Origin of Groundwater Salinity in the Draa Sfar Polymetallic Mine Area Using Conservative Elements (Morocco)
Abstract
1. Introduction
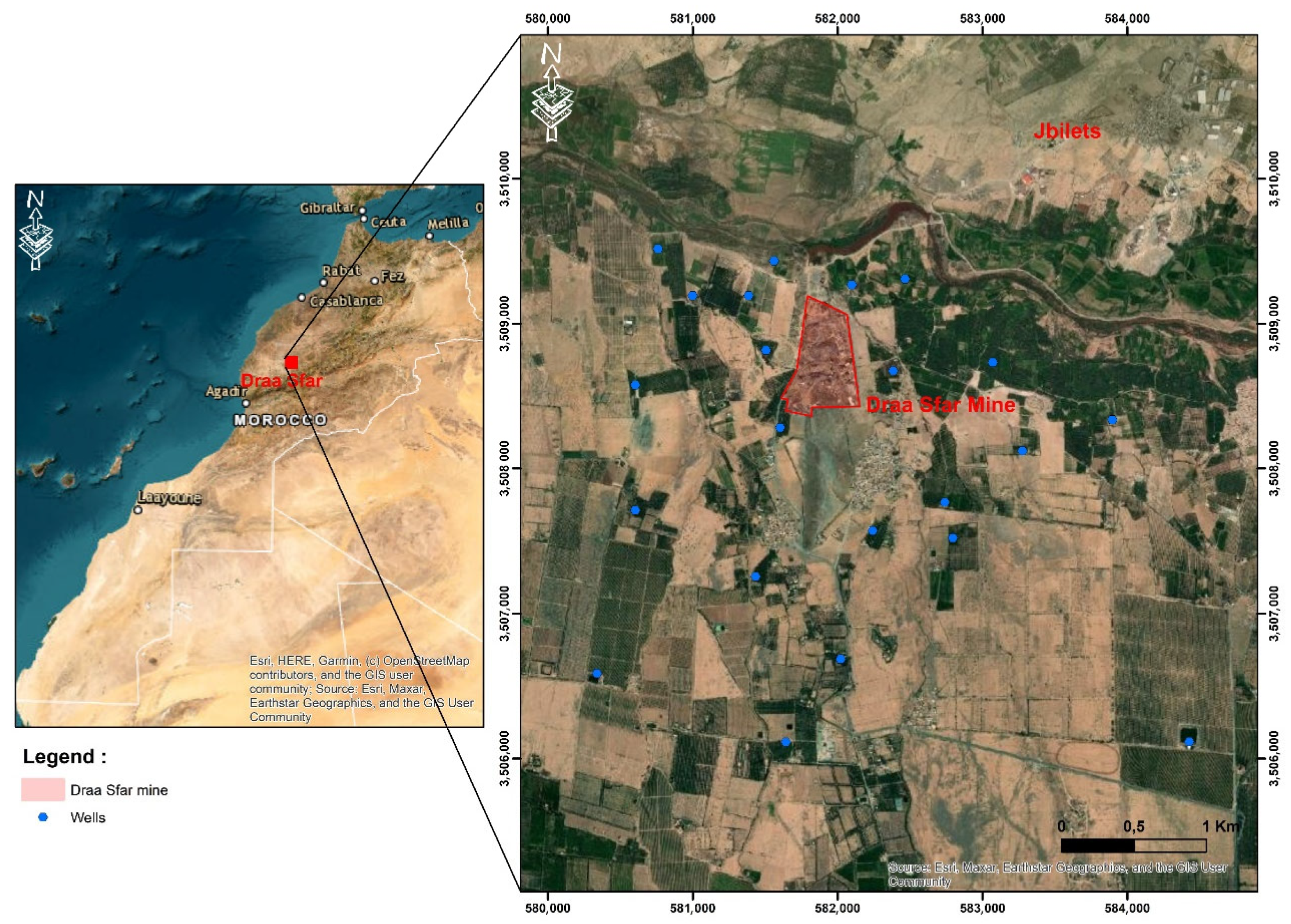
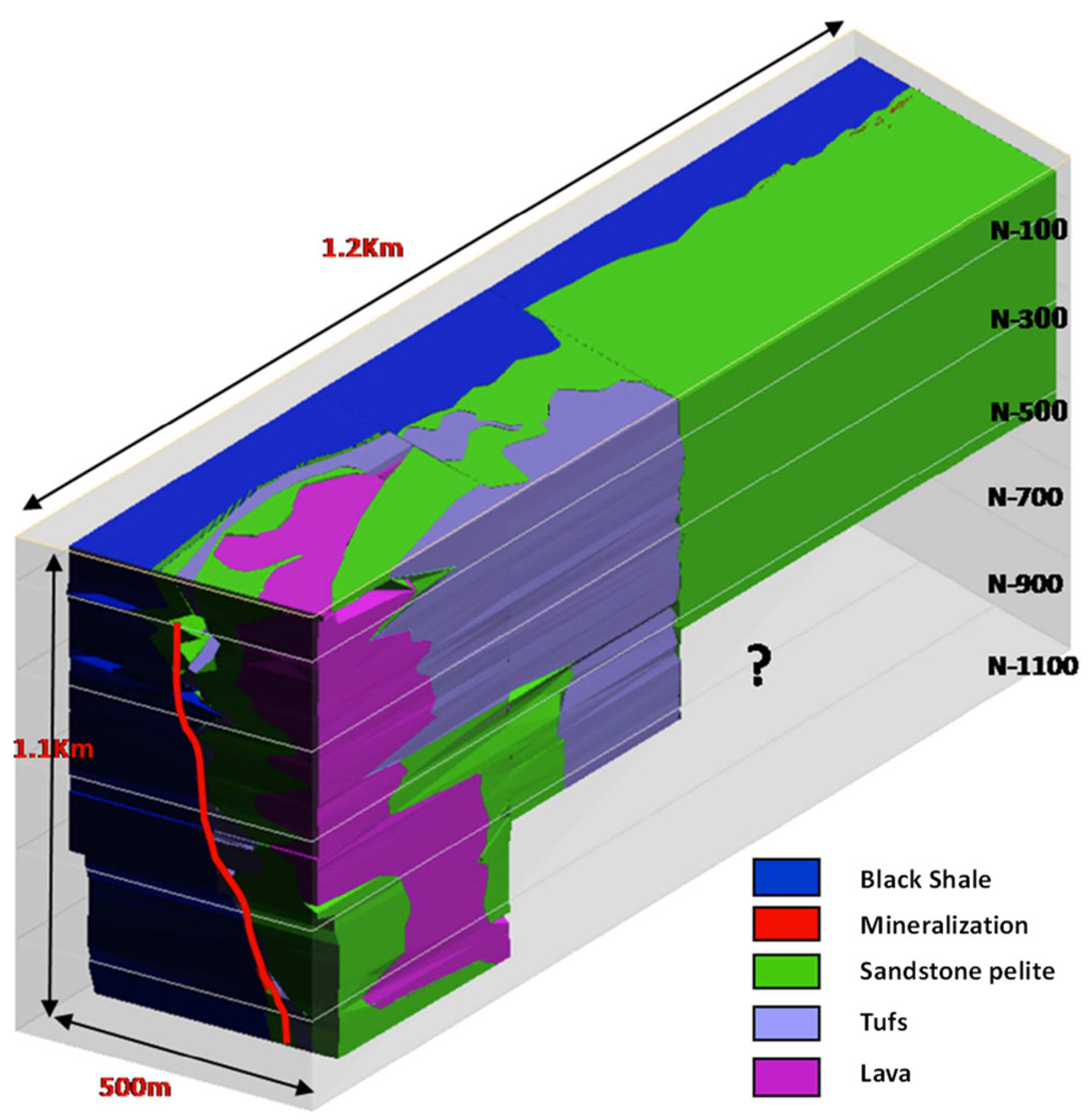
2. Geological Framework
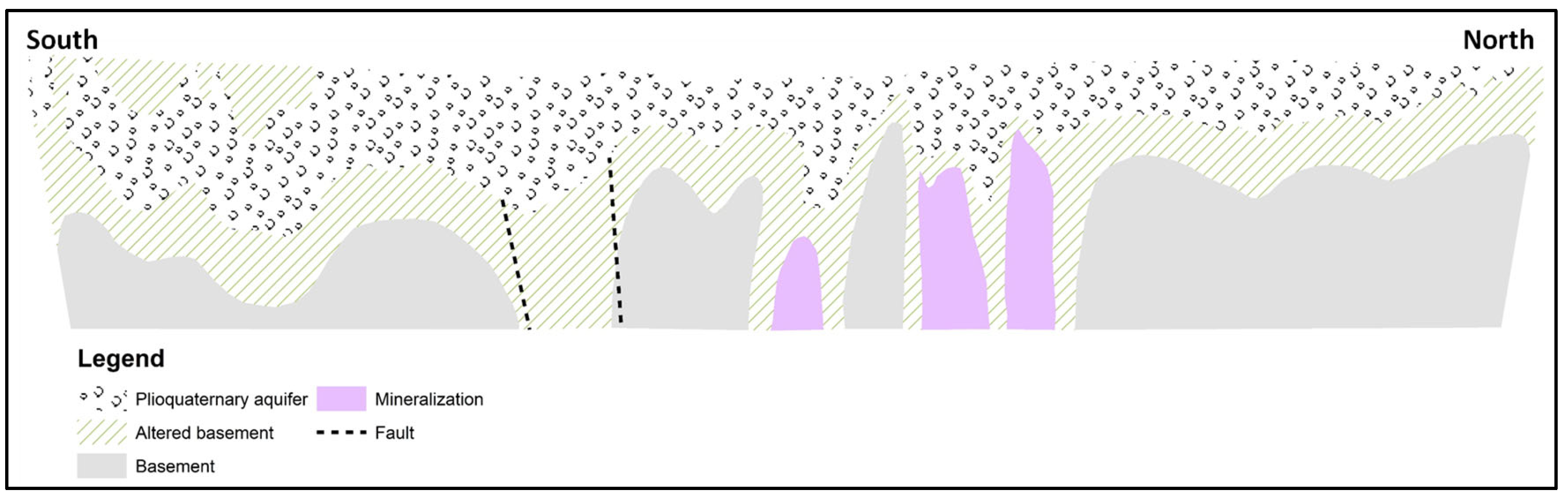
3. Hydrogeological Framework
4. Materials and Methods
4.1. Water Analysis
4.2. Rock Analysis
4.2.1. Porosity
- mssa: mass of the solid in saturated air (g)
- mssl: mass of the solid in the saturated liquid (g)
- Vtot: total volume of the solid (cm3)
- ρliq: liquid density (Kerdane) (g/cm3)
- ρair: air density (0.0012 g/cm3)
- Phcor: corrected buoyancy (0.99983)
4.2.2. Density
4.2.3. Leaching Tests
5. Results
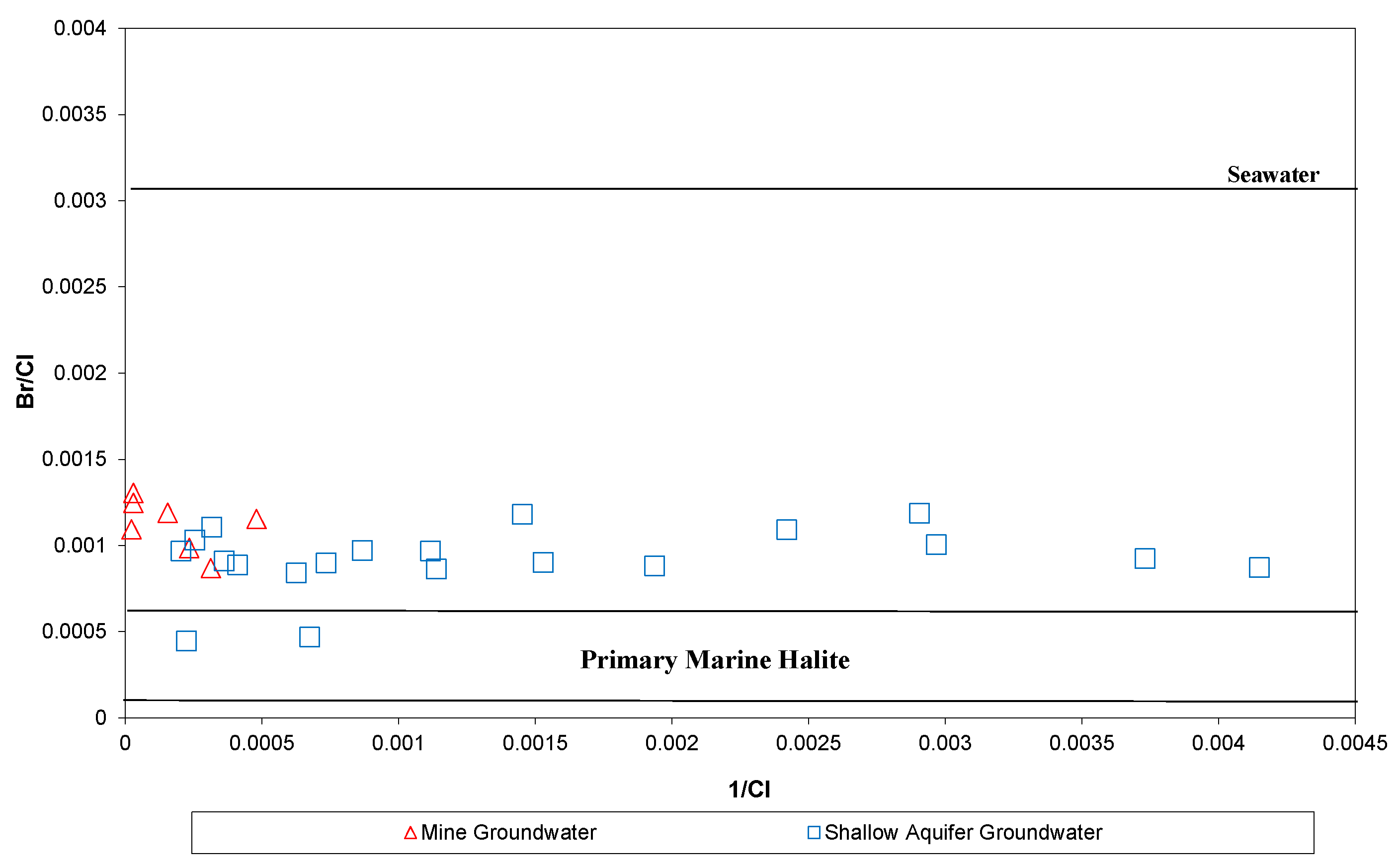
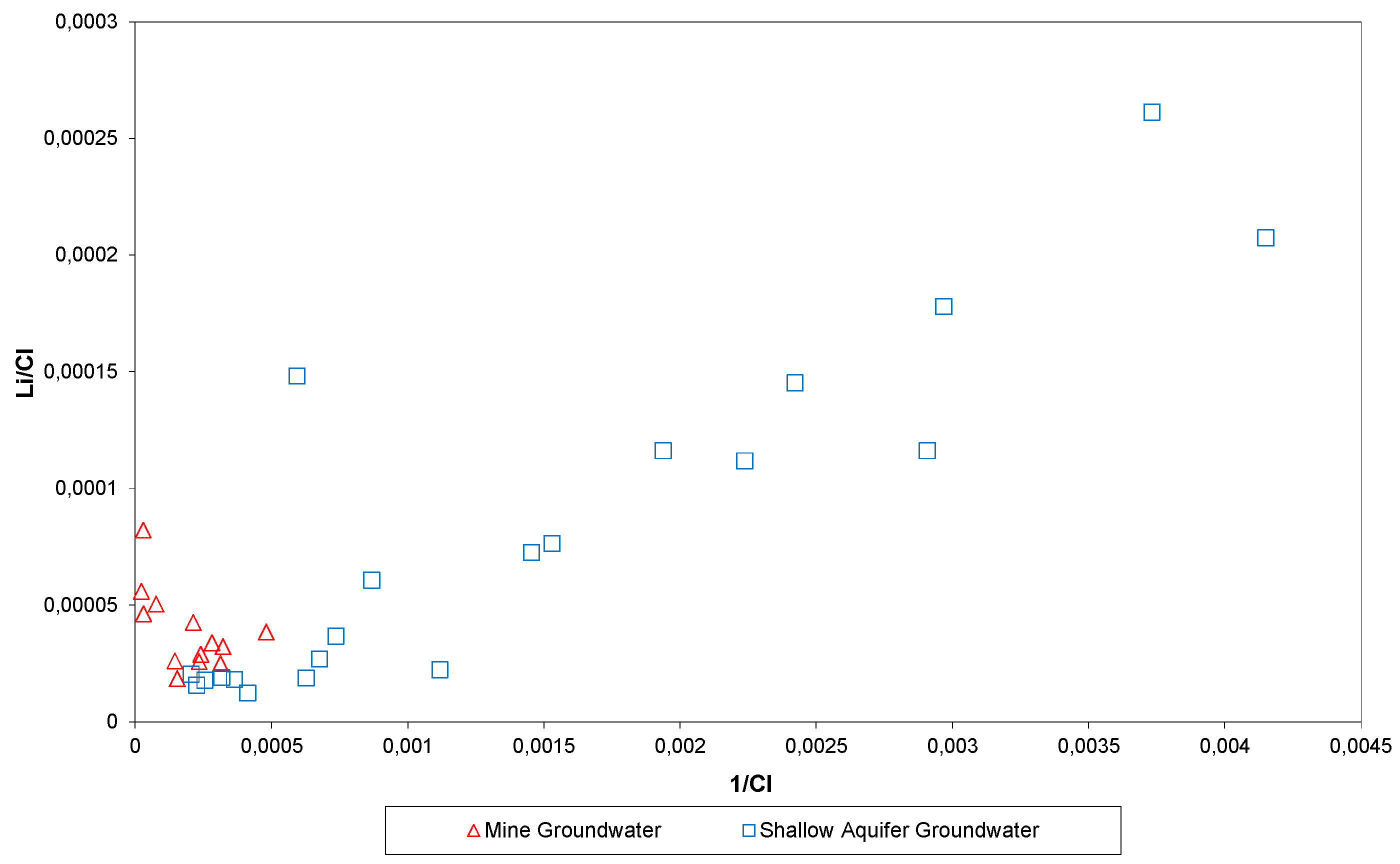
5.1. Water Analysis
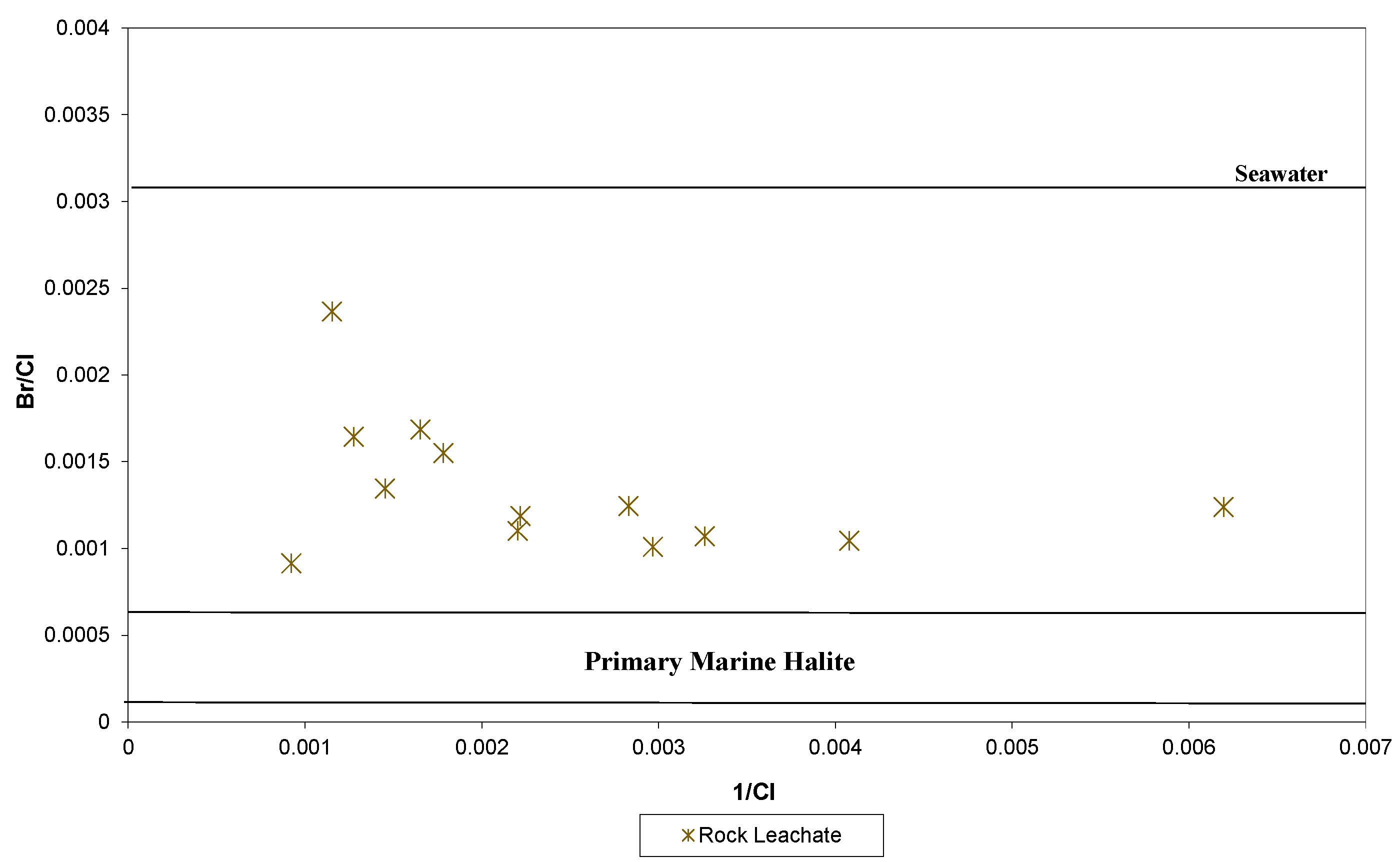
5.2. Porosity and Leaching Tests
- [Cl−]leach: chloride concentration in the leach solution
- [Cl−]R: chloride concentration in 100 g of the rock
- [Cl−]T: chloride concentration in the porosity if all was liquid
- VR: rock volume
- VE: water volume or pore volume
- dR: grain density g/cm3
6. Discussion
- High saline mine groundwater has [Cl−] = 44 g/L; [Br−] = 48.5 mg/L.
- As pore water, the average of the two high saline pore waters has been used: [Cl−]p = 240 g/L; [Br−]p = 267 mg/L.
- Meteoric water is assumed to contain negligible Cl− and Br− compared to pore waters: [Cl−]m = 0 g/L; [Br−]m = 0 mg/L.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boujghad, A.; Bouabdli, A.; Baghdad, B. Groundwater Quality Evaluation in the Vicinity of the Draa Sfar Mine in Marrakesh, Morocco. Euro-Mediterr. J. Environ. Integr. 2019, 4, 12. [Google Scholar] [CrossRef]
- Frape, S.K.; Fritz, P. Geochemical Trends from Groundwaters from Canadian Shield. In Geological Association of Canada Special Paper; Geological Association of Canada: St. John’s, NL, Canada, 1987. [Google Scholar]
- Pearson, F.J. Models of Mineral Controls on the Composition of Saline Groundwaters of the Canadian Shield. In Geological Association of Canada Special Paper; Geological Association of Canada: St. John’s, NL, Canada, 1987; pp. 39–52. [Google Scholar]
- Kamineni, D.C. Halogen Bearing Minerals in Plutonic Rocks: A Possible Source of Chlorine in Saline Groundwater in the Canadian Shield. In Geological Association of Canada Special Paper; Geological Association of Canada: St. John’s, NL, Canada, 1987; pp. 69–80. [Google Scholar]
- Walter, J.; Chesnaux, R.; Cloutier, V.; Gaboury, D. The Influence of Water/Rock−Water/Clay Interactions and Mixing in the Salinization Processes of Groundwater. J. Hydrol. Reg. Stud. 2017, 13, 168–188. [Google Scholar] [CrossRef]
- Lodemann, M.; Fritz, P.; Wolf, M.; Ivanovich, M.; Hansen, B.T. On the Origin of Saline Fluids in the KTB (Continental Deep Drilling Project of Germany). Appl. Geochem. 1998, 13, 651–671. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Kay, R.L.F.; McCartney, R.A. Origin of Saline Groundwaters in the Carnmenellis Granite (Cornwall, England): Natural Processes and Reaction during Hot Dry Rock Reservoir Circulation. Chem. Geol. 1985, 49, 287–301. [Google Scholar] [CrossRef]
- Nordstrom, D.; Olsson, T. Fluid Inclusions as a Source of Dissolved Salts in Deep Granitic Groundwaters. In Geological Association of Canada—Special Paper; Geological Association of Canada: St. John’s, NL, Canada, 1987; Volume 33, pp. 111–119. [Google Scholar]
- Savoye, S.; Aranyossy, J.-F.; Beaucaire, C.; Cathelineau, M.; Louvat, D.; Michelot, J.-L. Fluid Inclusions in Granites and Their Relationships with Present-Day Groundwater Chemistry. Eur. J. Miner. 1998, 10, 1215–1226. [Google Scholar] [CrossRef]
- Alexeev, S.V.; Alexeeva, L.P.; Vakhromeev, A.G. Brines of the Siberian Platform (Russia): Geochemistry and Processing Prospects. Appl. Geochem. 2020, 117, 104588. [Google Scholar] [CrossRef]
- Greene, S.; Battye, N.; Clark, I.; Kotzer, T.; Bottomley, D. Canadian Shield Brine from the Con Mine, Yellowknife, NT, Canada: Noble Gas Evidence for an Evaporated Palaeozoic Seawater Origin Mixed with Glacial Meltwater and Holocene Recharge. Geochim. Cosmochim. Acta 2008, 72, 4008–4019. [Google Scholar] [CrossRef]
- Bottomley, D.J.; Gregoire, D.C.; Raven, K.G. Saline Ground Waters and Brines in the Canadian Shield: Geochemical and Isotopic Evidence for a Residual Evaporite Brine Component. Geochim. Cosmochim. Acta 1994, 58, 1483–1498. [Google Scholar] [CrossRef]
- Shouakar-Stash, O.; Alexeev, S.V.; Frape, S.K.; Alexeeva, L.P.; Drimmie, R.J. Geochemistry and Stable Isotopic Signatures, Including Chlorine and Bromine Isotopes, of the Deep Groundwaters of the Siberian Platform, Russia. Appl. Geochem. 2007, 22, 589–605. [Google Scholar] [CrossRef]
- Michelot, J.L.; Beaucaire, C.; Kloppmann, W.; Louvat, D.; Matray, J.M.; Sacchi, E.; Savoye, S.; Trésonne, N. L’eau Du Système Granitique de La Vienne: Reconnaissance Hydrogéochimique et Isotopique. Actes Journées Sci. CNRS/ANDRA 1997, 181–201. [Google Scholar]
- Aquilina, L.; Landes, A.A.-L.; Ayraud-Vergnaud, V.; Labasque, T.; Roques, C.; Davy, P.; Pauwels, H.; Petelet-Giraud, E. Evidence for a Saline Component at Shallow Depth in the Crystalline Armorican Basement (W France). Procedia Earth Planet. Sci. 2013, 7, 19–22. [Google Scholar] [CrossRef]
- Louvat, D.; Michelot, J.L.; Franc, J. Origin and Residence Time of Salinity in the Äspö Groundwater System. Appl. Geochem. 1999, 14, 917–925. [Google Scholar] [CrossRef]
- Rinder, T.; Dietzel, M.; Stammeier, J.A.; Leis, A.; Bedoya-González, D.; Hilberg, S. Geochemistry of Coal Mine Drainage, Groundwater, and Brines from the Ibbenbüren Mine, Germany: A Coupled Elemental-Isotopic Approach. Appl. Geochem. 2020, 121, 104693. [Google Scholar] [CrossRef]
- Leybourne, M.I.; Goodfellow, W.D. Br/Cl Ratios and O, H, C, and B Isotopic Constraints on the Origin of Saline Waters from Eastern Canada. Geochim. Cosmochim. Acta 2007, 71, 2209–2223. [Google Scholar] [CrossRef]
- Leybourne, M.I.; Clark, I.D.; Goodfellow, W.D. Stable Isotope Geochemistry of Ground and Surface Waters Associated with Undisturbed Massive Sulfide Deposits; Constraints on Origin of Waters and Water–Rock Reactions. Chem. Geol. 2006, 231, 300–325. [Google Scholar] [CrossRef]
- Huvelin, P. Etude Géologique et Gitologique du Massif Hercynien des Jbilets (Maroc Occidental); Notes et Mémoires du Service Géologique; Service Géologique du Maroc: Rabat, Marroco, 1977.
- Marcoux, E.; Belkabir, A.; Gibson, H.L.; Lentz, D.; Ruffet, G. Draa Sfar, Morocco: A Visean (331 Ma) Pyrrhotite-Rich, Polymetallic Volcanogenic Massive Sulphide Deposit in a Hercynian Sediment-Dominant Terrane. Ore Geol. Rev. 2008, 33, 307–328. [Google Scholar] [CrossRef]
- Salama, L.; Mouguina, E.M.; Nahid, A.; Bachari, E.E.; Outhounjite, M.; Maacha, L.; Zouhair, M.; Naciri, A.E. Apport de la modélisation géologique 3D à l’exploration minière: Etude de cas du gisement de Draa Sfar (Jbilets centrales, Maroc). Int. J. Innov. Sci. Res. 2016, 22, 72–89. [Google Scholar]
- Hakkou, R.; Wahbi, M.; Bachnou, A.; Elamari, K.; Hanich, L.; Hibti, M. Impact de la décharge publique de Marrakech (Maroc) sur les ressources en eau. Bull. Eng. Geol. Environ. 2001, 60, 325–336. [Google Scholar] [CrossRef]
- Belkabir, A.; Gibson, H.L.; Marcoux, E.; Lentz, D.; Rziki, S. Geology and Wall Rock Alteration at the Hercynian Draa Sfar Zn–Pb–Cu Massive Sulphide Deposit, Morocco. Ore Geol. Rev. 2008, 33, 280–306. [Google Scholar] [CrossRef]
- Hogan, J.F.; Blum, J.D. Boron and Lithium Isotopes as Groundwater Tracers: A Study at the Fresh Kills Landfill, Staten Island, New York, USA. Appl. Geochem. 2003, 18, 615–627. [Google Scholar] [CrossRef]
- Paropkari, A.L. Geochemistry of Sediments from the Mangalore-Cochin Shelf and Upper Slope off Southwest India: Geological and Environmental Factors Controlling Dispersal of Elements. Chem. Geol. 1990, 81, 99–119. [Google Scholar] [CrossRef]
- Sanchez-Martos, F.; Pulido-Bosch, A.; Molina-Sanchez, L.; Vallejos-Izquierdo, A. Identification of the Origin of Salinization in Groundwater Using Minor Ions ž Lower Andarax, Southeast Spain. Sci. Total Environ. 2002, 297, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, W.M.; Smedley, P.L. Residence Time Indicators in Groundwater: The East Midlands Triassic Sandstone Aquifer. Appl. Geochem. 2000, 15, 737–752. [Google Scholar] [CrossRef]
- Leybourne, M.I.; Goodfellow, W.D.; Boyle, D.R. Hydrogeochemical, Isotopic, and Rare Earth Element Evidence for Contrasting Water–Rock Interactions at Two Undisturbed Zn–Pb Massive Sulphide Deposits, Bathurst Mining Camp, N.B., Canada. J. Geochem. Explor. 1998, 64, 237–261. [Google Scholar] [CrossRef]
- Bottomley, D.J.; Katz, A.; Chan, L.H.; Starinsky, A.; Douglas, M.; Clark, I.D.; Raven, K.G. The Origin and Evolution of Canadian Shield Brines: Evaporation or Freezing of Seawater? New Lithium Isotope and Geochemical Evidence from the Slave Craton. Chem. Geol. 1999, 155, 295–320. [Google Scholar] [CrossRef]
- Kloppmann, W.; Girard, J.-P.; Négrel, P. Exotic Stable Isotope Compositions of Saline Waters and Brines from the Crystalline Basement. Chem. Geol. 2002, 184, 49–70. [Google Scholar] [CrossRef]
- Möller, P.; Lüders, V.; De Lucia, M. Formation of Rotliegend Ca-Cl Brines in the North German Basin Compared to Analogues in the Geological Record. Chem. Geol. 2017, 459, 32–42. [Google Scholar] [CrossRef]
- Michelot, J.L. Hydrologie Isotopique Des Circulations Lentes En Milieu Cristallin Fracturé: Essai Méthodologique. Ph.D. Thesis, Université de Paris 11, Orsay, France, 1988. [Google Scholar]
- Karro, E.; Lahermo, P. Occurrence and Chemical Characteristics of Groundwater in Precambrian Bedrock in Finland. In Geological Survey of Finland, Special Paper 27; Geological Survey of Finland: Espoo, Finland, 1999. [Google Scholar]
- Essaifi, A.; Hibti, M. The Hydrothermal System of Central Jebilet (Variscan Belt, Morocco): A Genetic Association between Bimodal Plutonism and Massive Sulphide Deposits? J. Afr. Earth Sci. 2008, 50, 188–203. [Google Scholar] [CrossRef]
- Maacha, L.; Jaffal, M.; Jarni, A.; Kchikach, A.; Mouguina, E.M.; Zouhair, M.; Ennaciri, A.; Saddiqi, O. A Contribution of Airborne Magnetic, Gamma Ray Spectrometric Data in Understanding the Structure of the Central Jebilet Hercynian Massif and Implications for Mining. J. Afr. Earth Sci. 2017, 134, 389–403. [Google Scholar] [CrossRef]
- Cathles, L.M. An Analysis of the Hydrothermal System Responsible for Massive Sulfide Deposition in the Hokuroku Basin of Japan. In The Kuroko and Related Volcanogenic Massive Sulfide Deposits; Ohmoto, H., Skinner, B.J., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1983; Volume 5, ISBN 978-1-62949-000-7. [Google Scholar]
- Wood, S.A.; Samson, I.M. Solubility of Ore Minerals and Complexation of Ore Metals in Hydrothermal Solutions. In Techniques in Hydrothermal Ore Deposits Geology; Richards, J.P., Larson, P.B., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1998; Volume 10, ISBN 978-1-62949-017-5. [Google Scholar]
- Zhong, R.; Brugger, J.; Chen, Y.; Li, W. Contrasting Regimes of Cu, Zn and Pb Transport in Ore-Forming Hydrothermal Fluids. Chem. Geol. 2015, 395, 154–164. [Google Scholar] [CrossRef]
- Stewart, L.C.; Algar, C.K.; Fortunato, C.S.; Larson, B.I.; Vallino, J.J.; Huber, J.A.; Butterfield, D.A.; Holden, J.F. Fluid Geochemistry, Local Hydrology, and Metabolic Activity Define Methanogen Community Size and Composition in Deep-Sea Hydrothermal Vents. ISME J. 2019, 13, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Jambon, A.; Déruelle, B.; Dreibus, G.; Pineau, F. Chlorine and Bromine Abundance in MORB: The Contrasting Behaviour of the Mid-Atlantic Ridge and East Pacific Rise and Implications for Chlorine Geodynamic Cycle. Chem. Geol. 1995, 126, 101–117. [Google Scholar] [CrossRef]
- Nahnybida, T.; Gleeson, S.A.; Rusk, B.G.; Wassenaar, L.I. Cl/Br Ratios and Stable Chlorine Isotope Analysis of Magmatic–Hydrothermal Fluid Inclusions from Butte, Montana and Bingham Canyon, Utah. Miner. Depos. 2009, 44, 837. [Google Scholar] [CrossRef]



| Location | Sample | T °C | CE ms/cm | pH | Na+ mg/L | Cl− mg/L | Li+ mg/L | Br− mg/L |
|---|---|---|---|---|---|---|---|---|
| Surface wells | PJ | 21.4 | 15.50 | 7.15 | 1011.6 | 4449.0 | 0.07 | 2.0 |
| PK | 22.7 | 9.30 | 7.03 | 776.3 | 2428.3 | 0.03 | 2.2 | |
| PN | 23.4 | 6.39 | 7.21 | 389.9 | 1595.3 | 0.03 | 1.3 | |
| PC | 21.2 | 11.70 | 7.35 | 1189.1 | 3155.1 | 0.06 | 3.5 | |
| PI | 22.2 | 8.35 | 6.80 | 121.0 | 241.0 | 0.05 | 0.2 | |
| PG | 22.4 | 1.45 | 7.56 | 220.0 | 878.0 | 0.00 | 0.8 | |
| P 26 | 22.7 | 3.40 | 7.04 | 339.0 | 1686.0 | 0.25 | - | |
| PV | 23.4 | 7.90 | 7.30 | 114.0 | 337.0 | 0.06 | 0.3 | |
| PK1 | 21.5 | 1.88 | 7.10 | 371.0 | 1359.0 | 0.05 | 1.2 | |
| P X | 23.4 | 4.75 | 7.28 | 183.0 | 516.0 | 0.06 | 0.5 | |
| P 23 | 24.1 | 1.57 | 7.18 | 323.0 | 1480.0 | 0.04 | 0.7 | |
| P4 | 25.2 | 3.66 | 6.93 | 121.0 | 268.0 | 0.07 | 0.2 | |
| P 21 | 23.6 | 2.33 | 7.00 | 173.0 | 447.0 | 0.05 | - | |
| PL1 | 22.2 | 2.86 | 7.18 | 236.0 | 654.0 | 0.05 | 0.6 | |
| P2 | 29.3 | 2.34 | 8.09 | 288.0 | 413.0 | 0.06 | 0.5 | |
| PI1 | 25.9 | 4.32 | 7.20 | 404.0 | 1153.0 | 0.07 | 1.1 | |
| PP | 23.2 | 1.82 | 7.40 | 110.0 | 344.0 | 0.04 | 0.4 | |
| P 1 | 23.2 | 3.00 | 7.14 | 232.0 | 688.0 | 0.05 | 0.8 | |
| PB | 21.6 | 11.69 | 6.78 | 1420.0 | 3924.0 | 0.07 | 4.0 | |
| P ID | 22.3 | 14.30 | 6.64 | 1241.0 | 4887.0 | 0.10 | 4.7 | |
| PE | 24.2 | 8.62 | 6.69 | 643.0 | 2753.0 | 0.05 | 2.5 | |
| PF | 24.0 | 3.47 | 7.00 | 234.0 | 895.0 | 0.02 | 0.9 | |
| Mine Water | Niv-60 | 19.2 | 20.40 | 7.01 | 2029.0 | 6888.0 | 0.18 | - |
| N-67 | 19.9 | 18.10 | 7.30 | 2443.0 | 6470.0 | 0.12 | 7.7 | |
| N-520 | 28.3 | 12.75 | 6.90 | 1393.0 | 4268.0 | 0.11 | 4.2 | |
| N-69 | 22.3 | 10.36 | 7.47 | 1482.0 | 3201.0 | 0.08 | 2.8 | |
| N-300 | 26.5 | 6.56 | 6.90 | 522.0 | 2082.0 | 0.08 | 2.4 | |
| DF18 | 32.0 | 80.00 | 7.20 | 17,150.0 | 33,700.0 | 1.56 | 44.0 | |
| N -150 | - | 100.00 | 5.45 | 21,689.0 | 44,313.0 | 2.48 | - | |
| niv-67m | 19.4 | 9.56 | 7.48 | 1251.0 | 3106.0 | 0.10 | - | |
| niv-80m | 23 | 11.90 | 7.37 | 1727.0 | 3545.0 | 0.12 | - | |
| niv-240m | - | 12.98 | 7.23 | 889.0 | 4159.0 | 0.12 | 5.0 | |
| 400mBure | - | 14.67 | 7.50 | 2569.0 | 4694.0 | 0.20 | - | |
| Taille380 S-S | - | 34.40 | 6.74 | 6411.0 | 13,074.0 | 0.66 | 5.8 | |
| 400mDF18 | 33.0 | 83.00 | 5.78 | 18,130.0 | 34,353.0 | 2.82 | 42.9 |
| Rock Type/Sampling Level | Mass (g) | Mass in the Air after Saturation (g) | Mass in the Kerdane (g) | Mass after Drying at 150 °C (g) | Grains Density (g/cm3) | Total Porosity % |
|---|---|---|---|---|---|---|
| Lave Niv 640 | 44.23 | 44.24 | 31.11 | 44.22 | 2.67 | 0.4 |
| Lave Niv 640 | 17.59 | 17.60 | 12.37 | 17.58 | 2.67 | 0.6 |
| Tufs Niv 120 | 28.03 | 28.06 | 20.24 | 28.01 | 2.85 | 1.0 |
| Tufs Niv 120 | 19.40 | 19.41 | 14.01 | 19.39 | 2.85 | 0.8 |
| PN Niv 700 | 17.10 | 17.12 | 12.19 | 17.08 | 2.76 | 1.1 |
| PN Niv 700 | 16.57 | 16.57 | 11.82 | 16.56 | 2.76 | 0.6 |
| Rock Sample | Cl− mg/L | Br− mg/L |
|---|---|---|
| 700 m/PN | 1084.8 | 0.99 |
| Niv640/E26/PN | 605 | 1.02 |
| Niv500/E18/PN | 245.2 | 0.26 |
| Niv610/E21/PN | 353.4 | 0.44 |
| Niv110/E39/PN | 86.6 | - |
| Niv340/E8/tufs | 47.6 | - |
| Niv110/E33/Tufs | 450.8 | 0.54 |
| Niv340/E7/Lave | 337 | 0.34 |
| Niv110/E38/Lave | 688.6 | 0.93 |
| Niv150/E5/Lave | 257.6 | - |
| Niv640/E27/Pg | 867.8 | 2.05 |
| Niv340/E9/Pg | 561.2 | 0.87 |
| Niv420/E13/Pg | 161.4 | 0.2 |
| Niv120/E1/Pg | 306.6 | 0.33 |
| Niv340/E6/Pg | 454 | 0.5 |
| [Cl] Leach mg/L | [Cl] R mg/L | Density | Max Porosity | [Cl] T g/L Min | [Na] T g/L Min | NaCl g/L Min | Min Porosity | [Cl] T g/L Max | [Na] T g/L Max | NaCl Max | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 700 m/PN | 1084.8 | 54.2 | 2.76 | 0.011 | 133.0 | 86.2 | 219.2 | 0.006 | 259.1 | 167.9 | 427.0 |
| Niv640/E26/PN | 605.0 | 30.3 | 2.76 | 0.011 | 74.2 | 48.1 | 122.2 | 0.006 | 144.5 | 93.6 | 238.1 |
| Niv500/E18/PN | 245.2 | 12.3 | 2.76 | 0.011 | 30.1 | 19.5 | 49.5 | 0.006 | 58.6 | 38.0 | 96.5 |
| Niv610/E21/PN | 353.4 | 17.7 | 2.76 | 0.011 | 43.3 | 28.1 | 71.4 | 0.006 | 84.4 | 54.7 | 139.1 |
| Niv110/E39/PN | 86.6 | 4.3 | 2.76 | 0.011 | 10.6 | 6.9 | 17.5 | 0.006 | 20.7 | 13.4 | 34.1 |
| Niv340/E8/tufs | 47.6 | 2.4 | 2.85 | 0.010 | 7.0 | 4.6 | 11.6 | 0.008 | 8.4 | 5.5 | 13.9 |
| Niv110/E33/Tufs | 450.8 | 22.5 | 2.85 | 0.010 | 66.7 | 43.2 | 109.9 | 0.008 | 79.9 | 51.8 | 131.7 |
| Niv340/E7/Lave | 337.0 | 16.9 | 2.67 | 0.006 | 72.3 | 46.8 | 119.1 | 0.004 | 108.5 | 70.3 | 178.9 |
| Niv110/E38/Lave | 688.6 | 34.4 | 2.67 | 0.006 | 147.6 | 95.7 | 243.3 | 0.004 | 221.8 | 143.7 | 365.5 |
| Niv150/E5/Lave | 257.6 | 12.9 | 2.67 | 0.006 | 55.2 | 35.8 | 91.0 | 0.004 | 83.0 | 53.8 | 136.7 |
| Niv640/E27/Pg | 867.8 | 43.4 | 2.76 | 0.011 | 106.4 | 68.9 | 175.3 | 0.006 | 207.3 | 134.3 | 341.6 |
| Niv340/E9/Pg | 561.2 | 28.1 | 2.76 | 0.011 | 68.8 | 44.6 | 113.4 | 0.006 | 134.1 | 86.9 | 220.9 |
| Niv420/E13/Pg | 161.4 | 8.1 | 2.76 | 0.011 | 19.8 | 12.8 | 32.6 | 0.006 | 38.6 | 25.0 | 63.5 |
| Niv120/E1/Pg1 | 306.6 | 15.3 | 2.76 | 0.011 | 37.6 | 24.4 | 62.0 | 0.006 | 73.2 | 47.5 | 120.7 |
| Niv340/E6/Pg | 454.0 | 22.7 | 2.76 | 0.011 | 55.7 | 36.1 | 91.7 | 0.006 | 108.4 | 70.3 | 178.7 |
| Samples | Cl− g/L | Br− mg/L | α Min (Cl−) (%) | α Min Br− (%) | α Max Cl− (%) | α Max Br− (%) |
|---|---|---|---|---|---|---|
| N-67 | 6.5 | 7.7 | 95.4 | 95.2 | 97.3 | 97.1 |
| N-520 | 4.3 | 4.2 | 97.0 | 97.4 | 98.2 | 98.4 |
| N-69 | 3.2 | 2.8 | 97.7 | 98.3 | 98.7 | 99.0 |
| N-300 | 2.1 | 2.4 | 98.5 | 98.5 | 99.1 | 99.1 |
| DF18 | 33.7 | 44.0 | 75.9 | 72.5 | 86.0 | 83.5 |
| N-150 | 44.3 | 48.5 | 68.3 | 69.7 | 81.5 | 81.8 |
| Niv-240 m | 4.2 | 5.0 | 97.0 | 96.9 | 98.3 | 98.1 |
| Niv-363 m | 13.1 | 5.8 | 90.7 | 96.4 | 94.6 | 97.8 |
| 400mDF18 | 34.4 | 42.9 | 75.5 | 73.2 | 85.7 | 83.9 |
| Elements | Concentrations |
|---|---|
| Na+ g/L | 108.44 |
| K+ g/L | 0.65 |
| Mg2+ g/L | 7.25 |
| Ca2+ g/L | 21.02 |
| SO42− g/L | 1.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ait Lemkademe, A.; Michelot, J.-L.; Benkaddour, A.; Hanich, L.; Heddoun, O. Origin of Groundwater Salinity in the Draa Sfar Polymetallic Mine Area Using Conservative Elements (Morocco). Water 2023, 15, 82. https://doi.org/10.3390/w15010082
Ait Lemkademe A, Michelot J-L, Benkaddour A, Hanich L, Heddoun O. Origin of Groundwater Salinity in the Draa Sfar Polymetallic Mine Area Using Conservative Elements (Morocco). Water. 2023; 15(1):82. https://doi.org/10.3390/w15010082
Chicago/Turabian StyleAit Lemkademe, Anasse, Jean-Luc Michelot, Abdelfattah Benkaddour, Lahoucine Hanich, and Ouissal Heddoun. 2023. "Origin of Groundwater Salinity in the Draa Sfar Polymetallic Mine Area Using Conservative Elements (Morocco)" Water 15, no. 1: 82. https://doi.org/10.3390/w15010082
APA StyleAit Lemkademe, A., Michelot, J.-L., Benkaddour, A., Hanich, L., & Heddoun, O. (2023). Origin of Groundwater Salinity in the Draa Sfar Polymetallic Mine Area Using Conservative Elements (Morocco). Water, 15(1), 82. https://doi.org/10.3390/w15010082






