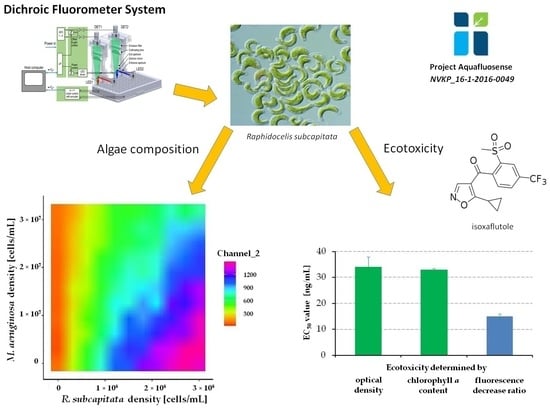
Application of a Fluorescence-Based Instrument Prototype for Chlorophyll Measurements and Its Utility in an Herbicide Algal Ecotoxicity Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalga Monocultures
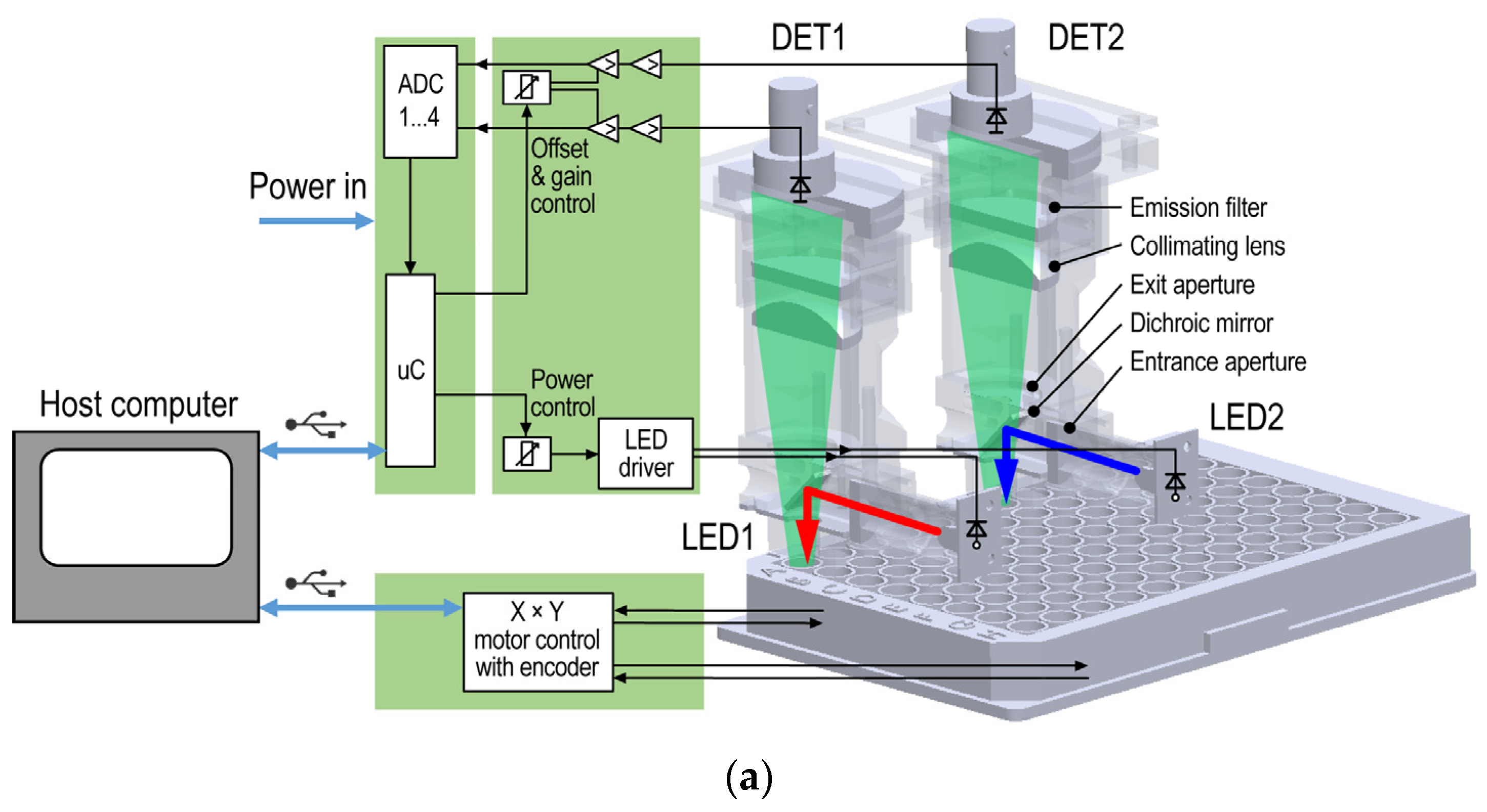
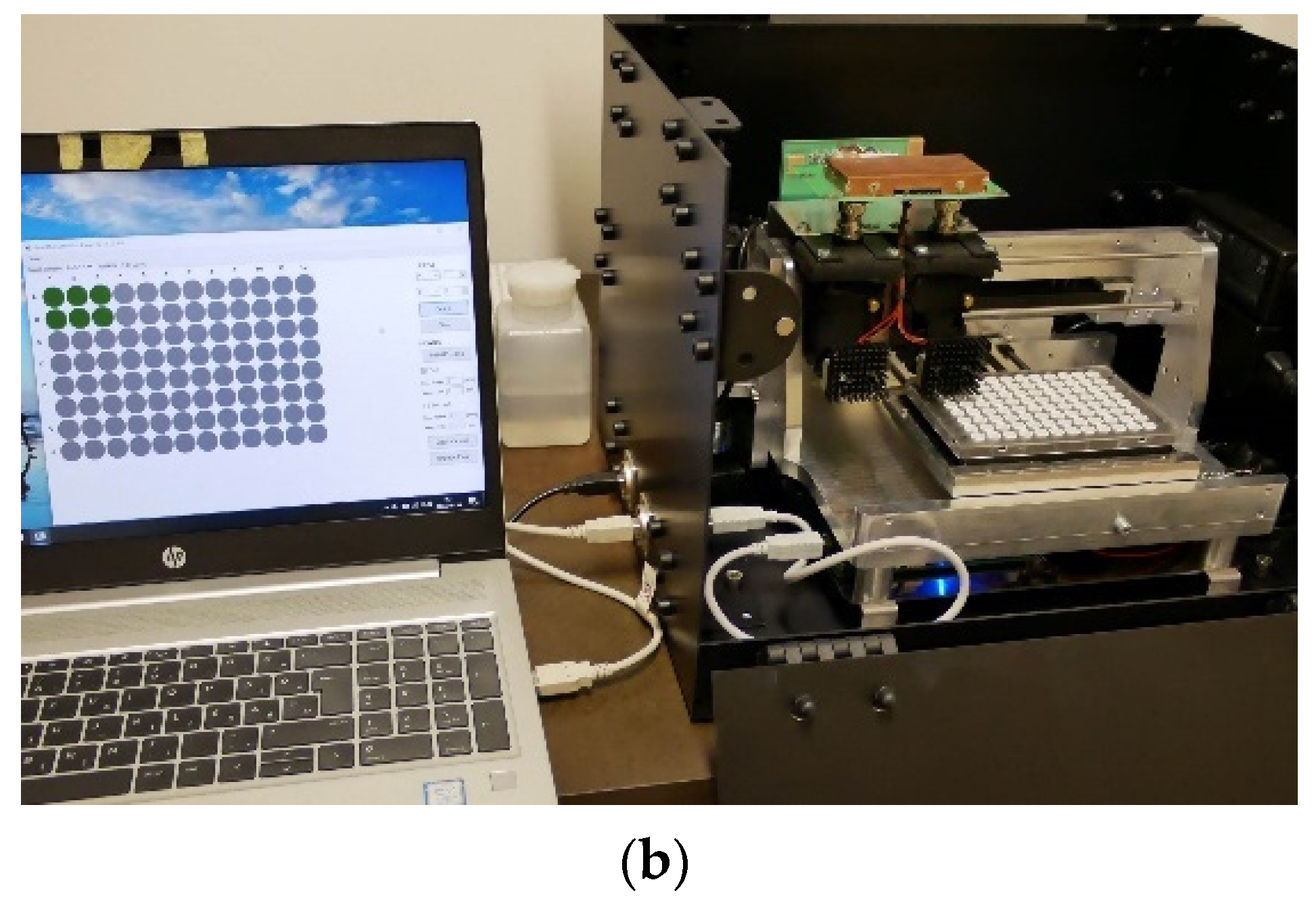
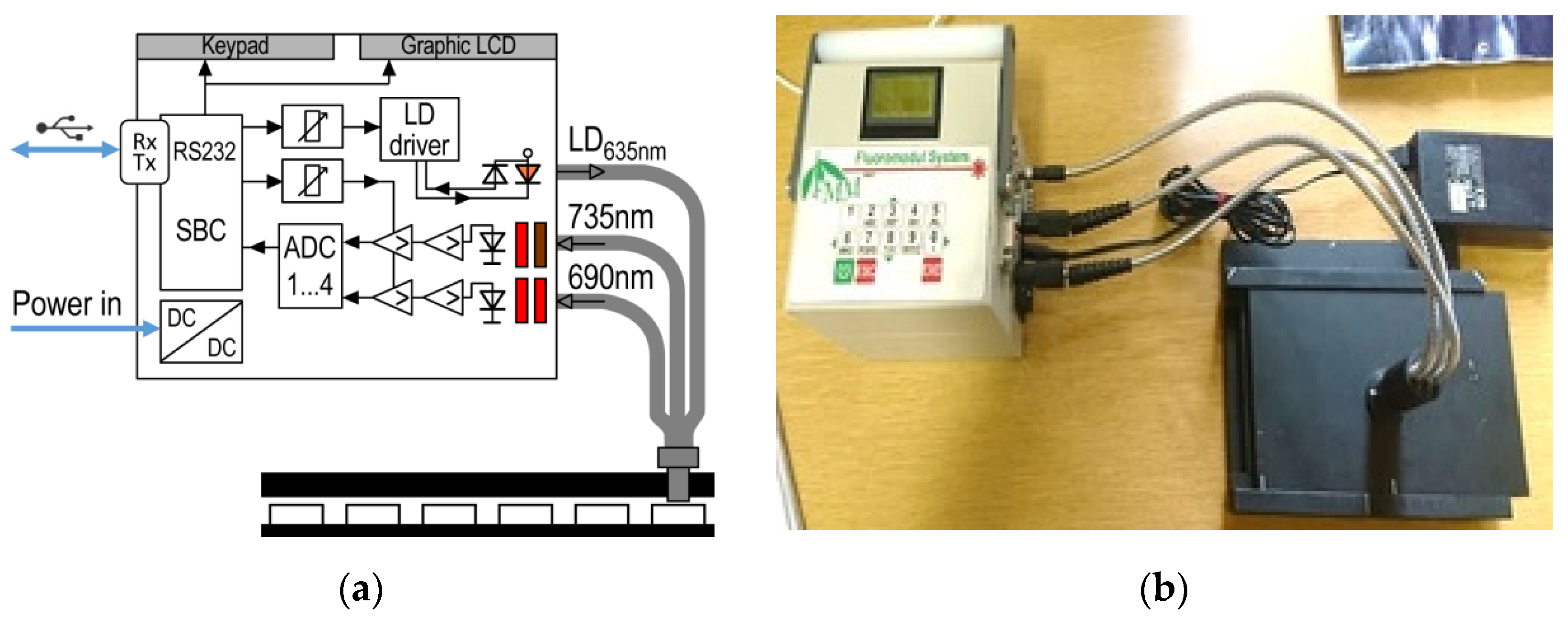
2.2. Instrumentation
2.3. Optimization of the Measurement Parameters
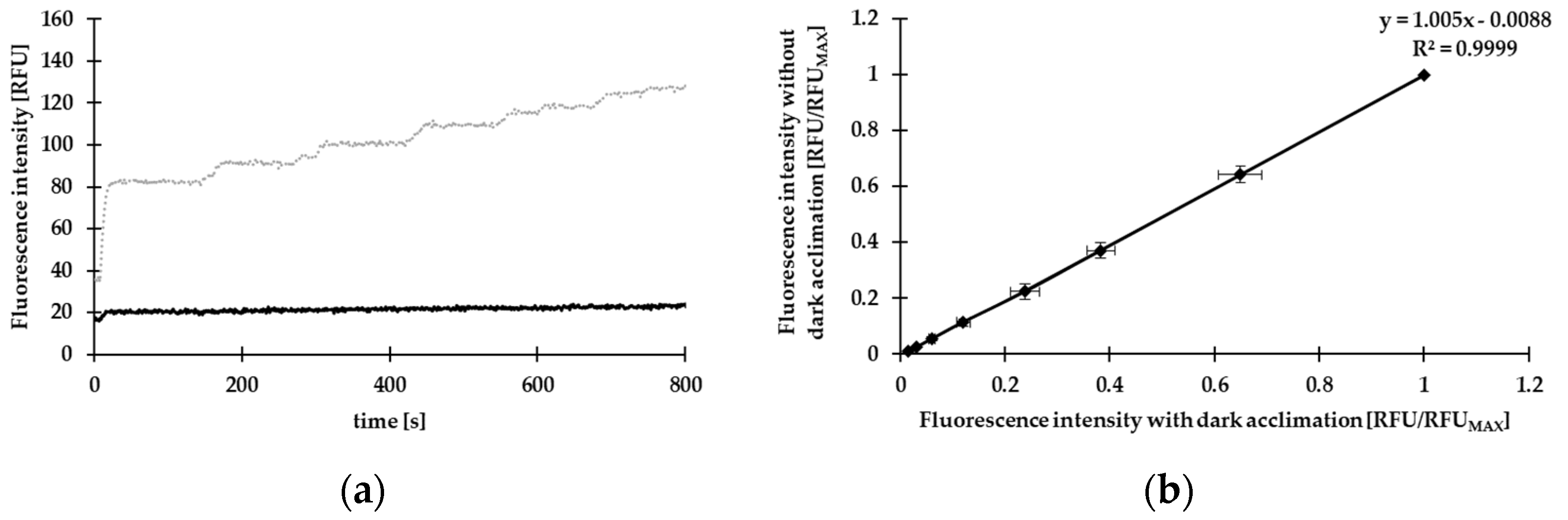
2.3.1. Reflection
2.3.2. Dark Acclimation
2.4. Comparison with Conventional Methods Most Commonly Applied for Algal Biomass Estimation
2.5. Application of Dichroic Fluorometer System on Surface Water
2.6. Examination of Algae Group Ratios by Dichroic Fluorometer System
2.7. Application of FluoroMeter Module in Ecotoxicology Tests
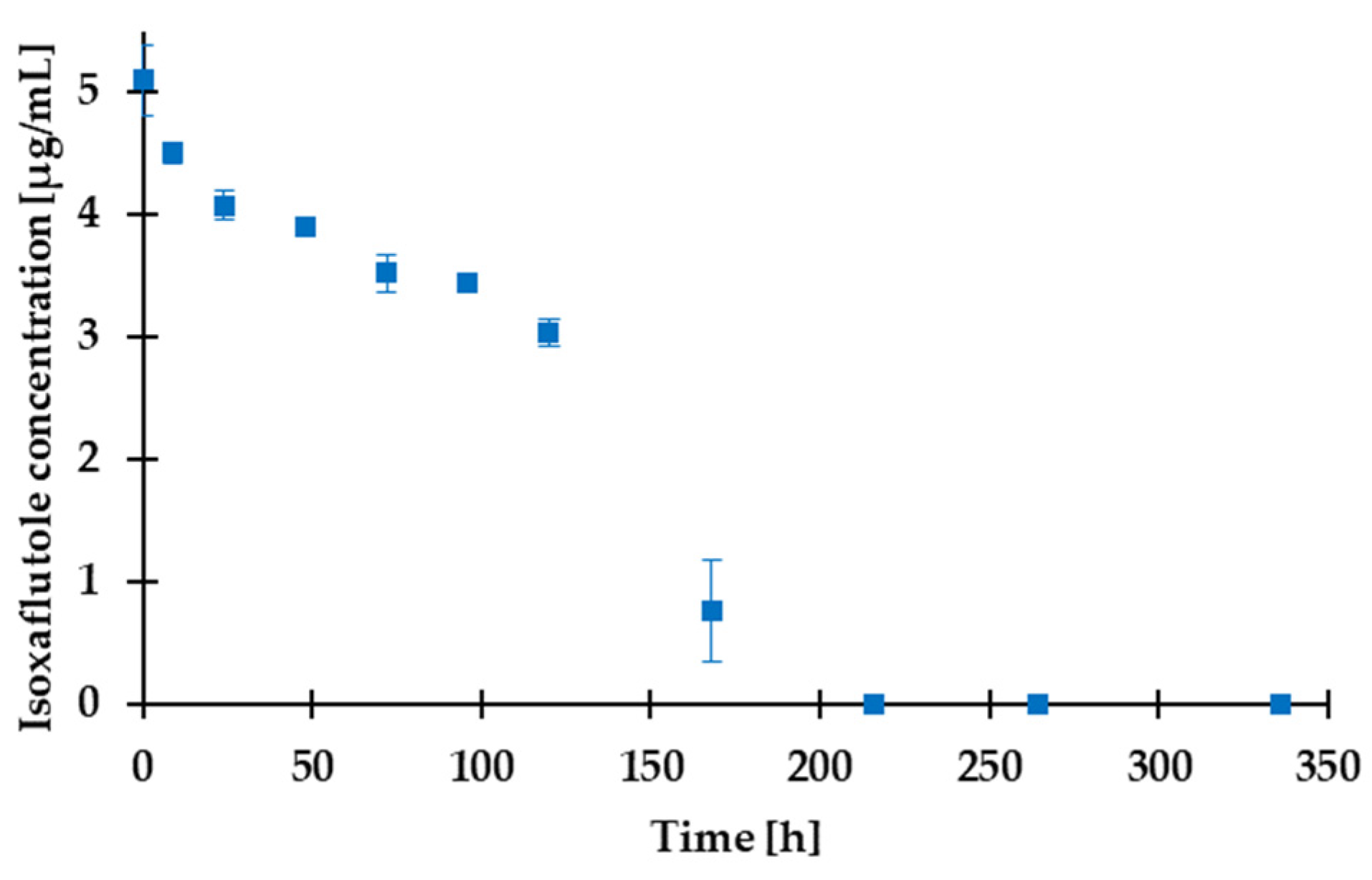
2.7.1. Degradation of Herbicide Active Ingredient Isoxaflutole
2.7.2. Ecotoxicity Test
2.8. Statistical Evaluation
3. Results and Discussion
3.1. Results for the Preliminary Experiments
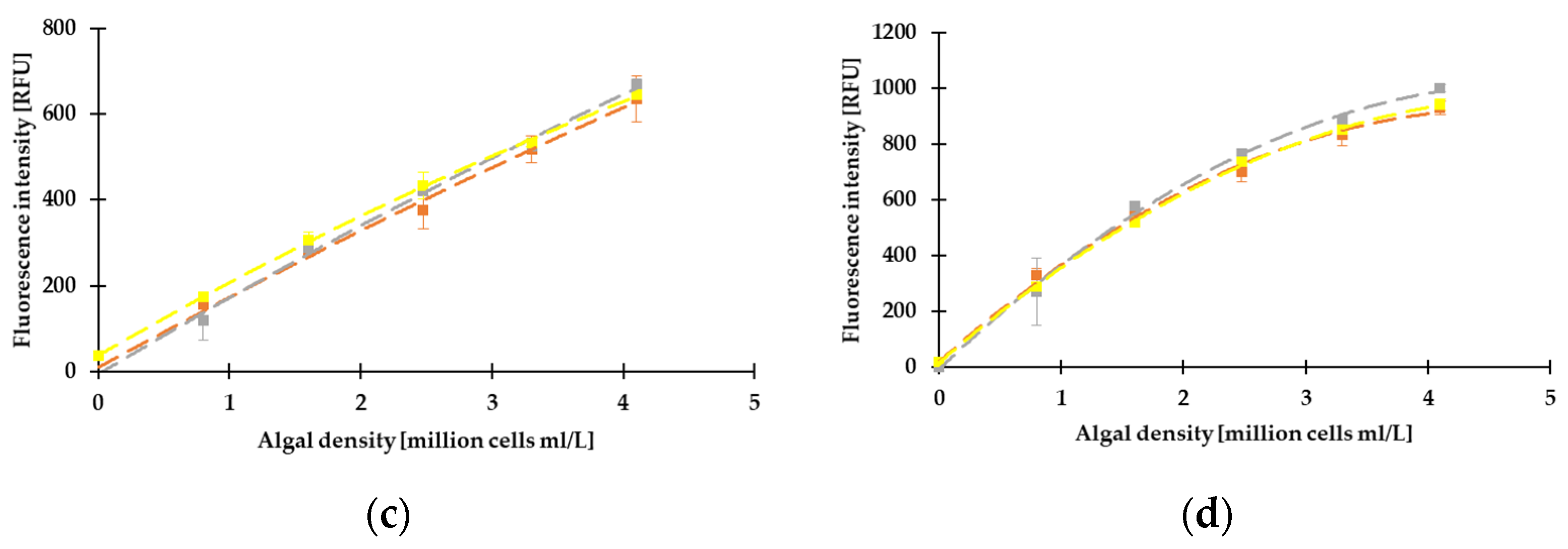
3.2. Correlation between the Different Methods, Determination of LOD, LLOQ, and ULOQ
3.3. Results of Fluorescence Measurements on Green and Blue-Green Algae by Dichroic Fluorometer System
3.4. Examination of Algae Group Ratios by the Dichroic Fluorometer System
3.5. Assessment of the Degradation and Algal Toxicity of a Potential Water-Contaminant Herbicide Active Ingredient by FluoroMeter Module
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verro, R.; Finizio, A.; Otto, S.; Vighi, M. Predicting Pesticide Environmental Risk in Intensive Agricultural Areas. II: Screening Level Risk Assessment of Complex Mixtures in Surface Waters. Environ. Sci. Technol. 2009, 43, 530–537. [Google Scholar] [CrossRef]
- Brock, T.C. Priorities to Improve the Ecological Risk Assessment and Management for Pesticides in Surface Water. Integr. Environ. Assess. Manag. 2013, 9, e64–e74. [Google Scholar] [CrossRef]
- Silva, E.; Daam, M.A.; Cerejeira, M.J. Predicting the Aquatic Risk of Realistic Pesticide Mixtures to Species Assemblages in Portuguese River Basins. J. Environ. Sci. 2015, 31, 12–20. [Google Scholar] [CrossRef]
- Sellner, K.G.; Doucette, G.J.; Kirkpatrick, G.J. Harmful algal blooms: Causes, impacts and detection. J. Ind. Microbiol. Biotechnol. 2003, 30, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Mirnasab, M.A.; Hashemi, H.; Samaei, M.R.; Azhdarpoor, A. Advanced removal of water NOM by Pre-ozonation, Enhanced coagulation and Bio-augmented Granular Activated Carbon. Int. J. Environ. Sci. Technol. 2021, 18, 3143–3152. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Padisák, J. Ecosystem services provided by marine and freshwater phytoplankton. Hydrobiologia 2022, 28, 1–16. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The Rise of Harmful Cyanobacteria Blooms: The Potential Roles of Eutrophication and Climate Change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Klátyik, S.; Takács, E.; Mörtl, M.; Földi, A.; Trábert, Z.; Ács, É.; Darvas, B.; Székács, A. Dissipation of the Herbicide Active Ingredient Glyphosate in Natural Water Samples in the Presence of Biofilms. Int. J. Environ. Anal. Chem. 2017, 97, 901–921. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Jin, X.; Xu, J.; Zhang, H.; Yu, J.; Sun, Q.; Gao, C.; Wang, L. Analysis of Algae Growth Mechanism and Water Bloom Prediction under the Effect of Multi-Affecting Factor. Saudi J. Biol. Sci. 2017, 24, 556–562. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Butterwick, C.; Heaney, S.I.; Talling, J.F. A Comparison of Eight Methods for Estimating the Biomass and Growth of Planktonic Algae. Br. Phycol. J. 1982, 17, 69–79. [Google Scholar] [CrossRef]
- OECD (Organisation for Economic Cooperation and Development). Freshwater Alga and Cyanobacteria, Growth Inhibition Test (201); OECD Guidelines for the Testing of Chemicals, Section 2; OECD: Paris, France, 2011; Volume 2. [Google Scholar]
- Chioccioli, M.; Hankamer, B.; Ross, I.L. Flow Cytometry Pulse Width Data Enables Rapid and Sensitive Estimation of Biomass Dry Weight in the Microalgae Chlamydomonas Reinhardtii and Chlorella Vulgaris. PLoS ONE 2014, 9, e97269. [Google Scholar] [CrossRef] [PubMed]
- Catlett, D.; Siegel, D.A.; Matson, P.G.; Wear, E.K.; Carlson, C.A.; Lankiewicz, T.S.; Iglesias-Rodriguez, M.D. Integrating phytoplankton pigment and DNA metabarcoding observations to determine phytoplankton composition in the coastal ocean. Limol. Oceanogr. 2022, 68, 361–376. [Google Scholar] [CrossRef]
- Kaplan-Levy, R.N.; Alster-Gloukhovski, A.; Benyamini, Y.; Zohary, T. Lake Kinneret phytoplankton: Integrating classical and molecular taxonomy. Hydrobiologia 2016, 764, 283–302. [Google Scholar] [CrossRef]
- Zhu, C.J.; Lee, Y.K. Determination of Biomass Dry Weight of Marine Microalgae. J. Appl. Phycol. 1997, 9, 189–194. [Google Scholar] [CrossRef]
- Jahnke, J.; Mahlmann, D.M.; Jacobs, P.; Priefer, U.B. The Influence of Growth Conditions on the Cell Dry Weight per Unit Biovolume of Klebsormidium Flaccidum (Charophyta), a Typical Ubiquitous Soil Alga. J. Appl. Phycol. 2011, 23, 655–664. [Google Scholar] [CrossRef]
- Suggett, D.J.; Ondrej, P. Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Borowitzka, M.A., Ed.; Springer: Dordrecht, The Netherlands, 2010; Volume 4, pp. 293–309. [Google Scholar]
- Fernandez-Jaramillo, A.A.; Duarte-Galvan, C.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Romero-Troncoso, R.J.; Guevara-Gonzalez, R.G.; Jesus, R.; Millan-Almaraz, J.R. Instrumentation in Developing Chlorophyll Fluorescence Biosensing: A Review. Sensors 2012, 12, 11853–11869. [Google Scholar] [CrossRef]
- Kahlert, M.; McKie, B.G. Comparing New and Conventional Methods to Estimate Benthic Algal Biomass and Composition in Freshwaters. Environ. Sci. Process. Impacts 2014, 16, 2627–2634. [Google Scholar] [CrossRef]
- Lenk, S.; Gádoros, P.; Kocsányi, L.; Barócsi, A. Teaching Laser-Induced Fluorescence of Plant Leaves. Eur. J. Phys. 2016, 37, 064003. [Google Scholar] [CrossRef]
- Marcek Chorvatova, A.; Uherek, M.; Mateasik, A.; Chorvat, D., Jr. Time-resolved Endogenous Chlorophyll Fluorescence Sensitivity to pH: Study on Chlorella sp. Algae. Methods Appl. Fluoresc. 2020, 8, 024007. [Google Scholar] [CrossRef]
- Mueller, T.C.; Moorman, T.B.; Locke, M.A. Detection of Herbicides Using Fluorescence Spectroscopy. Weed Sci. 1992, 40, 270–274. [Google Scholar] [CrossRef]
- Hunsche, M.; Bürling, K.; Noga, G. Spectral and Time-resolved Fluorescence Signature of Four Weed Species as Affected by Selected Herbicides. Pestic. Biochem. Physiol. 2011, 101, 39–47. [Google Scholar] [CrossRef]
- De Dayan, F.E.M.; Zaccaro, L.M. Chlorophyll Fluorescence as a Marker for Herbicide Mechanisms of Action. Pestic. Biochem. Physiol. 2012, 102, 189–197. [Google Scholar] [CrossRef]
- Levine, M. Fluorescence-based Sensing of Pesticides Using Supramolecular Chemistry. Front. Chem. 2021, 9, 616815. [Google Scholar] [CrossRef] [PubMed]
- Barócsi, A.; Kocsányi, L.; Várkonyi, S.; Richter, P.; Csintalan, Z.; Szente, K. Two-Wavelength, Multipurpose, Two-wavelength, multipurpose, truly portable chlorophyll fluorometer and its application in field monitoring of phytoremediation. Meas. Sci. Technol. 2000, 11, 717–729. [Google Scholar] [CrossRef]
- Malapascua, J.R.F.; Jerez, C.G.; Sergejevová, M.; Figueroa, F.L.; Masojídek, J. Photosynthesis Monitoring to Optimize Growth of Microalgal Mass Cultures: Application of Chlorophyll Fluorescence Techniques. Aquat. Biol. 2014, 22, 123–140. [Google Scholar] [CrossRef]
- Schaffer, J.D.; Sebetich, M.J. Effects of Aquatic Herbicides on Primary Productivity of Phytoplankton in the Laboratory. Bull. Environ. Contam. Toxicol. 2004, 72, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, K.; Krishna Prasad, M.; Mohan Narasimha Rao, G. Algae in Fresh Water Ecosystem. Phykos 2016, 46, 25–31. [Google Scholar]
- Pesce, S.; Bouchez, A.; Montuelle, B. Effects of Organic Herbicides on Phototrophic Microbial Communities in Freshwater Ecosystems. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2011; pp. 87–124. [Google Scholar] [CrossRef]
- Ma, J.; Wang, S.; Ma, L.; Chen, X.; Xu, R. Toxicity assessment of 40 herbicides to the green alga Raphidocelis subcapitata. Ecotox Environ. Saf. 2006, 63, 456–462. [Google Scholar] [CrossRef]
- Carbajal-Hernández, A.L.; Arzate-Cárdenas, M.A.; Valerio-García, R.C.; Martínez-Jerónimo, F. Commercial pesticides for urban applications induced population growth and subcellular alterations in Raphidocelis subcapitata (Chlorophyceae) at concerning environmental concentrations. Ecotoxicology 2022, 31, 1–15. [Google Scholar] [CrossRef]
- Lanasa, S.; Niedzwiecki, M.; Reber, K.P.; East, A.; Sivey, J.D.; Salice, C.D. Comparative toxicity of herbicide active ingredients, safener additives, and commercial formulations to the Nontarget Alga Raphidocelis subcapitata. Environ. Toxicol Chem. 2022, 41, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Pallett, K.E. The Mode of Action of Isoxaflutole: A Case Study of an Emerging Target Site. In Herbicides and Their Mechanism of Action; Cobb, A.H., Kirkwood, R.C., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 215–238. [Google Scholar]
- DeLorenzo, M.E.; Scott, G.I.; Ross, P.E. Toxicity of Pesticides to Aquatic Microorganisms: A Review. Environ. Toxicol. Chem. 2001, 20, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Trebst, A.; Depka, B.; Jäger, J.; Oettmeier, W. Reversal of the Inhibition of Photosynthesis by Herbicides Affecting Hydroxyphenylpyruvate Dioxygenase by Plastoquinone and Tocopheryl Derivatives in Chlamydomonas Reinhardtii. Pest Manag. Sci. 2004, 60, 669–674. [Google Scholar] [CrossRef] [PubMed]
- MacBean, C. The Pesticide Manual: A World Compendium; British Crop Production Council: Alton, UK, 2012; pp. 677–678. [Google Scholar]
- Kaur, H.; Bhowmik, P.C. Phytotoxicity of Isoxaflutole to Phalaris Minor Retz. Plant. Soil. 2004, 258, 161–168. [Google Scholar] [CrossRef]
- Pallett, K.E.; Cramp, S.M.; Little, J.P.; Veerasekaran, P.; Crudace, A.J.; Slater, A.E. Isoxaflutole: The Background to Its Discovery and the Basis of Its Herbicidal Properties. Pest Manag. Sci. 2001, 57, 133–142. [Google Scholar] [CrossRef]
- King, O.; Smith, R.; Mann, R.; Warne, M. Proposed Aquatic Ecosystem Protection Guideline Values for Pesticides Commonly Used in the Great Barrier Reef Catchment Area: Part 1–2,4-D, Ametryn, Diuron, Glyphosate, Hexazinone, Imazapic, Imidacloprid, Isoxaflutole, Metolachlor, Metribuzin, Metsulfuron-Methyl, Simazine and Tebuthiuron; Department of Science, Information Technology and Innovation: Brisbane, QLD, Australia, 2017; pp. 176–184. [Google Scholar]
- Ramanarayanan, T.; Narasimhan, B.; Srinivasan, R. Characterization of Fate and Transport of Isoxaflutole, a Soil-applied Corn Herbicide, in Surface Water Using a Watershed Model. J. Agric. Food Chem. 2005, 53, 8848–8858. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.T.; Scribner, E.A.; Kalkhoff, S.J. Comparison of Fate and Transport of Isoxaflutole to Atrazine and Metolachlor in 10 Iowa Rivers. Environ. Sci. Technol. 2007, 41, 6933–6939. [Google Scholar] [CrossRef]
- Da Silva Santarossa, M.A.; Coleone, A.C.; de Mello, N.P.; Ignácio, N.F.; Machado, A.A.; Marques Silva, J.R.; Velini, E.D.; Machado Neto, J.G. Contamination of Fee-fishing Ponds with Agrochemicals Used in Sugarcane Crops. SN Appl. Sci. 2020, 2, 1498. [Google Scholar] [CrossRef]
- US EPA. Pesticide Fact Sheet. Isoxaflutole. United States Environmental Protection Agency: Washington, DC, USA, 1998; pp. 1–15. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-123000_15-Sep-98.pdf (accessed on 12 May 2023).
- De Santo, F.B.; Ramos, G.A.; Filho, A.M.R.; Marchioro, C.A.; Niemeyer, J.C. Ecotoxicity of the Isoxaflutole Herbicide to Soil InvertebratesEcotoxicity of Isoxaflutole Herbicide to Soil Invertebrates. Rev. Ciênc. Agrovet. 2020, 19, 217–223. [Google Scholar] [CrossRef]
- Trebst, A.; Depka, B.; Holländer-Czytko, H. A Specific Role for Tocopherol and of Chemical Singlet Oxygen Quenchers in the Maintenance of Photosystem II Structure and Function in Chlamydomonas Reinhardtii. FEBS Lett. 2002, 516, 156–160. [Google Scholar] [CrossRef]
- Chamsi, O.; Pinelli, E.; Faucon, B.; Perrault, A.; Lacroix, L.; Sánchez-Pérez, J.-M.; Charcosset, J.-Y. Effects of Herbicide Mixtures on Freshwater Microalgae with the Potential Effect of a Safener. Ann. Limnol. Int. J. Lim. 2019, 55, 3. [Google Scholar] [CrossRef]
- Peer Review of the Pesticide Risk Assessment of the Active Substance Isoxaflutole. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4416 (accessed on 12 May 2023).
- US Geological Survey. Estimated Annual Agricultural Pesticide Use. Pesticide Use Maps—Isoxaflutole. United States US Geological Survey: Reston, VA, USA, 2022. Available online: https://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2017&map=ISOXAFLUTOLE&hilo=L&disp=Isoxaflutole (accessed on 12 May 2023).
- US EPA. Public Participation for Isoxaflutole: New Use on Herbicide Resistant Soybeans. EPA-HQ-OPP-2019-0398. United States Environmental Protection Agency: Washington, DC, USA, 2020; pp. 1–12. Available online: https://www.regulations.gov/docket/EPA-HQ-OPP-2019-0398 (accessed on 12 May 2023).
- Allen, M.M. Simple Conditions for Growth of Unicellular Blue-Green Algae on Plates1, 2. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef]
- Z8 Medium. Available online: https://www-cyanosite.bio.purdue.edu/media/table/Z8.html (accessed on 12 May 2023).
- Aguilera, A.; Berrendero Gómez, E.; Kaštovský, J.; Echenique, R.O.; Salerno, G.L. The polyphasic analysis of two native Raphidiopsis isolates supports the unification of the genera Raphidiopsis and Cylindrospermopsis (Nostocales, Cyanobacteria). Phycologia 2018, 57, 130–146. [Google Scholar] [CrossRef]
- Chekanov, K.; Lukyanov, A.; Boussiba, S.; Aflalo, C.; Solovchenko, A. Modulation of photosynthetic activity and photoprotection in Haematococcus pluvialis cells during their conversion into haematocysts and back. Photosynth. Res. 2016, 128, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Barócsi, A.; Lenk, S.; Kocsányi, L.; Buschmann, C. Excitation Kinetics during Induction of Chlorophyll a Fluorescence. Photosynthetica 2009, 47, 104–111. [Google Scholar] [CrossRef]
- Kautsky, H.; Hirsch, A. Neue versuche zur kohlensäureassimilation. Naturwissenschaften 1931, 19, 964. [Google Scholar] [CrossRef]
- Huang, Y.; Thomson, S.J.; Molin, W.T.; Reddy, K.N.; Yao, H. Early Detection of Soybean Plant Injury from Glyphosate by Measuring Chlorophyll Reflectance and Fluorescence. J. Agric. Sci. 2012, 4, 117–124. [Google Scholar] [CrossRef]
- Vredenberg, W.J. On the quantitative relation between dark kinetics of NPQ-induced changes in variable fluorescence and the activation state of the CF0·CF1·ATPase in leaves. Photosynthetica 2018, 56, 139–149. [Google Scholar] [CrossRef]
- Wetzel, R.G.; Likens, G.E. Limnol Analys; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 978-1-4757-3250-4. [Google Scholar]
- WHO. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Welker, M., Eds.; E & FN Spon: London, UK, 1999. [Google Scholar]
- Carvalho, L.; McDonald, C.; de Hoyos, C.; Mischke, U.; Phillips, G.; Borics, G.; Poikane, S.; Skjelbred, B.; Solheim, A.L.; Van Wichelen, J.; et al. Sustaining recreational quality of European lakes: Minimizing the health risks from algal blooms through phosphorus control. J. Appl. Ecol. 2013, 50, 315–323. [Google Scholar] [CrossRef]
- T-Krasznai, E.; Lerf, V.; Tóth, I.; Kisantal, T.; Várbíró, G.; Vasas, G.; B-Béres, V.; Görgényi, J.; Lukács, Á.; Kókai, Z.; et al. Uncertainties of cell number estimation in cyanobacterial colonies and the potential use of sphere packing. Harmful Algae 2022, 117, 102290. [Google Scholar] [CrossRef]
- Székács, A.; Mörtl, M.; Darvas, B. Monitoring Pesticide Residues in Surface and Ground Water in Hungary: Surveys in 1990–2015. J. Chem. 2015, 2015, 717948. [Google Scholar] [CrossRef]
- Utermöhl, H. Methods of Collecting Plankton for Various Purposes Are Discussed. SIL Commun. 1953–1996 1958, 9, 1–38. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Fondation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2016, 132, 13–66. [Google Scholar] [CrossRef]
- Sobiechowska-Sasim, M.; Stoń-Egiert, J.; Kosakowska, A. Quantitative analysis of extracted phycobilin pigments in cyanobacteria-an assessment of spectrophotometric and spectrofluorometric methods. J. Appl. Phycol. 2014, 26, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Gregor, J.; Maršálek, B. Freshwater phytoplankton quantification by chlorophyll a: A comparative study of in vitro, in vivo and in situ methods. Water Res. 2004, 38, 517–522. [Google Scholar] [CrossRef]
- Volpe, C.; Vadstein, O.; Andersen, G.; Andersen, T. Nanocosm: A well plate photobioreactor for environmental and biotechnological studies. Lab. Chip. 2021, 21, 2027–2039. [Google Scholar] [CrossRef]
- Schreiber, U.; Klughammer, C. Evidence for variable chlorophyll fluorescence of photosystem I in vivo. Photosynth. Res. 2021, 149, 213–231. [Google Scholar] [CrossRef]
- Cadondon, J.G.; Ong, P.M.B.; Vallar, E.A.; Shiina, T.; Galvez, M.C.D. Chlorophyll-a Pigment Measurement of Spirulina in Algal Growth Monitoring Using Portable Pulsed LED Fluorescence Lidar System. Sensors 2022, 22, 2940. [Google Scholar] [CrossRef]
- Albrecht, M.; Khanipour Roshan, S.; Fuchs, L.; Karsten, U.; Schumann, R. Applicability and limitations of high-throughput algal growth rate measurements using in vivo fluorescence in microtiter plates. J. Appl. Phycol. 2022, 34, 2037–2049. [Google Scholar] [CrossRef]
- Siedlewicza, G.; Zakb, A.; Sharmaa, L.; Kosakowskaa, A.; Pazdroa, K. Effects of oxytetracycline on growth and chlorophyll a fluorescence in green algae (Chlorella vulgaris), diatom (Phaeodactylum tricornutum) and cyanobacteria (Microcystis aeruginosa and Nodularia spumigena). Oceanologia 2020, 62, 214–225. [Google Scholar] [CrossRef]
- Cook, A.H. Algal pigments and their significance. Biol. Rev. 1945, 20, 115–132. [Google Scholar] [CrossRef]
- Macintyre, H.L.; Lawrenz, E.; Richardson, T.L. Taxonomic Discrimination of Phytoplankton by Spectral Fluorescence. Taxonomic Discrimination of Phytoplankton by Spectral Fluorescence. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Sugget, D., Prášil, O., Borowitzka, M., Eds.; Springer Science + Business Media B.V.: Dordrecht, The Netherlands, 2010; pp. 129–169. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal. Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Pagels, F.; Pereira, R.N.; Vicente, A.A.; Guedes, A.C. Extraction of Pigments from Microalgae and Cyanobacteria—A Review on Current Methodologies. Appl. Sci. 2021, 11, 5187. [Google Scholar] [CrossRef]
- Schagerl, M.; Siedler, R.; Konopáčová, E.; Ali, S.S. Estimating Biomass and Vitality of Microalgae for Monitoring Cultures: A Roadmap for Reliable Measurements. Cells 2022, 11, 2455. [Google Scholar] [CrossRef]
- Oláh, V.; Hepp, A.; Irfan, M.; Mészáros, I. Chlorophyll Fluorescence Imaging-Based Duckweed Phenotyping to Assess Acute Phytotoxic Effects. Plants 2021, 10, 2763. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Miehé, J.A. Fluorescence Imaging as a Diagnostic Tool for Plant Stress. Trends Plant. Sci. 1997, 2, 316–320. [Google Scholar] [CrossRef]
- Lichtentaler, H.K.; Babani, F. Detection of photosynthetic activity and water stress by imaging the red chlorophyll fluorescence. Plant. Physiol. Biochem. 2000, 38, 889–895. [Google Scholar] [CrossRef]
- Canora, D.D.; Guasch, L.L.; Zuazo, R.S. Species-specific responses of Antarctic terrestrial microalgae to salinity stress. Comparative study in Klebsormidium sp. and Stigeoclonium sp. Czech Polar Rep. 2022, 12, 89–102. [Google Scholar] [CrossRef]
- Fodorpataki, L.; Geráj, J.; Deák, H.; Barna, S.; Kovács, B. Influence of inorganic nutrients on parameters of biomass production in a local strain of the microalga Scenedesmus acuminatus. Contrib. Bot. 2013, 48, 83–94. Available online: http://contributiibotanice.reviste.ubbcluj.ro/materiale/2013/Contrib_Bot_vol_48_pp_083-094.pdf (accessed on 12 May 2023).
- Santos, A.M.D.; Vitorino, L.C.; Cruvinel, B.G.; Ávila, R.G.; Vasconcelos Filho, S.D.C.; Batista, P.F.; Bessa, L.A. Impacts of Cd Pollution on the Vitality, Anatomy and Physiology of Two Morphologically Different Lichen Species of the Genera Parmotrema and Usnea, Evaluated under Experimental Conditions. Diversity 2022, 14, 926. [Google Scholar] [CrossRef]
- National Registration Authority for Agricultural and Veterinary Chemicals (Australia). Public Release Summary on Evaluation of the New Active Isoxaflutole in the Product Balance 750WG Herbicide; The Authority: Canberra, NSW, Australia, 2001; p. 35. [Google Scholar]


| Instrument Features | Instrument Type | ||||||
|---|---|---|---|---|---|---|---|
| FMM 1 (This Study) | DFS 2 (This Study) | Phyto -PAM-II/ED 3 | AlgaeTorch 4 | AquaFluor 5 | AquaPen AP 110-C 6 | YSI 6025 7 | |
| Detection mode * | CE | CE | PM | CE | CE | PM | CE |
| PM measuring source ** Peak wavelength (nm) Number of wavelengths | LED 440–625 5 | LED | |||||
| Actinic (saturation) source Peak wavelength (nm) Number of wavelengths | LD 635 1 | LED 470, 630 2 | LED 440–640 6 | LED 470–610 3 | LED 350–530 2 | LED 455, 630 2 | LED 470 1 |
| Actinic (saturation) peak level (μmol/m2/s) | 770 | 1500 | 1500 (5000) | 1000 (3000) | |||
| Detection wavelength (FWHM bandpass) (nm) | 690 (10) 735 (10) | 708 (75) 716(43) | >650 (LP) *** | >660 (LP) | 708 (83) | 675 (50) | |
| Detector type **** Number of channels | PD 2 | PD 2 | PMT 1 | PD 1 | PD 2 | PD 1 | PD 1 |
| Chlorophyll a range (resolution) (ng/mL) | 80–8000 | 0–200 | 0–500 | 0–300 | 0–400 | ||
| (0.1) | (0.1) | (0.1) | (0.5) | (0.5) | (0.1) | ||
| Liquid-sample holder ***** | P | MP | C | P | C | C | P |
| Recorded data type ****** | K | M | F, L, K | M | M | F, L, M | M |
| Symbol | Definition | Description |
|---|---|---|
| Fo | observed | Non-variable (original) fluorescence intensity |
| Fp | observed | Peak fluorescence intensity, maximum fluorescence at a non-saturating light pulse |
| Fv* | Fp–Fo | Variable fluorescence in terms of Fp |
| Fv*/Fp | Fv*/Fp | Proxy of quantum efficiency of photosystem II |
| Fs | observed | Steady-state (terminal) fluorescence |
| Fd | Fp–Fs | Fluorescence decrease in terms of Fp |
| Rfd* | Fd/Fs | Fluorescence decrease ratio |
| Prototype | Raphidocelis subcapitata | Microcystis aeruginosa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FMM 1 | DFS 2 | FMM | DFS | ||||||
| r * | r2 | r | r2 | r | r2 | r | ρ ** | r2 | |
| Optical density (determined spectrophotometrically) | 0.992 (p < 0.001) *** | 0.984 | Channel 1 | 0.989 (p = 0.001) | 0.978 | Channel 1 | |||
| 0.999 (p < 0.001) | 0.998 | - | 1 (p < 0.001) | 0.999 | |||||
| Channel 2 | Channel 2 | ||||||||
| 0.997 (p < 0.001) | 0.994 | - | 0.976 (p < 0.001) | 0.962 | |||||
| Cell number (determined in a Bürker chamber) | 0.992 (p < 0.001) | 0.984 | Channel 1 | 0.989 (p = 0.001) | 0.978 | Channel 1 | |||
| 0.999 (p < 0.001) | 0.998 | 0.993 (p < 0.001) | - | 0.987 | |||||
| Channel 2 | Channel 2 | ||||||||
| 0.998 (p < 0.001) | 0.996 | 0.921 (p < 0.001) | - | 0.858 | |||||
| Chlorophyll-a content (obtained by extraction) | 0.992 (p < 0.001) | 0.984 | Channel 1 | 0.988 (p = 0.002) | 0.976 | Channel 1 | |||
| 0.999 (p < 0.001) | 0.998 | - | 1 (p < 0.001) | 0.998 | |||||
| Channel 2 | Channel 2 | ||||||||
| 0.991 (p = 0.001) | 0.982 | - | 0.976 (p < 0.001) | 0.962 | |||||
| Analytical Feature | Cell Number (Cells/mL) | |||||
|---|---|---|---|---|---|---|
| FMM 1 | DFS 2 | |||||
| R. subcapitata | M. aeruginosa | R. subcapitata | M. aeruginosa | |||
| Channel 1 | Channel 2 | Channel 1 | Channel 2 | |||
| LOD * | 4.01 × 106 | 8.26 × 107 | 7.58 × 104 | 3.70 × 103 | 3.80 × 104 | 1.13 × 105 |
| LLOQ ** | 8.12 × 106 | 1.51 × 108 | 9.34 × 104 | 6.10 × 103 | 8.72 × 104 | 9.97 × 105 |
| ULOQ *** | ND **** | ND | 7.22 × 106 | 8.06 × 106 | 1.27 × 108 | 2.4 × 109 |
| Taxon | Phylum | Concentration (106 cells/m3) | Fresh Weight (mg/m3) | Biomass (%) |
|---|---|---|---|---|
| Chrysochromulina parva | Haptophyta | 916.30 | 989.60 | 8.31 |
| Keratococcus sp. | Chlorophyta | 257.12 | 168.29 | 1.41 |
| Raphidocelis subcapitata | Chlorophyta | 4.67 | 13.22 | 0.11 |
| Scenedesmus sp. | Chlorophyta | 23.37 | 13.22 | 0.11 |
| Monoraphidium sp. | Chlorophyta | 28.05 | 27.49 | 0.23 |
| Pediastrum duplex | Chlorophyta | 79.47 | 149.81 | 1.26 |
| Cryptomonas sp. | Cryptista | 0.93 | 53.97 | 0.45 |
| Rhodomonas sp. | Cryptista | 130.90 | 1605.11 | 13.48 |
| Geitlerinema sp. | Cyanobacteria | 37.40 | 25.07 | 0.21 |
| Glaucospira sp. | Cyanobacteria | 4.67 | 2.57 | 0.02 |
| Gloeocapsa sp. | Cyanobacteria | 902.27 | 896.88 | 7.53 |
| Limnothrix redekei | Cyanobacteria | 23.37 | 3.30 | 0.03 |
| Merismopedia sp. | Cyanobacteria | 1477.30 | 3828.88 | 32.15 |
| Merismopedia elegans | Cyanobacteria | 205.70 | 8.62 | 0.07 |
| Merismopedia glauca | Cyanobacteria | 247.77 | 162.17 | 1.36 |
| Planktolyngbya circumcreta | Cyanobacteria | 9452.83 | 1336.36 | 11.22 |
| Planktolyngbya limnetica | Cyanobacteria | 579.70 | 227.65 | 1.91 |
| Planktothrix rubescens | Cyanobacteria | 776.05 | 1219.01 | 10.23 |
| Pseudanabaena sp. | Cyanobacteria | 37.40 | 505.23 | 4.24 |
| EC50 (µg/mL) | Standard Deviation (SD) (µg/mL) | |
|---|---|---|
| Optical density | 0.034 | 0.004 |
| Chlorophyll a content | 0.033 | 0.000 |
| Rfd* | 0.015 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lázár, D.; Takács, E.; Mörtl, M.; Klátyik, S.; Barócsi, A.; Kocsányi, L.; Lenk, S.; Domján, L.; Szarvas, G.; Lengyel, E.; et al. Application of a Fluorescence-Based Instrument Prototype for Chlorophyll Measurements and Its Utility in an Herbicide Algal Ecotoxicity Assay. Water 2023, 15, 1866. https://doi.org/10.3390/w15101866
Lázár D, Takács E, Mörtl M, Klátyik S, Barócsi A, Kocsányi L, Lenk S, Domján L, Szarvas G, Lengyel E, et al. Application of a Fluorescence-Based Instrument Prototype for Chlorophyll Measurements and Its Utility in an Herbicide Algal Ecotoxicity Assay. Water. 2023; 15(10):1866. https://doi.org/10.3390/w15101866
Chicago/Turabian StyleLázár, Diána, Eszter Takács, Mária Mörtl, Szandra Klátyik, Attila Barócsi, László Kocsányi, Sándor Lenk, László Domján, Gábor Szarvas, Edina Lengyel, and et al. 2023. "Application of a Fluorescence-Based Instrument Prototype for Chlorophyll Measurements and Its Utility in an Herbicide Algal Ecotoxicity Assay" Water 15, no. 10: 1866. https://doi.org/10.3390/w15101866
APA StyleLázár, D., Takács, E., Mörtl, M., Klátyik, S., Barócsi, A., Kocsányi, L., Lenk, S., Domján, L., Szarvas, G., Lengyel, E., & Székács, A. (2023). Application of a Fluorescence-Based Instrument Prototype for Chlorophyll Measurements and Its Utility in an Herbicide Algal Ecotoxicity Assay. Water, 15(10), 1866. https://doi.org/10.3390/w15101866