Developing the Ascorbic Acid Test: A Candidate Standard Tool for Characterizing the Intrinsic Reactivity of Metallic Iron for Water Remediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solutions
2.2. Iron Materials
2.3. Experimental Methods
2.3.1. Batch Experiments
2.3.2. Column Experiments
2.4. Analytical Method
2.5. Experimental Results
3. Results and Discussion
3.1. Suitability of the Experimental Protocol
3.2. Deciphering the Processes of Iron Dissolution in Fe0/AA Systems
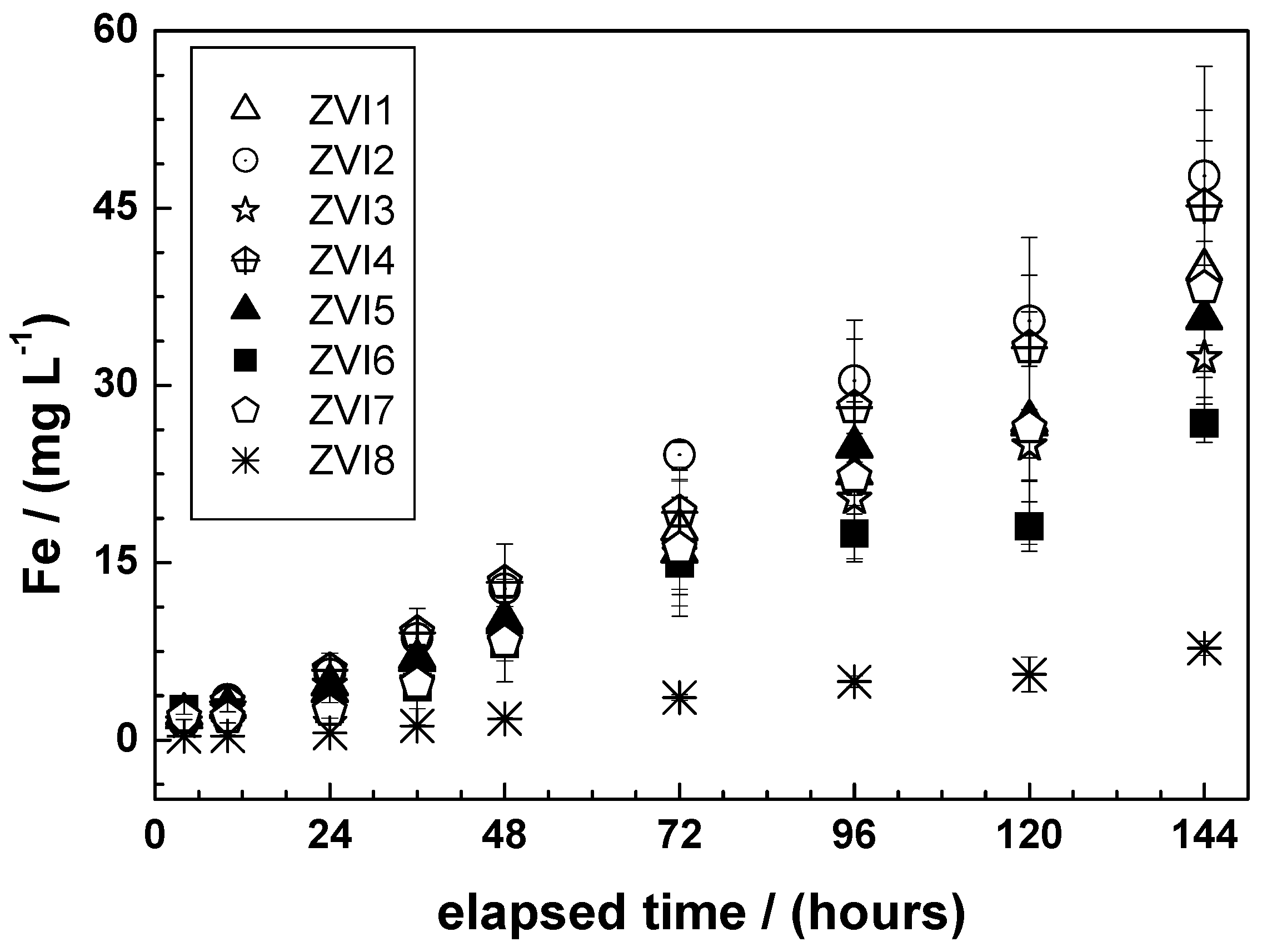
3.3. Characterizing Fe0 Dissolution in 2 mM Ascorbic Acid (AA)
3.4. Characterizing the Long-Term Fe0 Dissolution in Column Studies
4. Significance of the Results
4.1. Fe0 Quality as a Stand-Alone Operational Parameter
4.2. Other Key Operational Parameters
4.3. Current Approaches to Address Fe0 Quality
4.4. The AA Method as a Quality Control Tool for Fe0 Materials
- (1)
- Add 0.1 g of Fe0 to 50 mL of a 2 mM AA solution and monitor the concentration of dissolved Fe for 0.3, 1.0, 2.0., 3.0, 4.0 and 5.0 days.
- (2)
- Use the iron concentration after 8 h to estimate the amount of iron corrosion products and the remaining data to determine the kAA value. kAA is the slope of the line dissolved [Fe] versus time t for t ≥ 24 h.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henderson, A.D.; Demond, A.H. Long-term performance of zero-valent iron permeable reactive barriers: A critical review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.C.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef]
- Plessl, K.; Russ, A.; Vollprecht, D. Application and development of zero-valent iron (ZVI) for groundwater and wastewater treatment. Int. J. Environ. Sci. Technol. 2023, 20, 6913–6928. [Google Scholar] [CrossRef]
- Sbahi, S.; Mandi, L.; Masunaga, T.; Ouazzani, N.; Hejjaj, A. Multi-soil-layering, the emerging technology for wastewater treatment: Review, bibliometric analysis, and future directions. Water 2022, 14, 3653. [Google Scholar] [CrossRef]
- Bilardi, S.; Calabrò, P.S.; Moraci, N. A review of the hydraulic performance of permeable reactive barriers based on granular zero valent iron. Water 2023, 15, 200. [Google Scholar] [CrossRef]
- Fairbairn, D.J.; Trojan, M.D. Iron-enhanced sand filters: Multi-year urban runoff (stormwater) quality performance. Sci. Tot. Environ. 2023, 859, 160177. [Google Scholar] [CrossRef]
- Lawrinenko, M.; Kurwadkar, S.; Wilkin, R.T. Long–term performance evaluation of zero-valent iron amended permeable reactive barriers for groundwater remediation—A mechanistic approach. Geosci. Front. 2023, 14, 101494. [Google Scholar] [CrossRef]
- Singh, R.; Chakma, S.; Birke, V. Performance of field-scale permeable reactive barriers: An overview on potentials and possible implications for in-situ groundwater remediation applications. Sci. Total Environ. 2023, 858, 158838. [Google Scholar] [CrossRef]
- Matheson, L.J.; Tratnyek, P.G. Reductive dehalogenation of chlorinated methanes by iron metal. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, G.W.; Hoff, J.T.; Gillham, R.W. Sampling bias caused by materials used to monitor halocarbons in groundwater. Environ. Sci. Technol. 1990, 24, 135–142. [Google Scholar] [CrossRef]
- Gillham, R.W.; O’Hannesin, S.F. Enhanced degradation of halogenated aliphatics by zero-valent iron. Ground Water 1994, 32, 958–967. [Google Scholar] [CrossRef]
- O’Hannesin, S.F.; Gillham, R.W. Long-term performance of an in situ “iron wall” for remediation of VOCs. Ground Water 1998, 36, 164–170. [Google Scholar] [CrossRef]
- Whitney, W.R. The corrosion of iron. J. Am. Chem. Soc. 1903, 25, 394–406. [Google Scholar] [CrossRef]
- Khudenko, B.M. Feasibility evaluation of a novel method for destruction of organics. Water Sci. Technol. 1991, 23, 1873–1881. [Google Scholar] [CrossRef]
- Hu, R.; Gwenzi, W.; Sipowo Hu, R.; Ndé-Tchoupé, A.I.; Cao, V.; Gwenzi, W.; Noubactep, C. Metallic iron for environmental remediation: The fallacy of the electron efficiency concept. Front. Environ. Chem. 2021, 2, 677813. [Google Scholar] [CrossRef]
- Lawrinenko, M.; Kurwadkar, S.; Wilkin, R.T. Reply to comment by C. Noubactep on “Long-term performance evaluation of zero-valent iron amended permeable reactive barriers for groundwater remediation: A mechanistic approach”. Geosci. Front. 2023, 14, 101583. [Google Scholar] [CrossRef]
- Noubactep, C. Comments on “Long-term performance evaluation of zero-valent iron amended permeable reactive barriers for groundwater remediation: A mechanistic approach” by Lawrinenko et al., Geoscience Frontiers 14 (2023) 101494. Geosci. Front. 2023, 14, 101582. [Google Scholar] [CrossRef]
- Makota, S.; Ndé-Tchoupé, A.I.; Mwakabona, H.T.; Tepong-Tsindé, R.; Noubactep, C.; Nassi, A.; Njau, K.N. Metallic iron for water treatment: Leaving the valley of confusion. Appl. Water Sci. 2017, 7, 4177–4196. [Google Scholar] [CrossRef]
- Mines, P.D.; Kaarsholm, K.M.S.; Droumpali, A.; Andersen, H.R.; Hwang, Y. Estimating dehalogenation reactivity of nanoscale zero-valent iron by simple colorimetric assay by way of 4-chlorophenol reduction. Environ. Eng. Res. 2020, 25, 197–204. [Google Scholar] [CrossRef]
- Nyström, S. Long-term Effect of Metallic Iron Sorbents on Arsenic Mobility in an Anoxic Aquifer—An Assessment Based on Long-Term Column Experiments. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2022. [Google Scholar]
- Miyajima, K.; Noubactep, C. Characterizing the impact of sand addition on the efficiency of granular iron for water treatment. Chem. Eng. J. 2015, 262, 891–896. [Google Scholar] [CrossRef]
- Gheju, M. Progress in understanding the mechanism of CrVI Removal in Fe0-based filtration systems. Water 2018, 10, 651. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I. Effect of sand co-presence on CrVI removal in Fe0-H2O system. Water 2023, 15, 777. [Google Scholar] [CrossRef]
- Westerhoff, P. Reduction of nitrate, bromate, and chlorate by zero valent iron (Fe0). J. Environ. Eng. 2003, 129, 10–16. [Google Scholar] [CrossRef]
- Miehr, R.; Tratnyek, G.P.; Bandstra, Z.J.; Scherer, M.M.; Alowitz, J.M.; Bylaska, J.E. Diversity of contaminant reduction reactions by zerovalent iron: Role of the reductate. Environ. Sci. Technol. 2004, 38, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Ghauch, A.; Abou Assi, H.; Bdeir, S. Aqueous removal of diclofenac by plated elemental iron: Bimetallic systems. J. Hazard. Mater. 2010, 182, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Comba, S.; Di Molfetta, A.; Sethi, R. A Comparison between field applications of nano-, micro-, and millimetric zero-valent iron for the remediation of contaminated aquifers. Water Air Soil Pollut. 2011, 215, 595–607. [Google Scholar] [CrossRef]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Noubactep, C.; Caré, S. On nanoscale metallic iron for groundwater remediation. J. Hazard. Mater. 2010, 182, 923–927. [Google Scholar] [CrossRef]
- Noubactep, C.; Caré, S.; Crane, R.A. Nanoscale metallic iron for environmental remediation: Prospects and limitations. Water Air Soil Pollut. 2012, 223, 1363–1382. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Licha, T.; Scott, T.B.; Fall, M.; Sauter, M. Exploring the influence of operational parameters on the reactivity of elemental iron materials. J. Hazard. Mater. 2009, 172, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Btatkeu, K.B.D.; Miyajima, K.; Noubactep, C.; Caré, S. Testing the suitability of metallic iron for environmental remediation: Discoloration of methylene blue in column studies. Chem. Eng. J. 2013, 215–216, 959–968. [Google Scholar] [CrossRef]
- Hwang, Y.; Mines, P.D.; Jakobsen, M.H.; Andersen, H.R. Simple colorimetric assay for dehalogenation reactivity of nanoscale zero-valent iron using 4-chlorophenol. Appl. Catal. B Environ. 2015, 166–167, 18–24. [Google Scholar] [CrossRef]
- Reardon, E.J. Anaerobic corrosion of granular iron: Measurement and interpretation of hydrogen evolution rates. Environ. Sci. Technol. 1995, 29, 2936–2945. [Google Scholar] [CrossRef]
- Reardon, E.J. Zerovalent irons: Styles of corrosion and inorganic control on hydrogen pressure buildup. Environ. Sci. Tchnol. 2005, 39, 7311–7317. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Sauter, M.; Merkel, B. Testing the suitability of zerovalent iron materials for reactive Walls. Environ. Chem. 2005, 2, 71–76. [Google Scholar] [CrossRef]
- Kim, H.; Yang, H.; Kim, J. Standardization of the reducing power of zerovalent iron using iodine. Environ. Lett. 2014, 49, 514–523. [Google Scholar]
- Li, S.; Ding, Y.; Wang, W.; Lei, H. A facile method for determining the Fe(0) content and reactivity of zero valent iron. Anal. Methods 2016, 8, 1239–1248. [Google Scholar] [CrossRef]
- Hu, R.; Cui, X.; Xiao, M.; Qiu, P.; Lufingo, M.; Gwenzi, W.; Noubactep, C. Characterizing the suitability of granular Fe0 for the water treatment industry. Processes 2019, 7, 652. [Google Scholar] [CrossRef]
- Lufingo, M.; Ndé-Tchoupé, A.I.; Hu, R.; Njau, K.N.; Noubactep, C. A novel and facile method to characterize the suitability of metallic iron for water treatment. Water 2019, 11, 2465. [Google Scholar] [CrossRef]
- Ndé-Tchoupé, A.I.; Hu, R.; Gwenzi, W.; Nassi, A.; Noubactep, C. Characterizing the reactivity of metallic iron for water treatment: H2 evolution in H2SO4 and uranium removal efficiency. Water 2020, 12, 1523. [Google Scholar] [CrossRef]
- Elmagirbi, A.; Sulistyarti, H. Atikah Study of ascorbic acid as iron(III) reducing agent for spectrophotometric iron speciation. J. Pure App. Chem. Res. 2012, 1, 11–17. [Google Scholar] [CrossRef]
- Hynes, M.J.; Kelly, D.F. The reduction of iron(III) by ascorbic acid. J. Chem. Soc. Chem. Commun. 1988, 13, 849–850. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, X.; Fan, F.; Li, Y. Factors affecting the determination of iron species in the presence of ferric iron. Appl. Water Sci. 2018, 8, 228. [Google Scholar] [CrossRef]
- Hsieh, Y.H.P.; Hsieh, Y.P. Valence state of iron in the presence of ascorbic acid and ethylenediaminetetraacetic acid. J. Agric. Food Chem. 1997, 45, 1126–1129. [Google Scholar] [CrossRef]
- Fujita, Y.; Mori, I.; Yamaguchi, T.; Hoshino, M.; Shigemura, Y.; Shimano, M. Spectrophotometric determination of ascorbic acid with iron(III) and p-carboxyphenylfluorone in a cationic surfactant micellar medium. Anal. Sci. 2001, 17, 853–857. [Google Scholar] [CrossRef]
- Larsen, O.; Postma, D.; Jakobsen, R. The reactivity of iron oxides towards reductive dissolution with ascorbic acid in a shallow sandy aquifer (Rømø, Denmark). Geochim. Cosmochim. Acta 2006, 70, 4827–4835. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Liang, C. Carbon tetrachloride degradation by alkaline ascorbic acid solution. Environ. Sci. Technol. 2013, 47, 3299–3307. [Google Scholar] [CrossRef]
- Savasari, M.; Emadi, M.; Bahmanyar, M.A.; Biparva, P. Optimization of Cd (II) removal fromaqueous solution by ascorbic acidstabilized zero valent iron nanoparticles using response surface methodology. J. Ind. Eng. Chem. 2015, 21, 1403–1409. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Liu, H.; Ma, J. Ascorbic acid/Fe0 composites as an effective persulfate activator for improving the degradation of rhodamine B. RSC Adv. 2018, 8, 12791–12798. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, L.; Liu, X.; Xie, S.; Yang, Z.; Zhu, P. Ascorbic acid enhanced the zero-valent iron/peroxymonosulfate oxidation: Simultaneous chelating and reducing. Sep. Purif. Technol. 2022, 298, 121599. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Xiao, J.; Li, L.; Hou, Y.; Chu, D.; Hou, X.; Xiang, S.; Dong, Q. Ascorbic acid-assisted iron silicate composite activated peroxydisulfate for enhanced degradation of aqueous contaminants: Accelerated Fe(III)/Fe(II) cycle and the interaction between iron and silicate. Chem. Eng. J. 2023, 455, 140773. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Y.; Zhang, Z.; Ai, F.; Zhang, H.; Li, Y.; Wang, Y.; Zhang, Q. Ascorbic acid-mediated zero-valent iron enhanced hydrogen production potential of bean dregs and corn stover by photo fermentation. Bioresour. Technol. 2023, 374, 128761. [Google Scholar] [CrossRef] [PubMed]
- Plug, C.M.; Dekker, D.; Bult, A. Complex stability of ferrous ascorbate in aqueous solution and its significance for iron absorption. Pharm. Weekbl. Sci. Ed. 1984, 6, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Griffiths, P.T.; Campbell, S.J.; Utinger, B.; Kalberer, M.; Paulson, S.E. Ascorbate oxidation by iron, copper and reactive oxygen species: Review, model development, and derivation of key rate constants. Sci. Rep. 2021, 11, 7417. [Google Scholar] [CrossRef]
- Constantinou, D.; Samanides, C.G.; Koutsokeras, K.; Constantinides, G.; Vyrides, I. Hydrogen generation by soluble CO2 reaction with zero-valent iron or scrap iron and the role of weak acids for controlling FeCO3 formation. Sustain. Energy Technol. Assess. 2023, 56, 103061. [Google Scholar] [CrossRef]
- Rizvi, M.A.; Syed, R.M.; Khan, B. Complexation e_ect on redox potential of iron(III)–iron(II) couple: A simple potentiometric experiment. J. Chem. Educ. 2011, 88, 220–222. [Google Scholar] [CrossRef]
- Rizvi, M.A. Complexation modulated redox behavior of transition metal systems. Russ. J. Gen. Chem. 2015, 85, 959–973. [Google Scholar] [CrossRef]
- Nowack, B.; Sigg, L. Dissolution of Fe(III) (hydr) oxides by metal-EDTA complexes. Geochim. Cosmochim. Acta 1997, 61, 951–963. [Google Scholar] [CrossRef]
- Ritter, K.; Odziemkowski, M.S.; Gillham, R.W. An in situ study of the role of surface films on granular iron in the permeable iron wall technology. J. Contam. Hydrol. 2002, 55, 87–111. [Google Scholar] [CrossRef] [PubMed]
- Ritter, K.; Odziemkowski, M.S.; Simpgraga, R.; Gillham, R.W.; Irish, D.E. An in situ study of the effect of nitrate on the reduction of trichloroethylene by granular iron. J. Contam. Hydrol. 2003, 65, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Odziemkowski, M.S.; Simpraga, R.P. Distribution of oxides on iron materials used for remediation of organic groundwater contaminants—Implications for hydrogen evolution reactions. Can. J. Chem. Rev. Can. Chim. 2004, 82, 1495–1506. [Google Scholar] [CrossRef]
- Chaves, L.H.G. The role of green rust in the environment: A review. Rev. Bras. Eng. Agríc. Ambient. 2005, 9, 284–288. [Google Scholar] [CrossRef]
- Odziemkowski, M. Spectroscopic studies and reactions of corrosion products at surfaces and electrodes. In Spectroscopic Properties of Inorganic and Organometallic Compounds; Royal Society of Chemistry: London, UK, 2009; Volume 40, pp. 385–450. [Google Scholar]
- Byrne, R.H.; Kester, D.R. Solubility of hydrous ferric oxide and iron speciation in seawater. Mar. Chem. 1976, 4, 255–274. [Google Scholar] [CrossRef]
- Schwertmann, U. Solubility and dissolution of iron oxides. Plant Soil 1991, 130, 1–25. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, Y.; Palchoudhury, S.; Bao, Y. Water-Soluble iron oxide nanoparticles with high stability and selective surface functionality. Langmuir 2011, 27, 8990–8997. [Google Scholar] [CrossRef]
- Han, J.; Kim, M.; Ro, H.M. Factors modifying the structural configuration of oxyanions and organic acids adsorbed on iron (hydr)oxides in soils. A review. Environ. Chem. Lett. 2020, 18, 631–662. [Google Scholar] [CrossRef]
- Vanchikova, E.V.; Shamrikova, E.V.; Korolev, M.A.; Kyzyurova, E.V.; Mikhailov, V.I. Application of model systems containing exchangeable iron(III) to study acidity characteristics of strongly acid soils (pHKCl < 3.3). Eurasian Soil Sci. 2021, 54, 189–200. [Google Scholar]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Heron, G.; Crouzet, C.; Bourg, C.M.A.; Christensen, H.T. Oxidation capacity of aquifer sediments. Environ. Sci. Technol. 1994, 28, 1698–1705. [Google Scholar] [CrossRef]
- Ford, R.G.; Bertsch, P.M.; Farley, K.J. Changes in transition and heavy metal partitioning during hydrous iron oxide aging. Environ. Sci. Technol. 1997, 31, 2028–2033. [Google Scholar] [CrossRef]
- Ford, R.G. Rates of hydrous ferric oxide crystallization and the influence on coprecipitated arsenate. Environ. Sci. Technol. 2002, 36, 2459–2463. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. Investigations on Passive In-Situ Immobilization of U(VI) from Water. Ph.D. Thesis, TU Bergakademie, Freiberg, Germany, 2003. (In German). [Google Scholar]
- Noubactep, C.; Fall, M.; Meinrath, G.; Merkel, B. A simple method to select zero valent iron material for groundwater remediation. In Proceedings of the Quebec 2004, 57TH Canadian Geotechnical Conference, 5TH Joint CGS/IAH-CNC Conference, Session 1A, Quebec City, PQ, Canada, 24–27 October 2004; pp. 6–13. [Google Scholar]
- Li, J.; Dou, X.; Qin, H.; Sun, Y.; Yin, D.; Guan, X. Characterization methods of zerovalent iron for water treatment and remediation. Water Res. 2019, 148, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Melchers, R.E.; Petersen, R.B. A reinterpretation of the Romanoff NBS data for corrosion of steels in soils. Corros. Eng. Sci. Technol. 2018, 53, 131–140. [Google Scholar] [CrossRef]
- Angst, U.M. A critical review of the science and engineering of cathodic protection of steel in soil and concrete. Corrosion 2019, 75, 1420–1433. [Google Scholar] [CrossRef]
- Nesic, S. Key issues related to modelling of internal corrosion of oil and gas pipelines—A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Stefanoni, M.; Angst, U.; Elsener, B. Electrochemistry and capillary condensation theory reveal the mechanism of corrosion in dense porous media. Sci. Rep. 2018, 8, 7407. [Google Scholar] [CrossRef]
- Moraci, N.; Lelo, D.; Bilardi, S.; Calabrò, P.S. Modelling long-term hydraulic conductivity behaviour of zero valent iron column tests for permeable reactive barrier design. Can. Geotech. J. 2016, 53, 946–961. [Google Scholar] [CrossRef]
- Yang, H.; Hu, R.; Ndé-Tchoupé, A.I.; Gwenzi, W.; Ruppert, H.; Noubactep, C. Designing the next generation of Fe0-based filters for decentralized safe drinking water treatment. Processes 2020, 8, 745. [Google Scholar] [CrossRef]
- Yang, H.; Hu, R.; Ruppert, H.; Noubactep, C. Modeling porosity loss in Fe0-based permeable reactive barriers with Faraday’s law. Sci. Rep. 2021, 11, 16998. [Google Scholar] [CrossRef]
- Yang, H. Modelling the Porosity Loss of Metallic Iron Beds for Water Treatment. Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2022. [Google Scholar]
- Yang, H.; Tao, R.; Hu, R.; Liu, Q.; Taherdangkoo, R.; Liu, Y.; Ruppert, H.; Noubactep, C. Porosity loss in iron-based permeable reactive barriers: A review. J. Hydrol. 2023; submitted. [Google Scholar]
- Westerhoff, P.; James, J. Nitrate removal in zero-valent iron packed columns. Water Res. 2003, 37, 1818–1830. [Google Scholar] [CrossRef] [PubMed]
- Velimirovic, M.; Carniatoc, L.; Simons, Q.; Schoups, G.; Seuntjens, P.; Bastiaens, L. Corrosion rate estimations of microscale zerovalent iron particles via direct hydrogen production measurements. J. Hazard. Mater. 2014, 270, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Li, F.; Chen, X.; Tian, C.; Liu, C.; Liu, D. Assessment of the use of a zero-valent iron permeable reactive barrier for nitrate removal from groundwater in the alluvial plain of the Dagu River, China. Environ. Earth Sci. 2019, 78, 244. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Hou, J.; Wang, P.; You, G.; Miao, L.; Lv, B.; Yang, Y.; Zhang, F. Application of zero valent iron coupling with biological process for wastewater treatment: A review. Rev. Environ. Sci. Biotechnol. 2017, 16, 667–693. [Google Scholar] [CrossRef]
- Tepong-Tsindé, R.; Ndé-Tchoupé, A.I.; Noubactep, C.; Nassi, A.; Ruppert, H. Characterizing a newly designed steel-wool-based household filter for safe drinking water provision: Hydraulic conductivity and efficiency for pathogen removal. Processes 2019, 7, 966. [Google Scholar] [CrossRef]
- Tepong-Tsindé, R. Designing and Piloting a household filter for the peri-urban population of Douala (Cameroon). Freiberg Online Geosci. 2021, 61, 1–80. [Google Scholar]
- Phillips, D.H.; Van Nooten, T.; Bastiaens, L.; Russell, M.I.; Dickson, K.; Plant, S.; Ahad, J.M.E.; Newton, T.; Elliot, T.; Kalin, R.M. Ten year performance evaluation of a field-scale zero-valent iron permeable reactive barrier installed to remediate trichloroethene contaminated groundwater. Environ. Sci. Technol. 2010, 44, 3861–3869. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Acree, S.D.; Ross, R.R.; Puls, R.W.; Lee, T.R.; Woods, L.L. Fifteen-year assessment of a permeable reactive barrier for treatment of chromate and trichloroethylene in groundwater. Sci. Total Environ. 2014, 468–469, 186–194. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Lee, T.R.; Sexton, M.R.; Acree, S.D.; Puls, R.W.; Blowes, D.W.; Kalinowski, C.; Tilton, J.M.; Woods, L.L. Geochemical and isotope study of trichloroethene degradation in a zero-valent iron permeable reactive barrier: A twenty-two-year performance evaluation. Environ. Sci. Technol. 2019, 53, 296–306. [Google Scholar] [CrossRef]
- Sista, K.S.; Kumar, D.; Sinha, G.R.; Moon, A.P.; Dwarapudi, S. Iron powders as a potential material for arsenic removal in aqueous systems. ISIJ Int. 2021, 61, 2687–2702. [Google Scholar] [CrossRef]
- Fisher, B.A.; Feinberg, J.M. Formation Pathways for Iron Oxide Minerals and Geochemical Conditions for Phosphate Retention in Iron Enhanced Sand Filters. UMN Water Resources Center: St. Paul, MN, USA, 2019; Available online: https://conservancy.umn.edu/handle/11299/216623 (accessed on 28 March 2023).
- McGuire, M.M.; Carlson, D.L.; Vikesland, P.J.; Kohn, T.; Grenier, A.C.; Langley, L.A.; Roberts, A.L.; Fairbrother, D.H. Applications of surface analysis in the environmental sciences: Dehalogenation of chlorocarbons with zero-valent iron and iron-containing mineral surfaces. Anal. Chim. Acta 2003, 496, 301–313. [Google Scholar] [CrossRef]
- Wielinski, J.; Jimenez-Martinez, J.; Göttlicher, J.; Steininger, R.; Mangold, S.; Hug, S.J.; Berg, M.; Voegelin, A. Spatiotemporal mineral phase evolution and arsenic retention in microfluidic models of zerovalent iron-based water treatment. Environ. Sci. Technol. 2022, 56, 13696–13708. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Kaegi, R.; Voegelin, A.; Hussam, A.; Munir, A.K.M.; Hug, S.J. Arsenic removal with composite iron matrix filters in Bangladesh: A field and laboratory study. Environ. Sci. Technol. 2013, 47, 4544–4554. [Google Scholar] [CrossRef] [PubMed]
- Pavelková, A.; Stejskal, V.; Vološčuková, O.; Nosek, J. Cost-effective remediation using microscale ZVI: Comparison of commercially available products. Ecol. Chem. Eng. 2020, 27, 211–224. [Google Scholar] [CrossRef]
- Landis, R.L.; Gillham, R.W.; Reardon, E.J.; Fagan, R.; Focht, R.M.; Vogan, J.L. An examination of zero-valent iron sources used in permeable reactive barriers. In Proceedings of the 3rd International Containment Technology Conference, Orlando, FL, USA, 10–13 June 2001; Florida State University: Tallahassee, FL, USA, 2001. [Google Scholar]
- Birke, V.; Schuett, C.; Burmeier, H.; Friedrich, H.-J. Impact of trace elements and impurities in technical zero-valent iron brands on reductive dechlorination of chlorinated ethenes in groundwater. In Permeable Reactive Barrier Sustainable Groundwater Remediation; Naidu, R., Birke, V., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 87–98. ISBN 978-1-4822-2447-4. [Google Scholar]
- Lan, L.E.; Reina, F.D.; De Seta, G.E.; Meichtry, J.M.; Litter, M.I. Comparison between different technologies (zerovalent iron, coagulation-flocculation, adsorption) for arsenic treatment at high concentrations. Water 2023, 15, 1481. [Google Scholar] [CrossRef]
- Ren, Y.; Cui, M.; Zhou, Y.; Lee, Y.; Ma, J.; Han, Z.; Khim, J. Zero-valent iron based materials selection for permeable reactive barrier using machine learning. J. Hazard. Mater. 2023, 453, 131349. [Google Scholar] [CrossRef]
- Chen, Q.; Fan, G.; Na, W.; Liu, J.; Cui, J.; Li, H. Past, present, and future of groundwater remediation research: A scientometric analysis. Int. J. Environ. Res. Public Health 2019, 16, 3975. [Google Scholar] [CrossRef] [PubMed]
- Antia, D.D.J. Water treatment and desalination using the eco-materials n-Fe0 (ZVI), n-Fe3O4, n-FexOyHz[mH2O], and n-Fex[Cation]nOyHz[Anion]m [rH2O]. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Martínez, L., Kharisov, B., Eds.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Du, C.; Tian, Z.; Zhu, Q.; Li, G.; Shen, Q.; Li, C.; Li, J.; Li, W.; et al. Bibliometric analysis of zerovalent iron particles research for environmental remediation from 2000 to 2019. Environ. Sci. Pollut. Res. 2021, 28, 4200–34210. [Google Scholar] [CrossRef]
- Velimirovic, M.; Larsson, P.-O.; Simons, Q.; Bastiaens, L. Effect of boron on reactivity and apparent corrosion rate of microscale zerovalent irons. J. Environ. Chem. Eng. 2017, 5, 1892–1898. [Google Scholar] [CrossRef]
- Konadu-Amoah, B.; Hu, R.; Cui, X.; Tao, R.; Ndé-Tchoupé, A.I.; Gwenzi, W.; Noubactep, C. Characterizing the process of phosphate removal in Fe0/H2O systems. Chem. Eng. J. 2023, 465, 143042. [Google Scholar] [CrossRef]
- Pelekani, C.; Snoeyink, V.L. Competitive adsorption in natural water: Role of activated carbon pore size. Water Res. 1999, 33, 1209–1219. [Google Scholar] [CrossRef]
- Girgis, B.S.; Khalil, L.B.; Tawfik, T.A.M. Porosity characteristics of activated carbons from olive wastes impregnated with H3PO4. Adsorpt. Sci. Technol. 2000, 18, 373–383. [Google Scholar]
- Chen, J.P.; Yoon, J.T.; Yiacoumi, S. Effect of chemical and physical properties of influent on copper sorption onto activated carbon fixed-bed columns. Carbon 2003, 41, 1632–1644. [Google Scholar]
- Attia, A.A.; Girgis, B.S.; Fathy, N.A. Removal of methylene blue by carbons derived from peach stones by H3PO4 activation: Batch and column studies. Dye. Pigment. 2008, 76, 282–289. [Google Scholar] [CrossRef]
- Bergna, D.; Varila, T.; Romar, H.; Lassi, U. Comparison of the properties of activated carbons produced in one-stage and two-stage processes. Carbon 2018, 4, 41. [Google Scholar] [CrossRef]
- Hussam, A.; Munir, A.K.M. A simple and effective arsenic filter based on composite iron matrix: Development and deployment studies for groundwater of Bangladesh. J. Environ. Sci. Health A 2007, 42, 1869–1878. [Google Scholar] [CrossRef]
- Giles, D.E.; Mohapatra, M.; Issa, T.B.; Anand, S.; Singh, P. Iron and aluminium based adsorption strategies for removing arsenic from water. J. Environ. Manag. 2011, 92, 3011–3022. [Google Scholar] [CrossRef]
- Ghauch, A. Iron-based metallic systems: An excellent choice for sustainable water treatment. Freiberg Online Geosci. 2015, 32, 1–38. [Google Scholar]
- Ogata, R.; Dangol, B.; Sakamoto, M. Sustainability assessment of long-term, widely used household Kanchan Arsenic Filters in Nepal. J. Environ. Sci. Healtht A 2020, 55, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Nya, E.L.; Mwamila, T.B.; Komguem-Poneabo, L.; Njomou-Ngounou, E.L.; Fangang-Fanseu, J.; Tchoumbe, R.R.; Tepong-Tsindé, R.; Gwenzi, W.; Noubactep, C. Integrated water management in mountain communities: The case of Feutap in the Municipality of Bangangté, Cameroon. Water 2023, 15, 1467. [Google Scholar] [CrossRef]
- Hering, J.G.; Maag, S.; Schnoor, J.L. A call for synthesis of water research to achieve the sustainable development goals by 2030. Environ. Sci. Technol. 2016, 50, 6122–6123. [Google Scholar] [CrossRef]
- Hutton, G.; Chase, C. The knowledge base for achieving the sustainable development goal targets on water supply, sanitation and hygiene. Int. J. Environ. Res. Public Health 2016, 13, 536. [Google Scholar] [CrossRef] [PubMed]
- Banerji, T.; Chaudhari, S. A cost-effective technology for arsenic removal: Case study of zerovalent iron-based IIT Bombay arsenic filter in West Bengal. In Water and Sanitation in the New Millennium; Nath, K., Sharma, V., Eds.; Springer: New Delhi, India, 2017. [Google Scholar]
- Bangert, M.; Molyneux, D.H.; Lindsay, S.W.; Fitzpatrick, C.; Engels, D. The cross-cutting contribution of the end of neglected tropical diseases to the sustainable development goals. Infect. Dis. Poverty 2017, 6, 73. [Google Scholar] [CrossRef] [PubMed]




| Code | Shape | Size | Color | Specific Surface Area | Fe | Supplier |
|---|---|---|---|---|---|---|
| (mm) | (m2/g) | (%) | ||||
| ZVI1 | granular | 0.05–5.00 | black | n.s. | n.s. | iPutec GmbH |
| ZVI2 | sponge | 0.68–1.00 | black | n.s. | 90.0 | ISPAT GmbH |
| ZVI3 | sponge | 1.00–2.00 | black | n.s. | 90.0 | ISPAT GmbH |
| ZVI4 | scrap | 0.05–5.00 | black | n.s. | n.s. | Metallaufbereitung Zwickau |
| ZVI5 | scrap | 0.05–2.00 | black | n.s. | n.s. | Metallaufbereitung Zwickau |
| ZVI6 | granulate | 0.05–10.0 | black | n.s. | n.s. | Connelly |
| ZVI7 | spherical | 0.05–1.00 | grey | 0.74–1.26 | 99.99 | Tongda Alloy Material Factory |
| ZVI8 | spherical | 2.00 | grey | 0.39 | 99.99 | Tongda Alloy Material Factory |
| Sample | b | Δ(b) | a | Δa | R2(7) | R2(9) |
|---|---|---|---|---|---|---|
| (μg) | (μg) | (μg h−1) | (μg h−1) | (-) | (-) | |
| Using AA | ||||||
| ZVI1 | 110.3 | 11.9 | 13.2 | 0.5 | 0.99 | 0.95 |
| ZVI2 | 108.5 | 10.4 | 17.2 | 1.1 | 0.98 | 0.92 |
| ZVI3 | 92.5 | 9.8 | 11.5 | 1.3 | 0.94 | 0.88 |
| ZVI4 | 118. 9 | 16.1 | 14.8 | 0.5 | 0.99 | 0.98 |
| ZVI5 | 119.4 | 15.6 | 12.3 | 0.7 | 0.99 | 0.94 |
| ZVI6 | 126.1 | 8.1 | 10.3 | 0.8 | 0.97 | 0.78 |
| ZVI7 | 96.9 | 1.0 | 13.4 | 0.6 | 0.99 | 0.57 |
| ZVI8 | 16.9 | 2.1 | 2.8 | 0.1 | 0.99 | 0.90 |
| ZVI1 using AA, EDTA, and Phen | ||||||
| AA | 110.3 | 11.9 | 13.2 | 0.5 | 0.99 | 0.95 |
| EDTA | 56.3 | 76.7 | 18.6 | 1.3 | 0.98 | 0.99 |
| Phen | 66.4 | 35.1 | 8.1 | 0.5 | 0.98 | 0.99 |
| Rate (Unit) | ZVI1 | ZVI3 | ZVI5 | |
|---|---|---|---|---|
| Daily | (mg) | 3.7 | 4.1 | 3.9 |
| Total | (mg) | 475 | 530 | 497 |
| Total | (%) | 47.5 | 53.0 | 49.7 |
| Event | Time | ZVI1 | ZVI3 | ZVI5 |
|---|---|---|---|---|
| (-) | (d) | (mg) | (mg) | (mg) |
| 2 | 2 | 7.0 | 10.1 | 11.2 |
| 10 | 10 | 7.9 | 10.4 | 8.8 |
| 20 | 22 | 9.4 | 11.2 | 12.4 |
| 30 | 44 | 10.6 | 12.4 | 12.0 |
| 40 | 68 | 7.8 | 8.2 | 8.0 |
| 50 | 96 | 13.0 | 11.2 | 10.8 |
| 51 | 111 | 12.6 | 10.9 | 10.5 |
| 52 | 112 | 10.4 | 9.5 | 7.1 |
| Anno | Title | Citations | Citations |
|---|---|---|---|
| (Total) | (per Year) | ||
| 1995 | Anaerobic corrosion of granular iron: Measurement and interpretation of hydrogen evolution rates | 386 | 13.8 |
| 2005 | Testing the suitability of zerovalent iron materials for reactive walls | 110 | 6.1 |
| 2014 | Standardization of the reducing power of zerovalent iron using iodine | 30 | 3.3 |
| 2015 | Simple colorimetric assay for dehalogenation reactivity of nanoscale zero-valent iron using 4-chlorophenol | 35 | 4.4 |
| 2016 | A facile method for determining the Fe(0) content and reactivity of zero valent iron | 47 | 6.7 |
| 2019 | A novel and facile method to characterize the suitability of metallic iron for water treatment | 37 | 9.3 |
| 2020 | Characterizing the reactivity of metallic iron for water treatment: H2 evolution in H2SO4 and uranium removal efficiency | 8 | 2.7 |
| 2020 | Cost-effective remediation using microscale ZVI: comparison of commercially available products | 6 | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Xiao, M.; Tao, R.; Hu, R.; Ruppert, H.; Gwenzi, W.; Noubactep, C. Developing the Ascorbic Acid Test: A Candidate Standard Tool for Characterizing the Intrinsic Reactivity of Metallic Iron for Water Remediation. Water 2023, 15, 1930. https://doi.org/10.3390/w15101930
Cui X, Xiao M, Tao R, Hu R, Ruppert H, Gwenzi W, Noubactep C. Developing the Ascorbic Acid Test: A Candidate Standard Tool for Characterizing the Intrinsic Reactivity of Metallic Iron for Water Remediation. Water. 2023; 15(10):1930. https://doi.org/10.3390/w15101930
Chicago/Turabian StyleCui, Xuesong, Minhui Xiao, Ran Tao, Rui Hu, Hans Ruppert, Willis Gwenzi, and Chicgoua Noubactep. 2023. "Developing the Ascorbic Acid Test: A Candidate Standard Tool for Characterizing the Intrinsic Reactivity of Metallic Iron for Water Remediation" Water 15, no. 10: 1930. https://doi.org/10.3390/w15101930
APA StyleCui, X., Xiao, M., Tao, R., Hu, R., Ruppert, H., Gwenzi, W., & Noubactep, C. (2023). Developing the Ascorbic Acid Test: A Candidate Standard Tool for Characterizing the Intrinsic Reactivity of Metallic Iron for Water Remediation. Water, 15(10), 1930. https://doi.org/10.3390/w15101930









