Abstract
Providing safe drinking water to people in developing countries is an urgent worldwide water problem and a main issue in the UN Sustainable Development Goals. One of the most efficient and cheapest methods to attain these goals is to promote the use of slow sand filters. This review shows that slow sand filters can efficiently provide safe drinking water to people living in rural communities not served by a central water supply. Probably, the most important aspect of SSF for developing and less-developed countries is its function as a biological filter. WASH problems mainly relate to the spread of viruses, bacteria, and parasites. The surface and shallow groundwater in developing countries around urban areas and settlements are often polluted by domestic wastewater containing these microbes and nutrients. Thus, SSF’s function is to treat raw water in the form of diluted wastewater where high temperature and access to nutrients probably mean a high growth rate of microbes and algae but probably also high predation and high efficiency of the SSF. However, factors that may adversely affect the removal of microbiological constituents are mainly low temperature, high and intermittent flow rates, reduced sand depth, filter immaturity, and various filter amendments. Further research is thus needed in these areas, specifically for developing countries.
1. Introduction
In view of population increase and climate change, the issue of providing everyone with safe drinking water is one of the most acute problems in the world. In addition, the rapid development of industry and emerging pollutants increase the risk of water pollution by substances harmful to human health. More than 1 billion people do not have access to safe drinking water, and 80% of them live in rural areas [1,2]. The greatest risk associated with the ingestion of water is harmful microbial infection risk due to human and/or animal fecal contamination. Drinking contaminated water and poor hygiene are the major causes of death among children worldwide, after respiratory disease [3]. Annually, more than half a million deaths in low- and middle-income countries are due to drinking poor-quality water [4]. Water, sanitation, and hygiene (WASH) problems cause stunted children and great economic losses in the developing world.
Slow sand filtration (SSF) has historically been one of the most important methods to treat water for drinking and eradicate WASH problems. Due to its efficiency and low cost, SSF is still considered an effective and inexpensive way to provide clean drinking water in developing countries with limited resources. SSF is recognized by the U.S. Environmental Protection Agency (USEPA) and WHO as an inexpensive and reliable way to provide safe drinking water [5,6]. Therefore, this method is still used in rural areas and even in some larger cities of the world to provide the population with good-quality drinking water [7]. Characteristic features of SSF are simple construction, low energy consumption, low filtration rate, no chemical pre-treatment of water, and cleaning of filtering layers by scraping the surface or sand removal [8]. However, surprisingly, only about half a million people in developing countries at present use SSF for drinking purposes [7].
In view of the above, the objective of this review article was to critically summarize and synthesize the features and advantages of SSF methods for developing countries to improve the quality of drinking water. Recently, Maiyo et al. [9] provided a comprehensive review of SSF with applications to drinking water. Their review prioritized aspects on the removal of turbidity and microbes with general applications in developing countries. However, our review particularly emphasizes developing countries and specific aspects of SSF that are important for these countries such as temperature effects, pollutant load, and modifications to the filter media. Many developing countries are located in the tropics and subtropics. However, less-developed countries are also located in temperate and continental climates where diurnal or seasonal temperature may go well below 0 °C. This is expected to significantly affect the biological function of SSF methods; therefore, it is important to summarize the existing literature on temperature effects. Since surface and groundwater are increasingly affected by wastewater pollutants, especially in developing countries where wastewater often is not properly treated before discharge, it is important to review the effects of varying pollutant loads on SSF methods. For example, algae blooms and related pollutants can be pronounced phenomena in warm and sunny climates. Finally, intermittent raw water delivery to the SSFs may be expected in developing countries due to the appearance of long dry periods and high-intensive rainfalls. This will also significantly affect the function of SSF. As well, optimal sand quality with proper particle size distribution may not be readily available, and the pollutant kind and load of raw water may differ distinctly between different regions and geologic and anthropogenic settings. It is therefore pertinent to review how amendments to SSF can be made.
The literature search was carried out using keywords related to the above discussion using Web of Science and Google Scholar including SCI and SSCI indexed papers, research reports, and studies up to 2023. The reference lists of the research literature found were further studied to complement the Internet search. These searches included both the English and Russian languages. This resulted in about 300 potentially interesting publications, out of which 147 were finally selected (see References). Consequently, we start by giving a brief introduction to the main raw water purification methods that are often used in combination with SSF. Then, SSF characteristics and function depending specifically on temperature, raw material of filter media, and typical pollutant load characteristics for less-developed countries are summarized and reviewed. We close with a conclusion and a reflection on research needs to further improve SSF methods with application to developing countries.
2. Contemporary Raw Water Purification Methods
To date, there are various methods of treatment of raw water for drinking purposes. These can be combined with SSF depending on the type of pollutant and pollutant load and can be applied in developed as well as developing countries. Which treatment method to be used to treat the water depends on its chemical composition, turbidity, size of particles (impurities) present, and purpose of use and distribution system to end users. Below follows a brief description of the main methods used to treat raw water.
2.1. Mechanical Filtration
Mechanical filtration, such as SSF, is considered the simplest among the known methods of water purification. This method is usually used to purify water of turbidity and various insoluble substances. For this purpose, the water to be treated is passed through a porous medium constituting a filter or a grid. Various solids and filters (sand, gravel, clays, zeolites, bentonites, activated carbon, etc.) are used as a permeable porous medium [10,11,12]. Normally, the size of the detained (not passing through the filter) particles must be larger than the diameter of “holes” between the filtering particles or grid gaps. However, if the diameter of homogeneous spherical filter particles is equal to d, particles with a diameter more than 0.15 × d may not pass through the filter pores. When passing water through a column filled with powdered activated carbon with particle sizes of 0.1–1 mm, particles of about the same size are detained.
With purely mechanical filtration, it is often difficult to treat water contaminated with microorganisms, bacteria, and viruses due to their size range of 0.005 to 3 × 10−3 mm. The mechanical filtration method is usually used for the pre-treatment of water taken from open water bodies (rivers, lakes, reservoirs) containing relatively large particles of pollutants. In mechanical filtration, the treated water usually passes through the filter by gravity [13].
2.2. Reverse Osmosis
In reverse osmosis, water is passed through a membrane under pressure. The water passes freely through the membrane while other substances present in the water are retained [14]. Using this method, water can be purified of various (even from monovalent) ions, obtaining water of high quality (by composition close to distilled water). However, this method has several drawbacks [15,16]. First, this method has low selectivity, i.e., all “useful” and “harmful” substances for the human body are retained during water purification by the membrane. Therefore, to use water purified by this method as drinking water, it will be necessary to repeatedly add salts needed for the body. Secondly, the cost of reverse osmosis units is relatively high, and the productivity of the process is usually rather low (20–30 L/day). Thirdly, before using reverse osmosis, the water must be cleaned of relatively large mechanical impurities by filtration. Because large particles clog the pores of the membrane, the performance of the process drops, and the service life of the installation is dramatically reduced.
2.3. Ion Exchange
This method is based on the ion-exchange process occurring between water and the sorbent (ion-exchange resin) [17,18]. The ion exchange method can selectively purify water of ions. For this purpose, the raw water is passed through the sorbent (ion exchanger). In this case, the ions present in the water are adsorbed on the surface of the sorbent, and the water from the ion-exchange resins is transferred to an equivalent number of ions with the same charge with respect to the adsorbed ions. For example, the ion exchange process is often used to eliminate water hardness (to reduce the concentration of Mg2+ and Ca2+). For this purpose, ion exchangers (cation exchangers) containing a harmless cation (e.g., Na+) are used. When hard water is passed through the cation exchange resin, an ion exchange process occurs between the water and the ion exchange resin, because of which the calcium and magnesium cations present in the water are adsorbed on the surface of the cation exchange resin, and sodium cations from the ion exchange resin are transferred to the water. The ion exchange process is often used to remove heavy metal cations from water and to extract various ions from industrially polluted water [19,20,21]. The efficiency of the ion-exchange process for water treatment largely depends on the exchange capacity of the sorbent, i.e., the ability of the sorbent to adsorb a certain amount of ions from the solution composition, and on the cost of regeneration of the spent sorbent.
2.4. Electrochemical Purification
Electrochemical treatment is based on passing a strong electric current through the water to be treated [22,23]. When an electric current is applied, substances in the water participate in redox reactions (electrolysis), because of which they are transformed into other “harmless” substances. The electrochemical method is more efficient in terms of economy, and its performance is very high. With this method, it is possible to purify water of almost all microorganisms and obtain high-quality water [24]. However, if the water contains various organic substances, under the influence of a strong current, they can undergo complex changes, resulting in the formation of harmful substances to the environment. Therefore, before using this method, it is necessary to know in advance what substances the impurities present in the composition of water can be transformed into during electrolysis.
2.5. Distillation
The distillation method is based on the conversion of water to steam by heating the solution and then condensing the water vapor [25,26]. With this method, water can be cleaned of dissolved solid impurities, resulting in chemically pure (distilled) water. However, this method is expensive and the components (salts) necessary for human organism should be added to the distilled water to be used as drinking water. The main disadvantage of this method is the inability to purify water of low volatile organic substances by distillation. Therefore, to remove volatile organic compounds, the water is usually first passed through an adsorbent (e.g., activated carbon).
2.6. Sorption
Sorption refers to the adsorption by solid particles of components of gas mixtures and liquid solutions [27,28,29]. In this method, for the purpose of treatment, contaminated water is passed through a vessel filled with sorbent medium. The impurities in the water are adsorbed on the surface of the sorbent particles, and the purified water flows out from the bottom of the sorbent. In this method, the degree of water purification depends on many factors: size of the particles (specific surface area) of the adsorbent, nature of the interaction of components present in the water with the adsorbent surface, pressure, and temperature. With decreasing particle size (with increasing specific surface area), the sorption capacity of a solid increases dramatically. Various substances can be used as adsorbents, e.g., zeolites or activated carbon [30,31]. To date, the most common sorbent for water treatment is activated carbon. By activation, the specific surface area of carbon can be increased up to 1000–1500 m2/g. Activated carbon can be used to purify water of substances of different chemical natures. Therefore, activated carbon is one of the main sorbents used in many commercial filtration plants today.
2.7. Coagulation and Flocculation
Coagulation and flocculation are processes of precipitation of suspended dispersed particles present in solutions by adding electrolytes (coagulants) and water-soluble polymers (flocculants) [32,33,34,35]. They can be used to concentrate impurities in a flocculent form, which can be easily removed by sedimentation. Introduction of coagulants into a suspension leads to a reduction in the electrostatic repulsion force of dispersed particles due to the neutralization of surface charges and reduction in electro-kinetic (zeta) potential of particles. Flocculation is a form of coagulation, when fine suspended dispersed particles in a liquid or gaseous medium form loose flocculated clusters, i.e., floccules. Natural [36,37] and synthetic water-soluble polymers [38,39] and their polycomplexes [40,41] are used as flocculants for raw water treatment.
2.8. Disinfection
Disinfection is performed to kill off remaining microbes such as bacteria, parasites, and viruses after standard treatment. Chlorination is used to prevent the spreading of waterborne diseases such as cholera, dysentery, and typhoid. Raw water chlorination is usually performed by adding chlorine or chlorine compounds such as sodium hypochlorite.
3. Slow Sand Filtration
3.1. History
Filtration methods are traditional techniques of water purification used by mankind since ancient times. By filtering, water can be cleaned of sand, silt, turbidity, scale, and other suspended particles. According to [42], people have used sand and gravel filters as early as 2000 BC in ancient India. In ancient times, the Romans built canals near lakes to take advantage of natural filtration through the canal walls.
Modern slow sand filters (SSFs) for water purification were first used in the 19th century in England. Therefore, they are often called English filters. The first slow filter was built by the English engineer James Simpson in 1829 in London to purify water from the river Thames [43,44]. However, various designs of sand filters were used for water purification in earlier years in several Scottish cities: Paisley (1804), Glasgow (1807), and Greenock (1827) [45,46]. In Berlin, slow filters were built in 1853, in Warsaw in 1880, and in Moscow in 1902 [47]. In the United States, the first SSFs were built in 1872 at Poughkeepsie, New York [48,49], which operated until 1959 [50]. Thus, slow filtration of water has been an effective way to prevent the spread of various gastrointestinal diseases through drinking water for over 150 years [51,52]. In 1855, John Snow, in his essay “On the Means of Transmitting Cholera”, suggested a correlation between the spread of the cholera epidemic and the quality of the water supply in Soho [53].
According to Wegelin [54], “no other simple purification process can improve the physical, chemical, and bacteriological quality of surface waters better than SSF.” In 19th century Europe, SSF of water was recommended as one of the effective ways to prevent the spread of an infectious disease, the cholera epidemic [55]. SSFs can eliminate 90–99% of bacteria and viruses, remove 93.3% of fecal coliforms, and completely remove Giardia lamblia cysts and Cryptosporidium oocysts [1]. In view of its efficiency for basic raw water treatment and low-cost characteristics, it is noteworthy that only about half a million people in developing countries use SSFs to obtain a basic quality of drinking water [7,51]. Obviously, SSF has a much larger role to play in this regard to help reach the UN Sustainable Development Goals.
3.2. SSF Requirements
A distinction can be made between rapid sand filtration and SSF of water [56,57,58]. SSFs have an effective particle size diameter of 0.15–0.35 mm and a uniformity factor of 1.5–3.0. The effective particle size for trapping in fast filters is greater than 0.55 mm, with a uniformity factor of less than 1.5. The water filtration rate in fast filters varies between 4 and 21 m/h (100–475 m3 × m−2 × day−1) [59] and in SSF varies from 0.1 to 0.4 m/h (1–8 m3 × m−2 × day−1) [60]. The difference between these two methods is not only in the filtration rate but most importantly in the technology of water purification. Table 1 provides a list of particles frequently present in raw water [61]. Table 1 can represent a typical surface water source in a developing country affected by untreated wastewater since the contents include various kinds of microbial pollutants.

Table 1.
Examples of elements found in raw water [61].
SSF refers to biological water treatment methods, although filtering also refers to a mechanical and chemical (inertial collision and attachment, diffusion, adsorption, and sedimentation) separation of dispersed particles [61]. Fast sand filtration is a purely mechanical method of water treatment. Fast sand filters remove mainly relatively large, suspended particles. Fast sand filters can be either operated by gravity or pressure. SSF is an effective way to remove microbial contaminants and bacteria as well [62,63]. Particles are mainly removed in the upper part of the sand layer (schmutzdecke layer—German for “dirt layer”) [64]. Nonpathogenic aerobic microorganisms deposited on the surface of the sand filter can metabolize organic matter that enters the filter with the incoming water. These microorganisms can prey on bacteria and viruses present in the water [9].
The biological treatment functioning of the SSF is especially important in developing countries where wastewater and greywater usually are discharged without prior treatment. However, most surface water microbial quality studies have been performed for developed countries and temperate climates [65]. Thus, the dynamic distribution of pathogens is poorly quantified for developing countries. Usually, the same indicator organisms (commonly fecal coliform, E. coli and Enterococci) are used in both developed and developing countries. However, the indicator organisms for, e.g., temperate regions, may not be completely relevant for tropical regions. In warmer climates, the foremost waterborne pathogens can be V. cholerae, Salmonella, Shigella, C. perfringens, cyanobacteria, Entamoeba, rotavirus, and Giardia [65,66].
SSFs represent many advantages over other water treatment methods. They do not require chemical reagents and qualified specialists, are easy to operate, and have minimal maintenance and manpower requirements, low capital and operating costs, and low energy requirements [67,68,69]. For this reason, SSF has found widespread use in rural areas to provide good-quality drinking water [70]. However, there are some limitations, e.g., SSF is not recommended for water treatment with turbidity greater than five nephelometric turbidity units (NTU), because high turbidity can lead to filter clogging and thereby shorten the life of the filter [71]. Apart from turbidity, for successful application of SSF treatment, chlorophyll content in feed water must be <0.05 μg/L; iron and manganese must not exceed 0.3 and 0.05 mg/L, respectively. The quantity of dissolved heavy metals, pesticides, and colorants must be minimal, and the presence of residual oxidant before filtration is not desired [71]. At the same time, SSFs are better at purifying water contaminated with non-clayey impurities [72].
In Saskatchewan, Canada [73], a modular SSF polyethylene system was developed and tested that incorporated pre-treatment and post-treatment processes such as ozone oxidation, pre-treatment, and biological activated carbon (BAC) filters to provide significant reduction in turbidity, heavy metals, color, and organics. In the initial period, the filtration efficiency without the schmutzdecke layer may not be more than 60% [52]. Several studies [74,75] summarize work on the modification of SSFs, which help to eliminate the limitations of the application of this method.
Currently, for the preparation of potable water in many cases, chemical methods of treatment are used. However, the use of reagent methods at small treatment plants may create problems associated with the lack of qualified specialists and with the high cost of equipment and chemical reagents used for water treatment. These facts lead to the conclusion that reagent-free water treatment methods often are better-suited for rural areas in developing countries.
3.3. SSF Biological Processes
There are two important mechanisms regarding the filtration of particles and microorganisms through a slow sand layer: the transport mechanism and the attachment mechanism [75]. According to the transport mechanism, particles in water that are larger than the pore diameter of the sand layer cannot pass through the filter and are retained on the surface of the sand layer. Larger particles are mainly retained by the transport mechanism. However, as the particles settle and the biofilm schmutzdecke “matures” on the surface of the sand layer, the pore diameter of the sand filter gradually decreases. Because of this, particles and microorganisms much smaller than the pore diameter of the sand bed can be retained on the surface of the sand bed [76]. The particles (microorganisms) present in the water adhere to the sand layer surface through Van der Waals or electrostatic forces of attraction [77,78]. In this case, the formation of chemical (e.g., hydrogen) bonds between particles and solid surface cannot be excluded as well [79,80]. Bacteria (size 0.01–10 µm) [81], viruses (0.01–0.1 µm) [82,83], and colloidal particles (0.001–1 µm) [77] are mainly retained by this mechanism.
3.4. General Design of SSF
Traditional slow filters are usually tanks up to 6 m wide, up to 60 m long, and consisting of four layers (Figure 1) [9]. Drainage is placed on the bottom of the tank. Hollow pipes, bricks, or concrete slabs with gaps are usually used as drainage [75,80]. A supporting layer (approximate thickness of 0.5 m) of gravel, pebbles, or crushed stone is placed on the surface of the drainage. The particle size of the supporting layer can vary from 2 to 30 mm. Above the supporting layer, a filtering layer of sand (thickness 450–1250 mm) is placed with a developed surface and high porosity. The sand particle size can vary from 0.2 to 2 mm [84,85]. On the surface of the filtration layer, the supernatant water is located. The supernatant layer must provide the necessary head to filter water through the porous sand layer [8]. The flow rate can be regulated by changing the difference between the head of the supernatant water and the height at which the discharge pipe is open to the atmosphere.
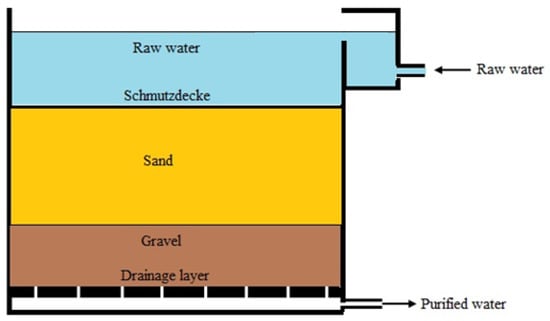
Figure 1.
Schematic of a general SSF design (adapted from Wikimedia Commons).
It is regarded that a sand layer thickness of 0.3 m is sufficient for the proper removal of turbidity and coliform bacteria and a thickness of 0.6 m for the significant removal of virus from the water composition [80]. Changing the thickness of the sand layer affects the removal rates of bacteria and viruses. For example, a decrease in sand layer thickness from 0.6 m to 0.3 m resulted in a 0.04% decrease in poliovirus removal (from 99.98% to 99.94%) [9,86] and a 2% decrease in coliform removal (from 97% to 95%) observed when filter layer thickness was reduced from 0.97 m to 0.48 m [61,87].
Depending on the weather conditions, the slow-filter tank can be located outdoors or indoors. During the cold winter period, it is recommended to conduct the filtration process indoors, especially at subzero temperatures when the filter may not work at all. Over time, as the biofilm thickens, the SSFs gradually lose their efficiency and the flow rate through the filter decreases. In this case, it is necessary to rebuild the filter. As a rule, the duration of an SSF is from 30 to 60 days, but sometimes it can reach more than 100 days [75,80]. This depends on the water flow and pollutant load. Water containing algae is known to clog up SSF in short periods of time. This may be a specific problem in developing countries with surface water containing nutrients.
3.5. SSF Regeneration
There are two main methods of filter layer regeneration: (1) removal of the upper contaminated layer of sand and (2) washing of the contaminated sand surface layer directly in the filter by mechanical or hydraulic loosening and removal of contaminants by a stream of clean water (wet harrowing) [88,89]. In the first method, the top layer of sand is periodically (2–3 times a month) removed and washed several times with clean water. After that, the cleaned sand is loaded back into the tank. After cleaning the filter, it takes some time for the filter to regain its full treatment capacity. Depending on which of the above methods are used, it is expected that this time is several weeks to about a month depending on the external environment [9].
3.6. SSF Speed Mode
The slow filtration rate depends on the suspended solids content of the raw water. At a particle concentration of not more than 25 mg/L, the filtration speed is 0.08–0.4 m/h [88], and at a particle concentration exceeding 25 mg/L, the filtration speed varies from 0.1 to 0.2 m/h.
Contaminated water in slow filters is purified with the help of the biological schmutzdecke film or hypogeal layer that forms on the surface of the filtering sand layer of algae, bacteria, and settled contaminant particles [61,76,90,91,92,93,94]. The duration of filter maturation significantly affects the rate and degree of removal of microbial and organic contaminants by the filter [7,86]. An effective biological film forms during the first 10–40 days of the SSF process of water [7,95,96,97] as mentioned above and provides detention of up to 90–98% of highly dispersed solids, bacteria [88], reduction in fecal coliform bacteria, turbidity per log10 [94], and reduction in total coliforms and turbidity to 97% [98]. A low filtration rate is necessary for complete biological processes in the filter [99,100].
SSF can remove pathogenic microorganisms, suspended organic and inorganic contaminants [84,101], turbidity [101], bacteria, viruses, and enteroparasite cysts [86,101,102]. Meanwhile, the main biological mechanisms responsible for the removal of bacteria in slow sand filters are predation by algae, eating detritus by aquatic worms, natural mortality, inactivation, metabolic breakdown, and adsorption on the sticky zoogleal surface of the sand [92,100,101,102,103].
The sorption capacity of the schmutzdecke layer is estimated through the sorption coefficient (Kd), which is calculated using [104]:
where Cs is the milligram of sorbed antimicrobial per kilogram of solid, mg/kg; Ce is the aqueous antimicrobial concentration mg/L after 24 h equilibration. Sorption coefficients are normalized to the share of organic carbon (Koc = Kd/foc) and organic matter (Kom = Kd/fom) where foc and fom are the mass fraction of organic carbon and organic matter in the schmutzdecke layer, respectively.
3.7. Influence of Filter Media and Hydraulic Residence Time
The size and homogeneity of sand particles essentially influence the efficiency of water purification with an SSF [45]. The homogeneity of the particles is determined by the homogeneity coefficient. The homogeneity coefficient of sand is defined as the ratio: coarseness at which 60% (by weight) of the sand sample passes through the sieve divided by the coarseness at which 10% of the same sample (by weight) passes through the sieve, i.e., K60/10 = d60/d10. A uniformity factor of one means that all particles are the same size. As the uniformity of the sand particles increases, the filtration efficiency increases. If the sand particles vary greatly in size, the smaller sand particles will fill the gaps between the larger particles, resulting in filter clogging [105]. The most effective sand particle size for slow filtration is 0.15–0.35 mm and a uniformity factor of less than two [106].
The thickness of the sand layer has a significant influence on the degree of removal of contaminants from the water composition by the method of SSF. It is generally assumed that the thicker the sand layer, the greater the retention of fine and colloidal particles and viruses and the better the discoloration of water. According to [107], a sand layer 200 mm thick removes 99.5% of fecal bacteria. The minimum thickness of the sand layer to remove turbidity and coliform bacteria is 300 mm, while 600 mm sand thickness is sufficient to remove all viruses [80].
According to [9], the key design parameter of SSF controlling water quality is the filter’s hydraulic residence time (HRT). HRT is determined by:
where Q is the water volume flow rate, m3/h; V is the total sand volume, m3; and n is the sand porosity. The porosity of sand usually ranges from 0.35 to 0.50. This means that 35 to 50% of the volume of the active filter is water in contact with microorganisms attached to the sand grains. Reducing the sand particle size increases the water–sand contact surface area and the porosity of the material. On the other hand, a wide range of particle sizes reduces the porosity of the sand layer, which leads to lower HRT. Therefore, the sand must have a sufficiently high homogeneity. According to [9], the use of a sand layer consisting of particles with a size of 0.35–1.5 mm provides a high degree of water purification at HRT from 8 to 12 h.
3.8. Purification of Water of Ions, Bacteria, and Microbes
SSF can also be used to purify water of ions. However, there are chemical impurities that cannot be effectively removed by SSF alone. These include sulfate (SO42−), nitrate (NO3−), sodium (Na+), calcium (Ca2+), and magnesium (Mg2+) ions and water hardness (as CaCO3) [108,109]. According to [109], biological treatment converts most ammonium ions (NH4+) to nitrate ions (NO3−). In addition, stable colloidal particles are also difficult to remove by SSF [72,73].
In the last two decades, so-called bio-sand filters (BSFs) have become widespread. For example, the company CAWST (Center for Accessible Water Supply and Sanitation Technology) in Calgary, Canada, has developed concrete filters made of bio-sand, which are used in 450 organizations in more than 55 countries [52,74]. Triple Quest of Grand Rapids, USA, offers bio-sand filters: 60 L HydrAid filters made of plastic [9]. Plastic bio-sand filters are relatively cheap and lighter than concrete BSFs [110,111,112]. The authors [9] proposed a modified household plastic filter (BSF). In the new filter design, the gravel layer is replaced by a thin porous plastic plate placed in a plastic bag. This replacement reduces the required filter media and increases the total pore volume in the core. As a result, the cost and labor required to install and maintain the filter is reduced.
A study [113] proposed a household SSF for the removal of As, Fe, and Mn from the composition of groundwater for rural areas in Vietnam. The sand for filtration was collected from the banks of the Red River. It was found that nitrate-reducing, Fe(II)-oxidizing, and Fe(III)-reducing bacteria were present in the dry sand, while microaerophilic Fe(II)-oxidizing bacteria were absent. Mn-oxidizing bacteria were found in the composition of the dry sand. Based on the analysis of the composition of the microbial community, the authors concluded that the abiotic processes of oxidation of Fe(II) prevail over the biotic oxidation of Fe(II) on the filter. Moreover, Mn-oxidizing bacteria played an important role in Mn(II) oxidation and deposition of Mn(III/IV) oxide in a separate layer of the sand filter. The formation of Mn(III/IV) oxides promoted abiotic oxidation of As(III) and immobilization of As(V) by sorption onto (oxy-hydro) oxides of Fe(III). This resulted in a significant reduction in As, Fe, and Mn concentrations in filtered groundwater.
In several studies [114,115,116], the design and principle of operation of a slow self-cleaning filter for natural water deferrization were proposed. A Birm Regular filter was used as a filter load, which simultaneously acts as a catalyst for the reaction of oxidation of Fe2+ by oxygen to Fe3+. Trivalent iron cations are hydrolyzed to Fe(OH)3, and then positively charged colloidal particles of Fe(III) hydroxide are formed [117]. Positively charged colloidal particles of iron (III) hydroxide are adsorbed on the negatively charged surface of the particles of filter media, resulting in the formation of a dense, gel-like adsorption layer on the surface. Such a layer is an effective filtering material. The concentration of Fe(OH)2 varied from 6.0 to 16 mg/L in the model’s natural water (simulant). It was established that the output of the filter to the working mode at Fe3+ concentration in the model solution of 16.0 mg/L was not more than 2.0 h. The analysis of the experimental data obtained for water with an iron concentration of 16.0 mg/L showed that at the first stage of filter operation the Fe3+ concentration in the treated water decreased from 16.0 to 0.9 mg/L after 20 min of filtration, and after 1.5 h it was 0.1 mg/L. The maximum allowable concentration for Fe3+ in drinking water is 0.3 mg/L [118]. According to the authors, the use of the proposed design for the pre-treatment of water contaminated with iron ions will significantly reduce the load on the stage of the final purification of water of iron.
In [119], the possibility of removing cyanobacterial hepatotoxins (microcystins) from the composition of water taken from Berlin lakes using SSFs was studied. Two full-scale experiments were performed: One experiment was performed with dissolved microcystins extracted from a cyanobacterial flower in one of the Berlin lakes. The second experiment was performed with a longer exposure of live cyanobacterial cells (collected from the same lake) to the filter. It was found that the experiment with dissolved microcystins revealed high rates of microcystin elimination (95%) within the sand filter bed and with a half-life for microcystins of about 1 h. In the second experiment, where cell-bound microcystins were used, rather good results (elimination of 85%) were also obtained in the first days after application of cyanobacteria. However, as the temperature decreased to 4 °C, elimination decreased to 60%, which, according to the authors, is associated with a slowing down of bacterial biodegradation at low temperature. Thus, it was concluded that at moderate plus temperatures, slow filtration through sand can serve as an effective method of removing microcystins from drinking water composition.
In [104], the efficiency of removal of water-soluble antimicrobials such as sulfamethazine (SMZ), tylosin (TYL), sulfamethoxazole (SMX), trimethoprim (TRI), and lincomycin (LIN) from water in rural areas by SSF was studied. Basalt sand was used as filtering material. Water-soluble antimicrobials are used in livestock and poultry production to promote growth and prevent bacterial infections. In rural areas, surface water may be contaminated by antimicrobials from wastewater or by diffuse contamination from the application of manure and processed biological solids containing antimicrobial residues to the soil [120,121,122]. Experiments were carried out using coarse (fast) and SSF methods. The coarse filter showed low antimicrobial removal efficiency. SSF showed effectiveness in removing antimicrobials, with the sorption of drugs on the surface of the filter layer changing as follows: TYL > TRI > LIN > SMX > SMZ. At the end of the 14-day period of the SSF study, the following results were obtained: >99% TRI removal, <25% LIN removal, and <4% sulfonamide antimicrobial removal from the contaminated river water.
In [60], slow and fast sand filtration methods were used to remove Triactinomyxon actinospores (Tams) of the salmon parasite Myxobolus cerebralis from contaminated water. Sand with a particle diameter of 0.180 mm was used as the filter material. The sand cushion of the filter was 17.8 cm, and the support gravel was 17.8 cm. Aquarium fish were used as targets of Tams infestation. Tams were introduced into fish-rearing systems over sand filters. The rapid filtration method was tested with two backwashing regimes. In the first, a continuous backwash was performed, and in the second, flow was diverted past the fish tanks for 5 min after backwashing. SSF through a sand filter without backwashing served as a control for the two fast filters. After 60 days, clinical signs of circling behavior and black tails were seen among the positive controls. Polymerase chain reaction (PCR) analysis for Myxobolus cerebralis showed that infections were absent in both fast sand filter water treatments, whereas 1.6% of all fish were infected with the SSF treatment. Based on these results, the authors concluded that both fast and SSFs can be used to remove Tams from the water composition, and the backwash method is important for the reliable functioning of fast sand filters [60].
Studies have indicated different removal mechanisms for bacteria and viruses in SSF. In [123], it was found that most of the E. coli was removed through filtration by the schmutzdecke. Consequently, the residence time in the SSF’s biologically active part had no significant effect on the E. coli removal. On the other hand, most MS-2 viruses were removed through longer residence time and effects in the biologically active layer. The schmutzdecke filtration did not have a significant effect on the MS-2 virus removal. However, ZVI (zero-valent iron as a waste byproduct from the iron industry)-amended filtering removed 100% of both E. coli and MS-2.
3.9. Temperature Effects
Temperature effects on a variety of pollutants for different SSF designs have not been extensively studied. Table 2 shows a summary of temperature effects on treatment efficiency for SSF. As seen from the table, temperature has significant effects on the treatment efficiency. The references mainly contain results for microbiological constituents.

Table 2.
Temperature effects on SSF treatment efficiency.
However, [128] states clear effects on pH, BOD, COD, and TOC removal as well (50% decrease by temperature decrease from 14 °C to 2 °C). SSF is highly efficient by means of removing enteroviruses from contaminated water [86]. Factors affecting this removal rate in a negative way are temperature, high flow rates, reduced sand depth, and filter immaturity [86]. Variation in removal rates is also stated to be mostly determined by temperature and the age of the schmutzdecke [127]. Change in filtration rate had a small effect on microorganism removal [127]. It has been suggested that for normal temperature, predation of bacteria is the most important of all biological removal mechanisms [93]. Consequently, at normal temperature, adsorption to biomass is the least significant mechanism due to reduced biological activity [130].
3.10. Modifications to the Filter Media
Different sand particle size distributions and various additions to the sand will affect the HRT and adsorption properties of the SSF. Adding biologically or chemically active amendments to the filter can improve the treatment efficiency. In this section, we review various amendments to the filter media. In [131], the effect of modifying a slow sand filter with quartz sand or Anadara granosa shells on the removal efficiency of turbidity, total suspended solids, and iron from the water composition of the Kali Jagir Surabaya River (Indonesia) was investigated. The data were processed using the Design Expert 11 software. The SSF reactor was operating continuously for 6 days. The optimum results were obtained in the SSF reactor plant filled with quartz sand and with a filtration rate of 0.1 m/h. The efficiency of removing turbidity was 82.1%, total suspended solids was 89.5%, and iron was 50.1%.
The possibility of using wood pellets and granulated cork as carbon sources in laboratory biofilters working under water-saturated and water-unsaturated conditions was studied in [132]. The efficiency of biofilters was monitored by determining the reduction in nitrate ions (200 mg/L) and pesticides (mecoprop, diuron, atrazine, and bromacil, each at a concentration of 5 μg/L) and by determining the formation of nitrite and pesticide transformation products. Microbiological characterization of each biofilter was also carried out. It was found that the highest nitrate removal (>99%) occurred in water-saturated wood biofilters, while cork biofilters lost all denitrifying capacity over time (38% to no removal). Unsaturated bio-filter columns were ineffective for nitrate removal (20–30% removal). Regarding pesticides, all biofilters showed high removal of mecoprop and diuron (>99% and >75%, respectively). Atrazine removal in wood pellet biofilters was better than in granulated cork (68–96% vs. 31–38%). Bromacil was removed only in the water-saturated granulated cork biofilter (67%). However, a product of bromacil transformation was formed. It should be noted that the water-saturated wood biofilter contained the largest number of de-nitrifying microorganisms, the characteristic representative of which was Methyloversatilis. Overall, the results showed that biofilters based on wood pellets operate under water-saturated conditions and can be applied for the treatment of groundwater polluted by nitrates and pesticides.
Prospects for the use of organic coagulant–flocculant for the pre-treatment of water to improve the reduction in microbial contamination and turbidity in combination with sand filtration for domestic conditions (point-of-use, POU) were studied by [133]. Chitosan was used as a flocculant. In this case, tabletop periodic sand filters with a 16 cm layer of sand and two different grain sizes, representing slow and fast sand filters, were dosed daily for 57 days with the addition of microbes to the surface water. E. coli bacteria and MS2 coliphage virus counts were determined every two weeks (N = 17) using culture methods. The removal of bacteria and viruses was found to be significantly improved compared to sand filtration without pre-treatment with chitosan (Wilcoxon Rank-Sum, p < 0.05). When water was pre-treated with an optimum dose of chitosan (10 mg/L) followed by filtration through the sand, a log10 decrease in the number of bacteria and viruses in the water was observed. The reduction in microbial activity and turbidity generally improved over the life of the filter but was independent of the filtration rate.
The effect of sand particle size, filter thickness, and filtration rate on the disinfection efficiency, bacterial community, and metabolic function of slow bio-sand filters was studied in [134]. It was shown that the average removal efficiency of fine sand was about 4% higher than that of coarse sand and that the thick filter layer showed a more stable performance. In water treatment, the schmutzdecke layer played an overwhelming role and removed most of the turbidity and organic contaminants. The filtration rate was a key factor in shaping the bacterial community structure. As filtration rate increased, the relative abundance of Proteobacteria and Cyanobacteria decreased and increased significantly, respectively. Co-occurrence patterns were dominant in the bacterial communities. Functional bacteria (e.g., Hyphomicrobium and Methylophilus) and rare genera (Curvibacter and Simplicispira) were identified as nodule genera in the networks. Bacterial communities exhibited metabolic versatility. Some secondary metabolic pathways shifted significantly under different conditions, such as biodegradation and xenobiotic metabolism. Moreover, the filtration rate and predominant species strongly influenced the efficiency of contaminant removal.
In [135], the effectiveness of four models of domestic slow sand filters (HSSFs) to remove microorganisms from river water throughout their biological development in the schmutzdecke was investigated. Two models were designed for continuous operation (HSSF-CC and HSSF-CT) and two models intermittently (HSSF-ID and HSSF-IF). The filters were fed with 48 L of pre-treated river water daily. Coarse solids in the river water were sedimented for 24 h, and then the water was passed through a non-woven synthetic blanket. The water samples were quantified with E. coli group bacteria and analyzed using light-field microscopy to visualize the microorganisms. Microorganisms such as algae, protozoa, and helminths were detected in raw water and pre-treated water. After passing through the sand filters, the total reduction in coliform bacteria in the water was between 1.42 ± 0.59 log and 2.96 ± 0.58 log, with continuous models showing better performance (p < 0.004). Escherichia coli reduction ranged from 1.49 ± 0.58 log to 2.09 ± 0.66 log, and HSSF-IF, HSSF-CC, and HSSF-CT showed similar performance (p > 0.06), slightly better than that represented by HSSF-ID (p = 0.04). The results of the study confirmed the feasibility of using HSSF in rural communities in domestic settings (POU) to reduce microbiological risk from river water.
The effect of the household sand filter process mode on the effectiveness of turbidity and color reduction, as well as on the reduction in E. coli and E. coli concentrations in the water after treatment, was studied in [136]. Two PVC house slow sand filters (HSSFs) were operated in continuous (C-HSSF) and intermittent (I-HSSF) flow regimes for eight consecutive months. A non-woven blanket was placed on top of the fine sand to facilitate cleaning. The results of the experiment showed that there were no differences between the continuous-flow and intermittent-flow modes in physicochemical parameters and overall E. coli reduction parameters. However, C-HSSF showed a better result in the reduction in E. coli in water (p = 0.02). Measurement of dissolved oxygen concentration in the adherent biofilm using a Clark microsensor also showed no significant difference between I-HSSF and C-HSSF (p = 0.98).
The effectiveness of the application of sand coated with graphene oxide on the degree of removal of two representative micropollutants (MPs)—atrazine (ATZ) and atenolol (ATL)—from the composition of groundwater by the SSF method was studied in [137]. A layer of graphene oxide (GO) on the surface of sand particles was applied using a simple thermal method. The results showed that the GO-coated sand removes ATZ, ATL, and total organic carbon (TOC) better and reduces water turbidity stronger than the simple sand. From this, it is assumed that the enhanced removal capacity of coated sand with respect to ATZ, ATL, and TOC may be mainly due to the GO coating layer and not to the formation of a biofilm (schmutzdecke). Consequently, the application of GO-coated sand in the SSF field to remove organic contaminants can eliminate the schmutzdecke biofilm growth phase.
In [138], to improve the efficiency of bacteria removal from water, biochar produced at different temperatures (400 °C, 550 °C, and 700 °C) and arginine-modified biochar were added (0.5 and 1 wt. %) to sand filtration columns as filter layers. The addition of biochar to the sand columns was shown to increase the removal efficiency of Escherichia coli and Bacillus subtilis under both slow (4 m/day) and fast (240 m/day) filtration conditions. At the same time, the removal efficiency of bacteria in sand columns with the addition of biochar made at 700 °C was higher than that of columns with the addition of biochar made at 400 °C and 550 °C. Moreover, the modification of biochar with arginine further improved bacteria removal efficiency. For example, complete removal of bacteria (1.35 × 107 ± 10% cells/mL) was achieved under both slow and fast filtration conditions in sand columns with the addition of biochar modified with 1 wt. % arginine. Increased adsorption capacity of bacteria was observed in columns with the addition of biochar modified with arginine. Bacteria are more closely associated with arginine-modified biochar than with simple biochar. Moreover, complete removal of bacteria in the combined presence of 5 mg/L humic acid in suspensions was achieved in columns with the addition of 1 wt. % arginine-modified biochar. The results of this study showed that arginine-modified biochar has great potential for cleaning water contaminated by pathogenic bacteria.
The effect of exposure to solar energy in combination with HSSF on the quality of drinking water was considered in [139]. For this purpose, a filter was built from PVC tubes, sand, and gravel. Solar water disinfection was performed according to the Solar Water Disinfection (SODIS) methodology. At a filtration rate of 2.38 m3/(m2 day), turbidity removal was 97%, and for all E. coli it was 99.9% and E. coli 99.1%.
In [140], the possibility of using a mixed layer of sand with activated carbon for the post-treatment of wastewater containing surfactants was investigated. The activated carbon was obtained from waste coffee grounds and the surfactant concentration in the wastewater in the Sewage Treatment Plant (STP)-Vila City (Brazil) varied from 21 to 39 mg/L. The slow filtration rate was 15 m3/(m2 day). The removal of surfactants was about 9% and 7% in Upflow Anaerobic Sludge Bed reactors (UASB-RALF) and in secondary treatment, respectively, in STP-Vila City plants. At the subsequent stage of water treatment by filtration/adsorption through a mixed layer of sand with activated carbon, a reduction of 94% turbidity (NTU) and 95% surfactant removal was achieved.
The possibility of treating natural water taken from the Blue Nile and White Nile (Egypt) with a domestic slow sand filter (HSSF) using local materials was investigated in [141]. Two filters were used to purify natural water. The first filter consisted of the following layers: standing water (30 cm), fine sand column (40 cm), and gravel (20 cm). The second filter consisted of the following layers: standing water (30 cm), fine sand column (25 cm), coarse (natural river) sand column (20 cm), and gravel (20 cm). The results showed that both filters were very effective in removing E. coli and all E. coli. The mean log10 removal of all E. coli and E. coli for the first filter ranged from 1.9 log to 1.7 log compared to a range of 1.1 log to 1.2 log for the second filter. The relationship of total coliform log10 with turbidity and TSS changed dramatically after filtration. In this case, the best performance of filter 1 was noted for bacteria removal, turbidity, iron (Fe), TSS, K, NO2, and Zn, respectively, compared to NO2, Fe, and Zn for filter 2 in the same order. All soluble ions after filtration did not exceed WHO limits. It is assumed that the first filter is more effective for treating natural water than the second filter.
4. Conclusions
SSF is in many ways well suited to treat raw water for drinking purposes in developing and less-developed countries. SSF is highly efficient in removing bacteria, viruses, and chemicals to produce safe drinking and household water to improve the UN SDGs. If SSF is designed properly with the right kind of sand material, sand depth, HRT, and reasonable knowledge of potential pollutants, filters will be reliable, easily operated, inexpensive, and not dependent on imported chemicals. Some disadvantages include the requirements of a relatively large land area. Moreover, in warm and sunny regions, the occurrence of algae in the raw water can clog the filter. Color and odor in the water may not be easily removed, and intermittent operation of the SSF can decrease the filter´s efficiency. Besides this, the SSF needs to be periodically cleaned by cleaning the layer of the matured schmutzdecke on top of the filter. However, this can be performed by unskilled labor, which makes it adaptable to developing countries.
The SSF works both as a mechanical and biological filter. Probably, the most important aspect of SSF for developing and less-developed countries is its function as a biological filter. WASH problems mainly relate to the spread of viruses, bacteria, and parasites. The surface and shallow groundwater in developing countries around urban areas and settlements are often polluted by domestic wastewater containing these microbes and nutrients. Thus, SSF’s function would be to treat raw water in the form of diluted wastewater where high temperature and access to nutrients probably mean a high growth rate of microbes and algae but probably also high predation and high efficiency of the SSF. As seen from this review, factors that may adversely affect the removal of microbiological constituents are mainly low temperature, high flow rates, reduced sand depth, and filter immaturity.
As surface and shallow groundwater are becoming increasingly affected by wastewater from households, industry, and agriculture, it is important that SSF techniques are continuously developed for a variety of existing and emerging pollutants. SSF technology has an important role in providing safe drinking and household water. Besides treating surface and groundwater, SSF has a relevant function in providing clean water from rainwater harvesting and greywater. Thus, continued research into these processes is needed.
5. Future Perspectives
The above review shows that there are ample possibilities for simple, cost-effective, yet effective applications for the extended use of slow sand filtration (SSF), especially in rural areas that are difficult to reach by central raw water treatment plants. This is a typical situation in developing and less-developed countries. The efficiency and cost-effectiveness of SSF make this method especially suited for these countries. Filters are easy to manufacture from local material and do not require expensive additional chemicals or complicated operation and maintenance. The following reflections regarding future perspectives and research needs can be made regarding SSF and possibilities to extend its use:
- SSF functions well as bio-filters that are especially important for developing and less-developed countries. However, to further continue to develop methods for SSF, combined use with disinfection techniques for additional water purification from microbiological pollutants such as bacteria, microbes, viruses, and parasites can be performed. These techniques can be applied before or after the SSF application. It is important to develop these techniques using local materials.
- Studies are needed to investigate SSF using other types of basic material in areas where suitable sand and gravel are not readily available. Possible local material may be constituted by inert or semi-inert material from processes such as ash from energy use and biomaterial from agricultural waste.
- It is becoming important, especially for developing and less-developed countries, to test and advance SSF methods to reduce the contents of emerging environmental pollutants such as protozoa, cyanobacteria, surfactants, and microplastics. Thus, further studies are needed to determine design criteria (particle size distribution, depth of media, residence time, temperature, etc.) for different types of pollutants, existing and emerging.
- Further research is needed to advise on life-cycle time, operation (e.g., batch or continuous flow), and maintenance procedures (cleaning of media, backflushing, etc.) for used porous media in SSF. This is especially important in developing countries where intermittent raw water input may affect the function of the SSF due to drought and wet periods.
- Surprisingly few people in developing and less-developed countries still have no access to SSF to obtain safe drinking and household water. This is noteworthy in view of SSF’s simplicity and efficiency in preventing typical WASH diseases. Obviously, SSF has a much larger role to play in helping to reach the UN Sustainable Development Goals. Further research is needed on experiences with SSF in developing countries such as SFF media materials, process removal efficiency, regeneration time, etc.
Author Contributions
Conceptualization, S.A. and K.A.; methodology, S.K. and B.K.; software, R.B. and S.A.; formal analysis, A.K., D.Y. and S.S.; investigation, S.K. and S.A.; resources, E.K.; writing—original draft preparation, K.A.; writing—review and editing, S.A. and R.B.; visualization, B.K.; supervision, E.K.; project administration, S.A.; funding acquisition, E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (program number BR11765599). Program title “Development and improvement of natural water purification technologies and improvement of drinking water quality in the regions of Kazakhstan”.
Data Availability Statement
Data used in this study can be received upon request.
Conflicts of Interest
The authors declare no potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| ATL | Atenolol |
| ATZ | Atrazine |
| BSF | Bio-Sand Filters |
| BOD | Biological Oxygen Demand |
| CAWST | Center for Accessible Water Supply and Sanitation Technology |
| Ce | Aqueous antimicrobial concentration mg/L after 24 h equilibration |
| Cs | Sorbed antimicrobial per kilogram of solid, mg/kg |
| C-HSSF | Continuous Household SSF |
| COD | Chemical Oxygen Demand |
| d | Diameter for typical particles in filter media |
| d60 | Diameter at which 60% (by weight) of sand passes through the sieve |
| d10 | Diameter at which 10% (by weight) of sand passes through the sieve |
| foc | Mass fraction of organic carbon matter in the schmutzdecke layer |
| fom | Mass fraction of organic matter in the schmutzdecke layer |
| GO | Graphene Oxide |
| HRT | Hydraulic Residence Time (V × n/Q) |
| HSSF | Household SSF |
| HSSF-CC | Household SSF Continuous Compact |
| HSSF-CT | Household SSF Continuous Traditional |
| HSSF-ID | Household SSF Intermittent Diffusor |
| HSSF-IF | Household SSF Intermittent Float |
| I-HSSF | Intermittent Household SSF |
| K60/10 | Homogeneity coefficient of sand (d60/d10) |
| Kd | Sorption coefficient |
| Koc | Normalized sorption coefficient to the share of organic carbon (Kd/foc) |
| Kom | Normalized sorption coefficient to the share of organic matter (Kd/fom) |
| LIN | Lincomycin |
| MS2 virus | Bacteriophage Emesvirus zinderi |
| n | Sand porosity |
| NTU | Nephelometric Turbidity Unit |
| PCR | Polymerase Chain Reaction |
| POU | Point Of Use |
| PVC | Polyvinyl Chloride |
| Q | Water flow rate (m3/h) |
| SCI | Science Citation Index |
| SMX | Sulfamethoxazole |
| SMZ | Sulfamethazine |
| SODIS | Solar Water Disinfection |
| SSCI | Social Sciences Citation Index |
| SSF | Slow Sand Filtration |
| STP | Sewage Treatment Plant |
| Tams | Triactinomyxon actinospores |
| TOK | Total Organic Carbon |
| TRI | Trimethoprim |
| TYL | Tylosin |
| UASB-RALF | Upflow Anaerobic Sludge Bed Reactor |
| UN SDGs | United Nations Sustainable Development Goals |
| USEPA | U.S. Environmental Protection Agency |
| V | Total sand volume (m3) |
| WASH | Water, sanitation, and hygiene |
| WHO | World Health Organization |
| ZVI | Zero-Valent Iron |
References
- Erazo-Oliveras, A.; May Ol-Bracero, O.L.; Ríos-Dávila, R.A. Improving slow sand filters for water-limited communities. Opflow 2012, 38, 24–27. [Google Scholar] [CrossRef]
- WHO. World Health Report; World Health Organization: Geneva, Switzerland, 2004; Volume 1, pp. 1–540.
- WHO. WHO Guidelines for Drinking Water Quality, 3rd ed.; World Health Organization: Geneva, Switzerland, 2004; p. 143.
- WHO. Preventing Diarrhoea through Better Water, Sanitation and Hygiene: Exposures and Impacts in Low- and Middle-Income Countries; World Health Organization: Geneva, Switzerland, 2014.
- Slezak, L.A.; Sims, R.C. The Application and Effectiveness of slow sand filtration in the United States. J. Am. Water Work. Assoc. 1984, 76, 38. [Google Scholar] [CrossRef]
- Agrawal, A.; Sharma, N.; Sharma, P. Designing an economically slow sand filter for households to improve water quality parameters. Mater. Today Proc. 2021, 43, 1582–1586. [Google Scholar] [CrossRef]
- Elliott, M.A.; Stauber, C.E.; Koksal, F.; DiGiano, F.A.; Sobsey, M.D. Reductions of E-coli, echovirus type 12 and bacteriophages in an intermittently operated household-scale slow sand filter. Water Res. 2008, 42, 2662–2670. [Google Scholar] [CrossRef]
- Haig, S.J.; Collins, G.; Davies, R.L.; Dorea, C.C.; Quince, C. Biological aspects of slow sand filtration: Past, present and future. Water Sci. Technol. Water Supply 2011, 11, 468–472. [Google Scholar] [CrossRef]
- Maiyo, J.K.; Dasika, S.; Jafvert, C.T. Slow Sand Filters for the 21st Century: A Review. Int. J. Environ. Res. Public Health 2023, 20, 1019. [Google Scholar] [CrossRef]
- Barkay-Arbel, Y.; Hatukai, S.; Asheri, T.; Vaizel-Ohayon, D.; Rebhun, M. Performance and process mechanisms of a high-rate direct filtration plant targeting 0.1 ntu. J. Am. Water Work. Assoc. 2012, 104, E653–E663. [Google Scholar] [CrossRef]
- Wen-Yong, W.; Yan, H.; Hong-Lu, L.; Shi-Yang, Y.; Yong, N. Reclaimed Water Filtration Efficiency and Drip Irrigation Emitter Performance with Different Combinations of Sand and Disc Filters. Irrig. Drain. 2015, 64, 362–369. [Google Scholar] [CrossRef]
- Zielina, M. Particle Shapes in the Drinking Water Filtration Process. CLEAN Soil Air Water 2011, 39, 941–946. [Google Scholar] [CrossRef]
- Schraer, A. Amiad Water Systems Filtration Technologies. Water Purif. Water Treat. Water Supply 2021, 4, 36–41. (In Russian) [Google Scholar]
- Lior, N.; El-Nashar, A.; Sommariva, C. Advanced Instrumentation, Measurement, Control, and Automation (IMCA) in Multistage Flash (MSF) and Reverse-Osmosis (RO) Water Desalination. In Advances in Water Desalination; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 453–658. [Google Scholar] [CrossRef]
- Uragami, T. Reverse Osmosis. In Science and Technology of Separation Membranes, 1st ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; Chapter 9; pp. 259–295. [Google Scholar] [CrossRef]
- Zargar, M.; Jin, B.; Dai, S. Development and application of reverse osmosis for separation. In Membrane Processing for Dairy Ingredient Separation, 1st ed.; Kang, H., James, M., Eds.; Dickson. John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; Chapter 6; pp. 139–175. [Google Scholar] [CrossRef]
- SenGupta, A.K. Ion Exchange Fundamentals. In Ion Exchange in Environmental Processes: Fundamentals, Applications and Sustainable Technology, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; Chapter 2; pp. 50–129. [Google Scholar] [CrossRef]
- Berrios, M.; Siles, J.A.; Martín, M.A.; Martín, A. Ion Exchange. In Separation and Purification Technologies in Biorefineries, 1st ed.; Ramaswamy, S., Ramarao, B.V., Huang, H.-J., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2013; Chapter 6; pp. 149–156. [Google Scholar] [CrossRef]
- Bornak, B. Desalination by Ion Exchange. In Desalination, 1st ed.; Kucera, J., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2014; Chapter 11; pp. 503–520. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, T. Ion Exchange Membranes. In Encyclopedia of Membrane Science and Technology; Eric, M.V., Hoek, V., Tarabara, V., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 1–58. [Google Scholar] [CrossRef]
- Ichihashi, K.; Konno, D.; Maryunina, K.Y.; Inoue, K.; Toyoda, K.; Kawaguchi, S.; Nishihara, S. Selective Ion Exchange in Supramolecular Channels in the Crystalline State. Angew. Chem. Int. Ed. 2019, 58, 4169–4172. [Google Scholar] [CrossRef]
- Baker, R.W. Ion Exchange Membrane Processes—Electrodialysis. In Membrane Technology and Applications, 3rd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; Chapter 10. [Google Scholar] [CrossRef]
- Conforti, K.M.; Bazant, M.Z. Continuous ion-selective separations by shock electrodialysis. AIChE J. 2019, 66, e16751. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, B.; Gao, C.; Wu, Y. Ion transfer modeling based on Nernst–Planck theory for saline water desalination during electrodialysis process. Asia Pac. J. Chem. Eng. 2020, 15, e2410. [Google Scholar] [CrossRef]
- Gong, F.; Li, H.; Yuan, X.; Huang, J.; Xia, D.; Papavassiliou, V.P.; Xiao, R.; Yamauchi, Y.; Kevin, C.-W.; Wu, K.C.-W.; et al. Recycling Polymeric Solid Wastes for Energy-Efficient Water Purification, Organic Distillation, and Oil Spill Cleanup. Nano Micro Small 2021, 17, 2102459. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, Y.; Zhang, Y.; Song, X.; Peng, H.; Jiang, H. Bilayer rGO-Based Photothermal Evaporator for Efficient Solar-Driven Water Purification. Chem. A Eur. J. 2021, 27, 17428–17436. [Google Scholar] [CrossRef] [PubMed]
- Taraskin, K.A.; Orlov, D.S.; Kanaev, B.A.; Shcherbakov, D.A.; Vorobyov, M.V. Adsorption treatment of wastewater from the production of noise-insulating composite materials from sulfur-containing compounds. Water Purif. Water Treat. Water Supply 2021, 5, 32–38. (In Russian) [Google Scholar]
- Zubkov, A.A.; Bagrov, V.V.; Kamrukov, A.S.; Kostritsa, V.N.; Krylov, V.I. Natural sorbents and their use for wastewater treatment. Water Purif. Water Treat. Water Supply 2020, 2, 36–44. (In Russian) [Google Scholar]
- Korosteleva, Y.A.; Fetyukhina, E.G.; Ignarina, L.M. Sorbent based on diatomite Diamix Aqua is an effective and profitable alternative to carbon filters in power plant water treatment. Water Purif. Water Treat. Water Supply 2020, 6, 44–51. [Google Scholar]
- Yap, P.L.; Nine, M.J.; Hassan, K.; Tung, T.T.; Tran, D.N.H.; Losic, D. Graphene-Based Sorbents for Multipollutants Removal in Water: A Review of Recent Progress. Adv. Funct. Mater. 2020, 31, 2007356. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.; Liu, K.; Miao, R.; Fang, Y. Gel-Emulsion Templated Polymeric Aerogels for Water Treatment through Organic Liquid Removing and Solar Vapor Generation. ChemSusChem 2019, 13, 749–755. [Google Scholar] [CrossRef]
- Eremeev, D.N.; Voropaev, S.V. The use of cationic flocculants to improve the efficiency of dehydration of urban sewage sludge. Water Purif. Water Treat. Water Supply 2021, 3, 40–45. (In Russian) [Google Scholar]
- Abdiyev, K.Z.; Maric, M.; Orynbayev, B.Y.; Toktarbay, Z.; Zhursumbaeva, M.B.; Seitkaliyeva, N.Z. Flocculating properties of 2-acrylamido-2-methyl-1-propane sulfonic acid-co-allylamine polyampholytic copolymers. Polym. Bull. 2022, 79, 10741–10756. [Google Scholar] [CrossRef]
- Shachneva, E.Y.; Khentov, V.Y.; Kudinova, D.E. Removal of zinc ions using H-600 flocculant. Water Purif. Water Treat. Water Supply 2020, 4, 20–27. (In Russian) [Google Scholar]
- Dauletov, Y.; Abdiyev, K.Z.; Toktarbay, Z.; Nuraje, N.; Zhursumbaeva, M.; Kenzhaliyev, B. Radical Polymerization and Kinetics of N,N-diallyl-N,N-dimethylammonium Chloride and Vinyl Ether of Monoethanolamine. Fibers Polym. 2018, 19, 2023–2029. [Google Scholar] [CrossRef]
- Courtney, M.; Weijue, G.; Pedram, F. Cationic lignin polymers as flocculants for municipal wastewater. Water Environ. J. 2023, 37, 95–102. [Google Scholar]
- Xiwen, L.; Qiaoxia, G.; Shenyong, R.; Junkang, G.; Chongbin, W.; Jiaxin, C.; Baojian, S. Synthesis of starch-based flocculant by multi-component grafting copolymerization and its application in oily wastewater treatment. J. Appl. Polym. Sci. 2023, 140, e53356. [Google Scholar]
- Bouras, B.; Tennouga, H. Flocculation of Clay Suspensions Using Copolymers Based on Acrylamide and Biopolymer. Phys. Chem. Res. 2023, 11, 221–230. [Google Scholar]
- Abdiyev, K.Z.; Maric, M.; Orynbayev, B.; Zhursumbaeva, M.; Seitkaliyeva, N.; Toktarbay, Z. Novel Cationic Polymer Surfactant for Regulation of the Rheological and Biocidal Properties of the Water-Based Drilling Muds. Polymers 2023, 15, 330. [Google Scholar] [CrossRef]
- Shaikhutdinov, E.M.; Khussain, S.K.; Abdiyev, K.Z.; Seitkaliyeva, N.Z. Complexation of Sodium 2-Acrylamido-2-Methylpropanesulfonate-Monoethanolamine Vinyl Ether Copolymer with Polyelectrolytes in Aqueous Medium. Polym. Sci. Ser. A 2007, 49, 584–592. [Google Scholar] [CrossRef]
- Abdiyev, K.Z.; Shaikhutdinov, E.M.; Zhursumbaeva, M.B.; Khussain, S.K. Effect of polymer concentration on the surface properties of polyacid-poly(N-vinylpyrrolidone) complexes. Colloid J. 2003, 65, 399–402. [Google Scholar] [CrossRef]
- Shanahan, P. Water and Wastewater Treatment Engineering. Massachusetts Institute of Technology: MIT OpenCourseWare. 2006. Available online: https://ocw.mit.edu (accessed on 3 January 2023).
- Brief History during the Snow Era. Available online: https://www.ph.ucla.edu/epi/snow/1859map/Chelsea_waterworks_a2.html (accessed on 24 February 2023).
- Christman, K. The history of chlorine. Waterworld 1998, 14, 66–67. [Google Scholar]
- Huisman, L.; Wood, W.E. Slow Sand Filtration; World Health Organization: Geneva, Switzerland, 1974. Available online: https://apps.who.int/iris/bitstream/handle/10665/38974/9241540370.pdf?sequence=1&isAllowed=y (accessed on 23 January 2023).
- Buchan, J. Crowed with Genius: The Scottish Enlightenment: Edinburgh’s Moment of the Mind; Harper Collins: New York, NY, USA, 2003. [Google Scholar]
- Crittenden, J.; Trussell, R.R.; Hand, D.W.; Howe, K.; Tchobanoglous, G. Water Treatment: Principles and Design, 2nd ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Johnson, G. Present Day Water Filtration Practice. J. Am. Water Work. Assoc. 1914, 1, 31–80. [Google Scholar] [CrossRef]
- National Drinking Water Clearinghouse. Slow sand filtration. Municipal Water Supply. In Water Encyclopedia; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- History|Poughkeepsies Water Treatment Facility. Retrieved 18 May 2017. Available online: www.pokwater.com (accessed on 13 February 2023).
- Lacey, M. Filtration to privatization, utility issues are universal. J. Am. Water Work. Assoc. 2009, 101, 2. [Google Scholar] [CrossRef]
- Manz, D. New horizons for slow sand filtration. In Proceedings of the Eleventh Canadian National Conference and Second Policy Forum on Drinking Water and the Biennial Conference of the Federal-Provincial-Territorial Committee on Drinking Water, Promoting Public Health through Safe Drinking Water, Calgary, AB, Canada, 3–6 April 2004; pp. 682–692. [Google Scholar]
- Gunn, S.; William, A.; Masellis, M. Concepts and Practice of Humanitarian Medicine. 23 October 2007. Available online: https://books.google.com/books?id=t1exE1cfKXIC&pg=PA87 (accessed on 19 January 2023).
- Wegelin, M. Roughing gravel filters for suspended solids removal. In Slow Sand Filtration: Recent Developments in Water Treatment Technology; Graham, N.J.D., Ed.; Ellis Horwood Ltd.: England, UK, 1988; p. 86. [Google Scholar]
- Logsdon, G.S.; Lippy, E.C. The role of filtration in preventing waterborne disease. J. Am. Water Work. Assoc. 1982, 74, 649–655. [Google Scholar] [CrossRef]
- Collins, M.R. Experiences introducing “new” technology: Slow sand filtration. In Providing Safe Drinking Water in Small Systems: Technology, Operations, and Economics; Cotruvo, J.A., Craun, G.F., Hearn, N., Eds.; CRC Press: Boca Raton, FL, USA, 1998; pp. 213–224. [Google Scholar]
- Letterman, R.D.; Cullen, T.R. Slow Sand Filter Maintenance: Costs and Effects on Water Quality; Report 600/S2-85/056; U.S. Environmental Protection Agency: Washington, DC, USA, 1985.
- Al-Ani, M.; McElroy, J.M.; Hibler, C.P.; Hendricks, D.W. Filtration of Giardia Cysts and Other Substances: Volume 3, Rapid-Rate Filtration; Report 600/S2-85/027; U.S. Environmental Protection Agency: Washington, DC, USA, 1985.
- Droste, R.L. The Theory and Practice of Water and Wastewater Treatment; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Arndt, R.; Wagner, E. Rapid and slow sand filtration Techniques and Their Efficacy at Filtering Triactinomyxons of Myxobolus cerebralis from Contaminated Water. N. Am. J. Aquac. 2004, 66, 261–270. [Google Scholar] [CrossRef]
- Bellamy, W.D.; Silverman, G.P.; Hendricks, D.W.; Logsdon, G.S. Removing Giardia cysts with slow sand filtration. J. Am. Water Work. Assoc. 1985, 77, 52–60. [Google Scholar] [CrossRef]
- Frederick, W. Drinking Water Regulation and Health, 1st ed.; Pontius, Wiley and Science: New York, NY, USA, 2003; ISBN 13: 978-0471415541/10: 0471415545. [Google Scholar]
- Yildiz, B.S. Water and wastewater treatment: Biological processes. In Metropolitan Sustainability; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 406–428. [Google Scholar]
- Lubarsky, H.; Fava, N.D.M.N.; Souza Freitas, B.L.; Terin, U.C.; Oliveira, M.; Lamon, A.W.; Pichel, N.; Byrne, J.A.; Sabogal-Paz, L.P.; Fernandez-Ibañez, P. Biological Layer in Household Slow Sand Filters: Characterization and Evaluation of the Impact on Systems Efficiency. Water 2022, 14, 1078. [Google Scholar] [CrossRef]
- Islam, M.M.M.; Iqbal, M.S.; D’Souza, N.; Islam, M.A. A review on present and future microbial surface water quality worldwide. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100523. [Google Scholar] [CrossRef]
- Liang, L.; Goh, S.G.; Vergara, G.G.R.V.; Fang, H.M.; Rezaeinejad, S.; Chang, S.Y.; Bayen, S.; Lee, W.A.; Sobsey, M.D.; Rose, J.B.; et al. Alternative fecal indicators and their empirical relationships with Enteric Viruses, Salmonella enterica, and Pseudomonas aeruginosa in surface waters of a tropical urban catchment. Appl. Environ. Microbiol. 2015, 81, 850–860. [Google Scholar] [CrossRef]
- Clark, P.A.; Pinedo, C.A.; Fadus, M.; Capuzzi, S. Slow-sand water filter: Design, implementation, accessibility and sustainability in developing countries. Med. Sci. Monit. 2012, 18, RA105–RA117. [Google Scholar] [CrossRef]
- Visscher, J.T. Slow sand filtration: Design, Operation, and Maintenance. J. Am. Water Work. Assoc. 1990, 82, 67–71. [Google Scholar] [CrossRef]
- Logsdon, G.S.; Sorg, T.J.; Clark, R.M. Capability and Cost of Treatment Technologies for Small Systems. J. Am. Water Work. Assoc. 1990, 82, 60–66. [Google Scholar] [CrossRef]
- Verma, S.; Daverey, A.; Sharma, A. Slow sand filtration for water and wastewater treatment—A review. Environ. Technol. Rev. 2017, 6, 47–58. [Google Scholar] [CrossRef]
- Logsdon, G.S.; Kohne, R.; Abel, S.; LaBonde, S. Slow sand filtration for small water systems. J. Environ. Eng. Sci. 2002, 68, 100–108. [Google Scholar] [CrossRef]
- Cleary, S. Sustainable Drinking Water Treatment for Small Communities using Multistage Slow Sand Filtration. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2005. [Google Scholar]
- Gottinger, A.M.; McMartin, D.W.; Price, D.; Hanson, B. The effectiveness of slow sand filters to treat Canadian rural prairie water. Can. J. Civ. Eng. 2011, 38, 455–463. [Google Scholar] [CrossRef]
- Collins, M.R.; Eighmy, T.T.; Malley, J.P. Evaluating Modifications to Slow Sand Filters. J. Am. Water Work. Assoc. 1991, 83, 62–70. [Google Scholar] [CrossRef]
- Guchi, E. Review on slow sand filtration in removing microbial contamination and particles from drinking water. Research 2015, 3, 47–55. [Google Scholar] [CrossRef]
- Weber-Shirk, M.; Dick, R. Physical–chemical mechanisms in slow sand filters. J. Am. Water Work. Assoc. 1997, 89, 87–100. [Google Scholar] [CrossRef]
- Montgomery, J.M. Water Treatment: Principles and Design; John Wiley & Sons: Hoboken, NJ, USA, 1985; pp. 1–432. [Google Scholar]
- Galvis, G.; Latorre, J.; Visscher, J.T. Multi-Stage Filtration: An Innovative Water Treatment Technology; TP Series; IRC International Water and Sanitation Center: The Hague, The Netherlands, 1998; Volume 34. [Google Scholar]
- Mc Connell, L.J. Evaluation of the Slow Rate Sand Filtration Process for Treatment of Drinking Water Containing Virus and Bacteria. Master’s Thesis, Utah State University, Logan, UT, USA, 1984. [Google Scholar]
- Ellis, K.V.; Wood, W.E. Slow sand filtration. CRC Crit. Rev. Environ. Control. 1985, 15, 315–354. [Google Scholar] [CrossRef]
- Van Dijk, J.C.; Ooman, J.H.C. Slow Sand Filtration for Community Water Supply in Developing Countries: A Design and Construction manual; WHO International Reference Center for Community Water Supply: The Hague, Netherlands, 1978; Chapter 4. [Google Scholar]
- Troyan, J.J.; Hansen, S.P. Treatment of Microbial Contaminants in Potable Water Supplies; Noyes Data Corporation: Park Ridge, IL, USA, 1989; pp. 5–54. [Google Scholar]
- Yahya, M.T.; Cluff, C.B.; Gerba, C.P. Virus removal by slow sand filtration and nanofiltration. Water Sci. Technol. 1993, 27, 445–448. [Google Scholar] [CrossRef]
- Cullen, T.R.; Letterman, R.D. The Effect of Slow Sand Filter Maintenance on Water Quality. J. Am. Water Work. Assoc. 1985, 77, 48–55. [Google Scholar] [CrossRef]
- Campos, L.C.; Su, M.F.; Graham, N.J.; Smith, S.R. Biomass development in slow sand filters. Water Res. 2002, 18, 4543–4551. [Google Scholar] [CrossRef]
- Poynter, S.F.B.; Slade, J.S. The removal of viruses by slow sand filtration. Prog. Water Technol. 1977, 9, 75–88. [Google Scholar]
- Bellamy, W.D.; Silverman, G.P.; Hendricks, D.W. Filtration of Giardia Cysts and Other Substances: Volume 2, Slow Sand Filtration; U.S. Environmental Protection Agency, Water Engineering Research Laboratory: Cincinnati, OH, USA, 1985. [Google Scholar]
- Slow Sand Filtration; Tech Brief Fourteen; National Drinking Water Clearinghouse (U.S.): Morgantown, WV, USA, 2000.
- Eighmy, T.T.; Collins, M.R. Modifications to the slow rate Filtration Process for Improved Trihalometane precursor Removal. In Slow Sand Filtration: Recent Developments in Water Treatment Technology; Graham, N.J.D., Ed.; Ellis Horwood Ltd.: England, UK, 1988; pp. 1–97. [Google Scholar]
- Edzwald, J.K. American Water Works Association. Water Quality & Treatment, a Handbook on Drinking Water, 6th ed.; McGraw-Hill: New York, NY, USA, 2010. [Google Scholar]
- Biosand Filter Manual: Design, Construction, & Installation; Centre for Affordable Water and Sanitation Technology: Calgary, AB, Canada, 2007.
- Haarhoff, J.; Cleasby, J.L. Biological and Physical Mechanisms in Slow Sand Filtration; Logsdon, G.S., Ed.; SSF American Society of Civil Engineers: New York, NY, USA, 1991; pp. 19–68. [Google Scholar]
- Weber-Shirk, M.; Dick, R. Biological mechanisms in slow sand filters. J. Am. Water Work. Assoc. 1997, 89, 72–83. [Google Scholar] [CrossRef]
- Weber-Shirk, M.; Dick, R. Bacterivory by a chrysophyte in slow sand filters. Water Res. 1999, 33, 631–638. [Google Scholar] [CrossRef]
- Bauer, R.; Dizer, H.; Graeber, I.; Rosenwinkel, K.-H.; López-Pilaa, J.M. Removal of bacterial fecal indicators, coliphages and enteric adenoviruses from waters with high fecal pollution by slow sand filtration. Water Res. 2011, 45, 439–452. [Google Scholar] [CrossRef]
- Kennedy, T.J.; Anderson, T.A.; Hernandez, E.A.; Morse, A.N. Determining the operational limits of the biosand filter. Water Sci. Technol.-Water Supply 2013, 13, 56–65. [Google Scholar] [CrossRef]
- Fewster, E.; Mol, A.; Wiessent-Brandsma, C. The Bio-sand Filter. Long term sustainability: User habits and technical performance evaluated. In Proceedings of the Presentation Given at the 2003 International Symposium on Household Technologies for Safe Water, Nairobi, Kenya, 16–17 June 2004. [Google Scholar]
- Mahmood, Q.; Baig, S.A.; Nawab, B.; Shafqat, M.N.; Pervez, A.; Zeb, B.S. Development of low cost household drinking water treatment system for the earthquake affected communities in Northern Pakistan. Desalination 2011, 273, 316–320. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). Technologies for Upgrading Existing or Designing New Drinking Water Treatment Facilities. Document No. EPA/625/4-89/023. 1990. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/300048WU.TXT (accessed on 2 March 2023).
- HDR Engineering. Handbook of Public Water Systems; John Wiley and Sons: New York, NY, USA, 2001; p. 353. ISBN 978-0-471-29211-1. Available online: https://www.wiley.com/en-ie/Handbook+of+Public+Water+Systems,+2nd+Edition-p-9780471292111 (accessed on 28 March 2023).
- Bellamy, W.D.; Hendricks, D.W.; Logsdon, G.S. Slow Sand Filtration: Influences of Selected Process Variables. J. Am. Water Work. Assoc. 1985, 77, 62–66. [Google Scholar] [CrossRef]
- Burman, N.P. Biological control of slow sand filtration. Effl. Water Treat. J. 1962, 2, 674. [Google Scholar]
- Weber-Shirk, M.L. Enhancing slow sand filter performance with an Acid- Soluble Seston Extract. Water Res. 2002, 36, 4753–4756. [Google Scholar] [CrossRef] [PubMed]
- Rooklidge, S.J.; Miner, J.R.; Kassim, T.A.; Nelson, P.O. Antimicrobial contaminant removal by multistage slow sand filtration. J. Am. Water Work. Assoc. 2005, 97, 92–100. [Google Scholar] [CrossRef]
- Lesikar, B. Sand Filters for Home Use—Texas Agricultural Extension Service. Scribd. Available online: http://www.scribd.com/doc/34621075/Sand-filters-for-home-use-Texas-Agricultural-Extension-Service (accessed on 25 January 2023).
- Logan, A.J.; Stevik, T.K.; Siegrist, R.L.; Rønn, R.M. Transport and fate of Cryptosporidium parvum oocysts in intermittent sand filters. Water Res. 2001, 35, 4359–4369. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.G. A study of bacteria reduction by slow sand filtration. In Paper Presented at the 1987 IWPC Biennial Conference, Port Elizabeth, South Africa, 12–15 May 1987; National Institute for water Research: Pretoria, South Africa, 1987. [Google Scholar]
- Peterson, H.; Corkal, D. Biological Treatment of Ground Water. Prairie Farm Rehabilitation Administration. Water Quality Matters. Available online: http://www5.agr.gc.ca/resources/prod/doc/pfra/pdf/bio_treat_groundwater_e.pdf (accessed on 25 January 2023).
- Peterson, H.; Broley, T.; Sketchell, J.; Corkal, D. ADD Board 15 and 43 Project Report: Biological Treatment of Ground Water; Publication No. R-1640-6-E-97; Saskatchewan Research Council: Saskatoon, SK, Canada, 1997. [Google Scholar]
- Kennedy, T.J.; Hernandez, E.A.; Morse, A.N.; Anderson, T.A. Hydraulic Loading Rate Effect on Removal Rates in a BioSand Filter: A Pilot Study of Three Conditions. Water Air Soil Pollut. 2012, 223, 4527–4537. [Google Scholar] [CrossRef]
- Bradley, I.; Straub, A.; Maraccini, P.; Markazi, S.; Nguyen, T.H. Iron oxide amended biosand filters for virus removal. Water Res. 2011, 45, 4501–4510. [Google Scholar] [CrossRef]
- Hussain, G.; Haydar, S.; Bari, A.J.; Aziz, J.A.; Anis, M.; Asif, Z. Evaluation of Plastic Household Biosand Filter (BSF) In Combination with Solar Disinfection (SODIS) For Water Treatment. J. Chem. Soc. Pak. 2015, 37, 352–362. [Google Scholar]
- Nitzsche, K.S.; Weigold, P.; Losekann-Behrens, T.; Kappler, A.; Behrens, S. Microbial community composition of a household sand filter used for arsenic, iron, and manganese removal from groundwater in Vietnam. Chemosphere 2015, 138, 47–59. [Google Scholar] [CrossRef]
- Lukasheva, G.N.; Yurovsky, A.V. Slow self-cleaning filter for deferrization of natural waters. Bull. Assoc. Univ. Tour. Serv. 2010, 4, 56–63. (In Russian) [Google Scholar]
- Yurovsky, A.V.; Lukasheva, G.N. Study of the efficiency of a slow self-cleaning filter. In Materials of the All-Russian Scientific Conference of Graduate Students and Young Scientists “Modern Problems of Tourism and Service”; FGSHUVPO “RGUTiS”: Moscow, Russia, 2010; pp. 270–274. (In Russian) [Google Scholar]
- Yurovsky, A.V.; Lukasheva, G.N. Formation of a chemisorption layer of the filter bed of a slow self-cleaning iron removal filter. In Materials of the All-Russian Scientific Conference of Graduate Students and Young Scientists “Modern Problems of Tourism and Service”; FGSHUVPO “RGUTiS”: Moscow, Russia, 2010; pp. 274–278. (In Russian) [Google Scholar]
- Sultanov, F.; Daulbayev, C.; Azat, S.; Kuterbekov, K.; Bekmyrza, K.; Bakbolat, B.; Bigaj, M.; Mansurov, Z. Influence of Metal Oxide Particles on Bandgap of 1D Photocatalysts Based on SrTiO3/PAN Fibers. Nanomaterials 2020, 10, 1734. [Google Scholar] [CrossRef]
- Rakhmanin, Y.A.; Cheskis, A.B. Drinking Water. Quality Standards. Handbook; VINITI: Moscow, Russia, 1993; pp. 1–7, 19, 26, 46. [Google Scholar]
- Grützmacher, G.; Böttcher, G.; Chorus, I.; Bartel, H. Removal of microcystins by slow sand filtration. Environ. Toxicol. 2002, 17, 386–394. [Google Scholar] [CrossRef]
- Onan, L.; LaPara, T. Tylosin-resistant Bacteria Cultivated from Agricultural Soil. FEMS Toxicol. Lett. 2003, 220, 15–24. [Google Scholar] [CrossRef] [PubMed]
- De Liguoro, M.; Cibin, V.; Capolongo, F.; Halling-Sørensen, B.; Montesissa, C. Use of oxytetracycline and tylosin in intensive calf farming: Evaluation of transfer to manure and soil. Chemosphere 2003, 52, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Inglezakis, V.J.; Azat, S.; Tauanov, Z.; Mikhalovsky, S.V. Functionalization of biosourced silica and surface reactions with mercury in aqueous solutions. Chem. Eng. J. 2021, 423, 129745. [Google Scholar] [CrossRef]
- Pachocka, M. Intermittent Slow Sand Filters: Improving their Design for Developing World Applications. Master’s Thesis, University of Delaware, Newark, DE, USA, 2010. [Google Scholar]
- Partinoudi, V.; Collins, M.R.; Dwyer, P.L.; Martin-Doole, M. Assessing Temperature Influences on Slow Sand Filtration Treatment Performance. Project Summary, New England Water Treatment Technology Assistance Center, Univ. of New Hampshire. 2007. Available online: http://www.unh.edu/wttac/Project_Summaries/assessing_temperature_slow_sand.pdf (accessed on 14 January 2023).
- Fogel, D.; Isaac-Renton, J.; Guasparini, R.; Moorehead, W.; Ongerth, J. Removing giardia and cryptosporidium by slow sand filtration. J. Am. Water Work. Assoc. 1993, 85, 77–84. [Google Scholar] [CrossRef]
- Liu, J.; Cao, X.; Meng, X. Effects of temperature on performances of a slow sand filter used for advanced wastewater treatment. Chin. J. Environ. Eng. 2010, 4, 2437–2440. [Google Scholar]
- Schijven, J.F.; van den Berg, H.H.J.L.; Colin, M.; Dullemont, Y.; Hijnen, W.A.M.; Magic-Knezev, A.; Oorthuizen, W.A.; Wubbels, G. A mathematical model for removal of human pathogenic viruses and bacteria by slow sand filtration under variable operational conditions. Water Res. 2013, 47, 2592–2602. [Google Scholar] [CrossRef]
- Jabur, H.S.; Gimbel, R.; Graham, N.J.D.; Collins, M.R. The Effect of Water Temperature on the Slow Sand Filter Process. Recent Progress in Slow Sand and Alternative Biofiltration Processes; IWA: London, UK, 2006; Volume 5, p. 582. [Google Scholar]
- Unger, M.; Collins, M.R. Assessing Escherichia coli removal in the schmutzdecke of slow-rate biofilters. J. Am. Water Works Ass. 2008, 100, 60–73. [Google Scholar] [CrossRef]
- Welte, B.; Montiel, A. Removal of BDOC by Slow Sand Filtration: Comparison with granular activated carbon and effect of temperature. In Advances in Slow Sand and Alternative Biological Filtration; Graham, N., Collins, R., Eds.; John Wiley & Sons Ltd.: England, UK, 1996; p. 60. [Google Scholar]
- Fitriani, N.; Wahyudianto, F.E.; Salsabila, N.F.; Mohamed, R.M.S.R.; Kurniawan, S.B. Performance of modified slow sand filter to reduce turbidity, total suspended solids, and iron in river water as water treatment in disaster areas. J. Ecol. Eng. 2023, 24, 1–18. [Google Scholar] [CrossRef]
- Escola Casas, M.; Guivernau, M.; Vinas, M.; Fernandez, B.; Caceres, R.; Biel, C.; Matamoros, V. Use of wood and cork in biofilters for the simultaneous removal of nitrates and pesticides from groundwater. Chemosphere 2023, 313, 137502. [Google Scholar] [CrossRef]
- Holmes, E.B.; Oza, H.H.; Bailey, E.S.; Sobsey, M.D. Evaluation of chitosans as coagulants—Flocculants to improve sand filtration for drinking water treatment. Inter. J. Mol. Sci. 2023, 24, 1295. [Google Scholar] [CrossRef]
- Liu, H.-L.; Li, X.; Li, N. Application of bio-slow sand filters for drinking water production: Linking purification performance to bacterial community and metabolic functions. J. Water Process Eng. 2023, 53, 103622. [Google Scholar] [CrossRef]
- Fava, N.M.N.; Terin, U.C.; Freitas, B.L.S.; Sabogal-Paz, L.P.; Fernandez-Ibanez, P.; Byrne, J.A. Household slow sand filters in continuous and intermittent flows and their efficiency in microorganism’s removal from river water. Environ. Technol. 2022, 43, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Lamon, A.W.; Faria Maciel, P.M.; Campos, J.R.; Corbi, J.J.; Dunlop, P.S.M.; Fernandez-Ibanez, P.; Byrne, J.A.; Sabogal-Paz, L.P. Household slow sand filter efficiency with schmutzdecke evaluation by microsensors. Environ. Technol. 2022, 43, 4042–4053. [Google Scholar] [CrossRef]
- Vu, C.T.; Wu, T. Enhanced slow sand filtration for the removal of micropollutants from groundwater. Sci. Total Environ. 2022, 809, 152161. [Google Scholar] [CrossRef]
- Zhang, M.; He, L.; Zhang, X.; Wang, S.; Zhang, B.; Hsieh, L.; Yang, K.; Tong, M. Improved removal performance of Gram-negative and Gram-positive bacteria in sand filtration system with arginine modified biochar amendment. Water Res. 2022, 211, 118006. [Google Scholar] [CrossRef]
- Rosa e Silva, G.O.; Loureiro, H.O.; Soares, L.G.; de Andrade, L.H.; Santos, R.G.L. Evaluation of an alternative household water treatment system based on slow filtration and solar disinfection. J. Water Health 2022, 20, 157–166. [Google Scholar] [CrossRef]
- Ribeiro, M.P.; Botari, A. Evaluation of effluent post-treatment by slow filtration and adsorption with activated carbon produced from spent coffee grounds in surfactant removal in sewage treatment. Rev. Ambiente Agua 2022, 17, E2756. [Google Scholar] [CrossRef]
- Mohammed, M.O.A.; Solumon, A.A.M. Two models of household sand filters for small scale water purification. Pol. J. Environ. Stud. 2022, 31, 2737–2748. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).