Tributyltin in Wastewater: Influence on the Performance of Suspended Growth Biological Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. TBT Characteristics and Analysis
2.2. Sludge Origin and Biological Activity Characterisation
2.2.1. Origin of the Sludge
2.2.2. Biological Activity Characterisation
2.3. Influence of the Presence of TBT on the Biological Activity of Sludge
2.3.1. Batch-Reactors Methodology
2.3.2. Continuous Reactor Methodology (Immersed Membrane Bioreactor System)
3. Results and Discussion
3.1. Influence of TBT Addition on the Biological Activity of Sludge in Batch Reactors
3.1.1. Endogenous Conditions
3.1.2. Exogenous Conditions
3.2. Influence of the Addition of TBT to the Sludge Biological Activity in a Continuous Reactor (MBRi System)
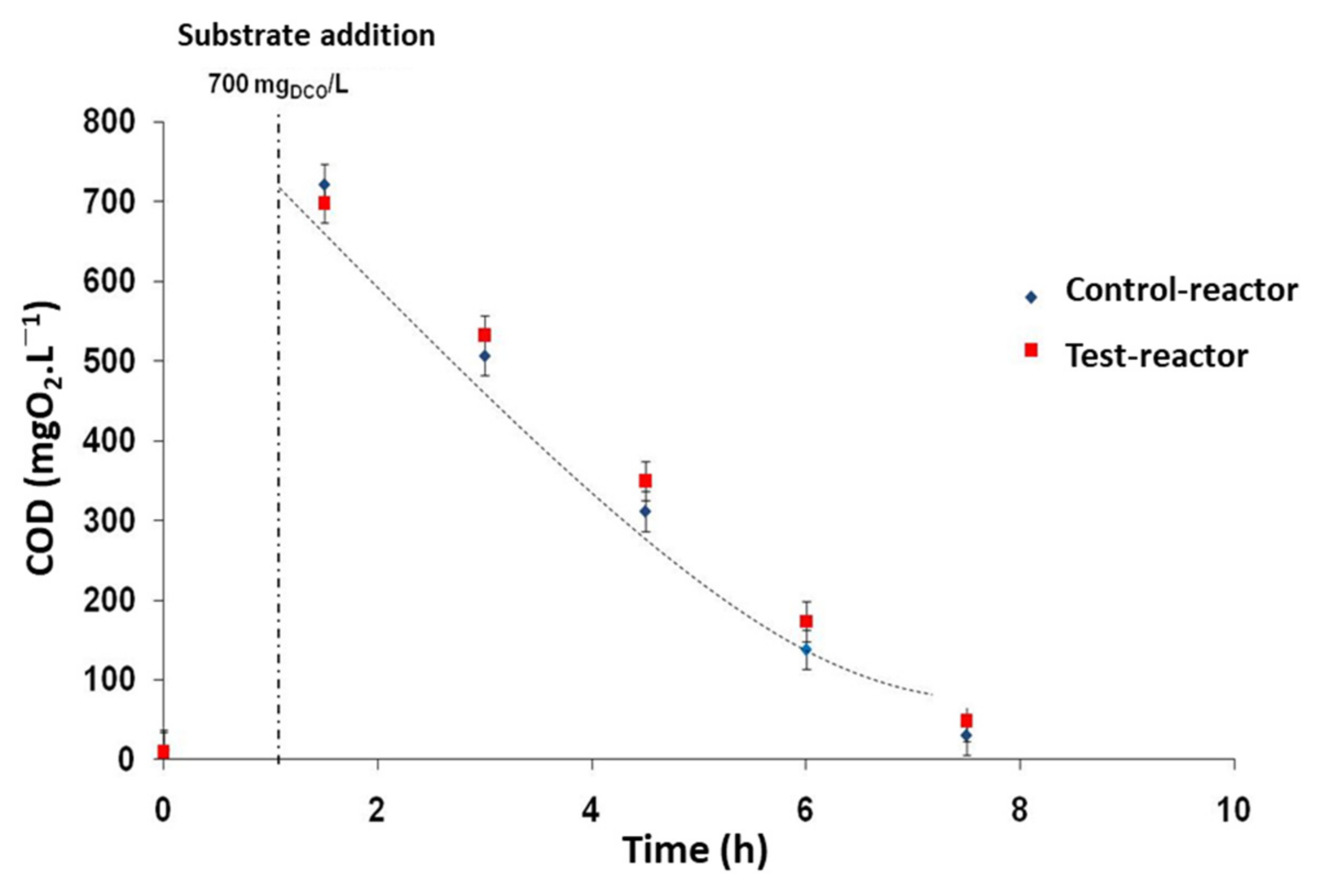
3.2.1. TBT Effect on COD Removal Rate
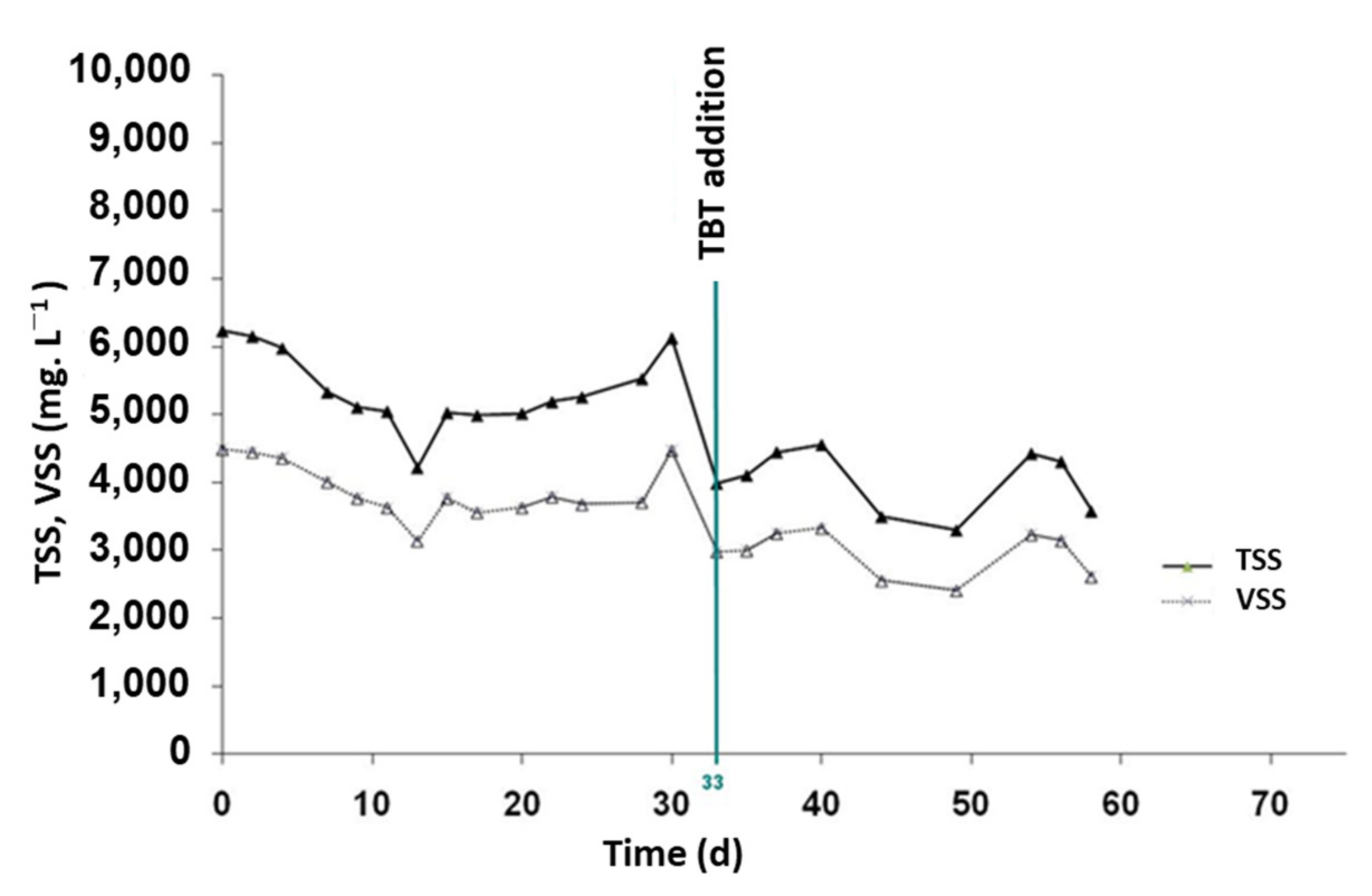
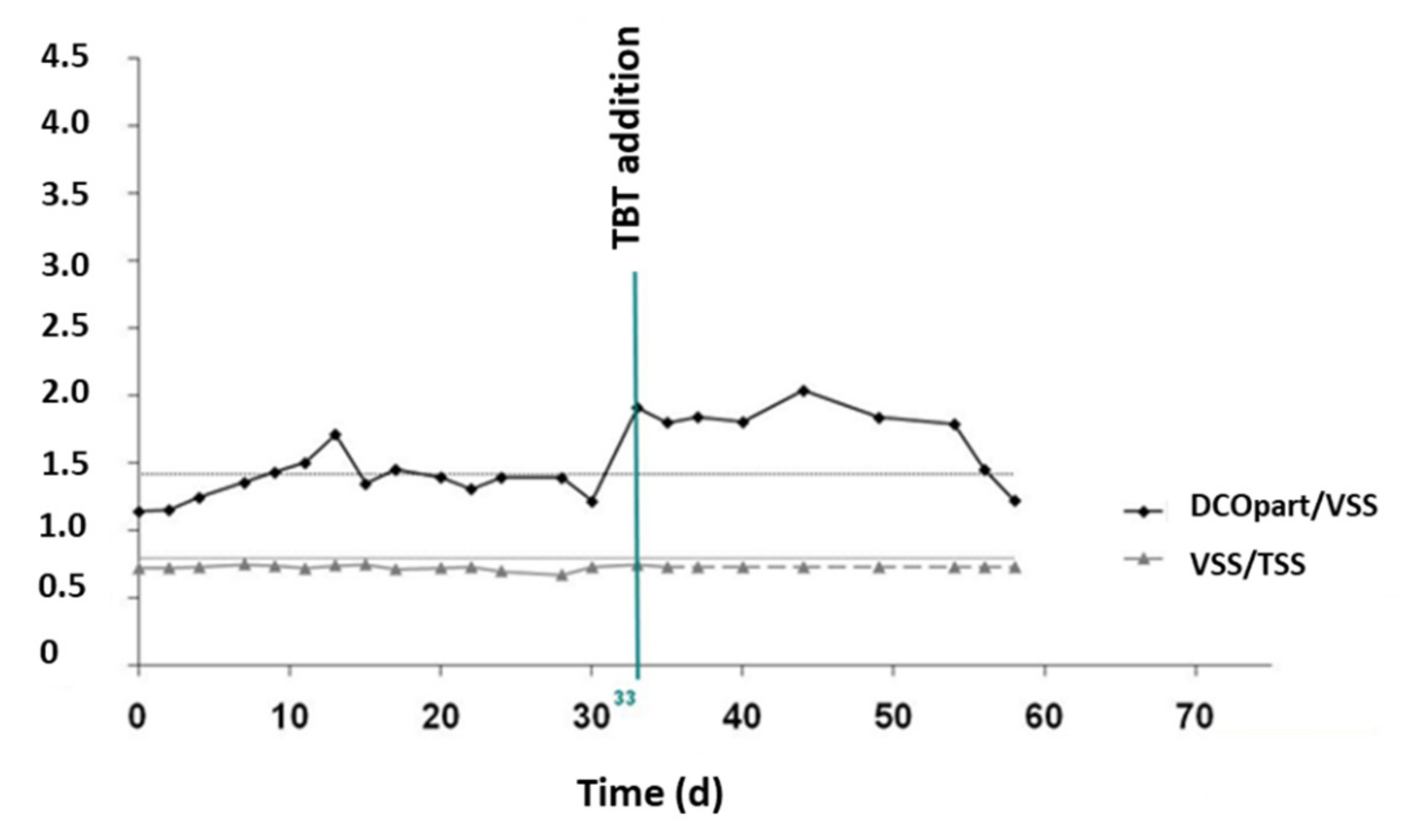
3.2.2. TBT Effect on Sludge Production
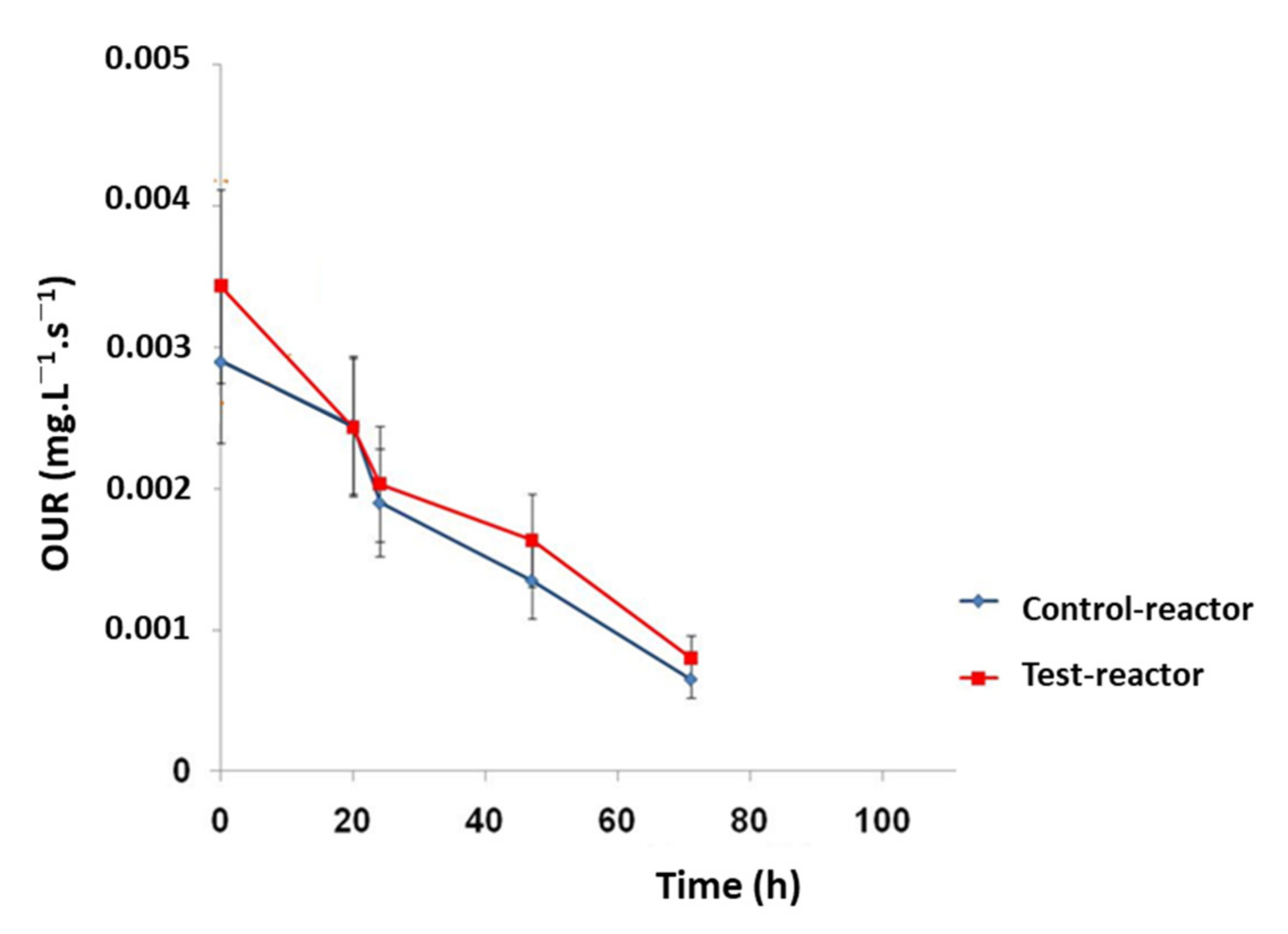
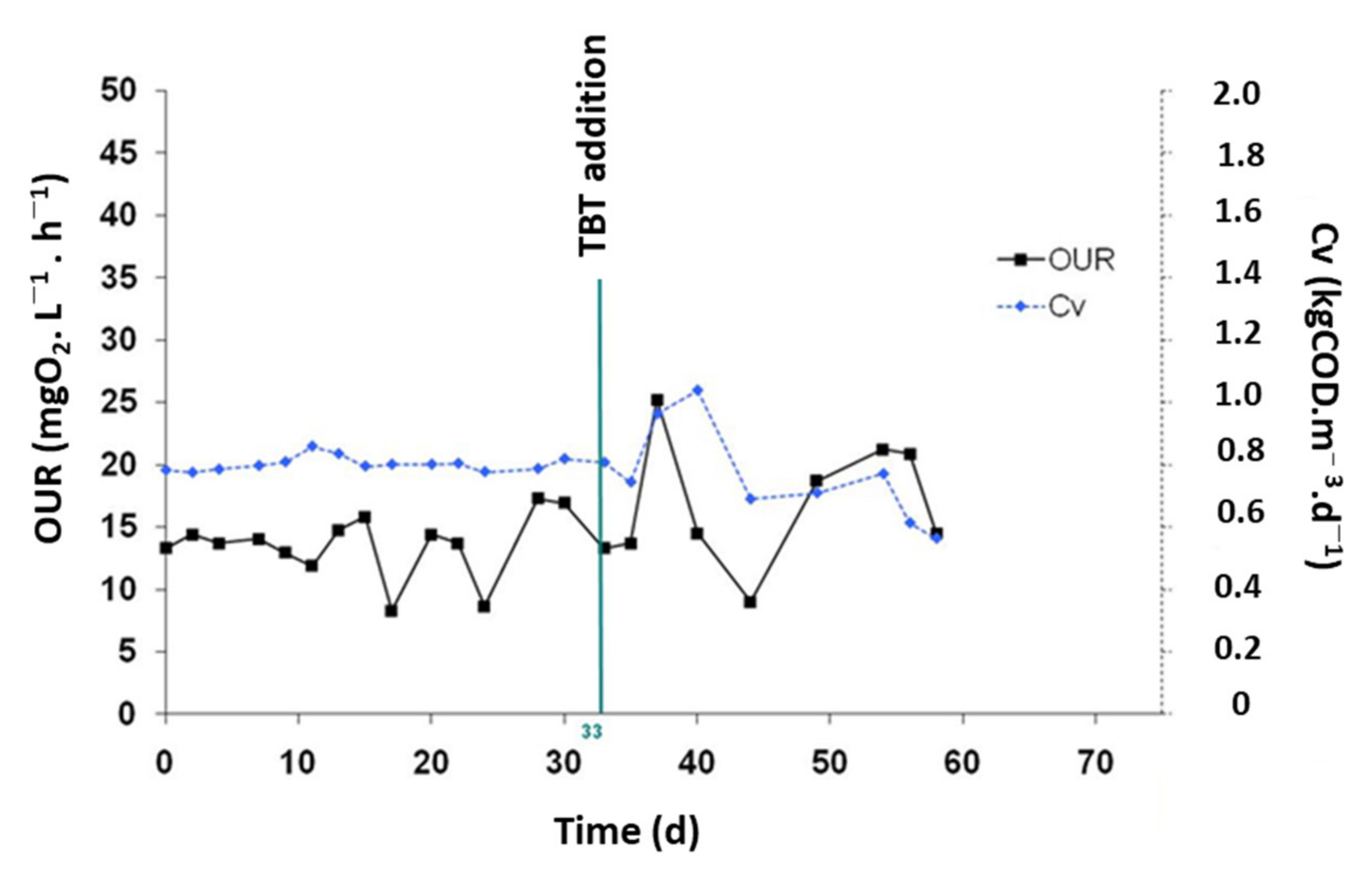
3.2.3. TBT Effect on the Oxygen Uptake Rate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hoch, M. Organotin Compounds in the Environment—An Overview. Appl. Geochem. 2001, 16, 719–743. [Google Scholar] [CrossRef]
- Okoro, H.K.; Fatoki, O.S.; Adekola, F.A.; Ximba, B.J.; Snyman, R.G. Organotin Compounds. Encycl. Toxicol. Third Ed. 2014, 720–725. [Google Scholar] [CrossRef]
- Alzieu, C. Environmental Impact of TBT: The French Experience. Sci. Total Environ. 2000, 258, 99–102. [Google Scholar] [CrossRef]
- Brancato, M.S.; Cardwell, R.D.; McKay, S. Chambering in the Pacific oyster, Crassostrea gigas, and its relationship to tributyltin. Mar. Environ. Res. 2000, 50, 437. [Google Scholar] [CrossRef]
- Shinoda, S.; Ohnogi, H.; Tanaka, M.; Korenaga, T. Effect of Tributyltin on Environmental Microorganisms. J. Antibact. Antifung. Agents 1996, 24, 15–20. [Google Scholar]
- De Mora, S.J. Tributyltin: Case Study of an Environmental Contaminant; Cambridge University Press: Cambridge, UK, 1996; Volume 8, pp. 1–317. [Google Scholar]
- OSPAR Background Document on Organic Tin Compounds (Update 2004; 103/2000). 2000. Available online: https://www.ospar.org/documents?v=7271 (accessed on 23 March 2022).
- David, A.; Bancon-Montigny, C.; Salles, C.; Rodier, C.; Tournoud, M.-G. Contamination of Riverbed Sediments by Hazardous Substances in the Mediterranean Context: Influence of Hydrological Conditions. J. Hydrol. 2012, 468–469. [Google Scholar] [CrossRef]
- Chahinian, N.; Bancon-Montigny, C.; Brunel, V.; Aubert, G.; Salles, C.; Marchand, P.; Rodier, C.; Seidel, J.L.L.; Gayrard, E.; Hernandez, F.; et al. Temporal and Spatial Variability of Organotins in an Intermittent Mediterranean River. J. Environ. Manag. 2013, 128, 173–181. [Google Scholar] [CrossRef]
- Bancon-Montigny, C.; Seidel, J.L.J.-L.; Brissaud, F.; Elbaz-Poulichet, F. Organotins in a Medium-Size Mediterranean Basin (the Herault River). J. Environ. Monit. 2008, 10, 638–647. [Google Scholar] [CrossRef]
- Bancon-Montigny, C.; Lespes, G.; Potin-Gautier, M. Organotin Survey in the Adour-Garonne Basin. Water Res. 2004, 38, 933–946. [Google Scholar] [CrossRef]
- Gao, J.M.; Chen, X.L.; Ren, C.R.; Qiu, H.; Zhang, K.; Guo, J.S.; Tang, Z.H.; Wu, W.N.; Zhang, Y.L. Organotins in the Aquatic Media of Secondary Anabranches in the Three Gorges Reservoir Region, China. Chemosphere 2019, 217, 232–242. [Google Scholar] [CrossRef]
- Directive 2008/105/EC of the European Parliament and of the Council (16 December 2008) on Environmental Quality Standards in the Field of Water Policy. Off. J. Eur. Union 2008, L 348, 84–97.
- Olofsson, U.; Bignert, A.; Haglund, P. Time-Trends of Metals and Organic Contaminants in Sewage Sludge. Water Res. 2012, 46, 4841–4851. [Google Scholar] [CrossRef] [PubMed]
- Mailler, R.; Gasperi, J.; Chebbo, G.; Rocher, V. Priority and Emerging Pollutants in Sewage Sludge and Fate during Sludge Treatment. Waste Manag. 2014, 34, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Sabah, A.; Bancon-Montigny, C.; Rodier, C.; Marchand, P.; Delpoux, S.; Ijjaali, M.; Tournoud, M.-G. Occurrence and Removal of Butyltin Compounds in a Waste Stabilisation Pond of a Domestic Waste Water Treatment Plant of a Rural French Town. Chemosphere 2016, 144, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Ophithakorn, T.; Sabah, A.; Delalonde, M.; Bancon-Montigny, C.; Suksaroj, T.T.; Wisniewski, C. Organotins’ Fate in Lagoon Sewage System: Dealkylation and Sludge Sorption/Desorption. Environ. Sci. Pollut. Res. 2016, 23, 22832–22842. [Google Scholar] [CrossRef] [PubMed]
- Martí, N.; Aguado, D.; Segovia-Martínez, L.; Bouzas, A.; Seco, A. Occurrence of Priority Pollutants in WWTP Effluents and Mediterranean Coastal Waters of Spain. Mar. Pollut. Bull. 2011, 62, 615–625. [Google Scholar] [CrossRef]
- Clara, M.; Windhofer, G.; Weilgony, P.; Gans, O.; Denner, M.; Chovanec, A.; Zessner, M. Identification of Relevant Micropollutants in Austrian Municipal Wastewater and Their Behaviour during Wastewater Treatment. Chemosphere 2012, 87, 1265–1272. [Google Scholar] [CrossRef]
- Fent, K. Organotin Compounds in Municipal Wastewater and Sewage Sludge: Contamination, Fate in Treatment Process and Ecotoxicological Consequences. Sci. Total Environ. 1996, 185, 151–159. [Google Scholar] [CrossRef]
- Fent, K.; Müller, M.D. Occurrence of Organotins in Municipal Wastewater and Sewage Sludge and Behavior in a Treatment Plant. Environ. Sci. Technol. 1991, 25, 489–493. [Google Scholar] [CrossRef]
- Aubenneau, M.; Tahar, A.; Casellas, C.; Wisniewski, C. Membrane Bioreactor for Pharmaceutically Active Compounds Removal: Effects of Carbamazepine on Mixed Microbial Communities Implied in the Treatment. Process Biochem. 2010, 45, 1826–1831. [Google Scholar] [CrossRef]
- Delgado, N.; Navarro, A.; Marino, D.; Peñuela, G.A.; Ronco, A. Removal of Pharmaceuticals and Personal Care Products from Domestic Wastewater Using Rotating Biological Contactors. Int. J. Environ. Sci. Technol. 2019, 16, 1–10. [Google Scholar] [CrossRef]
- Chiavola, A.; Boni, M.R.; di Marcantonio, C.; Cecchini, G.; Biagioli, S.; Frugis, A. A Laboratory-Study on the Analytical Determination and Removal Processes of THC-COOH and Bezoylecgonine in the Activated Sludge Reactor. Chemosphere 2019, 222, 83–90. [Google Scholar] [CrossRef]
- Bancon-Montigny, C.; Lespes, G.; Potin-Gautier, M. Optimisation Using Experimental Designs of the Sample Pretreatment: Application to the Control of the Organotins in Sewage Sludge by GC-FPD. Analyst 1999, 124, 1265–1270. [Google Scholar] [CrossRef]
- Brosillon, S.; Bancon-Montigny, C.; Mendret, J. Study of Photocatalytic Degradation of Tributyltin, Dibutylin and Monobutyltin in Water and Marine Sediments. Chemosphere 2014, 109, 173–179. [Google Scholar] [CrossRef]
- Montigny, C.; Lespes, G.; Potin-Gautier, M. Matrix effects and selectivity of the detector in the determination of butyl-and phenyltins by gas chromatography–flame photometric detection. J. Chromatogr. A 1998, 819, 221–230. [Google Scholar] [CrossRef]
- OECD. Organisation for Economic Co-Operation and Development—Test No. 209: Activated Sludge, Respiration Inhibition Test (Carbon and Ammonium Oxidation)-OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2010; ISBN 9789264070080. [Google Scholar]
- Kraigher, B.; Kosjek, T.; Heath, E.; Kompare, B.; Mandic-Mulec, I. Influence of Pharmaceutical Residues on the Structure of Activated Sludge Bacterial Communities in Wastewater Treatment Bioreactors. Water Res. 2008, 42, 4578–4588. [Google Scholar] [CrossRef]
- Henriques, I.D.S.; Holbrook, R.D.; Kelly, R.T.; Love, N.G. The Impact of Floc Size on Respiration Inhibition by Soluble Toxicants—A Comparative Investigation. Water Res. 2005, 39, 2559–2568. [Google Scholar] [CrossRef]
- Pitter, P.; Chudoba, J. Biodegradability of Organic Substances in the Aquatic Environment; CRC, Ed.; CRC Press: Boca Raton, FL, USA, 1990; ISBN 9780849351310. [Google Scholar]
- Ely, R.L.; Williamson, K.J.; Hyman, M.R.; Arp, D.J. Cometabolism of Chlorinated Solvents by Nitrifying Bacteria: Kinetics, Substrate Interactions, Toxicity Effects, and Bacterial Response. Biotechnol Bioeng. 1997, 54, 520–534. [Google Scholar] [CrossRef]
- Ray, S.; Peters, C.A. Changes in Microbiological Metabolism under Chemical Stress. Chemosphere 2008, 71, 474–483. [Google Scholar] [CrossRef]
- Avella, A.C.; Delgado, L.F.; Görner, T.; Albasi, C.; Galmiche, M.; de Donato, P. Effect of Cytostatic Drug Presence on Extracellular Polymeric Substances Formation in Municipal Wastewater Treated by Membrane Bioreactor. Bioresour. Technol. 2010, 101, 518–526. [Google Scholar] [CrossRef]
- Davey, M.E.; O’toole, G.A. Microbial Biofilms: From Ecology to Molecular Genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef] [PubMed]
- Aquino, S.F.; Stuckey, D.C. Soluble Microbial Products Formation in Anaerobic Chemostats in the Presence of Toxic Compounds. Water Res. 2004, 38, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Bott, C.B.; Love, N.G. Investigating a Mechanistic Cause for Activated-Sludge Deflocculation in Response to Shock Loads of Toxic Electrophilic Chemicals. Water Environ. Res. 2002, 74, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. Microbial Polysaccharide Products. Biotechnol. Genet. Eng. Rev. 1999, 16, 217–230. [Google Scholar] [CrossRef]
- Hsieh, K.M.; Murgel, G.A.; Lion, L.W.; Shuler, M.L. Interactions of Microbial Biofilms with Toxic Trace Metals: 1. Observation and Modeling of Cell Growth, Attachment, and Production of Extracellular Polymer. Biotechnol. Bioeng. 1994, 44, 219–231. [Google Scholar] [CrossRef]
- Avery, S.V.; Miller, M.E.; Gadd, G.M.; Codd, G.A.; Cooney, J.J. Toxicity of Organotins towards Cyanobacterial Photosynthesis and Nitrogen Fixation. FEMS Microbiol. Lett. 1991, 84, 205–210. [Google Scholar] [CrossRef][Green Version]



| Average Removal Efficiency (%) | Average Removal Rate rs (mgDCO·L−1·j−1) | |
|---|---|---|
| Without TBT | 97 ± 1 | 789 ± 39 |
| With TBT | 96 ± 3 | 797 ± 88 |
| rx (mgVSS·L−1·j−1) | Yobs | |
|---|---|---|
| Without TBT | 69 ± 8 | 0.09 |
| With TBT | 62 ± 14 | 0.08 |
| OUR (mg·L−1·d−1) | a′* (mgO2/mgCODremoved) | |
|---|---|---|
| Without TBT | 326 ± 10 | 0.40 ± 0.08 |
| With TBT | 377 ± 17 | 0.5 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montigny, C.; Delpoux, S.; Nurit, J.; Wisniewski, C. Tributyltin in Wastewater: Influence on the Performance of Suspended Growth Biological Processes. Water 2022, 14, 1483. https://doi.org/10.3390/w14091483
Montigny C, Delpoux S, Nurit J, Wisniewski C. Tributyltin in Wastewater: Influence on the Performance of Suspended Growth Biological Processes. Water. 2022; 14(9):1483. https://doi.org/10.3390/w14091483
Chicago/Turabian StyleMontigny, Chrystelle, Sophie Delpoux, Josiane Nurit, and Christelle Wisniewski. 2022. "Tributyltin in Wastewater: Influence on the Performance of Suspended Growth Biological Processes" Water 14, no. 9: 1483. https://doi.org/10.3390/w14091483
APA StyleMontigny, C., Delpoux, S., Nurit, J., & Wisniewski, C. (2022). Tributyltin in Wastewater: Influence on the Performance of Suspended Growth Biological Processes. Water, 14(9), 1483. https://doi.org/10.3390/w14091483







