o-b | 1.5 | e | ats |
| Cymbella neocistula Krammer | 0 | 0 | 1 | B | - | - | ind | i | - | - | - | - | hne |
| Cymbella parva (W.Smith) Kirchner | 0 | 1 | 0 | B | - | - | ind | i | - | b | 2.0 | o-m | - |
| Cymbella proxima Reimer | 0 | 0 | 1 | B | - | - | alf | hb | es | o | 1.0 | o-m | - |
| Cymbella sp. | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Cymbella cistula (Ehrenberg) O.Kirchner | 0 | 0 | 1 | B | - | st-str | alf | i | sx | o-b | 1.5 | e | ats |
| Cymbella compacta Østrup | 0 | 0 | 1 | B | - | - | - | - | - | b-a | 2.4 | - | - |
| Cymbella laevis Nägeli | 0 | 0 | 1 | B | cool | - | ind | i | sx | - | - | - | - |
| Cymbella lanceolata C.Agardh | 0 | 0 | 1 | B | - | st-str | alf | i | es | x-b | 0.9 | e | ate |
| Cymbopleura lata (Grunow) Krammer | 0 | 1 | 0 | B | - | - | ind | i | sx | - | - | - | - |
| Detonula confervacea (Cleve) Gran | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Diatoma elongata var. actinastroides Krieger | 0 | 1 | 0 | B | - | st-str | alf | hl | - | a | - | e | ate |
| Diatoma hiemalis (Lyngbye) Heiberg | 0 | 1 | 0 | P-B | cool | st-str | ind | hb | sx | b-o | 1.7 | - | ats |
| Diatoma moniliformis (Kützing) D.M.Williams | 0 | 0 | 1 | P-B | - | st-str | alf | i | - | - | - | o-m | - |
| Diatoma vulgare Bory | 1 | 1 | 1 | P-B | - | st-str | ind | i | sx | b | 2.2 | me | ate |
| Diploneis elliptica (Kützing) Cleve | 1 | 0 | 1 | B | temp | str | alf | i | sx | o-x | 0.6 | m | ats |
| Diploneis ovalis (Hilse) Cleve | 0 | 0 | 1 | B | - | str | alf | i | sp | x-b | 0.9 | o-m | ats |
| Diploneis subadvena Hustedt | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Discostella stelligera (Cleve et Grunow) Houk et Klee | 0 | 1 | 0 | P | - | st-str | ind | i | - | b | 2.3 | e | ate |
| Ditylum brightwellii (T.West) Grunow | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Ellerbeckia sol (Ehrenberg) R.M.Crawford et P.A.Sims | 0 | 1 | 0 | - | - | - | - | i | - | - | - | - | - |
| Encyonema caespitosum Kützing | 0 | 0 | 1 | B | - | - | - | i | sx | o | 1.3 | o-e | - |
| Encyonema leibleinii (C.Agardh) W.J.Silva, R.Jahn, T.A.V.Ludwig et M.Menezes | 0 | 0 | 1 | P-B | - | str | alb | i | es | o | 1.3 | e | ats |
| Encyonema ventricosum (C.Agardh) Grunow | 1 | 1 | 0 | B | - | st-str | ind | i | sx | x-o | 1.3 | o-e | ate |
| Encyonopsis microcephala (Grunow) Krammer | 0 | 0 | 1 | B | - | str | alf | i | es | o | 1.3 | me | ats |
| Entomoneis alata (Ehrenberg) Reimer | 1 | 1 | 0 | P-B | - | st | alf | mh | - | b | 2.0 | - | - |
| Entomoneis paludosa (W.Smith) Reimer | 1 | 1 | 0 | P-B | - | - | alf | hl | - | b-a | 2.5 | m | - |
| Epithemia adnata (Kützing) Brébisson | 0 | 1 | 1 | B | temp | st | alb | i | sx | o | 1.2 | me | ats |
| Epithemia gibba (Ehrenberg) Kützing | 0 | 0 | 1 | B | temp | - | alf | i | es | o-b | 1.4 | o-m | - |
| Epithemia parallela (Grunow) Ruck & Nakov | 0 | 0 | 1 | B | - | str | alf | i | es | b | 2.0 | o-m | ats |
| Epithemia sorex Kützing | 0 | 1 | 1 | B | temp | st-str | alf | i | sx | o | 1.1 | me | ats |
| Epithemia turgida (Ehrenberg) Kützing | 0 | 1 | 1 | B | temp | st | alf | i | sx | x-b | 0.9 | me | ats |
| Fallacia pygmaea (Kützing) A.J.Stickle et D.G.Mann | 0 | 0 | 1 | P-B | - | st-str | alf | mh | es | a-o | 2.7 | e | hne |
| Fallacia clepsidroides Witkowski | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Fallacia reichardtii (Grunow) Witkowski, Lange-Bertalot et Metzeltin | 0 | 1 | 1 | P-B | - | st | alf | i | sx | b-o | 1.7 | o-m | ate |
| Fragilaria capucina Desm. | 0 | 1 | 0 | P-B | - | - | ind | i | es | b-o | 1.6 | m | - |
| Fragilaria capucina subsp. rumpens (Kützing) Lange-Bertalot | 0 | 1 | 0 | P-B | eterm | st-str | acf | i | - | b-o | 1.6 | o-m | - |
| Fragilaria capucina var. mesolepta (Rabenhorst) Rabenhorst | 0 | 1 | 0 | P-B | - | - | alf | i | sx | - | - | - | - |
| Fragilaria crotonensis Kitton | 1 | 1 | 1 | P | - | st-str | alf | i | es | o-b | 1.5 | m | ate |
| Fragilaria sp. | 1 | 1 | 1 | - | - | - | - | - | - | - | - | - | - |
| Fragilaria perminuta (Grunow) Lange-Bertalot | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Fragilaria microvaucheriae (Kützing) D.M.Williams & Round | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Fragilaria nevadensis J.E.Linares-Cuesta & P.M.Sánchez-Castillo | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Fragilariforma virescens (Ralfs) D.M.Williams et Round | 0 | 1 | 0 | P-B | - | st | ind | i | es | x-o | 0.4 | o-m | ats |
| Frustulia creuzburgensis (Krasske) Hustedt | 0 | 0 | 1 | B | - | - | alf | hl | - | - | - | - | - |
| Gomphonema acuminatum Ehrenberg | 0 | 1 | 0 | B | - | st | ind | i | es | o-b | 1.4 | o-m | ats |
| Gomphonema coronatum Ehrenberg | 0 | 0 | 1 | B | - | st | ind | i | - | o-b | 1.4 | o-m | - |
| Gomphonema italicum Kützing | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Gomphonema olivaceum (Lyngbye) Desmazières | 1 | 1 | 0 | B | - | st-str | alf | i | es | o-b | 1.45 | e | ate |
| Gomphonema parvulum Kützing | 0 | 0 | 1 | B | temp | str | ind | i | es | b | 2.35 | o-m | hne |
| Gomphonema sp. | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Gomphonema truncatum Ehrenberg | 0 | 0 | 1 | B | - | st-str | ind | i | es | o-b | 1.4 | me | ats |
| Gomphonema utae Lange-Bertalot et E.Reichardt | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Gomphonema augur Ehrenberg | 0 | 0 | 1 | B | - | str | ind | i | es | o-b | 1.5 | me | ats |
| Grunowia tabellaria (Grunow) Rabenhorst | 0 | 0 | 1 | B | - | str | ind | i | sx | o-b | 1.4 | m | ats |
| Gyrosigma acuminatum (Kützing) Rabenhorst | 1 | 1 | 1 | B | cool | st-str | alf | i | es | o-a | 1.95 | me | ate |
| Gyrosigma balticum (Ehrenberg) Rabenhorst | 1 | 0 | 0 | B | - | - | - | hl | - | - | - | e | - |
| Gyrosigma distortum (W.Smith) Cleve | 0 | 1 | 0 | B | - | - | ind | hl | es | o | 1.0 | - | - |
| Gyrosigma fasciola (Ehrenberg) J.W.Griffith et Henfrey | 1 | 0 | 0 | B | - | - | alf | mh | - | o | 1.0 | - | - |
| Gyrosigma recta var. minuta (Donkin) Cleve | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Gyrosigma sp. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Gyrosigma strigilis (W.Smith) Cleve | 1 | 0 | 0 | B | - | - | - | mh | - | - | - | - | - |
| Gyrosigma wormleyi (Sullivant) Boyer | 0 | 1 | 0 | B | - | - | alf | hl | - | b | 2.0 | o-m | - |
| Gyrosigma acuminatum var. gallicum (Grunow) Cleve | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Gyrosigma arcuatum (Donkin) Sterrenburg | 1 | 0 | 0 | B | - | - | - | mh | - | - | - | - | - |
| Gyrosigma attenuatum (Kützing) Rabenhorst | 0 | 0 | 1 | P-B | - | st | alf | i | - | o-a | 1.8 | o-m | ate |
| Gyrosigma prolongatum (W.Smith) J.W.Griffith et Henfrey | 1 | 0 | 0 | B | - | - | - | mh | - | - | - | - | - |
| Hannaea arcus (Ehrenberg) R.M.Patrick | 1 | 0 | 0 | B | temp | str | alf | i | es | x | 0.3 | o-m | ats |
| Hantzschia amphioxys (Ehrbenb.) Grunow | 0 | 1 | 1 | B | temp | st-str | ind | I | es | o-a | 1.9 | o-e | ate |
| Hantzschia vivax (W.Smith) Grunow | 0 | 1 | 0 | B | - | - | alf | i | - | b | 2.0 | - | - |
| Hippodonta capitata (Ehrenberg) Lange-Bertalot, Metzeltin et Witkowski | 0 | 1 | 1 | B | temp | st-str | alf | hl | es | b | 2.1 | me | ate |
| Hippodonta hungarica (Grunow) Lange-Bertalot, Metzeltin et Witkowski | 0 | 1 | 1 | B | - | st-str | alf | hl | es | b | 2.3 | me | ate |
| Hippodonta costulata (Grunow) Lange-Bertalot, Metzeltin et Witkowski | 0 | 0 | 1 | B | - | - | alf | hl | sx | b-a | 2.5 | o-m | - |
| Hyalodiscus ambiguus (Grunow) Tempère et Peragallo | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Karayevia sp. | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Leptocylindrus danicus Cleve | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Leptocylindrus minimus Gran | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Lindavia bodanica (Eulenstein ex Grunow) T.Nakov, Guillory, Julius, Theriot et Alverson | 0 | 1 | 0 | P | - | st | ind | i | - | x | 1.0 | ot | ats |
| Mastogloia pseudosmithii Sylvia S. Lee, E.E. Gaiser, Van de Vijver, Edlund, S.A. Spauld Hustedt | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Mastogloia smithii Thwaites ex W.Smith | 0 | 0 | 1 | B | - | - | alf | mh | sx | o | 1.3 | me | - |
| Mayamaea atomus (Kützing) Lange-Bertalot | 0 | 0 | 1 | B | - | st-str | alf | i | es | a-o | 2.6 | he | hce |
| Melosira italica f. curvata Fäden | 1 | 0 | 0 | - | - | - | - | - | es | - | - | - | - |
| Melosira moniliformis (O.F.Müller) C.Agardh | 1 | 1 | 0 | P-B | - | str | - | hl | - | b | 2.0 | - | - |
| Melosira moniliformis var. subglobosa (Grunow) Hustedt | 1 | 0 | 0 | P-B | - | str | alf | hl | - | b | 2.0 | - | - |
| Melosira nummuloides C.Agardh | 1 | 0 | 0 | P-B | - | - | alf | mh | sp | b | 2.0 | - | - |
| Melosira sp. | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Melosira varians C. Agardh | 1 | 1 | 1 | P-B | temp | st-str | ind | hl | es | b | 2.1 | me | hne |
| Navicula capitatoradiata Germain | 1 | 0 | 0 | P-B | - | st-str | alf | mh | sx | b | 2.1 | me | ate |
| Navicula cari Ehrenberg | 0 | 0 | 1 | P-B | - | str | ind | i | es | b-a | 2.4 | o-m | ats |
| Navicula cryptocephala Kützing | 1 | 0 | 1 | P-B | temp | st-str | ind | i | es | b | 2.1 | o-e | ate |
| Navicula cryptotenella Lange-Bertalot | 0 | 0 | 1 | P-B | - | - | ind | i | es | o | 1.3 | - | - |
| Navicula directa (W.Smith) Ralfs | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Navicula distans (W.Smith) Ralfs | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Navicula germainii J.H.Wallace | 0 | 0 | 1 | B | - | - | - | - | - | - | - | - | - |
| Navicula gracilis Lauby | 1 | 0 | 0 | B | - | st-str | alf | i | es | b | 2.3 | - | - |
| Navicula gregaria Donkin | 0 | 0 | 1 | P-B | - | - | alf | I | es | b-a | 2.5 | me | ate |
| Navicula grevillei var. pararhombica Proshkina-Lavrenko | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Navicula longicephala Hustedt | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Navicula menisculus Schumann | 1 | 1 | 1 | P-B | - | st-str | alf | i | es | o-b | 1.45 | o-m | ate |
| Navicula minima Grunow | 0 | 0 | 1 | B | - | - | alf | hl | es | o-b | 1.4 | e | hne |
| Navicula radiosa Kützing | 1 | 0 | 0 | B | temp | st-str | ind | i | es | o | 1.3 | me | ate |
| Navicula recens (Lange-Bertalot) Lange-Bertalot | 0 | 0 | 1 | P-B | - | - | alf | hl | - | - | - | - | - |
| Navicula rhynchotella Lange-Bertalot | 0 | 0 | 1 | B | - | - | alf | hl | es | b-a | 2.55 | - | - |
| Navicula slesvicensis Grunow | 1 | 1 | 0 | P-B | - | st-str | alf | hl | es | a-o | 2.6 | o-m | ate |
| Navicula sp. | 1 | 1 | 1 | - | - | - | - | - | - | - | - | - | - |
| Navicula tripunctata (O.F.Müller) Bory | 0 | 0 | 1 | P-B | - | st-str | ind | i | es | b-o | 1.7 | e | ate |
| Navicula cf. vandamii Schoeman et R.E.M.Archibald | 0 | 0 | 1 | B | - | - | alf | i | - | - | - | e | - |
| Navicula veneta Kützing | 1 | 0 | 1 | P-B | - | - | alf | hl | es | a-o | 2.7 | me | ate |
| Navicula viridula (Kützing) Ehrenberg | 0 | 1 | 0 | P-B | - | st-str | alf | hl | es | b | 2.2 | me | ate |
| Navicula anglica var. minuta Cleve | 1 | 1 | 0 | B | - | - | - | i | - | - | - | - | - |
| Navicula antonii Lange-Bertalot | 0 | 0 | 1 | B | - | - | - | - | - | - | - | - | - |
| Navicula recens (Lange-Bertalot) Lange-Bertalot | 0 | 0 | 1 | P-B | - | - | alf | i | es | o-b | - | e | - |
| Neidium dubium (Ehenberg) Cleve | 0 | 0 | 1 | B | - | str | alf | i | - | b-o | 1.7 | me | ats |
| Nitzschia acicularis (Kützing) W. Sm. | 1 | 1 | 1 | P-B | temp | - | alf | i | es | a-o | 2.7 | e | hce |
| Nitzschia amphibia Grunow | 0 | 1 | 0 | P-B,S | temp | st-str | alf | i | sp | b | 2.1 | e | hne |
| Nitzschia angustata var. minuta Krasske | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Nitzschia archibaldii Lange-Bertalot | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Nitzschia capitellata Hustedt | 0 | 0 | 1 | B | - | - | ind | i | es | a | 3.0 | he | - |
| Nitzschia dissipata (Kützing) Rabenhorst | 0 | 0 | 1 | B | - | st-str | alf | i | sx | b-o | 1.7 | me | ate |
| Nitzschia filiformis (W.Smith) Van Heurck | 0 | 0 | 1 | P-B | - | st-str | alf | hl | es | b-a | 2.5 | e | hne |
| Nitzschia filiformis var. conferta (P.G.Richter) Lange-Bertalot | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Nitzschia frustulum (Kützing) Grunow | 0 | 0 | 1 | P-B | temp | st-str | alf | i | sp | b | 2.3 | e | hce |
| Nitzschia hantzschiana Rabenhorst | 0 | 1 | 0 | P-B | - | str | alf | i | es | x-o | 0.5 | m | ats |
| Nitzschia holsatica Hustedt | 1 | 1 | 0 | P-B | - | - | ind | i | es | b | 2.3 | - | - |
| Nitzschia incerta (Grunow) M.Peragallo | 0 | 1 | 0 | P-B | - | - | alf | hl | - | b-a | 2.5 | me | - |
| Nitzschia intermedia Hantzsch | 0 | 0 | 1 | P-B | - | - | ind | i | es | b | 2.0 | e | - |
| Nitzschia longissima (Brébisson) Ralfs | 1 | 1 | 0 | - | - | - | - | mh | - | - | - | - | - |
| Nitzschia lorenziana var. subtilis Grunow | 0 | 1 | 0 | B | - | - | - | mh | - | - | - | - | - |
| Nitzschia microcephala Grunow | 0 | 0 | 1 | P-B | - | st-str | alf | i | sx | b | 2.3 | e | hce |
| Nitzschia palea (Kützing) W. Sm. | 0 | 0 | 1 | P-B | temp | - | ind | i | sp | a-o | 2.8 | he | hce |
| Nitzschia palea var. debilis (Kützing) Grunow | 0 | 0 | 1 | B | - | - | neu | i | es | a-o | 2.8 | ot | - |
| Nitzschia paleacea (Grunow) Grunow | 0 | 1 | 1 | P-B | - | st-str | alf | i | es | b | 2.2 | e | hce |
| Nitzschia pusilla Grunow | 0 | 0 | 1 | P-B,S | - | st-str | alf | i | es | b-o | 1.7 | o-e | ate |
| Nitzschia reversa W.Smith | 1 | 1 | 1 | P | - | - | - | hl | - | - | - | - | - |
| Nitzschia scalpelliformis Grunow | 0 | 1 | 0 | B | - | - | alf | hl | sp | b | 2.0 | me | - |
| Nitzschia sigma (Kützing) W.Smith | 1 | 1 | 0 | B | temp | st-str | alf | mh | es | a | 3.0 | e | ate |
| Nitzschia sigmoidea (Nitzsch) W.Smith | 1 | 1 | 1 | P-B | - | st-str | alf | i | - | b-a | 2.5 | e | ate |
| Nitzschia sociabilis Hustedt | 0 | 0 | 1 | B | - | st-str | neu | hl | - | a-b | - | e | ate |
| Nitzschia sp. | 1 | 1 | 1 | - | - | - | - | - | - | - | - | - | - |
| Nitzschia sublinearis Hustedt | 1 | 1 | 0 | P-B | - | - | alf | i | es | a | 3.0 | me | - |
| Nitzschia tenuirostris Mer. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Nitzschia thermalis (Ehrenberg) Auerswald | 0 | 1 | 0 | P | - | - | ind | i | es | x | 0.3 | - | - |
| Nitzschia umbonata (Ehrenberg) Lange-Bertalot | 0 | 0 | 1 | P | - | st-str | ind | i | es | a-o | 2.8 | me | - |
| Nitzschia vermicularis (Kützing) Hantzsch | 1 | 1 | 0 | P-B | - | str | alf | i | - | b | 2.2 | m | - |
| Nitzschia inconspicua Grunow | 0 | 0 | 1 | B | - | st-str | alf | i | es | a-o | 2.7 | e | hne |
| Nitzschia reversa f. parva (Grunow) Bukhtiyarova | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Paralia sulcata (Ehrenberg) Cleve | 1 | 0 | 0 | B | - | - | alf | mh | - | a | 3.0 | - | - |
| Paraplaconeis minor (Grunow) Lange-Bertalot | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Paraplaconeis placentula (Ehrenberg) Kulikovskiy et Lange-Bertalot | 1 | 0 | 0 | B | temp | st-str | alf | i | sx | o-b | 1.5 | e | ate |
| Pinnularia sp. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Placoneis clementis (Grunow) E.J.Cox | 0 | 0 | 1 | B | - | str | alf | i | es | b | 2.0 | me | ate |
| Placoneis elginensis (Gregory) E.J.Cox | 0 | 1 | 0 | B | - | st-str | ind | i | sx | o-b | 1.4 | me | ate |
| Placoneis exigua (Gregory) Mereschkovsky | 0 | 0 | 1 | B | - | - | ind | i | es | o-b | 1.4 | o-m | - |
| Placoneis placentula (Ehrenberg) Mereschkowsky | 0 | 0 | 1 | B | temp | st-str | alf | i | sx | o-b | 1.5 | e | ate |
| Planothidium delicatulum (Kützing) Round et Bukht. | 0 | 0 | 1 | P-B | - | st | alb | hl | es | b | 2.0 | o-m | - |
| Planothidium lacustre Álvarez-Blanco, C. Cejudo-Figueiras & S. Blanco | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Planothidium lanceolatum (Bréb. ex Kützing) Lange-Bert. | 0 | 0 | 1 | P-B | warm | st-str | ind | - | sx | o | 1.6 | e | ate |
| Planothidium minutissimum (Krasske) E.A.Morales | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Pleurosigma angulatum (Queckett) W.Smith | 1 | 0 | 0 | B | - | st-str | alf | hl | - | b | 2.0 | - | - |
| Pleurosigma elongatum W.Smith | 1 | 1 | 1 | B | - | - | alf | hl | - | b | 2.0 | - | - |
| Pleurosigma formosum W.Smith | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Pleurosigma sp. | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Pleurosira laevis (Ehrenberg) Compère | 0 | 0 | 1 | B | temp | - | alf | mh | - | o | 1.0 | e | - |
| Podosira hormoides (Mont.) Kützing | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Pseudo-nitzschia delicatissima (Cleve) Heiden | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Pseudo-nitzschia seriata (Cleve) H.Peragallo | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Pseudostaurosira parasitica (W.Smith) E.Morales | 0 | 0 | 1 | P-B | - | str | alf | i | es | o-a | 1.9 | me | ats |
| Pseudostaurosira brevistriata (Grunow) D.M.Williams et Round | 0 | 0 | 1 | P-B | - | st-str | alf | i | - | o | 1.2 | o-e | ats |
| Rhizosolenia calcar-avis Schultze | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Rhoicosphenia abbreviata (C.Agardh) Lange-Bertalot | 0 | 1 | 1 | B | - | st-str | alf | i | es | o-a | 1.9 | me | ate |
| Sellaphora pupula (Kützing) Mereschk. | 0 | 0 | 1 | B | eterm | st | ind | hl | sx | o-a | 1.9 | me | ate |
| Skeletonema costatum (Greville) Cleve | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Skeletonema subsalsum (Cleve-Euler) Bethge | 1 | 0 | 0 | P | - | - | ind | i | - | o | 1.0 | me | - |
| Stauroforma exiguiformis (Lange-Bertalot) R.J.Flower, V.J.Jones et Round | 0 | 0 | 1 | - | - | - | - | i | - | o | 1.0 | o-m | - |
| Stauroneis acuta W.Smith | 0 | 0 | 1 | B | - | st-str | alf | i | - | o | 1.0 | o-m | - |
| Stauroneis anceps Ehrenberg | 0 | 1 | 0 | P-B | - | st-str | ind | i | sx | o | 1.3 | me | ate |
| Staurosira construens Ehrenberg | 0 | 1 | 1 | P-B | temp | st-str | alf | i | sx | o | 1.3 | me | ats |
| Staurosira leptostauron (Ehrenberg) Kulikovskiy et Genkal | 0 | 0 | 1 | P-B | - | st | alf | hb | es | o | 1.1 | me | ats |
| Staurosira venter (Ehrenberg) H.Kobayasi | 0 | 1 | 1 | P-B | warm | st-str | alf | i | sx | o | 1.3 | me | ate |
| Staurosira construens Ehrenberg | 0 | 0 | 1 | P-B | temp | st-str | alf | i | sx | o | 1.3 | me | ats |
| Staurosirella martyi (Héribaud-Joseph) E.A.Morales et K.M.Manoylov | 0 | 0 | 1 | P-B | - | st-str | alf | i | es | o | 1.1 | o-m | - |
| Stephanodiscus astraea (Ehrenberg) Grunow | 1 | 1 | 0 | P | temp | st | alb | i | es | b | 2.0 | - | - |
| Stephanodiscus binderanus (Kützing) Krieger | 0 | 1 | 0 | P | - | - | ind | hl | - | b | 2.3 | e | - |
| Stephanodiscus hantzschii Grunow | 1 | 1 | 1 | P | temp | st | alf | i | es | a-o | 2.7 | o-m | hne |
| Stephanodiscus minutulus (Kützing) Cleve et Möller | 1 | 1 | 1 | P | temp | st | alb | i | es | b | 2.2 | o-m | ate |
| Stephanodiscus rotula (Kützing) Hendey | 0 | 0 | 1 | P-B | temp | st | alf | i | es | b | 2.2 | o-m | - |
| Stephanodiscus subtilis (Goor) A.Cleve | 0 | 1 | 0 | - | - | st-str | - | i | - | - | - | he | - |
| Surirella didyma Kützing | 0 | 1 | 0 | B | - | - | alf | i | - | o | 1.0 | o-m | - |
| Surirella minuta Brébisson | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Surirella ovalis Brébisson | 1 | 1 | 0 | P-B | - | st-str | alf | i | es | a | 3.0 | me | ate |
| Surirella robusta Ehrenberg | 0 | 1 | 0 | P-B | - | st-str | ind | i | es | x-o | 0.5 | ot | - |
| Surirella sp. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Surirella striatula Turpin | 0 | 1 | 0 | P-B | temp | - | alf | hl | - | b | 2.0 | e | - |
| Surirella librile (Ehrenberg) Ehrenberg | 0 | 0 | 1 | P-B | - | - | alf | i | - | b | 2.1 | - | - |
| Surirella ovata f. constricta (Hustedt) Cleve-Euler | 0 | 1 | 0 | B | - | - | ind | i | - | - | - | - | - |
| Surirella ovata var. pseudopinnata (Ant.Mayer) Proshkina-Lavrenko | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Tabellaria fenestrata (Lyngb.) Kützing | 0 | 1 | 1 | P-B | - | st-str | ind | i | es | x | 0.3 | o-m | ats |
| Tabellaria fenestrata var. asterionelloides Grunow | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Tabellaria flocculosa (Roth) Kützing | 0 | 0 | 1 | P-B | eterm | st-str | acf | i | es | o-x | 0.6 | ot | ats |
| Tabularia fasciculata (C.Agardh) D.M.Williams et Round | 0 | 0 | 1 | P-B | - | st | ind | mh | es | b-a | 2.5 | e | ate |
| Thalassionema frauenfeldii (Grunow) Tempère et Peragallo | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Thalassionema nitzschioides (Grunow) Mereschkowsky | 1 | 0 | 0 | P | - | - | - | i | - | - | - | - | - |
| Thalassiosira decipiens (Grunow) E.G.Jørgensen | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Thalassiosira eccentrica (Ehrenberg) Cleve | 1 | 0 | 0 | P | - | - | ind | i | - | - | - | - | - |
| Thalassiosira parva Proshkina-Lavrenko | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Thalassiosira sp. | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Thalassiosira baltica (Grunow) Ostenfeld. | 1 | 0 | 0 | - | - | - | - | hl | - | - | - | - | - |
| Tryblionella acuminata W.Smith | 1 | 0 | 0 | - | - | st | alf | hl | sx | a-o | 2.9 | me | - |
| Tryblionella apiculata Gregory | 0 | 0 | 1 | B | - | - | alf | hl | es | a-o | 2.7 | e | - |
| Tryblionella circumsuta (Bailey) Ralfs | 1 | 0 | 0 | B | - | - | alf | mh | - | - | - | e | - |
| Tryblionella compressa (Bailey) Poulin | 0 | 0 | 1 | B | eterm | - | - | mh | - | - | - | - | - |
| Tryblionella hantzschiana Grunow | 0 | 0 | 1 | B | - | st-str | alf | hl | - | a-o | 2.6 | me | ate |
| Tryblionella hungarica (Grunow) Frenguelli | 1 | 1 | 1 | P-B | - | - | alf | mh | sp | a-o | 2.9 | e | ate |
| Tryblionella levidensis W.Smith | 0 | 1 | 1 | P-B | - | st-str | ind | mh | sp | a-o | 2.6 | e | ate |
| Tryblionella hantzschiana Grunow. | 0 | 1 | 1 | B | - | st-str | alf | hl | - | a-o | 2.6 | me | ate |
| Tryblionella punctata W.Smith | 1 | 1 | 0 | B | eterm | - | - | mh | - | - | - | - | - |
| Ulnaria acus (Kütz.) Aboal | 1 | 1 | 1 | P | - | st-str | alb | i | es | o-a | 1.8 | - | - |
| Ulnaria capitata (Ehrenberg) P.Compère | 0 | 1 | 0 | P-B | - | st-str | alf | i | es | o-b | 1.5 | e | ats |
| Ulnaria danica (Kützing) Compère et Bukhtiyarova | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Ulnaria delicatissima (W.Smith) M.Aboal et P.C.Silva | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Ulnaria delicatissima var. angustissima (Grunow) M.Aboal et P.C.Silva | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Ulnaria oxyrhynchus (Kützing) M.Aboal | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Ulnaria ulna (Nitzsch) Compere | 1 | 1 | 1 | P-B | temp | st-str | ind | i | es | b | 2.25 | o-e | ate |
| Charophyta | |||||||||||||
| Closterium acerosum (Schrank) Ehr. | 0 | 1 | 0 | P-B | - | st-str | ind | i | - | a-o | 2.6 | e | - |
| Closterium aciculare T.West | 0 | 1 | 1 | P | - | st-str | ind | - | - | b-o | 1.7 | me | - |
| Closterium acutum Brébisson | 1 | 0 | 1 | P-B | - | st-str | ind | - | - | b | 2.05 | m | - |
| Closterium exiguum West et G.S.West | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Closterium gracile Brèb. ex Ralfs | 0 | 1 | 0 | P | - | st-str | ind | hb | - | o-b | 1.5 | o-m | - |
| Closterium parvulum Nägeli | 0 | 0 | 1 | P-B | - | - | ind | i | - | b | 2.0 | m | - |
| Closterium sp. | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Closterium strigosum Brébisson | 0 | 1 | 0 | P-B | - | st-str | ind | - | - | o-a | 1.9 | e | - |
| Closterium tumidum L.N.Johnson | 0 | 1 | 0 | B | - | ae | acf | - | - | - | - | o-m | - |
| Closterium dianae Ehrenberg ex Ralfs | 0 | 0 | 1 | P-B | - | st-str | acf | - | - | x-b | 0.8 | m | - |
| Cosmarium bioculatum Brébisson ex Ralfs | 0 | 0 | 1 | P-B | - | st-str | ind | hb | - | x-o | 0.5 | m | - |
| Cosmarium formosulum Hoff | 0 | 1 | 0 | P-B | - | - | ind | - | - | o-a | 1.8 | me | - |
| Cosmarium granulatum W. West | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Cosmarium impressulum Elfving | 0 | 0 | 1 | P-B | - | - | ind | hb | - | b-o | 1.6 | m | - |
| Cosmarium perforatum P.Lundell | 0 | 1 | 0 | B | - | - | acf | - | - | - | - | m | - |
| Cosmarium sp. | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Gonatozygon monotaenium De Bary | 1 | 1 | 0 | B | - | st-str | acf | hb | - | x-b | 0.8 | me | - |
| Klebsormidium sp. | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Spirogyra sp. | 1 | 1 | 0 | B | - | - | - | - | - | - | - | - | - |
| Staurastrum gracile Ralfs | 0 | 1 | 0 | P | - | st | acf | i | - | o | 1.3 | m | - |
| Staurastrum paradoxum Meyen ex Ralfs | 0 | 0 | 1 | P | - | st | ind | i | - | - | - | ot | - |
| Zygnema sp. | 1 | 1 | 0 | B | - | - | - | - | - | o | 1.0 | - | - |
| Chlorophyta | |||||||||||||
| Actinastrum aciculare Playfair | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Actinastrum hantzschii var. gracile V.K.Tschernov | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Actinastrum hantzschii var. hantzschii Lagerh. | 1 | 1 | 1 | P-B | - | st-str | - | i | - | b | 2.3 | - | - |
| Acutodesmus acutiformis (Schröder) P.M.Tsarenko et D.M.John | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Ankistrodesmus falcatus (Corda) Ralfs | 0 | 1 | 0 | P-B | - | st-str | - | hb | - | b | 2.3 | - | - |
| Ankistrodesmus fusiformis Corda ex Korschikov | 0 | 0 | 1 | P-B | - | st-str | - | i | - | b | 2.0 | - | - |
| Ankistrodesmus arcuatus Korshikov | 1 | 1 | 1 | P-B | - | st-str | - | i | - | b | 2.1 | - | - |
| Binuclearia lauterbornii (Schmidle) Proschkina-Lavrenko | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chlamydomonas acuta Korshikov | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chlamydomonas atactogama Korshikov | 1 | 0 | 0 | P | - | - | - | i | - | - | - | - | - |
| Chlamydomonas elliptica Korshikov | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chlamydomonas globosa J.W.Snow | 1 | 0 | 0 | P,S | - | - | - | - | - | o-a | 1.9 | - | - |
| Chlamydomonas reinhardtii P.A.Dangeard | 0 | 1 | 0 | P-B | - | st-str | - | oh | - | a | 3.1 | - | - |
| Chlamydomonas sp. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chlorangiella basiannulata (Skuja) P.C.Silva | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chlorella vulgaris Beyerinck [Beijerinck] | 1 | 0 | 0 | P-B, pb,S | - | - | - | hl | - | a | 3.1 | - | - |
| Chlorotetraedron incus (Teiling) Komárek et Kováček | 0 | 1 | 1 | P-B | - | st-str | - | i | - | o-a | 1.9 | - | - |
| Chodatella subsalsa Lemmermann | 0 | 1 | 0 | P-B | - | st-str | - | - | - | b | 2.0 | - | - |
| Closteriopsis longissima (Lemmermann) Lemmermann | 1 | 0 | 0 | P | - | st-str | - | i | - | o-a | 1.8 | - | - |
| Coelastrum astroideum De Not. | 0 | 0 | 1 | P | - | st-str | - | - | - | b | 2.2 | - | - |
| Coelastrum microporum Nägeli | 1 | 1 | 1 | P-B | - | st-str | ind | i | - | b | 2.3 | - | - |
| Coelastrum pseudomicroporum Korschikov | 0 | 0 | 1 | P | - | - | - | - | - | o-a | 1.9 | - | - |
| Coelastrum sphaericum Nägeli | 1 | 1 | 0 | P-B,Ep | - | st-str | - | i | - | o-b | 1.4 | - | - |
| Coenococcus planctonicus Korschikov | 0 | 0 | 1 | P | - | - | - | - | - | - | - | - | - |
| Colemanosphaera charkowiensis (Korshikov) H.Nozaki, T.K.Yamada, F.Takahashi, R.Matsuzaki et T.Nakada | 0 | 1 | 0 | P | - | st-str | - | - | - | b | 2.2 | - | - |
| Crucigenia fenestrata (Schmidle) Schmidle | 0 | 1 | 1 | P-B,Ep | - | st-str | - | - | - | o-a | 1.8 | - | - |
| Crucigenia lauterbornei (Schmidle) Schmidle | 0 | 0 | 1 | P-B | - | st-str | - | - | - | b-o | 1.7 | - | - |
| Crucigenia quadrata Morren | 1 | 1 | 1 | P-B | - | st-str | acf | i | - | o-a | 1.9 | - | - |
| Crucigenia tetrapedia (Kirchn.) West et G.S. West | 1 | 1 | 1 | P-B,Ep | - | st-str | ind | i | - | b | 2.0 | - | - |
| Desmodesmus abundans (Kirchn.) E. Hegew. | 1 | 1 | 0 | P-B,Ep | - | st-str | - | - | - | o-a | 1.9 | - | - |
| Desmodesmus abundans var. parvus (G.M. Sm.) Bourr. | 0 | 0 | 1 | P-B,Ep | - | st-str | - | - | - | o-a | 1.9 | - | - |
| Desmodesmus aculeolatus (Reinsch) P. Tsarenko | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Desmodesmus armatus (Chodat) E. Hegew. | 0 | 0 | 1 | P-B,Ep | - | st-str | - | - | - | b | 2.2 | - | - |
| Desmodesmus bicaudatus (Dedus.) P. Tsarenko | 0 | 0 | 1 | P-B,Ep | - | - | - | - | - | b | 2.2 | - | - |
| Desmodesmus brasiliensis (Bohlin) E. Hegew. | 0 | 0 | 1 | P-B | - | st-str | - | - | - | b | 2.0 | - | - |
| Desmodesmus communis var. communis (E. Hegew.) E. Hegew. | 1 | 1 | 1 | P-B,Ep | - | st-str | ind | i | - | b | 2.15 | - | - |
| Desmodesmus costato-granulatus (Skuja) E. Hegew. | 0 | 0 | 1 | P-B | - | st-str | - | - | - | b | 2.1 | - | - |
| Desmodesmus granulatus (West et G.S. West) P. Tsarenko | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Desmodesmus hystrix (Lagerh.) E. Hegew. | 0 | 0 | 1 | P-B,Ep | - | - | - | - | - | b | 2.0 | - | - |
| Desmodesmus intermedius var. acutispinus (Y.V. Roll) E. Hegew. | 0 | 0 | 1 | P-B | - | st-str | - | - | - | b | 2.0 | - | - |
| Desmodesmus intermedius var. inflatus (Svirenko) E. Hegew. | 0 | 0 | 1 | P-B | - | st-str | - | - | - | b | 2.0 | - | - |
| Desmodesmus intermedius var. intermedius (Chodat) E. Hegew. | 1 | 1 | 1 | P-B | - | st-str | - | - | - | b | 2.0 | - | - |
| Desmodesmus intermedius var. balatonicus (Hortob.) P. Tsarenko | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Desmodesmus magnus (Meyen) P. Tsarenko | 1 | 0 | 1 | P,Ep | - | - | - | - | - | o | 1.3 | - | - |
| Desmodesmus opoliensis var. opoliensis (P. G. Richter) E. Hegew. | 0 | 0 | 1 | P-B,Ep | - | st-str | - | - | - | b | 2.2 | - | - |
| Desmodesmus protuberans (F.E. Fritsch. et Rich) E. Hegew. | 0 | 1 | 1 | P-B,Ep | - | st-str | - | - | - | - | - | - | - |
| Desmodesmus spinosus (Chodat) E. Hegew. | 0 | 0 | 1 | P-B | - | st-str | - | - | - | o-b | 1.4 | - | - |
| Desmodesmus subspicatus var. subspicatus (Chodat) E. Hegew. et A. Schmidt | 0 | 0 | 1 | P-B | - | st-str | - | - | - | o | 1.3 | - | - |
| Dictyosphaerium ehrenbergianum Nägeli | 1 | 1 | 1 | P-B,Ep | - | st-str | - | - | - | o-b | 1.5 | - | - |
| Dictyosphaerium granulatum Hindák | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Dictyosphaerium simplex Korshikov | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Elakatothrix gelatinosa Wille | 0 | 1 | 1 | P | - | st-str | - | i | - | o | 1.3 | - | - |
| Enallax costatus (Schmidle) Pascher | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Eudorina elegans Ehrenberg | 0 | 1 | 0 | P | - | st-str | - | i | - | b | 2.3 | - | - |
| Eudorina illinoisensis (Kofoid) Pascher | 0 | 1 | 0 | P | - | - | - | hl | - | b | 2.2 | - | - |
| Golenkinia radiata Chodat | 0 | 0 | 1 | P | - | st-str | - | i | - | o-a | 1.9 | - | - |
| Golenkiniopsis longispina (Korschikov) Korschikov | 1 | 0 | 0 | P-B | - | st-str | - | - | - | - | - | - | - |
| Golenkiniopsis solitaria (Korschikov) Korschikov | 0 | 0 | 1 | P-B | - | - | - | i | - | - | - | - | - |
| Gonium pectorale O. F. Müll. | 0 | 1 | 0 | P | - | st | - | i | - | a-o | 2.8 | - | - |
| Granulocystopsis decorata (Svirenko) P.M.Tsarenko | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Hindakia tetrachotoma (Printz) C. Bock, Pröschold et Krienitz | 0 | 0 | 1 | P | - | st | - | i | - | b | 2.3 | - | - |
| Hyaloraphidium contortum Pascher et Korshikov ex Korshikov | 0 | 1 | 0 | P-B | - | - | - | i | - | b | - | - | - |
| Kirchnariella sp. | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Kirchneriella irregularis (G.M.Smith) Korshikov | 1 | 0 | 0 | P-B,Ep | - | st-str | - | i | - | o-a | 1.8 | - | - |
| Kirchneriella lunaris (Kirchn.) Moeb. | 1 | 0 | 1 | P-B,Ep | - | st-str | - | i | - | o-a | 1.8 | - | - |
| Kirchneriella obesa (West) Schmidle | 1 | 1 | 0 | P-B,Ep | - | st-str | - | i | - | o-a | 1.8 | - | - |
| Koliella longiseta (Vischer) Hindák | 1 | 1 | 0 | P | - | st | - | i | - | b | 2.0 | - | - |
| Korshikoviella michailovskoensis (Elenkin) P.C.Silva | 0 | 1 | 0 | Ep | - | - | - | - | - | o-a | 1.8 | - | - |
| Korshikoviella sp. | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Lacunastrum gracillimum (West et G.S.West) H.A.McManus | 0 | 0 | 1 | P | - | - | - | - | - | b | 2.1 | - | - |
| Lagerheimia ciliata (Lagerheim) Chodat | 0 | 0 | 1 | P-B,Ep | - | st-str | - | - | - | b | 2.0 | - | - |
| Lagerheimia citriformis (Snow) Collins | 0 | 1 | 0 | P,Ep | - | st-str | - | - | - | - | - | - | - |
| Lagerheimia genevensis (Chodat) Chodat | 0 | 0 | 1 | P | - | - | - | i | - | b | 2.2 | - | - |
| Lagerheimia longiseta (Lemmermann) Printz | 0 | 1 | 1 | P-B,Ep | - | st-str | - | i | - | b | 2.1 | - | - |
| Lagerheimia wratislaviensis Schröder | 0 | 1 | 1 | P-B | - | st-str | - | - | - | b | 2.1 | - | - |
| Lemmermannia komarekii (Hindák) C.Bock et Krienitz | 0 | 0 | 1 | P-B,Ep | - | st-str | - | - | - | o-a | 1.85 | - | - |
| Lemmermannia triangularis (Chodat) C.Bock et Krienitz | 0 | 0 | 1 | P-B,Ep | - | st-str | - | - | - | b | 2.2 | - | - |
| Messastrum gracile (Reinsch) T.S.Garcia | 0 | 0 | 1 | P-B,Ep | - | st-str | - | - | - | o-a | 1.9 | - | - |
| Micractinium pusillum Fresen. | 0 | 1 | 1 | P-B,Ep | - | st-str | - | - | - | a-o | 2.6 | - | - |
| Micractinium quadrisetum (Lemmermann) G.M.Smith | 0 | 1 | 0 | P | - | st-str | - | - | - | - | - | - | - |
| Monactinus simplex (Meyen) Corda | 0 | 0 | 1 | P-B,Ep | - | st-str | - | - | - | b | 2.0 | - | - |
| Monoraphidium contortum (Thuret) Komárková-Legnerová | 1 | 1 | 1 | P-B | - | st-str | - | i | - | b | 2.2 | - | - |
| Monoraphidium griffithii (Berkeley) Komárková-Legnerová | 1 | 1 | 1 | P-B | - | st-str | - | i | - | b | 2.2 | - | - |
| Monoraphidium irregulare (G.M.Smith) Komárková-Legnerová | 0 | 1 | 1 | P-B | - | st-str | - | i | - | - | - | - | - |
| Monoraphidium minutum (Nägeli) Komárková-Legnerová | 0 | 0 | 1 | P,Ep | - | st-str | - | i | - | b-a | 2.5 | - | - |
| Monoraphidium mirabile (West & G.S.West) Pankow | 1 | 0 | 0 | P,Ep | - | st | - | oh | - | b-a | 2.5 | - | - |
| Mucidosphaerium pulchellum (H.C.Wood) C. Bock, Proschold et Krienitz | 1 | 1 | 1 | P-B | - | st-str | ind | i | - | b | 2.3 | - | - |
| Neglectella solitaria (Wittrock) Stenclová et Kastovsky | 0 | 1 | 1 | P-B | - | st | ind | i | - | b-o | 1.7 | - | - |
| Oocystidium ovale Korshikov | 0 | 1 | 0 | P | - | st | - | - | - | - | - | - | - |
| Oocystis borgei J.W.Snow | 1 | 1 | 0 | P-B,S | - | st-str | ind | i | - | o-a | 1.9 | - | - |
| Oocystis lacustris Chodat | 0 | 0 | 1 | P-B,Ep | - | st-str | - | hl | - | b-o | 1.7 | - | - |
| Oocystis sp. | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Oocystis submarina Lagerheim | 0 | 1 | 0 | P-B,Ep | - | st | - | i | - | - | - | - | - |
| Pandorina morum (O.F.Müller) Bory de Saint-Vincent | 1 | 1 | 0 | P | - | st | - | i | - | b | 2.3 | - | - |
| Pectinodesmus pectinatus (Meyen) E.Hegewald, M.Wolf, Al.Keller, Friedl et Krienitz | 1 | 1 | 1 | P-B | - | st-str | - | - | - | - | - | - | - |
| Pediastrum duplex var. duplex Meyen | 0 | 1 | 1 | P | - | st-str | ind | i | - | b | 2.1 | - | - |
| Phacotus coccifer Korshikov | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Pseudodidymocystis planctonica (Korshikov) E.Hegewald et Deason | 0 | 1 | 0 | P-B,Ep | - | - | - | - | - | o-a | 1.8 | - | - |
| Pseudopediastrum boryanum var. boryanum (Turpin) E. Hegew. | 1 | 1 | 1 | P-B | - | st-str | ind | i | - | b | 2.1 | - | - |
| Pseudopediastrum boryanum var. cornutum (Racib.) P. Tsarenko | 0 | 0 | 1 | P-B | - | - | - | - | - | - | - | - | - |
| Pseudopediastrum boryanum var. longicorne (Reinsch) P. Tsarenko | 0 | 0 | 1 | P-B | - | st-str | - | - | - | b | 2.1 | - | - |
| Pseudopediastrum kawraiskyi (Schmidle) E. Hegew. | 0 | 1 | 0 | P | - | - | - | - | - | o-b | 1.4 | - | - |
| Pseudoschroederia robusta (Korshikov) E.Hegewald et E.Schnepf | 0 | 1 | 0 | P-B | - | st-str | - | i | - | o-a | 1.9 | - | - |
| Pteromonas aculeata Lemmermann | 0 | 1 | 0 | P | - | - | - | - | - | b | 2.2 | - | - |
| Quadricoccus ellipticus Hortob. | 0 | 0 | 1 | P | - | - | - | - | - | - | - | - | - |
| Radiococcus polycoccus (Korshikov) I.Kostikov, T.Darienko, A.Lukesová et L.Hoffmann | 0 | 1 | 1 | P | - | - | - | i | - | - | - | - | - |
| Raphidocelis sigmoidea Hindak | 0 | 0 | 1 | P | - | st-str | - | - | - | - | - | - | - |
| Raphidocelis subcapitata (Korshikov) Nygaard, Komárek, J.Kristiansen et O.M.Skulberg | 0 | 1 | 0 | P-B,Ep | - | st-str | - | i | - | o-b | 1.5 | - | - |
| Rhaphoneis amphiceros (Ehrenberg) Ehrenberg | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Scenedesmus arcuatus (Lemmermann) Lemmermann | 0 | 1 | 1 | P-B | - | st-str | - | i | - | o-a | 1.9 | - | - |
| Scenedesmus ellipticus Corda | 1 | 1 | 1 | P-B,S | - | st-str | - | - | - | b-o | 1.7 | - | - |
| Scenedesmus obtusus Meyen | 0 | 0 | 1 | P-B | - | st-str | - | - | - | o-a | 1.8 | - | - |
| Scenedesmus obtusus var. apiculatus (West et G.S. West) P. Tsarenko | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Scenedesmus papillosum Pankow | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Scenedesmus sp. | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Schroederia setigera (Schröd.) Lemmermann | 0 | 1 | 1 | P | - | st-str | - | i | - | b-o | 1.7 | - | - |
| Schroederia spiralis (Printz) Korshikov | 0 | 1 | 0 | P-B,Ep | - | - | - | - | - | o-a | 1.8 | - | - |
| Siderocelis ornata (Fott) Fott | 1 | 1 | 0 | P-B,Ep | - | st-str | - | i | - | b | 2.2 | - | - |
| Siderocystopsis punctifera (Boloch.) E. Hegew. et Schnepf | 0 | 1 | 0 | P-B | - | st-str | - | i | - | - | - | - | - |
| Sphaerocystis planctonica (Korschikov) Bourr. | 0 | 0 | 1 | P-B | - | - | - | i | - | - | - | - | - |
| Sphaerocystis schroeteri Chodat | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Stauridium tetras (Ehrenberg) E. Hegew. | 1 | 1 | 1 | P-B | - | st-str | ind | i | - | b | 2.1 | - | - |
| Tetradesmus lagerheimii M.J.Wynne et Guiry | 1 | 1 | 1 | P-B | - | st-str | ind | i | - | b | 2.15 | - | - |
| Tetradesmus obliquus (Turpin) M.J.Wynne | 0 | 1 | 1 | - | - | - | - | - | - | - | - | - | - |
| Tetraedron caudatum (Corda) Hansg. | 0 | 1 | 1 | P-B | - | st-str | ind | i | - | b | 2.0 | - | - |
| Tetraedron minimum var. minimum f. minimum (A. Braun) Hansg. | 1 | 1 | 1 | P-B,Ep | - | st-str | - | i | - | b | 2.1 | - | - |
| Tetraedron triangulare Korschikov | 0 | 0 | 1 | P-B,Ep | - | st-str | - | i | - | b | 2.0 | - | - |
| Tetrastrum glabrum (Y.V.Roll) Ahlstrom & Tiffany | 1 | 1 | 0 | P | - | - | ind | i | - | - | - | - | - |
| Tetrastrum staurogeniaeforme (Schröd.) Lemmermann | 1 | 1 | 1 | P-B,Ep | - | st-str | - | i | - | b | 2.2 | - | - |
| Treubaria triappendiculata C. Bernard | 0 | 0 | 1 | P-B,Ep | - | st-str | - | - | - | - | - | - | - |
| Ulothrix zonata (Weber & Mohr) Kützing | 1 | 0 | 0 | P-B | - | st-str | ind | i | - | o-a | 1.8 | - | - |
| Volvox polychlamys Korshikov | 0 | 1 | 0 | P | - | - | - | hb | - | - | - | - | - |
| Westella botryoides (W. West) De Wild. | 0 | 0 | 1 | P | - | st-str | - | - | - | o-a | 1.8 | - | - |
| Willea apiculata (Lemmermann) D.M.John, M.J.Wynne et P.M.Tsarenko | 0 | 1 | 1 | P-B,Ep | - | st-str | - | - | - | b | 2.2 | - | - |
| Willea rectangularis (A.Braun) D.M. John, M.J. Wynne et P. Tsarenko | 1 | 1 | 0 | P | - | - | ind | i | - | b | 2.1 | - | - |
| Willea irregularis (Wille) Schmidle | 1 | 0 | 0 | P-B | - | st-str | ind | i | - | - | - | - | - |
| Cryptista | |||||||||||||
| Cryptomonas erosa Ehrenberg | 0 | 1 | 0 | P | - | st-str | - | - | - | b | 2.3 | - | - |
| Cryptomonas rostrata Skuja | 0 | 1 | 0 | P | - | - | - | - | - | o-a | 1.8 | - | - |
| Cryptomonas sp. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Cyanobacteria | |||||||||||||
| Anabaena sphaerica f. conoidea Elenkin | 0 | 1 | 0 | P,S | - | - | - | - | - | o-b | 1.5 | - | - |
| Anabaenopsis arnoldii Aptekar | 1 | 1 | 0 | P-B | - | st-str | - | - | - | b-o | 1.7 | me | - |
| Anabaenopsis elenkinii V.V.Miller | 0 | 1 | 0 | P-B | - | st | - | - | - | o-b | 1.5 | me | - |
| Anabaenopsis raciborskii Woloszynska | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Anagnostidinema amphibium (C.Agardh ex Gomont) Struneckỳ, Bohunická, J.R. Johansen et Komárek Gomont | 0 | 0 | 1 | P-B,S | - | st-str,H2S | - | hl | - | a-o | 2.6 | m | - |
| Aphanizomenon flosaquae (L.) Ralfs ex Bornet et Flahault | 1 | 1 | 1 | P | - | - | - | hl | - | o-a | 1.95 | m | - |
| Aphanocapsa planctonica (G.M. Sm.) Komárek et Anagn. | 1 | 1 | 1 | P | - | - | - | i | - | - | - | ot | - |
| Chroococcus minutus (Kützing) Nägeli | 1 | 0 | 1 | P-B | - | - | ind | i | - | o-a | 1.8 | o-m | - |
| Cuspidothrix issatschenkoi (Usačev) Rajaneimi et al. | 1 | 0 | 1 | P | - | - | - | - | - | b | 2.3 | me | - |
| Dactylococcopsis rhaphidioides f. falciformis Printz | 0 | 1 | 0 | P | - | st-str | - | - | - | - | - | - | - |
| Dactylococcopsis rhaphidioides f. pannonica (Hortob.) Hollerbach | 0 | 1 | 0 | P | - | st-str | - | - | - | - | - | - | - |
| Dactylococcopsis rhaphidioides Hansgirg | 0 | 1 | 0 | P | - | st-str | - | - | - | - | - | - | - |
| Dolichospermum flos-aquae (Lyngb.) Wacklin, Hoffmann et Komarek | 0 | 1 | 1 | P | - | st | - | i | - | b | 2.0 | e | - |
| Dolichospermum sp. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Dolichospermum spiroides (Klebanh) Wackkin, Hoffmann et Komarek | 0 | 1 | 0 | P | - | st-str | - | i | - | b | 2.0 | e | - |
| Gloeocapsa gelatinosa Kützing | 0 | 0 | 1 | B | warm | - | - | - | - | - | - | - | - |
| Gloeocapsa minor (Kutzing) Hollerbach | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Gloeocapsa punctata Nägeli | 0 | 0 | 1 | Ep,S | - | ae | - | hl | - | - | - | - | - |
| Gloeocapsa sp. | 0 | 1 | 1 | - | - | - | - | - | - | - | - | - | - |
| Jaaginema geminatum (Schwabe ex Gomont) Anagnostidis et Komárek | 1 | 1 | 0 | P-B | warm | st | - | i | - | - | - | - | - |
| Jaaginema quadripunctulata (Bruhl. Eet Biswas) Anagn. et Komarek | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Leibleinia subtilis (Holden) Anagnostidis et Komárek | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Limnothrix planctonica (Wołosz.) Meffert | 0 | 0 | 1 | P | - | - | - | i | - | o-b | 1.5 | me | - |
| Lyngbya confervoides C.Agardh ex Gomont | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Merismopedia convoluta Brébisson ex Kützing | 0 | 1 | 0 | P-B | - | - | - | - | - | - | - | - | - |
| Merismopedia glauca (Ehrenberg) Kützing | 1 | 1 | 0 | P-B | - | - | ind | i | - | b-o | 1.75 | o-m | - |
| Merismopedia minima Beck | 1 | 1 | 0 | B,S | - | ae | - | - | - | - | - | ot | - |
| Merismopedia sp. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Merismopedia tenuissima Lemmermann | 1 | 1 | 1 | P-B | - | - | - | hl | - | b-a | 2.4 | e | - |
| Merismopedia warmingiana Lagerh. | 0 | 0 | 1 | P | - | - | - | - | - | - | - | e | - |
| Merismopedia tranquilla (Ehrenberg) Trevisan | 0 | 0 | 1 | P-B | - | - | ind | i | - | o-a | 1.8 | me | - |
| Microcystis aeruginosa (Kützing) Kützing | 1 | 1 | 1 | P | - | - | - | hl | - | b | 2.1 | e | - |
| Microcystis firma (Bréb. et Lenorm.) Schmidle | 0 | 0 | 1 | P | - | - | - | - | - | o-b | 1.5 | - | - |
| Microcystis flosaquae (Wittr.) Kirchn. emend. Wesenb.-Lund. | 0 | 0 | 1 | P | - | - | - | i | - | b | 2.0 | e | - |
| Microcystis pulverea (Wood) Forti | 0 | 0 | 1 | P-B,S | - | - | - | i | - | o-b | 1.5 | - | - |
| Microcystis viridis (A.Braun) Lemmermann | 0 | 0 | 1 | P | - | - | - | - | - | b | 2.2 | e | - |
| Microcystis wesenbergii (Komárek) Komárek | 0 | 0 | 1 | P | - | - | - | - | - | o-a | 1.9 | e | - |
| Oscillatoria limosa C.Agardh ex Gomont | 1 | 1 | 1 | P-B | - | st-str | - | hl | - | b | 2.3 | e | - |
| Oscillatoria limosa f. laeteaeruginosa Kützing ex Elenkin | 0 | 1 | 0 | P-B,S | - | st-str | - | - | - | - | - | - | - |
| Oscillatoria margaritifera Kützing ex Gomont | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Oscillatoria planctonica Woloszynska | 1 | 1 | 1 | P | - | - | - | i | - | o-b | 1.5 | me | - |
| Oscillatoria princeps Vaucher ex Gomont | 0 | 0 | 1 | P-B,S | - | st-str | - | - | - | a-o | 2.8 | o-m | - |
| Oscillatoria punctata Corda | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Oscillatoria sp. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Oscillatoria tenuis C.Agardh ex Gomont | 1 | 0 | 1 | P-B,S | - | st-str | - | hl | - | a-o | 2.6 | me | - |
| Oscillatoria terebriformis f. amphigranulata Elenkin et Kossinskaja | 0 | 1 | 0 | B,S | eterm | st-str | - | - | - | b-p | 2.9 | - | - |
| Oscillatoria ucrainica Vladimirova | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Phormidium chalybeum (Mertens ex Gomont) Anagnostidis et Komárek | 0 | 1 | 0 | P-B,S | - | st-str | - | - | - | a | 3.3 | e | - |
| Phormidium nigroviride (Thwaites ex Gomont) Anagnostidis et Komárek | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Phormidium nigrum (Vaucher ex Gomont) Anagnostidis et Komárek | 1 | 0 | 0 | P-B | warm | - | - | - | - | b | 2.2 | m | - |
| Phormidium solitare (Kützing ex Gomont) Anagnostidis et Komárek | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Phormidium thwaitesii I.Umezaki & M.Watanabe | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Planktothrix agardhii (Gomont) Anagnostidis et Komárek | 1 | 1 | 0 | P-B | - | st | - | hl | - | b | 2.2 | e | - |
| Pleurocapsa minor Hansgirg | 1 | 1 | 0 | B | - | st-str | - | - | - | x-b | 0.9 | ot | - |
| Pleurocapsa minuta Geitler | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Rhabdogloea elenkinii (Y.V. Roll) Komárek et Anagn. | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Rivularia sp. | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Snowella lacustris (Chodat) Komárek et Hindák | 0 | 1 | 1 | P | - | - | - | i | - | b-o | 1.6 | me | - |
| Spirulina adriatica Hansgirg | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Spirulina laxissima G.S.West | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Spirulina sp. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Woronichinia compacta (Lemmermann) Komárek et Hindák | 0 | 0 | 1 | P-B | - | - | - | - | - | - | - | o-m | - |
| Miozoa | |||||||||||||
| Apocalathium aciculiferum (Lemmermann) Craveiro, Daugbjerg, Moestrup & Calado | 0 | 0 | 1 | P | - | - | - | - | - | o-b | 1.5 | - | - |
| Biceratium furca (Ehrenberg) Vanhoeffen | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Ceratium fusus (Ehrenberg) Dujardin | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Ceratium hirundinella (O.F.Müller) Dujardin | 0 | 0 | 1 | P | - | st-str | - | i | - | o | 1.3 | - | - |
| Ceratium tripos (O.F.Müller) Nitzsch | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Dinophysis saccula Stein | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Diplosalis acuta (Apstein) Entz | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Diplosalis acuta var. halophila Er. Lindem. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Ellobiopsis chattonii Caullery | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Glenodinium paululum Lindernann | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Glenodinium rotundatum Skvortzov | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Glenodinium sp. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Glochidinium pernardiforme (Lindemann) Boltovskoy | 0 | 0 | 1 | - | - | - | - | - | - | o-b | 1.4 | - | - |
| Gonyaulax apiculata (Pénard) Entz | 0 | 1 | 0 | - | - | - | - | - | - | o | 1.1 | - | - |
| Gonyaulax polygramma Stein | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Gymnodinium paradoxum A.J. Schill | 0 | 0 | 1 | P | - | - | - | - | - | o-b | 1.5 | - | - |
| Gymnodinium sp. | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Lingulodinium polyedra (F.Stein) J.D.Dodge | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Noctiluca scintillans (Macartney) Kofoid & Swezy | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Peridiniella danica (Paulsen) Y.B.Okolodkov & J.D.Dodge | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Peridiniopsis penardiformis (Lindemann) Bourrelly | 0 | 1 | 0 | P | - | st | - | - | - | o-b | 1.4 | - | - |
| Peridiniopsis thompsonii Bourrelly | 1 | 1 | 0 | P | - | - | - | - | - | - | - | - | - |
| Peridinium cinctum (O.F.Müller) Ehrenberg | 0 | 1 | 0 | P-B | - | st-str | - | i | - | b-o | 1.6 | - | - |
| Peridinium sp. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Prorocentrum balticum (Lohmann) Loeblich | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Prorocentrum cordatum (Ostenfeld) Dodge | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Prorocentrum lima (Ehrenberg) F.Stein | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Prorocentrum micans Ehrenberg | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Protoperidinium bipes (Paulsen) Balech | 1 | 0 | 0 | P | - | st-str | - | oh | - | o | 1.3 | - | - |
| Protoperidinium crassipes (Kofoid) Balech | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Protoperidinium decipiens (Jörgensen) Parke & Dodge | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Protoperidinium depressum (Bailey) Balech | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Protoperidinium divergens (Ehrenberg) Balech | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Protoperidinium granii (Ostenfeld) Balech | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Protoperidinium ovatum Pouchet | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Protoperidinium pallidum (Ostenfeld) Balech | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Protoperidinium quarnerense (B.Schröder) Balech | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Protoperidinium steinii (Jørgensen) Balech | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Protoperidinium knipowitschii (Usachev) Balech | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Pyrophacus horologicum Stein | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Scrippsiella acuminata (Ehrenberg) Kretschmann, Elbrächter, Zinssmeister, S.Soehner, Kirsch, Kusber & Gottschling | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Unruhdinium penardii (Lemmermann) Gottschling | 0 | 1 | 0 | P | - | - | - | hl | - | o | 1.3 | - | - |
| Euglenozoa | |||||||||||||
| Astasia dangeardii Lemmermann | 1 | 0 | 0 | P-B | warm | st | ind | - | - | p | 4.0 | - | - |
| Astasia sp. | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Euglena geniculata Dujardin | 1 | 0 | 0 | P-B | eterm | st-str | alf | - | - | a | 3.4 | - | - |
| Euglena gracilis G.A. Klebs | 0 | 0 | 1 | P-B | eterm | st | ind | oh | - | b | 2.25 | - | - |
| Euglena granulata (G.A. Klebs) Schmitz | 1 | 1 | 0 | P-B | eterm | st-str | ind | mh | - | a-o | 2.75 | - | - |
| Euglena minima Francé | 0 | 1 | 0 | P-B | eterm | st | alb | mh | - | b | 2.2 | - | - |
| Euglena sp. | 1 | 1 | 1 | - | - | - | - | - | - | - | - | - | - |
| Euglena viridis Perty | 1 | 1 | 1 | P-B,S | eterm | st-str | ind | mh | - | i | 4.0 | - | - |
| Euglena agilis H.J.Carter | 0 | 1 | 0 | P-B | eterm | st-str | alf | mh | - | a | 3.0 | - | - |
| Eutreptia lanowii Steuer | 1 | 0 | 0 | - | - | - | - | mh | - | - | - | - | - |
| Lepocinclis ovum var. ovum (Ehrenberg) Lemmermann | 0 | 0 | 1 | P | eterm | st | ind | i | - | b-a | 2.4 | - | - |
| Lepocinclis sp. | 0 | 0 | 1 | P | warm | st | alf | - | - | - | - | - | - |
| Lepocinclis spirogyra Korshikov | 0 | 1 | 0 | P-B | - | - | - | - | - | b-a | 2.4 | - | - |
| Lepocinclis steinii Lemmermann | 0 | 1 | 0 | P | eterm | st | ind | i | - | b | 2.2 | - | - |
| Lepocinclis acus (O.F.Müller) B.Marin & Melkonian | 1 | 1 | 1 | P | eterm | st | ind | i | - | b | 2.2 | - | - |
| Lepocinclis caudata (A.M.Cunha) Pascher | 1 | 1 | 1 | P-B | warm | st-str | ind | mh | - | a-o | 2.8 | - | - |
| Lepocinclis gracillimoides B.Zakrys & K.Chaber | 0 | 1 | 0 | P | - | - | - | - | - | a-o | 2.7 | - | - |
| Lepocinclis oxyuris (Schmarda) B.Marin & Melkonian | 0 | 1 | 0 | P-B | - | st-str | ind | mh | - | a-o | 2.6 | - | - |
| Lepocinclis oxyuris var. skvortzovii (T.G.Popova) Taşkin & Alp | 0 | 1 | 0 | P | - | st-str | acf | - | - | a-o | 2.7 | - | - |
| Monomorphina pyrum (Ehrenberg) Mereschkowsky | 1 | 0 | 0 | P | eterm | st-str | ind | mh | - | b | 2.35 | - | - |
| Phacus caudatus Hübner | 0 | 0 | 1 | P-B | eterm | st-str | alf | i | - | b | 2.3 | - | - |
| Phacus longicauda var. longicauda f. longicauda (Ehrenberg) Dujard. | 0 | 1 | 0 | P-B | - | st | ind | i | - | a-o | 2.8 | - | - |
| Phacus pleuronectes (Ehrenberg) Dujard. | 0 | 1 | 0 | P-B | - | st-str | ind | i | - | a-o | 2.7 | - | - |
| Phacus sp. | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Phacus limnophilus (Lemmermann) E.W.Linton & Karnkowska | 0 | 1 | 0 | P-B | eterm | st-str | - | - | - | o-b | 1.5 | - | - |
| Phacus tortus (Lemmermann) Skvortsov | 1 | 1 | 0 | P-B | - | st-str | ind | i | - | a-o | 2.7 | - | - |
| Strombomonas acuminata (Schmarda) Deflandre | 0 | 1 | 1 | P | - | st-str | ind | i | - | b | 2.2 | - | - |
| Strombomonas fluviatilis (Lemmermann) Deflandre | 0 | 1 | 0 | P-B | eterm | st-str | ind | i | - | b | 2.25 | - | - |
| Trachelomonas armata (Ehrenberg) F.Stein | 0 | 1 | 0 | - | - | - | - | - | - | b | 2.1 | - | - |
| Trachelomonas hispida var. hispida (Perty) F.F. Stein | 1 | 1 | 0 | P-B | eterm | st-str | - | i | - | b | 2.2 | - | - |
| Trachelomonas intermedia f. intermedia P.A. Dang. | 1 | 0 | 0 | P-B | eterm | - | - | i | - | b | 2.2 | - | - |
| Trachelomonas nigra Svirenko | 0 | 0 | 1 | P | cool | st-str | - | hl | - | b | 2.2 | - | - |
| Trachelomonas oblonga Lemmermann | 1 | 0 | 0 | P | eterm | st-str | - | i | - | b-a | 2.4 | - | - |
| Trachelomonas sp. | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Trachelomonas volvocina var. volvocina Ehrenberg | 1 | 1 | 1 | B | eterm | st-str | ind | i | - | b | 2.0 | - | - |
| Ochrophyta (Chrysophyceae) | |||||||||||||
| Dinobryon divergens O.E. Imhof | 0 | 1 | 0 | P | - | st-str | ind | i | - | o-b | 1.45 | - | - |
| Dinobryon sertularia Ehrenberg | 1 | 0 | 0 | P | - | - | - | i | - | o | 1.3 | - | - |
| Kephirion sp. | 0 | 0 | 1 | B | - | - | - | - | - | o-b | 1.5 | - | - |
| Synochromonas gracilis Korshikov | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Synochromonas pallida Korshikov | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Ochrophyta (Xanthophyceae) | |||||||||||||
| Goniochloris fallax Fott | 0 | 1 | 0 | P | - | st-str | - | - | - | b | 2.1 | - | - |
| Goniochloris smithii (Bourr.) Fott | 0 | 0 | 1 | P,S | - | st-str | - | hb | - | b-o | 1.7 | - | - |
| Goniochloris spinosa Pascher | 0 | 0 | 1 | P | - | - | - | - | - | o-a | 1.8 | - | - |
| Ophiocytium capitatum Wolle | 0 | 1 | 1 | P | - | st | - | oh | - | o | 1.2 | - | - |
| Pseudostaurastrum subglobosum (Pascher) Bourrelly | 0 | 1 | 0 | - | - | - | - | - | - | x-b | 0.9 | - | - |
| Tribonema affine (Kützing) G.S.West | 1 | 0 | 0 | B | - | - | - | hb | - | x-b | 0.8 | - | - |
| Total: 613 | 259 | 295 | 289 |
| Variable | Stage I | Stage II | Stage III |
|---|---|---|---|
| Phylum | |||
| Bacillariophyta | 132 | 127 | 145 |
| Chlorophyta | 41 | 75 | 84 |
| Cyanobacteria | 28 | 37 | 30 |
| Euglenozoa | 17 | 22 | 11 |
| Miozoa | 33 | 12 | 4 |
| Charophyta | 5 | 13 | 9 |
| Ochrophyta (Xanthophyceae) | 1 | 3 | 3 |
| Cryptista | 1 | 3 | 0 |
| Ochrophyta (Chrysophyceae) | 1 | 3 | 1 |
| No. of Species | 259 | 295 | 289 |
| Habitat | |||
| P | 33 | 59 | 49 |
| P-B | 75 | 114 | 128 |
| B | 37 | 46 | 64 |
| Ep | 0 | 1 | 1 |
| Oxygen and water moving | |||
| H2S | 0 | 0 | 1 |
| st | 14 | 30 | 25 |
| st-str | 72 | 126 | 131 |
| str | 5 | 3 | 12 |
| aer | 1 | 2 | 1 |
| Water pH | |||
| acf | 2 | 7 | 3 |
| ind | 36 | 59 | 54 |
| alf | 41 | 55 | 80 |
| alb | 5 | 6 | 5 |
| Water salinity | |||
| hb | 2 | 5 | 5 |
| i | 79 | 126 | 139 |
| hl | 26 | 31 | 30 |
| mh | 21 | 13 | 10 |
| Nitrogen metabolism | |||
| ats | 2 | 12 | 22 |
| ate | 26 | 37 | 45 |
| hne | 3 | 4 | 9 |
| hce | 1 | 2 | 7 |
| Class of Water Quality | |||
| Class I | 2 | 6 | 2 |
| Class II | 25 | 44 | 58 |
| Class III | 73 | 112 | 127 |
| Class IV | 21 | 27 | 25 |
| Trophic state | |||
| ot | 3 | 5 | 5 |
| o-m | 7 | 19 | 31 |
| m | 7 | 9 | 11 |
| me | 25 | 37 | 38 |
| e | 16 | 23 | 39 |
| o-e | 4 | 3 | 7 |
| he | 0 | 1 | 4 |
| Temperature | |||
| cool | 3 | 4 | 3 |
| eterm | 10 | 13 | 10 |
| temp | 17 | 21 | 29 |
| warm | 4 | 3 | 5 |
| Environment | |||
| Water exchange period, day | 505 | 163 | 312 |
| Inflow through the cannel, mln m3 year−1 | 0 | 765 | 399 |
| Inflow of water from the sea, mln m3 year−1 | 142 | 0 | 0 |
| Evaporation, mln m3 year−1 | 173 | 156 | 193 |
| Cl-, mg dm−3 | 7790 | 603.5 | 491 |
References
- Ellis, E.C. Anthropogenic transformation of the terrestrial biosphere. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 1010–1035. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.C.; Wright, D.K.; Ivory, S.J.; Choi, J.H.; Nightingale, S.; Mackay, A.; Schilt, F.; Otárola-Castillo, E.; Mercader, J.; Forman, S.L.; et al. Early human impacts and ecosystem reorganization in southern-central Africa. Sci. Adv. 2021, 5, eabf9776. [Google Scholar] [CrossRef] [PubMed]
- Pichura, V.I.; Malchykova, D.S.; Ukrainskij, P.A.; Shakhman, I.A.; Bystriantseva, A.N. Anthropogenic Transformation of Hydrological Regime of The Dnieper River. Indian J. Ecol. 2018, 45, 445–453. [Google Scholar]
- Gâştescu, P. Razim-Sinoie lake complex, Romania. In Encyclopedia of Hydrology and Lakes; Encyclopedia of Earth Science; Springer: Dordrecht, The Netherlands, 1998. [Google Scholar] [CrossRef]
- Ng, P.X.; Tan, H.H. Fish diversity before and after construction of the Punggol and Serangoon reservoirs, Singapore. Nat. Singap. 2013, 6, 19–24. [Google Scholar]
- Toh, K.B.; Ng, C.S.L.; Leong, W.-K.G.; Jaafar, Z.; Chou, L.M. Assemblages and diversity of fishes in Singapore’s marinas. Raffles Bull. Zool. 2016, 32, 85–94. [Google Scholar]
- Sasyk Lake. Available online: http://www.ramsar.org/sasyk-lake (accessed on 28 November 2022).
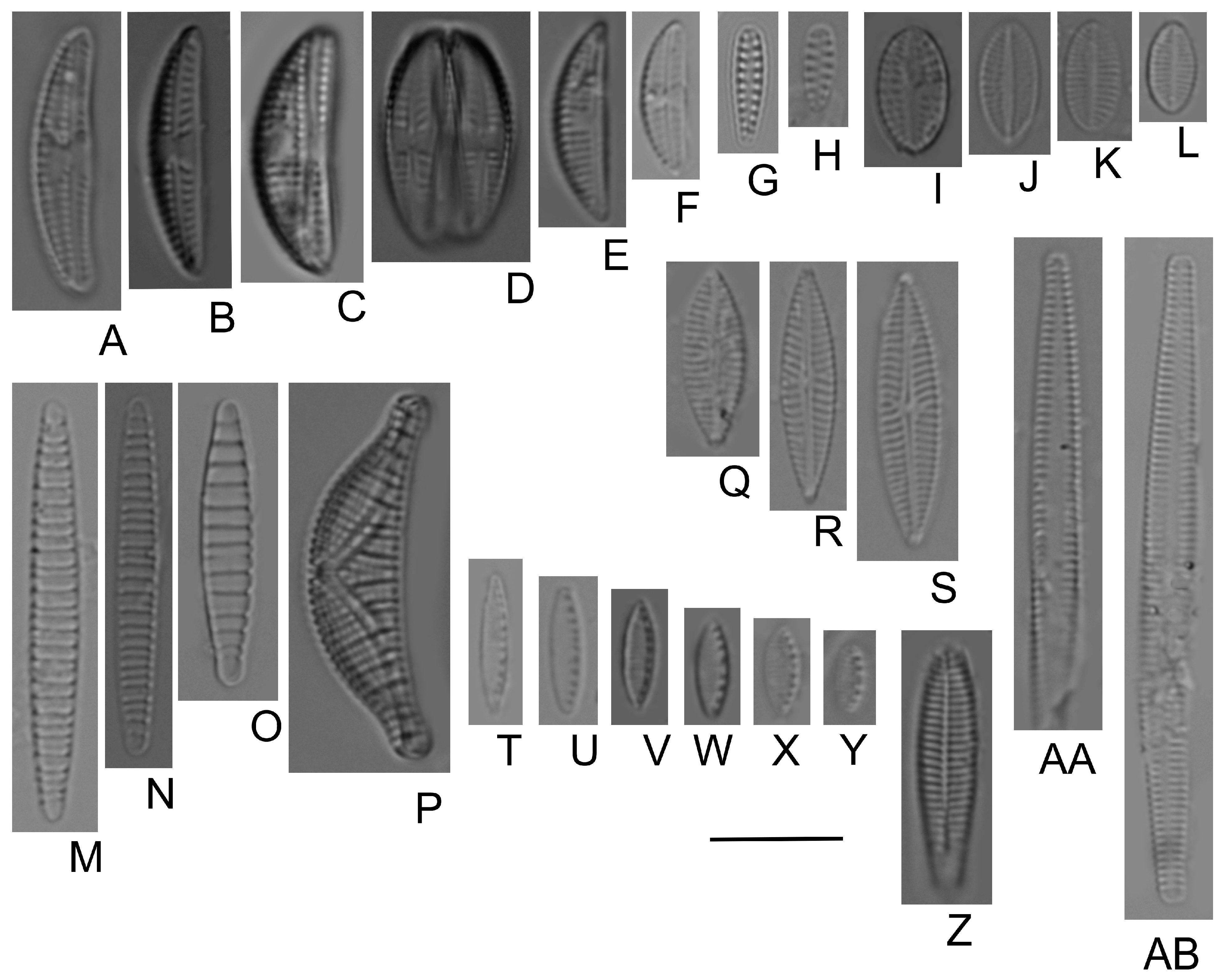
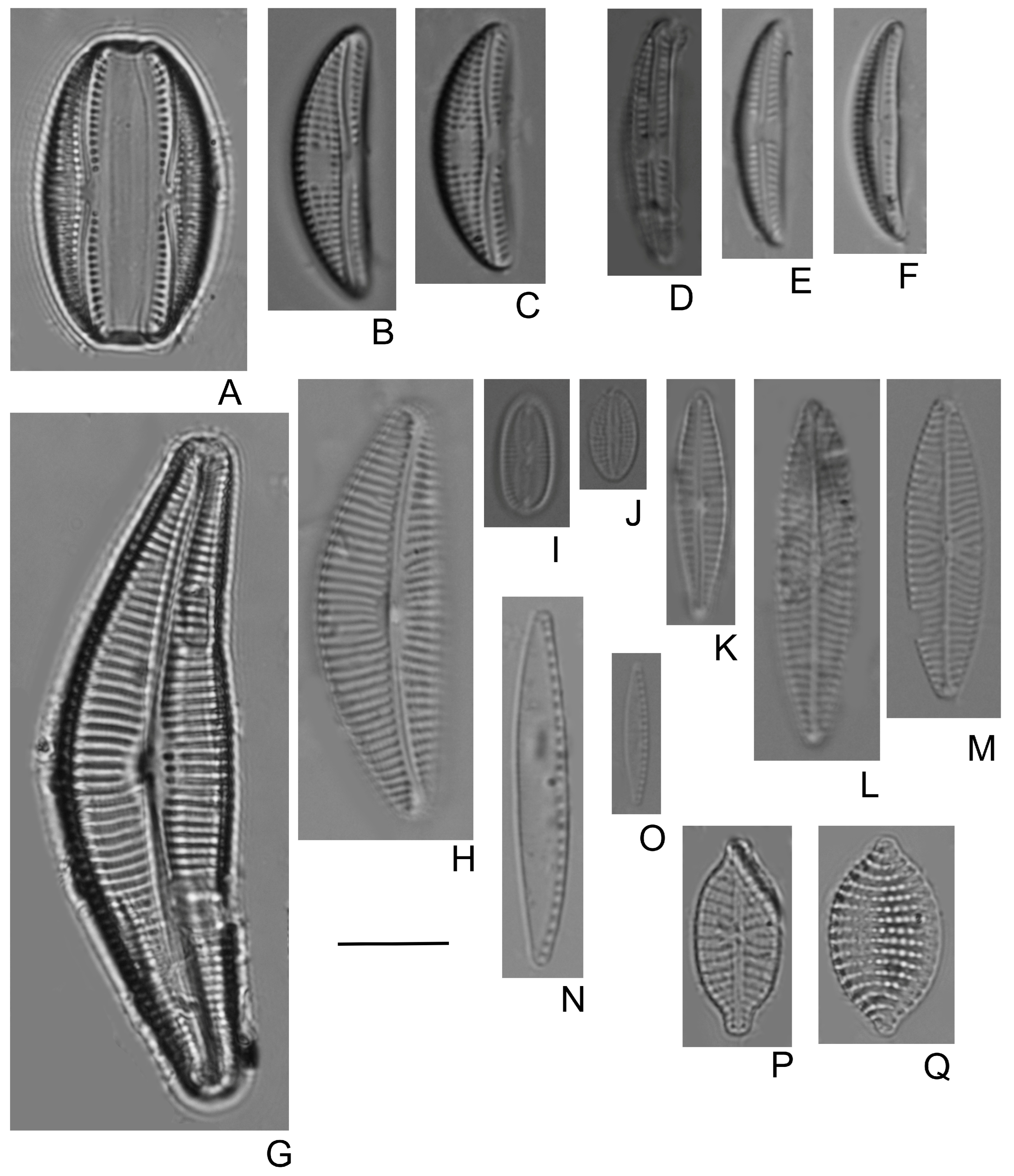
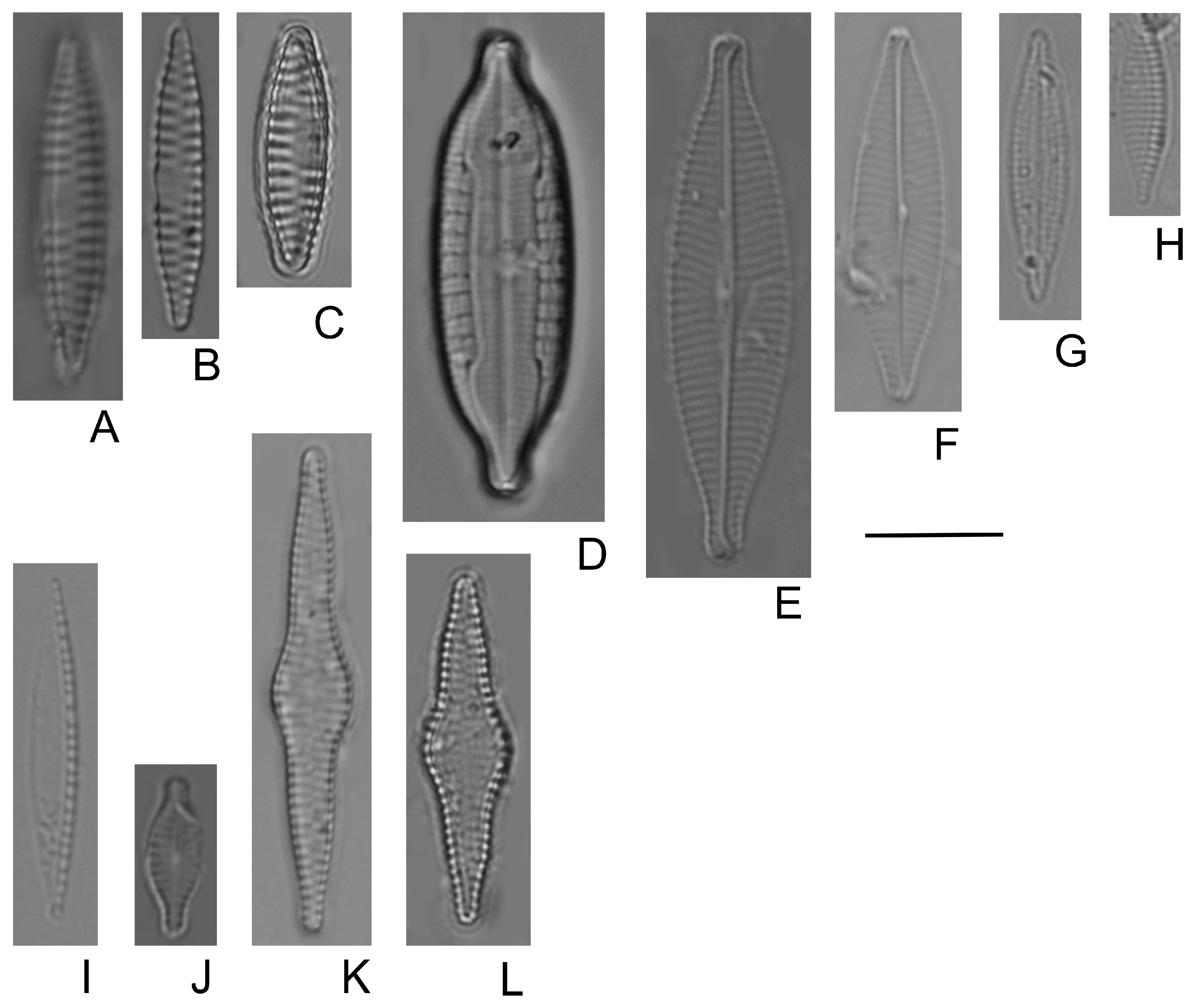
- Bilous, O.P.; Wojtal, A.Z.; Ivanova, N.O.; Tsarenko, P.M.; Burova, O.V.; Barinova, S. Benthic Diatom Composition in Coastal Zone of Black Sea, Sasyk Reservoir (Ukraine). Diversity 2020, 12, 458. [Google Scholar] [CrossRef]
- Ivanova, N.O. Transformation of the hydrological regime of the water body as a factor of discreteness of the ecosystem (on the example of the Sasyk reservoir). In Proceedings of the XVI International Scientific Conference Monitoring of Geological Processes and Ecological Condition of the Environment, Kyiv, Ukraine, 15–18 November 2022; ESI “Institute of Geology” of Taras Shevchenko National University of Kyiv: Kyiv, Ukraine, 2022; p. 5. [Google Scholar]
- Medinets, V.I.; Cherkez, E.A.; Pavlik, T.V.; Shatalin, S.M.; Kozlova, T.V.; Kuzmenko, A.Y.; Kuzmenko, O.Y.; Medinets, S.V.; Soltys, I.E. Long-Term Changes of the Shoreline Dynamics in the Ukrainian North-Western Black Sea During 1980–2020. In Proceedings of the 16th International Conference Monitoring of Geological Processes and Ecological Condition of the Environment, Kyiv, Ukraine, 15–18 November 2022; European Association of Geoscientists & Engineers: Dubai, United Arab Emirates, 2022; Volume 2022, pp. 1–5. [Google Scholar] [CrossRef]
- Kharchenko, T.A.; Timchenko, V.I.; Ivanov, A.I.; Kolesnik, M.P.; Novikov, B.I.; Enaki, I.G.; Ryabov, A.K.; Sirenko, L.A.; Klokov, V.M.; Bashmakova, I.C.; et al. Bioproductivnost’ i kachestvo vody Sasykskogo vodokhranilischa v usloviyakh ego opresneniya. In Bioproductivity and Water Quality Conditions in the Sasyk Reservoir on Its Desalination; Braginsky, L.P., Ed.; Nauk. Dumka: Kiev, Ukraine, 1990; 276p. (In Russian) [Google Scholar]
- Ivanova, N.O. Water exchange as a factor in the formation of modern conditions for the functioning of the ecosystem of the Sasyk Reservoir. Sci. Notes Volodymyr Hnatiuk Ternopil Natl. Pedagog. Univ. 2015, 3–4, 274–277. (In Ukrainian) [Google Scholar]
- Romanenko, V.D. (Ed.) Environmental Justification for the Creation of the Danube-Dnepr Water Management Complex (Final Report) Book 1; National Academy of Sciences of URSR, Institute of Hydrobiology: Kyiv, Ukraine, 1980; 501p. (In Russian) [Google Scholar]
- Shuisky, Y.D.; Vykhovanets, G.V. The Nature of the Black Sea Estuaries; Astroprint: Odessa, Ukraine, 2011; 275p. (In Russian) [Google Scholar]
- Shvebs, I.G. (Ed.) Liman-Estuarine Complexes of the Black Sea Region: Geographical Bases of Economic Development; Nauka: Leningrad, Ukraine, 1988; 304p. (In Russian) [Google Scholar]
- Enaki, I.G. Hydrochemical regime of the Sasyk estuary and the Sasyk reservoir [Hydrokhemicheskyi rezhim limana Sasyk I Sasykskogo vodokhranilischa]. In Hydrobiology of the Danube and Limans of the North-Western Black Sea Region: Sat. Scientific Works; Naukova Dumka: Kyiv, Ukraine, 1986; pp. 36–52. (In Russian) [Google Scholar]
- Ivanova, N.A. Studies of oxygen regime Sasyk Reservoir. In The Collection of Works of the VII International Scientific Conference of Young Scientists and Talented Students from the Federal State Institution of the Science Institute of Water Problems in Russian Academy of Sciences; Water Resources, Environment and Water Safety; IWP RAS: Moscow, Russia, 2013; pp. 171–173. [Google Scholar]
- Ivanova, N.O. Transparency and chromaticity of Sasyka water as abiotic components of its ecosystem. Hydrol. Hydrochem. Hydroecol. 2016, 40, 90–104. [Google Scholar]
- Bilous, O.P.; Barinova, S.S.; Ivanova, N.O.; Huliaieva, O.A. The use of phytoplankton as an indicator of internal hydrodynamics of a large seaside reservoir—case of the Sasyk Reservoir, Ukraine. Ecohydrol. Hydrobiol. 2016, 16, 160–174. [Google Scholar] [CrossRef]
- Bilous, O.P.; Ivanova, N.O. Phytoplankton Characteristics of the Sasyk Reservoir (Ukraine). Intern. J. Algae 2018, 20, 277–288. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2022. Available online: https://www.algaebase.org (accessed on 25 April 2023).
- Barinova, S. Essential and practical bioindication methods and systems for the water quality assessment. Int. J. Environ. Sci. Nat. Res. 2017, 2, 555588. [Google Scholar] [CrossRef]
- Barinova, S.S.; Belous, Y.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine; Haifa University Press: Haifa, Israel; Kiev, Ukraine, 2019; pp. 1–367. (In Russian) [Google Scholar]
- Tsarenko, P.M.; Bilous, O.P.; Kryvosheia-Zakharova, O.M.; Lilitska, H.H.; Barinova, S. Diversity of Algae and Cyanobacteria and Bioindication Characteristics of the Alpine Lake Nesamovyte (Eastern Carpathians, Ukraine) from 100 Years Ago to the Present. Diversity 2021, 13, 256. [Google Scholar] [CrossRef]
- Bilous, O.; Afanasyev, S.; Lietytska, O.; Manturova, O.; Polishchuk, O.; Nezbrytska, I.; Pohorielova, M.; Barinova, S. Preliminary Assessment of Ecological Status of the Siversky Donets River Basin (Ukraine) Based on Phytoplankton Parameters and Its Verification by Other Biological Data. Water 2021, 13, 3368. [Google Scholar] [CrossRef]
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.A.; Ly, A.; Gronau, F.Q.; Smira, M.; Epskamp, S.; et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination; Version 4.5; Microcomputer Power Press: Ithaca, NY, USA, 2002; p. 500. [Google Scholar]
- McAleece, N.; Gage, J.D.G.; Lambshead, P.J.D.; Paterson, G.L.J. BioDiversity Professional Statistics Analysis Software; Scottish Association for Marine Science and the London Natural History Museum: Oban, Scotland, 1997; Available online: https://biodiversity-pro.software.informer.com (accessed on 25 April 2023).
- Hustedt, F. Systematische und Ökologische Untersuchungen über die Diatomeenflora von Java, Bali und Sumatra. Arch. Hydrobiol. 1938, 15, 131–177. [Google Scholar]
- Hustedt, F. Die Diatomeenflora des Flußsystems der Weser im Gebiet der Hansestadt Bremen. Abhandlungen des Naturwissenschaftlichen Vereins zu Bremen 1957, 34, 181–440. [Google Scholar]
- Van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Netherland J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar]
- Bilous, O.; Barinova, S.; Klochenko, P. The role of phytoplankton in the ecological assessment of the Southern Bug River middle reaches (Ukraine). Fundam. Appl. Limnol. 2014, 184, 277–295. [Google Scholar] [CrossRef]
- Novikov, V.I. Bottom Sediments of Limans of the North-Western Black Sea Region and Their Influence on Water Quality during Desalination; Hydrobiology of the Danube and Estuaries of the North-Western Black Sea Region: Scientific Works; Naukova Dumka: Kyiv, Ukraine, 1986; pp. 67–80. [Google Scholar]
- Zaldivar, J.-M.; Cardoso, A.C.; Viaroli, P.; Newton, A.; de Wit, R.; Ibanez, C.; Reizopoulou, S.; Somma, F.; Razinkovas, A.; Basset, A.; et al. Eutrophication in transitional waters: An overview. Transitional Waters. Environ. Sci. 2008, 2, 78. [Google Scholar] [CrossRef]
- Hartnett, M.; Wilson, J.G.; Nash, S. Irish estuaries: Water quality status and monitoring implications under the water framework directive. Mar. Policy 2011, 35, 810–818. [Google Scholar] [CrossRef]
- Basset, A.; Barbone, E.; Elliott, M.; Li, B.-L.; Jorgensen, S.E.; Lucena-Moya, P.; Pardo, I.; Mouillot, D. A unifying approach to understanding transitional waters: Fundamental properties emerging from ecotone ecosystems. Estuar. Coast. Shelf Sci. 2013, 132, 5–16. [Google Scholar] [CrossRef]
- Facca, C. Ecological Status Assessment of Transitional Waters. Water 2020, 12, 3159. [Google Scholar] [CrossRef]
- Bilous, O.P.; Genkal, S.I.; Zimmermann, J.; Kusber, W.-H.; Jahn, R. Centric diatom diversity in the lower part of the Southern Bug River (Ukraine): The transitional zone at Mykolaiv city. PhytoKeys 2021, 178, 31–69. [Google Scholar] [CrossRef] [PubMed]
- Snigirova, A.; Bogatova, Y.; Barinova, S. Assessment of River-Sea Interaction in the Danube Nearshore Area (Ukraine) by Bioindicators and Statistical Mapping. Land 2021, 10, 310. [Google Scholar] [CrossRef]
- Tsarenko, P.M.; Ennan, A.A.; Shikhaleeva, G.N.; Barinova, S.S.; Gerasimyuk, V.P.; Ryzhko, V.E. Cyanoprokaryota of the Kuyalnyk estuary (Ukraine). Int. J. Algae 2016, 18, 337–352. [Google Scholar] [CrossRef]
- Bănăduc, D.; Simić, V.; Cianfaglione, K.; Barinova, S.; Afanasyev, S.; Öktener, A.; McCall, G.; Simić, S.; Curtean-Bănăduc, A. Freshwater as a Sustainable Resource and Generator of Secondary Resources in the 21st Century: Stressors, Threats, Risks, Management and Protection Strategies, and Conservation Approaches. Int. J. Environ. Res. Public Health 2022, 19, 16570. [Google Scholar] [CrossRef]
- Bănăduc, D.; Barinova, S.; Cianfaglione, K.; Curtean-Bănăduc, A. Editorial: Multiple freshwater stressors—Key drivers for the future of freshwater environments. Front. Environ. Sci. 2023, 11, 1143706. [Google Scholar] [CrossRef]
- Dedić, A.; Gerhardt, A.; Kelly, M.G.; Stanić-Koštroman, S.; Šiljeg, M.; Kalamujić Stroil, B.; Kamberović, J.; Mateljak, Z.; Pešić, V.; Vučković, I.; et al. Innovative methods and approaches for WFD: Ideas to fill knowledge gaps in science and policy. Water Solut. 2020, 3, 30–42. [Google Scholar]
- Barinova, S.; Bilous, O.; Ivanova, N. New Statistical Approach to Spatial Analysis of Ecosystem of the Sasyk Reservoir, Ukraine. Int. J. Ecotoxicol. Ecobiol. 2016, 1, 118–126. [Google Scholar] [CrossRef]
- Bănăduc, D.; Sas, A.; Cianfaglione, K.; Barinova, S.; Curtean-Bănăduc, A. The Role of Aquatic Refuge Habitats for Fish, and Threats in the Context of Climate Change and Human Impact, during Seasonal Hydrological Drought in the Saxon Villages Area (Transylvania, Romania). Atmosphere 2021, 12, 1209. [Google Scholar] [CrossRef]
- Pogrebnyak, I.I. Phytobenthos of Kuyalnyk eastuary. Pratsi Odes. Derz. Univ. 1949, 4, 123–133. (In Ukrainian) [Google Scholar]
- Pogrebnyak, I.I. Bottom Vegetation of the Estuaries of the North-Western Black Sea Region and Adjacent Water Areas of the Black Sea. Ph.D. Thesis, Odessa, Ukraine, 1965; 31p. (In Russian). [Google Scholar]
- Ennan, A.A.; Shikhaleeva, G.N.; Adobovskiy, V.V.; Gerasimyuk, V.P.; Shikhaleev, I.I.; Kiryushkina, A.N. Degradation of the aquatic ecosystem of the Kuyalnyk estuary and its ways recovery. Prychornomor. Ecol. Bull. 2012, 1, 75–85. [Google Scholar]
- Ennan, A.A.; Shikhaleeva, G.N.; Shikhaleev, I.I.; Adobovskiy, V.V.; Kiryushkina, A.N. Effects of Kuyalnik Estuary degradation (North-Western Black Sea region, Ukraine). Odesa Natl. Univ. Her. Chem. 2014, 19, 60–69. [Google Scholar] [CrossRef]
- Shikhaleeva, G.M.; Ennan, A.A.; Chursina, O.D.; Shikhaleev, I.I.; Yurchenko, Y.Y. Ecological and geochemical asse-sement of the Kuyalnyk estuary. Biol. Valeol. 2017, 19, 199–207. [Google Scholar] [CrossRef]
- Shikhaleyeva, G.M.; Ennan, A.A.-A.; Tsarenko, P.M.; Kiryushkina, G.M. Taxonomic Diversity and Ecological Characteristics of Chlorophyta and Charophyta in the Water Bodies of the Kuyalnyk Estuary (Ukraine, the Black Sea Northwestern Coast). Hydrobiol. J. 2023, 59, 25–40. [Google Scholar] [CrossRef]
- Tsarenko, P.M.; Ennan, A.A. Encyclopedia of the Kuyalnyk Estuary [Encyclopedia Kuyalnitskogo Limana]; Algae. Astroprint: Odesa, Ukraine, 2020; Volume 2, 446p. (In Ukrainian) [Google Scholar]
- Klymiuk, V.; Barinova, S.; Fatiukha, A. Algal bio-indication in assessment of hydrological impact on ecosystem in wetlands of “Slavyansky Resort”. Transylv. Rev. Syst. Ecol. Res. 2015, 17, 63–70. [Google Scholar] [CrossRef]
- Ivanova, N.O. Dynamics of the Sasyka water surface level at different stages of the reservoir’s existence. Hydrol. Hydrochem. Hydroecology 2018, 51, 63–75. (In Ukrainian) [Google Scholar]
- Yyryshynets, V.I.; Kornyushin, A.V. Bivalve species Sinanodonta woodiana (Bivalvia, Unionide), new to the fauna of Ukraine, its diagnostics and possible ways of introduction. Vestn. Zool. Zoodiversity 2000, 35, 79–84. (In Ukrainian) [Google Scholar]
- Bilous, O. Water Quality and Economy for Sustainable Growth. In Clean Water and Sanitation; Encyclopedia of the UN Sustainable Development Goals; Leal Filho, W., Azul, A.M., Brandli, L., Lange Salvia, A., Wall, T., Eds.; Springer: Cham, Germany, 2021. [Google Scholar] [CrossRef]
- Watanabe, T.; Asai, K.; Houki, A. Numerical estimation of organic pollution of flowing water by using the epilithic diatom assemblage—Diatom Assemblage Index (DAIpo). Sci. Total Environ. 1986, 55, 209–218. [Google Scholar] [CrossRef]
- Sládeček, V. Diatoms as indicators of organic pollution. Acta Hydrochim. Hydrobiol. 1986, 14, 555–566. [Google Scholar] [CrossRef]
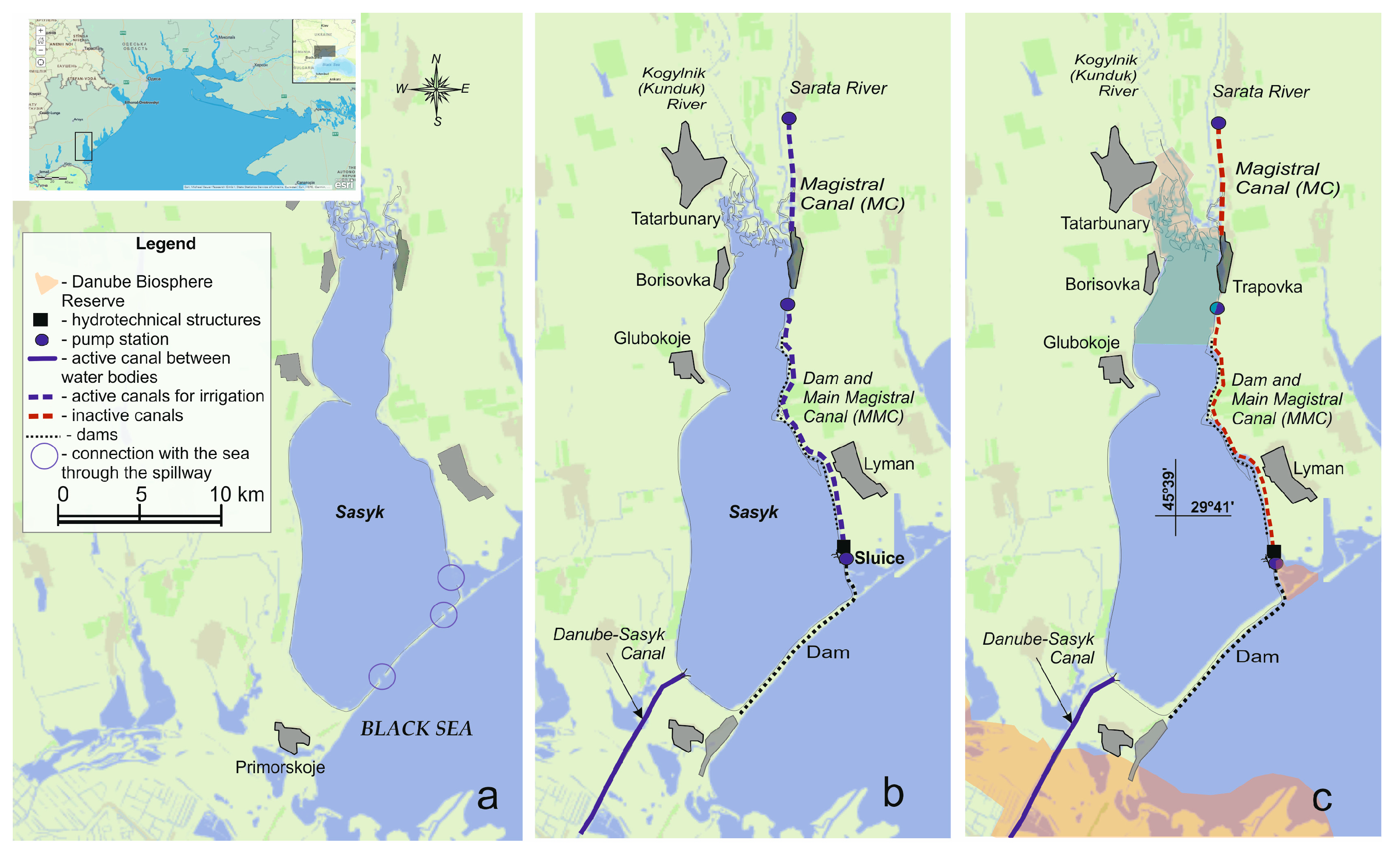
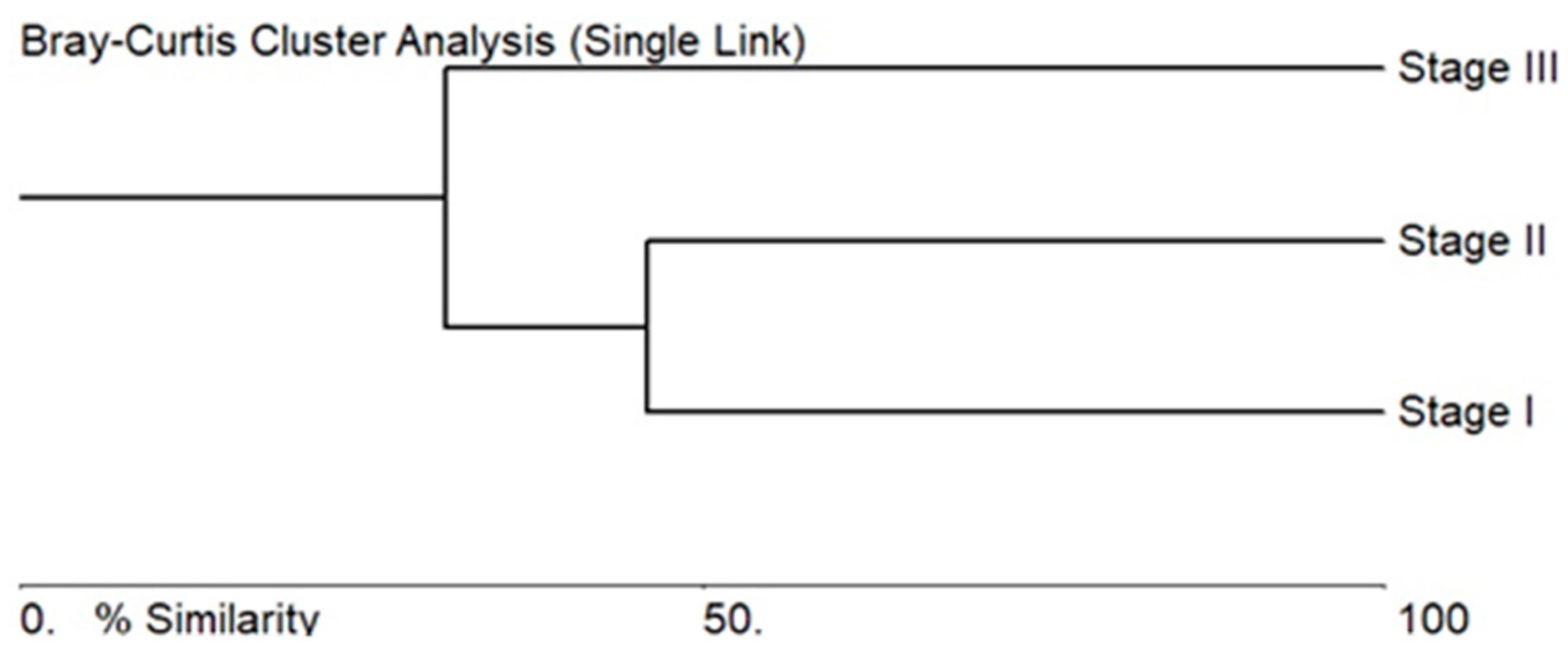

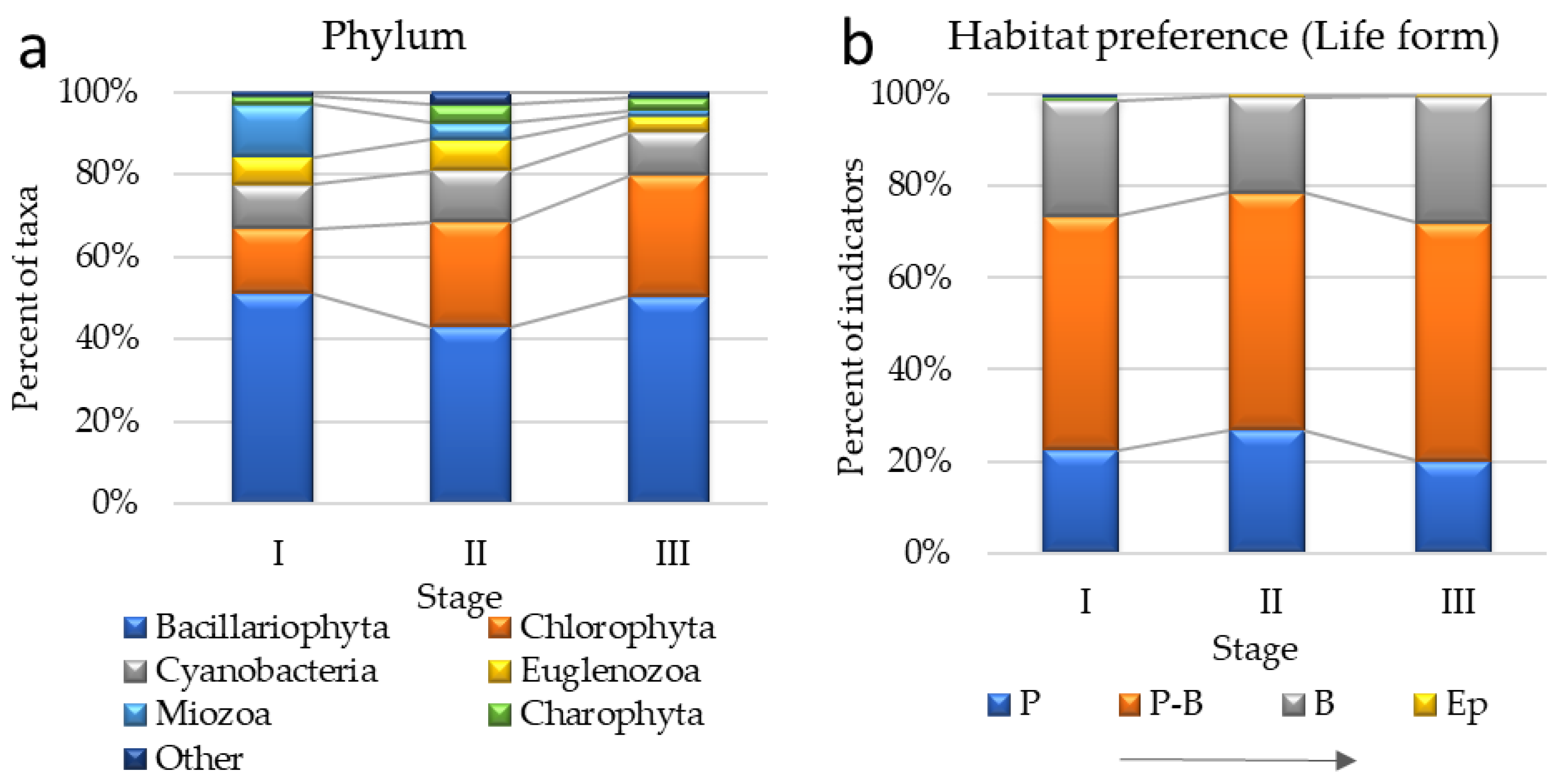
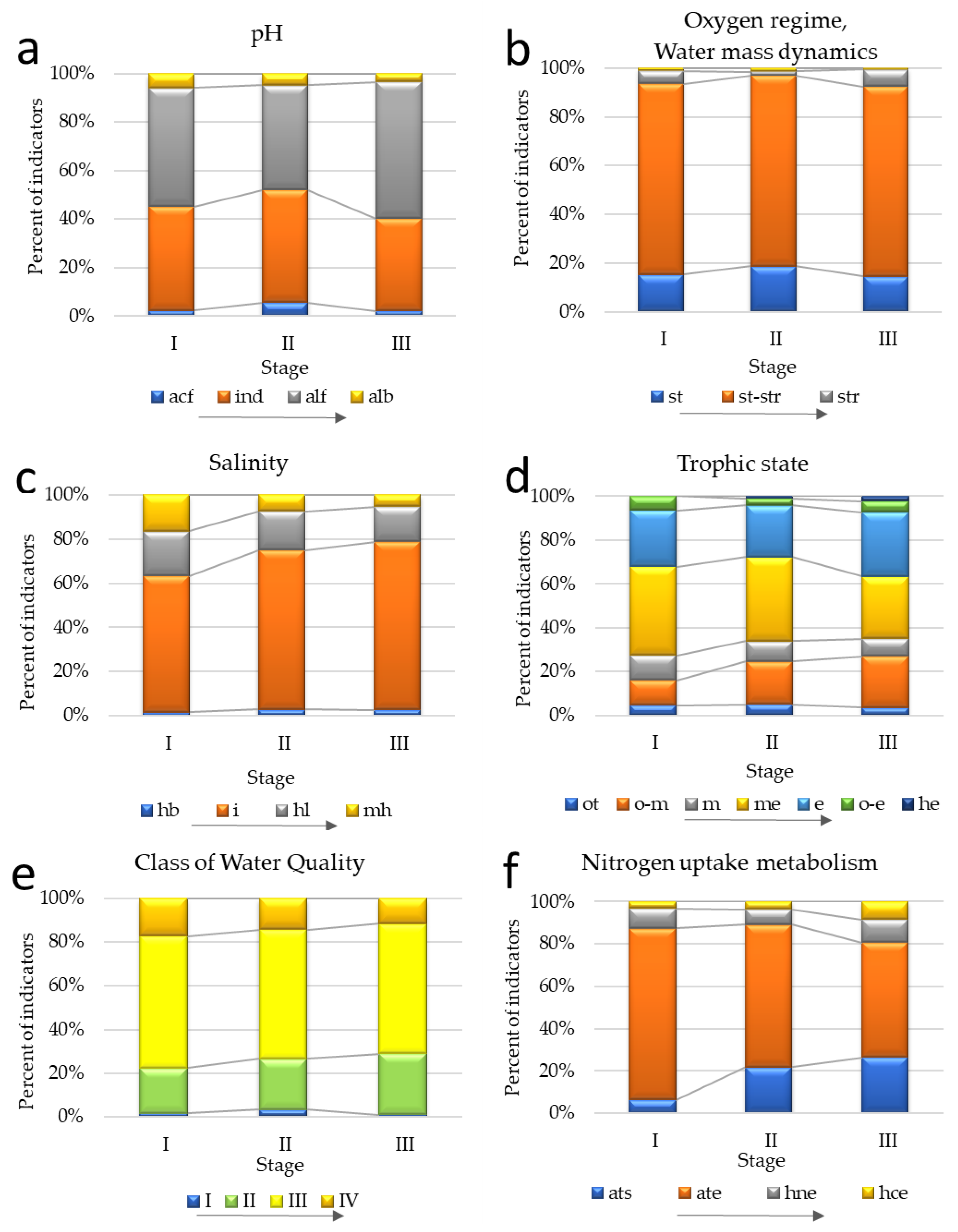
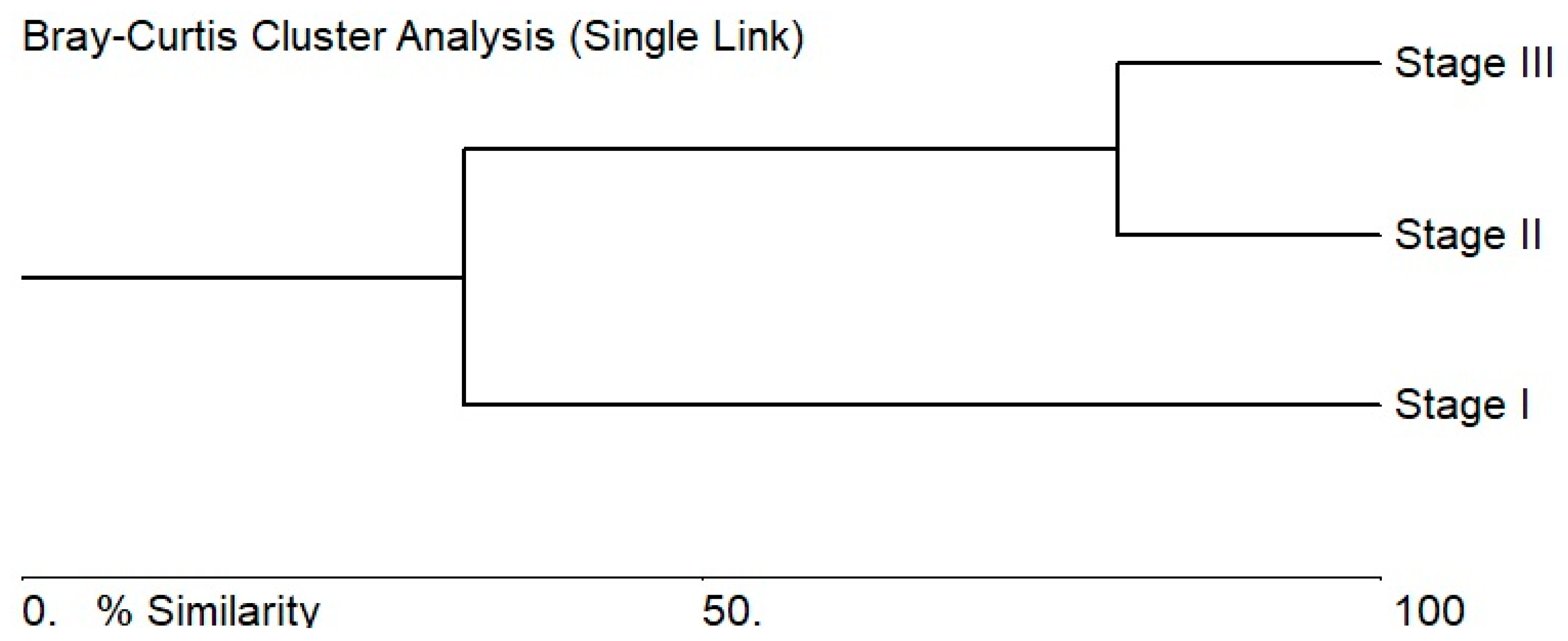
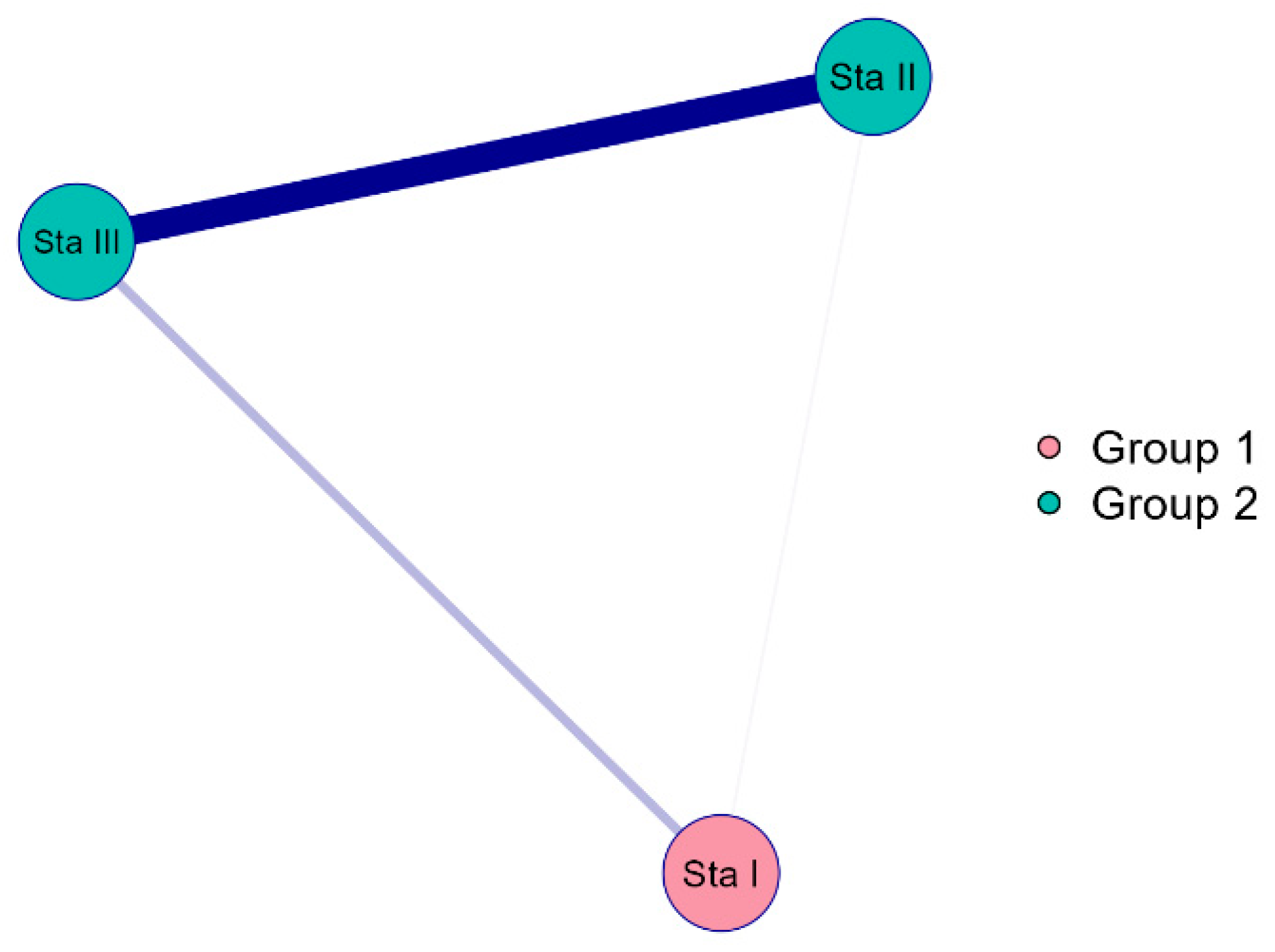
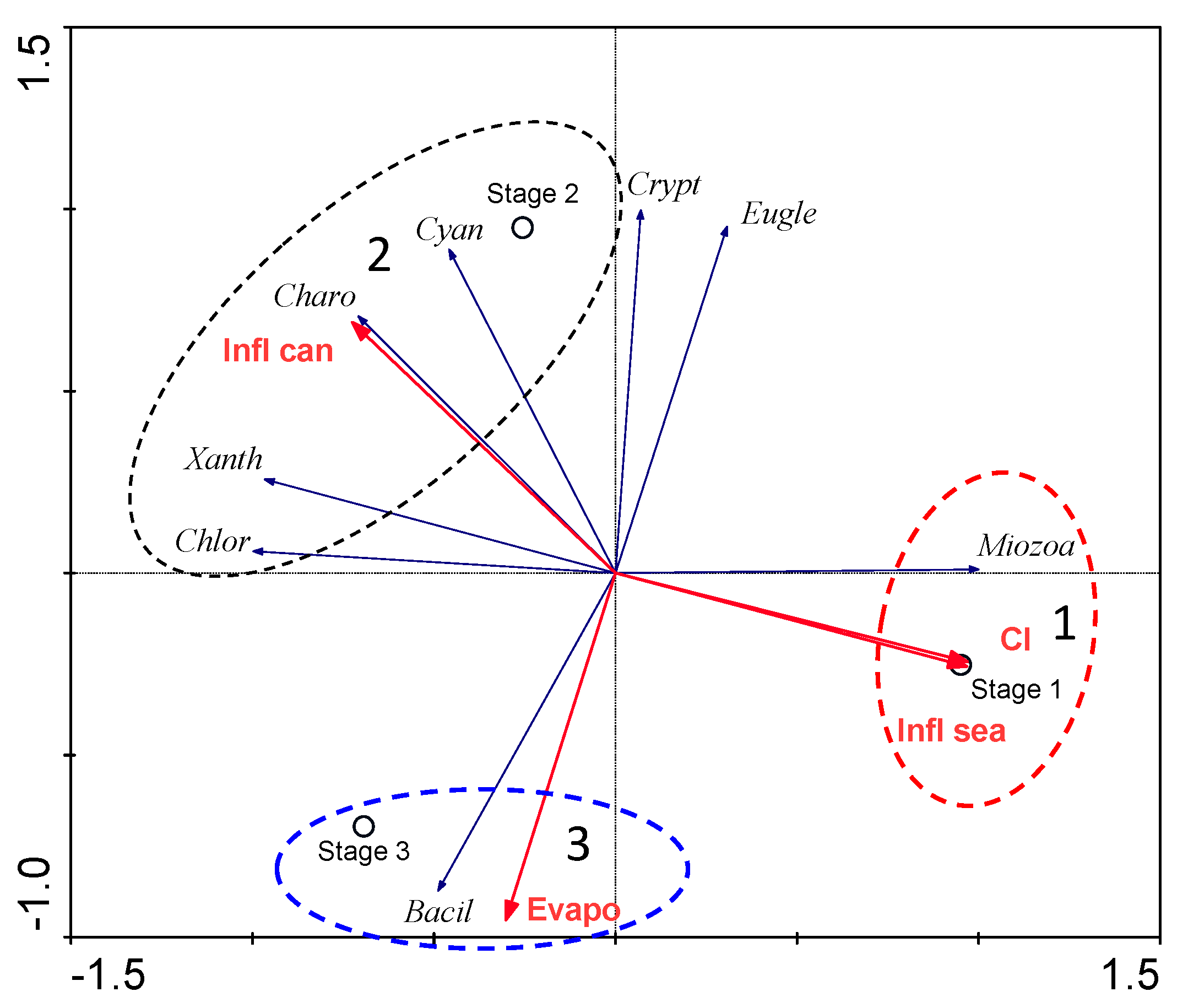
| Stage | I | II | III |
|---|---|---|---|
| Water-body type | Lake-estuary | Forming a reservoir | Reservoir |
| Monitoring years | 1967–1977 | 1980–1990 | 2013–2019 |
| Ranges of periods | 1950–1979 (connected with the Black Sea) | 1979–1999 (1979–1985: an active transformation; 1986–1994: irrigation; 1995–1999: stabilization) | 2000–now |
| Stage | I | II | III | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Average | Min. | Max. | Average | Min. | Max. | Average | Min. | Max. | |
| Water exchange period, day | 505 | nd | nd | 163 | nd | nd | 312 | 180 | 568 |
| Inflow through the channel, mln m3 year−1 | 0 | 0 | 0 | 765 | 480 | 1260 | 399 | 127.2 | 796.1 |
| Inflow of water from the sea, mln m3 year−1 | 142 | nd | nd | 0 | 0 | 0 | 0 | 0 | 0 |
| Evaporation, mln m3 year−1 | 173 | nd | nd | 156 | 106 | 219 | 193 | 182 | 206.5 |
| Suspended solids (sediments), mg dm−3 | nd | nd | nd | 44.4 | 10 | 89.4 | 186 | 27.1 | 655 |
| Water temperature, °C | nd | nd | nd | 14.2 | 0 | 24.9 | 14.3 | 0 | 26.3 |
| Water transparency, m | nd | nd | nd | 0.293 | nd | nd | 0.136 | 0.02 | 0.31 |
| pH | nd | nd | nd | 8.19 | 7.9 | 8.3 | 8.04 | 6.9 | 8.8 |
| Salinity, g dm−3 | nd | 2.39 | 17.6 | 2.29 | 1.4 | 3.45 | 1.79 | 0.96 | 2.96 |
| Cl-, mg dm−3 | 7790 | 6240 | 9410 | 603.5 | 396 | 793 | 491 | 212.8 | 2552 |
| O2, mgO2 dm−3 | nd | 3.9 | 21.2 | 10.4 | 6.17 | 14.8 | 9.23 | 6.27 | 14.7 |
| Saturation O2, % | nd | 43 | 150 | 89.7 | 66 | 114 | 83 | 52 | 120 |
| BOD, mgO2 dm−3 | nd | nd | nd | 2.6 | 0.39 | 7.83 | 3.6 | 0.61 | 9.53 |
| N-NO3, mg dm−3 | nd | nd | nd | 0.809 | 0.02 | 2.4 | 0.4 | 0.008 | 1.8 |
| N-NO2, mg dm−3 | nd | nd | nd | 0.0515 | 0.01 | 0.092 | 0.085 | 0.0016 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilous, O.P.; Wojtal, A.Z.; Ivanova, N.O.; Burova, O.V.; Barinova, S.; Maystrova, N.V.; Polishchuk, O.; Curtean-Bănăduc, A.; Tsarenko, P.M. Indication of Long-Term Changes of Algae Communities in a Hydrologically Transformed Estuary Sasyk, Black Sea, Ukraine. Water 2023, 15, 2078. https://doi.org/10.3390/w15112078
Bilous OP, Wojtal AZ, Ivanova NO, Burova OV, Barinova S, Maystrova NV, Polishchuk O, Curtean-Bănăduc A, Tsarenko PM. Indication of Long-Term Changes of Algae Communities in a Hydrologically Transformed Estuary Sasyk, Black Sea, Ukraine. Water. 2023; 15(11):2078. https://doi.org/10.3390/w15112078
Chicago/Turabian StyleBilous, Olena P., Agata Z. Wojtal, Natalia O. Ivanova, Olga V. Burova, Sophia Barinova, Nadiya V. Maystrova, Oleksandr Polishchuk, Angela Curtean-Bănăduc, and Petro M. Tsarenko. 2023. "Indication of Long-Term Changes of Algae Communities in a Hydrologically Transformed Estuary Sasyk, Black Sea, Ukraine" Water 15, no. 11: 2078. https://doi.org/10.3390/w15112078
APA StyleBilous, O. P., Wojtal, A. Z., Ivanova, N. O., Burova, O. V., Barinova, S., Maystrova, N. V., Polishchuk, O., Curtean-Bănăduc, A., & Tsarenko, P. M. (2023). Indication of Long-Term Changes of Algae Communities in a Hydrologically Transformed Estuary Sasyk, Black Sea, Ukraine. Water, 15(11), 2078. https://doi.org/10.3390/w15112078









