Three-Dimensional Biofilm Electrode Reactors with a Triple-Layer Particle Electrode for Highly Efficient Treatment of Micro-Polluted Water Sources
Abstract
:1. Introduction
2. Materials and Methods
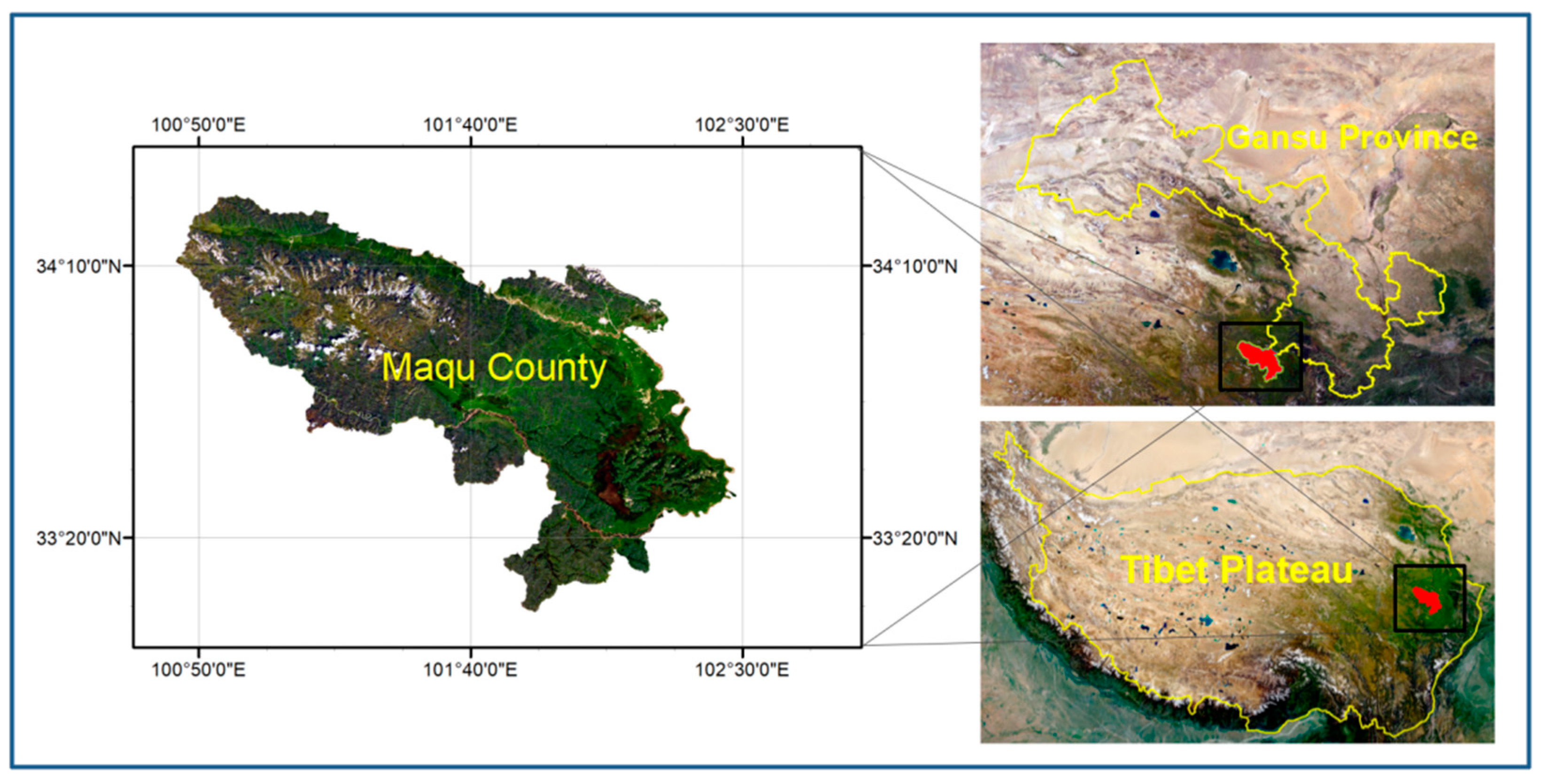
2.1. Wastewater Source
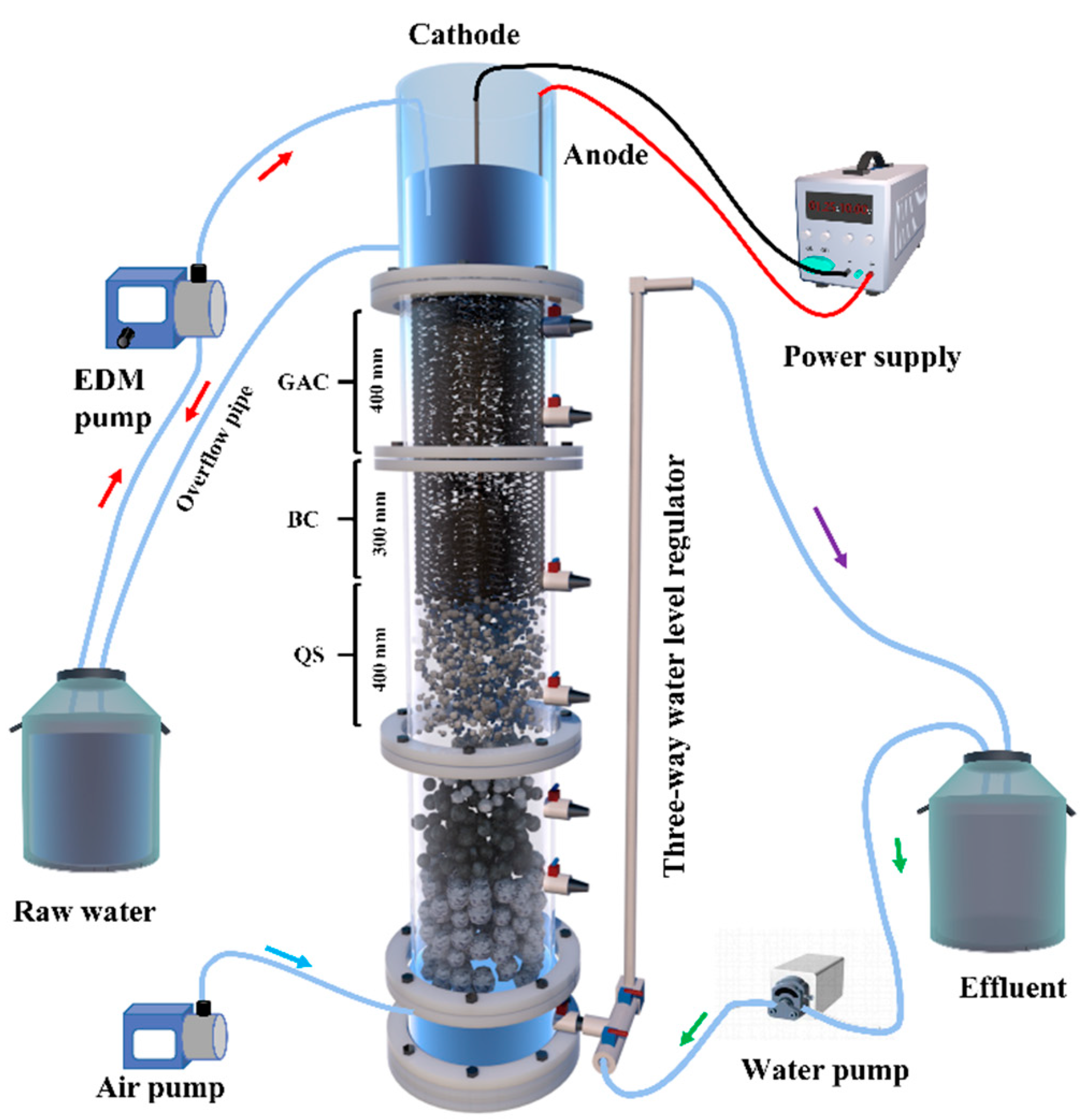
2.2. Experimental Setup and Start-Up
2.2.1. Experimental Setup
2.2.2. Experimental Procedure
2.3. Analytical Methods
3. Results and Discussion
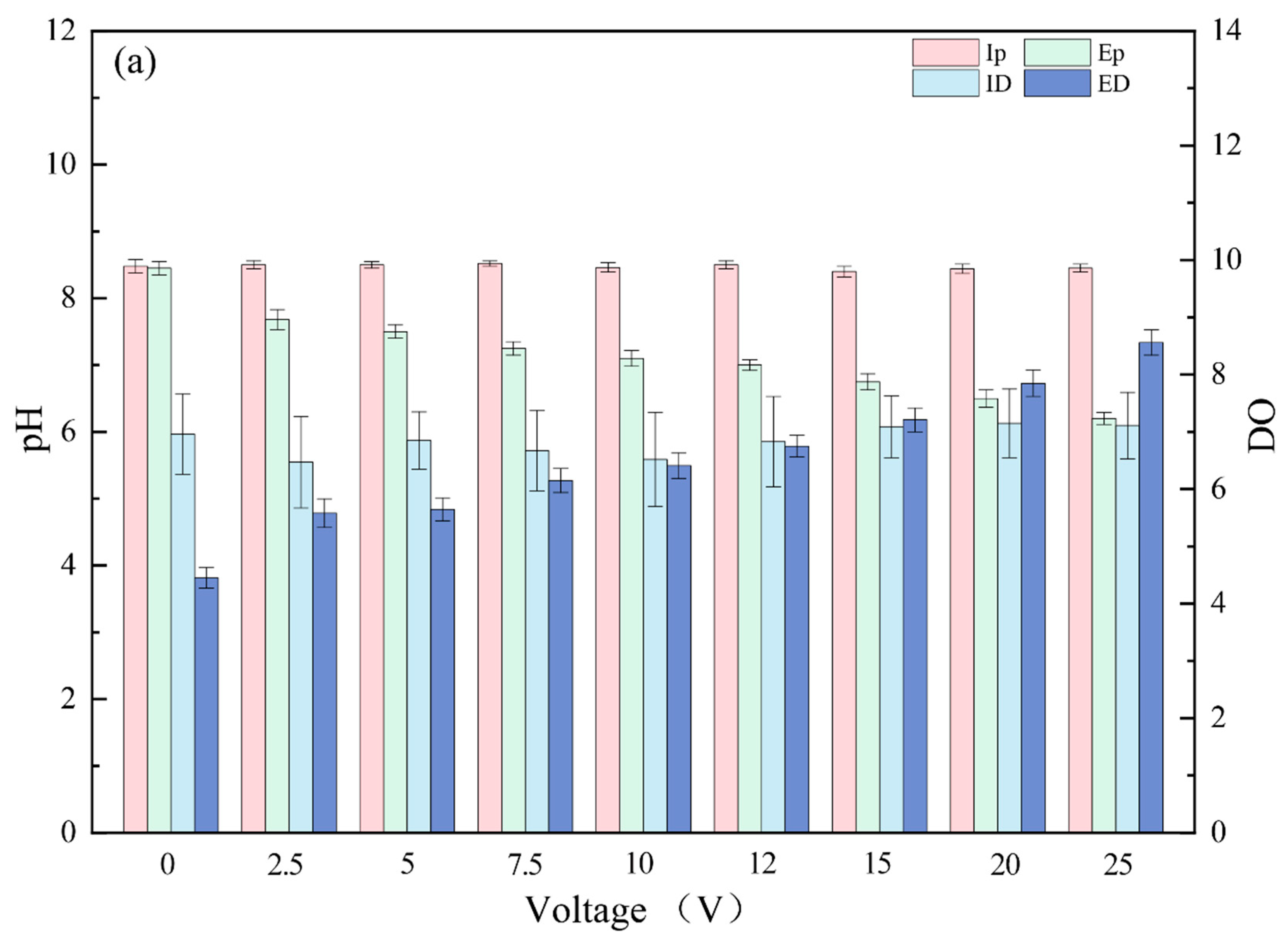
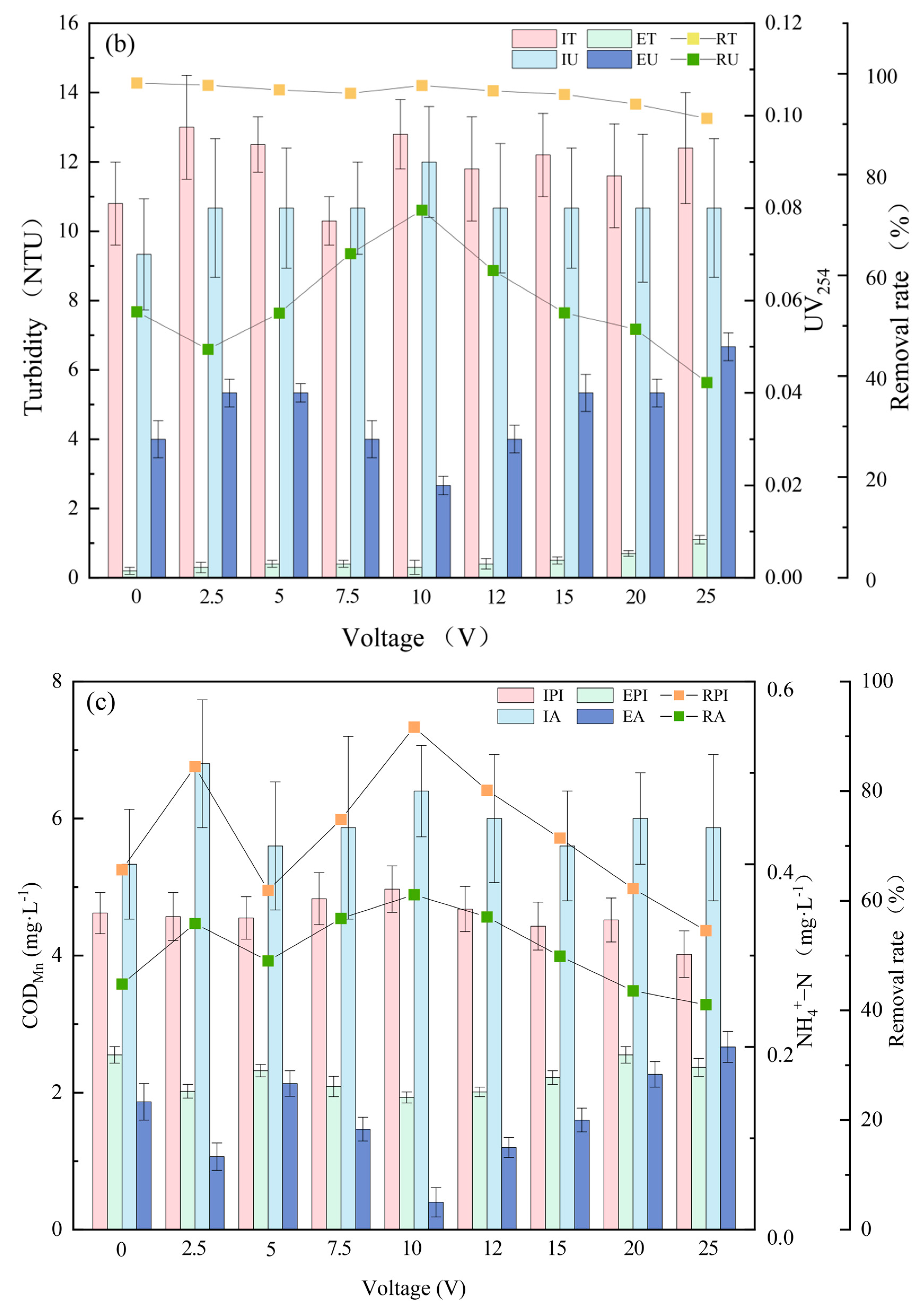
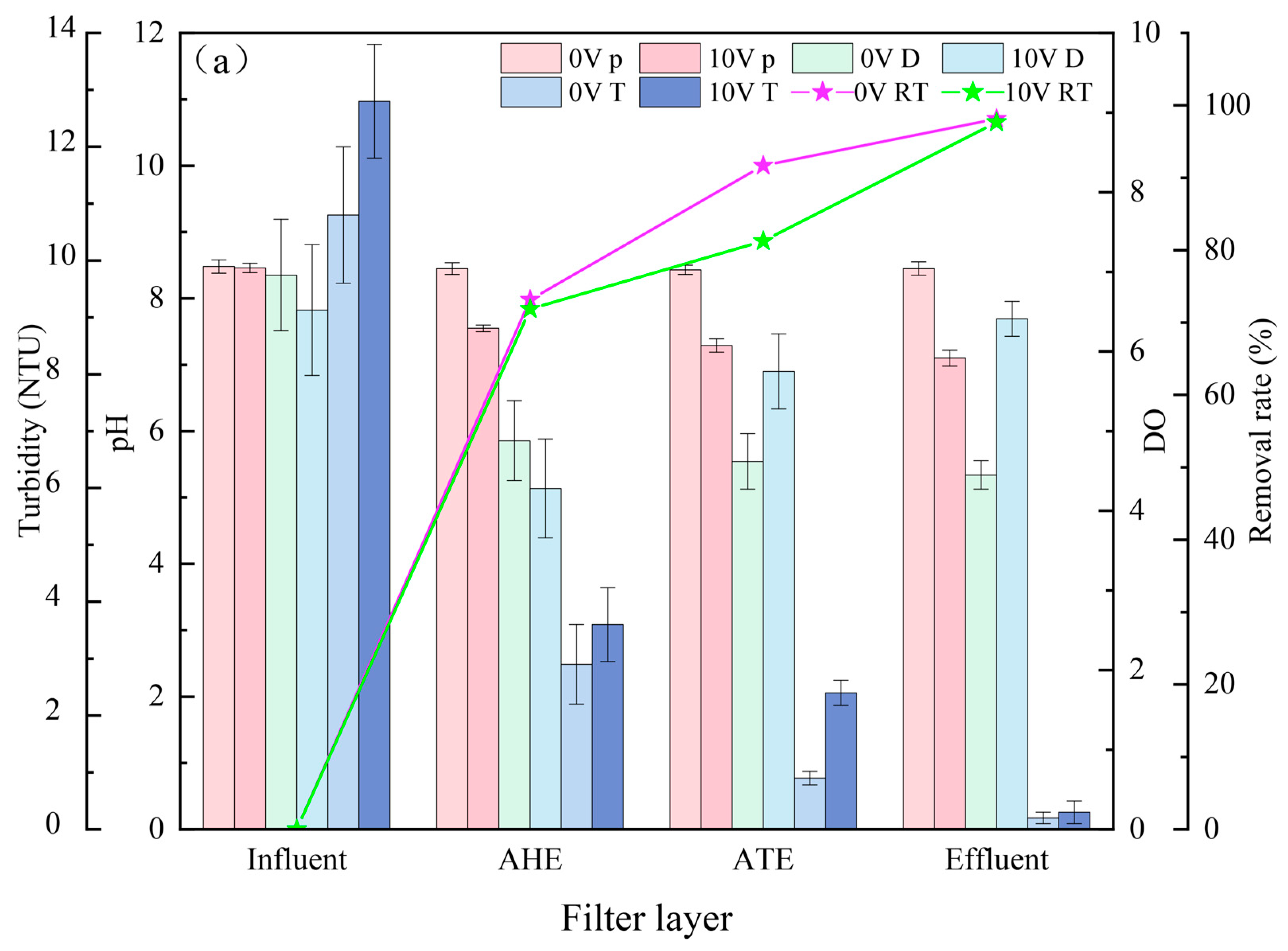
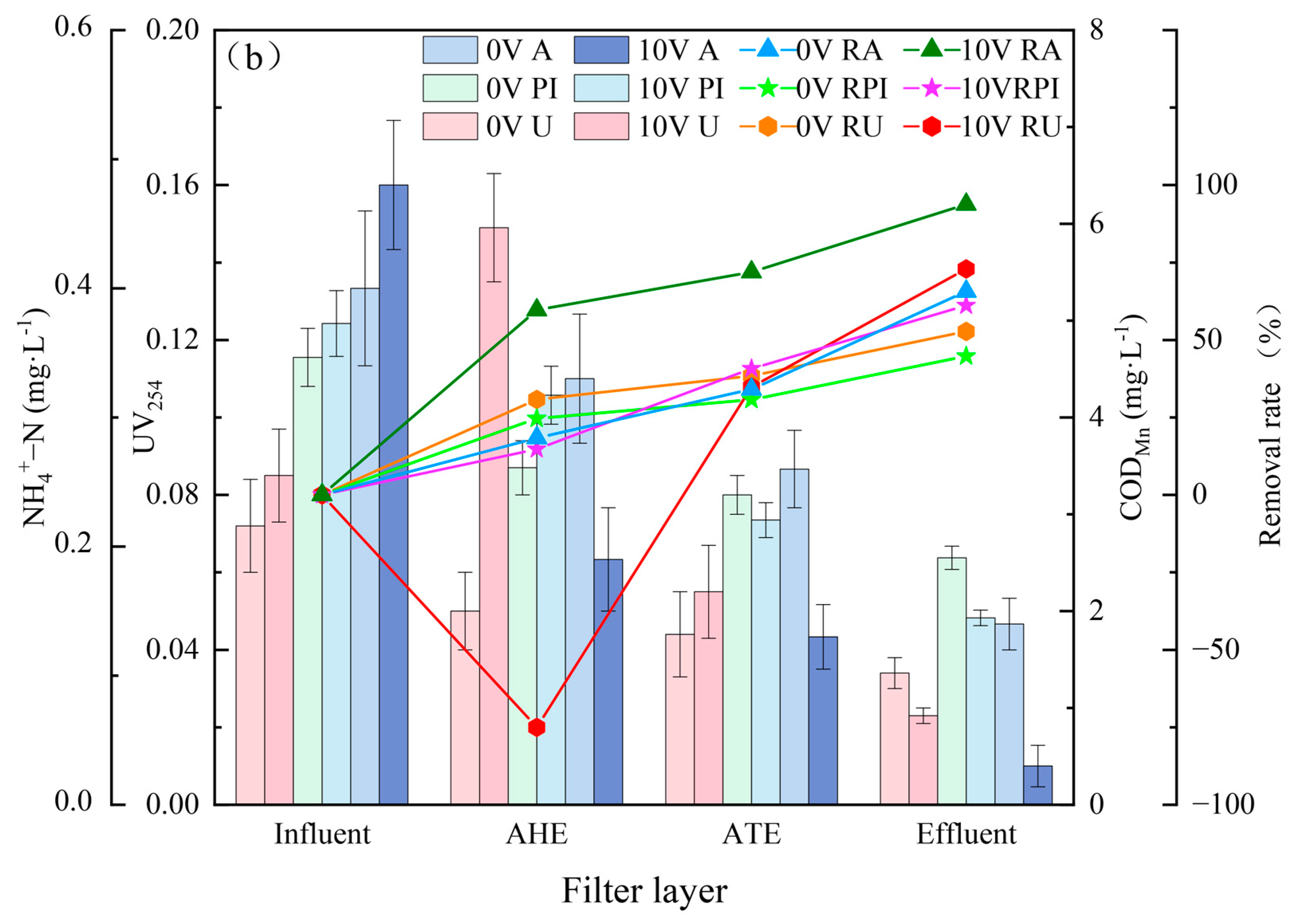
3.1. Pollutant Removal Effects under Different Voltages
3.2. Pollutant Removal Effects of Different Filter Layers
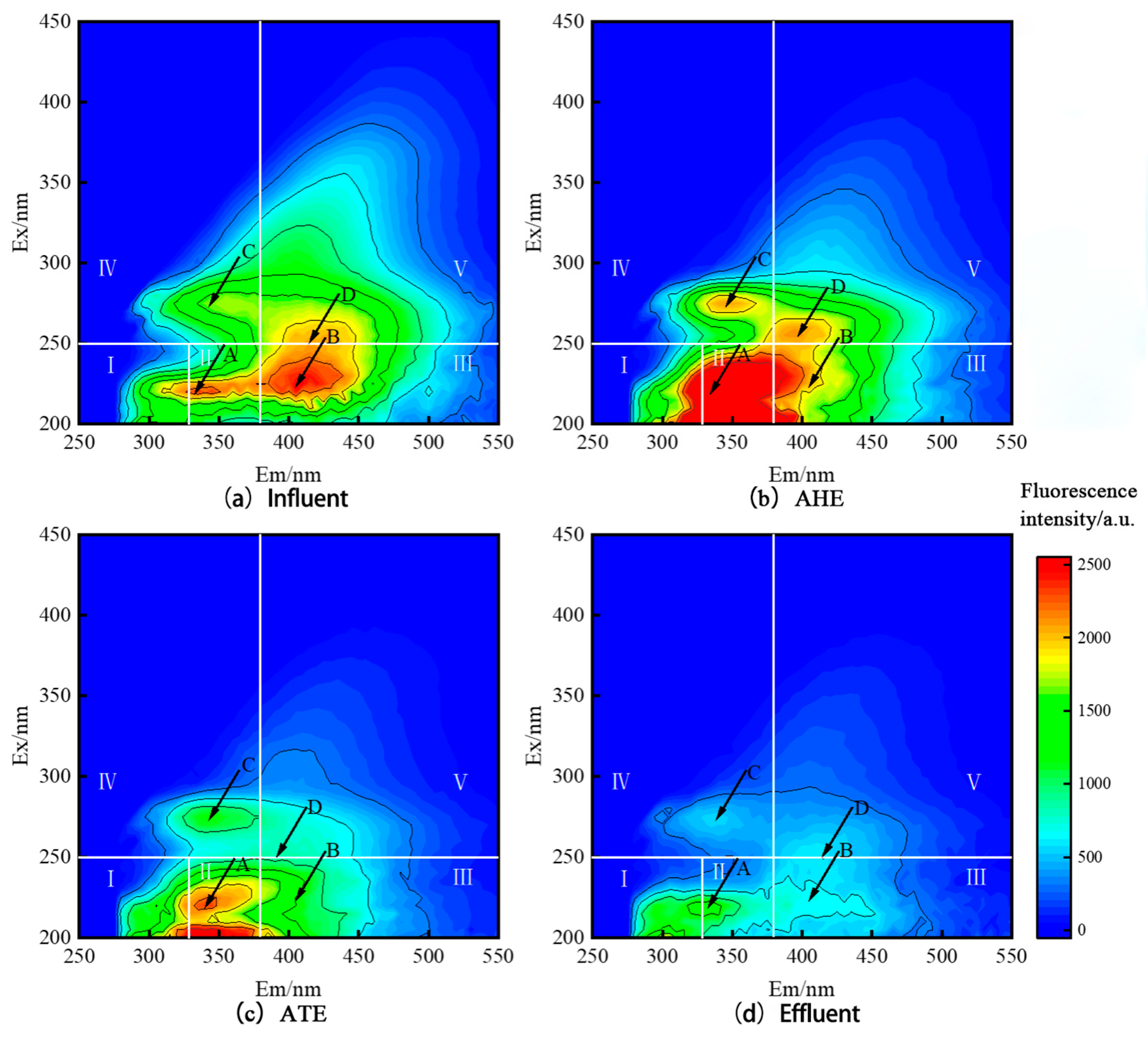
3.3. Analysis of the DOM Degradation Process in TL-BERs
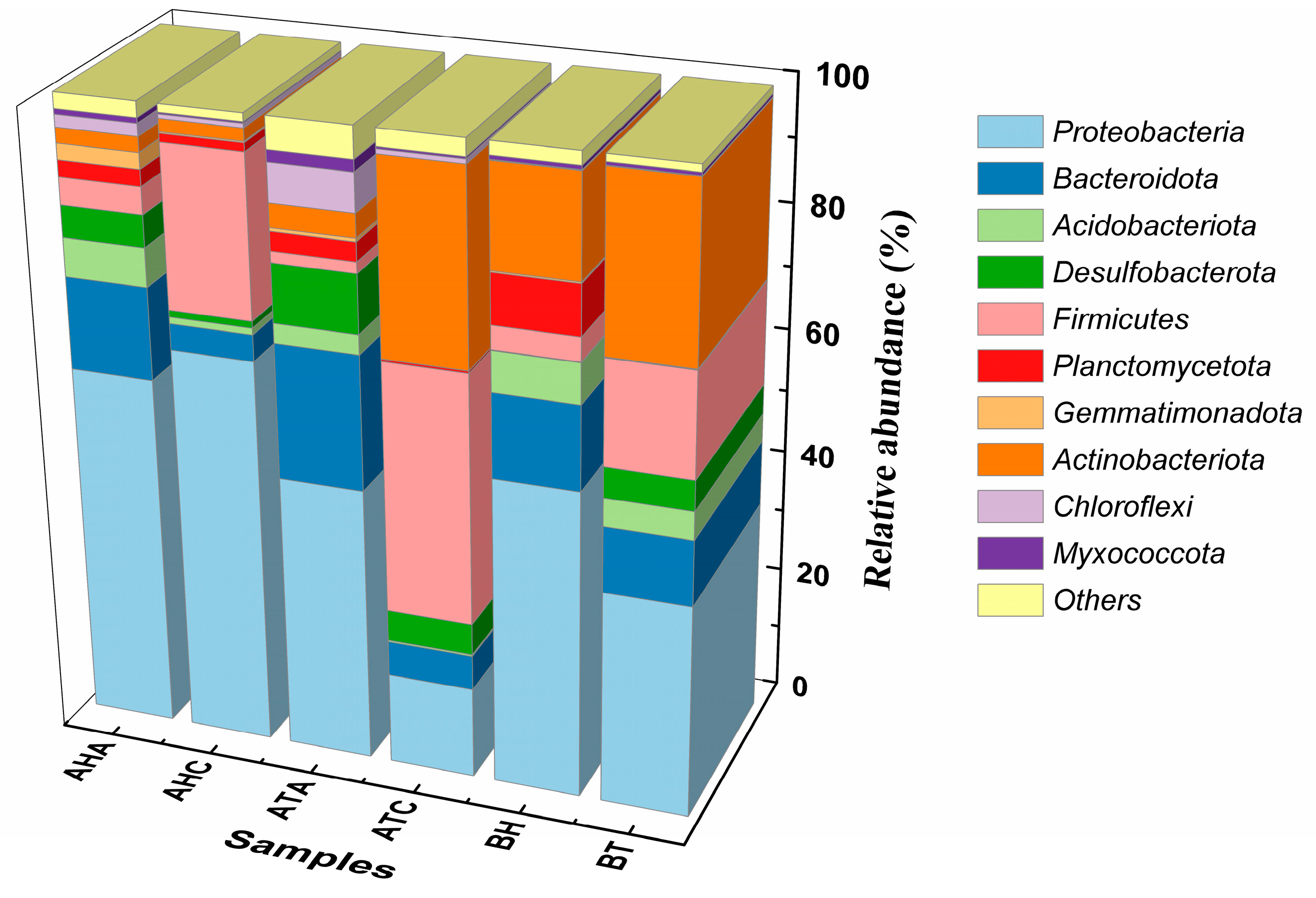
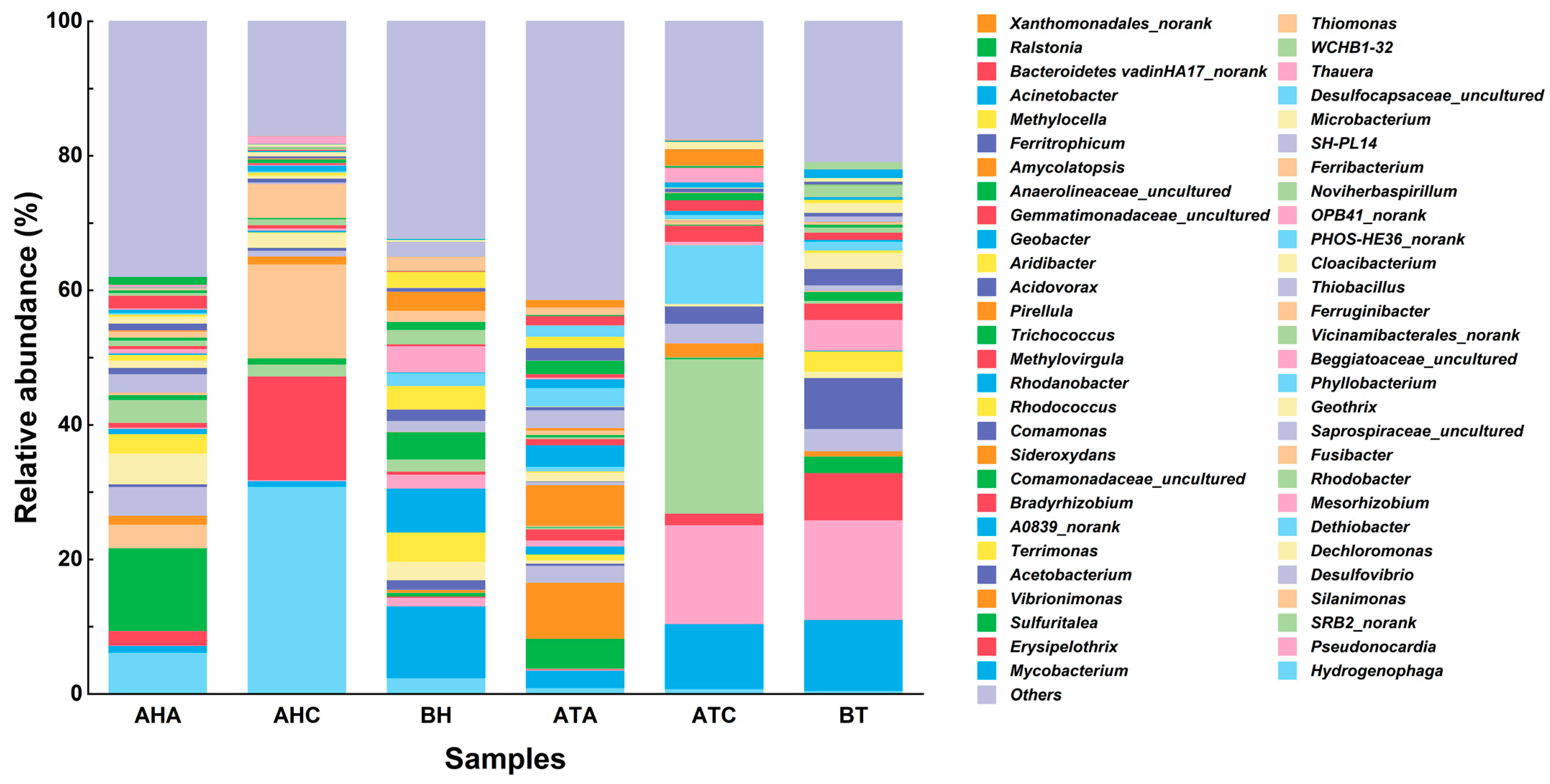
3.4. Analysis of High-Throughput Sequencing Results
3.4.1. Effect of TL-BERs on Microbiome Levels
3.4.2. Effect of TL-BERs on Microbial Genus Levels
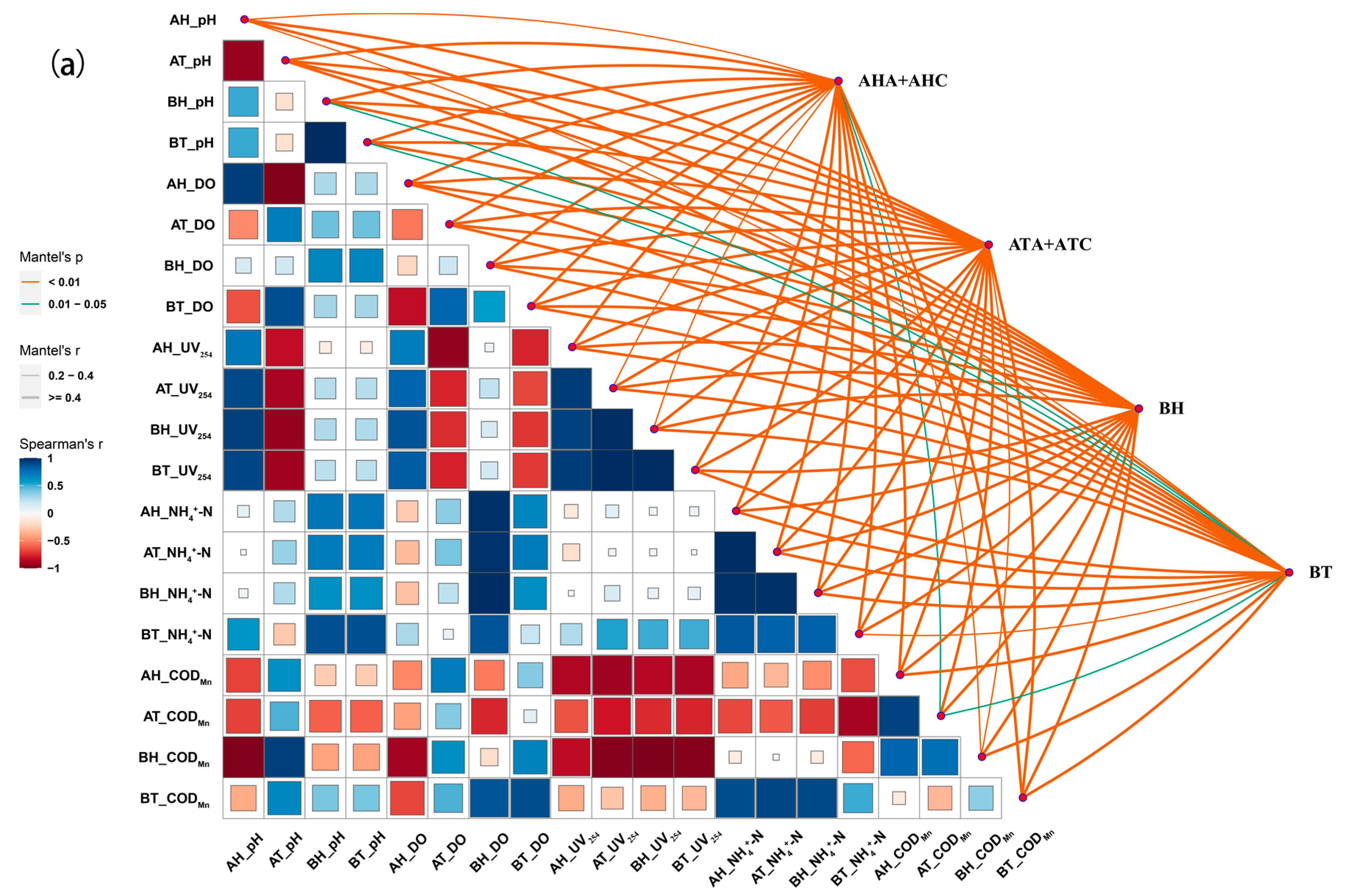
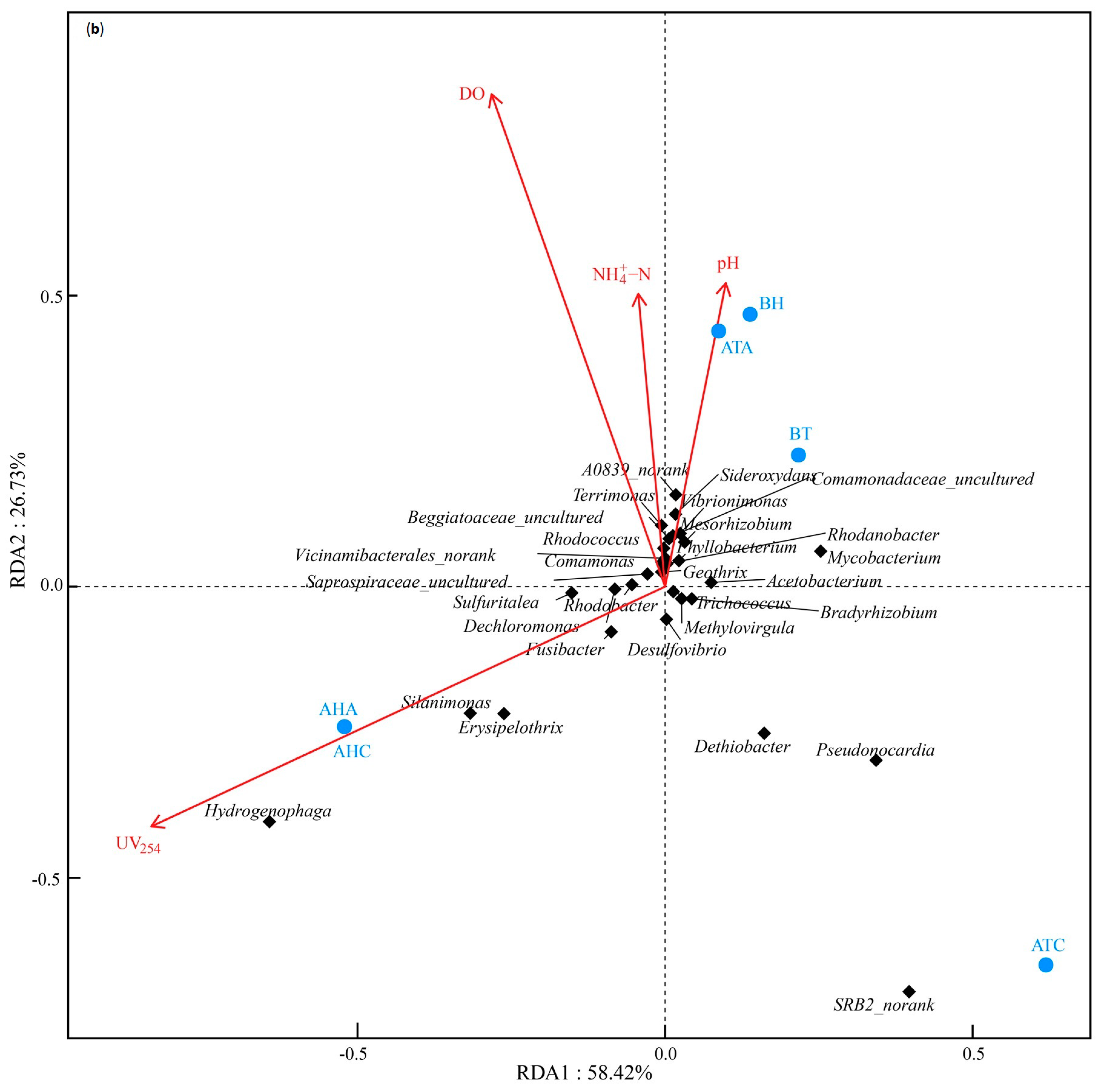
3.4.3. Correlation Analysis of Microbial Composition and Water Quality Physicochemical Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Geng, A.; Chen, Z.; Wang, Y. Application research of rotating cross-flow ultrafiltration water purifier in rural decentralized water supply project. IOP Conf. Ser. Earth Environ. Sci. 2019, 233, 052054. [Google Scholar] [CrossRef]
- Zeng, J.; Chabi, K.; Hu, Y.; Zhang, S.; Yu, X. Ammonium removal of biological roughing filter for rural drinking water pretreatment. Water Sci. Technol. Water Supply 2020, 20, 2768–2778. [Google Scholar] [CrossRef]
- Sun, X.; You, W.; Xuan, X.; Ji, L.; Xu, X.; Wang, G.; Zhao, S.; Boczkaj, G.; Yoon, J.Y.; Chen, S. Effect of the cavitation generation unit structure on the performance of an advanced hydrodynamic cavitation reactor for process intensifications. Chem. Eng. J. 2021, 412, 128600. [Google Scholar] [CrossRef]
- Gottinger, A.; McMartin, D.; Price, D.; Hanson, B. The effectiveness of slow sand filters to treat Canadian rural prairie water. Can. J. Civ. Eng. 2011, 38, 455–463. [Google Scholar] [CrossRef]
- Ji, X.; Zhao, C.; Lv, Y.; Yang, J.; Li, B. Influence of Particle Size of River Sand on the Decontamination Process in the Slow Sand Filter Treatment of Micro-Polluted Water. Water 2022, 14, 100. [Google Scholar] [CrossRef]
- Qin, W.; Luo, Y.; Zhao, W.; Luo, Y.; Du, X.; Wang, Z. Performance and microbial characteristics of a novel pilot-scale tubing biological contact oxidation reactor for rural drinking water. J. Water Process. Eng. 2021, 43, 102290. [Google Scholar] [CrossRef]
- García-Ávila, F.; Avilés-Anazco, A.; Sánchez-Cordero, E.; Valdiviezo-Gonzáles, L.; Ordnoez, M.D.T. The challenge of improving the efficiency of drinking water treatment systems in rural areas facing changes in the raw water quality. S. Afr. J. Chem. Eng. 2021, 37, 141–149. [Google Scholar] [CrossRef]
- Sun, X.; Liu, S.; Manickam, S.; Tao, Y.; Yoon, J.Y.; Xuan, X. Intensification of biodiesel production by hydrodynamic cavitation: A critical review. Renew. Sust. Energ. Rev. 2023, 179, 113277. [Google Scholar] [CrossRef]
- Xuan, X.; Wang, M.; You, W.; Manickam, S.; Tao, Y.; Yoon, J.Y.; Sun, X. Hydrodynamic cavitation-assisted preparation of porous carbon from garlic peels for supercapacitors. Ultrason. Sonochem. 2023, 94, 106333. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Wu, J.; Wang, Y.; Zheng, Z.; Jiang, Z. Mechanistic insight and rapid co-adsorption of nitrogen pollution from micro-polluted water over MgAl-layered double hydroxide composite based on zeolite. Sep. Purif. Technol. 2022, 297, 121484. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Zhang, Y.; Liu, B.; Liang, P. Catalytic oxidation of volatile organic compounds over manganese-based catalysts: Recent trends and challenges. J. Environ. Chem. Eng. 2022, 10, 108638. [Google Scholar] [CrossRef]
- Xie, W.; Song, W.; Li, J.; Zhang, X.; Dong, W.; Sun, F. Micro-polluted water resources treatment by PVDF-TiO2 membrane combined with Fe2+/sodium dithionite (DTN)/O2 pre-oxidation process. Chemosphere 2023, 311 Pt 1, 136998. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Chen, J.; Luo, Y.; Sun, B.; Chu, J. Performance and microbial analysis of a biotrickling filter inoculated by a specific bacteria consortium for removal of a simulated mixture of pharmaceutical volatile organic compounds. Chem. Eng. J. 2016, 304, 757–765. [Google Scholar] [CrossRef]
- Chen, L.; Zhai, Y.; van der Mark, E.; Liu, G.; van der Meer, W.; Medema, g. Microbial community assembly and metabolic function in top layers of slow sand filters for drinking water production. J. Clean. Prod. 2021, 294, 126342. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Li, N. Application of bio-slow sand filters for drinking water production: Linking purification performance to bacterial community and metabolic functions. J. Water Process Eng. 2023, 53, 103622. [Google Scholar] [CrossRef]
- Greenstein, K.; Lew, J.; Dickenson, E.; Wert, E. Investigation of biotransformation, sorption, and desorption of multiple chemical contaminants in pilot-scale drinking water biofilters. Chemosphere 2018, 200, 248–256. [Google Scholar] [CrossRef]
- Babaei, F.; Ehrampoush, M.; Eslami, H.; Ghaneian, M.; Fallahzadeh, H.; Talebi, P.; Fard, R.; Ebrahimi, A. Removal of linear alkylbenzene sulfonate and turbidity from greywater by a hybrid multi-layer slow sand filter microfiltration ultrafiltration system. J. Clean. Prod. 2019, 211, 922–931. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Liu, C.; Wang, S.; Wang, X.; Hou, H.; Wang, J.; Li, H. Purification of harvested rainwater using slow sand filters with low-cost materials: Bacterial community structure and purifying effect. Sci. Total Environ. 2019, 674, 344–354. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, G.; Pan, Z.; Zhou, X.; Lai, W.; Zheng, L.; Xu, Y. A novel Pb/PbO2 electrodes prepared by the method of thermal oxidation-electrochemical oxidation: Characteristic and electrocatalytic oxidation performance. J. Alloys Compd. 2020, 851, 156834. [Google Scholar] [CrossRef]
- Feng, L.; Li, X.; Gan, L.; Xu, J. Synergistic effects of electricity and biofilm on Rhodamine B (RhB) degradation in three-dimensional biofilm electrode reactors (3D-BERs). Electrochim. Acta 2018, 290, 165–175. [Google Scholar] [CrossRef]
- Zeyoudi, M.; Altenaiji, E.; Ozer, L.Y.; Ahmed, I.; Yousef, A.F.; Hasan, S.W. Impact of continuous and intermittent supply of electric field on the function and microbial community of wastewater treatment electro-bioreactors. Electrochim. Acta 2015, 181, 271–279. [Google Scholar] [CrossRef]
- Feng, Y.; Long, Y.; Wang, Z.; Wang, X.; Shi, N.; Suo, N.; Shi, Y.; Yu, Y. Performance and microbial community of an electric biological integration reactor (EBIR) for treatment of wastewater containing ibuprofen. Bioresour. Technol. 2019, 274, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Juan, X.; Wu, L.; Ni, B.J. Three-dimensional biofilm electrode reactors (3D-BERs) for wastewater treatment. Bioresour. Technol. 2022, 344 Pt B, 126274. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, W.; Chi, M. Enhancement on the simultaneous removal of nitrate and organic pollutants from groundwater by a three-dimensional bio-electrochemical reactor. Bioresour. Technol. 2009, 100, 4662–4668. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, G.; Yang, X.; Wu, F.; Liu, J. Application of rough and slow filtration techniques to treatment of water in rainwater harvesting pits. China Water Wastewater 2013, 29, 47–51. [Google Scholar] [CrossRef]
- Li, S.; Su, C.; Zhang, J. Experimental study on different biofilm formation methods in biological sand filter. China Water Wastewater 2007, 23, 60–63. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Jerry, A.L.; Booksh, K. Fluorescence excitation−emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.; Li, Y.; Hu, Z.; Zhou, L.; Zhou, M. Three-dimensional electrochemical process for wastewater treatment: A general review. Chem. Eng. J. 2013, 228, 455–467. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Liu, Y.; Wang, R.; Yang, Y.; Chen, J. A critical review of research progress for metal alloy materials in hydrogen evolution and oxygen evolution reaction. Environ. Sci. Pollut. Res. 2023, 30, 11302–11320. [Google Scholar] [CrossRef]
- Rahimi, Y.; Torabian, A.; Mehrdadi, N.; Habibi-Rezaie, M.; Pezeshk, H.; Nabi-Bidhendi, G.R. Optimizing aeration rates for minimizing membrane fouling and its effect on sludge characteristics in a moving bed membrane bioreactor. J. Hazard. Mater. 2011, 186, 1097–1102. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Li, Y.Y.; Liu, Y.; Zhao, Y. Influence of zero valent scrap iron (ZVSI) supply on methane production from waste activated sludge. Chem. Eng. J. 2015, 263, 461–470. [Google Scholar] [CrossRef]
- Dong, H.; Yue, L.; Cheng, J.; Xia, R.; Zhou, J. Microbial electrochemical degradation of lipids for promoting methane production in anaerobic digestion. Bioresour. Technol. 2022, 345, 126467. [Google Scholar] [CrossRef] [PubMed]
- Wei, V.; Oleszkiewicz, J.A.; Elektorowicz, M. Nutrient removal in an electrically enhanced membrane bioreactor. Water Sci. Technol. 2009, 60, 3159–3163. [Google Scholar] [CrossRef]
- Wang, B.; Chen, X.; Xu, Y.; Zhang, Z.; Zhang, Y. Three-Dimensional Biofilm Electrode Reactors with Polyurethane Sponge Carrier for Highly Efficient Treatment of Pharmaceuticals Wastewater Containing Tetrahydrofuran. Water 2022, 14, 3792. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; Pei, L.; Zhu, X.; Zhu, S.; Liu, Y.; Ye, Z. Synergistic removal of ammonia nitrogen by UV photo-electrocatalytic process: Heterogeneous reaction pathways and mechanism. J. Clean. Prod. 2023, 384, 135515. [Google Scholar] [CrossRef]
- Raju, G.B.; Karuppiah, M.T.; Latha, S.S.; Parvathy, S.; Prabhakar, S. Treatment of wastewater from synthetic textile industry by electrocoagulation–electrooxidation. Chem. Eng. J. 2008, 144, 51–58. [Google Scholar] [CrossRef]
- Peng, S.; He, X.; Pan, H. Spectroscopic study on transformations of dissolved organic matter in coal-to-liquids wastewater under integrated chemical oxidation and biological treatment process. J. Environ. Sci.-China 2018, 70, 206–216. [Google Scholar] [CrossRef]
- Cheng, Q.; Zheng, B.; Wang, S.; Jiao, X.; Huang, M. Optical signatures of chromophoric dissolved organic matter in water body of Tien Lake. Spectrosc. Spect. Anal. 2014, 34, 698–703. [Google Scholar] [CrossRef]
- Shen, J.; Liu, B.; Wu, J.; Chai, Y.; Cheng, C.; Liu, C.; Yan, R.; Saleem Khan, M.F. Characterization of fluorescent dissolved organic matters in metalworking fluid by fluorescence excitation-emission matrix and high-performance liquid chromatography. Chemosphere 2020, 239, 124703. [Google Scholar] [CrossRef]
- Guo, W.; Xu, J.; Wang, J.; Wen, Y.; Zhuo, J.; Yan, Y. Characterization of dissolved organic matter in urban sewage using excitation emission matrix fluorescence spectroscopy and parallel factor analysis. J. Environ. Sci.-China 2010, 22, 1728–1734. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; An, Q.; Wang, X.; Sun, S.; Li, X.; Zheng, N. Analysis on Diversity and Structure of Microbial Community in River Sediment of Siping Section of Liaohe River. Environ. Sci. 2022, 43, 2586–2594. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Li, Q.; Zhao, C.; Feng, Y.; Li, L. Analysis of microbial diversity and driving factors in coastal wetlands of the Yellow River Delta. Acta Ecol. Sin. 2021, 41, 6103–6114. [Google Scholar] [CrossRef]
- Hashimoto, K.; Matsuda, M.; Inoue, D.; Ike, M. Bacterial community dynamics in a full-scale municipal wastewater treatment plant employing conventional activated sludge process. J. Biosci. Bioeng. 2014, 118, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Liu, M.; Wang, H.; Fan, Y.; Zhang, H.; Liu, J.; Xing, E.; Xu, X.; Li, H. The distribution of active beta-glucosidase producing microbial communities in composting. Can. J. Microbiol. 2017, 63, 998–1008. [Google Scholar] [CrossRef] [Green Version]
- Speirs, L.B.M.; Rice, D.T.F.; Petrovski, S.; Seviour, R.J. The Phylogeny, Biodiversity, and Ecology of the Chloroflexi in Activated Sludge. Front. Microbiol. 2019, 10, 2015. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Peng, Y.; Guo, Y.; Zhao, M.; Wang, S. Illumina MiSeq sequencing reveals the key microorganisms involved in partial nitritation followed by simultaneous sludge fermentation, denitrification and anammox process. Bioresour. Technol. 2016, 207, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.S.; Sundaravadivelu, D.; Techtmann, S.; Conmy, R.N.; Santo Domingo, J.W.; Campo, P. Microbial degradation of Cold Lake Blend and Western Canadian select dilbits by freshwater enrichments. J. Hazard. Mater. 2018, 352, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qin, H.; Sun, Q.; Wang, B.; Gao, R.; Guo, R.; Li, W. Microbial Diversity and Influencing Factors in a Small Watershed in Winter. Environ. Sci. 2020, 41, 5016–5026. [Google Scholar] [CrossRef]
| Parameter | Turbidity /NTU | Temperature/°C | pH | Conductivity /µS·cm−1 | DO /mg·L−1 | NH4+-N /mg·L−1 | UV254 | CODMn /mg·L−1 |
|---|---|---|---|---|---|---|---|---|
| Simulated water quality | ~10–50 | ~1–20 | ~8.2–8.6 | ~250–350 | ~5–8 | ~0.07–0.87 | ~0.050–0.110 | ~2.5–10 |
| Water quality | ~9–25 | ~8–15 | ~8.4–8.6 | ~350–420 | ~5.8–7.8 | ~0.28–0.65 | ~0.055–0.095 | ~3.2–5.5 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Yang, X.; Chen, X.; Tan, L.; Wang, G. Three-Dimensional Biofilm Electrode Reactors with a Triple-Layer Particle Electrode for Highly Efficient Treatment of Micro-Polluted Water Sources. Water 2023, 15, 1833. https://doi.org/10.3390/w15101833
Wang B, Yang X, Chen X, Tan L, Wang G. Three-Dimensional Biofilm Electrode Reactors with a Triple-Layer Particle Electrode for Highly Efficient Treatment of Micro-Polluted Water Sources. Water. 2023; 15(10):1833. https://doi.org/10.3390/w15101833
Chicago/Turabian StyleWang, Baoshan, Xiuxiu Yang, Xiaojie Chen, Lei Tan, and Guangzong Wang. 2023. "Three-Dimensional Biofilm Electrode Reactors with a Triple-Layer Particle Electrode for Highly Efficient Treatment of Micro-Polluted Water Sources" Water 15, no. 10: 1833. https://doi.org/10.3390/w15101833
APA StyleWang, B., Yang, X., Chen, X., Tan, L., & Wang, G. (2023). Three-Dimensional Biofilm Electrode Reactors with a Triple-Layer Particle Electrode for Highly Efficient Treatment of Micro-Polluted Water Sources. Water, 15(10), 1833. https://doi.org/10.3390/w15101833






