Health Risks in a Brazilian Cerrado Population Due to Pathogens Transmitted through Water and Land Use Conditions
Abstract
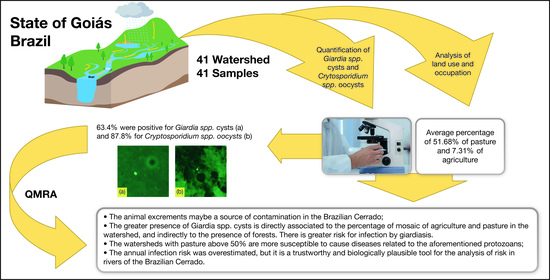
:1. Introduction
2. Materials and Methods
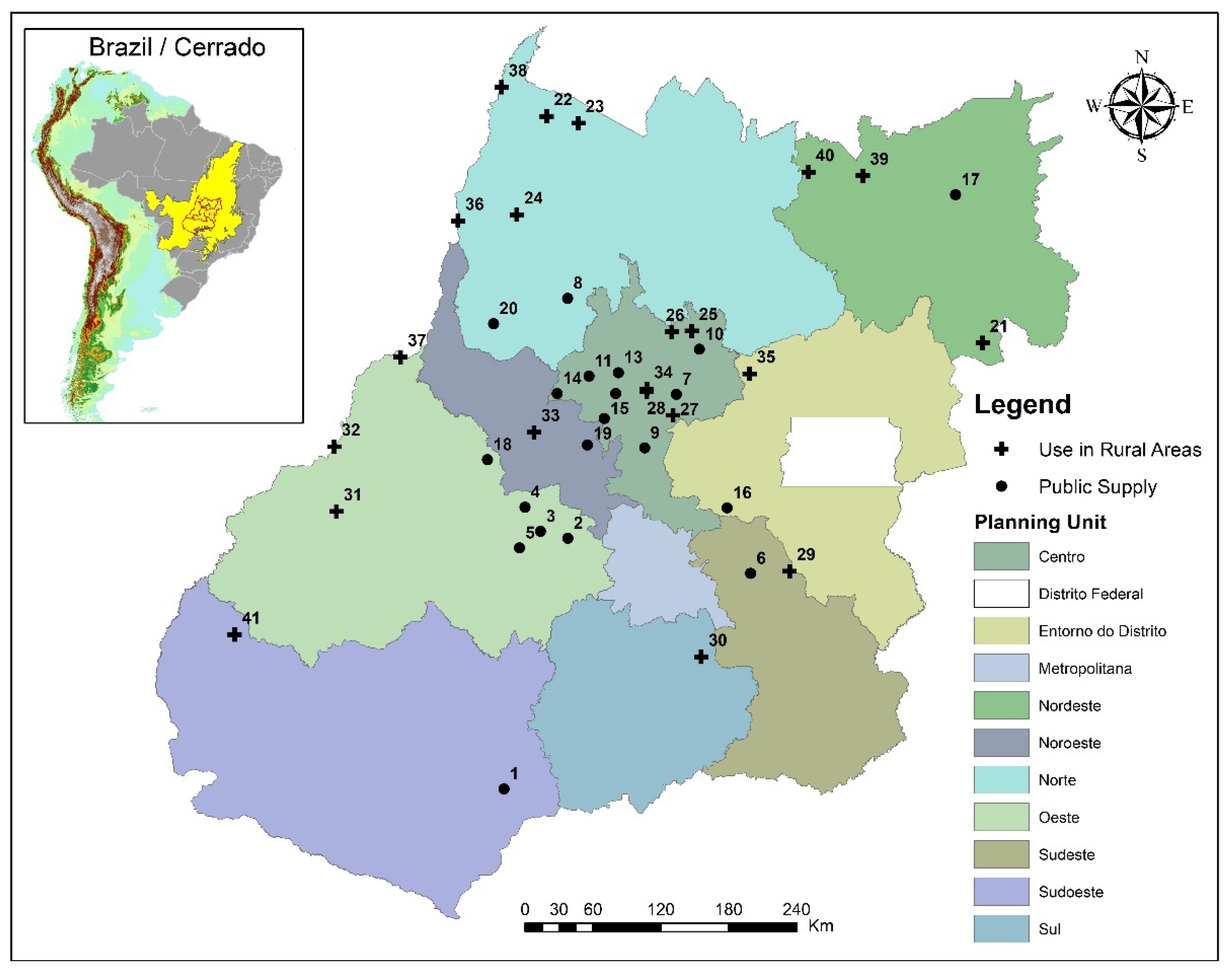
2.1. Area of Study and Sampling
2.2. Collection and Parasitological Analysis
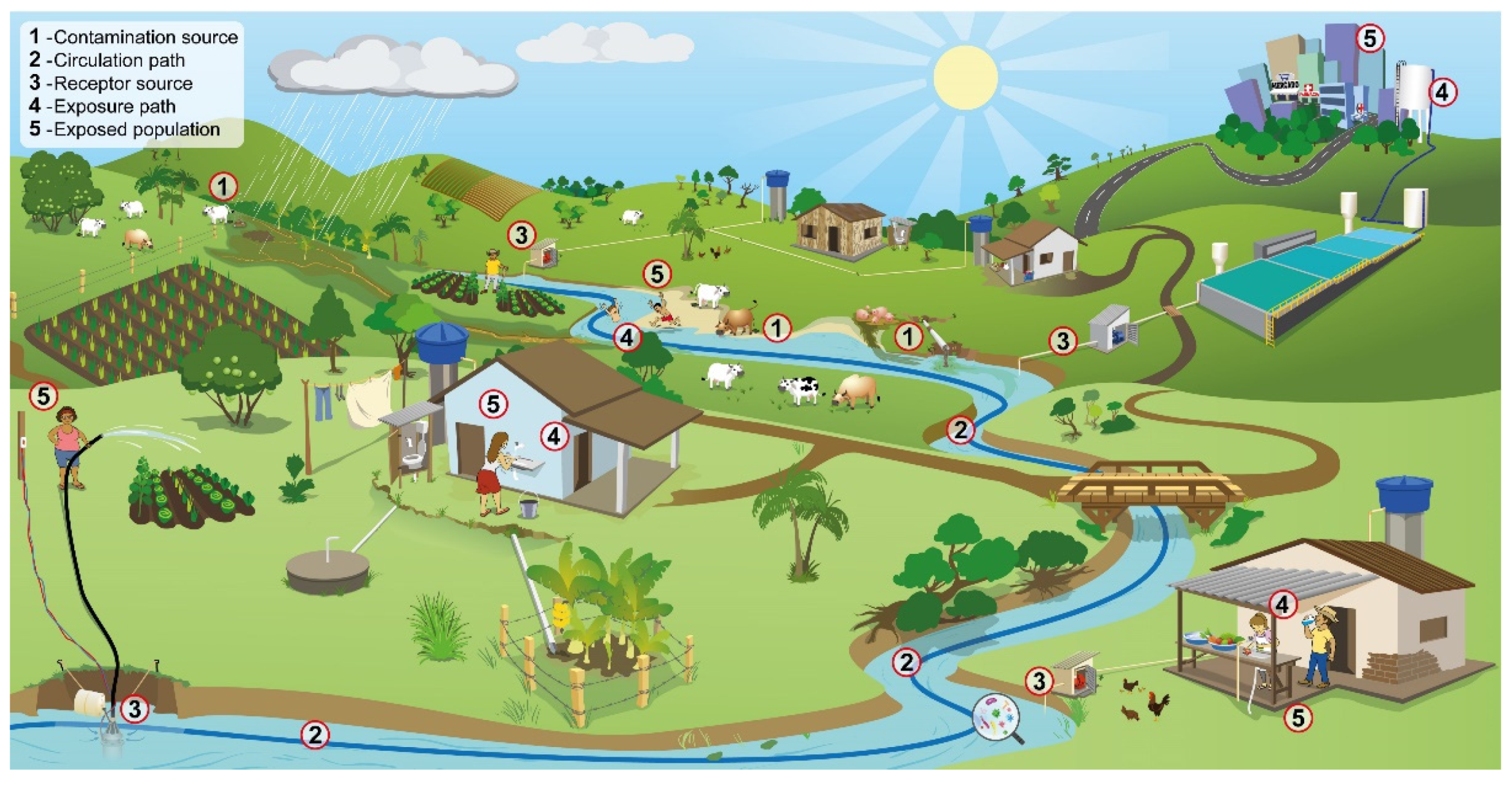
2.3. Probability of Infection
2.4. Characterization of Land Use and Occupation
2.5. Statistical Analysis
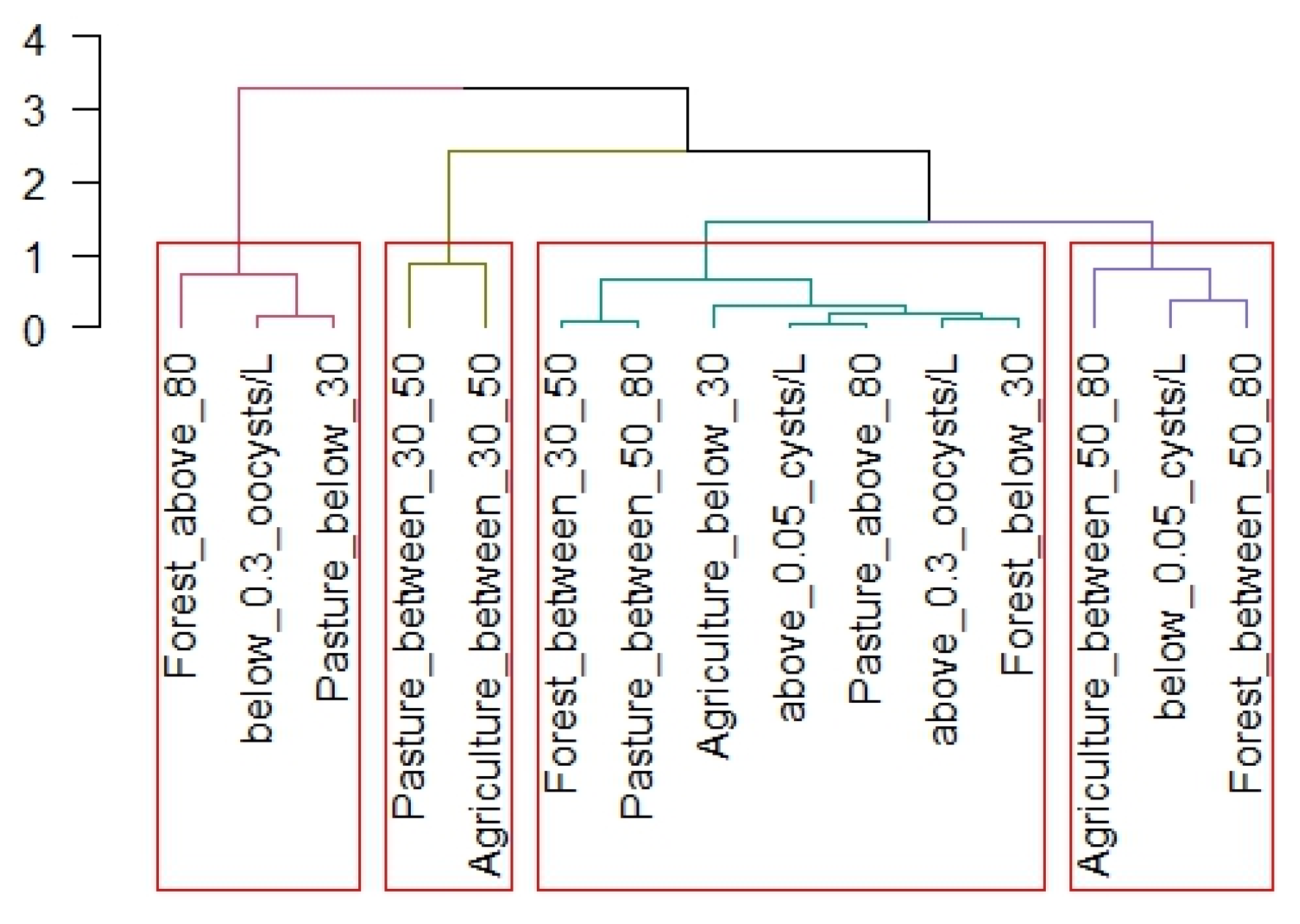
3. Results
4. Discussion
5. Conclusions
- Animal excrements may be a source of contamination in the Brazilian Cerrado, that is, a potential risk to public health. Given that, the greater presence of Cryptosporidium spp. oocysts is associated to watersheds with greater use by pasture and lesser presence of forests
- The greater presence of Giardia spp. cysts is directly associated to the percentage of mosaic of agriculture and pasture in the watershed, and indirectly to the presence of forests. There is greater risk for infection by giardiasis.
- The watersheds with pasture above 50% are more susceptible to cause cryptosporidiosis or giardiasis infection, and those with forest above 50% as safer due to a lower concentration of the protozoans. However, all waters are inadequate for consumption without previous treatment.
- The annual infection risk was overestimated, but it is a trustworthy and biologically plausible tool for the analysis of risk in rivers of the Brazilian Cerrado, which contribute to three massive South American watersheds.
6. Recommendations
- A detailed epidemiological investigation in the cases of ADD in the analyzed municipalities is recommended for validating the estimated risk of infection using the QMRA.
- It is recommended to amplify the temporal period for sample collection and the acquisition of data regarding use and occupation of land in better resolutions to improve the precision of the (oo)cysts estimator model.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministério do Meio Ambiente. Available online: https://www.icmbio.gov.br/cbc/conservacao-da-biodiversidade/biodiversidade.html (accessed on 15 November 2022).
- Brasil. Decreto nº 6.040, de 07 de Fevereiro de 2007. Available online: https://www.planalto.gov.br (accessed on 15 November 2022).
- Fundação Nacional de Saúde. Programa Nacional de Saneamento Rural; Ministério da Saúde e Fundação Nacional de Saúde: Brasília, Brazil, 2019.
- Sato, A.P.S.; Waldman, E.A. Trajetória das doenças infecciosas no Brasil nos últimos 50 anos: Um contínuo Desafio. Rev. Saúde Pública (RSP) 2016, 50, 68–85. [Google Scholar]
- Efstratiou, A.; Ongerth, J.E.; Karanis, P. Waterborne transmission of protozoan parasites: Review of worldwide outbreaks-An update 2011–2016. Water Res. 2017, 114, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Secretaria de Vigilância em Saúde. Sistema Nacional de Vigilância em Saúde: Relatório de Situação: Goiás, 5th ed.; Ministério da Saúde: Brasília, Brazil, 2011; 34p.
- Cheng, H.W.A.; Lucy, F.E.; Graczyk, T.K.; Broaders, M.A.; Tamang, L.; Connolly, M. Fate of Cryptosporidium parvum and Cryptosporidium hominis oocysts and Giardia duodenalis cysts during secondary wastewater treatments. Parasitol. Res. 2009, 105, 689. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Li, M.Y.; Qi, Z.Z.; Fu, M.; Sun, T.F.; Elsheikha, H.M.; Cong, W. Waterborne protozoan outbreaks: An update on the global, regional, and national prevalence from 2017 to 2020 and sources of contamination. Sci. Total Environ. 2022, 806, 150562. [Google Scholar] [CrossRef] [PubMed]
- Nações Unidas Brasil. Available online: https://brasil.un.org/pt-br/sdgs (accessed on 13 November 2022).
- Rodrigues, A.A.; Macedo, M.N.; Silvério, D.V.; Maracahipes, L.; Coe, M.T.; Brando, P.M.; Shimbo, J.Z.; Rajão, R.; Soares Filho, B.; Bustamante, M.M.C. Cerrado deforestation threatens regional climate and water availability for agriculture and ecosystems. Glob. Chang. Biol. 2022, 28, 6807–6822. [Google Scholar] [CrossRef]
- Dias, G.M.F.; Bevilacqua, P.D.; Bastos, R.K.X.; Oliveira, A.A.; Campos, G.M.M. Giardia spp. e Cryptosporidium spp. em agua de manancial superficial de abastecimento contaminada por dejetos humano e animal. Arq. Bras. De Med. Vet. E Zootec. 2008, 60, 1291–1300. [Google Scholar] [CrossRef]
- Brasil. Portaria nº 888 de 04 de maio de 2021. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2021/prt0888_07_05_2021.html (accessed on 13 November 2022).
- Haas, C.N.; Rose, J.B.; Gerba, C.P. Quantitative Microbial Risk Assessmen, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; p. 449. [Google Scholar]
- USEPA. National Primary Drinking Water Regulations: Disinfectants and Disinfection Byproducts; Office of the Federal Register: Washington, DC, USA, 1998. Available online: https://www.federalregister.gov/d/98-32887 (accessed on 1 September 2019).
- Gitter, A.; Mena, K.D.; Wagner, K.L.; Boellstorff, D.E.; Borel, K.E.; Gregory, L.F.; Gentry, T.J.; Karthikeyan, R. Human health risks associated with recreational waters: Preliminary approach of integrating quantitative microbial risk assessment with microbial source tracking. Water 2020, 12, 327. [Google Scholar] [CrossRef] [Green Version]
- Morales, E.; Ibarra, G.; Reyes, L.; Barrantes, K.; Achí, R.; Chacón, L. Disease burden from simultaneous exposure of Cryptosporidium sp. and Giardia sp. and land use vulnerability assessment in a Costa Rican drinking water system. Microb. Risk Anal. 2022, 21, 100213. [Google Scholar] [CrossRef]
- Bataiero, M.O.; Araujo, R.S.; Nardocci, A.C.; Matté, M.H.; Sato, M.I.Z.; Lauretto, M.D.S.; Razzolini, M.T.P. Quantification of Giardia and Cryptosporidium in surface water: A risk assessment and molecular characterization. Water Supply 2019, 19, 1823–1830. [Google Scholar] [CrossRef]
- Cristiane Pinto, K.; de Souza Lauretto, M.; Navarro Gonzalez, M.I.J.; Sato, M.I.Z.; Nardocci, A.C.; Razzolini, M.T.P. Assessment of health risks from recreational exposure to Giardia and Cryptosporidium in coastal bathing waters. Environ. Sci. Pollut. Res. 2020, 27, 23129–23140. [Google Scholar] [CrossRef]
- Silva, D.P.; Reis, Y.P.; Bezerra, N.R.; Carneiro, L.C.; Scalize, P.S. Risk to human health from protozoan (oo)cysts in water treatment plants. Eng. Sanitária E Ambient. 2021, 26, 845–854. [Google Scholar] [CrossRef]
- Krkosek, W.; Reed, V.; Gagnon, G.A. Assessing protozoan risks for surface drinking water supplies in Nova Scotia, Canada. J. Water Health 2016, 14, 155–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goiás. Plano Estadual de Recursos Hídricos do Estado de Goiás: Produto 5; Secretaria de Estado de Meio Ambiente e Desenvolvimento Sustentável: Goiânia, Brazil, 2015. [Google Scholar]
- Lima, J.E.F.W.; Silva, E.M. Estimativa da superficial do Cerrado brasileiro. In Cerrado: Ecologia, Biodiversidade e Conservação, 1st ed.; Scariot, A., Souza-Silva, J.C., Felfili, J.M., Eds.; Ministério do Meio Ambiente: Brasília, Brazil, 2005; pp. 61–72. [Google Scholar]
- Köppen, W. Climatologia: Com um Estudio de los Climas de la Tierra, 1st ed.; Fondo de Cultura Economica: Buenos Aires, Mexico, 1948; p. 478. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda, 4th ed.; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 15 November 2022).
- Goiás. Resolução nº 26 de 05 de Dezembro de 2012. Available online: https://www.meioambiente.go.gov.br/transpar%C3%AAncia/legisla%C3%A7%C3%A3o.html#cerhi (accessed on 15 November 2022).
- Franco, R.M.B.; Rocha-Eberhardt, R.; Cantusio Neto, R. Occurrence of Cryptosporidium oocysts and Giardia cysts in raw water from the Atibaia river, Campinas, Brazil. Rev. Do Inst. De Med. Trop. De São Paulo 2001, 43, 109–111. [Google Scholar] [CrossRef]
- Shepherd, K.M.; Wyn-Jones, A.P. An evaluation of methods for the simultaneous detection of Cryptosporidium oocysts and Giardia cysts from water. Appl. Environ. Microbiol. 1996, 62, 1317–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EPA 816 -R-12-001; Method 1623.1 Cruptosporidium an Giardia in Water by Filtration/IMS/FA. Office of Water (MS-140). United States Enviromental Protection Agency: Washington, DC, USA, 2012.
- Cantusio Neto, R.; Franco, R.M.B. Ocorrência de oocistos de Cryptosporidium. e cistos de Giardia em diferentes pontos do processo de tratamento de água, em Campinas, São Paulo, Brasil. Hig. Aliment. 2004, 18, 52–59. [Google Scholar]
- Lapen, D.R.; Schmidt, P.J.; Thomas, J.L.; Edge, T.A.; Flemming, C.; Keithlin, J.; Neumann, N.; Pollari, F.; Ruecker, N.; Simhom, A.; et al. Towards a more accurate quantitative assessment of seasonal Cryptosporidium infection risks in surface waters using species and genotype information. Water Res. 2016, 105, 625–637. [Google Scholar] [CrossRef]
- Murphy, H.M.; Thomas, M.K.; Schmidt, P.J.; Medeiros, D.T.; McFadyen, S.; Pintar, K.D.M. Estimating the burden of acute gastrointestinal illness due to Giardia, Cryptosporidium, Campylobacter, E. coli O157 and norovirus associated with private wells and small water systems in Canada. Epidemiol. Infect. 2016, 144, 1355–1370. [Google Scholar] [CrossRef] [Green Version]
- Razzolini, M.T.P.; Santos, T.F.S.; Bastos, V.K. Detecção de cistos/oocistos de Giardia e Cryptosporidium em bacias hidrográficas e fontes de água potável em áreas urbanas do Brasil. J. De Água E Saúde 2010, 8, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Gerba, C.P. Risk Assessment. In Environmental Microbiology, 1st ed.; Maier, R.M., Pepper, I.L., Gerba, C.P., Eds.; Academic Press: San Diego, CA, USA, 2000; pp. 557–570. [Google Scholar]
- MAPBIOMAS. Available online: https://mapbiomas.org/colecoes-mapbiomas-1?cama_set_language%20=pt-br (accessed on 1 August 2020).
- R: A Language and Environment for Statistical Computing. R. Available online: http://softlibre.unizar.es/manuales/aplicaciones/r/fullrefman.pdf (accessed on 6 November 2022).
- Wallis, P.M.; Erlandsen, S.L.; Isaac-Renton, J.L.; Olson, M.E.; Robertson, W.J.; Van Keulen, H. Prevalence of Giardia cysts and Cryptosporidium oocysts and characterization of Giardia spp. isolated from drinking water in Canada. Appl. Environ. Microbiol. 1996, 62, 2789–2797. [Google Scholar] [CrossRef] [Green Version]
- Haas, C.N.; Rose, J.B. Developing an action level for Cryptosporidium. J. Am. Water Work. Assoc. 1995, 87, 81–84. [Google Scholar] [CrossRef]
- MCA––Multiple Correspondence Analysis in R: Essentials. Available online: http://www.sthda.com/english/articles/31-principal-component-methods-in-r-practical-guide/114-mca-multiple-correspondence-analysis-in-r-essentials/ (accessed on 9 November 2022).
- Brasil. Resolução do CONAMA nº 357, de 17 de Março de 2005. Available online: https://www.icmbio.gov.br/cepsul/images/stories/legislacao/Resolucao/2005/res_conama_357_2005_classificacao_corpos_agua_rtfcda_altrd_res_393_2007_397_2008_410_2009_430_2011.pdf (accessed on 16 December 2022).
- USEPA. Use of Micobial Risk Assessment in Setting EPA Drinking Water Standards; Office of the Federal Register: Washington, DC, USA, 1992.
- Karanis, P. Giardia and Cryptosporidium: Occurrence in Water Supplies. Encycl. Environ. Health 2011, 1, 946–954. [Google Scholar] [CrossRef]
- Thompson, R.C.A. The zoonotic significance and molecular epidemiology of Giardia and Giardiasis. Vet. Parasitol. 2004, 126, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.M.B.; Hachich, E.M.; Sato, M.I.S.; Naveira, R.M.L.; Silva, E.D.C.; Campos, M.M.D.C.; Cantusio Neto, R.; Cerqueira, D.A.; Branco, N.; Leal, D.A.G. Performance evaluation of different methodologies for detection of Cryptosporidium spp. and Giardia spp. in water for human consumption to meet the demands of the Environmental Health Surveillance in Brazil. Epidemiol. E Serviços De Saúde 2012, 21, 233–242. [Google Scholar] [CrossRef]
- Hunter, P.R.; De Sylor, M.A.; Risebro, H.L.; Nichols, G.L.; Kay, D.; Hartemann, P. Quantitative microbial risk assessment of cryptosporidiosis and giardiasis from very small private water supplies. Risk Anal. Int. J. 2011, 31, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L. Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 2010, 1, 80–89. [Google Scholar] [CrossRef]
- Ryan, U.N.A.; Fayer, R.; Xiao, L. Cryptosporidium species in humans and animals: Current understanding and research needs. Parasitology 2014, 141, 1667–1685. [Google Scholar] [CrossRef] [Green Version]
- Lane, S.; Lloyd, D. Current Trends in Research into the Waterborne Parasite Giardia. Crit. Rev. Microbiol. 2002, 28, 123–147. [Google Scholar] [CrossRef]
- Quon, H.; Allaire, M.; Jiang, S.C. Assessing the Risk of Legionella Infection through Showering with Untreated Rain Cistern Water in a Tropical Environment. Water 2021, 13, 889. [Google Scholar] [CrossRef]
- Burch, T. Validation of quantitative microbial risk assessment using epidemiological data from outbreaks of waterborne gastrointestinal disease. Risk Anal. 2019, 39, 599–615. [Google Scholar] [CrossRef]
- Petterson, S.R.; Signor, R.S.; Ashbolt, N.J. Incorporating method recovery uncertainties in stochastic estimates of raw water protozoan concentrations for QMRA. J. Water Health 2007, 5, 51–65. [Google Scholar] [CrossRef] [Green Version]
- Bergion, V.; Sokolova, E.; Åström, J.; Lindhe, A.; Sörén, K.; Rosén, L. Hydrological modelling in a drinking water catchment area as a means of evaluating pathogen risk reduction. J. Hydrol. 2017, 544, 74–85. [Google Scholar] [CrossRef]


| Parameter | Pathogen-Reference | Reference | |
|---|---|---|---|
| Giardia ssp. | Cryptosporidium ssp. | ||
| V (L/day) 1 | 2 | 2 | [24] |
| Tr (%) | 78.1 | 60.6 | [19] |
| R (log) | 0 | 0 | NA |
| r * | 0.0199 | 0.0042 | [13] |
| n (days) | 365 | 365 | NA |
| ID | Surface Source Name | Turbidity (NTU) | Giardia (cysts/L) | Cryptosporidium (oocysts/L) | LD ((oo)cysts/L) | Pinf(a) Giardia 1 | Pinf(a) Cryptosporidium 1 |
|---|---|---|---|---|---|---|---|
| 1 | Pedras Creek PS | 5.98 | 116.67 | 133.33 | 8.33 | 1.00 | 1.00 |
| 2 | Boa Esperança Creek PS | 6.86 | 100.00 | 175.00 | 8.33 | 1.00 | 1.00 |
| 3 | São Manoel Creek PS | 6.80 | 66.67 | 208.33 | 8.33 | 1.00 | 1.00 |
| 4 | Cerrado Creek PS | 7.19 | 58.33 | 216.67 | 8.33 | 1.00 | 1.00 |
| 5 | Santana Creek PS | 12.40 | 66.67 | 66.67 | 8.33 | 1.00 | 1.00 |
| 6 | Dirceu Creek PS | 5.93 | 58.33 | 108.33 | 8.33 | 1.00 | 1.00 |
| 7 | Anda Só Creek PS | 11.90 | 16.67 | 108.33 | 8.33 | 1.00 | 1.00 |
| 8 | Forquilha Creek PS | 2.11 | 16.67 | 62.50 | 4.17 | 1.00 | 1.00 |
| 9 | Pari River PS | 7.38 | <LD | 8.33 | 8.33 | 1.00 | 1.00 |
| 10 | Barro Alto Creek PS | 7.47 | <LD | 58.33 | 8.33 | 1.00 | 1.00 |
| 11 | Novo River PS | 4.99 | 50.00 | 66.67 | 8.33 | 1.00 | 1.00 |
| 12 | Almas River PS | 4.84 | 58.33 | 66.67 | 8.33 | 1.00 | 1.00 |
| 13 | Jatobá Creek PS | 2.44 | 8.33 | 16.67 | 4.17 | 1.00 | 1.00 |
| 14 | Olho D’água Creek PS | 16.30 | 116.67 | 250.00 | 16.67 | 1.00 | 1.00 |
| 15 | Uru River PS | 2.64 | 79.17 | 54.17 | 4.17 | 1.00 | 1.00 |
| 16 | Poções Creek PS | 6.95 | <LD | <LD | 4.17 | 1.00 | 9.94 × 10−1 |
| 17 | Morcego Creek PS | 4.99 | <LD | 8.33 | 8.33 | 1.00 | 1.00 |
| 18 | Palmeiras Creek PS | 62.10 | 41.67 | 300.00 | 8.33 | 1.00 | 1.00 |
| 19 | Café Creek PS | 15.7 | <LD | 8.33 | 4.17 | 1.00 | 1.00 |
| 20 | Barreirinha Creek PS | 4.69 | 8.33 | 45.00 | 1.67 | 1.00 | 1.00 |
| 21 | Santa Maria Creek UR | 7.48 | <LD | 11.67 | 1.67 | 1.00 | 1.00 |
| 22 | Formiga Creek UR | 14.5 | 16.67 | 91.67 | 8.33 | 1.00 | 1.00 |
| 23 | Mata Creek UR | 15.4 | 8.33 | 208.33 | 8.33 | 1.00 | 1.00 |
| 24 | São Jorge Creek UR | 15.8 | 8.33 | 241.67 | 8.33 | 1.00 | 1.00 |
| 25 | Pombal River UR | 3.59 | <LD | <LD | 8.33 | 1.00 | 9.94 × 10−1 |
| 26 | Fundo Creek UR | 18.2 | 33.33 | 141.67 | 8.33 | 1.00 | 1.00 |
| 27 | Santa Família Creek UR | 3.57 | 1.67 | 10.00 | 1.67 | 1.00 | 1.00 |
| 28 | Pica Pau Creek UR | 1.37 | <LD | 8.33 | 1.67 | 1.00 | 1.00 |
| 29 | São Sebastião Creek UR | 2.62 | 4.17 | 4.17 | 4.17 | 1.00 | 1.00 |
| 30 | Sucuapara Creek UR | 4.14 | <LD | 4.17 | 4.17 | 1.00 | 1.00 |
| 31 | Retiro Creek UR | 19.3 | <LD | <LD | 8.33 | 1.00 | 9.94 × 10−1 |
| 32 | Araguaia River UR | 7.74 | <LD | 22.22 | 11.11 | 1.00 | 1.00 |
| 33 | Água Limpa Creek UR | 21.7 | 4.17 | 12.50 | 4.17 | 1.00 | 1.00 |
| 34 | Gameleira Creek UR | 3.67 | 8.33 | <LD | 4.17 | 1.00 | 9.94 × 10−1 |
| 35 | Affluent of Maranhão RiverUR | 2.11 | 8.33 | 4.17 | 4.17 | 1.00 | 1.00 |
| 36 | Landi Creek UR | 3.8 | <LD | 100.00 | 16.67 | 1.00 | 1.00 |
| 37 | Araguaia River UR | 11.8 | 16.67 | 83.33 | 16.67 | 1.00 | 1.00 |
| 38 | Araguaia River UR | 5.07 | <LD | 88.89 | 22.22 | 1.00 | 1.00 |
| 39 | Cachoeirinha Creek UR | 1.38 | 44.44 | 66.67 | 11.11 | 1.00 | 1.00 |
| 40 | Macaco Creek UR | 17.6 | <LD | <LD | 5.56 | 1.00 | 9.94 × 10−1 |
| 41 | Grande Creek UR | 26.4 | <LD | 16.67 | 8.33 | 1.00 | 1.00 |
| Average | 1.00 | 9.99 × 10−1 |
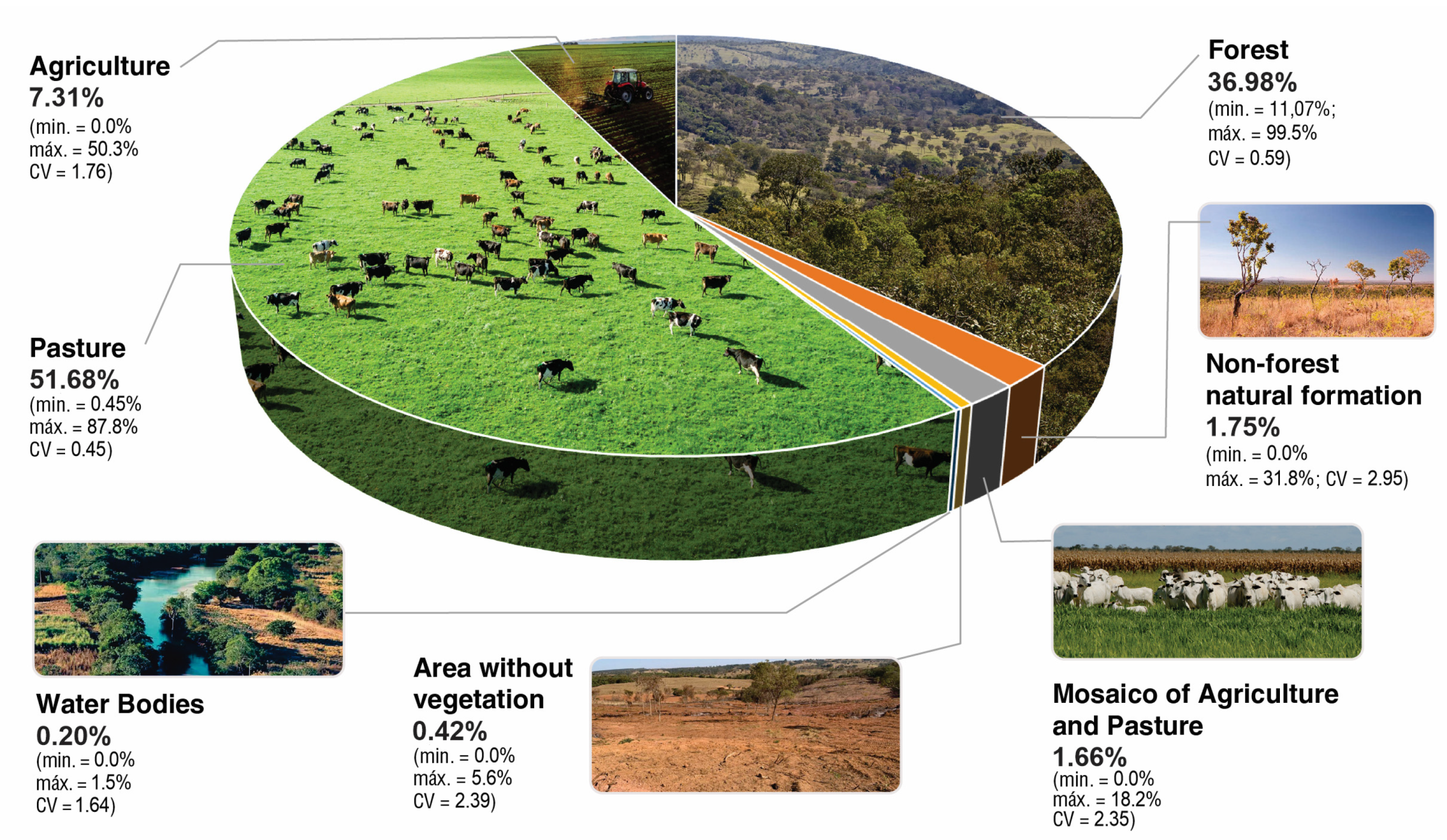
| ID | WS Area (km2) | Forest (%) | Non-Forest Natural Formation (%) | Pasture (%) | Agriculture (%) | Mosaic of Agriculture and Pasture (%) 1 | Area without Vegetation (%) | Water Bodies (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 216.01 | 24.34 | 0.70 | 45.61 | 11.20 | 18.16 | 0.00 | 0.00 |
| 2 | 28.51 | 23.95 | 0.33 | 75.33 | 0.39 | 0.00 | 0.00 | 0.00 |
| 3 | 132.21 | 15.50 | 0.03 | 71.61 | 4.75 | 8.02 | 0.00 | 0.10 |
| 4 | 39.34 | 12.53 | 0.01 | 86.32 | 0.91 | 0.23 | 0.00 | 0.00 |
| 5 | 27.67 | 14.40 | 0.03 | 83.12 | 2.43 | 0.00 | 0.03 | 0.00 |
| 6 | 14.93 | 26.29 | 0.68 | 35.95 | 28.49 | 7.57 | 0.93 | 0.10 |
| 7 | 91.22 | 28.28 | 0.73 | 51.83 | 13.67 | 4.83 | 0.00 | 0.66 |
| 8 | 11.75 | 62.70 | 0.12 | 37.17 | 0.00 | 0.00 | 0.00 | 0.00 |
| 9 | 908.53 | 29.82 | 0.37 | 66.40 | 2.92 | 0.37 | 0.13 | 0.00 |
| 10 | 33.83 | 51.81 | 4.20 | 40.88 | 0.16 | 2.72 | 0.16 | 0.07 |
| 11 | 217.69 | 29.45 | 0.17 | 61.14 | 2.52 | 6.67 | 0.01 | 0.03 |
| 12 | 10,984.71 | 32.40 | 0.69 | 52.23 | 6.93 | 7.12 | 0.38 | 0.25 |
| 13 | 18.29 | 12.20 | 0.11 | 24.16 | 50.27 | 11.73 | 0.00 | 1.53 |
| 14 | 25.12 | 28.05 | 0.86 | 71.10 | 0.00 | 0.00 | 0.00 | 0.00 |
| 15 | 3534.70 | 32.36 | 0.37 | 54.34 | 12.14 | 0.44 | 0.36 | 0.00 |
| 16 | 26.38 | 29.00 | 0.00 | 36.60 | 28.82 | 0.00 | 5.58 | 0.00 |
| 17 | 8.20 | 99.53 | 0.00 | 0.45 | 0.00 | 0.00 | 0.01 | 0.00 |
| 18 | 42.32 | 18.29 | 0.00 | 81.14 | 0.00 | 0.00 | 0.56 | 0.05 |
| 19 | 18.11 | 21.25 | 0.00 | 73.83 | 4.41 | 0.00 | 0.51 | 0.00 |
| 20 | 10.65 | 13.28 | 0.00 | 86.55 | 0.00 | 0.00 | 0.10 | 0.08 |
| 21 | 428.62 | 62.09 | 0.00 | 26.56 | 8.36 | 0.00 | 2.95 | 0.04 |
| 22 | 14.07 | 23.18 | 0.00 | 75.99 | 0.00 | 0.00 | 0.73 | 0.11 |
| 23 | 202.16 | 29.98 | 0.00 | 69.70 | 0.06 | 0.00 | 0.08 | 0.24 |
| 24 | 126.31 | 21.41 | 0.00 | 77.97 | 0.02 | 0.00 | 0.01 | 0.60 |
| 25 | 138.07 | 75.61 | 0.00 | 23.33 | 0.96 | 0.00 | 0.06 | 0.05 |
| 26 | 42.19 | 35.78 | 0.00 | 54.24 | 9.87 | 0.00 | 0.03 | 0.08 |
| 27 | 79.89 | 50.21 | 0.00 | 49.68 | 0.02 | 0.00 | 0.03 | 0.06 |
| 28 | 12.88 | 58.95 | 0.00 | 40.71 | 0.35 | 0.00 | 0.00 | 0.00 |
| 29 | 150.44 | 26.49 | 3.96 | 31.04 | 38.24 | 0.00 | 0.16 | 0.11 |
| 30 | 36.53 | 12.92 | 0.04 | 39.07 | 47.64 | 0.00 | 0.04 | 0.30 |
| 31 | 16.52 | 11.07 | 0.00 | 87.80 | 1.13 | 0.00 | 0.00 | 0.00 |
| 32 | 51,161.88 | 36.20 | 3.96 | 51.68 | 7.64 | 0.00 | 0.27 | 0.25 |
| 33 | 8.80 | 29.43 | 0.06 | 70.29 | 0.00 | 0.00 | 0.01 | 0.22 |
| 34 | 5.51 | 82.02 | 0.02 | 17.11 | 0.85 | 0.00 | 0.00 | 0.00 |
| 35 | 5.07 | 66.66 | 7.45 | 25.89 | 0.00 | 0.00 | 0.00 | 0.00 |
| 36 | 51.26 | 36.71 | 1.33 | 60.56 | 0.26 | 0.00 | 0.52 | 0.62 |
| 37 | 68,117.81 | 34.18 | 3.59 | 55.31 | 6.27 | 0.00 | 0.28 | 0.38 |
| 38 | 117,515.70 | 34.08 | 2.80 | 58.21 | 4.03 | 0.00 | 0.27 | 0.61 |
| 39 | 29.98 | 56.98 | 31.78 | 8.96 | 0.00 | 0.00 | 2.29 | 0.00 |
| 40 | 33.33 | 84.67 | 0.00 | 13.75 | 0.00 | 0.00 | 0.50 | 1.08 |
| 41 | 128.40 | 42.09 | 7.43 | 45.54 | 3.94 | 0.00 | 0.38 | 0.62 |
| Average | 36.98 | 1.75 | 51.68 | 7.31 | 1.66 | 0.42 | 0.20 |
| Variable | Giardia | Cryptosporidium |
|---|---|---|
| Area (km2) | −0.139 | −0.010 |
| Turbidity (NTU) | 0.027 | 0.455 |
| Giardia spp. (cysts/L) | 1.000 | 0.581 |
| Cryptosporidium spp. (oocysts/L) | 0.581 | 1.000 |
| LD ((oo)cysts/L) | 0.218 | 0.412 |
| Forest (%) | −0.327 | −0.418 |
| Non-forest natural formation (%) | 0.017 | −0.086 |
| Pasture (%) | 0.277 | 0.532 |
| Agriculture (%) | −0.066 | −0.232 |
| Mosaic of agriculture and pasture (%) | 0.452 | 0.132 |
| Area without vegetation (%) | −0.129 | −0.173 |
| Water bodies (%) | −0.290 | −0.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, D.P.d.; Bezerra, N.R.; Basso, R.E.; Vieira, M.d.N.; Scalize, P.S. Health Risks in a Brazilian Cerrado Population Due to Pathogens Transmitted through Water and Land Use Conditions. Water 2023, 15, 158. https://doi.org/10.3390/w15010158
Silva DPd, Bezerra NR, Basso RE, Vieira MdN, Scalize PS. Health Risks in a Brazilian Cerrado Population Due to Pathogens Transmitted through Water and Land Use Conditions. Water. 2023; 15(1):158. https://doi.org/10.3390/w15010158
Chicago/Turabian StyleSilva, Débora Pereira da, Nolan Ribeiro Bezerra, Raviel Eurico Basso, Maria da Natividade Vieira, and Paulo Sérgio Scalize. 2023. "Health Risks in a Brazilian Cerrado Population Due to Pathogens Transmitted through Water and Land Use Conditions" Water 15, no. 1: 158. https://doi.org/10.3390/w15010158
APA StyleSilva, D. P. d., Bezerra, N. R., Basso, R. E., Vieira, M. d. N., & Scalize, P. S. (2023). Health Risks in a Brazilian Cerrado Population Due to Pathogens Transmitted through Water and Land Use Conditions. Water, 15(1), 158. https://doi.org/10.3390/w15010158