Effects of Salinity, pH, and Cu(II) on the Adsorption Behaviors of Tetracycline onto Polyvinyl Chloride Microplastics: A Site Energy Distribution Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
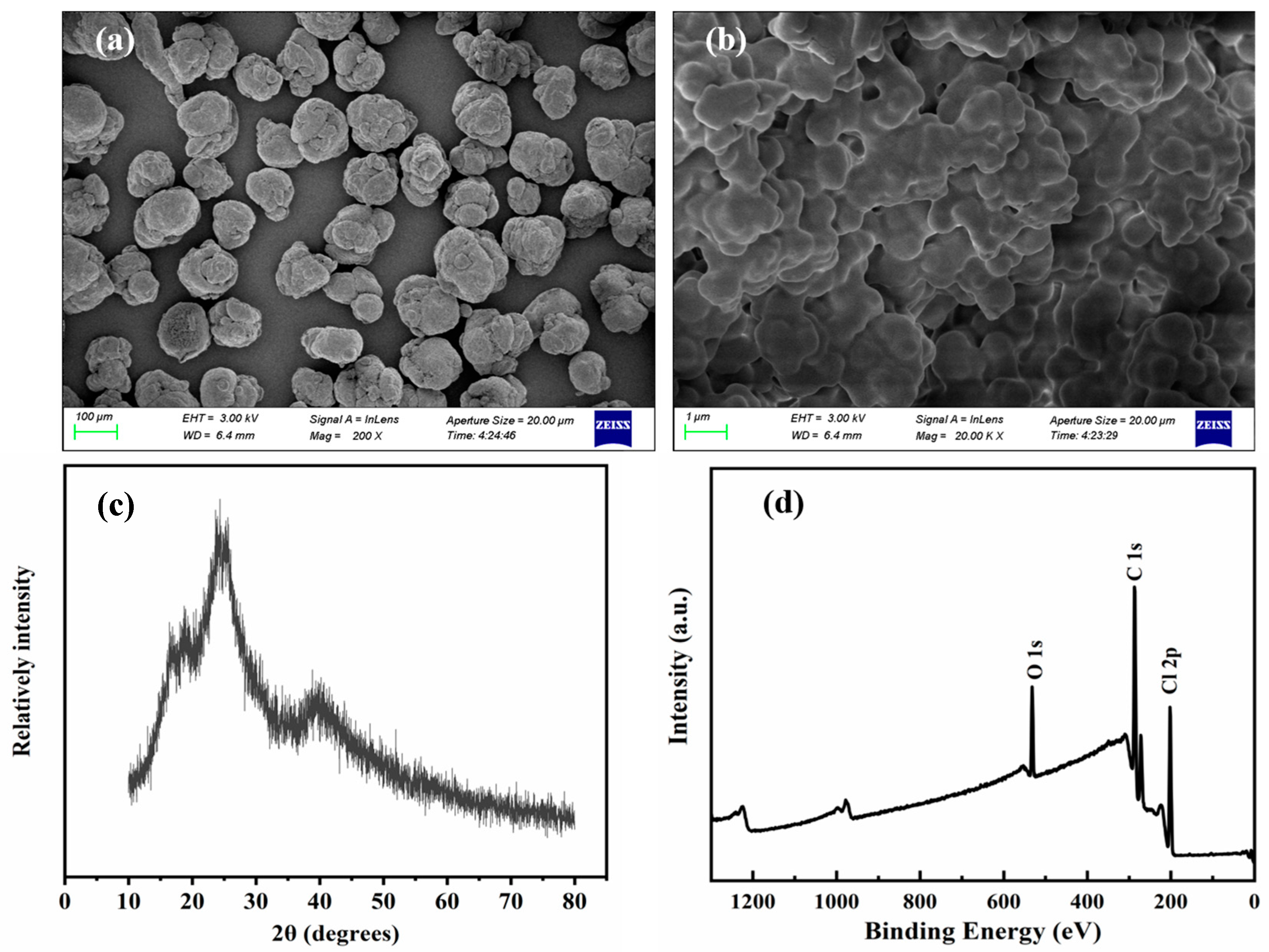
2.2. Characterization
2.3. Sorption Experiments
2.4. Adsorption Kinetics of TC onto MPs
2.5. The Site Energy Distribution (SED) Analysis
2.6. The Analysis Method of Antibiotics
3. Results and Discussion
3.1. The Adsorption Kinetics of TC by MPs
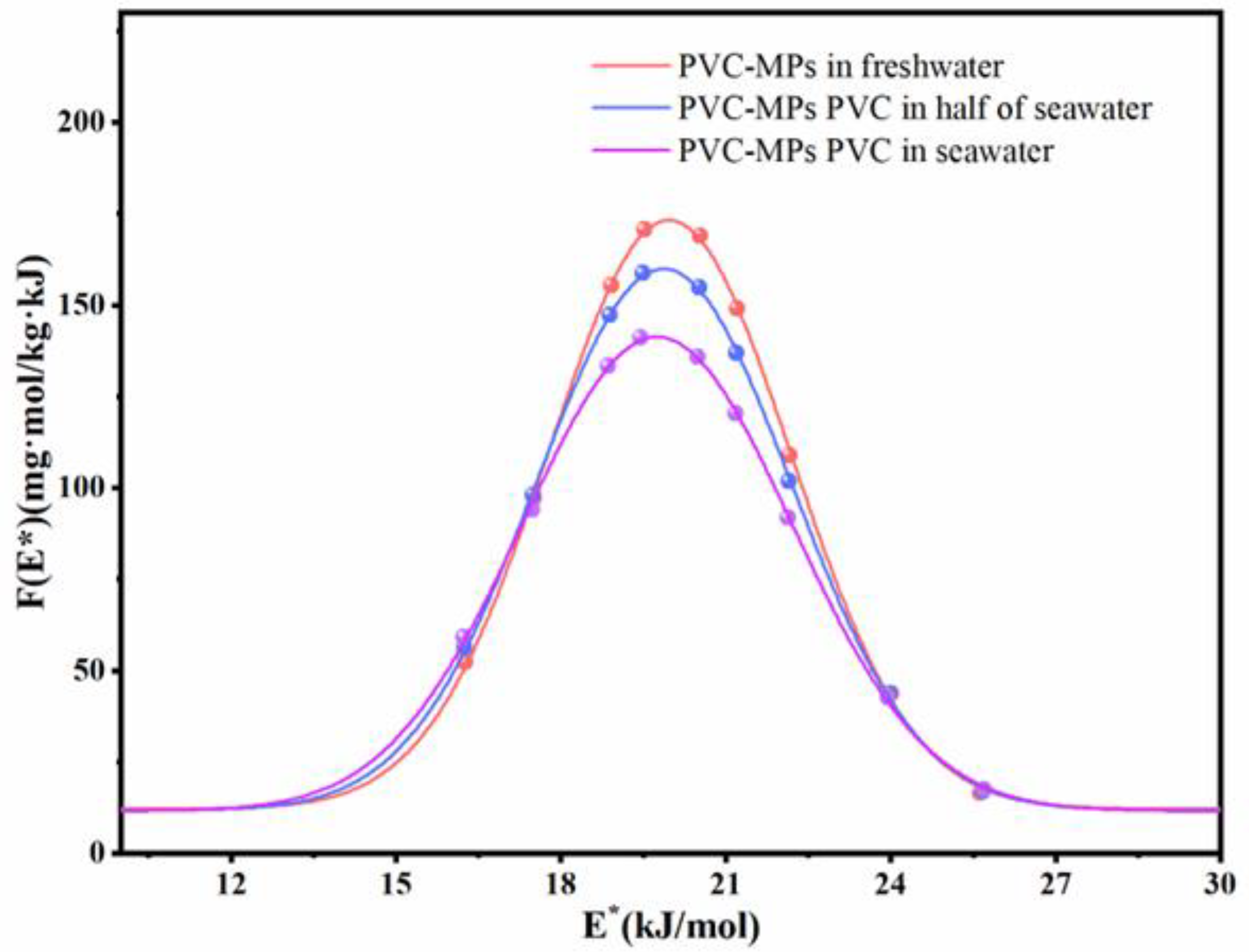
3.2. Effects of Salinity on Adsorption Kinetics of TC onto PVC MPs
3.3. Effects of pH on Adsorption of TC onto PVC MPs
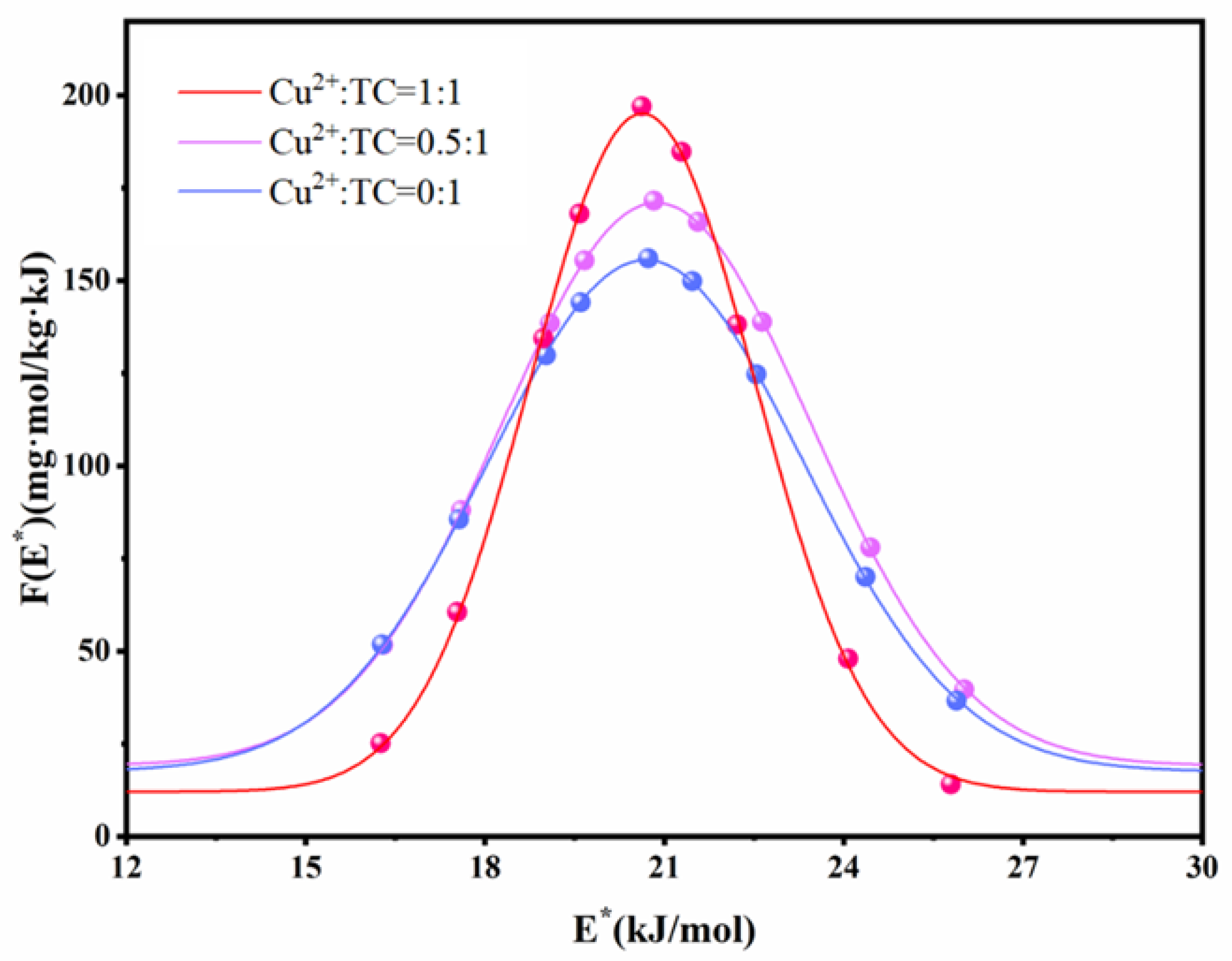
3.4. The Effects of Cu2+ on Adsorption of TC onto PVC MPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Law, K.L.; Morét-Ferguson, S.E.; Goodwin, D.S.; Zettler, E.R.; DeForce, E.; Kukulka, T.; Proskurowski, G. Distribution of surface plastic debris in the eastern Pacific Ocean from an 11-year data set. Environ. Sci. Technol. 2014, 48, 4732–4738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xiong, X.; Hu, H.; Wu, C.; Bi, Y.; Wu, Y.; Liu, J. Occurrence and characteristics of microplastic pollution in Xiangxi Bay of Three Gorges Reservoir, China. Environ. Sci. Technol. 2017, 51, 3794–3801. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.; Covernton, G.; Davies, H.; Dower, J.; Juanes, F.; Dudas, S. Human consumption of microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Cris, B.; Gomez, C.; Gomez, B.C.; Adzel, A.G.; Escalante, O. The occurrence of microplastics in the gastrointestinal tract of demersal fish species. Int. J. Biosci. 2020, 16, 152–162. [Google Scholar]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; De-la-Torre, G.E. Sorption of chemical contaminants on degradable and non-degradable microplastics: Recent progress and research trends. Sci. Total Environ. 2021, 757, 143875. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Schwarz, A.E.; Ligthart, T.N.; Boukris, E.; van Harmelen, T. Sources, transport, and accumulation of different types of plastic litter in aquatic environments: A review study. Mar. Pollut. Bull. 2019, 143, 92–100. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Green, D.S.; Boots, B.; O’Connor, N.E.; Thompson, R. Microplastics affect the ecological functioning of an important biogenic habitat. Environ. Sci. Technol. 2017, 51, 68–77. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Liu, Y.G.; Chen, Y.; Zhang, W.; Zhao, J.M.; He, S.Y.; Yang, C.P.; Zhang, T.; Tang, C.F.; Zhang, C. A review: Research progress on microplastic pollutants in aquatic environments. Sci. Total Environ. 2021, 766, 142572. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Tang, S.; Wang, X.S.; Sun, X.; Han, Z.; Chen, Y. Accumulation mechanism of tetracycline hydrochloride from aqueous solutions by nylon microplastics. Environ. Technol. Innov. 2020, 18, 100750. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Y.; Li, J.; Wang, F.; Xia, S.; Zhao, J. Biofilm alters tetracycline and copper adsorption behaviors onto polyethylene microplastics. Chem. Eng. J. 2020, 392, 123808. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Xu, Z.; Wei, Y.; Zhou, Y.; Yang, X.; Yang, Y.; Yang, J.; Zhang, J.; Luo, L.; Zhou, Z. Carbon-based materials as adsorbent for antibiotics removal: Mechanisms and influencing factors. J. Environ. Manag. 2019, 237, 128–138. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. Sorption of antibiotics onto aged microplastics in freshwater and seawater. Mar. Pollut. Bull. 2019, 149, 110511. [Google Scholar] [CrossRef]
- Razanajatovo, R.M.; Ding, J.; Zhang, S.; Jiang, H.; Zou, H. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics. Mar. Pollut. Bull. 2018, 136, 516–523. [Google Scholar] [CrossRef]
- Wang, Y.J.; Niu, J.F.; Li, Y.; Zheng, T.J.; Xu, Y.; Liu, Y. Performance and mechanisms for removal of perfluorooctanoate (PFOA) from aqueous solution by activated carbon fiber. RSC Adv. 2015, 5, 86927–86933. [Google Scholar] [CrossRef]
- Zhang, R.; Pei, J.; Zhang, R.; Wang, S.; Zeng, W.; Huang, D.; Wang, Y.; Zhang, Y.; Wang, Y.; Yu, K. Occurrence and distribution of antibiotics in mariculturefarms, estuaries and the coast of the Beibu Gulf, China: Bioconcentration and diet safety of seafood. Ecotoxicol. Environ. Saf. 2018, 154, 27–35. [Google Scholar] [CrossRef]
- Tolls, J. Sorption of veterinary pharmaceuticals in soils: A review. Environ. Sci. Technol. 2001, 35, 3397–3406. [Google Scholar] [CrossRef]
- Ying, G. Chinese Antibiotic Use and Watershed Pollution. Chin. Chem. Soc. 2016, 144, 26-I-005. (In Chinese) [Google Scholar]
- Quan, B.; Li, X.; Zhang, H.; Zhang, C.; Ming, Y.; Huang, Y.; Tang, Y. Technology and principle of removing triclosan from aqueous media: A review. Chem. Eng. J. 2019, 378, 122185. [Google Scholar] [CrossRef]
- Pulicharla, R.; Brar, S.K.; Rouissi, T.; Auger, S.; Drogui, P.; Verma, M.; Surampalli, R.Y. Degradation of chlortetracycline in wastewater sludge by ultrasonication, Fenton oxidation, and ferro-sonication. Ultrason. Sonochem. 2017, 34, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhu, T.; Fei, X. Enhanced adsorption performance of oxytetracycline by desugared reed residues. Int. J. Environ. Res. Public Health 2018, 15, 2229. [Google Scholar] [CrossRef]
- Puckowski, A.; Cwiek, W.; Mioduszewska, K.; Stepnowski, P.; Bialk-Bielinska, A. Sorption of pharmaceuticals on the surface of microplastics. Chemosphere 2021, 263, 127976. [Google Scholar] [CrossRef]
- Guo, X.; Chen, C.; Wang, J. Sorption of sulfamethoxazole onto six types of microplastics. Chemosphere 2019, 228, 300–308. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Huang, G.Q.; Yang, C.F.; Chen, C.; Zhou, T.; Zhao, Y.C.; Ma, J. Adsorption behavior of the antibiotic levofloxacin on microplastics in the presence of different heavy metals in an aqueous solution. Chemosphere 2020, 260, 127650. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Kang, J.; Fan, M.; Qu, J. Removal of tetracycline from water by Fe-Mn binary oxide. J. Environ. Sci. 2012, 24, 242–247. [Google Scholar] [CrossRef]
- Kuniki, H.; Tomonori, S.; Takafumi, M.; Fumiyoshi, U.; Satoshi, H.; Shigeki, K.; Takashi, T. Simulation for radiolytic products of seawater: Effects of seawater constituents, dilution rate, and dose rate. J. Nucl. Sci. Technol. 2016, 8, 1183–1191. [Google Scholar]
- Wu, X.; Liu, P.; Shi, H.; Wang, H.; Huang, H.; Shi, Y.; Gao, S. Photo aging and fragmentation of polypropylene food packaging materials in artificial seawater. Water Res. 2021, 188, 116456. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, P.; Wu, X.; Shi, H.; Huang, H.; Wang, H.; Gao, S. Insight into chain scission and release profiles from photodegradation of polycarbonate microplastics. Water Res. 2021, 195, 116980. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, P.; Chen, Q.; Ma, D.; Ge, W.; Jiang, T.; Chai, C. Effects of polymer aging on sorption of 2, 2′, 4, 4′-tetrabromodiphenyl ether by polystyrene microplastics. Chemosphere 2020, 253, 126706. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. Kungliga svenska vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Carter, M.C.; Kilduff, J.E.; Weber, W.J. Site energy distribution analysis of preloaded adsorbents. Environ. Sci. Technol. 1995, 29, 1773–1780. [Google Scholar] [CrossRef]
- Cerofolini, G.F. Localized adsorption on heterogeneous surfaces. Thin Solid Film. 1974, 23, 129–152. [Google Scholar] [CrossRef]
- Shen, X.; Guo, X.; Zhang, M.; Tao, S.; Wang, X. Sorption mechanisms of organic compounds by carbonaceous materials: Site energy distribution consideration. Environ. Sci. Technol. 2015, 49, 4894–4902. [Google Scholar] [CrossRef]
- He, J.; Guo, J.; Zhou, Q.; Yang, J.; Fang, F.; Yang, H. Analysis of 17a-ethinylestradiol and bisphenol A adsorption on anthracite surfaces by site energy distribution. Chemosphere 2019, 216, 59–68. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019, 246, 26–33. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, J.; Zhu, Z.; Li, L.; Yu, F. Effect of microplastic size on the adsorption behavior and mechanism of triclosan on polyvinyl chloride. Environ. Pollut. 2019, 254, 113104. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, T.; Tian, L.; Liu, X.; Qi, Z.; Ma, Y.; Chen, W. Aging significantly affects mobility and contaminant-mobilizing ability of nanoplastics in saturated loamy sand. Environ. Sci. Technol. 2019, 53, 5805–5815. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, Y.; Wang, J. Sorption of sulfamethazine onto different types of microplastics: A combined experimental and molecular dynamics simulation study. Mar. Pollut. Bull. 2019, 145, 547–554. [Google Scholar] [CrossRef]
- Aristilde, L.; Marichal, C.; Miéhé-Brendlé, J.; Lanson, B.; Charlet, L. Interactions of oxytetracycline with a smectite clay: A spectroscopic study with molecular simulations. Environ. Sci. Technol. 2010, 44, 7839–7845. [Google Scholar] [CrossRef]
- Guo, X.; Pang, J.; Chen, S.; Jia, H. Sorption properties of tylosin on four different microplastics. Chemosphere 2018, 209, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Pulicharla, R.; Hegde, K.; Brar, S.K.; Surampalli, R.Y. Tetracyclines metal complexation: Significance and fate of mutual existence in the environment. Environ. Pollut. 2017, 221, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Liu, X.; Zhao, J.; Liu, R.; Xing, B. Interaction of microplastics with antibiotics in aquatic environment: Distribution, adsorption, and toxicity. Environ. Sci. Technol. 2021, 55, 15579–15595. [Google Scholar] [CrossRef]
- Yan, B.; Niu, C.H.; Wang, J. Analyses of levofloxacin adsorption on pretreated barley straw with respect to temperature: Kinetics, π–π electron-donor–acceptor interaction and site energy distribution. Environ. Sci. Technol. 2017, 51, 8048–8056. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Zhou, B.; Zhou, Y.; Dai, Z.; Zhou, Q.; Luo, Y. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors. Environ. Pollut. 2018, 243, 1550–1557. [Google Scholar] [CrossRef]
- Ma, X.; Yang, C.; Jiang, Y.; Zhang, X.; Wang, Q.; Dang, Z. Desorption of heavy metals and tetracycline from goethite-coated sands: The role of complexation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 88–94. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, J.; Li, Y.; Xia, S.Q.; Zhao, J.F.; Minale, T.M.; Gu, Z.L. Coadsorption of tetracycline and copper (II) onto struvite loaded zeolite–An environmentally friendly product recovered from swine biogas slurry. Chem. Eng. J. 2019, 371, 366–377. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Ying, C.; Zhu, J.; Zhou, Q.; Sun, K.; Tian, Y.; Li, J. Effects of Salinity, pH, and Cu(II) on the Adsorption Behaviors of Tetracycline onto Polyvinyl Chloride Microplastics: A Site Energy Distribution Analysis. Water 2023, 15, 1925. https://doi.org/10.3390/w15101925
Liang Y, Ying C, Zhu J, Zhou Q, Sun K, Tian Y, Li J. Effects of Salinity, pH, and Cu(II) on the Adsorption Behaviors of Tetracycline onto Polyvinyl Chloride Microplastics: A Site Energy Distribution Analysis. Water. 2023; 15(10):1925. https://doi.org/10.3390/w15101925
Chicago/Turabian StyleLiang, Yifan, Chuhan Ying, Jianyu Zhu, Qian Zhou, Kuan Sun, Yajun Tian, and Jun Li. 2023. "Effects of Salinity, pH, and Cu(II) on the Adsorption Behaviors of Tetracycline onto Polyvinyl Chloride Microplastics: A Site Energy Distribution Analysis" Water 15, no. 10: 1925. https://doi.org/10.3390/w15101925
APA StyleLiang, Y., Ying, C., Zhu, J., Zhou, Q., Sun, K., Tian, Y., & Li, J. (2023). Effects of Salinity, pH, and Cu(II) on the Adsorption Behaviors of Tetracycline onto Polyvinyl Chloride Microplastics: A Site Energy Distribution Analysis. Water, 15(10), 1925. https://doi.org/10.3390/w15101925




