Removal of Cefixime from Wastewater Using a Superb nZVI/Copper Slag Nanocomposite: Optimization and Characterization
Abstract
:1. Introduction
2. Experimental
2.1. Materials
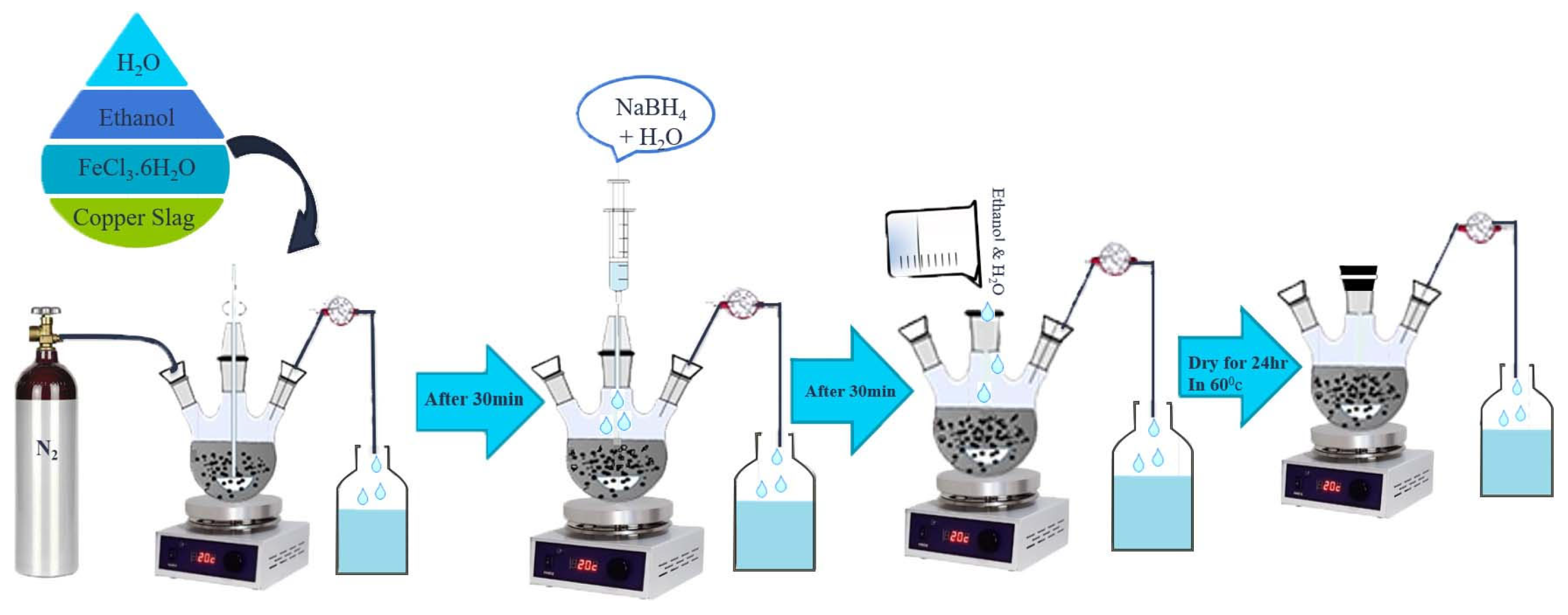
2.2. Preparation of nZVI/CS
2.3. Characterization of Nanocomposite
2.4. Experimental Design and Statistical Analysis of Cefixime Elimination
2.5. Adsorption Experiments
3. Results and Discussion
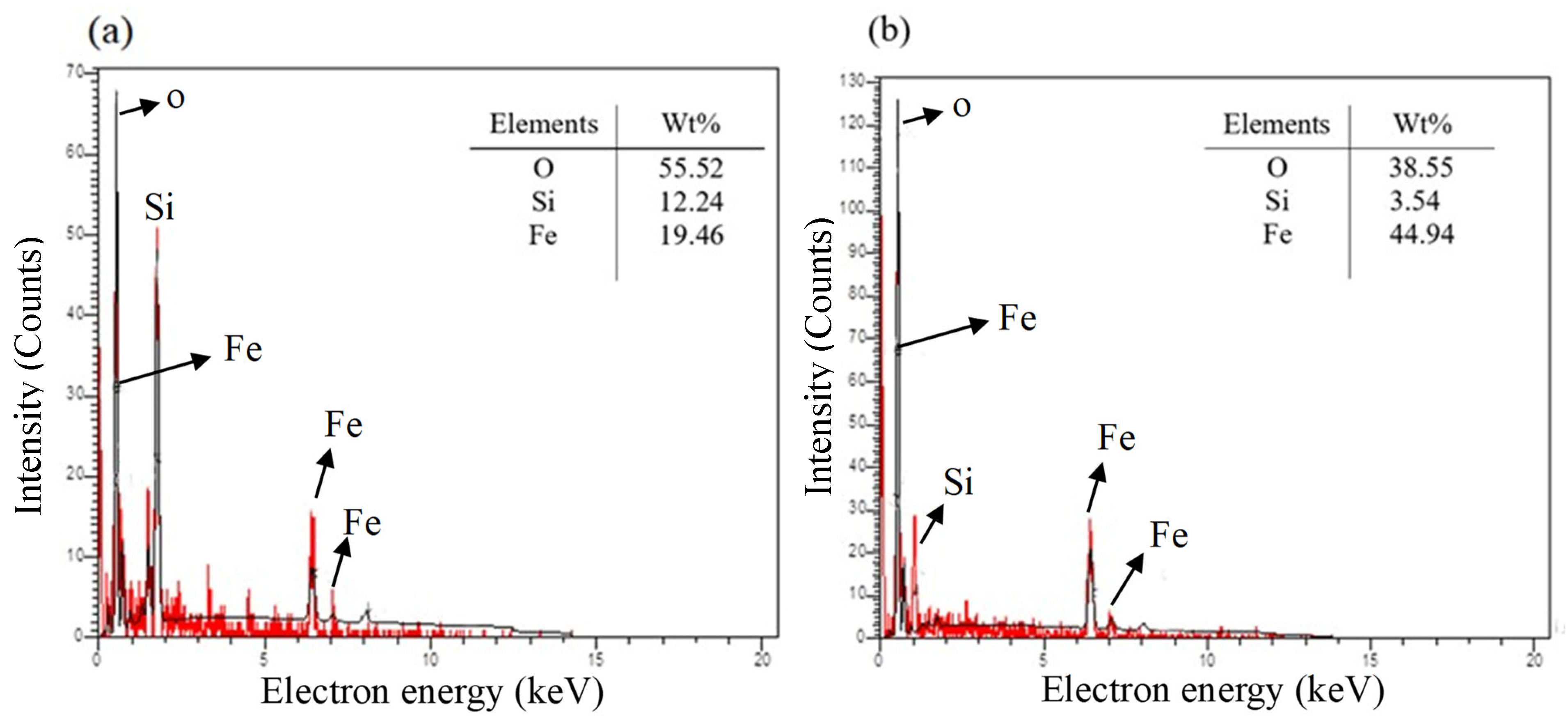
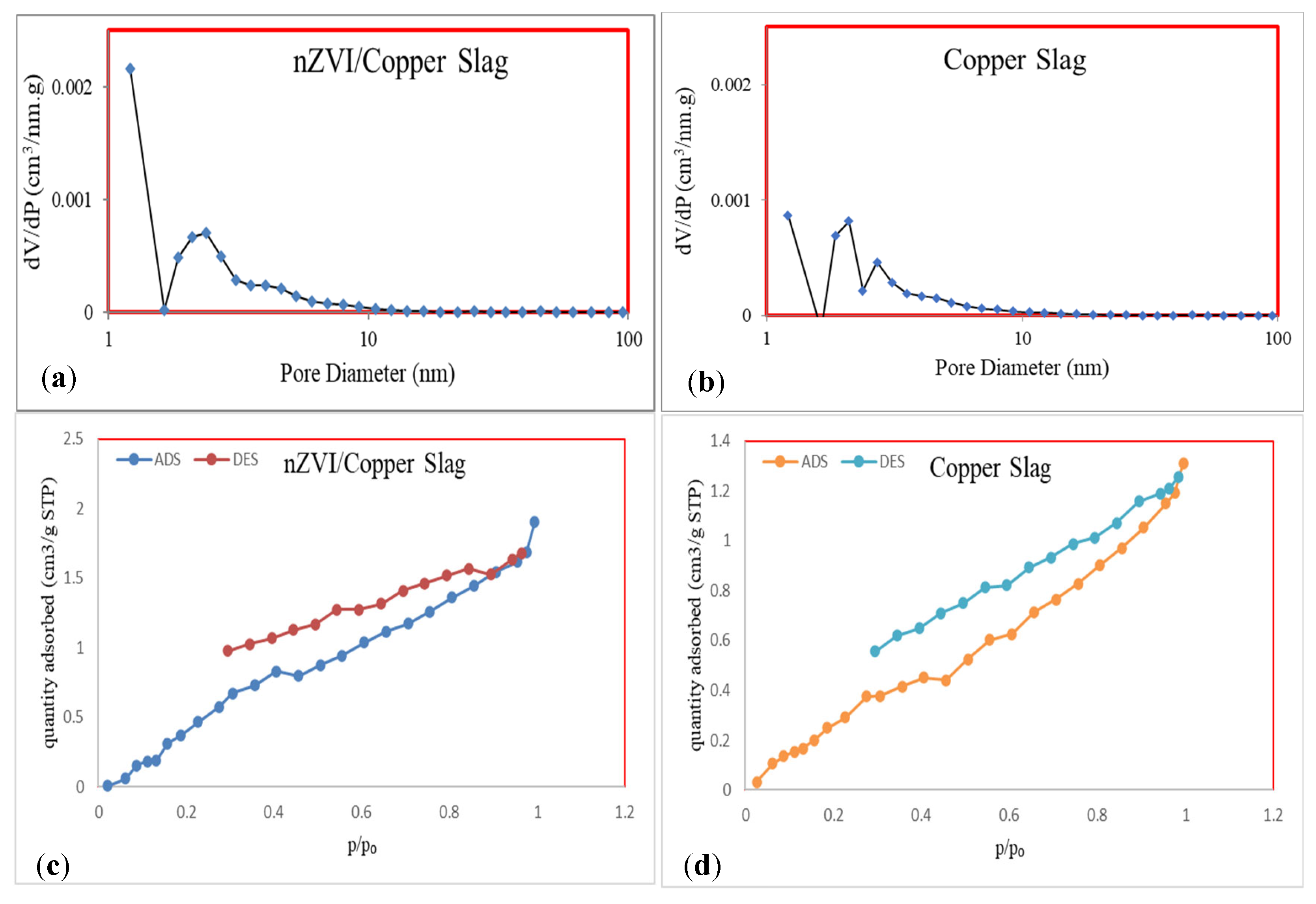
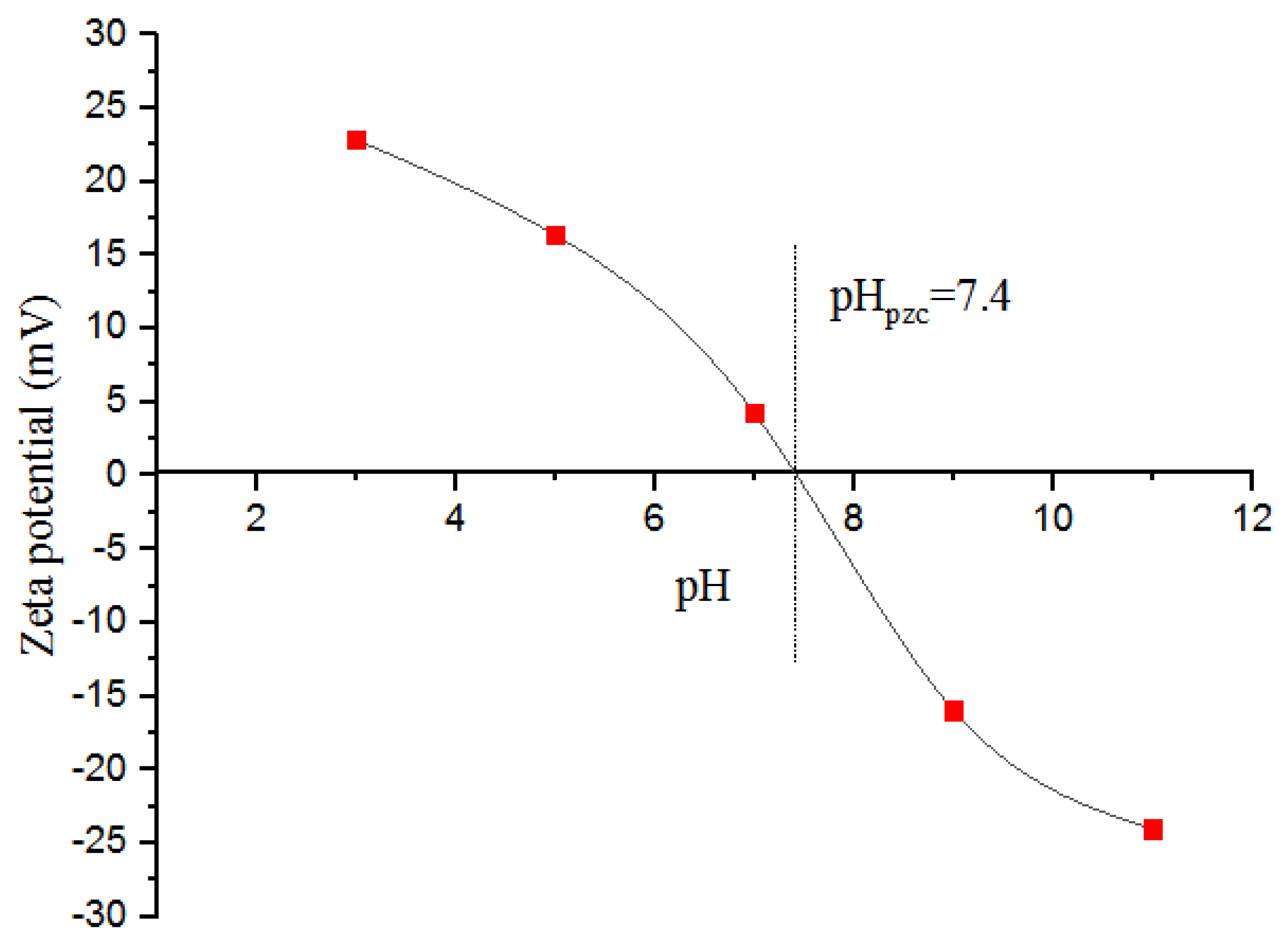
3.1. Characterization
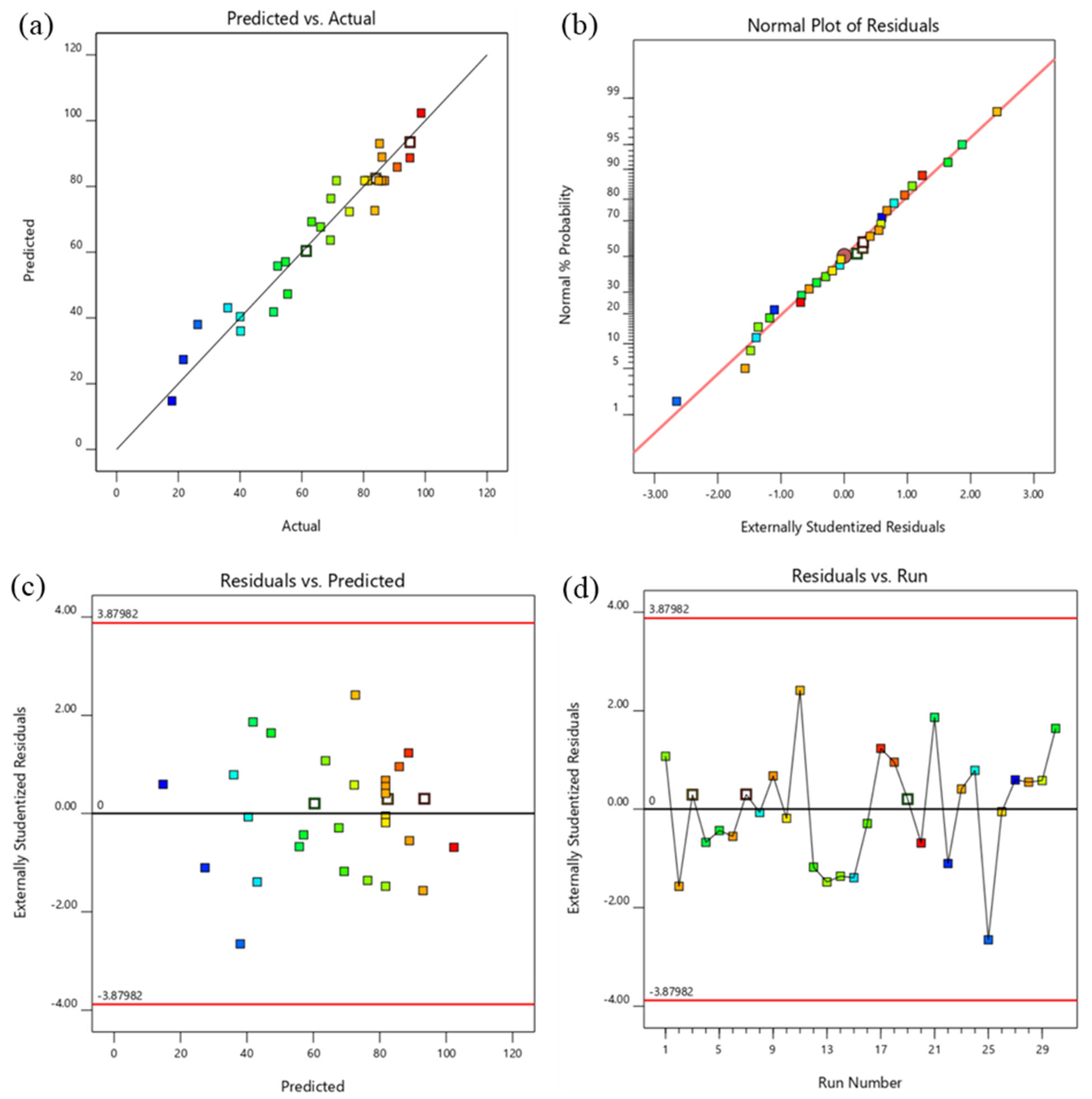
3.2. Statistical Analysis and Investigation of Experimental Data
3.3. Effect of Effective Variables on CFX Elimination
3.3.1. Examining the Effect of CFX Concentration on the Elimination Yield
3.3.2. Impact of Adsorbent Dosage on the Performance of CFX Elimination
3.3.3. Examining the Impact of the pH of the Solution on the CFX Elimination Percentage
3.3.4. Effect of Contact Time on CFX Removal Using NZVI/CS Nanocomposite
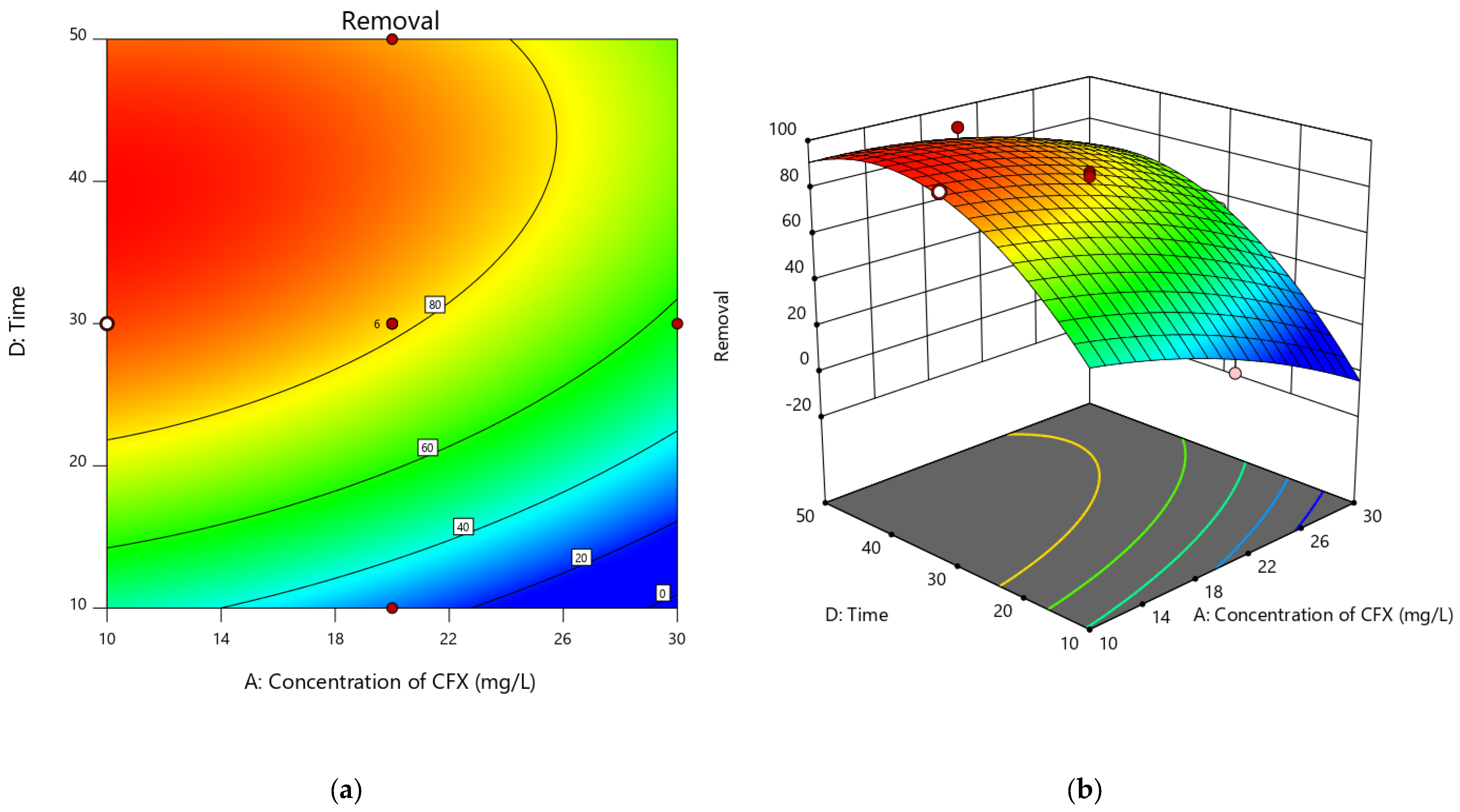
3.4. Interactive Influence of Process Factors
3.4.1. Adsorbent Dosage and CFX Concentration Impact on the Adsorption Efficiency
3.4.2. pH and CFX Concentration Impact on the Adsorption Efficiency
3.4.3. Contact Time and CFX Concentration Impact on the Adsorption Efficiency
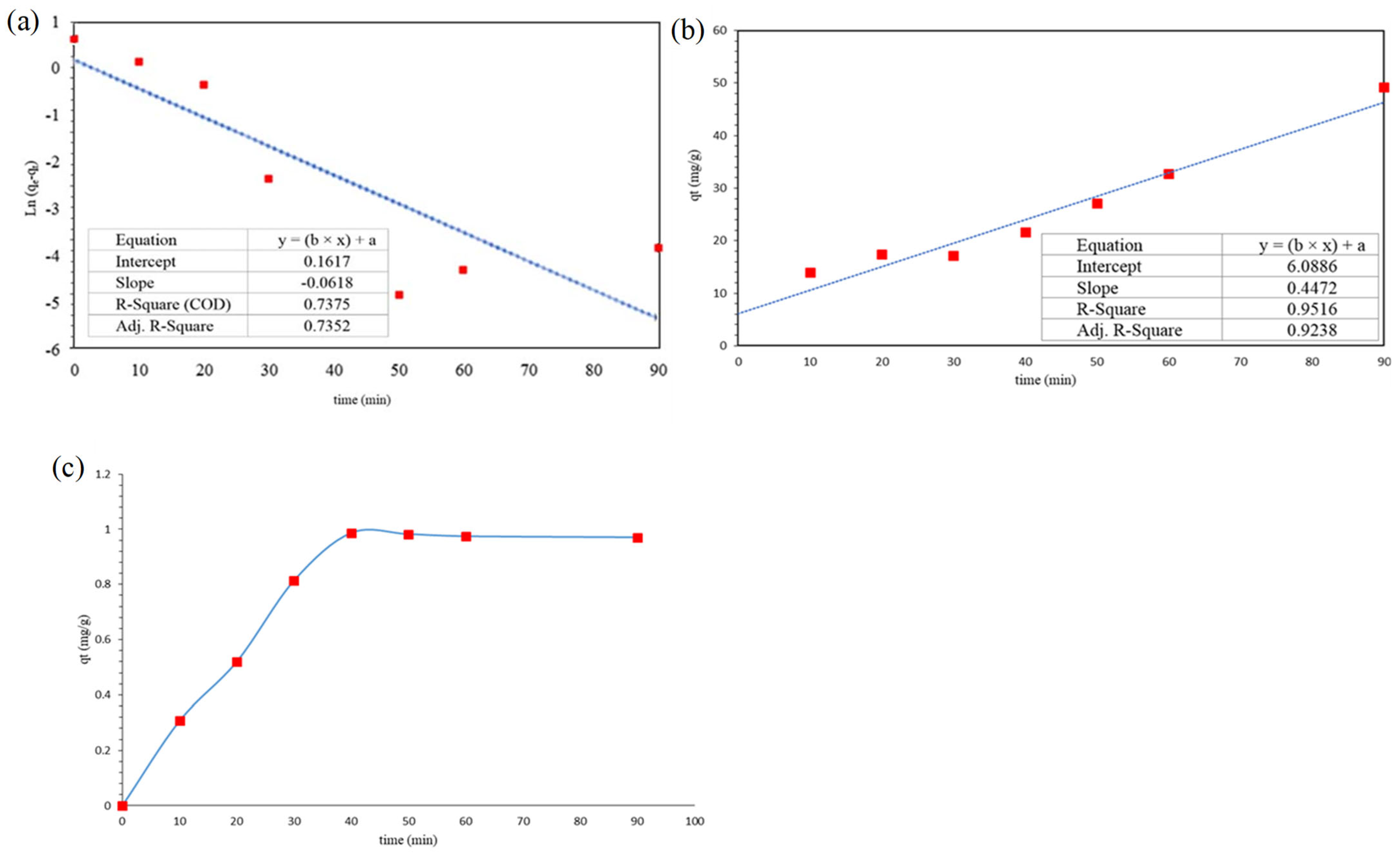
3.5. Adsorption Kinetic
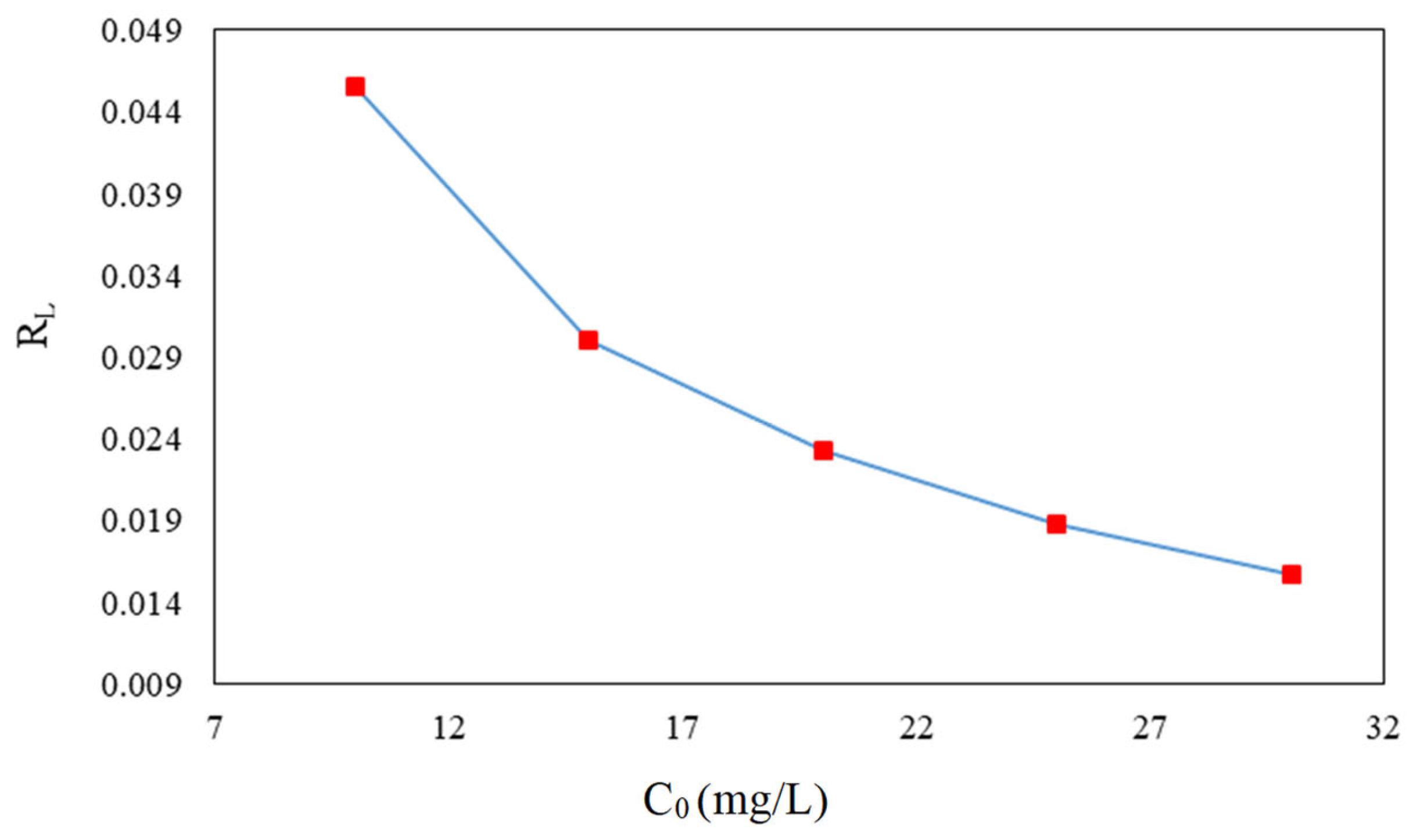
3.6. Adsorption Isotherms of CFX
3.7. Reusability and Regeneration of nZVI/CS Adsorbent
3.8. Comparison of the Removal Performance with the Previous Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fereidooni, M.; Esmaeilzadeh, F.; Zandifar, A. Innovatively-synthesized CeO2/ZnO photocatalysts by sono-photochemical deposition: Catalyst characterization and effect of operational parameters on high efficient dye removal. J. Mater. Sci. 2022, 57, 16228–16244. [Google Scholar] [CrossRef]
- Liang, J.; Lin, H.; Singh, B.; Wang, A.; Yan, Z. A global perspective on compositions, risks, and ecological genesis of antibiotic resistance genes in biofilters of drinking water treatment plants. Water Res. 2023, 233, 119822. [Google Scholar] [CrossRef] [PubMed]
- Madhav, S.; Ahamad, A.; Singh, A.K.; Kushawaha, J.; Chauhan, J.S.; Sharma, S.; Singh, P. Water Pollutants: Sources and Impact on the Environment and Human Health. In Sensors in Water Pollutants Monitoring: Role of Material; Pooja, D., Kumar, P., Singh, P., Patil, S., Eds.; Springer: Singapore, 2020; pp. 43–62. [Google Scholar]
- Benrighi, Y.; Nasrallah, N.; Chaabane, T.; Sivasankar, V.; Darchen, A.; Baaloudj, O. Photocatalytic performances of ZnCr2O4 nanoparticles for cephalosporins removal: Structural, optical and electrochemical properties. Opt. Mater. 2021, 115, 111035. [Google Scholar] [CrossRef]
- Mirbagheri, N.S.; Sabbaghi, S. A Ti-doped γ-Fe2O3/SDS nano-photocatalyst as an efficient adsorbent for removal of methylene blue from aqueous solutions. J. Environ. Manag. 2018, 213, 56–65. [Google Scholar] [CrossRef]
- Gonbadi, M.; Sabbaghi, S.; Saboori, R.; Derakhshandeh, A.; Narimani, M.; Fatemi, A. Sulfide adsorption by “green synthesized Fe3O4@ ZnO core/shell” nanoparticles from aqueous solution and industrial rich amine solution: Kinetic and equilibrium study. Int. J. Environ. Sci. Technol. 2023, 20, 1–20. [Google Scholar] [CrossRef]
- Asadi-Ghalhari, M.; Mostafaloo, R.; Ghafouri, N.; Kishipour, A.; Usefi, S.; Baaloudj, O. Removal of Cefixime from aqueous solutions via proxy electrocoagulation: Modeling and optimization by response surface methodology. React. Kinet. Mech. Catal. 2021, 134, 459–471. [Google Scholar] [CrossRef]
- Kafaei, R.; Papari, F.; Seyedabadi, M.; Sahebi, S.; Tahmasebi, R.; Ahmadi, M.; Sorial, G.A.; Asgari, G.; Ramavandi, B. Occurrence, distribution, and potential sources of antibiotics pollution in the water-sediment of the northern coastline of the Persian Gulf, Iran. Sci. Total Environ. 2018, 627, 703–712. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, R.; Wang, P.; Mai-Prochnow, A.; McConchie, R.; Li, W.; Zhou, R.; Thompson, E.W.; Ostrikov, K.; Cullen, P.J. Degradation of cefixime antibiotic in water by atmospheric plasma bubbles: Performance, degradation pathways and toxicity evaluation. Chem. Eng. J. 2021, 421, 127730. [Google Scholar] [CrossRef]
- Patel, S.; Sharma, J.; Gole, V.L. Removal of antibiotic cefixime from wastewater using UVC/Sodium persulphate system. Mater. Today Proc. 2023, 78, 69–73. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Dragoi, E.-N.; Almomani, F.; Le, V.T. Graphene-based materials for metronidazole degradation: A comprehensive review. Chemosphere 2022, 286, 131727. [Google Scholar] [CrossRef]
- Rasouli, K.; Alamdari, A.; Sabbaghi, S. Ultrasonic-assisted synthesis of α-Fe2O3@TiO2 photocatalyst: Optimization of effective factors in the fabrication of photocatalyst and removal of non-biodegradable cefixime via response surface methodology-central composite design. Sep. Purif. Technol. 2023, 307, 122799. [Google Scholar] [CrossRef]
- Mohammadi, M.; Sabbaghi, S.; Sadeghi, H.; Zerafat, M.; Pooladi, R. Preparation and characterization of TiO2/ZnO/CuO nanocomposite and application for phenol removal from wastewaters. Desalination Water Treat. 2016, 57, 799–809. [Google Scholar] [CrossRef]
- Mirzaei, M.; Sabbaghi, S.; Zerafat, M.M. Photo-catalytic degradation of formaldehyde using nitrogen-doped TiO2 nano-photocatalyst: Statistical design with response surface methodology (RSM). Can. J. Chem. Eng. 2018, 96, 2544–2552. [Google Scholar] [CrossRef]
- Khan, M.A.; Raza, N.; Manzoor, S.; Shuja, R.; Raza, H.; Khan, M.I.; Azam, M.; Shanableh, A. Experimental design by response surface methodology for efficient cefixime uptake from hospital effluents using anion exchange membrane. Chemosphere 2023, 311, 137103. [Google Scholar] [CrossRef]
- Malekshahi, M.; Sabbaghi, S.; Rasouli, K. Preparation of α-alumina/γ-alumina/γ-alumina-titania ceramic composite membrane for chloride ion removal. Mater. Chem. Phys. 2022, 287, 126218. [Google Scholar] [CrossRef]
- Ghaee, A.; Zerafat, M.; Askari, P.; Sabbaghi, S.; Sadatnia, B. Fabrication of polyamide thin-film nanocomposite membranes with enhanced surface charge for nitrate ion removal from water resources. Environ. Technol. 2017, 38, 772–781. [Google Scholar] [CrossRef]
- Nabizad, M.; Dadvand Koohi, A.; Erfanipour, Z. Removal of Cefixime Using Heterogeneous Fenton Catalysts: Alginate/Magnetite Hydroxyapatite Nanocomposite. J. Water Environ. Nanotechnol. 2022, 7, 14–30. [Google Scholar]
- Sereshti, H.; Beyrak-Abadi, E.; Esmaeili Bidhendi, M.; Ahmad, I.; Shahabuddin, S.; Rashidi Nodeh, H.; Sridewi, N.; Wan Ibrahim, W.N. Sulfide-Doped Magnetic Carbon Nanotubes Developed as Adsorbent for Uptake of Tetracycline and Cefixime from Wastewater. Nanomaterials 2022, 12, 3576. [Google Scholar] [CrossRef]
- Hashemi, S.F.; Sabbaghi, S.; Saboori, R.; Zarenezhad, B. Photocatalytic degradation of ammonia with titania nanoparticles under UV light irradiation. Environ. Sci. Pollut. Res. 2022, 29, 68600–68614. [Google Scholar] [CrossRef]
- Emami, N.; Farhadian, M.; Solaimany Nazar, A.R.; Tangestaninejad, S. Adsorption of cefixime and lamotrigine on HKUST-1/ZIF-8 nanocomposite: Isotherms, kinetics models and mechanism. Int. J. Environ. Sci. Technol. 2022, 20, 1645–1672. [Google Scholar] [CrossRef]
- Arizavi, A.; Mirbagheri, N.; Hosseini, Z.; Chen, P.; Sabbaghi, S. Efficient removal of naphthalene from aqueous solutions using a nanoporous kaolin/Fe3O4 composite. Int. J. Environ. Sci. Technol. 2020, 17, 1991–2002. [Google Scholar] [CrossRef]
- Salehia, F.; Sabbaghia, S.; Mirbagherib, N.S. Modification of graphitic carbon nitride photocatalyst by Pb-contaminated water for efficient removal of cefixime from aqueous media. Desalination Water Treat. 2021, 229, 331–342. [Google Scholar] [CrossRef]
- Moradi, H.; Sabbaghi, S.; Mirbagheri, N.S.; Chen, P.; Rasouli, K.; Kamyab, H.; Chelliapan, S. Removal of chloride ion from drinking water using Ag NPs-Modified bentonite: Characterization and optimization of effective parameters by response surface methodology-central composite design. Environ. Res. 2023, 223, 115484. [Google Scholar] [CrossRef] [PubMed]
- Mehrdoost, A.; Jalilzadeh Yengejeh, R.; Mohammadi, M.K.; Babaei, A.A.; Haghighatzadeh, A. Comparative analysis of UV-assisted removal of azithromycin and cefixime from aqueous solution using PAC/Fe/Si/Zn nanocomposite. J. Health Sci. Surveill. Syst. 2021, 9, 39–49. [Google Scholar]
- Truong, T.T.T.; Vu, T.N.; Dinh, T.D.; Pham, T.T.; Nguyen, T.A.H.; Nguyen, M.H.; Nguyen, T.D.; Yusa, S.-i.; Pham, T.D. Adsorptive removal of cefixime using a novel adsorbent based on synthesized polycation coated nanosilica rice husk. Prog. Org. Coat. 2021, 158, 106361. [Google Scholar] [CrossRef]
- Ciğeroğlu, Z.; Küçükyıldız, G.; Erim, B.; Alp, E. Easy preparation of magnetic nanoparticles-rGO-chitosan composite beads: Optimization study on cefixime removal based on RSM and ANN by using Genetic Algorithm Approach. J. Mol. Struct. 2021, 1224, 129182. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saini, V.K.; Jain, N. Adsorption of As(III) from aqueous solutions by iron oxide-coated sand. J. Colloid Interface Sci. 2005, 288, 55–60. [Google Scholar] [CrossRef]
- Mirhosseininia, J.; Sabbaghi, S.; Mirbagheri, N.S.; Zerafat, M.M. Treatment of As-contaminated drinking water using a nano zero-valent iron/copper slag nanocomposite. J. Water Process Eng. 2022, 49, 103011. [Google Scholar] [CrossRef]
- Bhowmick, S.; Chakraborty, S.; Mondal, P.; Van Renterghem, W.; Van den Berghe, S.; Roman-Ross, G.; Chatterjee, D.; Iglesias, M. Montmorillonite-supported nanoscale zero-valent iron for removal of arsenic from aqueous solution: Kinetics and mechanism. Chem. Eng. J. 2014, 243, 14–23. [Google Scholar] [CrossRef]
- Yadav, R.; Sharma, A.K.; Babu, J.N. Sorptive removal of arsenite [As(III)] and arsenate [As(V)] by fuller’s earth immobilized nanoscale zero-valent iron nanoparticles (F-nZVI): Effect of Fe0 loading on adsorption activity. J. Environ. Chem. Eng. 2016, 4, 681–694. [Google Scholar] [CrossRef]
- Pojananukij, N.; Wantala, K.; Neramittagapong, S.; Lin, C.; Tanangteerpong, D.; Neramittagapong, A. Improvement of As (III) removal with diatomite overlay nanoscale zero-valent iron (nZVI-D): Adsorption isotherm and adsorption kinetic studies. Water Sci. Technol.: Water Supply 2017, 17, 212–220. [Google Scholar] [CrossRef]
- Awang, N.A.; Wan Salleh, W.N.; Aziz, F.; Yusof, N.; Ismail, A.F. A review on preparation, surface enhancement and adsorption mechanism of biochar-supported nano zero-valent iron adsorbent for hazardous heavy metals. J. Chem. Technol. Biotechnol. 2022, 98, 22–44. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Z.; Wang, Z.; Wang, Z.-L.; Bush, R. Highly efficient and rapid removal of arsenic (iii) from aqueous solutions by nanoscale zero-valent iron supported on a zirconium 1, 4-dicarboxybenzene metal–organic framework (uio-66 mof). RSC Adv. 2019, 9, 39475–39487. [Google Scholar] [CrossRef]
- Wu, Y.; Li, H.; Zhao, Z.; Yi, X.; Deng, D.; Zheng, L.; Luo, X.; Cai, Y.; Luo, W.; Zhang, M. Low-cost and high-efficiency metallurgical copper slag@polyaniline core–shell composite as an adsorbent for the removal of Cr(VI) from aqueous solution. J. Alloys Compd. 2021, 851, 156741. [Google Scholar] [CrossRef]
- Mithun, B.; Narasimhan, M. Performance of alkali activated slag concrete mixes incorporating copper slag as fine aggregate. J. Clean. Prod. 2016, 112, 837–844. [Google Scholar] [CrossRef]
- Zeynolabedin, R.; Mahanpoor, K. Preparation and characterization of nano-spherical CoFe2O4 supported on copper slag as a catalyst for photocatalytic degradation of 2-nitrophenol in water. J. Nanostructure Chem. 2017, 7, 67–74. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, C.; Shen, H.; Du, J.; Li, Q.; Wu, W.; Guo, B.; Liu, G. Green synthesis of ceramsite from industrial wastes and its application in selective adsorption: Performance and mechanism. Environ. Res. 2022, 214, 113786. [Google Scholar] [CrossRef]
- Ji, R.; Liu, T.-J.; Kang, L.-L.; Wang, Y.-T.; Li, J.-G.; Wang, F.-P.; Yu, Q.; Wang, X.-M.; Liu, H.; Guo, H.-W. A review of metallurgical slag for efficient wastewater treatment: Pretreatment, performance and mechanism. J. Clean. Prod. 2022, 380, 135076. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When I’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Türk, T.; Alp, I.; Sezer, R.; Arslan, C. Removal of arsenic from water using copper slag. J. S. Afr. Inst. Min. Metall. 2020, 120, 313–318. [Google Scholar] [CrossRef]
- Kanel, S.R.; Choi, H.; Kim, J.-Y.; Vigneswaran, S.; Shim, W.G. Removal of arsenic (III) from groundwater using low-cost industrial by-products-blast furnace slag. Water Qual. Res. J. 2006, 41, 130–139. [Google Scholar] [CrossRef]
- Glocheux, Y.; Pasarín, M.M.; Albadarin, A.B.; Allen, S.J.; Walker, G.M. Removal of arsenic from groundwater by adsorption onto an acidified laterite by-product. Chem. Eng. J. 2013, 228, 565–574. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, W.; Ma, G. Mechanical properties of copper slag reinforced concrete under dynamic compression. Constr. Build. Mater. 2010, 24, 910–917. [Google Scholar] [CrossRef]
- Phiri, T.C.; Singh, P.; Nikoloski, A.N. The potential for copper slag waste as a resource for a circular economy: A review—Part I. Miner. Eng. 2022, 180, 107474. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, S.; Lu, X.-Q.; Chen, Z.-l. Removal of Pb(II) from water using synthesized kaolin supported nanoscale zero-valent iron. Chem. Eng. J. 2010, 163, 243–248. [Google Scholar] [CrossRef]
- Kanel, S.R.; Manning, B.; Charlet, L.; Choi, H. Removal of Arsenic(III) from Groundwater by Nanoscale Zero-Valent Iron. Environ. Sci. Technol. 2005, 39, 1291–1298. [Google Scholar] [CrossRef]
- Wu, C.; Tu, J.; Liu, W.; Zhang, J.; Chu, S.; Lu, G.; Lin, Z.; Dang, Z. The double influence mechanism of pH on arsenic removal by nano zero valent iron: Electrostatic interactions and the corrosion of Fe0. Environ. Sci. Nano 2017, 4, 1544–1552. [Google Scholar] [CrossRef]
- Rao, W.; Lv, G.; Wang, D.; Liao, L. Enhanced degradation of Rh 6G by zero valent iron loaded on two typical clay minerals with different structures under microwave irradiation. Front. Chem. 2018, 6, 463. [Google Scholar] [CrossRef]
- Zahedany, F.A.; Sabbaghi, S.; Saboori, R.; Rasouli, K. Investigation of the synergistic effect of TiO2 nanofluid and biomaterials derived from three bacteria in various culture media: Implications for enhanced oil recovery. Chem. Eng. Res. Des. 2022, 186, 50–63. [Google Scholar] [CrossRef]
- Sadeghalvaad, M.; Reza Razavi, S.; Sabbaghi, S.; Rasouli, K. Heating performance of a large-scale line heater by adding synthesized carbon- nanodots to the heater bath fluid: CFD simulation and experimental study. Adv. Powder Technol. 2023, 34, 103960. [Google Scholar] [CrossRef]
- Tandon, P.K.; Shukla, R.C.; Singh, S.B. Removal of Arsenic(III) from Water with Clay-Supported Zerovalent Iron Nanoparticles Synthesized with the Help of Tea Liquor. Ind. Eng. Chem. Res. 2013, 52, 10052–10058. [Google Scholar] [CrossRef]
- Hamdy, A. Experimental Study of the Relationship Between Dissolved Iron, Turbidity, and Removal of Cu(II) Ion From Aqueous Solutions Using Zero-Valent Iron Nanoparticles. Arab. J. Sci. Eng. 2021, 46, 5543–5565. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Khalaf Ali, M.M.; Saleh, M.M. Adsorption and removal of cationic and anionic surfactants using zero-valent iron nanoparticles. J. Mol. Liq. 2018, 268, 497–505. [Google Scholar] [CrossRef]
- Mantry, S.; Behera, D.; Satapathy, A.; Jha, B.B.; Mishra, B.K. Deposition of plasma sprayed copper slag coatings on metal substrates. Surf. Eng. 2013, 29, 222–227. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, J.; Wang, Y.; Jiang, B. Well-dispersed nanoscale zero-valent iron supported in macroporous silica foams: Synthesis, characterization, and performance in Cr (VI) removal. J. Mater. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Khasawneh, O.F.S.; Palaniandy, P.; Ahmadipour, M.; Mohammadi, H.; Bin Hamdan, M.R. Removal of acetaminophen using Fe2O3-TiO2 nanocomposites by photocatalysis under simulated solar irradiation: Optimization study. J. Environ. Chem. Eng. 2021, 9, 104921. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Ma, S.; Chen, M.; Tian, L.; Gou, G.; Chen, Y. A research on the removal characteristics of naphthol Green B from wastewater using nanomaterials nZVI/CS/APT. Phys. Chem. Liq. 2022, 60, 219–232. [Google Scholar] [CrossRef]
- Mansourian, R.; Mousavi, S.M.; Alizadeh, S.; Sabbaghi, S. CeO2/TiO2/SiO2 nanocatalyst for the photocatalytic and sonophotocatalytic degradation of chlorpyrifos. Can. J. Chem. Eng. 2022, 100, 451–464. [Google Scholar] [CrossRef]
- Tao, W.; Zhong, H.; Pan, X.; Wang, P.; Wang, H.; Huang, L. Removal of fluoride from wastewater solution using Ce-AlOOH with oxalic acid as modification. J. Hazard. Mater. 2020, 384, 121373. [Google Scholar] [CrossRef]
- Hasanzadeh, V.; Rahmanian, O.; Heidari, M. Cefixime adsorption onto activated carbon prepared by dry thermochemical activation of date fruit residues. Microchem. J. 2020, 152, 104261. [Google Scholar] [CrossRef]
- Al-husseiny, R.A.; Kareem, S.L.; Naje, A.S.; Ebrahim, S.E. Effect of green synthesis of Fe3O4 nanomaterial on the removal of cefixime from aqueous solution. Biomass Convers. Biorefin. 2023, 48, 1028. [Google Scholar] [CrossRef]
- Esmaeili Bidhendi, M.; Poursorkh, Z.; Sereshti, H.; Rashidi Nodeh, H.; Rezania, S.; Afzal Kamboh, M. Nano-size biomass derived from pomegranate peel for enhanced removal of cefixime antibiotic from aqueous media: Kinetic, equilibrium and thermodynamic study. Int. J. Environ. Res. Public Health 2020, 17, 4223. [Google Scholar] [CrossRef] [PubMed]





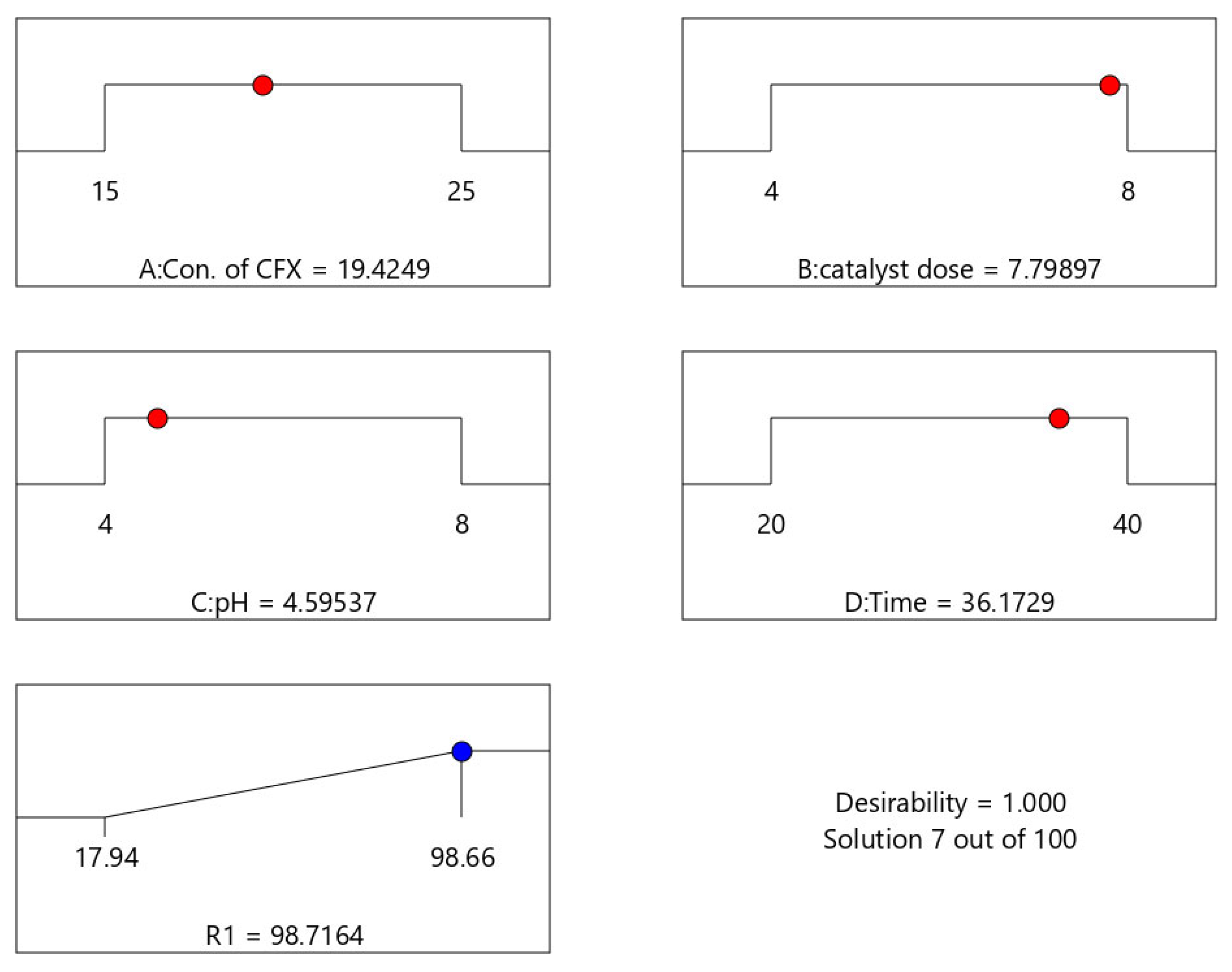

| Run No. | A | B | C | D |
|---|---|---|---|---|
| CFX Concentration (mg/L) | Adsorbent Dosage (g/L) | pH | Time (min) | |
| 1 | 15 | 8 | 8 | 20 |
| 2 | 25 | 8 | 4 | 40 |
| 3 | 15 | 4 | 4 | 40 |
| 4 | 15 | 4 | 4 | 20 |
| 5 | 30 | 6 | 6 | 30 |
| 6 | 15 | 8 | 8 | 40 |
| 7 | 10 | 6 | 6 | 30 |
| 8 | 25 | 8 | 8 | 20 |
| 9 | 20 | 6 | 6 | 30 |
| 10 | 20 | 6 | 6 | 30 |
| 11 | 20 | 6 | 2 | 30 |
| 12 | 25 | 4 | 4 | 40 |
| 13 | 20 | 6 | 6 | 30 |
| 14 | 15 | 8 | 4 | 20 |
| 15 | 20 | 2 | 6 | 30 |
| 16 | 15 | 4 | 8 | 40 |
| 17 | 20 | 10 | 6 | 30 |
| 18 | 20 | 6 | 6 | 50 |
| 19 | 25 | 8 | 4 | 20 |
| 20 | 15 | 8 | 4 | 40 |
| 21 | 15 | 4 | 8 | 20 |
| 22 | 20 | 6 | 6 | 10 |
| 23 | 20 | 6 | 6 | 30 |
| 24 | 25 | 4 | 4 | 20 |
| 25 | 20 | 6 | 10 | 30 |
| 26 | 20 | 6 | 6 | 30 |
| 27 | 25 | 4 | 8 | 20 |
| 28 | 20 | 6 | 6 | 30 |
| 29 | 25 | 8 | 8 | 40 |
| 30 | 25 | 4 | 8 | 40 |
| Parameter | BET Surface Area (m2/mg) | Langmuir Surface Area (m2/mg) | Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|---|
| CS | 1.8317 | 26.949 | 4.3225 | 0.0019 |
| CS-nZVI Absorbent | 3.3424 | 63.008 | 3.426 | 0.0028 |
| Run No. | CFX Concentration (mg/L) | Adsorbent Dosage (g/L) | pH | Time (min) | Removal of CFX (%) | Predicted Removal (%) |
|---|---|---|---|---|---|---|
| 1 | 15 | 8 | 8 | 20 | 69.27 | 63.67 |
| 2 | 25 | 8 | 4 | 40 | 85.19 | 93.02 |
| 3 | 15 | 4 | 4 | 40 | 83.97 | 82.38 |
| 4 | 15 | 4 | 4 | 20 | 52.18 | 55.78 |
| 5 | 30 | 6 | 6 | 30 | 54.72 | 57.07 |
| 6 | 15 | 8 | 8 | 40 | 85.96 | 88.93 |
| 7 | 10 | 6 | 6 | 30 | 95.06 | 93.44 |
| 8 | 25 | 8 | 8 | 20 | 40.03 | 40.41 |
| 9 | 20 | 6 | 6 | 30 | 86.85 | 81.76 |
| 10 | 20 | 6 | 6 | 30 | 80.33 | 81.76 |
| 11 | 20 | 6 | 2 | 30 | 83.64 | 72.65 |
| 12 | 25 | 4 | 4 | 40 | 63.19 | 69.28 |
| 13 | 20 | 6 | 6 | 30 | 71.22 | 81.76 |
| 14 | 15 | 8 | 4 | 20 | 69.4 | 76.33 |
| 15 | 20 | 2 | 6 | 30 | 36.02 | 43.09 |
| 16 | 15 | 4 | 8 | 40 | 66.09 | 67.67 |
| 17 | 20 | 10 | 6 | 30 | 95.03 | 88.69 |
| 18 | 20 | 6 | 6 | 50 | 90.89 | 85.88 |
| 19 | 25 | 8 | 4 | 20 | 61.45 | 60.36 |
| 20 | 15 | 8 | 4 | 40 | 98.66 | 99.33 |
| 21 | 15 | 4 | 8 | 20 | 50.86 | 41.82 |
| 22 | 20 | 6 | 6 | 10 | 21.63 | 27.36 |
| 23 | 20 | 6 | 6 | 30 | 84.88 | 81.76 |
| 24 | 25 | 4 | 4 | 20 | 40.19 | 36.01 |
| 25 | 20 | 6 | 10 | 30 | 26.28 | 37.99 |
| 26 | 20 | 6 | 6 | 30 | 81.36 | 81.76 |
| 27 | 25 | 4 | 8 | 20 | 17.94 | 14.76 |
| 28 | 20 | 6 | 6 | 30 | 85.92 | 81.76 |
| 29 | 25 | 8 | 8 | 40 | 75.43 | 72.32 |
| 30 | 25 | 4 | 8 | 40 | 55.41 | 47.27 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 14,312/50 | 14 | 1022/32 | 15/57 | <0.0001 |
| A- Cefixime concentration | 1984/53 | 1 | 1984/53 | 30/22 | <0.0001 |
| B-Adsorbent dosage | 3118/58 | 1 | 3118/58 | 47/49 | <0.0001 |
| C-pH | 1801/97 | 1 | 1801/97 | 27/44 | 0.0001 |
| D-Time | 5136/30 | 1 | 5136/30 | 78/22 | <0.0001 |
| AB | 14/10 | 1 | 14/40 | 0/2193 | 0/6463 |
| AC | 53/22 | 1 | 53/22 | 0/8104 | 0/3822 |
| AD | 44/36 | 1 | 44/36 | 0/6755 | 0/4240 |
| BC | 1/70 | 1 | 1/70 | 0/0055 | 0/8742 |
| BD | 0/3600 | 1 | 0/3600 | 0/0086 | 0/9420 |
| CD | 0/5625 | 1 | 0/5625 | 1/11 | 0/9275 |
| A2 | 72/61 | 1 | 72/61 | 6/58 | 0/3096 |
| B2 | 431/94 | 1 | 431/94 | 18/25 | 0/0216 |
| C2 | 1198/26 | 1 | 1198/26 | 16/50 | 0/0007 |
| D2 | 1083/32 | 1 | 1083/32 | - | 0/0010 |
| Residual | 984/95 | 15 | 65/66 | - | - |
| Lack of Fit | 818/71 | 10 | 81/87 | 2/46 | 0/1659 |
| Pure Error | 166/24 | 5 | 33/25 | - | - |
| Cor Total | 15,297/46 | 29 | - | - | - |
| Kinetic Model | Kobs (h−1) | K2 (g/mg/h) | R2 |
|---|---|---|---|
| Pseudo-first-order model | 0.62 | ---- | 0.738 |
| Pseudo-second-order model | ---- | 0.032 | 0.95 |
| Equilibrium Model | Parameters | nZVI/CS |
|---|---|---|
| Langmuir isotherm | qm (mg g−1) | 4.38 |
| b (L mg−1) | 0.09 | |
| R2 | 0.99 | |
| Freundlich isotherm | Kғ (L mg−1) | 1.88 |
| n | 2.97 | |
| R2 | 0.85 |
| Pollutant | Adsorbent | Pollutant Concentration (mg/L) | Adsorbent Dosage (g/L) | Contact Time (min) | pH | Adsorption Capacity (mg/g) | Removal Percentage (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cefixime | NAC KAC | 500 | 1 | 180 | 4 | 557.9 571.5 | - - | [61] |
| Cefixime | PAC/Fe/Si/Zn | 10 | 0.08 | 120 | 9–11 | 5.76 | 95.6 | [25] |
| Cefixime | HKUST-1/ZIF-8 | 200 | 0.1 | 240 | 7 | 110 | 90 | [21] |
| Cefixime | Fe3O4 and T-Fe3O4 | 40 50 | 0.005 | 120 | 5 | 41.32 56.49 | - - | [62] |
| Cefixime | Fe3O4@ MWCNT-CdS | 10 | 0.04 | 60 | 5 | 105.26 | 87.44 | [19] |
| Cefixime | Nano-sized activated carbon | 50 | 1 | 60 | 4 | 181.81 | 95 | [63] |
| Cefixime | nZVI/CS | 19.42 | 7.79 | 36.17 | 4.59 | 4.31 | 98.71 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moridi, A.; Sabbaghi, S.; Rasouli, J.; Rasouli, K.; Hashemi, S.A.; Chiang, W.-H.; Mousavi, S.M. Removal of Cefixime from Wastewater Using a Superb nZVI/Copper Slag Nanocomposite: Optimization and Characterization. Water 2023, 15, 1819. https://doi.org/10.3390/w15101819
Moridi A, Sabbaghi S, Rasouli J, Rasouli K, Hashemi SA, Chiang W-H, Mousavi SM. Removal of Cefixime from Wastewater Using a Superb nZVI/Copper Slag Nanocomposite: Optimization and Characterization. Water. 2023; 15(10):1819. https://doi.org/10.3390/w15101819
Chicago/Turabian StyleMoridi, Atefeh, Samad Sabbaghi, Jamal Rasouli, Kamal Rasouli, Seyyed Alireza Hashemi, Wei-Hung Chiang, and Seyyed Mojtaba Mousavi. 2023. "Removal of Cefixime from Wastewater Using a Superb nZVI/Copper Slag Nanocomposite: Optimization and Characterization" Water 15, no. 10: 1819. https://doi.org/10.3390/w15101819





