In Situ Polyaniline Immobilized ZnO Nanorods for Efficient Adsorptive Detoxification of Cr (VI) from Aquatic System
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthesis of Zinc Oxide Nanorods (ZnO NRs)
2.3. Synthesis of ZnO Immobilized PAni NRs
2.4. Apparatus Used for Characterization
2.5. Adsorption of Cr (VI) Experiments
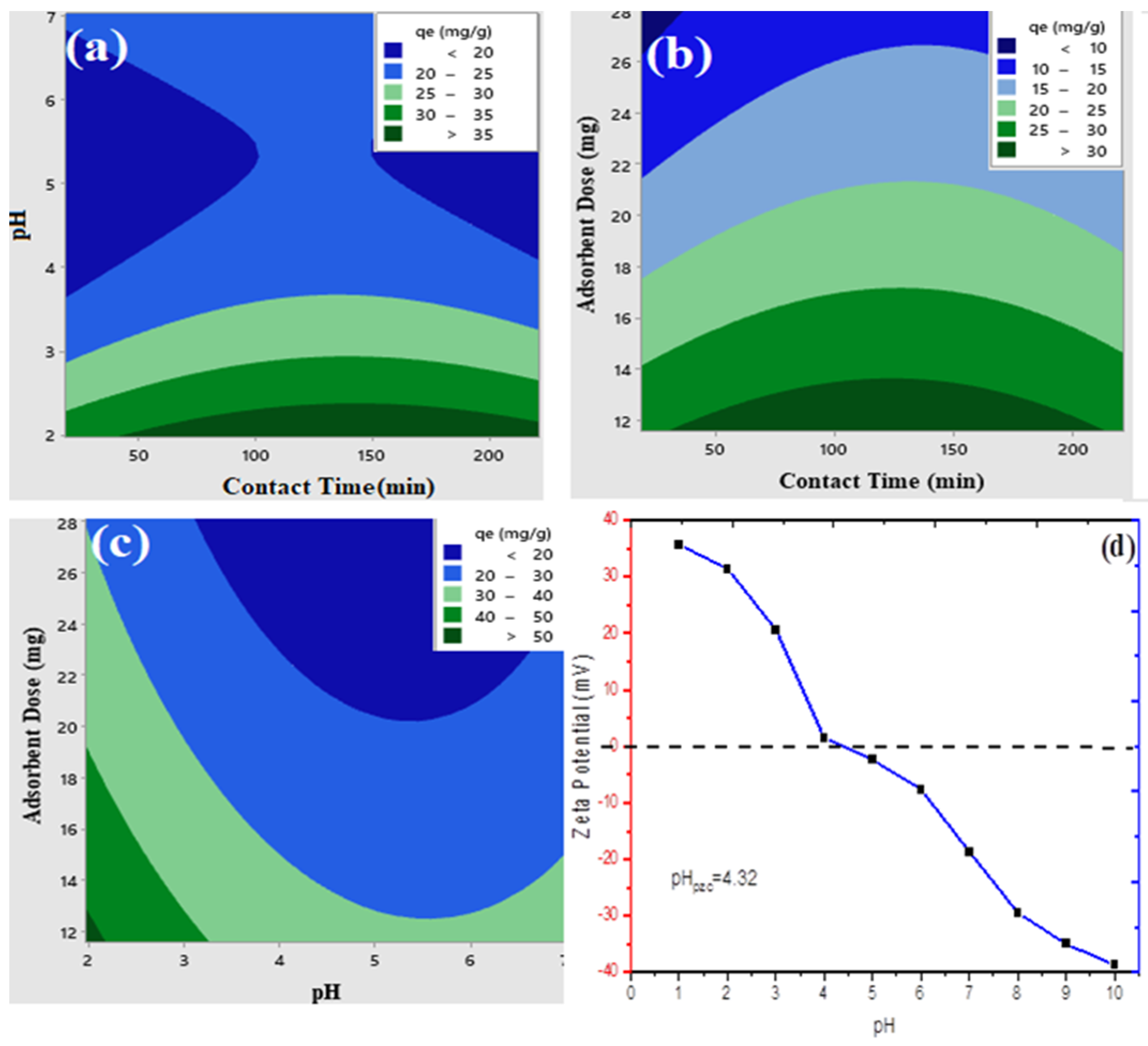
2.6. Effects of Variable Nanorod Size, Initial Cr (VI) Concentration, and Individual Constituents on Adsorption
2.7. Adsorption Isotherms
2.7.1. Langmuir Isotherm
2.7.2. Freundlich Isotherm
2.8. Adsorption Kinetics
2.8.1. Pseudo-First Order
2.8.2. Pseudo-Second Order
2.8.3. Elovich Model
2.8.4. Intraparticle Diffusion Model
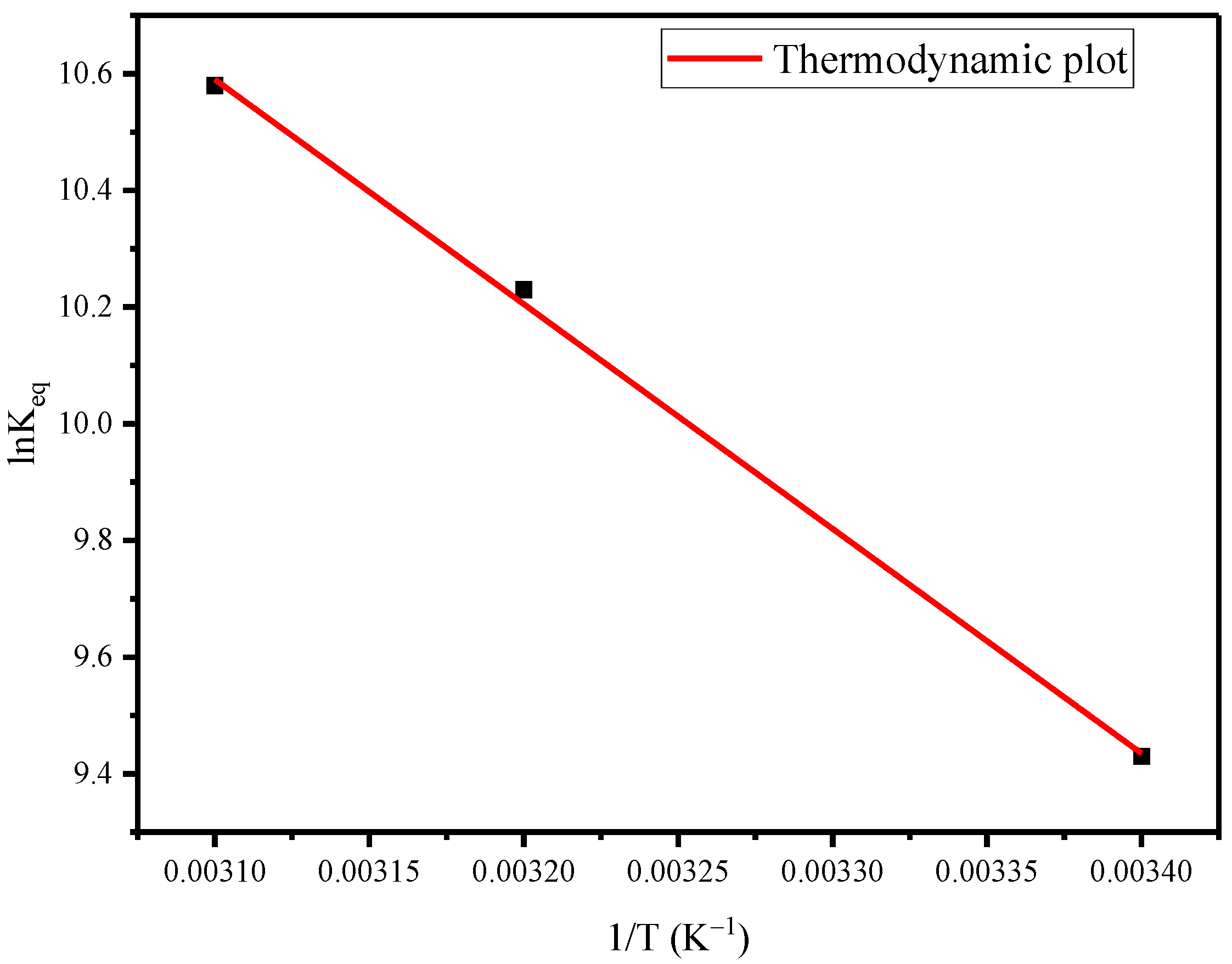
2.9. Adsorption Thermodynamics
2.10. Determination of Point of Zero Charge and Zeta Potential
3. Results and Discussion
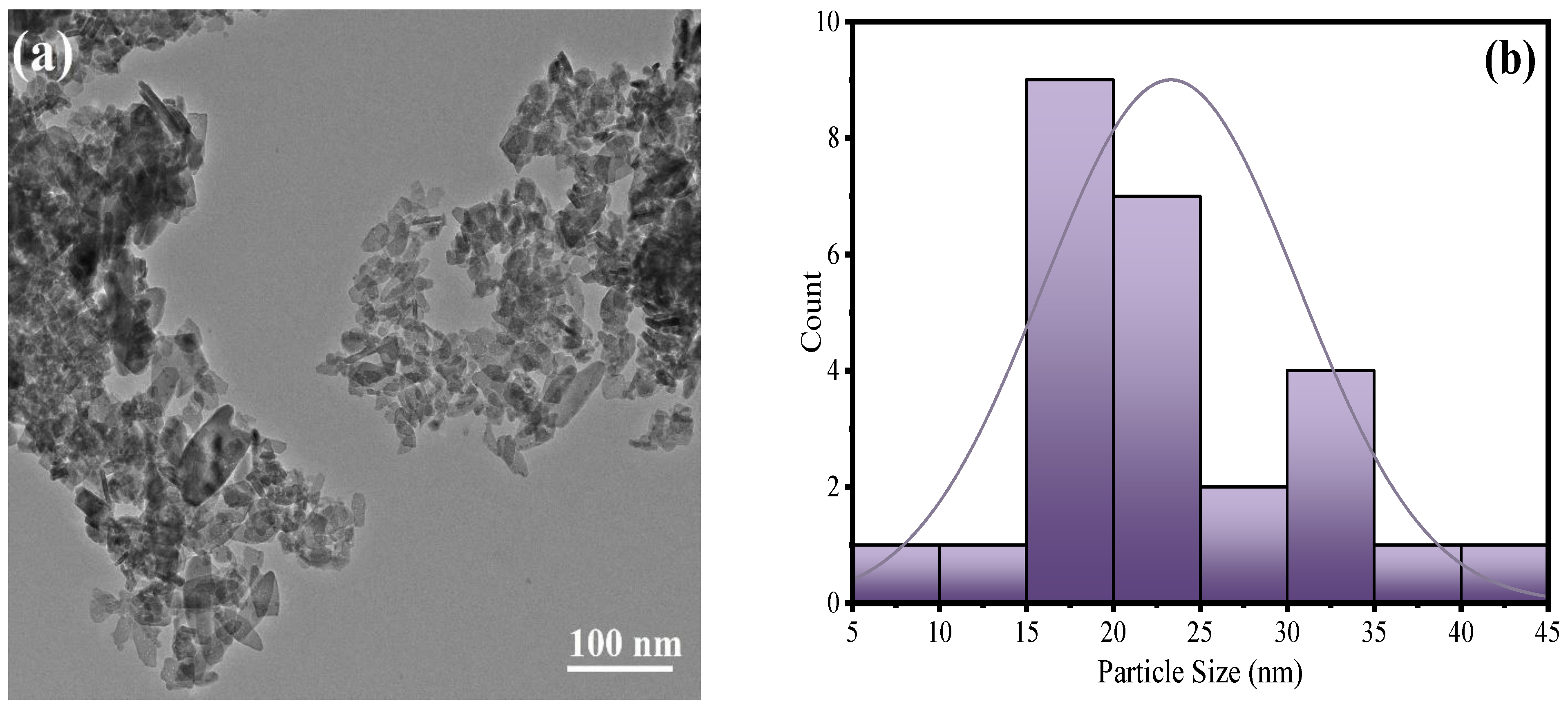
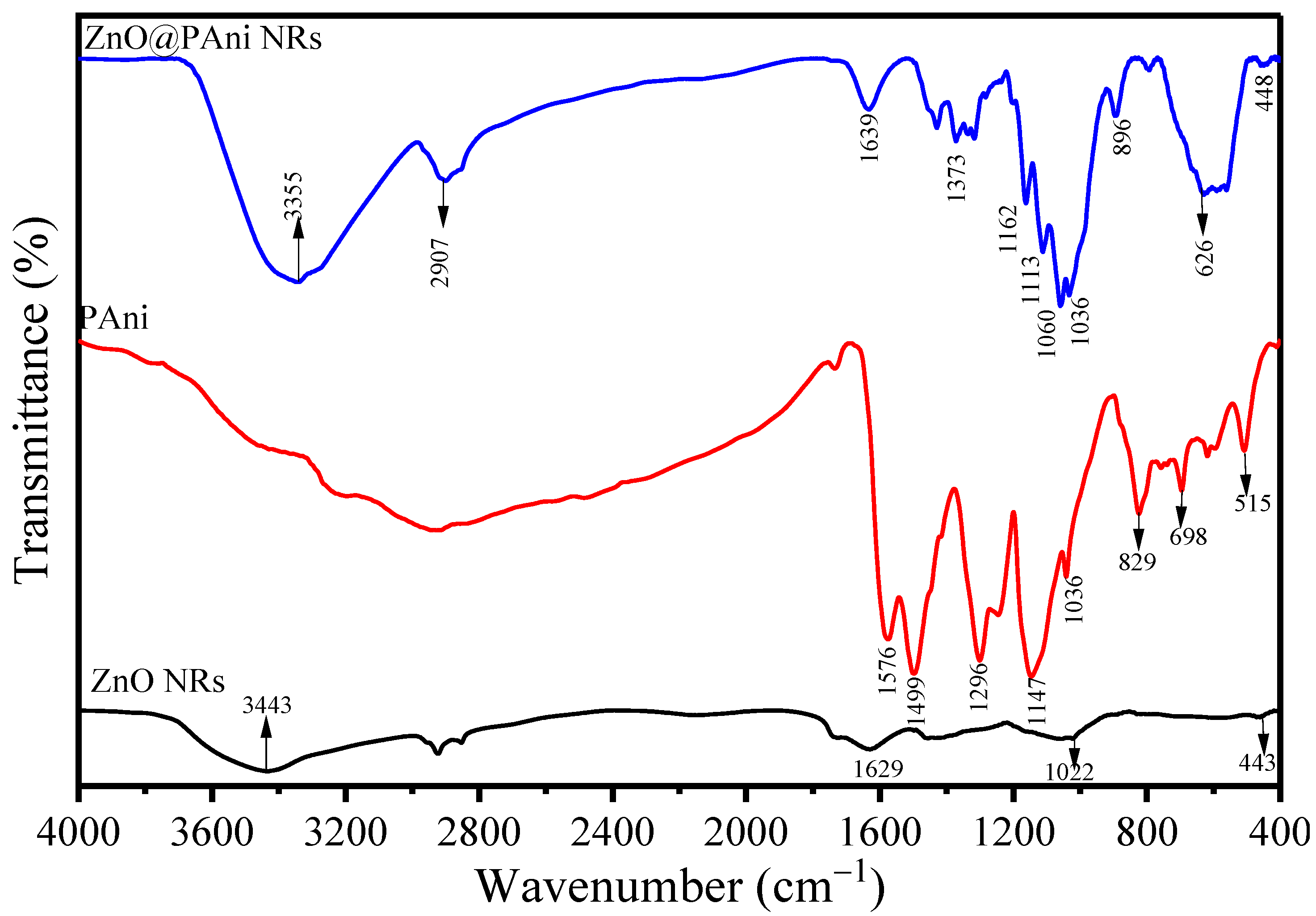
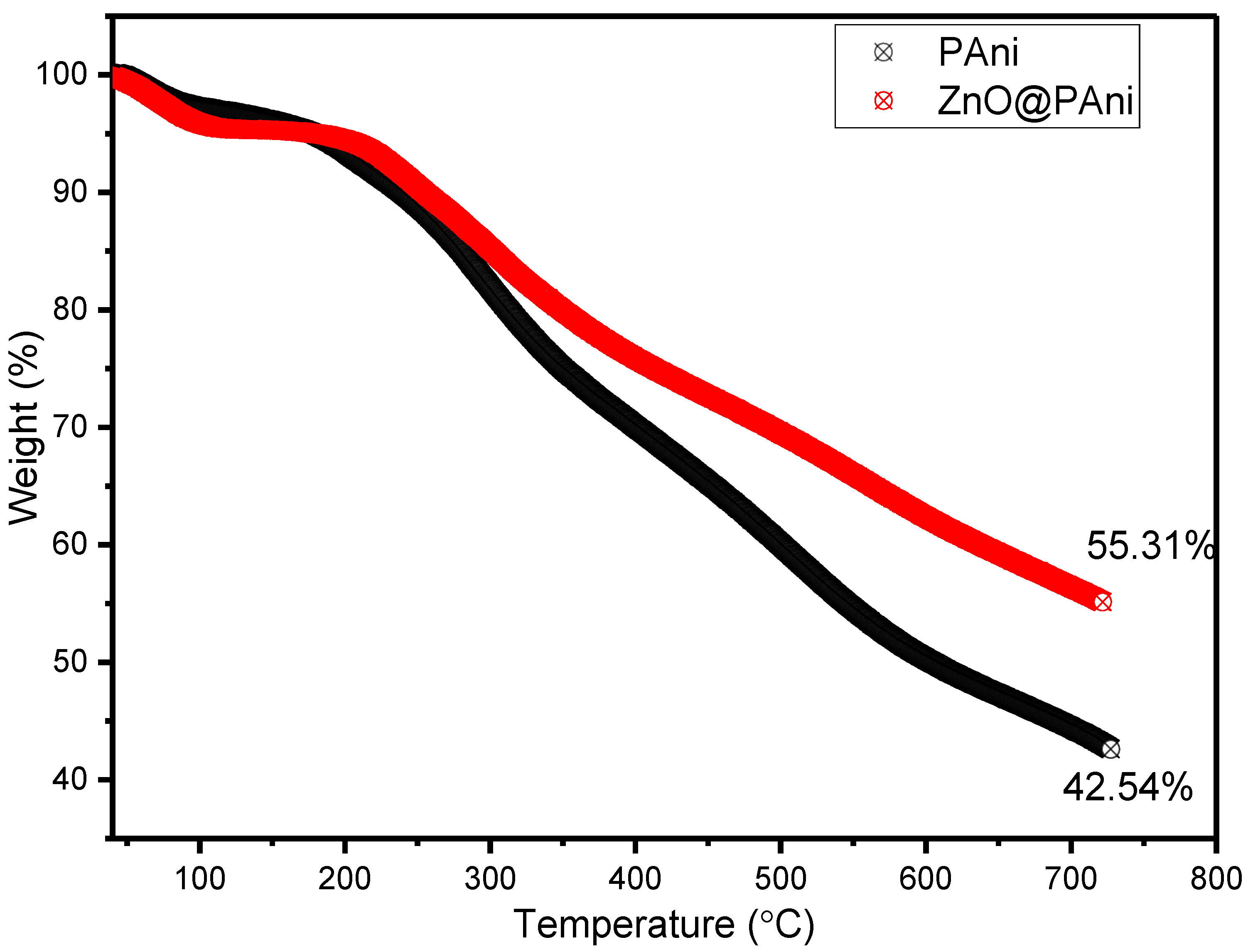
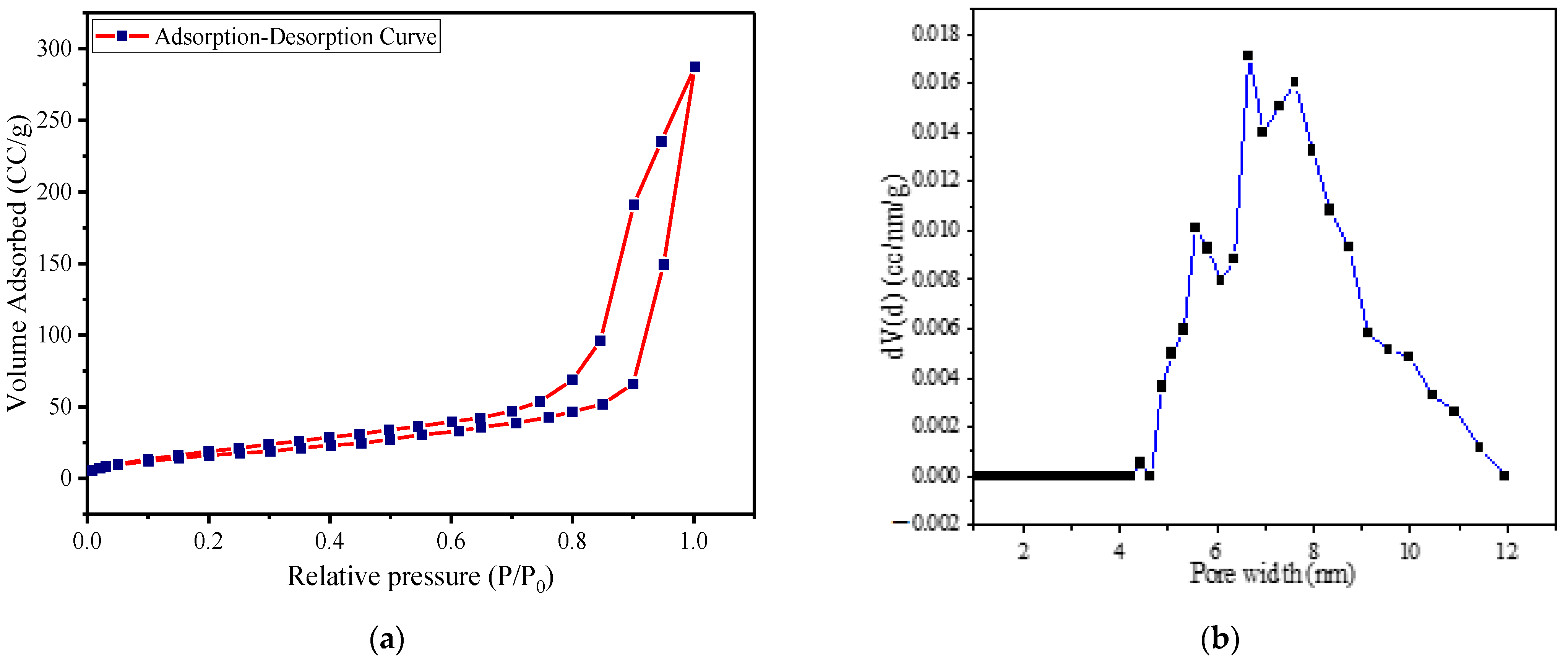
3.1. Characterization of ZnO@PAni
3.2. Adsorption Studies
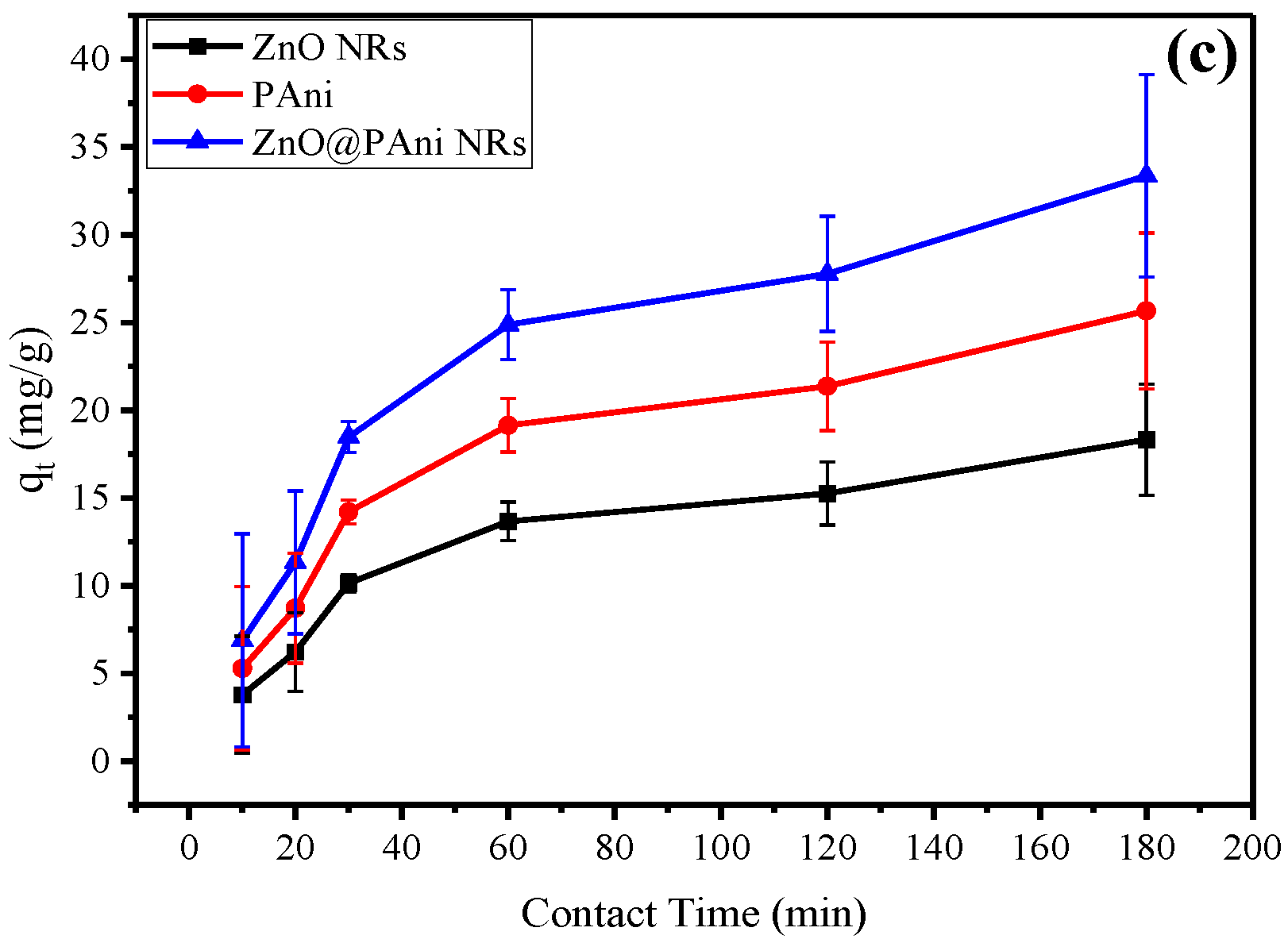
3.3. Effect of Varying Nanorod Size, Initial Cr (VI) Concentration, and Individual Adsorbent Material
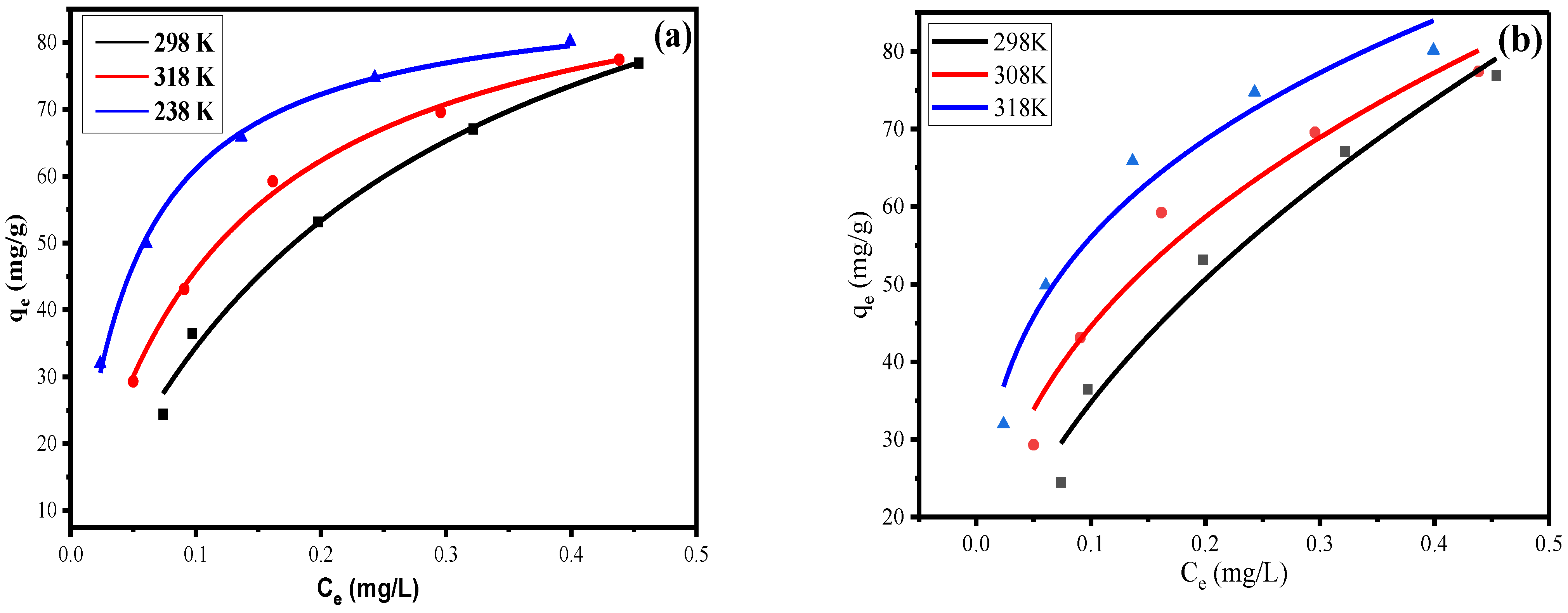
3.4. Adsorption Isotherm Studies
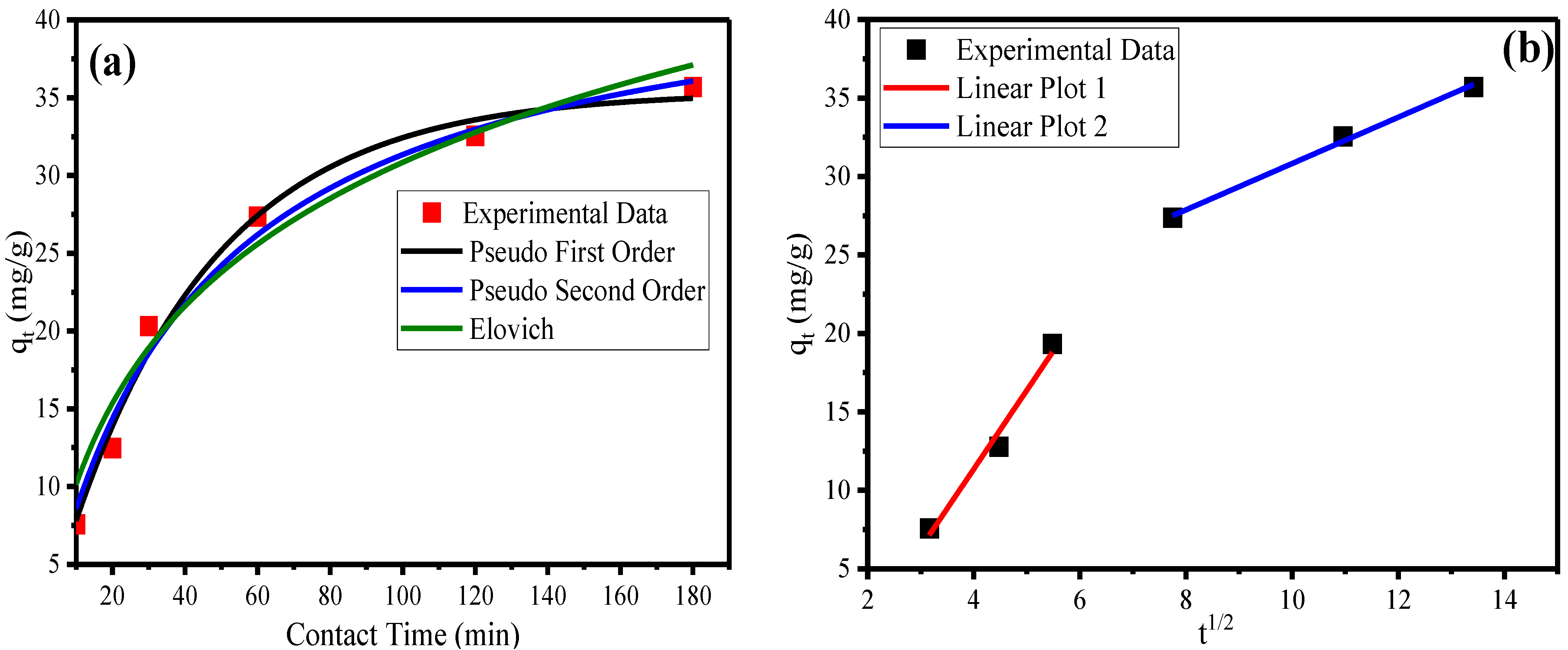
3.5. Adsorption Kinetics
3.6. Adsorption Thermodynamics
3.7. Adsorption Mechanism
3.8. Comparison with the Literature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barad, J.M.; Kohli, H.P.; Chakraborty, M. Adsorption of hexavalent chromium from aqueous stream by maghemite nanoparticles synthesized by the microemulsion method. Energy Nexus 2021, 5, 100035. [Google Scholar] [CrossRef]
- Nematollahzadeh, A.; Seraj, S.; Mirzayi, B. Catecholamine coated maghemite nanoparticles for the environmental remediation: Hexavalent chromium ions removal. Chem. Eng. J. 2015, 277, 21–29. [Google Scholar] [CrossRef]
- Marti, N.; Bouzas, A.; Seco, A.; Ferrer, J. Struvite precipitation assessment in anaerobic digestion processes. Chem. Eng. J. 2008, 141, 67–74. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Bollinger, J.C.; Belkhiri, L.; Tiri, A.; Bouzaza, A.; Mouni, L. Zeolite Waste Characterization and Use as Low-Cost, Ecofriendly, and Sustainable Material for Malachite Green and Methylene Blue Dyes Removal: Box;Behnken Design, Kinetics, and Thermodynamics. Appl. Sci. 2022, 12, 7587. [Google Scholar] [CrossRef]
- Mammar, A.C.; Mouni, L.; Bollinger, J.-C.; Belkhiri, L.; Bouzaza, A.; Assadi, A.A.; Belkacemi, H. Modeling and optimization of process parameters in elucidating the adsorption mechanism of Gallic acid on activated carbon prepared from date stones. Sep. Sci. Technol. 2019, 55, 3113–3125. [Google Scholar] [CrossRef]
- Bayuo, J. An extensive review on chromium (vi) removal using natural and agricultural wastes materials as alternative bio-sorbents. J. Environ. Health Sci. Eng. 2021, 19, 1193–1207. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Dweiri, R.; Khader, M.; Al-Sabbagh, S.; Al-Shannag, M.; Qasrawi, S.; Al-Halawani, M. Processing and character-ization of magnetic composites of activated carbon, fly ash, and beach sand as adsorbents for Cr (VI) removal. Case Stud. Chem. Environ. Eng. 2023, 7, 100333. [Google Scholar] [CrossRef]
- Al-Waqfi, M.S.E.-A.; Al-Qodah, Z. An iron rotating disk for the elimination of hexavalent chromium ion from industrial wastewaters: Putting it to work. Desalination Water Treat 2022, 270, 83–91. [Google Scholar] [CrossRef]
- Zhang, H.; Li, P.; Wang, Z.; Zhang, X.; Zheng, S.; Zhang, Y. In Situ synthesis of γ-AlOOH and synchronous adsorption separation of V(V) from highly concentrated Cr (VI) multiplex complex solutions. ACS Sustainable Chem. Eng. 2017, 5, 6674–6681. [Google Scholar] [CrossRef]
- Hu, Q.; Paudyal, H.; Zhao, J.; Huo, F.; Inoue, K.; Liu, H. Adsorptive recovery of vanadium(V) from chromium(VI)-containing effluent by Zr(IV)-loaded orange juice residue. Chem. Eng. J. 2014, 248, 79–88. [Google Scholar] [CrossRef]
- Sun, F.; Liu, M.; Yuan, B.; He, J.; Wu, P.; Liu, C.; Jiang, W. Separation of vanadium and chromium by selective adsorption by titanium-based microspheres. Chem. Eng. J. 2022, 450, 138039. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Selvasembian, R.; Ashiq, A.; Gunarathne, V.; Ekanayake, A.; Perera, V.; Wijesekera, H.; Mia, S.; Ahmad, M.; Vithanage, M.; et al. A systematic review on adsorptive removal of hexavalent chromium from aqueous solutions: Recent advances. Sci. Total. Environ. 2022, 809, 152055. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, X.; Wang, M. Separation and recovery of chromium and vanadium from vanadium-containing chromate solution by ion exchange. Hydrometallurgy 2013, 136, 31–35. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, X.; Tian, X.; Yang, Y.; Chen, Y. Leaching of vanadium from chromium residue. Hydrometallurgy 2010, 103, 7–11. [Google Scholar] [CrossRef]
- He, K.; Chen, P.; Yuan, B.; Sun, F.; He, J.; Wu, P.; Liu, C.; Jiang, W. Removing trace chromium from high concentration vanadium solution by photoreduction deposition with Ti–Zr solid solution. Sep. Purif. Technol. 2022, 290, 120855. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Hadadi, A.; Hamri, N.; Bollinger, J.-C.; Amrane, A.; Tahraoui, H.; Manseri, A.; Mouni, L. Adsorption Performance of Zeolite for the Removal of Congo Red Dye: Factorial Design Experiments, Kinetic, and Equilibrium Studies. Separations 2023, 10, 57. [Google Scholar] [CrossRef]
- Bouchelkia, N.; Tahraoui, H.; Amrane, A.; Belkacemi, H.; Bollinger, J.-C.; Bouzaza, A.; Zoukel, A.; Zhang, J.; Mouni, L. Jujube stones based highly efficient activated carbon for methylene blue adsorption: Kinetics and isotherms modeling, thermodynamics and mechanism study, optimization via response surface methodology and machine learning approaches. Process. Saf. Environ. Prot. 2023, 170, 513–535. [Google Scholar] [CrossRef]
- El Messaoudi, N.; El Mouden, A.; Fernine, Y.; El Khomri, M.; Bouich, A.; Faska, N.; Ciğeroğlu, Z.; Américo-Pinheiro, J.H.P.; Jada, A.; Lacherai, A. Green synthesis of Ag2O nanoparticles using Punica granatum leaf extract for sulfamethoxazole antibiotic adsorption: Characterization, experimental study, modeling, and DFT calculation. Environ. Sci. Pollut. Res. 2022, 1–18. [Google Scholar] [CrossRef]
- El Mouden, A.; El Guerraf, A.; El Messaoudi, N.; Haounati, R.; El Fakir, A.A.; Lacherai, A. Date Stone Functionalized with 3-Aminopropyltriethoxysilane as a Potential Biosorbent for Heavy Metal Ions Removal from Aqueous Solution. Chem. Afr. 2022, 5, 745–759. [Google Scholar] [CrossRef]
- El Mouden, A.; El Messaoudi, N.; El Guerraf, A.; Bouich, A.; Mehmeti, V.; Lacherai, A.; Jada, A.; Américo-Pinheiro, J.H.P. Removal of cadmium and lead ions from aqueous solutions by novel dolomite-quartz@Fe3O4 nanocomposite fabricated as nanoadsorbent. Environ. Res. 2023, 225, 115606. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Joshi, P.; Gusain, R.; Khatri, O.P. Recent advances in adsorptive removal of heavy metal and metalloid ions by metal oxide-based nanomaterials. Coord. Chem. Rev. 2021, 445, 214100. [Google Scholar] [CrossRef]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: A review. J. Water Process. Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- De Jesus, R.A.; de Assis, G.C.; de Oliveira, R.J.; Costa, J.A.S.; da Silva, C.M.P.; Bilal, M.; Iqbal, H.M.; Ferreira, L.F.R.; Figueiredo, R.T. Environmental remediation potentialities of metal and metal oxide nanoparticles: Mechanistic biosynthesis, influencing factors, and application standpoint. Environ. Technol. Innov. 2021, 24, 101851. [Google Scholar] [CrossRef]
- Mittal, A.; Ahmad, R.; Hasan, I. Iron oxide-impregnated dextrin nanocomposite: Synthesis and its application for the biosorption of Cr(VI) ions from aqueous solution. Desalination Water Treat. 2015, 57, 15133–15145. [Google Scholar] [CrossRef]
- Liu, M.; Yin, W.; Qian, F.-J.; Zhao, T.-L.; Yao, Q.-Z.; Fu, S.-Q.; Zhou, G.-T. A novel synthesis of porous TiO2 nanotubes and sequential application to dye contaminant removal and Cr(VI) visible light catalytic reduction. J. Environ. Chem. Eng. 2020, 8, 104061. [Google Scholar] [CrossRef]
- Sharma, P.; Prakash, J.; Palai, T.; Kaushal, R. Surface functionalization of bamboo leave mediated synthesized SiO2 nanoparti-cles: Study of adsorption mechanism, isotherms and enhanced adsorption capacity for removal of Cr (VI) from aqueous solution. Environ. Res. 2022, 214, 113761. [Google Scholar] [CrossRef]
- Wang, S.Z.; Zheng, M.; Zhang, X.; Zhuo, M.P.; Zhou, Q.Q.; Zheng, M.; Liao, L.S. Fine synthesis of hierarchical CuO/Cu(OH)2 urchin-like nanoparticles for efficient removal of Cr(Ⅵ). J. Alloys Compd. 2021, 884, 161052. [Google Scholar] [CrossRef]
- Sahu, U.K.; Zhang, Y.; Huang, W.; Ma, H.; Mandal, S.; Sahu, S.; Sahu, M.K.; Patel, R.K.; Pu, S. Nanoceria-loaded tea waste as bio-sorbent for Cr(VI) removal. Mater. Chem. Phys. 2022, 290, 126563. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Oyewo, O.A.; Ogunjinmi, O.E.; Ojelere, O. Hexavalent chromium reduction by ZnO, SnO2 and ZnO-SnO2 synthesized using biosurfactants from extract of Solanum macrocarpon. South Afr. J. Chem. Eng. 2021, 38, 21–33. [Google Scholar] [CrossRef]
- Pandey, M.; Tripathi, B.D. Synthesis, characterization and application of zinc oxide nano particles for removal of hexavalent chromium. Res. Chem. Intermed. 2017, 43, 121–140. [Google Scholar] [CrossRef]
- Rout, D.R.; Jena, H.M. Enhanced Cr(VI) adsorption using ZnO decorated graphene composite: Batch and continuous studies. J. Taiwan Inst. Chem. Eng. 2022, 140, 104534. [Google Scholar] [CrossRef]
- Ruotolo, L.; Gubulin, J. Chromium(VI) reduction using conducting polymer films. React. Funct. Polym. 2005, 62, 141–151. [Google Scholar] [CrossRef]
- Dognani, G.; Hadi, P.; Ma, H.; Cabrera, F.C.; Job, A.; Agostini, D.L.; Hsiao, B.S. Effective chromium removal from water by polyaniline-coated electrospun adsorbent membrane. Chem. Eng. J. 2019, 372, 341–351. [Google Scholar] [CrossRef]
- Herath, A.; Reid, C.; Perez, F.; Pittman, C.U.; Mlsna, T.E. Biochar-supported polyaniline hybrid for aqueous chromium and nitrate adsorption. J. Environ. Manag. 2021, 296, 113186. [Google Scholar] [CrossRef]
- Muhammad, A.; Shah, A.U.H.A.; Bilal, S. Effective Adsorption of Hexavalent Chromium and Divalent Nickel Ions from Water through Polyaniline, Iron Oxide, and Their Composites. Appl. Sci. 2020, 10, 2882. [Google Scholar] [CrossRef]
- Mohammad, N.; Atassi, Y. Enhancement of removal efficiency of heavy metal ions by polyaniline deposition on electrospun polyacrylonitrile membranes. Water Sci. Eng. 2021, 14, 129–138. [Google Scholar] [CrossRef]
- Mahmood, N.B.; Saeed, F.R.; Gbashi, K.R.; Mahmood, U.S. Synthesis and characterization of zinc oxide nanoparticles via ox-alate co-precipitation method. Mater. Lett. X 2022, 13, 100126. [Google Scholar]
- Hasanpoor, M.; Aliofkhazraei, M.; Delavari, H.J.P.M.S. Microwave-assisted Synthesis of Zinc Oxide Nanoparticles. Proc. Mater. Sci. 2015, 11, 320–325. [Google Scholar] [CrossRef]
- Gerbreders, V.; Krasovska, M.; Sledevskis, E.; Gerbreders, A.; Mihailova, I.; Tamanis, E.; Ogurcovs, A. Hydrothermal synthesis of ZnO nanostructures with controllable morphology change. Crystengcomm 2020, 22, 1346–1358. [Google Scholar] [CrossRef]
- Li, J.; Wu, Z.; Bao, Y.; Chen, Y.; Huang, C.; Li, N.; He, S.; Chen, Z. Wet chemical synthesis of ZnO nanocoating on the surface of bamboo timber with improved mould-resistance. J. Saudi Chem. Soc. 2017, 21, 920–928. [Google Scholar] [CrossRef]
- Al Abdullah, K.; Awad, S.; Zaraket, J.; Salame, C. Synthesis of ZnO Nanopowders by Using Sol-Gel and Studying Their Struc-tural and Electrical Properties at Different Temperature. Energy Procedia 2017, 119, 565–570. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Liu, Y.; Fan, Y.; Dang, F.; Qiu, Y.; Zhou, H.; Wang, W.; Liu, Y. Solvothermal Synthesis of ZnO Nanoparticles for Photocatalytic Degradation of Methyl Orange and p-Nitrophenol. Water 2021, 13, 3224. [Google Scholar] [CrossRef]
- Pineda-Reyes, A.M.; Olvera, M.D.L.L. Synthesis of ZnO nanoparticles from water-in-oil (w/o) microemulsions. Mater. Chem. Phys. 2018, 203, 141–147. [Google Scholar] [CrossRef]
- Banerjee, P.; Chakrabarti, S.; Maitra, S.; Dutta, B.K. Zinc oxide nano-particles—Sonochemical synthesis, characterization and application for photo-remediation of heavy metal. Ultrason. Sonochem. 2012, 19, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Chandraiahgari, C.R.; De Bellis, G.; Ballirano, P.; Balijepalli, S.K.; Kaciulis, S.; Caneve, L.; Sarto, F.; Sarto, M.S. Synthesis and characterization of ZnO nanorods with a narrow size distribution. RSC Adv. 2015, 5, 49861–49870. [Google Scholar] [CrossRef]
- Mostafaei, A.; Zolriasatein, A. Synthesis and characterization of conducting polyaniline nanocomposites containing ZnO nanorods. Prog. Nat. Sci. Mater. Int. 2012, 22, 273–280. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über Die Adsorption in Lösungen. Z. Für Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Lagergren, S.K. On the Theory of So-Called Adsorption of Materials. R. Swed. Acad. Sci. 1898, 24, 1–13. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Aharoni, C.; Ungarish, M. Kinetics of Activated Chemisorption. Part 1.—The Non-Elovichian Part of the Isotherm. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1976, 72, 400–408. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Gupta, A.K. Determination of thermodynamic parameters from Langmuir isotherm constant-revisited. J. Mol. Liq. 2017, 225, 137–146. [Google Scholar] [CrossRef]
- Raghav, S.; Kumar, D. Adsorption Equilibrium, Kinetics, and Thermodynamic Studies of Fluoride Adsorbed by Tetrametallic Oxide Adsorbent. J. Chem. Eng. Data 2018, 63, 1682–1697. [Google Scholar] [CrossRef]
- Primo, J.D.O.; Bittencourt, C.; Acosta, S.; Sierra-Castillo, A.; Colomer, J.-F.; Jaerger, S.; Teixeira, V.C.; Anaissi, F.J. Synthesis of Zinc Oxide Nanoparticles by Ecofriendly Routes: Adsorbent for Copper Removal from Wastewater. Front. Chem. 2020, 8, 1100. [Google Scholar] [CrossRef]
- Khan, M.; Naqvi, A.H.; Ahmad, M. Comparative study of the cytotoxic and genotoxic potentials of zinc oxide and titanium dioxide nanoparticles. Toxicol. Rep. 2015, 2, 765–774. [Google Scholar] [CrossRef]
- Taghizadeh, S.-M.; Lal, N.; Ebrahiminezhad, A.; Moeini, F.; Seifan, M.; Ghasemi, Y.; Berenjian, A. Green and Economic Fabrication of Zinc Oxide (ZnO) Nanorods as a Broadband UV Blocker and Antimicrobial Agent. Nanomaterials 2020, 10, 530. [Google Scholar] [CrossRef]
- Saravanan, S.; Joseph Mathai, C.; Anantharaman, M.; Venkatachalam, S.; Prabhakaran, P. Investigations on the electrical and structural properties of polyaniline doped with camphor sulphonic acid. J. Phys. Chem. Solids 2006, 67, 1496–1501. [Google Scholar] [CrossRef]
- Bera, S.; Khan, H.; Biswas, I.; Jana, S. Polyaniline hybridized surface defective ZnO nanorods with long-term stable photoelec-trochemical activity. Appl. Surf. Sci. 2016, 383, 165–176. [Google Scholar] [CrossRef]
- Mutlaq, S.; Albiss, B.; Al-Nabulsi, A.A.; Jaradat, Z.W.; Olaimat, A.N.; Khalifeh, M.S.; Holley, R.A. Con-ductometric Immunosensor for Escherichia coli O157:H7 Detection Based on Polyaniline/Zinc Oxide (PANI/ZnO) Nano-composite. Polymers 2021, 13, 3288. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, R.M.; Morsi, S.M.M.; Selim, M.M.; Ghoneim, A.M.; El-Sherif, H.M. Electrical, thermal, morphological, and antibacterial studies of synthesized polyaniline/zinc oxide nanocomposites. Polym. Bull. 2018, 76, 6135–6160. [Google Scholar] [CrossRef]
- Yusuff, A.S. Optimization of adsorption of Cr(VI) from aqueous solution by Leucaena leucocephala seed shell activated carbon using design of experiment. Appl. Water Sci. 2018, 8, 232. [Google Scholar] [CrossRef]
- Rouhaninezhad, A.A.; Hojati, S.; Masir, M.N. Adsorption of Cr (VI) onto micro- and nanoparticles of palygorskite in aqueous solutions: Effects of pH and humic acid. Ecotoxicol. Environ. Saf. 2020, 206, 111247. [Google Scholar] [CrossRef] [PubMed]
- Olad, A.; Farshi Azhar, F. study on the adsorption of chromium (VI) from aqueous solutions on the algi-nate-montmorillonite/polyaniline nanocomposite. Desalination Water Treat 2014, 52, 2548–2559. [Google Scholar] [CrossRef]
- Aliabadi, M.; Khazaei, I.; Fakhraee, H.; Mousavian, M.T.H. Hexavalent chromium removal from aqueous solutions by using low-cost biological wastes: Equilibrium and kinetic studies. Int. J. Environ. Sci. Technol. 2012, 9, 319–326. [Google Scholar] [CrossRef]
- Amaku, J.F.; Ogundare, S.A.; Akpomie, K.G.; Ngwu, C.M.; Conradie, J. Sequestered uptake of chromium (VI) by Irvingia gab-onensis stem bark extract anchored silica gel. Biomass Convers Biorefin. 2021, 1–13. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Mondal, M.K. Novel green strategy for CuO–ZnO–C nanocomposites fabrication using marigold (Tagetes spp.) flower petals extract with and without CTAB treatment for adsorption of Cr(VI) and Congo red dye. J. Environ. Manag. 2021, 290, 112615. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Bollinger, J.-C.; Tran, H.N.; Lima, E.C. Comments on “removal of methylene blue dye from aqueous solution using citric acid modified apricot stone”. Chem. Eng. Commun. 2022, 1–6. [Google Scholar] [CrossRef]
- Tran, H.N. Applying Linear Forms of Pseudo-Second-Order Kinetic Model for Feasibly Identifying Errors in the Initial Periods of Time-Dependent Adsorption Datasets. Water 2023, 15, 1231. [Google Scholar] [CrossRef]
- Salvestrini, S.; Ambrosone, L.; Kopinke, F.-D. Some mistakes and misinterpretations in the analysis of thermodynamic adsorption data. J. Mol. Liq. 2022, 352, 118762. [Google Scholar] [CrossRef]
- Murali, A.; Sarswat, P.K.; Free, M.L. Adsorption-coupled reduction mechanism in ZnO-Functionalized MWCNTs nanocom-posite for Cr (VI) removal and improved anti-photocorrosion for photocatalytic reduction. J. Alloys Compd. 2020, 843, 155835. [Google Scholar] [CrossRef]
- Naseem, T.; Bibi, F.; Arif, S.; Waseem, M.; Haq, S.; Azra, M.N.; Liblik, T.; Zekker, I. Adsorption and Kinetics Studies of Cr (VI) by Graphene Oxide and Reduced Graphene Oxide-Zinc Oxide Nanocomposite. Molecules 2022, 27, 7152. [Google Scholar] [CrossRef]
- Taha, A.; Da’Na, E.; Hassanin, H.A. Modified activated carbon loaded with bio-synthesized Ag/ZnO nanocomposite and its application for the removal of Cr (VI) ions from aqueous solution. Surf. Interfaces 2021, 23, 100928. [Google Scholar] [CrossRef]
- Dinari, M.; Haghighi, A. Ultrasound-assisted synthesis of nanocomposites based on aromatic polyamide and modified ZnO nanoparticle for removal of toxic Cr(VI) from water. Ultrason. Sonochem. 2018, 41, 75–84. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Hota, G. Amine-Functionalized GO Decorated with ZnO-ZnFe2O4 Nanomaterials for Remediation of Cr(VI) from Water. ACS Appl. Nano Mater. 2019, 2, 983–996. [Google Scholar] [CrossRef]
- Samuel, M.S.; Subramaniyan, V.; Bhattacharya, J.; Parthiban, C.; Chand, S.; Singh, N.P. A GO-CS@MOF [Zn(BDC)(DMF)] material for the adsorption of chromium(VI) ions from aqueous solution. Compos. Part B Eng. 2018, 152, 116–125. [Google Scholar] [CrossRef]



| Method | Precursor | Solvent | Features | Advantages/Cost | Disadvantages | References |
|---|---|---|---|---|---|---|
| Chemical coprecipitation | Zinc acetate | Double distilled water | 30–60 nm of nanorod | Low energy input/low cost | High cost of precursors | [38] |
| Microwave decomposition | Zinc acetate dehydrate | 1-Butyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide [bmim][NTf2] | 37–47 nm Sphere | Industrial-scale production/low cost | Parameter control | [39] |
| Hydrothermal process | Zinc acetate dihydrate | Polyvinylpyrrolidone (PVP) | 50–200 nm of nanorod | Uncomplicated equipment/low cost | Nanoparticle stability | [40] |
| Wet chemical Method | Zinc nitrate hexahydrate | Sodium hydroxide (NaOH) as precursors and soluble starch as stabilizing agent | 20–30 nm Acicular | Easy parameter tailoring, low cost | Nanoparticle stability | [41] |
| Sol–gel method | Zinc nitrate | Distilled water and gelatin as substrate | 30–60 nm Circular and hexagonal | Inexpensive and easy to handle chemical reagents | [42] | |
| Solvothermal | Zinc acetate dihydrate | Polyethylene glycol, absolute ethanol | 10–20 nm Quasi-spherical | Industrial-scale production | High cost of precursors | [43] |
| Micro-emulsion | Zn (AOT)2 | Heptane, diethyl oxalate, chloroform, methanol | 10–20 nm Quasi-spherical | Easy parameter tailoring | Surfactant use | [44] |
| Sono-chemical | zinc nitrate hexahydrate | Potassium hydroxide, cetyltrimethylammonium bromide | 200–400 nm flakes | Industrial-scale production | Parameter control | [45] |
| Model | Parameters | 298 K | 308 K | 318 K |
|---|---|---|---|---|
| Langmuir | qm (mg/g) | 142.27 | 219.18 | 310.47 |
| KL (L/mg) | 0.412 | 0.767 | 0.849 | |
| R2 | 0.99 | 0.99 | 0.99 | |
| χ2 | 4.13 | 3.55 | 3.41 | |
| Freundlich | KF (mg/g) (mg/L)1/n | 109.79 | 111.17 | 121.44 |
| n | 1.84 | 2.52 | 3.42 | |
| R2 | 0.98 | 0.97 | 0.96 | |
| χ2 | 21.61 | 19.04 | 15.13 |
| Model | Parameters | 318 K |
|---|---|---|
| Pseudo-first order | qe,exp (mg/g) | 35.69 |
| qe,cal (mg/g)k1 (1/min) | 41.36 | |
| R2 | 0.98 | |
| χ2 | 1.63 | |
| Pseudo-second order | qe,exp (mg/g) | 35.69 |
| qe,cal (mg/g)k2 (g/mg × min) | 36.27 | |
| R2 | 0.99 | |
| χ2 | 0.36 | |
| Elovich | α (mg/g × min) | 1.64 |
| β (g/mg) | 0.089 | |
| R2 | 0.96 | |
| χ2 | 2.36 | |
| Intraparticle diffusion | kp1 (mg/g × min1/2) | 5.02 |
| kp2 (mg/g × min1/2) | 1.48 | |
| R12 | 0.98 | |
| R22 | 0.96 | |
| χ2 | 1.38 |
| Temperature (K) | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (kJ/mol·K) |
|---|---|---|---|
| 298 | −23.79 | 32.01 | 0.187 |
| 308 | −25.67 | ||
| 318 | −27.54 |
| Adsorbent | Cr (VI) Removal Capacity (mg/g) | pH | Contact Time (min) | References |
|---|---|---|---|---|
| CuO–ZnO–C | 201.56 | 2 | 600 | [68] |
| ZnO-MWCNTs | 152.2 | 2 | 120 | [73] |
| rGO-ZnO | 25.45 | 3 | 240 | [74] |
| Ag/ZnO-AC | 4.17 | 2.5 | 1200 | [75] |
| PA/TSC-ZnO | 90.83 | 4 | 250 | [76] |
| GO ZnO-ZnFe2O4 | 109.89 | 4 | 120 | [77] |
| GO-CS@MOF [Zn(BDC)(DMF)] | 144.92 | 2 | 360 | [78] |
| ZnO@PAni NRs | 310.47 | 2 | 120 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharthi, F.A.; Alshammari, R.H.; Hasan, I. In Situ Polyaniline Immobilized ZnO Nanorods for Efficient Adsorptive Detoxification of Cr (VI) from Aquatic System. Water 2023, 15, 1949. https://doi.org/10.3390/w15101949
Alharthi FA, Alshammari RH, Hasan I. In Situ Polyaniline Immobilized ZnO Nanorods for Efficient Adsorptive Detoxification of Cr (VI) from Aquatic System. Water. 2023; 15(10):1949. https://doi.org/10.3390/w15101949
Chicago/Turabian StyleAlharthi, Fahad A., Riyadh H. Alshammari, and Imran Hasan. 2023. "In Situ Polyaniline Immobilized ZnO Nanorods for Efficient Adsorptive Detoxification of Cr (VI) from Aquatic System" Water 15, no. 10: 1949. https://doi.org/10.3390/w15101949
APA StyleAlharthi, F. A., Alshammari, R. H., & Hasan, I. (2023). In Situ Polyaniline Immobilized ZnO Nanorods for Efficient Adsorptive Detoxification of Cr (VI) from Aquatic System. Water, 15(10), 1949. https://doi.org/10.3390/w15101949








