Recent Changes in Composition and Distribution Patterns of Summer Mesozooplankton off the Western Antarctic Peninsula
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
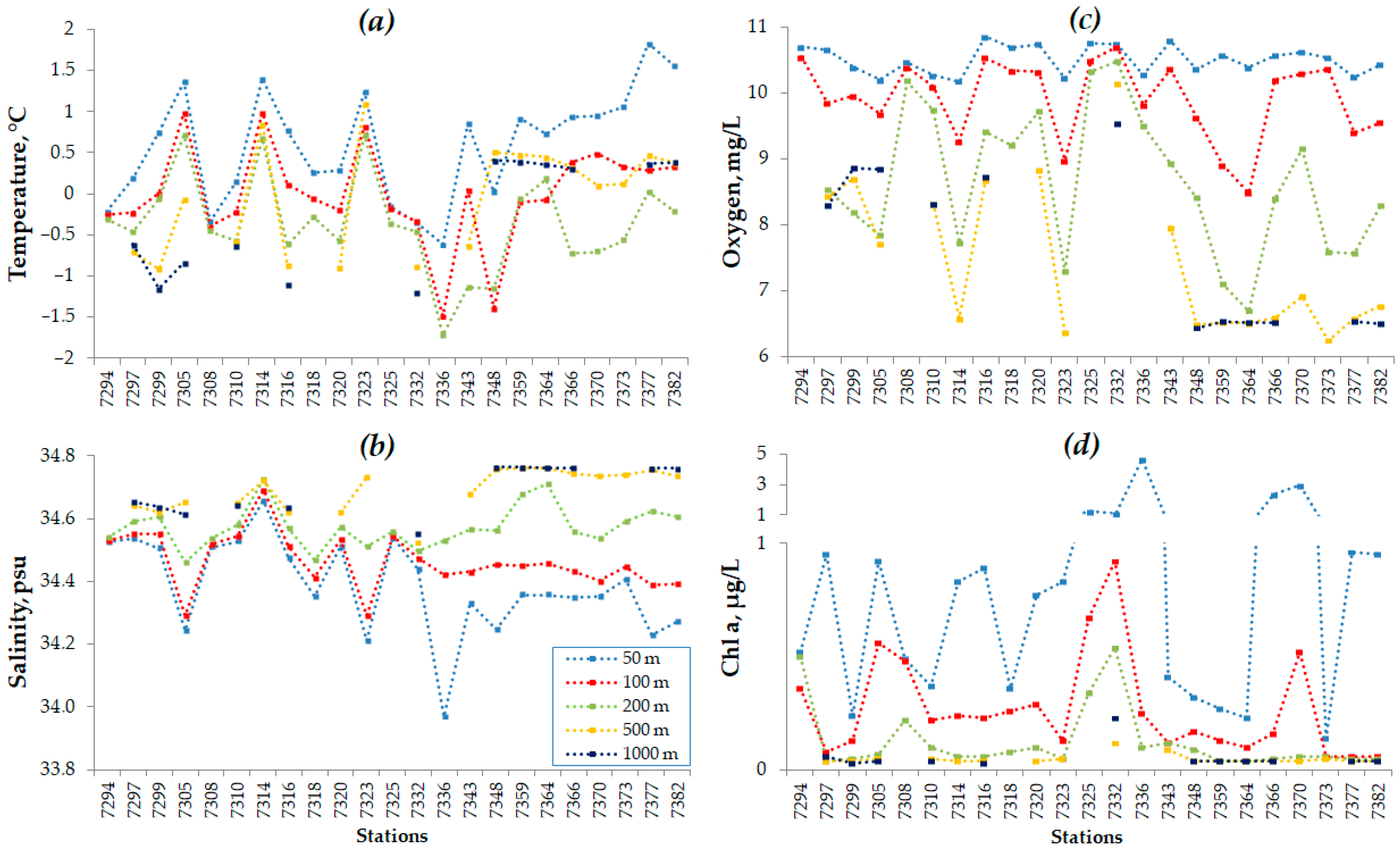
3.1. Dynamics of Environmental Conditions
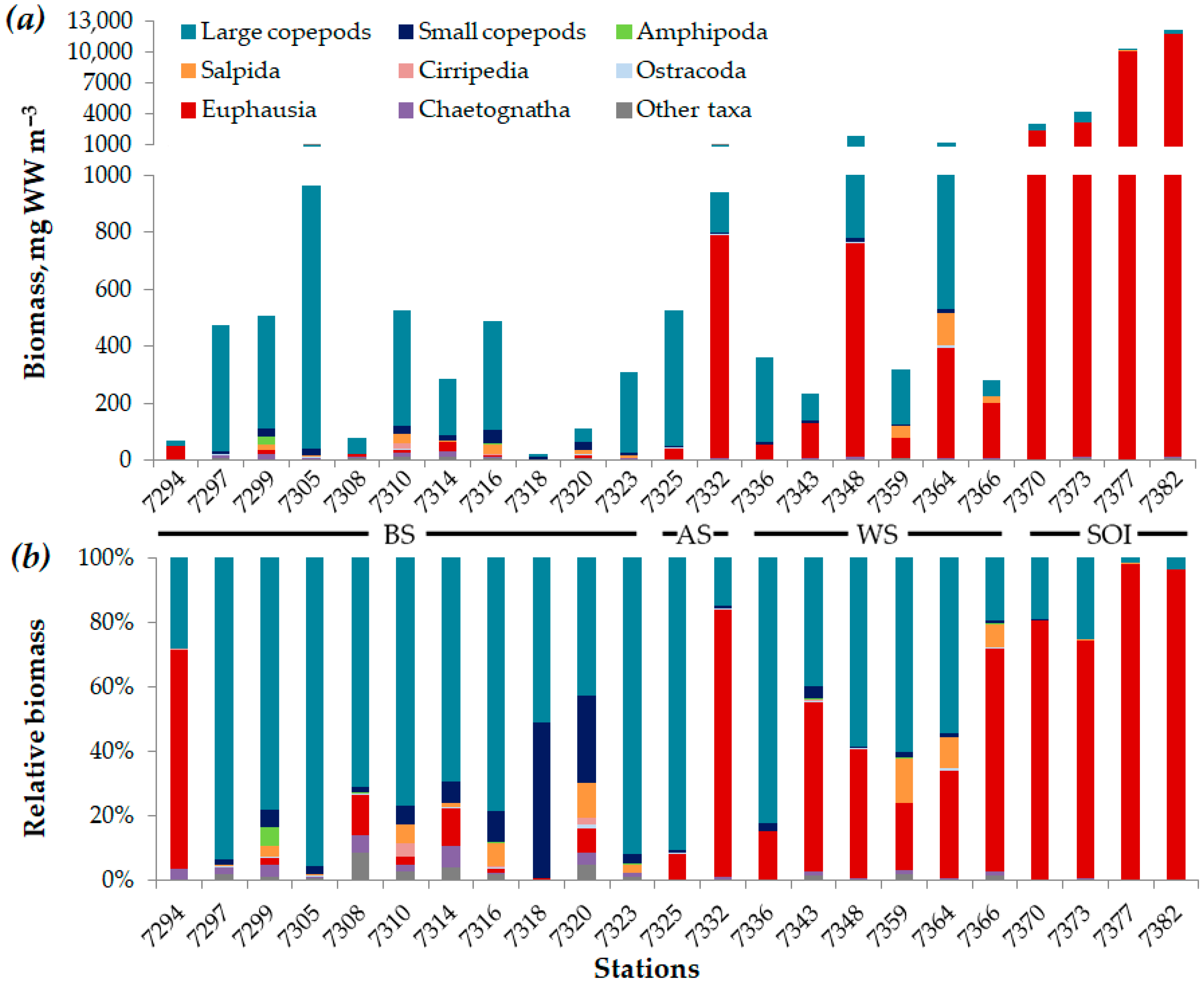
3.2. Composition and Distribution Patterns of Mesozooplankton Abundance and Biomass
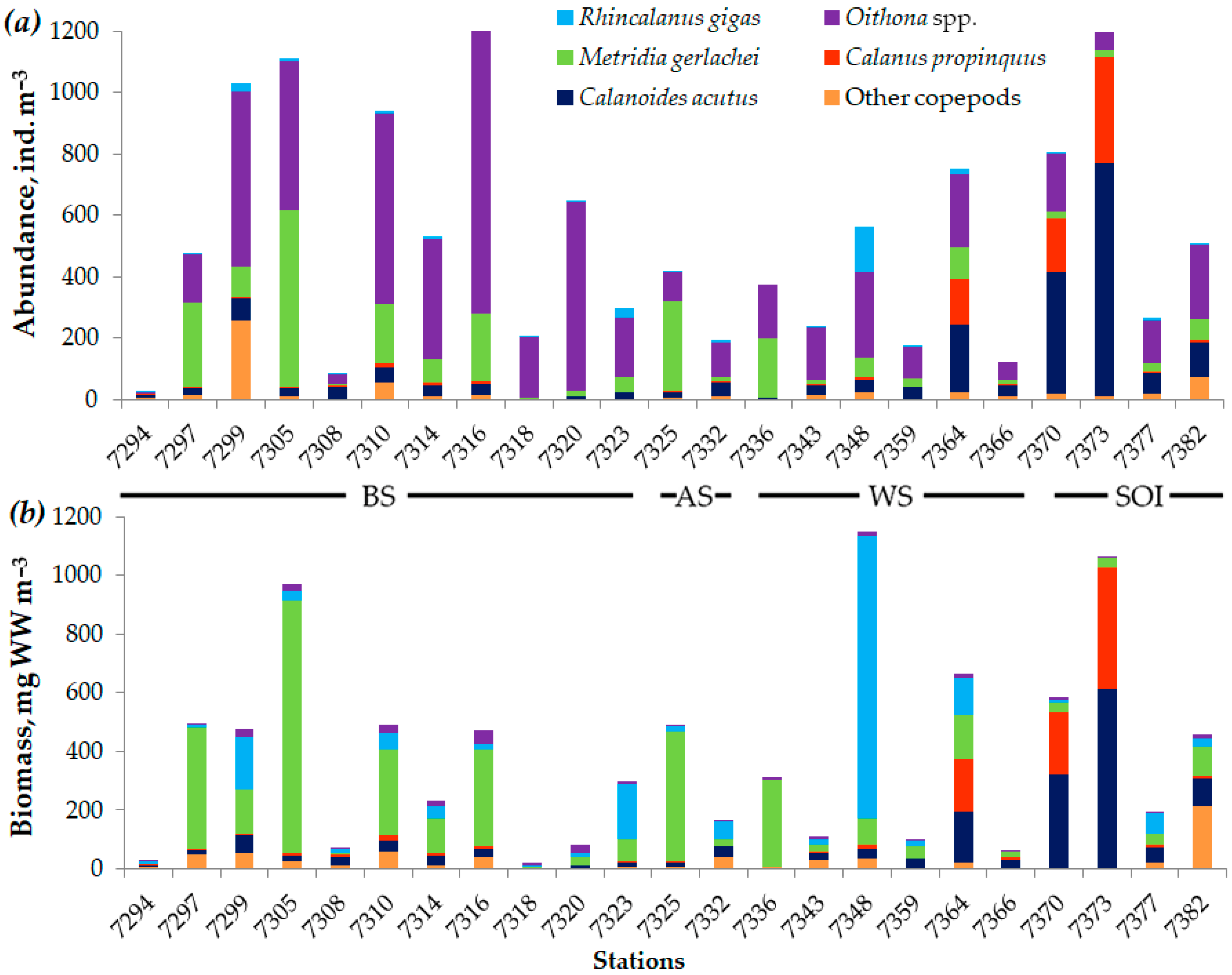
3.3. Dynamics of Copepod Community
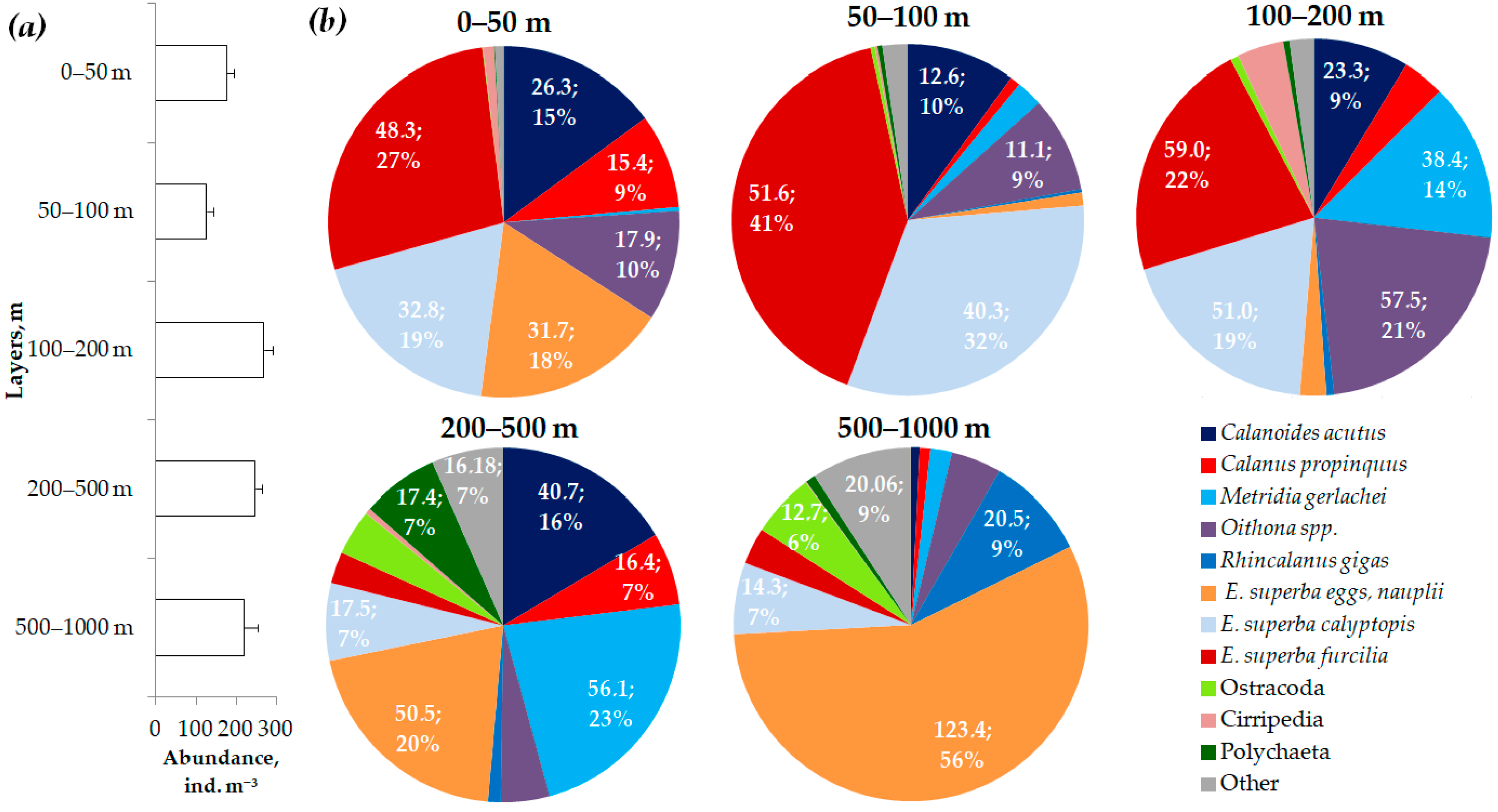
3.4. Vertical Distribution Patterns of Mesozooplankton Abundance
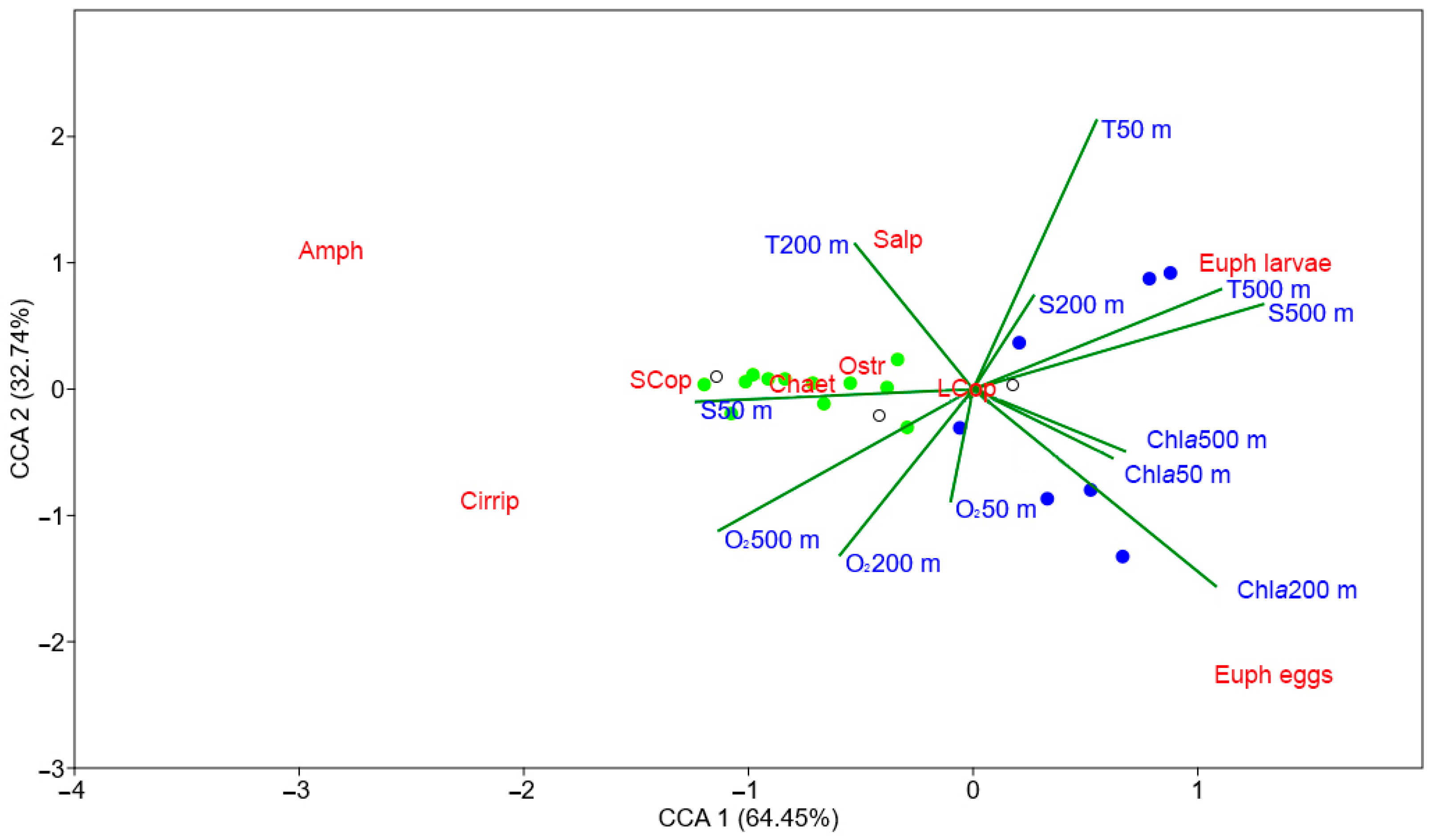
3.5. Effects of Environmental Variables on Distribution of Summer Mesozooplankton
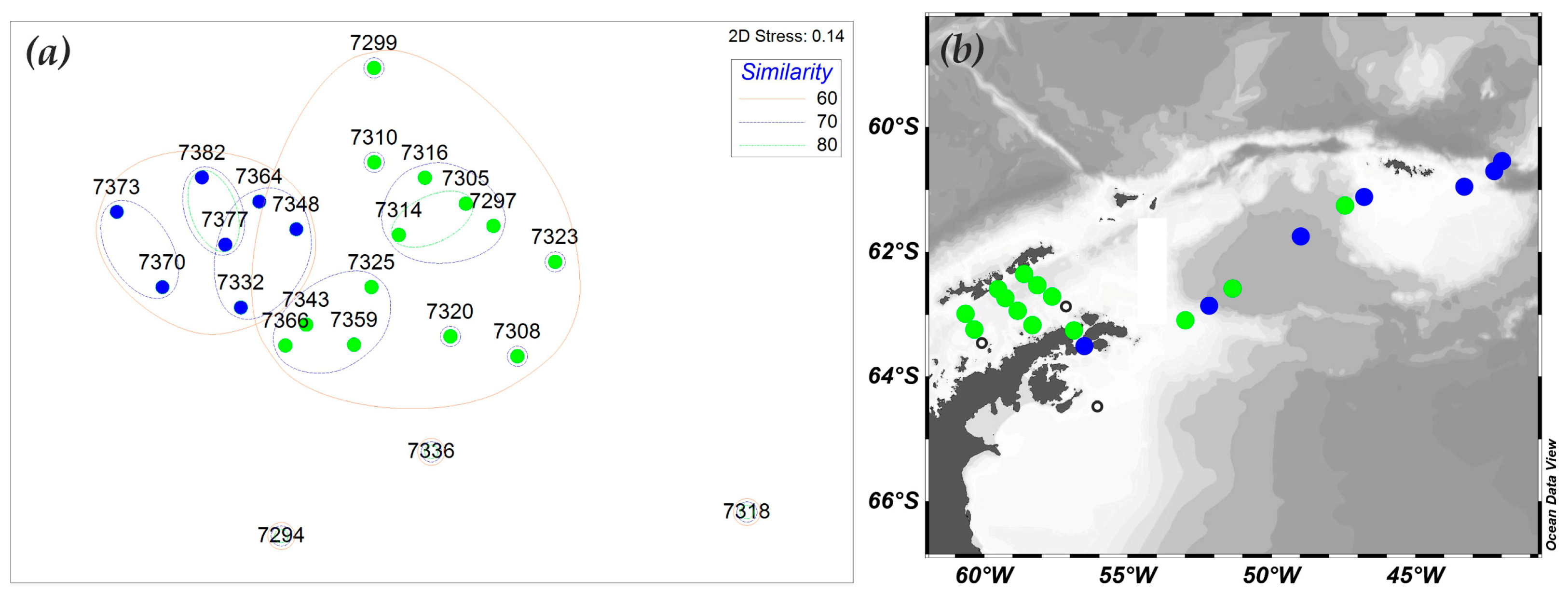
3.6. Mesozooplankton Community Structure
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaughan, D.; Marshall, G.; Connolley, W.; Parkinson, C.; Mulvaney, R.; Hodgson, D.; King, J.C.; Pudsey, C.J.; Turner, J. Recent rapid regional climate warming on the Antarctic Peninsula. Clim. Chang. 2003, 60, 243–274. [Google Scholar] [CrossRef]
- Cook, A.J.; Fox, A.J.; Vaughan, D.G.; Ferrigno, J.G. Retreating Glacier fronts on the Antarctic Peninsula over the past half-century. Science 2005, 308, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C. Changes in Antarctic climate system: Past, present and future. Adv. Clim. Chang. Res. 2008, 4, 1–7. [Google Scholar]
- Constable, A.J.; Melbourne-Thomas, J.; Corney, S.P.; Arrigo, K.R.; Barbraud, C.; Barnes, D.K.A.; Bindoff, N.L.; Boyd, P.W.; Brandt, A.; Costa, D.P.; et al. Climate change and Southern Ocean ecosystems I: How changes in physical habitats directly affect marine biota. Glob. Chang. Biol. 2014, 20, 3004–3025. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.P.; King, J.C. Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys. Res. Lett. 2005, 32, L19604. [Google Scholar] [CrossRef]
- Turner, J.; Colwell, S.; Marshall, G.; Lachlan-Cope, T.; Carleton, A.; Jones, P.; Lagun, V.; Reid, P.A.; Iagovkina, S. Antarctic climate change during the last 50 years. Int. J. Climatol. 2005, 25, 279–294. [Google Scholar] [CrossRef]
- Hill, S.L.; Phillips, T.; Atkinson, A. Potential climate change effects on the habitat of Antarctic krill in the Weddell Quadrant of the Southern Ocean. PLoS ONE 2013, 8, e72246. [Google Scholar] [CrossRef] [PubMed]
- Stammerjohn, S.; Martinson, D.; Smith, R.; Iannuzzi, R. Sea ice in the western Antarctic Peninsula region: Spatio-temporal variability from ecological and climate change perspectives. Deep-Sea Res. II 2008, 55, 2041–2058. [Google Scholar] [CrossRef]
- NOAA National Centers for Environmental Information, Monthly Global Climate Report for January 2022, Published Online February 2022. Available online: https://www.ncei.noaa.gov/access/monitoring/monthly-report/global/202201 (accessed on 13 March 2023).
- Constable, A.J.; Nicol, S.; Strutton, P.G. Southern Ocean productivity in relation to spatial and temporal variation in the physical environment. J. Geophys. Res. 2003, 108, 8079. [Google Scholar]
- Montes-Hugo, M.; Vernet, M.; Martinson, D.; Smith, R.; Iannuzzi, R. Variability on phytoplankton size structure in the western Antarctic Peninsula (1997–2006). Deep Sea Res. II 2008, 55, 2106–2117. [Google Scholar] [CrossRef]
- Deppeler, S.L.; Davidson, A.T. Southern Ocean phytoplankton in a changing climate. Front. Mar. Sci. 2017, 4, 40. [Google Scholar] [CrossRef]
- Sapozhnikov, P.V.; Kalinina, O.Y.; Morozova, T.V. Phytopelagic сommunities of the Powell Basin in the summer of 2020. In Antarctic Peninsula Region of the Southern Ocean; Morozov, E.G., Flint, M.V., Spiridonov, V.A., Eds.; Springer: Cham, Switzerland, 2021; Volume 6, pp. 279–306. [Google Scholar] [CrossRef]
- Rombola, E.F.; Franzosi, C.A.; Tosonotto, G.V.; Alder, V.A.; Marschoff, E.R. Variability of euphausiid larvae densities during the 2011, 2012, and 2014 summer seasons in the Atlantic sector of the Antarctic. Polar. Sci. 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Garcia, M.D.; Sofia Dutto, M.; Chazarreta, C.J.; Berasategui, A.A.; Schloss, I.R.; Hoffmeyer, M.S. Micro- and mesozooplankton successions in the Antarctic coastal environment during a warm year. PLoS ONE 2020, 15, e0232614. [Google Scholar] [CrossRef]
- Pakhomov, E.; Pshenichnov, L.; Krot, A.; Paramonov, V.; Slypko, I.; Zabroda, P. Zooplankton distribution and community structure in the Pacific and Atlantic Sectors of the Southern Ocean during austral summer 2017–2018: A Pilot Study Conducted from Ukrainian Long-Liners. J. Mar. Sci. Eng. 2020, 8, 488. [Google Scholar] [CrossRef]
- Pinkerton, M.H.; Decima, M.; Kitchener, J.A.; Takahashi, K.T.; Robinson, K.V.; Stewart, R.; Hosie, G.W. Zooplankton in the Southern Ocean from the continuous plankton recorder: Distributions and long-term change. Deep Sea Res. 2020, 162, 103303. [Google Scholar] [CrossRef]
- Yakovenko, V.A.; Spiridonov, V.A.; Gorbatenko, K.M.; Shadrin, N.V.; Samyshev, E.Z.; Minkina, N.I. Macro- and mesozooplankton in the Powell Basin (Antarctica): Species composition and distribution of abundance and biomass in February 2020. In Antarctic Peninsula Region of the Southern Ocean; Morozov, E.G., Flint, M.V., Spiridonov, V.A., Eds.; Springer: Cham, Switzerland, 2021; Volume 6, pp. 131–141. [Google Scholar] [CrossRef]
- Criales–Hernandez, M.I.; Jerez–Guerrero, M.; Latandret–Solana, S.A.; Gomez–Sanchez, M.D. Spatial distribution of meso- and macrozooplankton in the Bransfield Strait and around Elephant Island, Antarctic Peninsula, during the 2019–2020 austral summer. Polar. Sci. 2022, 32, 100821. [Google Scholar] [CrossRef]
- Kasyan, V.V.; Bitiutskii, D.G.; Mishin, A.V.; Zuev, O.A.; Murzina, S.A.; Sapozhnikov, P.V.; Kalinina, O.Y.; Syomin, V.L.; Kolbasova, G.D.; Voronin, V.P.; et al. Composition and Distribution of Plankton Communities in the Atlantic Sector of the Southern Ocean. Diversity 2022, 14, 923. [Google Scholar] [CrossRef]
- Loeb, V.J.; Santora, J.A. Population dynamics of Salpa thompsoni near the Antarctic Peninsula: Growth rates and interannual variations in reproductive activity (1993–2009). Prog. Oceanogr. 2012, 96, 93–107. [Google Scholar] [CrossRef]
- Siegel, V.; Watkins, J.L. Distribution, Biomass and Demography of Antarctic Krill, Euphausia superba. In Biology and Ecology of Antarctic Krill; Advances in Polar Ecology; Siegel, V., Ed.; Springer: Cham, Switzerland, 2016; pp. 21–100. [Google Scholar] [CrossRef]
- Pakhomov, E.A.; Froneman, P.W.; Perissinotto, R. Salp/krill interactions in the Southern Ocean: Spatial segregation and implications for the carbon flux. Deep Sea Res. II 2002, 49, 1881–1907. [Google Scholar] [CrossRef]
- Atkinson, A.; Hill, S.L.; Pakhomov, E.A.; Siegel, V.; Reiss, C.S.; Loeb, V.J.; Steinberg, D.K.; Schmidt, K.; Tarling, G.A.; Gerrish, L.; et al. Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nat. Clim. Chang. 2019, 9, 142–147. [Google Scholar] [CrossRef]
- McBride, M.M.; Schram Stokke, O.; Renner, A.H.H.; Krafft, B.A.; Bergstad, O.; Biuw, M.; Lowther, A.; Stiansen, J. Antarctic krill Euphausia superba: Spatial distribution, abundance, and management of fisheries in a changing climate. Mar. Ecol. Prog. Ser. 2021, 668, 185–214. [Google Scholar] [CrossRef]
- Bitiutskii, D.G.; Samyshev, E.Z.; Minkina, N.I.; Melnikov, V.V.; Chudinovskih, E.S.; Usachev, S.I.; Salyuk, P.A.; Serebrennikov, A.N.; Zuev, O.A.; Orlov, A.M. Distribution and demography of Antarctic krill and salps in the Atlantic sector of the Southern Ocean during austral summer 2021–2022. Water 2022, 14, 3812. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, Z.; Huang, H.; Chen, X.; Feng, C.; Li, L. Geographical distribution and age composition of Euphausia superba larvae (Crustacea: Euphausiacea) in the South Shetland Islands region and southern Scotia Sea in relation to environmental conditions. Acta Oceanol. Sin. 2013, 32, 59–67. [Google Scholar] [CrossRef]
- Sologub, D.O. Distribution and age structure of euphausiids larvae at the Western Antarctic Peninsula (Subarea 48.1). Trudy VNIRO 2015, 154, 3–15. [Google Scholar]
- Kasyan, V.V. Age Structure and Spatial Distribution of Euphausia superba Larvae off the Antarctic Peninsula, Southern Ocean. Water 2022, 14, 3196. [Google Scholar] [CrossRef]
- Voronina, N.M. Pelagic Ecosystems of the Southern Ocean; Nauka: Moscow, Russia, 1984; pp. 5–206. [Google Scholar]
- McBride, M.; Dalpadado, P.; Drinkwater, K.; Godo, O.; Hobday, A.; Hollowed, A.; Kristiansen, T.; Murphy, E.; Ressler, P.; Subbey, S.; et al. Krill, climate, and contrasting future scenarios for Arctic and Antarctic fisheries. ICES J. Mar. Sci. 2014, 71, 1934–1955. [Google Scholar] [CrossRef]
- Takahashi, K.; Takamura, T.; Odate, T. Zooplankton communities along a Southern Ocean monitoring transect at 110 E from three CPR surveys (Dec 2014, Jan 2015, Mar 2015). Polar. Biol. 2021, 44, 1069–1081. [Google Scholar] [CrossRef]
- Johnston, N.M.; Murphy, E.J.; Atkinson, A.; Constable, A.J.; Cotte, C.; Cox, M.; Daly, K.L.; Driscoll, R.; Flores, H.; Halfter, S.; et al. Status, change, and futures of zooplankton in the Southern Ocean. Front. Ecol. Evol. 2022, 9, 624692. [Google Scholar] [CrossRef]
- Quetin, L.B.; Ross, R.M.; Frazer, T.K.; Haberman, K.L. Factors affecting distribution and abundance of zooplankton, with an emphasis on Antarctic krill, Euphausia superba. In Antarctic Research Series; Hofmann, E.E., Ross, R.M., Quetin, L.B., Eds.; American Geophysical Union: Washington, DC, USA, 1996; pp. 357–371. [Google Scholar] [CrossRef]
- Atkinson, A. Life cycle strategies of epipelagic copepods in the Southern Ocean. J. Mar. Syst. 1998, 15, 289–311. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Nicol, S. Learning about Antarctic krill from the fishery. Antarct. Sci. 2007, 19, 219–230. [Google Scholar] [CrossRef]
- Siegel, V.; Reiss, C.S.; Dietrich, K.S.; Haraldsson, M.; Rohardt, G. Distribution and abundance of Antarctic krill (Euphausia superba) along the Antarctic Peninsula. Deep Res. Part I 2013, 77, 63–74. [Google Scholar] [CrossRef]
- Steinberg, D.K.; Ruck, K.E.; Gleiber, M.R.; Garzio, L.M.; Cope, J.S.; Bernard, K.S.; Stammerjohn, S.E.; Schofield, O.M.E.; Quetin, L.B.; Ross, R.M. Long-term (1993–2013) changes in macrozooplankton off the Western Antarctic Peninsula. Deep Sea Res. I 2015, 101, 54–70. [Google Scholar] [CrossRef]
- Weikert, H.; John, H.C. Experiences with a modified be multiple opening–closing plankton net. J. Plankton Res. 1981, 3, 167–176. [Google Scholar] [CrossRef]
- Tranter, D.J. (Ed.) Reviews on zooplankton sampling methods. In Zooplankton Sampling; Part I (2); UNESCO: Paris, France, 1968; pp. 11–144. [Google Scholar]
- Boltovskoy, D. South Atlantic Zooplankton; Backhuys Publishers: Leiden, The Netherlands, 1999; p. 868. [Google Scholar]
- WoRMS Editorial Board. World Register of Marine Species. 2015. Available online: http://www.marinespecies.org (accessed on 29 August 2022).
- Razouls, C.; De Bovee, F.; Kouwenberg, J.; Desreumaux, N. Diversity and Geographic Distribution of Marine Planktonic Copepods; CNRS—Sorbonne University: Paris, France, 2005–2022; Available online: http://copepodes.obs–banyuls.fr/en/ (accessed on 3 February 2022).
- Makarov, R.R. Larvae of Euphausia superba Dana in plankton from the Sea of Scotia. Tr. Vses. Nauchno-Issled. Ryb. Okeanogr. 1974, 99, 84–103. [Google Scholar]
- Pertsova, K.N. Larvae of euphausiids of the Antarctic. In Ecology and Biogeography of Plankton: Proceedings of the Institute of Oceanology RAS; Nauka: Moscow, Russia, 1976; Volume 105, pp. 147–170. [Google Scholar]
- Suntov, A.P. Manual on Quantitative Processing of Marine Net Plankton; TINRO: Vladivostok, Russia, 1982; pp. 1–29. (In Russian) [Google Scholar]
- Mizdalski, E. Weight and length data of zooplankton in the Weddell Sea in austral spring 1986 (ANT V/3). Ber. Polarforsch. 1988, 55, 1–72. [Google Scholar]
- Heywood, K.J.; Garabato, A.C.N.; Stevens, D.P.; Muench, R.D. On the fate of the Antarctic Slope Front and the origin of the Weddell Front. J. Geophys. Res. 2004, 109, C06021. [Google Scholar] [CrossRef]
- Thompson, A.F.; Heywood, K.J.; Thorpe, S.E.; Renner, A.H.H.; Trasvica, A. Surface circulation at the tip of the Antarctic Peninsula from drifters. J. Phys. Oceanog. 2009, 39, 3–26. [Google Scholar] [CrossRef]
- Morozov, E.G.; Flint, M.V.; Spiridonov, V.A. Antarctic Peninsula region of the Southern Ocean; Advances in Polar Ecology; Springer: Cham, Switzerland, 2021; Volume 6, p. 433. [Google Scholar] [CrossRef]
- Clark, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; PRIMER–E Ltd.: Plymouth, UK, 2001; p. 144. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Clarke, K.R.; Ainsworth, M. A method of linking multivariate community structure to environmental variables. Mar. Ecol. Prog. Ser. 1993, 92, 205–219. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER–E Ltd.: Plymouth, UK, 2006; p. 192. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 262. Available online: http://palaeo–electronica.org/2001_1/past/issue1_01.htm (accessed on 1 March 2023).
- Schlitzer, R. Ocean Data View; Alfred Wegener Institute: Bremerhaven, Germany, 2012; p. 151. Available online: http://odv.awi.de (accessed on 13 February 2023).
- Zhou, X.Q.; Zhu, G.P.; Hu, S. Influence of tides on mass transport in the Bransfield Strait and the adjacent areas. Antarct. Polar. Sci. 2020, 23, 100506. [Google Scholar] [CrossRef]
- Fedotova, A.A.; Stepanova, S.V. Water mass transformation in the Powell Basin. In Antarctic Peninsula Region of the Southern Ocean; Morozov, E.G., Flint, M.V., Spiridonov, V.A., Eds.; Springer: Cham, Switzerland, 2021; Volume 6, pp. 115–129. [Google Scholar] [CrossRef]
- Krechik, V.A.; Frey, D.I.; Morozov, E.G. Peculiarities of water circulation in the central part of the Bransfield Strait in January 2020. Dokl. Earth Sci. 2021, 496, 92–95. [Google Scholar] [CrossRef]
- Frey, D.I.; Krechik, V.A.; Morozov, E.G.; Drozd, I.D.; Gordey, A.S.; Latushkin, A.A.; Mekhova, O.S.; Mukhametianov, R.Z.; Murzina, S.A.; Ostroumova, S.A.; et al. Water Exchange between Deep Basins of the Bransfield Strait. Water 2022, 14, 3193. [Google Scholar] [CrossRef]
- Lee, W.; Kim, S.; Kang, S.; Bang, H.; Lee, K.; Kwak, I. Distribution and abundance of zooplankton in the Bransfield Strait and the western Weddell Sea during austral summer. Ocean Polar. Res. 2004, 26, 607–618. [Google Scholar] [CrossRef]
- Atkinson, A.; Ward, P.; Hunt, B.P.V.; Pakhomov, E.A.; Hosie, G.W. An overview of Southern Ocean zooplankton data: Abundance, biomass, feeding and functional relationships. CCAMLR Sci. 2012, 19, 171–218. [Google Scholar]
- Edwards, M.; Bresnan, E.; Cook, K.; Heath, M.; Helaouet, P.; Lynam, C.; Raine, R.; Widdicombe, C. Impacts of climate change on plankton. MCCIP Sci. Rev. 2013, 4, 98–112. [Google Scholar] [CrossRef]
- Sallee, J.B. Southern Ocean warming. Oceanography 2018, 31, 52–62. [Google Scholar] [CrossRef]
- Schofield, O.; Brown, M.; Kohut, J.; Nardelli, S.; Saba, G.; Waite, N.; Ducklow, H. Changing upper ocean mixed layer depth and phytoplankton productivity along the West Antarctic Peninsula. Philos. Trans. R. Soc. A 2018, 376, 20170174. [Google Scholar] [CrossRef]
- Moline, M.A.; Claustre, H.; Frazer, T.K.; Schofield, O.; Vernet, M. Alteration of the food web along the Antarctic Peninsula in response to a regional warming trend. Glob. Chang. Biol. 2004, 10, 1973–1980. [Google Scholar] [CrossRef]
- Montes-Hugo, M.; Doney, S.C.; Ducklow, H.W.; Fraser, W.; Martinson, D.; Stammerjohn, S.E.; Schofield, O. Recent changes in phytoplankton communities associated with rapid regional climate change along the Western Antarctic Peninsula. Science 2009, 323, 1470–1473. [Google Scholar] [CrossRef]
- Biggs, T.E.G.; Alvarez-Fernandez, S.; Evans, C.; Mojica, K.D.A.; Rozema, P.D.; Venables, H.J.; Pond, D.W.; Brussaard, C.P.D. Antarctic phytoplankton community composition and size structure: Importance of ice type and temperature as regulatory factors. Polar. Biol. 2019, 42, 1997–2015. [Google Scholar] [CrossRef]
- Atkinson, A.; Hill, S.L.; Pakhomov, E.A.; Siegel, V.; Anadon, R.; Chiba, S.; Daly, K.L.; Downie, R.; Fielding, S.; Fretwell, P.; et al. KRILLBASE: A circumpolar database of Antarctic krill and salp numerical densities, 1926–2016. Earth Syst. Sci. Data 2017, 9, 193–210. [Google Scholar] [CrossRef]
- Morozov, E.G.; Krechik, V.A.; Frey, D.I.; Polukhin, A.A.; Artemiev, V.A.; Kasyan, V.V.; Sapozhnikov, P.V.; Mukhametianov, R.Z. Frontal Zone between Relatively Warm and Cold Waters in the Northern Weddell Sea. In Antarctic Peninsula Region of the Southern Ocean; Morozov, E.G., Flint, M.V., Spiridonov, V.A., Eds.; Springer: Cham, Switzerland, 2021; Volume 6, pp. 31–54. [Google Scholar] [CrossRef]
- Gallienne, C.P.; Robins, D.B. Is Oithona the most important copepod in the world’s oceans? J. Plankton Res. 2001, 23, 1421–1432. [Google Scholar] [CrossRef]
- Skjoldal, H.R.; Wiebe, P.H.; Postel, L.; Knutsen, T.; Kaartvedt, S.; Sameoto, D.D. Intercomparison of zooplankton (net) sampling systems: Results the ICES/GLOBEC sea-going workshop. Prog. Oceanogr. 2013, 108, 1–42. [Google Scholar] [CrossRef]
- Morozov, E.G.; Flint, M.V.; Orlov, A.M.; Frey, D.I.; Molodtsova, T.N.; Krechik, V.A.; Latushkin, A.A.; Salyuk, P.A.; Murzina, S.A.; Minin, K.V.; et al. Oceanographic and Ecosystem Studies in the Atlantic Sector of Antarctica (Cruise 87 of the Research Vessel Akademik Mstislav Keldysh). Oceanology 2022, 62, 825–827. [Google Scholar] [CrossRef]
- Hopkins, T.L.; Torres, J.J. The zooplankton community in the vicinity of the ice edge, western Weddell Sea, March 1986. Polar. Biol. 1988, 9, 79–87. [Google Scholar] [CrossRef]
- Voronina, N.M.; Kosobokova, K.N.; Pakhomov, E.A. Composition and biomass of summer metazoan plankton in the 0–200 m layer of the Atlantic sector of the Antarctic. Polar. Biol. 1994, 14, 91–95. [Google Scholar] [CrossRef]
- Fransz, H.G.; Gonzalez, S.R. The production of Oithona similis (Copepoda: Cyclopoida) in the Southern Ocean. ICES (Int. Counc. Explor. Sea) J. Mar. Sci. 1995, 52, 549–555. [Google Scholar]
- Pinkerton, M.H.; Smith, A.N.H.; Raymond, B.; Hosie, G.W.; Sharp, B.; Leathwick, J.R.; Bradford-Grieve, J.M. Spatial and seasonal distribution of adult Oithona similis in the Southern Ocean: Predictions using boosted regression trees. Deep Sea Res. I 2010, 57, 469–485. [Google Scholar] [CrossRef]
- Takahashi, M.; Nemoto, T. The food of some Antarctic fish in the western Ross Sea in summer 1979. Polar. Biol. 1984, 3, 237–239. [Google Scholar] [CrossRef]
- Spiridonov, V.A.; Zalota, A.K.; Yakovenko, V.A.; Gorbatenko, K.M. Composition of population and transport of juveniles of Antarctic krill in Powell Basin region (northwestern Weddell Sea) in January 2020. Tr. VNIRO 2020, 181, 33–51. [Google Scholar] [CrossRef]
- Tarling, G.A.; Peck, V.; Ward, P.; Ensor, N.; Achterberg, E.; Tynan, E.; Poulton, A.J.; Mitchell, E.; Zubkov, M.V. Effects of acute ocean acidification on spatially–diverse polar pelagic foodwebs: Insights from on–deck microcosms. Deep-Sea Res. II 2016, 127, 75–92. [Google Scholar] [CrossRef]
- Trifoglio, N.L.; Olguin Salinas, H.F.; Franzosi, C.A.; Alder, V.A. Annual cycle of phytoplankton, protozoa and diatom species from Scotia Bay (South Orkney Islands, Antarctica): Community structure prior to, during and after an anomalously low sea ice year. Prog. Oceanogr. 2022, 204, 102807. [Google Scholar] [CrossRef]
- Quetin, L.B.; Ross, R.M. Feeding by Antarctic krill, Euphausia superba: Does size matter? In Antarctic Nutrient Cycles and Food Webs; Siegfried, W.R., Condy, P.R., Laws, R.M., Eds.; Springer: Berlin, Germany, 1985; pp. 372–377. [Google Scholar]
- Granli, E.; Graneli, W.; Rabbani, M.M.; Daugbjerg, N.; Fransz, G.; Roudy, J.C.; Alder, V.A. The influence of copepod and krill grazing on the species composition of phytoplankton communities from the Scotia Weddell Sea. Polar. Biol. 1993, 13, 201–213. [Google Scholar] [CrossRef]
- Froneman, P.W.; Perissinotto, R. Microzooplankton grazing and protozooplankton community structure in the South Atlantic and in the Atlantic sector of the Southern Ocean. Deep Sea Res. 1996, 43, 703–721. [Google Scholar] [CrossRef]
- Perissinotto, R.A.; Pakhomov, E.A. The trophic role of the tunicate Salpa thompsoni in the Antarctic marine ecosystem. J. Mar. Syst. 1998, 17, 361–374. [Google Scholar] [CrossRef]
- Flores, H.; Hunt, B.P.V.; Kruse, S.; Pakhomov, E.A.; Siegel, V.; van Franeker, J.A.; Strass, V.; Van de Putte, A.P.; Meesters, E.H.W.G.; Bathmann, U. Seasonal changes in the vertical distribution and community structure of Antarctic macrozooplankton and micronekton. Deep Sea Res. 2014, 84, 127–141. [Google Scholar] [CrossRef]
- Takahashi, K.T.; Ojima, M.; Tanimura, A.; Odate, T.; Fukuchi, M. The vertical distribution and abundance of copepod nauplii and other micro- and mesozooplankton in the seasonal ice zone of Lützow-Holm Bay during austral summer 2009. Polar. Biol. 2017, 40, 79–93. [Google Scholar] [CrossRef]
- Conroy, J.A.; Steinberg, D.K.; Thibodeau, P.S.; Schofield, O. Zooplankton diel vertical migration during Antarctic summer. Deep Sea Res. I 2020, 162, 103324. [Google Scholar] [CrossRef]
- Delgado, L.E.; Jana, R.; Mari, V.H. Testing hypotheses on life-cycle models for Antarctic calanoid copepods, using qualitative, winter, zooplankton samples. Polar. Biol. 1998, 20, 74–76. [Google Scholar] [CrossRef]
- Tarling, G.A.; Ward, P.; Thorpe, S.E. Spatial distributions of Southern Ocean mesozooplankton communities have been resilient to long-term surface warming. Glob. Chang. Biol. 2018, 24, 132–142. [Google Scholar] [CrossRef]
- Mackintosh, N.A. Life cycle of Antarctic krill in relation to ice and water conditions. Discov. Rep. 1972, 36, 1–94. [Google Scholar]
- Ross, R.M.; Quetin, L.B.; Kirsch, E. Effect of temperature on developmental times and survival of early larval stages of Euphausia superba Dana. J. Exp. Mar. Biol. Ecol. 1988, 121, 55–71. [Google Scholar] [CrossRef]
- Ross, R.M.; Quetin, L.B.; Baker, K.S.; Vernet, M.; Smith, R.C. Growth limitation in young Euphausia superba under field conditions. Limnol. Oceanogr. 2000, 45, 31–43. [Google Scholar] [CrossRef]
- Kouwenberg, J.H.M.; Razouls, C.; Desreumaux, N. Southern Ocean pelagic copepods. In The Biogeographic Atlas of the Southern Ocean; De Broyer, C., Koubbi, P., Griffith, H.J., Raymond, B., d’Udekem d’Acoz, C., Van de Putte, A.D., Danis, B., David, B., Grant, S., Gutt, J., et al., Eds.; SCAR: Cambridge, UK, 2014; pp. 209–296. [Google Scholar]
- Cornils, A.; Sieger, R.; Mizdalski, E.; Schumacher, S.; Grobe, H.; Schnack-Schiel, S.B. Copepod species abundance from the Southern Ocean and other regions (1980–2005)—A legacy. Earth Syst. Sci. Data 2018, 10, 1457–1471. [Google Scholar] [CrossRef]



| Variables | Study Region | |||
|---|---|---|---|---|
| BS | AS | WS | SOI | |
| 50 m S | 34.46 ± 0.14 | 34.49 ± 0.07 | 34.27 ± 0.15 | 34.32 ± 0.08 |
| 200 m S | 34.56 ± 0.07 | 34.53 ± 0.04 | 34.60 ± 0.07 | 34.59 ± 0.04 |
| 500 m S | 34.62 ± 0.08 | 34.54 ± 0.03 | 34.70 ± 0.09 | 34.74 ± 0.01 |
| 50 m T | 0.53 ± 0.61 | −0.25 ± 0.14 | 0.47 ± 0.63 | 1.35 ± 0.41 |
| 200 m T | −0.11 ± 0.55 | −0.41 ± 0.07 | −0.77 ± 0.72 | −0.37 ± 0.33 |
| 500 m T | −0.28 ± 0.68 | −0.63 ± 0.37 | −0.10 ± 0.91 | 0.27 ± 0.19 |
| 50 m O2 | 10.49 ± 0.25 | 10.76 ± 0.01 | 10.50 ± 0.19 | 10.46 ± 0.16 |
| 200 m O2 | 8.75 ± 0.95 | 10.41 ± 0.12 | 8.17 ± 1.08 | 8.15 ± 0.75 |
| 500 m O2 | 8.12 ± 0.90 | 9.74 ± 0.59 | 7.09 ± 0.9 | 6.63 ± 0.29 |
| 50 m Chl a | 0.65 ± 0.26 | 1.17 ± 0.09 | 1.38 ± 1.82 | 1.15 ± 1.1 |
| 200 m Chl a | 0.12 ± 0.14 | 0.44 ± 0.14 | 0.07 ± 0.03 | 0.06 ± 0.01 |
| 500 m Chl a | 0.07 ± 0.08 | 0.11 ± 0.01 | 0.06 ± 0.03 | 0.04 ± 0.01 |
| Number of Variables | Correlation Coefficient | Selections |
|---|---|---|
| 2 | 0.400 | 500 m S, 500 m Chl a |
| 3 | 0.385 | 500 m S, 50 m Chl a, 500 m Chl a |
| 3 | 0.361 | 500 m S, 200 m T, 500 m Chl a |
| 3 | 0.353 | 500 m S, 50 m S, 500 m Chl a |
| 50 m T | 50 m S | 50 m O2 | 50 m Chl a | 200 m T | 200 m S | 200 m O2 | 200 m Chl a | 500 m T | 500 m S | 500 m O2 | 500 m Chl a | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abn Meso | 0.38 | −0.16 | −0.20 | 0.05 | −0.06 | 0.35 | −0.13 | −0.23 | 0.11 | 0.40 | −0.24 | −0.46 |
| Bm Meso | 0.37 | −0.28 | −0.24 | 0.12 | 0.01 | 0.31 | −0.27 | −0.34 | 0.21 | 0.50 | −0.31 | −0.41 |
| Abn Cop | 0.29 | 0.08 | −0.21 | −0.19 | 0.00 | 0.26 | −0.13 | −0.23 | −0.10 | 0.21 | −0.12 | −0.43 |
| Bm Cop | 0.14 | −0.07 | −0.32 | −0.10 | −0.02 | 0.18 | −0.21 | −0.25 | 0.11 | 0.38 | −0.29 | −0.29 |
| Abn Euph | 0.08 | −0.36 | −0.03 | 0.12 | −0.39 | 0.34 | −0.09 | −0.02 | 0.21 | 0.44 | −0.28 | −0.14 |
| Bm Euph | 0.17 | −0.26 | 0.02 | 0.40 | −0.26 | 0.36 | −0.17 | −0.07 | 0.27 | 0.49 | −0.33 | −0.15 |
| Taxa | OSAP | ISAP | OSAP vs. ISAP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | SIM/SD | Contr, % | N | SIM/SD | Contr, % | DISS/SD | Contr, % | Cum, % | |
| Similarity: 68.78 | Similarity: 64.81 | Dissimilarity: 41.82 | |||||||
| E. superba calyptopis | 5.58 | 9.42 | 14.81 | 1.33 | 2.84 | 12.48 | 12.48 | ||
| E. superba furcilia | 4.73 | 1.73 | 9.91 | 0.77 | 1.82 | 11.89 | 24.37 | ||
| E. superba eggs | 4.08 | 0.92 | 6.82 | 1.33 | 1.43 | 10.30 | 34.67 | ||
| Calanus propinquus | 3.42 | 2.04 | 6.89 | 1.67 | 3.24 | 6.45 | 1.13 | 5.57 | 40.24 |
| Calanoides acutus | 4.93 | 6.74 | 12.45 | 3.39 | 4.56 | 14.67 | 1.31 | 4.62 | 44.86 |
| Rhincalanus gigas | 2.19 | 1.64 | 1.81 | 5.18 | 1.28 | 4.32 | 49.18 | ||
| Cirripedia | 0.79 | 1.45 | 1.20 | 4.21 | 53.39 | ||||
| Polychaeta | 2.08 | 1.05 | 0.85 | 4.02 | 57.42 | ||||
| Metridia gerlachei | 3.63 | 8.77 | 9.41 | 4.22 | 3.30 | 15.77 | 1.45 | 3.95 | 61.37 |
| Oncea spp. | 1.00 | 0.53 | 0.92 | 3.54 | 64.91 | ||||
| Ostracoda | 3.00 | 3.78 | 7.13 | 2.38 | 1.98 | 8.46 | 1.10 | 3.26 | 68.17 |
| Calanus simillimus | 1.03 | 1.25 | 1.28 | 2.99 | 71.15 | ||||
| Oithona spp. | 5.08 | 11.43 | 13.95 | 5.42 | 5.48 | 22.66 | 1.51 | 2.90 | 74.06 |
| Metridia spp. | 0.88 | 0.22 | 0.69 | 2.81 | 76.87 | ||||
| Salpa thompsoni | 0.96 | 0.74 | 1.27 | 2.63 | 82.01 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasyan, V.V. Recent Changes in Composition and Distribution Patterns of Summer Mesozooplankton off the Western Antarctic Peninsula. Water 2023, 15, 1948. https://doi.org/10.3390/w15101948
Kasyan VV. Recent Changes in Composition and Distribution Patterns of Summer Mesozooplankton off the Western Antarctic Peninsula. Water. 2023; 15(10):1948. https://doi.org/10.3390/w15101948
Chicago/Turabian StyleKasyan, Valentina V. 2023. "Recent Changes in Composition and Distribution Patterns of Summer Mesozooplankton off the Western Antarctic Peninsula" Water 15, no. 10: 1948. https://doi.org/10.3390/w15101948





