Role of Seasons in the Fate of Dissolved Organic Carbon and Nutrients in a Large-Scale Surface Flow Constructed Wetland
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Chemical Analyses
2.4. Spectroscopy
2.5. Irradiation Experiments
2.6. Statistics
3. Results and Discussion
3.1. Weather Conditions and Vegetation
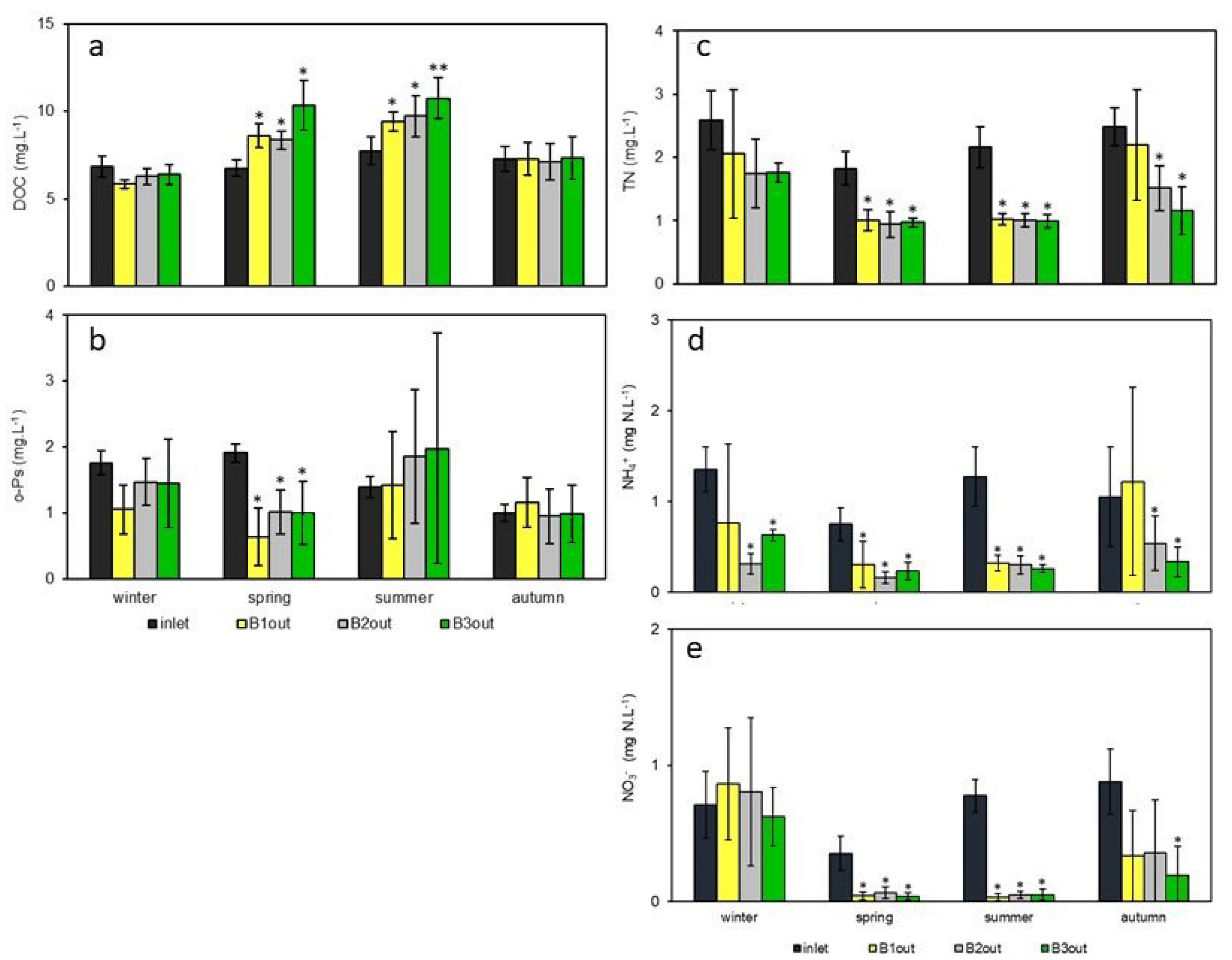
3.2. Evolution of pH, Conductivity, DOC, Nitrogen Species and o-Ps
3.3. DOM Characterization
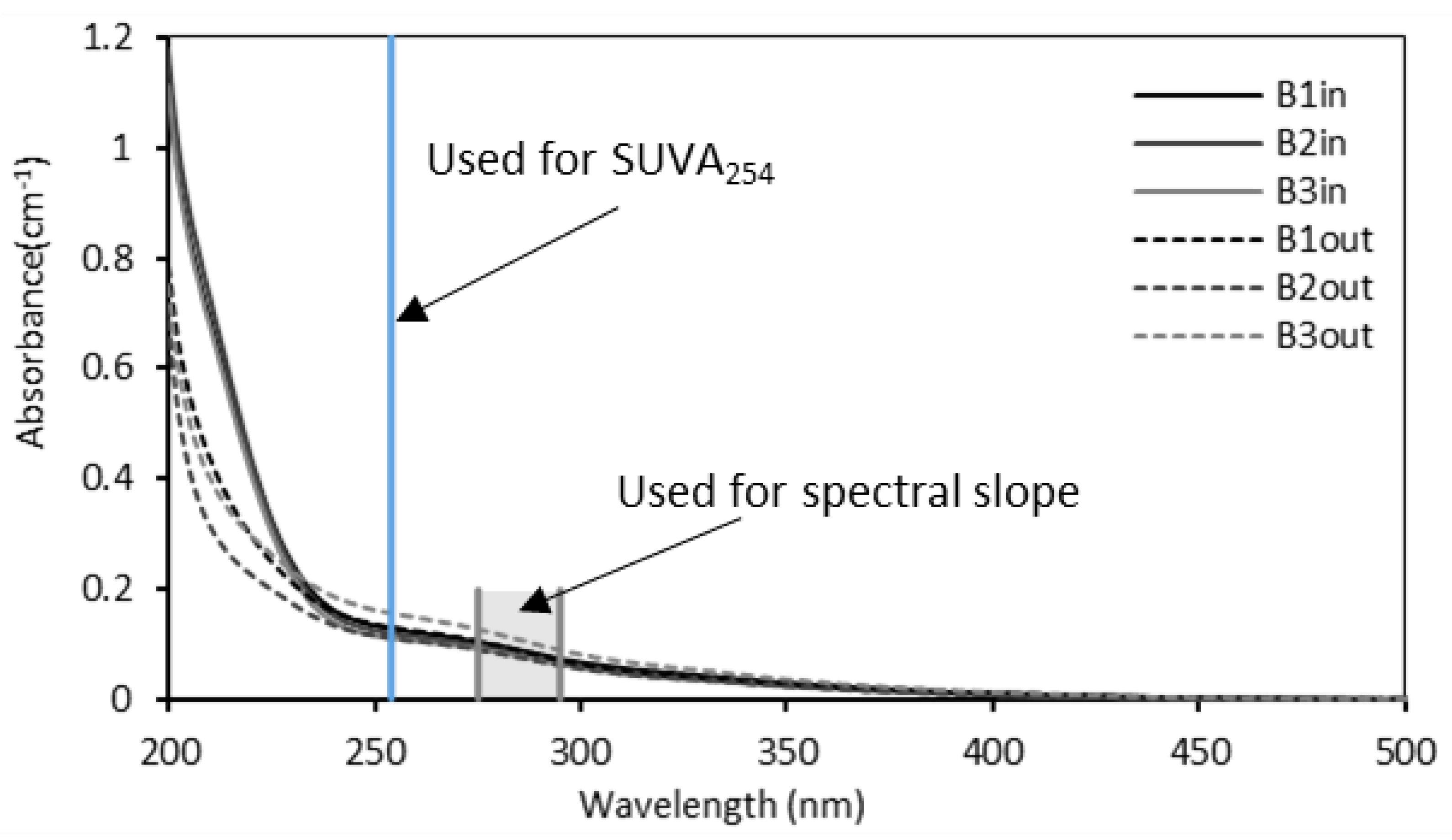
3.3.1. UV-Visible Spectra
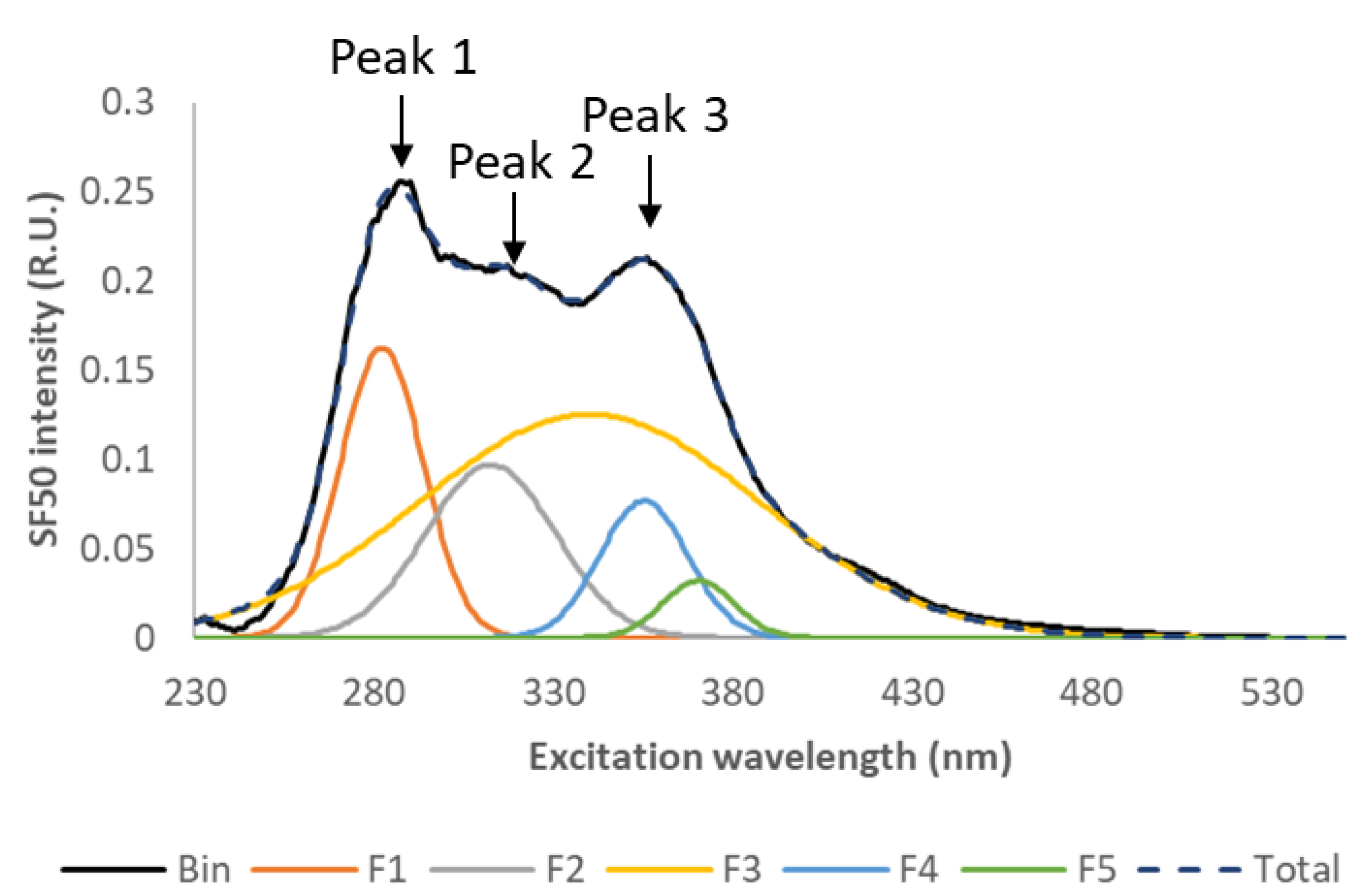
3.3.2. Fluorescence Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Geißen, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Marrs, C.F.; Simon, C.; Xi, C. Wastewater treatment contributes to selective increase of antibiotic resistance among Acinetobacter spp. Sci. Total Environ. 2009, 407, 3702–3706. [Google Scholar] [CrossRef]
- Matamoros, V.; García, J.; Bayona, J.M. Organic micropollutant removal in a full-scale surface flow constructed wetland fed with secondary effluent. Water Res. 2008, 42, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Hijosa-Valsero, M.; Matamoros, V.; Sidrach-Cardona, R.; Martín-Villacorta, J.; Bécares, E.; Bayona, J.M. Comprehensive assessment of the design configuration of constructed wetlands for the removal of pharmaceuticals and personal care products from urban wastewaters. Water Res. 2010, 44, 3669–3678. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A. Constructed wetland for wastewater treatment and reuse: A case study of developing country. Int. J. Environ. Sci. Dev. 2013, 4, 20–24. [Google Scholar] [CrossRef][Green Version]
- Matamoros, V.; Rodríguez, Y.; Bayona, J.M. Mitigation of emerging contaminants by full-scale horizontal flow constructed wetlands fed with secondary treated wastewater. Ecol. Eng. 2017, 99, 222–227. [Google Scholar] [CrossRef]
- Ergaieg, K.; Miled, T.B. Full-scale hybrid constructed wetlands monitoring for decentralized tertiary treatment of municipal wastewater. Arab. J. Geosci. 2021, 14, 1407. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef]
- Khan, S.; Ahmad, I.; Shah, M.T.; Rehman, S.; Khaliq, A. Use of constructed wetland for the removal of heavy metals from industrial wastewater. J. Environ. Manag. 2009, 90, 3451–3457. [Google Scholar] [CrossRef]
- Dunne, E.J.; Coveney, M.F.; Marzolf, E.R.; Hoge, V.R.; Conrow, R.; Naleway, R.; Lowe, E.F.; Battoe, L.E. Efficacy of a large-scale constructed wetland to remove phosphorus and suspended solids from Lake Apopka, Florida. Ecol. Eng. 2012, 42, 90–100. [Google Scholar] [CrossRef]
- Mathon, B.; Coquery, M.; Miège, C.; Vandycke, A.; Choubert, J.-M. Influence of water depth and season on the photodegradation of micropollutants in a free-water surface constructed wetland receiving treated wastewater. Chemosphere 2019, 235, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Talan, A.; Tiwari, B.; Pilli, S.; Sellamuthu, B.; Tyagi, R.D. Constructed wetlands for the removal of organic micro-pollutants. In Current Developments in Biotechnology and Bioengineering; Varjani, S., Pandey, A., Tyagi, R.D., Ngo, H.H., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 87–140. [Google Scholar] [CrossRef]
- Cory, R.M.; Harrold, K.H.; Neilson, B.T.; Kling, G.W. Controls on dissolved organic matter (DOM) degradation in a headwater stream: The influence of photochemical and hydrological conditions in determining light-limitation or substrate-limitation of photo-degradation. Biogeosciences 2015, 12, 6669–6685. [Google Scholar] [CrossRef]
- Reitsema, R.E.; Meire, P.; Schoelynck, J. The future of freshwater macrophytes in a changing world: Dissolved organic carbon quantity and quality and its interactions with macrophytes. Front. Plant Sci. 2018, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Ghosh, S.; Xing, B. Nonideal binding between dissolved humic acids and polyaromatic hydrocarbons. Environ. Sci. Technol. 2007, 41, 6472–6478. [Google Scholar] [CrossRef] [PubMed]
- Doig, L.E.; Liber, K. Influence of dissolved organic matter on nickel bioavailability and toxicity to Hyalella azteca in water-only exposures. Aquat. Toxicol. 2006, 76, 203–216. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xia, X.; Zhai, Y.; Lin, H.; Wen, W.; Wang, Z. Dissolved organic matter affects both bioconcentration kinetics and steady-state concentrations of polycyclic aromatic hydrocarbons in zebrafish (Danio rerio). Sci. Total Environ. 2018, 639, 648–656. [Google Scholar] [CrossRef]
- Liu, S.; Feng, W.; Song, F.; Li, T.; Guo, W.; Wang, B.; Wang, H.; Wu, F. Photodegradation of algae and macrophyte-derived dissolved organic matter: A multi-method assessment of DOM transformation. Limnologica 2019, 77, 125683. [Google Scholar] [CrossRef]
- He, W.; Chen, M.; Schlautman, M.A.; Hur, J. Dynamic exchanges between DOM and POM pools in coastal and inland aquatic ecosystems: A review. Sci. Total Environ. 2016, 551–552, 415–428. [Google Scholar] [CrossRef]
- Ward, C.P.; Cory, R.M. Complete and partial photo-oxidation of dissolved organic matter draining permafrost soils. Environ. Sci. Technol. 2016, 50, 3545–3553. [Google Scholar] [CrossRef]
- Castillo, C.R.; Sarmento, H.; Álvarez-Salgado, X.A.; Gasol, J.M.; Marraséa, C. Production of chromophoric dissolved organic matter by marine phytoplankton. Limnol. Oceanogr. 2010, 55, 446–454. [Google Scholar] [CrossRef]
- Hur, J.; Lee, B.-M.; Shin, H.-S. Microbial degradation of dissolved organic matter (DOM) and its influence on phenanthrene–DOM interactions. Chemosphere 2011, 85, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.M.; Guéguen, C. Binding interactions of algal-derived dissolved organic matter with metal ions. Chemosphere 2013, 90, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, Y.; Meng, W.; He, Z.; Feng, W.; Zhang, C.; Giesy, J.P. Characteristics and degradation of carbon and phosphorus from aquatic macrophytes in lakes: Insights from solid-state 13C NMR and solution 31P NMR spectroscopy. Sci. Total Environ. 2016, 543, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Xue, Y.; Wang, X. Release and microbial degradation of dissolved organic carbon and nitrogen from Phragmites australis and Suaeda salsa in the wetland of the Yellow River Estuary. J. Oceanogr. Mar. Res. 2017, 5, 160. [Google Scholar] [CrossRef]
- Maie, N.; Jaffé, R.; Miyoshi, T.; Childers, D.L. Quantitative and qualitative aspects of dissolved organic carbon leached from senescent plants in an oligotrophic wetland. Biogeochemistry 2006, 78, 285–314. [Google Scholar] [CrossRef]
- Xiaobing, L.; Jianming, Z.; Congqiang, L.; Zhongqing, W.; Fushun, W.; Guojiang, W.; Ronggui, H. Enzymatic and microbial degradation of organic matter in Lake Hongfeng, Guizhou Province, China. Chin. J. Geochem. 2004, 23, 81–88. [Google Scholar] [CrossRef]
- Kirchman, D.L. Microbial proteins for organic material degradation in the deep ocean. Proc. Natl. Acad. Sci. USA 2018, 115, 445–447. [Google Scholar] [CrossRef]
- Kosinkiewicz, B. Humic-like substances of bacterial origin. I. Some aspects of the formation and nature of humic-like substances produced by Pseudomonas. Acta Microbiol. Pol. 1977, 26, 377–386. [Google Scholar]
- Shimotori, K.; Omori, Y.; Hama, T. Bacterial production of marine humic-like fluorescent dissolved organic matter and its biogeochemical importance. Aquat. Microb. Ecol. 2009, 58, 55–66. [Google Scholar] [CrossRef]
- Lou, T.; Xie, H. Photochemical alteration of the molecular weight of dissolved organic matter. Chemosphere 2006, 65, 2333–2342. [Google Scholar] [CrossRef]
- Wilske, C.; Herzsprung, P.; Lechtenfeld, O.J.; Kamjunke, N.; von Tümpling, W. Photochemically induced changes of dissolved organic matter in a humic-rich and forested stream. Water 2020, 12, 331. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of Specific Ultraviolet Absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- De Haan, H.; De Boer, T. Applicability of light absorbance and fluorescence as measures of concentration and molecular size of dissolved organic carbon in humic Lake Tjeukemeer. Water Res. 1987, 21, 731–734. [Google Scholar] [CrossRef]
- Assaad, A.; Pontvianne, S.; Corriou, J.-P.; Pons, M.-N. Spectrophotometric characterization of dissolved organic matter in a rural watershed: The Madon River (N-E France). Environ. Monit. Assess. 2015, 187, 188. [Google Scholar] [CrossRef]
- Weaver, A.J.; Eby, M.; Wiebe, E.C.; Bitz, C.M.; Duffy, P.B.; Ewen, T.L.; Fanning, A.F.; Holland, M.M.; MacFadyen, A.; Matthews, H.D.; et al. The UVic earth system climate model: Model description, climatology, and applications to past, present and future climates. Atmos. Ocean 2001, 39, 361–428. [Google Scholar] [CrossRef]
- Reid, A.M.; Chapman, W.K.; Prescott, C.E.; Nijland, W. Using excess greenness and green chromatic coordinate colour indices from aerial images to assess lodgepole pine vigour, mortality and disease occurrence. Forest Ecol. Manag. 2016, 374, 146–153. [Google Scholar] [CrossRef]
- Soetaert, K.; Hoffmann, M.; Meire, P.; Starink, M.; van Oevelen, D.; Van Regenmortel, S.; Cox, T. Modeling growth and carbon allocation in two reed beds (Phragmites australis) in the Scheldt estuary. Aquat. Bot. 2004, 79, 211–234. [Google Scholar] [CrossRef]
- ISO10304-1; Water Quality—Determination of Dissolved Anions by Liquid Chromatography of Ions—Part 1: Determination of Bromide, Chloride, Fluoride, Nitrate, Nitrite, Phosphate and Sulfate. International Organization for Standardization: Geneva, Switzerland, 2017.
- Standard Methods Committee of the American Public Health Association, American Water Works Association, Water Environment Federation. 4500-p phosphorus. In Standard Methods For the Examination of Water and Wastewater; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- NF T90-015; Determination of Ammonium. Association Française de Normalisation: La Plaine Saint-Denis, France, 1975.
- ISO 21793; Water Quality—Determination of Total Organic Carbon (TOC), Dissolved Organic Carbon (DOC), Total Bound Nitrogen (TNb), Dissolved Bound Nitrogen (DNb), Total Bound Phosphorus (TPb) and Dissolved Bound Phosphorus (DPb) after Wet Chemical Catalysed Ozone Hydroxyl Radical Oxidation (COHR). International Organization for Standardization: Geneva, Switzerland, 2020.
- Peuravuori, J.; Pihlaja, K. Molecular size distribution and spectroscopic properties of aquatic humic substances. Anal. Chim. Acta 1997, 337, 133–149. [Google Scholar] [CrossRef]
- Lawaetz, A.J.; Stedmon, C.A. Fluorescence intensity calibration using the Raman scatter peak of water. Appl. Spectrosc. 2009, 63, 936–940. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Instrumentation for Fluorescence Spectroscopy. In Principles of Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2006; pp. 27–61. [Google Scholar] [CrossRef]
- Lee, C.; Fletcher, T.D.; Sun, G. Nitrogen removal in constructed wetland systems. Eng. Life Sci. 2009, 9, 11–22. [Google Scholar] [CrossRef]
- Hua, Y.; Peng, L.; Zhang, S.; Heal, K.V.; Zhao, J.; Zhu, D. Effects of plants and temperature on nitrogen removal and microbiology in pilot-scale horizontal subsurface flow constructed wetlands treating domestic wastewater. Ecol. Eng. 2017, 108, 70–77. [Google Scholar] [CrossRef]
- Van Oostrom, A.J.; Russell, J.M. Denitrification in constructed wastewater wetlands receiving high concentrations of nitrate. Water Sci. Technol. 1994, 29, 7–14. [Google Scholar] [CrossRef]
- Mietto, A.; Politeo, M.; Breschigliaro, S.; Borin, M. Temperature influence on nitrogen removal in a hybrid constructed wetland system in Northern Italy. Ecol. Eng. 2015, 75, 291–302. [Google Scholar] [CrossRef]
- Huang, J.; Cai, W.; Zhong, Q.; Wang, S. Influence of temperature on micro-environment, plant eco-physiology and nitrogen removal effect in subsurface flow constructed wetland. Ecol. Eng. 2013, 60, 242–248. [Google Scholar] [CrossRef]
- Best, E.P.H. Effects of nitrogen on the growth and nitrogenous compounds of Ceratophyllum demersum. Aquat. Bot. 1980, 8, 197–206. [Google Scholar] [CrossRef]
- Cedergreen, N.; Madsen, T.V. Nitrogen uptake by the floating macrophyte Lemna minor. N. Phytol. 2002, 155, 285–292. [Google Scholar] [CrossRef]
- Tylova-Munzarova, E.; Lorenzen, B.; Brix, H.; Votrubova, O. The effects of NH4+ and NO3− on growth, resource allocation and nitrogen uptake kinetics of Phragmites australis and Glyceria maxima. Aquat. Bot. 2005, 81, 326–342. [Google Scholar] [CrossRef]
- Eliašová, A.; Hrivnák, R.; Štefánová, P.; Svitok, M.; Kochjarová, J.; Oťaheľová, H.; Novikmec, M.; Pal’ove-Balang, P. Effects of ammonium levels on growth and accumulation of antioxidative flavones of the submerged macrophyte Ceratophyllum demersum. Aquat. Bot. 2021, 171, 103376. [Google Scholar] [CrossRef]
- Mostofa, K.M.G.; Yoshioka, T.; Konohira, E.; Tanoue, E. Photodegradation of fluorescent dissolved organic matter in river waters. Geochem. J. 2007, 41, 323–331. [Google Scholar] [CrossRef]
- Lee, M.-H.; Hur, J. Photodegradation-induced changes in the characteristics of dissolved organic matter with different sources and their effects on disinfection by-product formation potential: Photodegradation-induced changes in the characteristics of dissolved organic matter. CLEAN Soil Air Water 2014, 42, 552–560. [Google Scholar] [CrossRef]
- Hansen, A.M.; Kraus, T.E.C.; Pellerin, B.A.; Fleck, J.A.; Downing, B.D.; Bergamaschi, B.A. Optical properties of dissolved organic matter (DOM): Effects of biological and photolytic degradation. Limnol. Oceanogr. 2016, 61, 1015–1032. [Google Scholar] [CrossRef]
- Bowen, J.C.; Kaplan, L.A.; Cory, R.M. Photodegradation disproportionately impacts biodegradation of semi-labile DOM in streams. Limnol. Oceanogr. 2020, 65, 13–26. [Google Scholar] [CrossRef]
- Amorim, C.A.; de Moura-Falcão, R.H.; Valença, C.R.; de Souza, V.R.; Moura, A.N. Allelopathic effects of the aquatic macrophyte Ceratophyllum demersum L. on phytoplankton species: Contrasting effects between cyanobacteria and chlorophytes. Acta Limnol. Bras. 2019, 31, e21. [Google Scholar] [CrossRef]
- Gross, E.M.; Erhard, D.; Iványi, E. Allelopathic activity of Ceratophyllum demersum L. and Najas marina ssp. intermedia (Wolfgang) Casper. Hydrobiologia 2003, 506–509, 583–589. [Google Scholar] [CrossRef]
- Dong, J.; Chang, M.; Li, C.; Dai, D.; Gao, Y. Allelopathic effects and potential active substances of Ceratophyllum demersum L. on Chlorella vulgaris Beij. Aquat. Ecol. 2019, 53, 651–663. [Google Scholar] [CrossRef]
- Moran, M.A.; Hodson, R.E. Dissolved humic substances of vascular plant origin in a coastal marine environment. Limnol. Oceanogr. 1994, 39, 762–771. [Google Scholar] [CrossRef]
- Vymazal, J.; Kröpfelová, L. Wastewater Treatment in Constructed Wetlands with Horizontal Sub-Surface Flow; Environmental Pollution; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Saeed, T.; Al-Muyeed, A.; Afrin, R.; Rahman, H.; Sun, G. Pollutant removal from municipal wastewater employing baffled subsurface flow and integrated surface flow-floating treatment wetlands. J. Environ. Sci. 2014, 26, 726–736. [Google Scholar] [CrossRef]
- Feijoó, C.; Giorgi, A.; Ferreiro, N. Phosphate uptake in a macrophyte-rich Pampean stream. Limnologica 2011, 41, 285–289. [Google Scholar] [CrossRef][Green Version]
- Gao, J.; Xiong, Z.; Zhang, J.; Zhang, W.; Obono Mba, F. Phosphorus removal from water of eutrophic Lake Donghu by five submerged macrophytes. Desalination 2009, 242, 193–204. [Google Scholar] [CrossRef]
- Song, M.; Li, M.; Liu, J. Uptake characteristics and kinetics of inorganic and organic phosphorus by Ceratophyllum demersum. Water Air Soil Pollut. 2017, 228, 407. [Google Scholar] [CrossRef]
- Güsewell, S.; Koerselman, W. Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect. Plant Ecol. Evol. Syst. 2002, 5, 37–61. [Google Scholar] [CrossRef]
- Jesus, J.M.; Danko, A.S.; Fiúza, A.; Borges, M.-T. Effect of plants in constructed wetlands for organic carbon and nutrient removal: A review of experimental factors contributing to higher impact and suggestions for future guidelines. Environ. Sci. Pollut. Res. Int. 2018, 25, 4149–4164. [Google Scholar] [CrossRef]
- Sillanpää, M. Natural Organic Matter in Water: Characterization and Treatment Methods; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Osborne, T.Z.; Inglett, P.W.; Reddy, K.R. The use of senescent plant biomass to investigate relationships between potential particulate and dissolved organic matter in a wetland ecosystem. Aquat. Bot. 2007, 86, 53–61. [Google Scholar] [CrossRef]
- Pellerin, B.A.; Hernes, P.J.; Saraceno, J.; Spencer, R.G.M.; Bergamaschi, B.A. Microbial degradation of plant leachate alters lignin phenols and trihalomethane precursors. J. Environ. Qual. 2010, 39, 946–954. [Google Scholar] [CrossRef]
- Nishimura, S.; Maie, N.; Baba, M.; Sudo, T.; Sugiura, T.; Shima, E. Changes in the quality of chromophoric dissolved organic matter leached from senescent leaf litter during the early decomposition. J. Environ. Qual. 2012, 41, 823–833. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Guo, X.; Zhai, L.; Wang, X.; Wang, J.; Cui, Y.; He, L.; Yan, C.; Kou, Y. Impact of hydrophyte decomposition on the changes and characteristics of dissolved organic matter in lake water. Ecol. Indic. 2020, 116, 106482. [Google Scholar] [CrossRef]
- Cuassolo, F.; Bastidas Navarro, M.; Balseiro, E.; Modenutti, B. Leachates and elemental ratios of macrophytes and benthic algae of an Andean high altitude wetland. J. Limnol. 2011, 70, 168. [Google Scholar] [CrossRef]
- Cuassolo, F.; Navarro, M.B.; Balseiro, E.; Modenutti, B. Effect of light on particulate and dissolved organic matter production of native and exotic macrophyte species in Patagonia. Hydrobiologia 2016, 766, 29–42. [Google Scholar] [CrossRef]
- Qu, X.; Xie, L.; Lin, Y.; Bai, Y.; Zhu, Y.; Xie, F.; Giesy, J.P.; Wu, F. Quantitative and qualitative characteristics of dissolved organic matter from eight dominant aquatic macrophytes in Lake Dianchi, China. Environ. Sci. Pollut. Res. 2013, 20, 7413–7423. [Google Scholar] [CrossRef]
- Coble, P. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Hur, J.; Hwang, S.-J.; Shin, J.-K. Using synchronous fluorescence technique as a water quality monitoring tool for an urban river. Water Air Soil Pollut. 2008, 191, 231–243. [Google Scholar] [CrossRef]
- Chupakova, A.A.; Chupakov, A.V.; Neverova, N.V.; Shirokova, L.S.; Pokrovsky, O.S. Photodegradation of river dissolved organic matter and trace metals in the largest European Arctic estuary. Sci. Total Environ. 2018, 622–623, 1343–1352. [Google Scholar] [CrossRef]
- Palma, D.; Sleiman, M.; Voldoire, O.; Beauger, A.; Parlanti, E.; Richard, C. Study of the dissolved organic matter (DOM) of the Auzon cut-off meander (Allier River, France) by spectral and photoreactivity approaches. Environ. Sci. Pollut. Res. 2020, 27, 26385–26394. [Google Scholar] [CrossRef]
- Sardana, A.; Cottrell, B.; Soulsby, D.; Aziz, T.N. Dissolved organic matter processing and photoreactivity in a wastewater treatment constructed wetland. Sci. Total Environ. 2019, 648, 923–934. [Google Scholar] [CrossRef]
- Hur, J.; Jung, K.-Y.; Jung, Y.M. Characterization of spectral responses of humic substances upon UV irradiation using two-dimensional correlation spectroscopy. Water Res. 2011, 45, 2965–2974. [Google Scholar] [CrossRef]
- Clark, C.D.; De Bruyn, W.J.; Brahm, B.; Aiona, P. Optical properties of chromophoric dissolved organic matter (CDOM) and dissolved organic carbon (DOC) levels in constructed water treatment wetland systems in southern California, USA. Chemosphere 2020, 247, 125906. [Google Scholar] [CrossRef]
- Yao, Y.; Li, Y.; Guo, X.; Huang, T.; Gao, P.; Zhang, Y.; Yuan, F. Changes and characteristics of dissolved organic matter in a constructed wetland system using fluorescence spectroscopy. Environ. Sci. Pollut. Res. 2016, 23, 12237–12245. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Liu, L.; He, Z.; Giesy, J.P.; Bai, Y.; Sun, F.; Wu, F. Cation-induced coagulation of aquatic plant-derived dissolved organic matter: Investigation by EEM-PARAFAC and FT-IR spectroscopy. Environ. Pollut. 2018, 234, 726–734. [Google Scholar] [CrossRef]
- Fu, X.; Du, H.; Xu, H. Comparison in UV-induced photodegradation properties of dissolved organic matters with different origins. Chemosphere 2021, 280, 130633. [Google Scholar] [CrossRef]

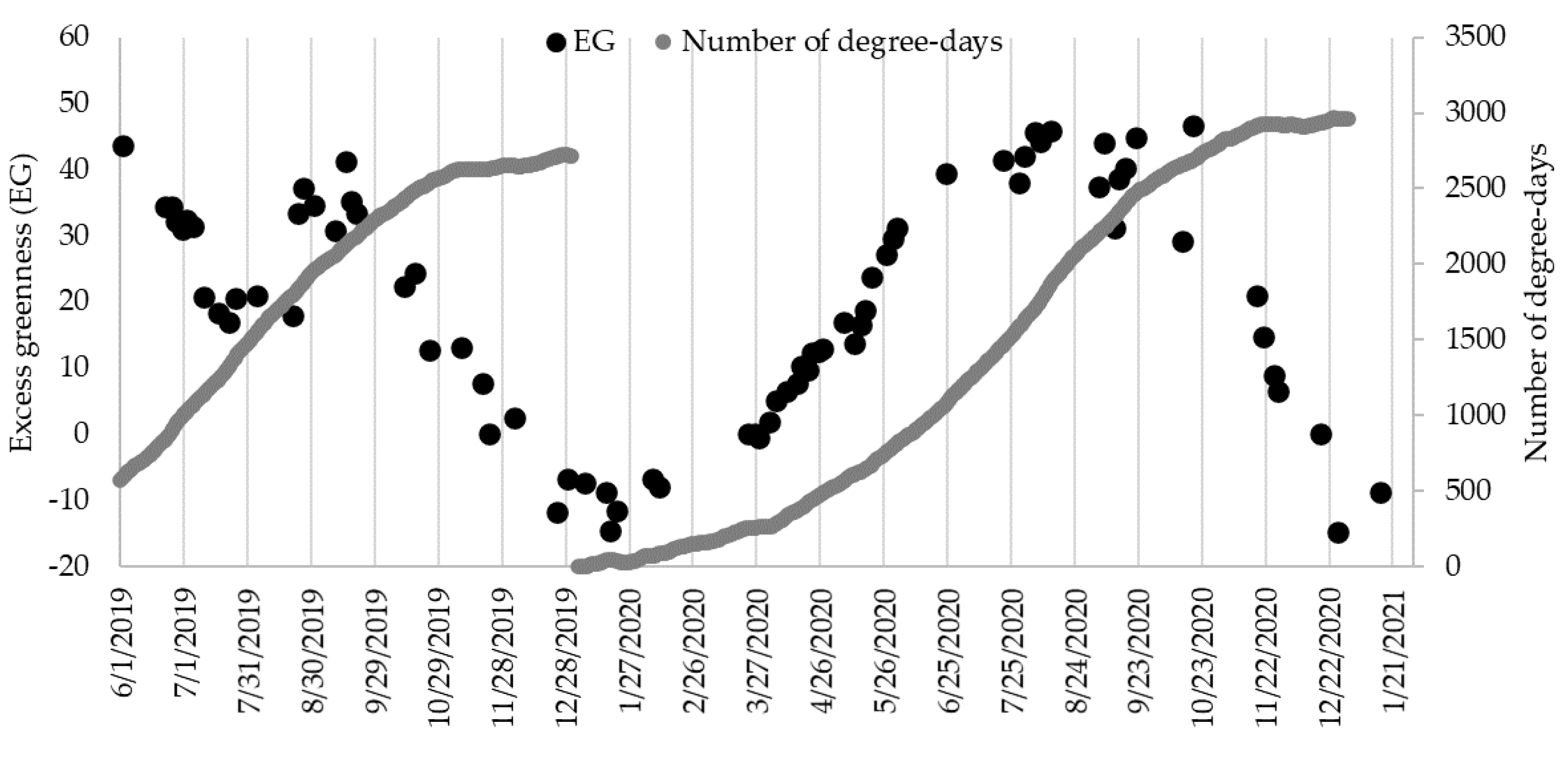

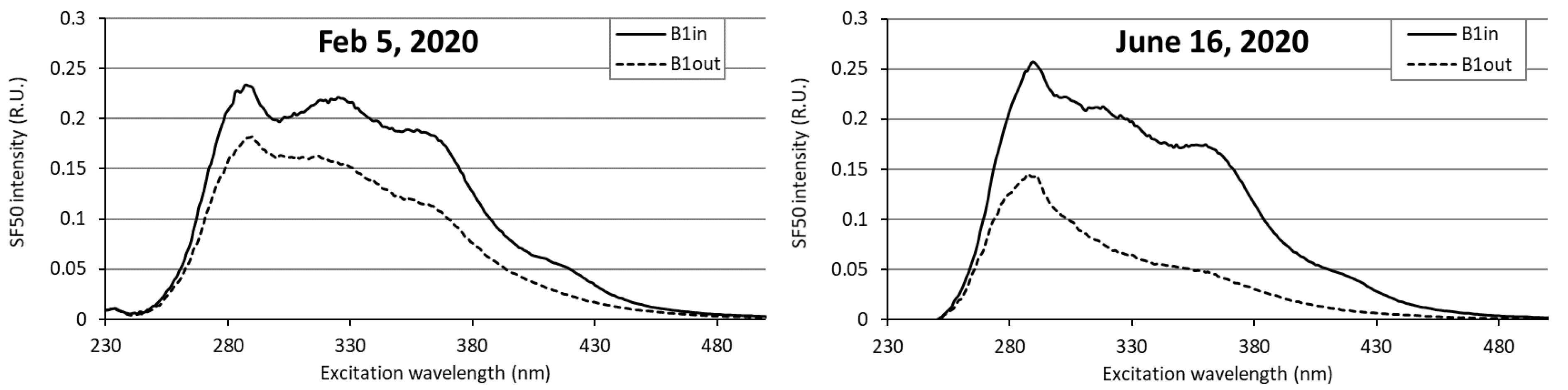

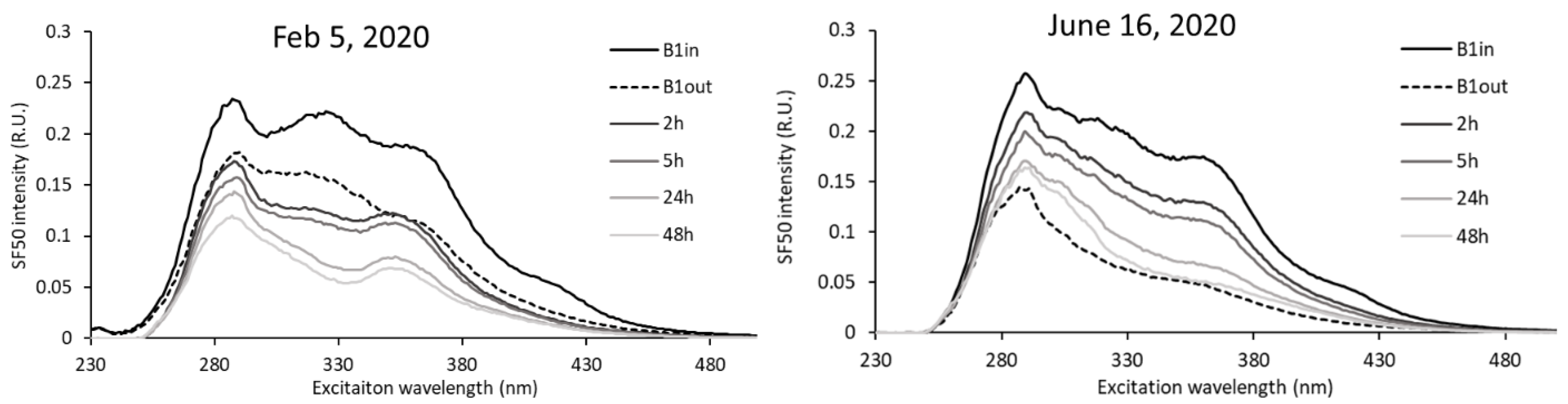

| Season | Months | Average Air Temperature (°C) | Number of Sunlight Hours (h·month−1) | Average Water Temperature (°C) (Min–Max) |
|---|---|---|---|---|
| Winter | December, January, February | 5.6 ± 1.4 | 79 ± 37 | 6.5 ± 2.5 (0.2–14.1) |
| Spring | March, April, May | 9.9 ± 2.4 | 216 ± 53 | 14.5 ± 4.5 (3.6–25.8) |
| Summer | June, July, August | 18.7 ± 1.3 | 232 ± 38 | 20.5 ± 2.0 (14.7–28.2) |
| Autumn | September, October, November | 11.7 ± 4.3 | 132 ± 60 | 12.5 ± 3.6 (3.6–22.3) |
| pH | Conductivity (µS·cm−1) | DOC (mgC·L−1) | TN (mgN·L−1) | N-NH4 (mgN·L−1) | N-NO3 (mgN·L−1) | o-Ps (mgPO4·L−1) | |
|---|---|---|---|---|---|---|---|
| Inlet | 7.8 ± 0.2 a | 928 ± 117 a | 7.3 ± 0.8 a | 2.3 ± 0.6 a | 0.9 ± 0.4 a | 0.9 ± 0.8 a | 1.4 ± 0.4 a |
| B1out | 7.9 ± 0.3 a | 849 ± 114 a | 8.1 ± 1.5 a | 1.6 ± 0.7 b | 0.7 ± 0.8 a | 0.3 ± 0.4 b | 1.2 ± 0.6 a |
| B2out | 7.9 ± 0.3 a | 854 ± 109 a | 7.9 ± 1.4 a | 1.2 ± 0.4 b | 0.4 ± 0.3 b | 0.3 ± 0.4 b | 1.4 ± 0.6 a |
| B3out | 7.9 ± 0.3 a | 832 ± 101 a | 8.5 ± 1.8 a | 1.1 ± 0.3 b | 0.3 ± 0.2 b | 0.2 ± 0.3 b | 1.4 ± 0.8 a |
| SUVA254 (L·mgC−1·nm−1) | S275–295 | E2/E3 | |
|---|---|---|---|
| Inlet | 2.1 ± 0.2 a | 0.015 ± 0.001 a | 5.1 ± 0.06 a |
| B1out | 2.2 ± 0.3 a | 0.016 ± 0.001 a | 5.1 ± 0.07 a |
| B2out | 2.1 ± 0.3 a | 0.015 ± 0.005 a | 5.3 ± 0.06 a |
| B3out | 2.2 ± 0.2 a | 0.016 ± 0.001 a | 5.3 ± 0.05 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurice, N.; Pochet, C.; Adouani, N.; Pons, M.-N. Role of Seasons in the Fate of Dissolved Organic Carbon and Nutrients in a Large-Scale Surface Flow Constructed Wetland. Water 2022, 14, 1474. https://doi.org/10.3390/w14091474
Maurice N, Pochet C, Adouani N, Pons M-N. Role of Seasons in the Fate of Dissolved Organic Carbon and Nutrients in a Large-Scale Surface Flow Constructed Wetland. Water. 2022; 14(9):1474. https://doi.org/10.3390/w14091474
Chicago/Turabian StyleMaurice, Nicolas, Cécile Pochet, Nouceiba Adouani, and Marie-Noëlle Pons. 2022. "Role of Seasons in the Fate of Dissolved Organic Carbon and Nutrients in a Large-Scale Surface Flow Constructed Wetland" Water 14, no. 9: 1474. https://doi.org/10.3390/w14091474
APA StyleMaurice, N., Pochet, C., Adouani, N., & Pons, M.-N. (2022). Role of Seasons in the Fate of Dissolved Organic Carbon and Nutrients in a Large-Scale Surface Flow Constructed Wetland. Water, 14(9), 1474. https://doi.org/10.3390/w14091474








