Abstract
The paper presents the results of physicochemical analyses of spring waters in the Postomia River valley (Northwest Poland). Multivariate statistical methods, i.e., cluster analysis (CA) and principal component analysis (PCA) were used to assess the spatial distribution of similarities and differences in the concentrations of individual elements. Concentrations of macro elements (MEs), trace elements (TEs) and rare-earth elements (REEs) were analysed concerning the spring’s typology, land use structure and the distance from roads. The results showed that the springs waters are of the Ca2+-HCO3− and Ca2+-HCO3−-SO42− types, medium hardness and low mineralisation. The study revealed differences between valley springs and scarp-foot springs in terms of electrical conductivity and concentrations of F−, SO42−, NO3−, Mg2+, Ba, Zn, and U. Greater variability was observed between the physical and chemical conditions of the spring waters due to their location in terms of land use. Springs located in agricultural areas had lower pH values than those in other areas, and higher NO3− concentrations. The pH values and concentrations of Fe, Mo, Rb, and Th in urban areas were higher than in agricultural areas. Moreover, the concentrations of F−, Cl−, K+, Na+, Mo, Sb, Se, and Sr were higher in urban areas than in forested areas. The study shows that only HCO3− values and SO42− concentrations were related to the distance from the road network. The concentrations of Cl−, SO42−, and K+ were higher in the waters of springs located more than 50 m from the road network. The Ca and PCA analysis did not permit the identification of a single dominant origin of pollutants, suggesting an interaction of different types of pollution sources.
1. Introduction
Springs are natural spontaneous outflows of underground waters to the ground surface [1]. They constitute an abiotic element of the environment, dependent on the geological structure, relief, and climatic conditions in a given area [2]. Springs are located at the interface between two systems [3], connecting the underground and surface elements in the water cycle. They are essential components of the environment, and need to be protected against anthropogenic impact [4], mainly due to their frequent use as sources of drinking water [5]. Their importance will grow with the vast depletion of water resources due to climate change [6]. Preserving adequate water value requires accurate identification of the spring status and the assessment of both natural and anthropogenic factors determining their quality [7].
Due to the small number of springs in its area, the Polish Lowland is less thoroughly explored in those terms than the mountain regions [8]. Springs in lowlands are more challenging to identify. They are usually point sources, and due to their limited output, they are considered of marginal importance, or even completely disregarded [9]. Their role is significant, however, through balancing river runoff and draining underground waters [10]. Moreover, they are important from an ecological point of view [4].
In the Postomia catchment, surface waters come into contact with underground waters. This is promoted by the geological structure, alternate arrangement of permeable and impermeable deposits, tunnel valleys, and lack of aquifer continuity [11]. The underground waters of the Quaternary and Paleogene–Neogene horizons are supplied by infiltrating precipitation waters. This causes a serious penetration of contaminants into deeper aquifer horizons. In the active water exchange zone, the underground waters are influenced by natural and anthropogenic processes [12]. Human pressure may have a negative effect on the quality of underground waters [13,14,15].
The area of the Postomia catchment has not been investigated in terms of physicochemical changes occurring in underground waters. In all locations, underground waters have a unique chemical composition resulting from geochemical and biological processes [16]. Literature sources describe many natural factors influencing the chemical character of waters [17,18,19]. In-depth knowledge on geochemistry is therefore necessary to identify processes determining the chemical composition of underground waters [12,20,21,22]. Spring water chemistry is influenced by a number of factors, including geology, climate, vegetation, and land use, determining groundwater residence times and water–rock interaction [23,24,25]. Gao et al. [26] indicate that accelerating urbanisation processes and intensifying human activities have significant impacts on spring waters. Anthropogenic activities are the dominant processes controlling the chemical evolution of spring waters in the region [27]. These include atmospheric pollution resulting from industrial activity [28] in the form of gases, dusts, and aerosols. They are transferred from the emission sites and penetrate to the hydrogeochemical cycle after precipitation, taking the form of large-area pollutant sources for underground waters [29]. Particularly dangerous contaminants result from unregulated water supply and sewage disposal [30,31], discharge of pollutants from nearby farms [29], or inappropriate agricultural practices [5,32]. Large amounts of sewage generated by urban areas, industry, and agricultural production contaminate the soil and groundwaters [13,33,34,35]. Moreover, the degradation of underground water resources may be connected with local factors, e.g., landfills in headwater areas [29]. The chemical composition of spring waters depends on the composition of tree stands covering their catchment area [36]. Studies also demonstrate the negative impact of roads and road transport on groundwater quality [37,38]. The effects of winter road maintenance substances on groundwater quality are also highlighted [39].
The objective of this study conducted in the Postomia River catchment was: (1) to determine concentrations of macro elements (MEs), trace elements (TEs), and rare-earth elements (REEs) in spring waters, (2) to divide springs into groups characterised by similar water chemical properties, (3) to correlate concentrations of MEs, TEs, and REEs with physical parameters, i.e., hydrodynamic type, morphometry, and land use, and (4) to identify the origin of individual elements and compounds in spring waters. The study of REEs in spring waters has a pioneering character.
2. Materials and Methods
2.1. Study Area
The Postomia River is located in Northwest Poland (Figure 1). It is a left tributary of the Warta River. The catchment covers an area of approximately 200 km2. The Postomia catchment is located in the temperate climate transition zone. The mean annual temperature here is 8.5 °C, and the mean annual precipitation total reaches 500 mm. Analyses were conducted in the catchment within the upper and middle reaches of the Postomia River. The area (the upper stretch is used as a military training area in Wędrzyn) is to a considerable degree covered by mixed broadleaved forests. Springs 7 to 15 are located within the military area (Table S1). Next, the river flows through a forest complex called Bory Postomskie (Postomia Coniferous Forests). In the town of Sulęcin, building development has altered water relations, hindered water outflow from the developed area, and cut off the links between small water bodies and the river. In the Sulęcin–Krzeszyce stretch, natural water bodies have been transformed into fishing ponds with recreational facilities located above.
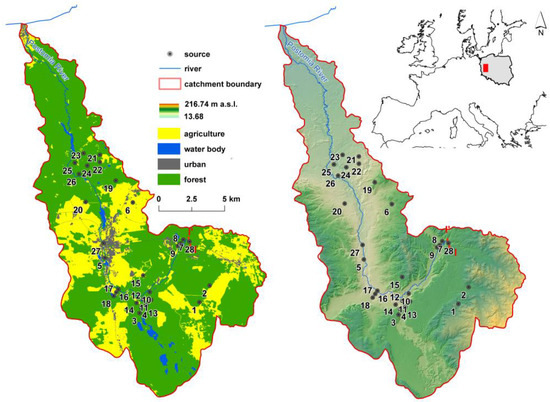
Figure 1.
Study area with sampling point locations.
In terms of its geological structure, the catchment is composed of Quaternary deposits, mainly of glacial origin, and to a lesser degree of Holocene deposits (formed primarily as a result of fluvial and aeolian processes). Among Pleistocene deposits connected with glacial accumulation, glacial tills, sands, and gravels are found along with loamy sands that frequently separate glaciofluvial and glaciolacustrine accumulation deposits [40]. Waters of the Quaternary deposits constitute the main available horizon of underground waters, connected with gravel and sandy postglacial and fluvial deposits. Aquifers are found in deposits filling contemporary river valleys such as the Warta and its major tributaries. The greatest water resources in this horizon are connected with fossil (buried) valleys and primal valleys. These structures contain aquifers as large as several dozen meters in thickness [41]. A shallow groundwater table, lack of isolation from the surface provided by poorly permeable deposits, the draining nature of the first aquifer, and numerous human settlements pose an anthropogenic threat to the waters.
2.2. Sample Collection and Preparation
Water samples for physicochemical analyses were collected in the period from 27 to 31 October 2017 from 28 springs in the upper and central parts of the Postomia catchment (Figure 1). All springs identified during the desktop and field studies were selected for analysis. The location of the investigated springs was determined using a GPS GARMIN eTrex 10 device by Garmin Ltd. Measurements taken on-site using a multi-functional measuring device by EIJKELKAMP included water temperature, pH, and electrolytic conductivity (Table 1). Water samples of 500 mL were collected to polyethylene bottles (HDPE) by Nalgene®. Samples used for chemical analyses were acidified in situ. High-purity 65% HNO3 and CHCl3 Pro Analysis® (Merck, Darmstadt, Germany) were used for trace element subsamples in an amount needed to obtain pH < 2. After collection, the samples were taken to a chemical laboratory in a mobile refrigerator at a temperature of 4 ± 2.5 °C. Adequate precautions were applied to avoid water contamination during sample collection, transport, and handling.

Table 1.
The values of descriptive statistics for physical parameters and the concentrations of macro elements, micro elements and rare-earth elements in spring waters.
2.3. Chemical Analysis
Spring water samples were also analysed for their cations (Na+, NH4+, K+, Ca2+, and Mg2+) and anions (Cl−, F−, NO3−, NO2−, SO42−, and PO43−) using a Metrohm ion chromatograph (IC), model 881Compact IC Pro (Metrohm, Switzerland) [42].
Concentrations of Al, As, Cd, Ce, Co, Cr, Cu, Fe, Li, Mn, Ni, Pb, Sb, Se, Sr, V, Mo, Zn, Rb, La, Pr, Nd, Eu, Gd, Tb, Dy, Ho, Er, Tm, Lu, Sc, Th, and U were determined by inductively coupled plasma mass spectrometry (ICP-QQQ 8800 Triple Quad, Agilent Technologies, Tokyo, Japan) [43,44,45].
Alkalinity was measured by in situ titration with HCl (0.1 N) using methyl orange as an indicator. Chemical oxygen demand (CODMn) was determined as a permanganate index. As a quality control measure, the ionic error balance was calculated. The calculated error did not exceed ±3%.
2.4. Reagents and Certified Reference Material
During ion chromatography (IC) assays, standard solutions from Merck (Merck, Darmstadt, Germany) and CPAchem (C.P.A. Ltd., Stara Zagora, Bulgaria) were used. The mobile phase for cations and anions was prepared using Fluka reagents (Sigma-Aldrich, Steinheim, Switzerland).
Assays using the ICP-QQQ technique were performed using calibration curves obtained from the diluted stock multi-element standard at 100 µg/mL (VHG Labs, Manchester, NH, USA). The used reagents were ultrapure, and the water was de-ionised to a resistivity of 18.2 MΩ·cm in a Direct-Q® UV3 Ultrapure Water System apparatus (Millipore, France). The analytical quality control was verified by the analysis of certified reference materials, i.e., SRM 1640a (National Institute of Standards and Technology, Gaithersburg, MD, USA), and SPS-SW2 (Spectra pure Standards As, Oslo, Norway). High compliance with reference values was found.
2.5. Statistical Analysis
In the first stage of the analysis, the minimum, mean, median and maximum values were calculated for all elements. Furthermore, lower and upper quartile values, standard deviation, skewness, and interquartile range (IQR) were calculated. In the second stage, the concentrations of macro elements (MEs), trace elements (TEs), and rare-earth elements (REEs) were analysed in reference to the spring’s typology and land use structure. Based on the direction of the hydrodynamic force, the springs were classified to the respective types: descending (27) and ascending (1). In terms of relief, the springs were grouped into those located in valleys (19), by riverbeds (2), scarp-foot (6), and slope (1). In terms of land use, the springs were situated in agricultural fields (4), forests (17), and urban areas (7). The assessment of differences in the concentrations of MEs, TEs, and REEs in the designated groups of springs employed a non-parametric Kruskal–Wallis test coupled with a post-hoc Dunn test at a significance level of p ≤ 0.05. In the third stage, the correlation analysis was carried out to assess the impact of roads on spring water pollution. The analysis aimed to answer the question whether the concentration of MEs, TEs, and REEs is related to the distance of the spring from the road. Moreover, the differences in ME, TE, and REE concentrations were analysed for two groups of springs at distances of up to 50 m (Group 1) and more than 50 m (Group 2).
In the fourth stage, to determine the enrichment of spring waters with REEs, their concentrations were standardised in reference to the average concentrations of REEs in chondrite [46]. Then, the results were visualised on graphs to show their pattern. This allowed the identification of anomalies, i.e., concentrations higher than the reference values and the preceding and following REEs. Anomalies in REE concentrations enable the identification of potential sources of pollution and the influence and type of natural processes [44].
In the fifth stage, prior to multivariate statistical analysis, the data were verified in terms of the incidence of outliers. The analysis employed a two-sided Grubbs test at a significance level of 0.05. According to Tabachnick and Fidell [47], outliers are easiest to eliminate by removing them from further analysis. However, especially in multidimensional statistical methods, their conversion should be considered. Elliott and Stettler [48] proposed to replace outliers with possible values. The outliers were replaced as follows: in the case of the highest values, the second highest value was taken, and it was increased by 1%, while in the case of the lowest values, the second lowest value was taken, and it was reduced by 1%. Subsequently the ME, TE, and REE concentrations in water samples before the statistical analysis applying multivariate statistical techniques were log-transformed to obtain a normal distribution. Misclassification due to differences in data dimensionality was avoided by applying cluster analysis (CA) and principal component analysis (PCA) on standardised data through z-scale transformation [49].
The cluster analysis (CA) was applied to select springs characterised by similar concentrations of macro elements (MEs), trace elements (TEs), and rare-earth elements (REEs). Ward’s method was employed to assess similarities and differences between springs in terms of ME, TE, and REE levels. The applied similarity measure was square Euclidean distance. Ward’s method for the estimation of distance between clusters employs the variance analysis approach, aiming at the minimisation of total square deviations within clusters [50]. The CA method provided dendrograms presenting the hierarchical structure of the studied springs.
The next stage of statistical analysis involved detrended component analysis (DCA). The obtained gradients of the first axis in DCA were shorter than 3.0 SD, indicating that the data were in linear distribution. In such cases, Principal Component Analysis (PCA) is recommended for further data analysis [51,52]. PCA is an ordination method facilitating the interpretation of complex correlations between the analysed MEs, TEs, and REEs, and environmental data. The following environmental data were considered in three groups: hydrodynamic type of spring (descending and ascending), morphometry (valley, by riverbeds, scarp-foot, and slope), and land use (agriculture, forest, and urban area). Principle Component Analysis (PCA) was employed to identify potential sources of MEs, TEs, and REEs. The number of significant principal components was selected based on the Kaiser criterion of eigenvalues greater than 1. Moreover, it was assumed that when factor loadings between the concentrations of selected MEs, TEs, and REEs and principal components are 0.75–1.00, 0.50–0.75, and 0.30–0.50, they are strongly, moderately, and weakly correlated, respectively [53]. CA and PCA were conducted using the Statistica 14.0 and Canoco 5.0 software, respectively.
3. Results
3.1. Physico-Chemical Properties of Springs Water
The physicochemical status of the spring waters was determined based on 51 parameters. The parameters present the physical properties and concentrations of macro elements (ME), trace elements (TEs), and rare-earth elements (REEs) in the spring waters. Descriptive statistics for the parameters are presented in Table 1. Descriptive statistics for specific land use types are shown in Table S2.
During the study, the spring water temperatures (WT) ranged from 7.5 °C to 10.0 °C, with a mean value of 9.3 °C. The WT changes resulted from the variability in the thick-ness and depth of the aquifer horizons. The electric conductivity (EC) and pH values ranged from 284 to 602 µS/cm and from 6.42 to 8.90 µS/cm, respectively. Water temperature and pH values in the springs were characterised by low variability. The distribution of water temperature was strongly left-skewed, and the coefficient of variation value was approximately 6%. The pH values, on the other hand, had a slightly left-skewed distribution, and the variability of this parameter was at a similar level to WT. The coefficient of variability of EC was 25%, and the distribution of this parameter was slightly right-skewed (skewness value 0.2). Similarly, the variability in the alkalinity and hardness of the spring waters was low. The coefficient of variation values were 22% and 15%, respectively. The alkalinity of the spring waters had a distribution analogous to that of the EC and the hardness of the water to that of the pH value.
Based on cation concentrations, waters of the analysed springs are of the Ca2+-HCO3− (sampling points: 1, 2, 4, 7–13, 18, 19, 27, 28–50% of spring water samples) and Ca2+-HCO3−-SO42− types (sampling points: 3, 5, 6, 14–17, 20–26–50% of spring water samples) (Figure 2).
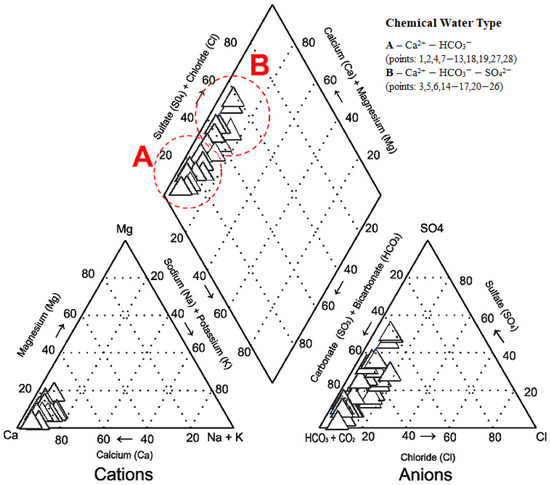
Figure 2.
Concentrations of cations and anions in spring waters.
A Piper diagram objectively shows the relative concentrations of various ions and the chemical characteristics of spring waters (Figure 2). The use of Piper plots in combination with information on geological and hydrogeological conditions allows the analyses of the laws of evolution of the chemical composition of spring waters. The major cation in the spring waters of the study area was identified as Ca2+. Processes contributing to the Ca2+ concentration in spring waters include long-term leaching of Ca2+ from Quaternary deposits, and particularly gravelly–sandy postglacial and fluvial deposits, by precipitation. The major anions in the spring waters of the study area were identified as weakly acidic ions (HCO3−) and strongly acidic ions (SO42−).
The concentrations of nitrate and nitrite varied strongly within the springs from 0.095 to 55.6 and from 0.003 to 0.441, respectively. The distribution of nitrates and nitrites was very strongly right-skewed, with skewness values of 2.7 and 3.2. Slightly lower variability was observed for ammonium nitrogen, which also had a strongly right-skewed distribution. Phosphate concentrations ranged from 0.07 to 0.21 mg/L with a mean value of 0.64 mg/L. Moreover, the variability of this compound was 36%, and was at a lower level than that of nitrogen compounds. Very high variability was also observed for Fe and Mn. The mean concentrations of these elements were 3.98 and 0.26 mg/L, respectively. Fe and Mn concentrations showed normal distribution, and very high positive skewness.
Mean concentrations of TEs in the spring waters can be arranged in descending order as follows: Sr > Al > Zn > Ba > Cu > Li > As > Rb > Pb > Ni > V > Ce > Mo > Co > Se > Cr > Sb > Be. Mean concentrations of these elements ranged widely from 0.029 to 95.5 mg/L. It should be emphasised that all TEs have right-skewed distributions. Concentrations of Ni and Se had right-skewed distributions. Concentrations of Ni and Se had right-skewed distributions. Other elements had strongly right-skewed distributions, while the concentrations of Sb, Be, and Ce were extremely right-skewed. The variability of the studied trace elements was characterised by calculating the IQR and median values. The values of the ratio ranged from 53% (Co) to 304% (Be).
According to their mean concentration value, rare-earth elements (REEs) in the spring waters can be arranged in descending order as follows: Ce > U > La > Nd > Pr > Gd > Sm > Dy > Er > Th > Eu > Ho > Tb > Tm > Lu. The range of REEs concentrations in the spring waters was 0.003 to 0.539 µg/L. Th and U concentrations in the waters had very strongly skewed distribution (skewness from 1 to 3), and other REEs had an extremely right-skewed distribution (skewness above 3). The range of variability expressed by the coefficient of variation and the ratio of IQR and median values was from 92% to 262%, and from 106% to 387%, respectively.
Analysis of the data in terms of the incidence of outliers revealed three outliers in physical parameters, 12 outliers in the group of MEs, 16 outliers in TEs, and 15 outliers in REEs, respectively. Among the analysed water parameters, no outliers were found in the case of EC, Alkalinity, Hardness HCO3−, F−, Cl−, SO42−, PO43−, K+, Mg2+, Ca2+, Cu, Ni, Pb, Se, or Sr. In the case of REEs, outliers were found within each element. Within the ME group, outliers were observed for water samples from springs 1, 2, 5, 6, 7, 14, 20, and 22. In the TE group, outliers were found for water samples from springs 1, 5, 7, 9, 15, and 20, while in the group of REEs in water samples from springs 9, 15, and 25. The greatest numbers of outliers were recorded for the water sample from spring 9, where concentrations of as many as 20 elements were considered outliers.
3.2. Statistical Analysis
In view of their location, the springs were divided into valley springs, springs formed by riverbeds and scarp-foot springs. The analysis for all analysed elements in the distinguished spring types showed statistically significant differences between valley springs and scarp-foot springs. Greater variations in physicochemical parameters were found in relation to land use. Analyses showed significant differences in pH value, EC, NO3− and Fe in spring waters located in agriculture and urban areas. The pH values and Fe concentrations were higher in urban areas; the opposite situation occurred for EC and NO3−. In turn, between waters from springs located in agriculture and forest areas differences were detected in WT, pH value, F−, NO3− and Fe. Higher WT, and also F− and NO3− concentrations were in the agriculture area, while the opposite situation occurred for pH values and Fe concentrations. In contrast, concentrations of F−, Cl−, K+ and Na+ were higher in the urban area than in the forest area. Slight variation in TE and REE concentrations was observed in waters of springs depending on land use. In the group of TEs differences were found between Mo and Rb concentrations in springs waters located in agriculture and urban areas. In turn, within the REE group, differences were recorded in concentrations of Th between springs waters located in agriculture and urban areas. The concentrations of the mentioned elements were higher in urbanised areas. In contrast, in urban areas, there were higher concentrations of Mo, Sb, Se and Sr than in forest areas. The analysis of ME, TE and REE concentrations in springs was performed in the context of distance from roads. The springs are located between 2 and 160 m from roads with an average value of 57 m. Correlation analysis showed relationships between the distance of the spring to the road and the concentration of SO42− in the waters (positive correlation) and alkalinity and HCO3− (negative correlation). A final analysis of the differences between the concentrations of MEs, TEs and REEs in the springs was performed for two groups—1 (springs up to 50 m from roads—14 springs) and 2 (springs more than 50 m from roads—14 springs). The results showed significant differences for Cl−, SO42− and K+. Higher values of these ions were found in springs located more than 50 m from the road.
In the next stage of the study, springs were grouped based on similarities observed in their concentrations of MEs (Figure 3a), TEs (Figure 3b) and REEs (Figure 3c).
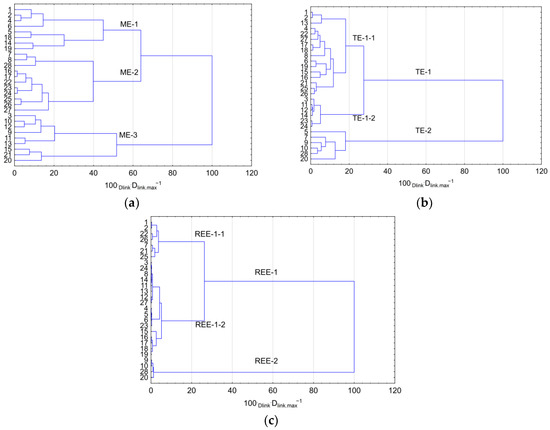
Figure 3.
Grouping of springs based on similarities in concentrations of (a) MEs; (b) TEs; (c) REEs.
The analysis provided the basis for the division of springs into three groups based on MEs. Group ME-1 comprised 8 springs, while group ME-2 consisted of 11 springs. Waters of springs classified into groups ME-1 and ME-2 showed higher concentrations compared to group ME-3, with the exception of Mn concentrations and pH values. In group ME-1, concentrations of F−, Cl−, NO3−, NO2−, and K+ were higher than in group ME-2, while values of CODMn, EC, alkalinity, HCO3−, SO42−, Mg2+, and Fe were lower. The lowest ME concentrations were observed in group ME-3, covering nine springs. No spatial relationship was found between concentrations of selected MEs in the springs. The CA analysis for TEs provided grounds for the division of springs into two principal groups, TE-1 and TE-2. As many as 22 springs were classified into group TE-1. Therefore, for a detailed description of TEs in waters, two subgroups were designated, namely TE-1-1 and TE-1-2. Group TE-2 included springs where the recorded TE concentrations were approximately 2-9-fold higher than in group TE-1. Springs classified to group TE-2 were mainly located in forest areas, whereas, in terms of relief, they were valley springs. In subgroup TE-1-1, TE concentrations were from 1.3 to 3.7-fold higher than in subgroup TE-1-2. The CA analysis was performed in reference to REEs, facilitating the division of springs into two groups, namely REE-1 and REE-2. As in the case of TEs in the latter group REE-2, the concentrations of REEs were on average 3.7 to 4.3 times higher than in group REE-1. The lowest REE concentrations were found in subgroup REE-1-2, and they were from 2.0 to as much as 8.1-fold lower than in subgroup REE-1-1. At the same time, in springs 9, 10, 20, and 28, the highest concentrations of TEs and REEs were recorded, suggesting the presence of local sources of anthropogenic pollution. It should be emphasised that among the analysed springs, the greatest variation was found in terms of MEs, while the smallest was found for REEs.
The chondrite normalised patterns for the spring water samples are plotted in Figure 4.
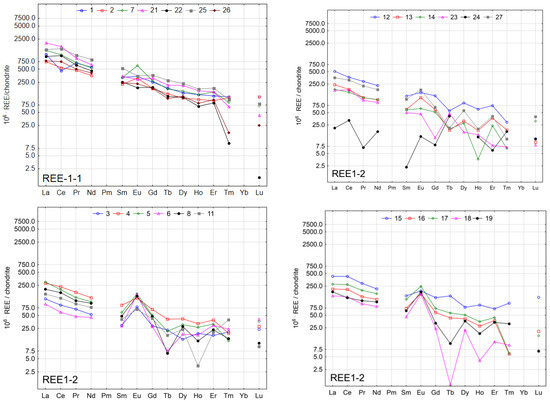
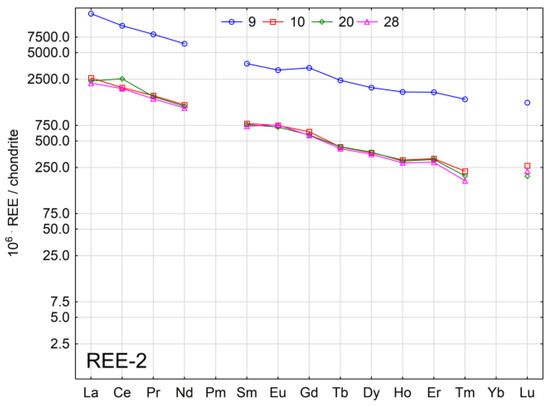
Figure 4.
Chondrite—normalised REE patterns in spring waters.
Normalised graphs for REE concentrations in the water of individual springs show high variability. The smallest variation was found in the case of waters from springs in group REE-2. Lower variation was observed in subgroup REE-1-1, particularly in the case of Tm and Lu. The greatest variation was found in subgroup REE-1-2, particularly in the group of elements from Sm to Lu. In the case of waters from springs 3, 4, 5, 6, 8, 11, 16, 17, 18, and 19, an anomaly in Eu was observed. Similar results in terms of the Eu anomaly were reported in a study on the Wadąg river-lake system [44].
The PCA was conducted in reference to the designated groups of water quality indices, i.e., MEs, TEs, and REEs. For MEs, 20 variables originally used to characterise the quality of water from springs were reduced to six PCA-derived principal components. According to the Kaiser criterion, the distinguished principal components had values exceeding 1, and explain 6.3% to 28.9% of the variability in the original data. Jointly identified principal components explain 85.6% of the variation. Strong positive correlations with the first principal component PCA1 were found for hardness, Cl−, Na+, and Ca2+, and the correlations were average for cations Mg2+, NH4+, and K+ (Figure 5a).
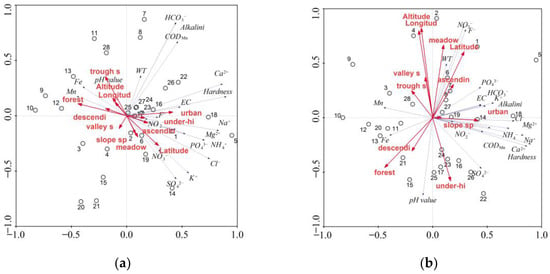
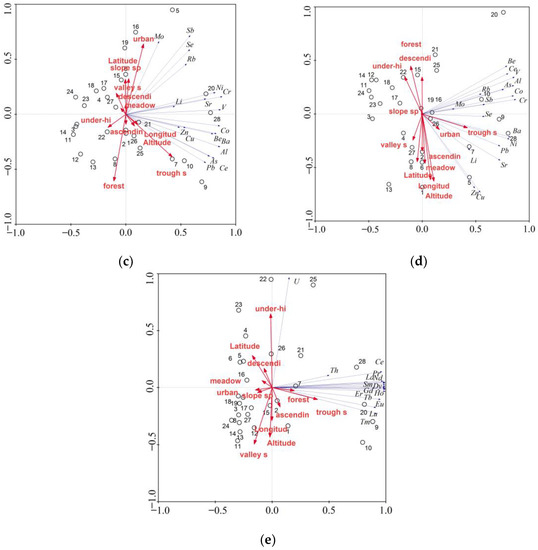
Figure 5.
Results of PCA analysis in reference to: (a) MEs—PCA1 vs. PCA2; (b) MEs—PCA1 vs. PCA3; (c) TEs—PCA1 vs. PCA2; (d) TEs—PCA1 vs. PCA3; (e) REEs—PCA1 vs. PCA2.
Strong positive correlations with the second principal component PCA2 were found for alkalinity and HCO3−, while the strength of the correlation with CODMn was average. In contrast, negative correlations of average strength were recorded for SO42− and K+ (Figure 5b). No environmental variables included in the analyses were correlated with PCA1 or PCA2. Three water quality parameters were correlated with PCA3. In the case of F− and NO3−, there was a strong positive correlation, and for pH value there was a negative correlation of average strength. With PCA3, strong positive correlations were found in the case of environmental variables investigated in this analysis. Positive correlations were obtained for latitude, longitude, and altitude, as well as the agriculture locations, while for the scarp-foot and forest locations the correlations were negative. In the case of the fourth and fifth principal components, explaining approximately 10% variation, correlations were found for PCA4—EC, Mg2+, Fe, and Mn (negative and average), PCA5—WT (positive and average), and PO43−, Fe, and Mn (negative and average). The last principal component PCA6 explains only 6.3% of the variability in the original data. It was positively strongly correlated with NO2− and negatively, with an average strength, with NH4+.
Lower spatial variation within group TE resulted in the identification of four principal components with values exceeding 1. The distinguished principal components explain 50.4%, 12.8%, 12.2%, and 7.0% of the internal variation. A strong positive correlation with PCA1 was recorded for Al, As, Ba, Be, Ce, Co, Cr, Ni, and V, whereas the correlations were of average strength in the cases of Cu, Pb, Rb, Sb, and Se (Figure 5c). A positive average correlation with PCA2 was found for Mo, Sb, and Se (Figure 5d), while with PCA3, the correlation was negative and of average strength for Cu and Zn. Only one correlation was found with PCA4, i.e., a negative strong correlation for Li. Among the environmental variables, urban and forest areas were positively correlated with PCA2, while longitude and altitude were correlated negatively with PCA3. The PCA analysis for REEs facilitated the identification of two principal components, which according to the Kaiser criterion, had values exceeding 1. The identified principal components explain 91.6% of the variation. Strong positive correlations with PCA1 were found for almost all analysed REEs except for Th and U. Only U concentration was strongly positively correlated with PCA2 (Figure 5e). The PCA results confirm slight variation within this group of elements and strong correlations between most parameters. It was also observed that the analysed environmental factors had no effect on the concentrations of most REEs in the waters of investigated springs. Negative correlations with the second principal component PCA2 were recorded for valley springs, while they were positive with scarp-foot springs. This shows higher U values detected as a rule for scarp-foot springs in comparison to valley springs.
4. Discussion
Waters of the investigated springs are of the Ca2+-HCO3− and Ca2+-HCO3−-SO42− types. Calcium in these waters may originate from the dissolution of carbonate minerals, as well as from geogenic and anthropogenic sources. Some hydrogen carbonates are derived from carbon dioxide dissolved in water [54]. The ionic composition of Ca2+-HCO3− is frequently found in headwaters of lowland rivers, and it is typical of shallow Quaternary groundwaters in the active exchange zone of waters [55]. This is confirmed by studies by Mazurek et al. [54] and Szczucińska [15]. These waters are medium hard (from 161 to 274) and weakly alkaline (from 1.8 to 4.6). An exception to the rule is found for waters from spring 1 (ascending), where a weakly acidic reaction is directly determined. In contrast, water pH in spring 22 was close to alkaline (pH value—6.42). Electrolytic conductivity is a parameter indicating the effect of anthropogenic factors on water quality [56]. The analysed waters showed relatively low values of conductivity, ranging from 282 µS/cm to 602 µS/cm. The highest values were recorded for spring waters from agricultural areas (536.5 µS/cm—602 µS/cm), i.e., areas most exposed to the supply of inorganic substances with fertilisers and pesticides. Water temperature fell within a range of values typical of natural outflows of underground waters (from 7.5 to 10.0 °C) with a mean of around 9.3 °C. The temperature of spring waters was influenced by the temperature of underground waters (depth of the aquifer horizon), by mixing with waters from the headwater area affected directly by current weather conditions, as well as spring yield [57].
Concentrations of NH4+ ions were high in waters of almost all springs regardless of the environment at the outflow or spring type. Elevated ammonium ion levels are obviously influenced by natural factors related to the presence of humic substances and the decomposition of organic matter. Other contributing factors include intensive human activity and the influx of domestic sewage. Nevertheless, in the case of NO3−, the highest mean values were recorded in agriculture areas, exceeding by almost 6-fold the mean for urban areas and by almost 15-fold the mean for forest areas. Nitrates in pure waters are found in very small amounts [58]. Their high concentrations may be explained by the mineralisation and nitrification processes taking place in the headwater area, or contamination of the aquifer horizon supplying springs with mineral fertilisers. At the same time, the presence of nitrates and the ammonium ion shows permanent water pollution [59]. All springs from urban areas and agricultural areas as well as springs 25 and 26 from forest areas had increased concentrations of NO2−. The presence of NO3− and NO2− in waters is a particularly adverse phenomenon [60]. Nitrate contamination is likely to be affected by pollution sources, i.e., agricultural activities and municipal sewage [61]. The primary cause of water pollution is related to chemical treatments in agriculture and improper water and sewage management.
In all springs in urban areas and forests, high Fe concentrations were recorded. High Mn concentrations were found locally in areas subject to anthropogenic pressure (spring 28 near the junction of provincial roads, and springs 7 and 9 with outflows in the military training area). Moreover, high Mn values were recorded in spring 20 in the Bory Postomskie coniferous forest. Concentrations of Fe and Mn are commonly reported in underground waters of the Quaternary horizon [62]. Their occurrence is primarily connected with changes in hydrogeochemical conditions and the presence of organic matter [54]. The presence of iron in underground waters supplying spring 9 is indicated by precipitated amorphous deposits. As in the case of nitrates, phosphorus compounds are mineralised and eventually transformed into phosphates. Considerable differences in concentrations in individual springs were revealed for sulphates. Extreme values of SO42− were reached in springs in the forest in the military training area (springs 11 and 15). In that area, increased concentrations of chlorides Cl− were recorded, potentially caused by the common source of anthropogenic pollution related to military activity. In the spring on arable land, higher Mg2+ concentrations were locally detected in comparison with the other springs. This may have been caused by the application of magnesium fertilisers [63].
Moreover, locally extreme As concentrations were recorded, reaching 12.6 µg/L. The element readily penetrates from the lithosphere to the hydrosphere, and it is a common component of water. Its concentration in waters varies and depends on the surrounding geological deposits, the degree of environmental pollution, as well as bio-methylation and demethylation processes. For comparison, mean As concentrations in waters of the Wielkopolska National Park and the Drawa National Park amounted to 0.40 µg/L and 0.95 µg/L, respectively [64]. Furthermore, in the case of Be, Li, and V concentrations, elevated values were observed in the military training area. A slightly different situation was recorded in the case of V. Its elevated levels were found in springs in urban areas as well as agriculture areas. In the case of heavy metals, their highest values were recorded in forest areas in the military training area. In the same springs, enrichment was found in the case of Be, Cr, Tm, and Al. Elevated Zn concentrations in the spring in the military training area suggest its anthropogenic origin. Its sources may have included municipal and industrial sewage, combustion of fuels, and runoff from urban areas as a consequence of zinc leaching from surfaces protected against corrosion [65]. Heavy metals may be found in underground waters as a result of penetration of pesticides and mineral fertilisers used in arable fields, but they may also originate from leachate from waste disposal sites as well as industrial and municipal sewage. Contamination of waters with trace elements is of particular importance in the context of the role played by waters in the circulation of chemical components between various elements of the environment. This contamination depends both on natural and anthropogenic conditions [66].
La, Ce, Pr, Nd, and Sm belong to the group of light rare-earth elements (LREEs) that include more readily soluble elements, whereas Er, Ho, and Lu belong to heavy rare-earth elements (HREEs), including less readily soluble elements [44]. Due to their limited permeability for aqueous solutions, REEs exhibit a more limited migration capacity [67]. In aquatic systems, REE concentrations are lower compared to their concentrations in rocks due to their slight permeability [68], but increasing consumption of REEs in advanced technologies leads to the release of increasing amounts of REEs into the environment [69]. In Poland, the potential sources of rare-earth elements include, e.g., combustion ash and biomass-coal co-combustion in power plants and road traffic. Their very small concentrations in most analysed springs indicate their geogenic origin. This is suggested by the presence of rare-earth elements at maximum levels in forest areas, such as in spring 9 (by the river bed), with waters containing maximum concentrations of all REEs. In urban areas and agriculturally utilised areas, their concentrations were lower and comparable. However, high Sc concentrations were recorded in urban areas.
The results are generally comparable to the range of hydrochemical backgrounds for springs in the Lubusz Plateau [15], south of the study area. Considerable differences are observed, however, particularly in terms of NH4+, NO2−, and NO3− concentrations, with elevated concentrations in this analysis. No elevated Pb values were recorded, however, as mentioned by Szczucińska in her analyses [15]. The study showed that the influence of roads on the concentrations of MEs, TEs, and REEs in spring waters was generally limited to only three elements (SO42−, alkalinity, and HCO3−). There were also no differences between spring water quality up to 50 m and above 50 m with the exception of K+, Cl−, and SO42−. The researchers Uliasz-Misiak et al. [37] showed that road transport can affect the quality of groundwater up to 50 m from roads. According to Wang et al. [38], Cu, Pb, Zn, Cr, Cd, Na+, K+, and Cl− in groundwater were negatively affected by road transportation. The maximum affected distance of these pollutants varied from 15 to 100 m.
5. Conclusions
The results of the analysis of water chemistry for springs in the area of the Postomia River revealed the following:
- i
- Waters of springs in the investigated area are of calcium-hydrogen carbonate (Ca2+-HCO3−) and calcium-hydrogen carbonate-sulphur (Ca2+-HCO3−-SO42−) types.
- ii
- The study showed differences between valley springs and scarp-foot springs in terms of electrical conductivity and concentrations of F−, SO42−, NO3−, Mg2+, Ba, Zn, and U.
- iii
- Greater variability was observed between the physical and chemical conditions of the spring waters due to their location in terms of land cover. The pH values and the concentrations of Fe, Mo, Rb, and Th were higher than in agricultural areas, and the concentrations of F−, Cl−, K+, Na+, Mo, Sb, Se, and Sr were higher than in forested areas.
- iv
- Springs in agricultural areas had lower pH values than those in other areas, and higher NO3− concentrations.
- v
- Road traffic had a limited effect on the physical and chemical status of water in the springs. Only HCO3− values and SO42− concentrations were related to the distance from the road network. The concentrations of Cl−, SO42− and K+ were higher in the waters of springs located more than 50 m from the road network.
- vi
- Cluster analysis permitted dividing the springs into groups with similar concentrations of MEs, TEs, and REEs in water. The designation of these groups, however, was not related to spring type or land use.
- vii
- Principal component analysis did not permit the identification of a single dominant origin with regard to most of the studied elements. This suggests an interaction of different pollution sources.
- viii
- The analysis of REE concentrations in relation to chondrite concentrations indicates a disturbing pattern of presence in springs 14, 18, and 24, potentially resulting from local anthropogenic factors. Furthermore, the concentrations of REEs were at low levels, indicating their geogenic origin.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15010157/s1, Table S1: Location of springs and physical parameters of their waters; Table S2. Statistical comparison of physicochemical parameters determination results for spring water samples.
Author Contributions
Conceptualisation—M.S. (Marcin Siepak) and A.L.; methodology—M.S. (Marcin Siepak) and M.S. (Mariusz Sojka); data collection—M.S. (Marcin Siepak) and A.L.; chemical analyses—M.S. (Marcin Siepak) and A.L.; statistical analysis and interpretation—M.S. (Mariusz Sojka); writing—original draft preparation, M.S. (Marcin Siepak); A.L.; M.S. (Mariusz Sojka); writing—review, M.S. (Marcin Siepak) and M.S. (Mariusz Sojka); editing manuscript—M.S. (Marcin Siepak); visualisation, M.S. (Mariusz Sojka) and M.S. (Marcin Siepak); supervision—M.S. (Marcin Siepak) and M.S. (Mariusz Sojka). All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by funds granted by the Ministry of Science and Higher Education; Research project No. 215862/E-336/SPUB/2017/1, and within the framework of the Ministry of Science and Higher Education programme as “Regional Initiative Excellence” in years 2019–2022, project No. 005/RID/2018/19.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bajkiewicz-Grabowska, E.; Mikulski, Z. General Hydrology; Wydawnictwo Naukowe PWN: Warszawa, Poland, 1999; p. 313. (In Polish) [Google Scholar]
- Wieczysty, A. Engineering Hydrogeology; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1982; pp. 619–623. (In Polish) [Google Scholar]
- Jokiel, P. Springs, their role in the environment and importance in water management. Folia Geogr. 1997, 2, 5–7. (In Polish) [Google Scholar]
- Pokładek, R.; Kowalczyk, T.; Orzepowski, W.; Pulikowski, K. Na, K, Ca and Mg concentrations in effluent water drained from agricultural catchment basins in Lower Silesia. J. Elem. 2012, 16, 467–479. [Google Scholar] [CrossRef]
- Wiśnios, M.; Kanownik, W.; Bogdał, A. Hydrochemistry of springs in the Ojców National Park. Acta Sci. Pol. Form. Circumiectus 2015, 14, 205–217. [Google Scholar] [CrossRef]
- Major, M.; Cieśliński, R. Impact of hydrometeorological conditions on the chemical composition of water in closed-basin kettle ponds: A comparative study of two postglacial areas. J. Elem. 2017, 22, 151–167. [Google Scholar] [CrossRef]
- Wolanin, A.; Żelazny, M. Seasonal changes in chemistry of Tatra spring waters in the Chochołowski and the Koscielski stream catchments in 2009. In Waters in Geographical Studies; Ciupa, T., Suligowski, R., Eds.; Uniwersytet Humanistyczno-Przyrodniczy w Kielcach: Kielce, Poland, 2010; pp. 347–355. (In Polish) [Google Scholar]
- Dynowska, I. Regional diversity of springs in Poland. Folia Geogr. 1986, 18, 5–30. (In Polish) [Google Scholar]
- Szumińska, D.; Fabianowska, K. Conditions of occurrence and dynamics of groundwater outflows in outwash plains based on the middle part of the Wda catchment. J. Health Sci. 2013, 3, 88–105. (In Polish) [Google Scholar]
- Maksymiuk, Z.; Moniewski, P. Hydrological and landscape role of springs in a small catchment in the western part of the Wzniesienie Łódzkie edge zone. Folia Geogr. 2000, 5, 67–87. (In Polish) [Google Scholar]
- Puk, K. Conditions of occurrence and the production and temperature regime for groundwater outflows in the Sierakowski Landscape Park and in the adjacent area. In Badania Fizjograficzne nad Polską Zachodnią; Wydawnictwo Poznańskiego Towarzystwa Przyjaciół Nauk: Poznań, Poland, 2005; pp. 137–143. (In Polish) [Google Scholar]
- Kazakis, N.; Vaudouris, K.S. Groundwater vulnerability and pollution risk assessment of porous aquifers to nitrate: Modifying the DRASTIC method using quantitative parameters. J. Hydrol. 2015, 525, 13–25. [Google Scholar] [CrossRef]
- Moosavirad, S.M.; Janardhana, M.R.; Khairy, H. Impact of anthropogenic activities on the chemistry and quality of groundwater: A case study from a terrain near Zarand City, Kerman Province, SE Iran. Environ. Earth Sci. 2013, 69, 2451–2467. [Google Scholar] [CrossRef]
- Amiri, V.; Sohrabi, N.; Dadgar, M.A. Evaluation of groundwater chemistry and its suitability for drinking and agricultural uses in the Lenjanat plain central Iran. Environ. Earth Sci. 2015, 74, 6163–6176. [Google Scholar] [CrossRef]
- Szczucińska, A. Spring water chemistry in a formerly glaciated area of western Poland the contribution of natural and anthropogenic factors. Environ. Earth Sci. 2016, 75, 712. [Google Scholar] [CrossRef]
- Rao, N.S.; Rao, P.S.; Reddy, G.V.; Nagamani, M.; Vidyasagar, G.; Satyanarayana, N.L.V.V. Chemical characteristics of groundwater and assessment of groundwater quality in Varaha River Basin, Visakhapatnam District, Andhra Pradesh, India. Environ. Monit. Assess. 2012, 184, 5189–5214. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Takahashi, A.; Weng, J.H.; Huang, L.F.; Kim, H.K.; Li, C.K.; Huang, F.T.C.; Jeng, F.T. Precipitation chemistry in East Asia. Atmos. Environ. 2000, 34, 525–537. [Google Scholar] [CrossRef]
- Hayashi, M.; Quinton, W.L.; Pietroniro, J.; Gibson, J.J. Hydrologic functions of wetlands in a discontinuous permafrost basin indicated by isotopic and chemical signatures. J. Hydrol. 2004, 296, 81–97. [Google Scholar] [CrossRef]
- Said-Pullicino, D.; Kaiser, K.; Guggenberger, G.; Gigliotti, G. Changes in the chemical composition of water-extractable organic matter during composting: Distribution between stable and labile organic matter pools. Chemosphere 2007, 66, 2166–2176. [Google Scholar] [CrossRef]
- Satora, S. Diversity of chemical composition of flysch Carpathian groundwater. Infrastrukt. Ekol. Teren. Wiej. 2009, 6, 157–160. (In Polish) [Google Scholar]
- Subramani, T.; Rajmohan, N.; Elango, L. Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environ. Monit. Assess. 2010, 162, 123–137. [Google Scholar] [CrossRef]
- Kozłowski, M.; Komisarek, J. Groundwater chemistry and hydrogeochemical processes in a soil catena of the Poznań Lakeland, central Poland. J. Elem. 2017, 22, 681–695. [Google Scholar] [CrossRef]
- Merk, M.; Goeppert, N.; Goldscheider, N. Processes controlling spatial and temporal dynamics of spring water chemistry in the Black Forest National Park. Sci. Total Environ. 2020, 723, 137742. [Google Scholar] [CrossRef]
- Meng, F.; Liang, X.; Xiao, C.; Wang, G. Hydrochemical characteristics and identification of pollution ions of the springs in the south of Yanbian City, China. Environ. Geochem. Health 2022, 44, 2215–2233. [Google Scholar] [CrossRef]
- Thapa, B.; Pant, R.R.; Thakuri, S.; Pond, G. Assessment of spring water quality in Jhimruk River Watershed, Lesser Himalaya, Nepal. Environ. Earth Sci. 2020, 79, 1–14. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, J.; Xu, X.; Wang, Q.; Wang, M.; Feng, J.; Fu, T. Temporal variations of spring water in karst areas: A case study of Jinan Spring Area, Northern China. Water 2020, 12, 1009. [Google Scholar] [CrossRef]
- Ansari, M.; Deodhar, A.; Kumar, U.S. Modeling of geochemical processes and multivariate statistical analysis for hydrochemical assessment of spring water of the Outer Himalaya, India. Environ. Earth Sci. 2019, 78, 1–17. [Google Scholar] [CrossRef]
- Bogdał, A.; Kowalik, T.; Kanownik, W.; Ostrowski, K.; Wiśnios, M. Assessment of physico-chemical status of meteoric water and outflow from the Wolninka stream catchment. Gaz Woda Tech. Sanit. 2012, 8, 362–365. (In Polish) [Google Scholar]
- Siwek, J. Springs in the Catchment Areas of Prądnik, Dłubnia and Szreniawa. Natural and Anthropogenic Conditioning of Water Quality; Uniwersytet Jagielloński: Kraków, Poland, 2004; pp. 7–98. (In Polish) [Google Scholar]
- Baścik, M.; Chełmicki, W.; Korska, A.; Pociask-Karteczka, J.; Siwek, J. Springs of the Krakowsko-Wieluńska and Miechowska Uplands. Changes in the Years 1973–2000; Uniwersytet Jagielloński: Kraków, Poland, 2001; pp. 7–107. (In Polish) [Google Scholar]
- Policht-Latawiec, A.; Kanownik, W.; Łukasik, D. Impact of point pollutions on water quality in the San River. Infrastrukt. Ekol. Teren. Wiej. 2013, 4, 253–269. (In Polish) [Google Scholar]
- Al-Khashman, O.A.; Jaradat, A.Q. Assessment of groundwater quality and its suitability for drinking and agricultural uses in arid environment. Stoch. Environ. Res. Risk Assess. 2014, 28, 743–753. [Google Scholar] [CrossRef]
- Naseems, S.; Hamza, S.; Bashir, E. Groundwater geochemistry of Winder agricultural farms, Balochistan, Pakistan and assessment for irrigation water quality. Eur. Water 2010, 31, 21–32. [Google Scholar]
- Abdesselam, S.; Halitim, A.; Jan, A.; Trolard, F.; Bourrie, G. Anthropogenic contamination of groundwater with nitrate in arid region: Case study of southern Hodna (Algeria). Environ. Earth. Sci. 2013, 70, 2129–2141. [Google Scholar] [CrossRef]
- Shirazi, S.M.; Adham, M.I.; Zardari, N.H.; Ismail, Z.; Imran, H.M.; Mangrio, M.A. Groundwater quality and hydrogeological characteristics of Malacca state in Malaysia. J. Water Land Dev. 2015, 24, 11–19. [Google Scholar] [CrossRef]
- Jasik, M.; Małek, S.; Żelazny, M. Effect of water stage and tree stand composition on spatiotemporal differentiation of spring water chemistry draining Carpathian flysch slopes (Gorce Mts). Sci. Total Environ. 2017, 599, 1630–1637. [Google Scholar] [CrossRef]
- Uliasz-Misiak, B.; Winid, B.; Lewandowska-Śmierzchalska, J.; Matuła, R. Impact of road transport on groundwater quality. Sci. Total Environ. 2022, 824, 153804. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Nie, L.; Xu, Y.; Du, C.; Zhang, T.; Wang, Y. Effects of highway-related pollutant on the groundwater quality of turfy swamps in the Changbai Mountain Area. Int. J. Environ. Res. Public Health 2018, 15, 1652. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, A.; Goodarzi, A.R.; Razmara, P. Long-term impacts of road salt application on the groundwater contamination in urban environments. Environ. Sci. Pollut. Res. 2020, 27, 30162–30177. [Google Scholar] [CrossRef]
- Pisarska-Jamroży, M. Physiographic characteristics and genesis of the Noteć-Warta proglacial stream valley. In Nature and Cultural Value of the Wista-Odra Waterway; Przyroda i Turystyka Regionu Pomorza i Kujaw; Wydawnictwo LOGO: Bydgoszcz, Poland, 2008; Volume II. (In Polish) [Google Scholar]
- Wacławski, M. Engineering Geology and Hydrogeology, Part II. Hydrogeology; Wydawnictwo Politechniki Krakowskiej: Kraków, Poland, 1999; pp. 178–184. (In Polish) [Google Scholar]
- Walna, B.; Siepak, M. Heavy metals: Their pathway from the ground, groundwater and springs to Lake Góreckie (Poland). Environ. Monit. Assess. 2012, 184, 3315–3340. [Google Scholar] [CrossRef] [PubMed]
- Siepak, M.; Sojka, M. Application of multivariate statistical approach to identify trace elements sources in surface waters: A case study of Kowalskie and Stare Miasto reservoirs, Poland. Environ. Monit. Assess. 2017, 189, 364. [Google Scholar] [CrossRef] [PubMed]
- Sojka, M.; Siepak, M.; Pietrewicz, K. Concentration of Rare Earth Elements in surface water and bottom sediments in Lake Wadąg, Poland. J. Elem. 2019, 24, 125–140. [Google Scholar]
- Sojka, M.; Choiński, A.; Ptak, M.; Siepak, M. The variability of lake water chemistry in the Bory Tucholskie National Park (northern Poland). Water 2020, 12, 394. [Google Scholar] [CrossRef]
- Nakamura, N. Determination of REE, Ba, Fe, Mg, Na and K in carbonaceous and ordinary chondrites. Geochim. Cosmochim. Acta 1974, 38, 757–775. [Google Scholar] [CrossRef]
- Tabachnick, B.; Fidell, L. Using Multivariate Statistics; Pearson: Boston, MA, USA, 2007; 815p. [Google Scholar]
- Elliott, M.; Stettler, N. Using a mixture model for multiple imputation in the presence of outliers: The ‘Healthy for life’ project. Appl. Statist. 2007, 56 Pt 1, 63–78. [Google Scholar] [CrossRef]
- Sojka, M.; Siepak, M.; Zioła, A.; Frankowski, M.; Murat Błażejewska, S.; Siepak, J. Application of multivariate statistical techniques to evaluation of water quality in the Mała Wełna River (Western Poland). Environ. Monit. Assess. 2008, 147, 159–170. [Google Scholar] [CrossRef]
- Ptak, M.; Sojka, M.; Choiński, A.; Nowak, B. Effect of environmental conditions and morphometric parameters on surface water temperature in Polish lakes. Water 2018, 10, 580. [Google Scholar] [CrossRef]
- Glińska-Lewczuk, K.; Gołaś, I.; Koc, J.; Gotkowska-Płachta, A.; Harnisz, M.; Rochwerge, A. The impact of urban areas on the water quality gradient along a lowland river. Environ. Monit. Assess. 2016, 188, 624. [Google Scholar] [CrossRef]
- Sojka, M.; Jaskuła, J.; Siepak, M. Heavy Metals in Bottom Sediments of Reservoirs in the Lowland Area of Western Poland: Concentrations, Distribution, Sources and Ecological Risk. Water 2019, 11, 56. [Google Scholar] [CrossRef]
- Liu, C.W.; Lin, K.H.; Kuo, Y.M. Application of factor analysis in the assessment of groundwater quality in blackfoot disease in Taiwan. Sci. Total Environ. 2003, 313, 77–89. [Google Scholar] [CrossRef]
- Mazurek, M.; Dobrowolski, R.; Osadowski, Z. Geochemistry of deposits from spring-fed fens in West Pomerania (Poland) and its significance for palaeoenvironmental reconstruction. Géomorphologie Relief Process. Environ. 2014, 20, 323–342. [Google Scholar] [CrossRef]
- Nowicki, Z.; Sadurski, A. Hydrogeological aspects of Quaternary sediments in Poland. Państwowy Inst. Geol.-Państwowy Inst. Badaw. 2010, 441, 123–229. [Google Scholar]
- Moniewski, P.; Stolarska, M. Natural and anthropogenic impact factors on basic physicochemical characteristics of water in a small catchment of the suburban area of Łódź. Woda-Sr.-Obsz. Wiej. 2007, 1, 105–122. (In Polish) [Google Scholar]
- Górniak, A.; Jekatierynczuk-Rudczyk, E.; Dobrzyń, P. Hydrochemistry of three dystrophic lakes in Northeastern Poland. Acta Hydrochim. Et Hydrobiol. 1999, 27, 12–18. [Google Scholar] [CrossRef]
- Chomutowska, H.; Wilamowski, K. Research on the physico-chemical status of waters in the Bialowieza Forest. In Ochrona Środowiska i Zasobów Naturalnych; IOŚ: Warszawa, Poland, 2012; Volume 54, pp. 190–199. (In Polish) [Google Scholar]
- Chełmicki, W. Water Resources, Degradation, Protection; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2001. (In Polish) [Google Scholar]
- Kowalski, J. Hydrogeology with Basics of Geology; Wydawnictwo Uniwersytetu Przyrodniczego: Wrocław, Poland, 2007; pp. 90–134. (In Polish) [Google Scholar]
- Lee, B.D.; Jeong, C.H.; Lee, Y.C.; Lee, Y.J.; Yang, J.H.; Choo, C.O.; Hong, J.W. Statistical analysis and thermodynamic equilibrium modelling for chemical composition of groundwater and spring water at Jeju Island, South Korea. Water 2020, 12, 777. [Google Scholar] [CrossRef]
- Macioszczyk, A.; Dobrzyński, D. Hydrogeochemistry of the Active Groundwater Exchange Zone; Wydawnictwo PWN: Warszawa, Poland, 2002; pp. 258–282. (In Polish) [Google Scholar]
- Grzebisz, W.; Przygocka-Cyna, K.; Szczepaniak, W.; Diatta, J.; Potarzycki, J. Sodesium as a nutritional tool of nitrogen efficient management—Plant production and environment. J. Elem. 2010, 15, 771–788. [Google Scholar]
- Niedzielski, P.; Siepak, J.; Siepak, M.; Kraska, M. Occurrence of arsenic, antimony and selenium in surface waters of Drawieński National Park. Pol. J. Environ. Stud. 2002, 11, 41–45. [Google Scholar]
- Niemiec, M. Accumulation of zinc in water, sediments and bleak fish (Alburnus alburnus L.) in the ecosystem of the Dunajec River. J. Elem. 2016, 21, 173–184. [Google Scholar] [CrossRef]
- Frankowski, M.; Sojka, M.; Zioła, A.; Siepak, M.; Murat-Błażejewska, S. Distribution of heavy metals in the Mała Wełna River system (Western Poland). Oceanol. Hydrobiol. Stud. 2009, 2, 1–11. [Google Scholar] [CrossRef]
- Chudaev, O.V.; Chelnokov, G.A.; Bragin, I.V.; Kharitonova, N.A.; Blokhin, M.G.; Aleksandrov, I.A. REE fractionation in the rivers of eastern and southern Sikhote Alin with natural and anthropogenic anomalies. Russ. J. Pac. Geol. 2015, 9, 428–438. [Google Scholar] [CrossRef]
- Noack, C.W.; Dzombak, D.A.; Karamalidis, A.K. Rare earth element distributions andtrends in natural waters with a focus on groundwater. Environ. Sci. Technol. 2014, 48, 4317–4326. [Google Scholar] [CrossRef]
- Kulaksız, S.; Bau, M. Anthropogenic dissolved and colloid/nanoparticle-bound samarium, lanthanum and gadolinium in the Rhine River and the impending destruction of the natural rare earth element distribution in rivers. Earth Planet. Sci. Lett. 2013, 362, 43–50. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).