The Effect of Nitrate-Contaminated Drinking Water and Vegetables on the Prevalence of Acquired Methemoglobinemia in Beit Lahia City in Palestine
Abstract
:1. Introduction
2. Materials and Methods
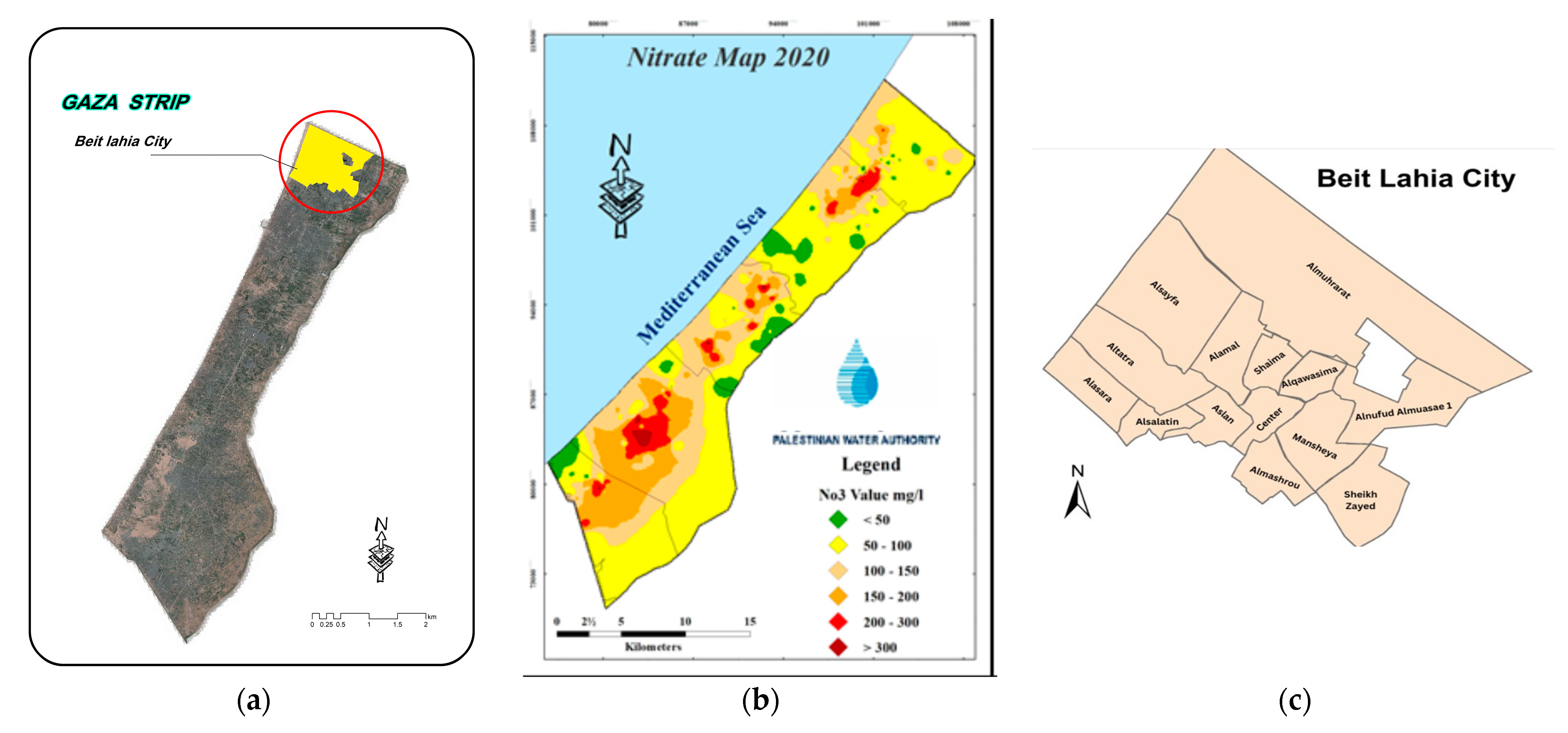
2.1. Description of the Study Area
2.2. Study Setting and Population Characteristics
2.3. Questionnaire Tools Development
2.4. Water Sampling and Analysis
2.5. Vegetable Sampling and Analysis
2.6. Sample Collection and Analysis of Blood
2.7. Risk Assessment
2.8. Statistical Analysis
3. Results
3.1. Nitrate Levels in Drinking Water
Risk Assessment Results
3.2. Nitrate Levels in Vegetables
3.3. Prevalence of Methemoglobinemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sachs, J.; Kroll, C.; Lafortune, G.; Fuller, G.; Woelm, F. Sustainable Development Report 2022; Cambridge University Press: Cambridge, UK, 2022; ISBN 978-1-00-921008-9. [Google Scholar]
- Ward, M.; Jones, R.; Brender, J.; de Kok, T.; Weyer, P.; Nolan, B.; Villanueva, C.; van Breda, S. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [PubMed]
- Picetti, R.; Deeney, M.; Pastorino, S.; Miller, M.R.; Shah, A.; Leon, D.A.; Dangour, A.D.; Green, R. Nitrate and Nitrite Contamination in Drinking Water and Cancer Risk: A Systematic Review with Meta-Analysis. Environ. Res. 2022, 210, 112988. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Marapana, R. Nitrate and Nitrite Content of Vegetables: A Review. J. Pharmacogn. Phytochem. 2018, 7, 322–328. [Google Scholar]
- van den Brand, A.D.; Beukers, M.; Niekerk, M.; van Donkersgoed, G.; van der Aa, M.; van de Ven, B.; Bulder, A.; van der Voet, H.; Sprong, C.R. Assessment of the Combined Nitrate and Nitrite Exposure from Food and Drinking Water: Application of Uncertainty around the Nitrate to Nitrite Conversion Factor. Food Addit. Contam. Part A 2020, 37, 568–582. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Nitrate and Nitrite in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Aires, A.; Carvalho, R.; Rosa, E.A.S.; Saavedra, M.J. Effects of Agriculture Production Systems on Nitrate and Nitrite Accumulation on Baby-leaf Salads. Food Sci. Nutr. 2013, 1, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Wagh, V.M.; Panaskar, D.B.; Mukate, S.V.; Aamalawar, M.L.; Laxman Sahu, U. Nitrate Associated Health Risks from Groundwater of Kadava River Basin Nashik, Maharashtra, India. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 654–672. [Google Scholar] [CrossRef]
- Golaki, M.; Azhdarpoor, A.; Mohamadpour, A.; Derakhshan, Z.; Conti, G.O. Health Risk Assessment and Spatial Distribution of Nitrate, Nitrite, Fluoride, and Coliform Contaminants in Drinking Water Resources of Kazerun, Iran. Environ. Res. 2022, 203, 111850. [Google Scholar] [CrossRef]
- Karunanidhi, D.; Aravinthasamy, P.; Subramani, T.; Kumar, M. Human Health Risks associated with Multipath Exposure of Groundwater Nitrate and Environmental Friendly Actions for Quality Improvement and Sustainable Management: A Case Study from Texvalley (Tiruppur Region) of India. Chemosphere 2021, 265, 129083. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, G.; Subramani, T.; Sathya, U.; Roy, P.D. Seasonal Changes in Groundwater Composition in an Industrial Center of South India and Quality Evaluation for Consumption and Health Risk Using Geospatial Methods. Geochemistry 2020, 80, 125651. [Google Scholar] [CrossRef]
- Ludlow, J.T.; Wilkerson, R.G.; Nappe, T.M. Methemoglobinemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Maran, J.; Guan, Y.; Ou, C.-N.; Prchal, J.T. Heterogeneity of the Molecular Biology of Methemoglobinemia: A Study of Eight Consecutive Patients. Haematologica 2005, 90, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Fossen Johnson, S. Methemoglobinemia: Infants at Risk. Curr. Probl. Pediatr. Adolesc. Health Care 2019, 49, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J. Hematology: Basic Principles and Practice. Bone Marrow Transplant. 2013, 48, 1144. [Google Scholar] [CrossRef]
- Shomar, B. Retraction Note: Groundwater Contaminations and Health Perspectives in Developing World Case Study: Gaza Strip. Environ. Geochem. Health 2012, 34, 151. [Google Scholar] [CrossRef] [PubMed]
- Al-Najar, H.; Al-Dalou, F.; Snounu, I.; Al-Dadah, J. Framework Analysis of Socio-Economic and Health Aspects of Nitrate Pollution from Urban Agricultural Practices: The Gaza Strip as a Case Study. J. Agric. Environ. Sci. 2014, 3, 355–370. [Google Scholar]
- Qrenawi, L.I.; Shomar, R.A. Health Risk Assessment of Groundwater Contamination Case Study: Gaza Strip. J. Eng. Res. Technol. 2020, 7. [Google Scholar] [CrossRef]
- Palestinian Central Bureau of Statistics-PCBS. Preliminary Results of the Population, Housing and Establishments Census 2017; Palestinian Central Bureau of Statistics-PCBS: Ramallah, Palestine, 2018. [Google Scholar]
- Water Resources Directorate. 2020 Water Resources Status Summary Report/Gaza Strip; Unpublished Report; Water Resources Directorate: Ramallah, Palestine, 2021. [Google Scholar]
- Baird, R.; Eaton, A.D.; Rice, E.W.; Bridgewater, L. (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; ISBN 978-0-87553-287-5. [Google Scholar]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Statement on Possible Public Health Risks for Infants and Young Children from the Presence of Nitrates in Leafy Vegetables|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/1935 (accessed on 17 February 2023).
- Qasemi, M.; Afsharnia, M.; Farhang, M.; Bakhshizadeh, A.; Allahdadi, M.; Zarei, A. Health Risk Assessment of Nitrate Exposure in Groundwater of Rural Areas of Gonabad and Bajestan, Iran. Environ. Earth Sci. 2018, 77, 551. [Google Scholar] [CrossRef]
- US EPA. Conducting a Human Health Risk Assessment. Available online: https://www.epa.gov/risk/conducting-human-health-risk-assessment (accessed on 17 February 2023).
- Assessment, U.E.N.C. for ENitrate CASRN 14797-55-8|IRIS|US EPA, ORD. Available online: https://iris.epa.gov/ChemicalLanding/&substance_nmbr=76 (accessed on 17 February 2023).
- Rezaei, H.; Jafari, A.; Kamarehie, B.; Fakhri, Y.; Ghaderpoury, A.; Karami, M.A.; Ghaderpoori, M.; Shams, M.; Bidarpoor, F.; Salimi, M. Health-Risk Assessment Related to the Fluoride, Nitrate, and Nitrite in the Drinking Water in the Sanandaj, Kurdistan County, Iran. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1242–1250. [Google Scholar] [CrossRef]
- Module Three—Lecture Notes|Toxicology Curriculum for Communities Trainer’s Manual|ATSDR. Available online: https://www.atsdr.cdc.gov/training/toxmanual/modules/3/lecturenotes.html (accessed on 21 February 2023).
- Calculating Hazard Quotients and Cancer Risk Estimates. Available online: https://www.atsdr.cdc.gov/pha-guidance/conducting_scientific_evaluations/epcs_and_exposure_calculations/hazardquotients_cancerrisk.html (accessed on 21 February 2023).
- Sanchez-Echaniz, J.; Benito-Fernández, J.; Mintegui-Raso, S. Methemoglobinemia and Consumption of Vegetables in Infants. Pediatrics 2001, 107, 1024–1028. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No.1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. UNEP Law and Environment Assistance Platform. Available online: https://leap.unep.org/countries/eu/national-legislation/commission-regulation-ec-no-18812006-setting-maximum-levels (accessed on 17 February 2023).
- Hord, N.G.; Ghannam, J.S.; Garg, H.K.; Berens, P.D.; Bryan, N.S. Nitrate and Nitrite Content of Human, Formula, Bovine, and Soy Milks: Implications for Dietary Nitrite and Nitrate Recommendations. Breastfeed. Med. 2011, 6, 393–399. [Google Scholar] [CrossRef]
- Colla, G.; Kim, H.-J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in Fruits and Vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Pagana, K.D. 1952—Mosby’s Diagnostic and Laboratory Test Reference, 7th ed.; Elsevier Mosby: St. Louis, MO, USA, 2005. [Google Scholar]
- Palestinian Ministry of Health. Hospital Reports; Unpublished Report; Palestinian Ministry of Health: Ramallah, Palestine, 2022. [Google Scholar]
- Abu Naser, A.A.; Ghbn, N.; Khoudary, R. Relation of Nitrate Contamination of Groundwater with Methemoglobin Level among Infants in Gaza. EMHJ—East. Mediterr. Health J. 2007, 13, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Sadeq, M.; Moe, C.L.; Attarassi, B.; Cherkaoui, I.; ElAouad, R.; Idrissi, L. Drinking Water Nitrate and Prevalence of Methemoglobinemia among Infants and Children Aged 1–7 Years in Moroccan Areas. Int. J. Hyg. Environ. Health 2008, 211, 546–554. [Google Scholar] [CrossRef]
- Abu-Dayeh, A.G.H. Determination of Nitrate and Nitrite Content in Several Vegetables in Tulkarm District. 2007. Available online: https://hdl.handle.net/20.500.11888/7664 (accessed on 10 April 2023).
- Sebaei, A.S.; Refai, H.M. Hazard Index: Probabilistic Risk Exposure of Nitrate and Nitrite in Egyptian Fruits and Vegetables. Int. J. Environ. Anal. Chem. 2021, 101, 1477–1484. [Google Scholar] [CrossRef]
- Taneja, P.; Labhasetwar, P.; Nagarnaik, P. Nitrate in Drinking Water and Vegetables: Intake and Risk Assessment in Rural and Urban Areas of Nagpur and Bhandara Districts of India. Environ. Sci. Pollut. Res. 2019, 26, 2026–2037. [Google Scholar] [CrossRef]
- Zendehbad, M.; Mostaghelchi, M.; Mojganfar, M.; Cepuder, P.; Loiskandl, W. Nitrate in Groundwater and Agricultural Products: Intake and Risk Assessment in Northeastern Iran. Environ. Sci. Pollut. Res. 2022, 29, 78603–78619. [Google Scholar] [CrossRef]
- Salehzadeh, H.; Maleki, A.; Rezaee, R.; Shahmoradi, B.; Ponnet, K. The Nitrate Content of Fresh and Cooked Vegetables and Their Health-Related Risks. PLoS ONE 2020, 15, e0227551. [Google Scholar] [CrossRef]
- McNulty, R.; Kuchi, N.; Xu, E.; Gunja, N. Food-induced Methemoglobinemia: A Systematic Review. J. Food Sci. 2022, 87, 1423–1448. [Google Scholar] [CrossRef]
- Cannata, G.; Abate, L.; Scarabello, C.; Rubini, M.; Giacometti, A.; Principi, N.; Esposito, S.; Dodi, I. The Dose Makes the Poison: A Case Report of Acquired Methemoglobinemia. Int. J. Environ. Res. Public Health 2020, 17, 1845. [Google Scholar] [CrossRef]
- Savino, F.; Maccario, S.; Guidi, C.; Castagno, E.; Farinasso, D.; Cresi, F.; Silvestro, L.; Mussa, G.C. Methemoglobinemia Caused by the Ingestion of Courgette Soup Given in Order to Resolve Constipation in Two Formula-Fed Infants. Ann. Nutr. Metab. 2006, 50, 368–371. [Google Scholar] [CrossRef]
- Murone, A.-J.B.; Stucki, P.; Roback, M.G.; Gehri, M. Severe Methemoglobinemia Due to Food Intoxication in Infants. Pediatr. Emerg. Care 2005, 21, 536–538. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.Y.K. Vegetable-Borne Nitrate and Nitrite and the Risk of Methemoglobinemia. Toxicol. Lett. 2011, 200, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Shomar, R.T.A.; Qrenawi, L.I. Mapping Potential Health Risk Vulnerability in Relation to Nitrate Pollution of Water in Gaza, Palestine. Res. Sq. 2022, in review. [Google Scholar] [CrossRef]
| Variable | Category | N | Percent % | Significance p-Value 1 |
|---|---|---|---|---|
| Gender | Male | 51 | 58.6 | 0.496 |
| Female | 36 | 41.4 | ||
| Age group (month) | 1 month | 3 | 3.4 | 0.798 |
| 2 months | 2 | 2.3 | ||
| 3 months | 7 | 8 | ||
| 6 months | 21 | 24.1 | ||
| 6–12 months | 54 | 62.1 | ||
| Location | Al-Shaimaa health center | 41 | 47.2 | |
| Al-Atatra health center | 46 | 52.8 |
| Desalination Plant Unit | Production (m3/h) | Local Coordination | Nitrate Concentration (mg/L) | ||
|---|---|---|---|---|---|
| X | Y | In | Out | ||
| El-barka | 12.6 | 103,994 | 105,111 | 144.0 | 16.7 |
| El-weam | 4.8 | 103,994 | 105,738 | 100.2 | 7.2 |
| Aladham | 8.4 | 104,753 | 106,103 | 169.4 | 6.8 |
| Alzaeem | 12.6 | 102,872 | 106,124 | 100.6 | 10.2 |
| Dar al-salam | 5.4 | 101,818 | 107,255 | 161.9 | 5.2 |
| El-neama | 11.1 | 100,429 | 106,892 | 180.6 | 12.7 |
| Desalination Plant Unit | Examined Water | Minimum (mg/L) | Maximum (mg/L) | X ± 2SD (95%) |
|---|---|---|---|---|
| El-Barka | Feeding water | 20.6 | 199.00 | 144.75 ± 49.02 |
| Treated water | 1 | 23.70 | 16.47 ± 7.38 | |
| El-weam | Feeding water | 66.5 | 143.60 | 100.25 ±21.08 |
| Treated water | 0 | 10.00 | 7.29 ± 2.60 | |
| Aladham | Feeding water | 136.3 | 209.00 | 169.4± 18.24 |
| Treated water | 0 | 9.60 | 6.87 ± 2.53 | |
| Alzaeem | Feeding water | 9.0 | 132.80 | 100.66 ± 32.83 |
| Treated water | 0 | 17.00 | 10.24 ± 4.21 | |
| Dar al-salam | Feeding water | 123.7 | 182.00 | 161.94 ± 16.83 |
| Treated water | 0 | 11.90 | 5.22 ± 2.72 | |
| El-neama | Feeding water | 145.0 | 323.30 | 180.60 ± 46.61 |
| Treated water | 0 | 16.23 | 12.75 ± 4.86 |
| Desalination Plants | Drinking Water | Groundwater | ||
|---|---|---|---|---|
| CDI | HQ | CDI | HQ | |
| El-Barka | 1.3392 | 0.8370 | 11.5800 | 7.2375 |
| El-weam | 0.5832 | 0.3645 | 8.0200 | 5.0125 |
| Aladham | 0.5496 | 0.3435 | 13.5552 | 8.4720 |
| Alzaeem | 0.8192 | 0.5120 | 8.0528 | 5.0330 |
| Dar al-salam | 0.4176 | 0.2610 | 12.9552 | 8.0970 |
| El-neama | 1.0200 | 0.6375 | 3.7288 | 2.3305 |
| NO3− mg/kg Fresh Weight | |||
|---|---|---|---|
| Vegetable (n = 15) | Minimum | Maximum | Average Std Dev. |
| Carrot | 204.0 | 298.6 | 237.20 ± 53.23 |
| Potatoes | 175.6 | 343.6 | 246.80 ± 81.42 |
| Zucchini | 177.1 | 327.5 | 275.86 ± 58.87 |
| Infants Age (Months) | Total (n = 87) | |||
|---|---|---|---|---|
| Maximum < 1.5% | Methemoglobin Level > 1.5% | |||
| % | N | % | N | |
| 0–1 | 2.3 | 2 | 1.1 | 1 |
| 1–2 | 2.3 | 2 | 0.0 | 0 |
| 2–3 | 3.4 | 3 | 4.6 | 4 |
| 3–6 | 18.4 | 16 | 5.7 | 5 |
| 6–12 | 41.4 | 36 | 20.7 | 18 |
| Total | 67.8 | 59 | 32.2 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaban, J.; Al-Najar, H.; Kocadal, K.; Almghari, K.; Saygi, S. The Effect of Nitrate-Contaminated Drinking Water and Vegetables on the Prevalence of Acquired Methemoglobinemia in Beit Lahia City in Palestine. Water 2023, 15, 1989. https://doi.org/10.3390/w15111989
Shaban J, Al-Najar H, Kocadal K, Almghari K, Saygi S. The Effect of Nitrate-Contaminated Drinking Water and Vegetables on the Prevalence of Acquired Methemoglobinemia in Beit Lahia City in Palestine. Water. 2023; 15(11):1989. https://doi.org/10.3390/w15111989
Chicago/Turabian StyleShaban, Jehad, Husam Al-Najar, Kumsal Kocadal, Khaled Almghari, and Sahan Saygi. 2023. "The Effect of Nitrate-Contaminated Drinking Water and Vegetables on the Prevalence of Acquired Methemoglobinemia in Beit Lahia City in Palestine" Water 15, no. 11: 1989. https://doi.org/10.3390/w15111989
APA StyleShaban, J., Al-Najar, H., Kocadal, K., Almghari, K., & Saygi, S. (2023). The Effect of Nitrate-Contaminated Drinking Water and Vegetables on the Prevalence of Acquired Methemoglobinemia in Beit Lahia City in Palestine. Water, 15(11), 1989. https://doi.org/10.3390/w15111989






