Ecological and Health Risk Assessment of Heavy Metals in Soils from Recycled Lead Smelting Sites
Abstract
:1. Introduction
2. Materials and Methods
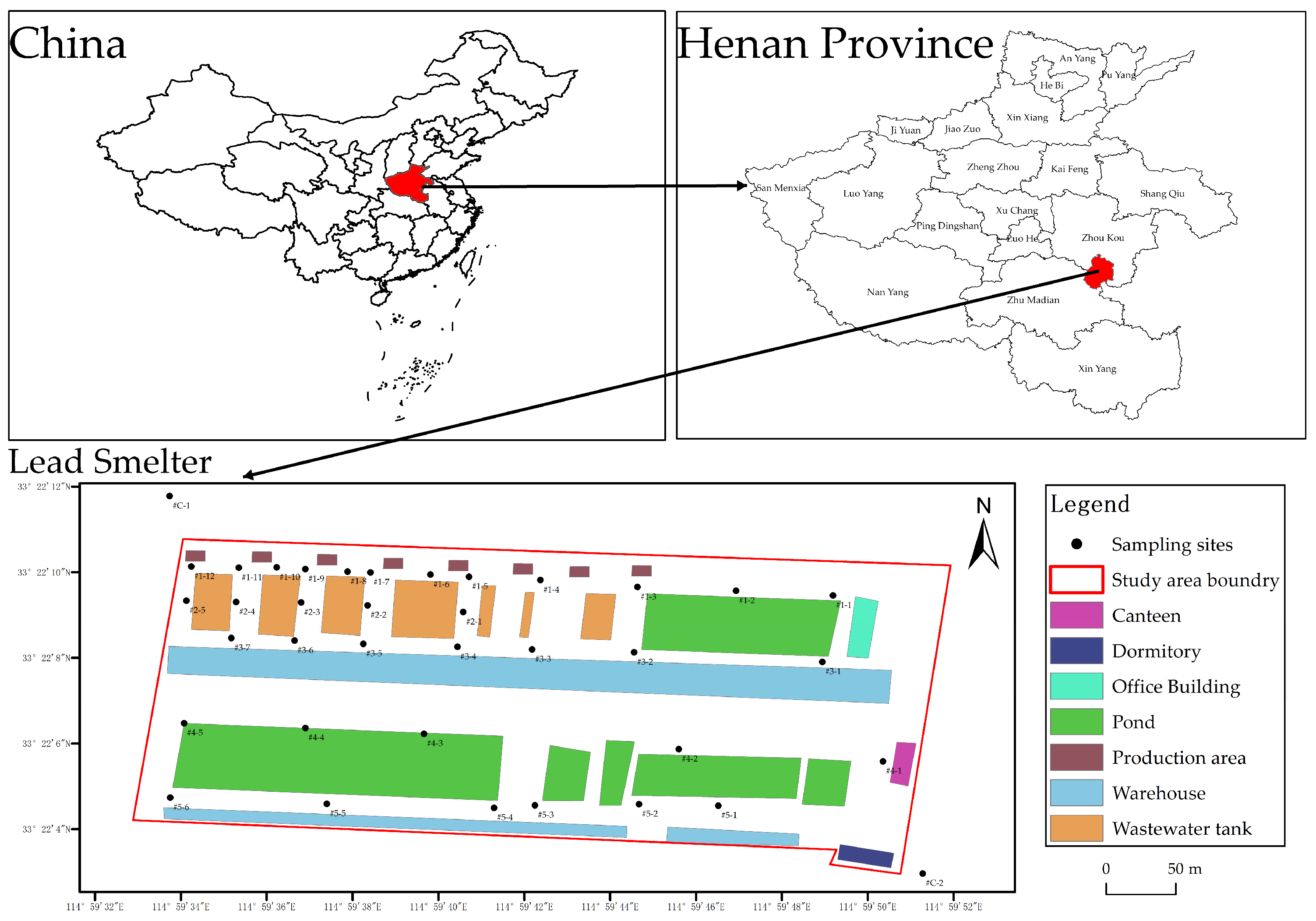
2.1. Study Area
2.2. Sample Collection and Pretreatment
2.3. Laboratory Analysis
2.3.1. Total HM Analysis
2.3.2. HM Speciation Analysis
2.3.3. Quality Control
2.4. Soil Risk Assessment
2.4.1. Geo-Accumulation Index
2.4.2. RAC
2.4.3. Human Health Risk Assessment
2.5. Statistical Analysis
3. Results and Discussion
3.1. Soil Concentration of HMs
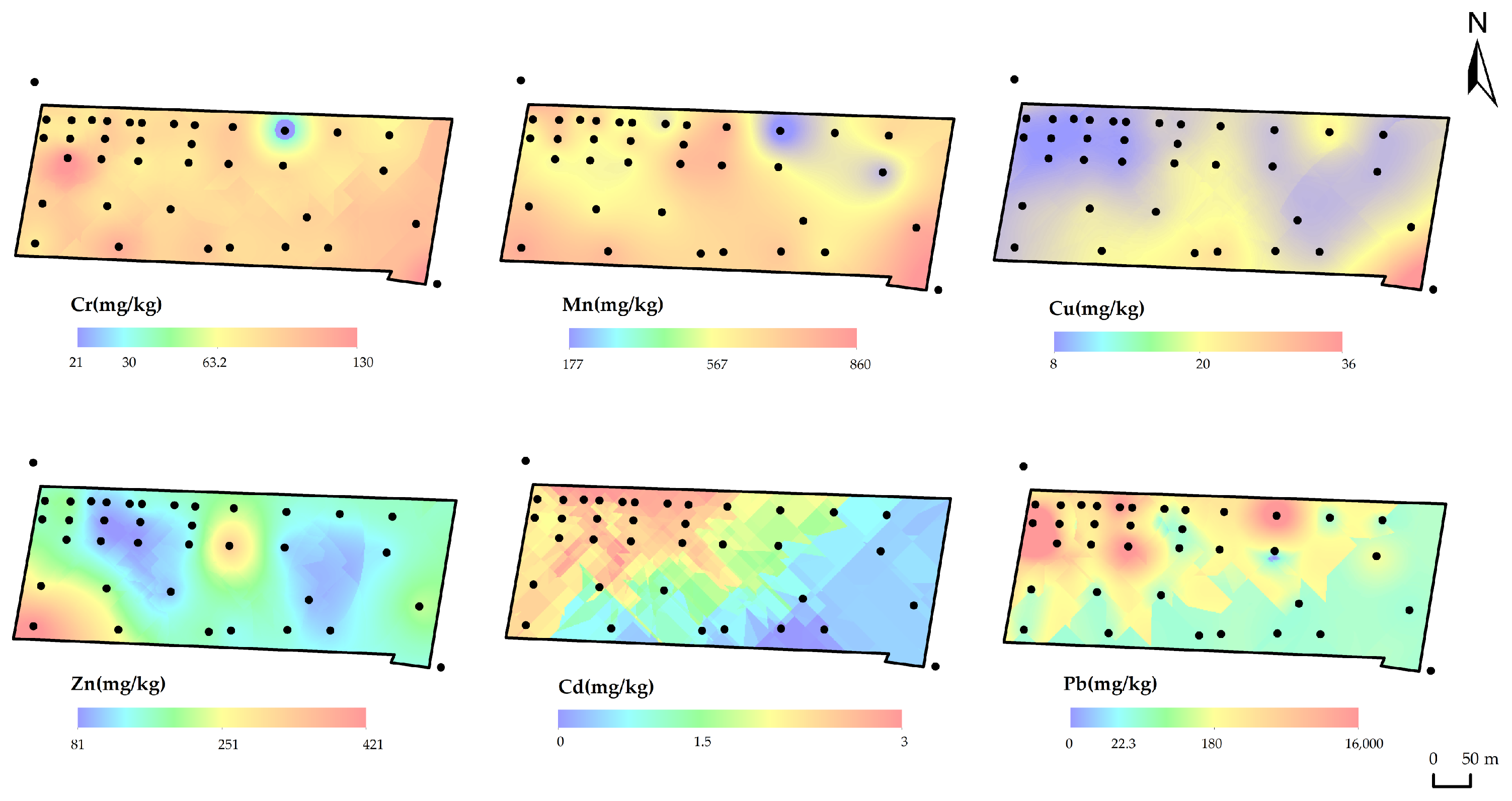
3.1.1. Characteristics of HM Distributions in Surface Soil (0–0.2 m)
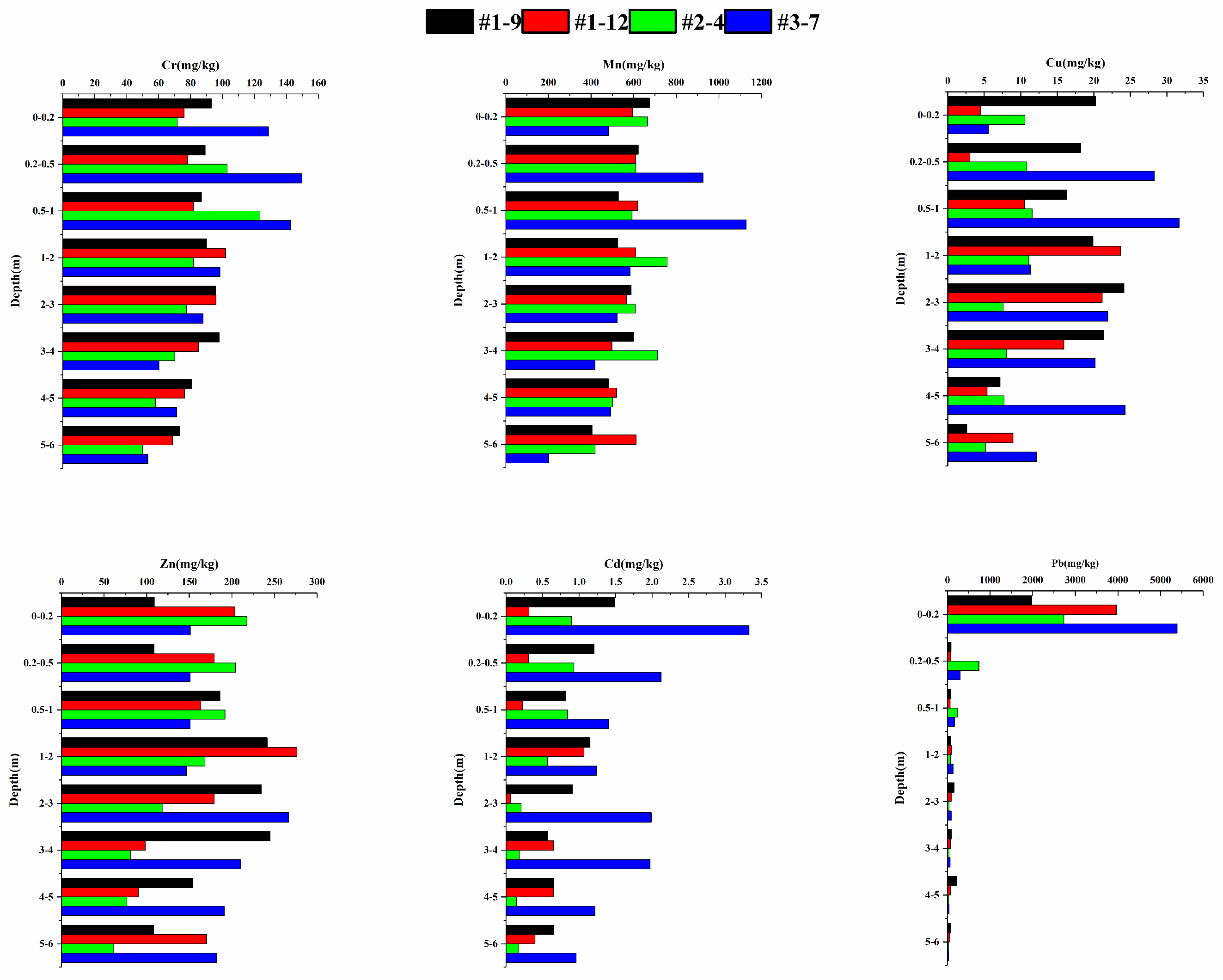
3.1.2. Vertical Distribution of HM Concentration
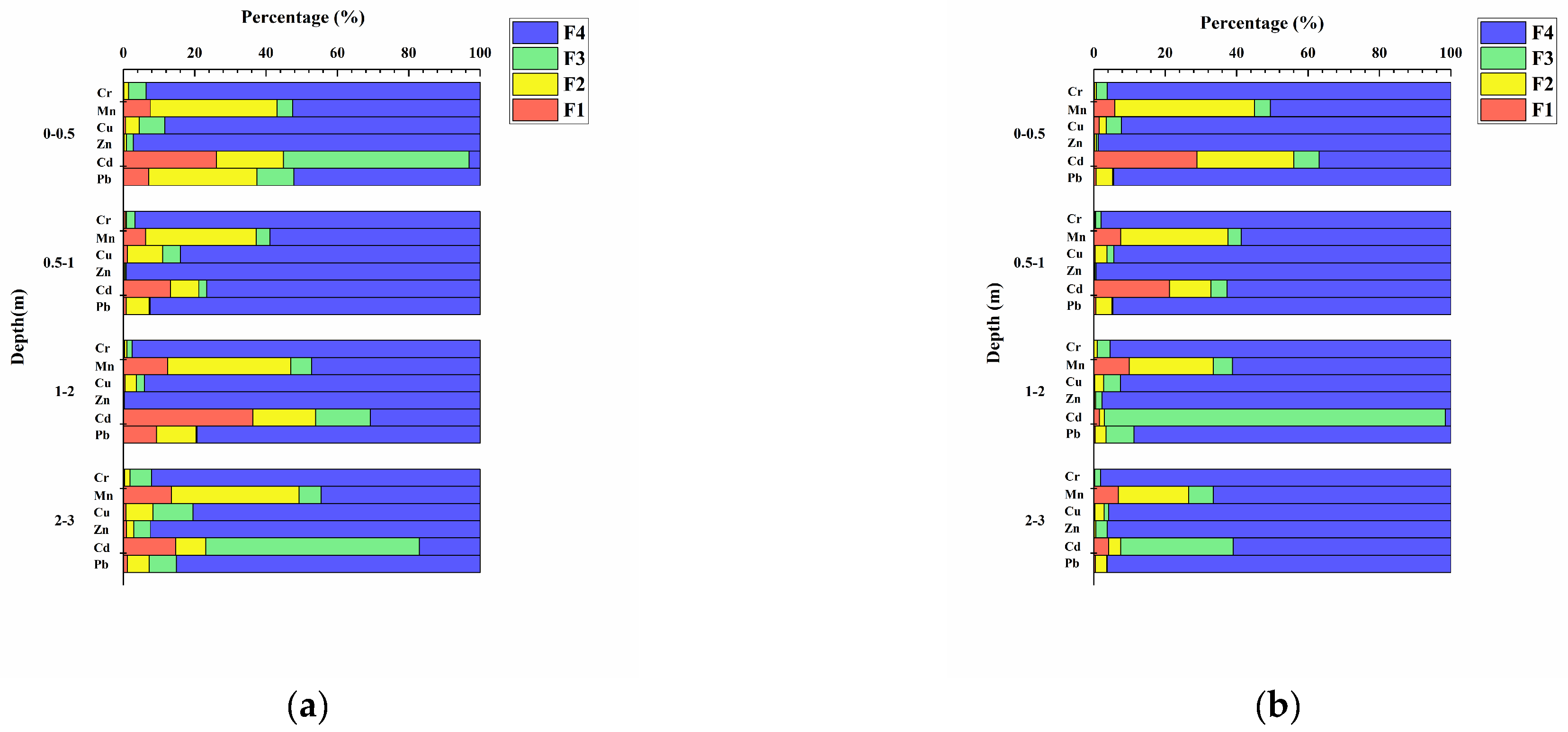
3.1.3. Speciation Analysis of HMs in Soil of the Study Area
3.2. Ecological Risk Assessment
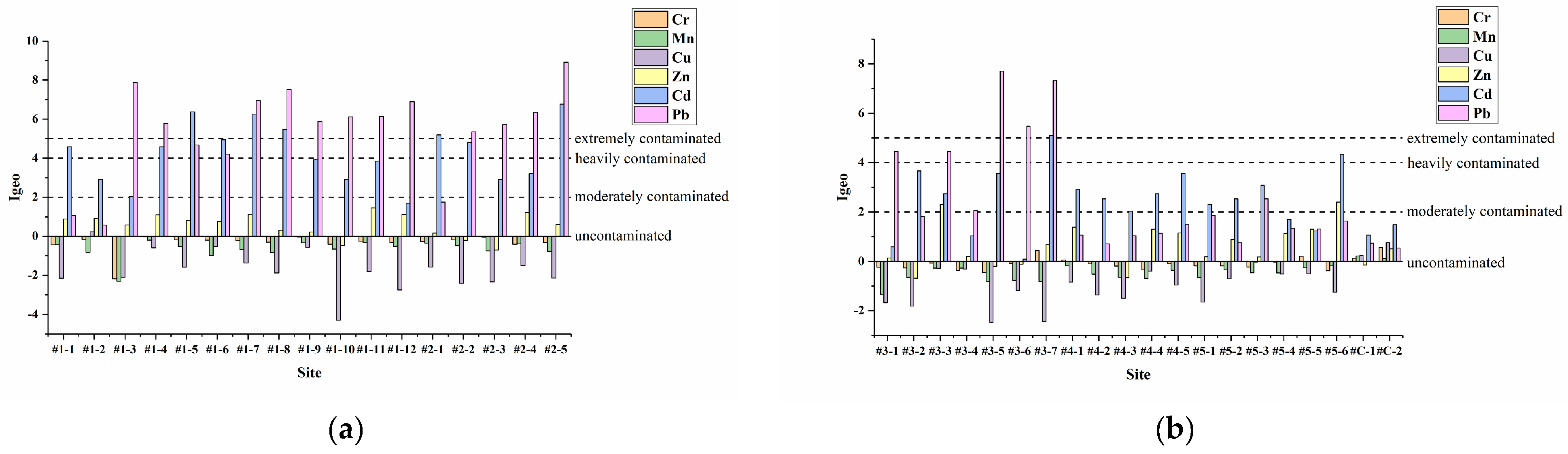
3.2.1. Geo-Accumulation Index (Igeo)
3.2.2. RAC
3.3. Health Risk Assessment
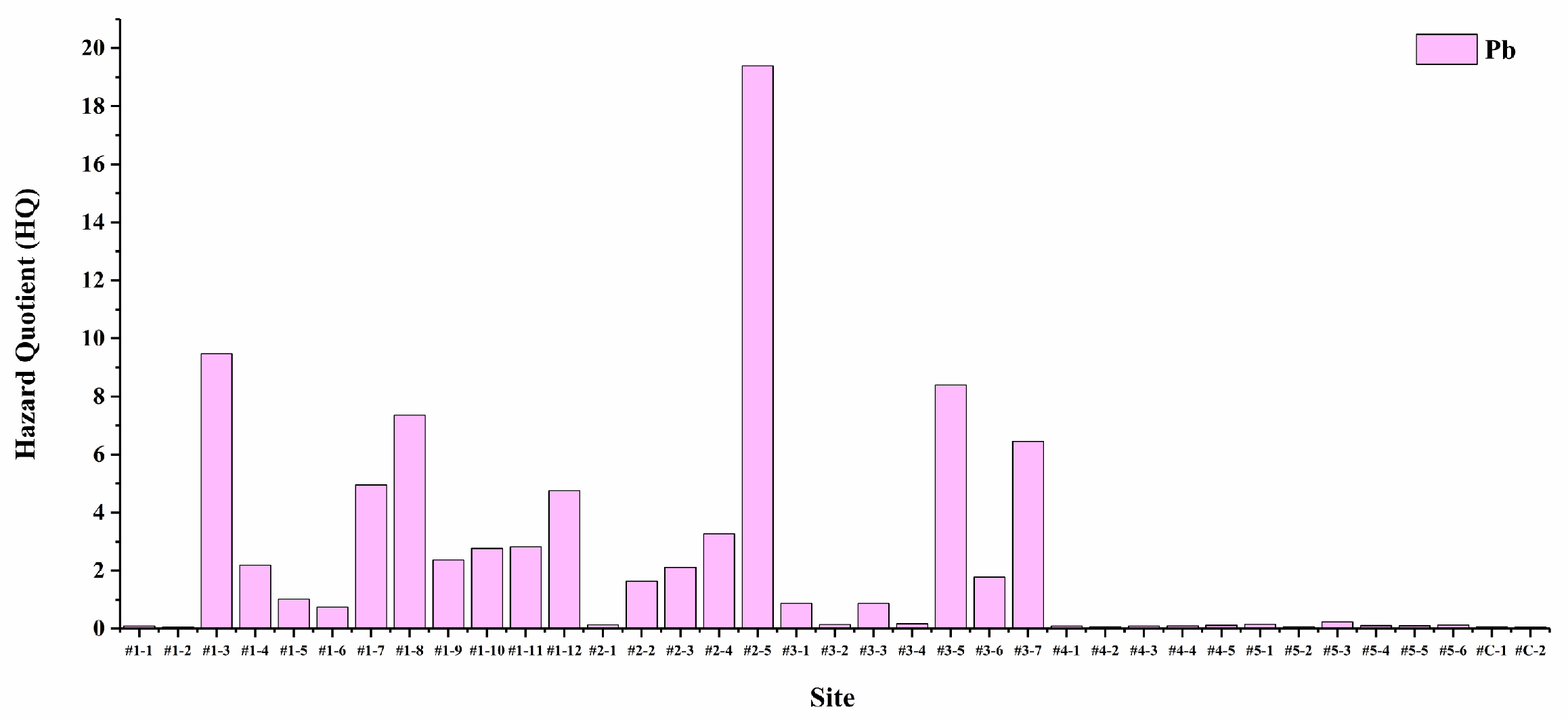
3.3.1. Hazard Quotient (HQ)
3.3.2. Carcinogenic Risk (CR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akter, S.; Rahman, M.O.; Hasan, M.; Tushar, S.I.; Sarkar, M.H.; Mamun, K.M.; Kabir, M.J.; Rahman, M.S.; Abedin, M.J.; Begum, B.A.; et al. Source Apportionment of Heavy Metal Contaminated Soil Around a Urea Fertilizer Factory and Probabilistic Risk Assessment of Human Health. Available online: https://www.researchgate.net/publication/352387464_Source_Apportionment_of_Heavy_Metal_Contaminated_Soil_Around_a_Urea_Fertilizer_Factory_and_Probabilistic_Risk_Assessment_of_Human_Health (accessed on 12 April 2022).
- Guemiza, K.; Coudert, L.; Metahni, S.; Mercier, G.; Besner, S.; Blais, J.-F. Treatment technologies used for the removal of As, Cr, Cu, PCP and/or PCDD/F from contaminated soil: A review. J. Hazard. Mater. 2017, 333, 194–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2016, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.Y. The Current Status on the Recycling of Lead-acid Batteries in China. Int. J. Electrochem. Sci. 2013, 8, 6457–6466. [Google Scholar]
- Wu, Z.-Y.; Zhang, L.-N.; Xia, T.-X.; Jia, X.-Y.; Li, H.-Y.; Wang, S.-J. Quantitative Assessment of Human Health Risks Based on Soil Heavy Metals and PAHs Sources: Take a Polluted Industrial Site of Beijing as an Example. Environ. Sci. 2020, 41, 4180–4196. [Google Scholar]
- Wu, Z.; Zhang, L.; Xia, T.; Jia, X.; Wang, S. Heavy metal pollution and human health risk assessment at mercury smelting sites in Wanshan district of Guizhou Province, China. RSC Adv. 2020, 10, 23066–23079. [Google Scholar] [CrossRef]
- Sungur, A.; Soylak, M.; Ozcan, H. Fractionation, Source Identification and Risk Assessments for Heavy Metals in Soils near a Small-Scale In-dustrial Area (Çanakkale-Turkey). Soil Sediment Contam. Int. J. 2019, 28, 213–227. [Google Scholar] [CrossRef]
- Sterckeman, T.; Douay, F.; Proix, N.; Fourrier, H. Vertical distribution of Cd, Pb and Zn in soils near smelters in the North of France. Environ. Pollut. 2000, 107, 377–389. [Google Scholar] [CrossRef]
- Sterckeman, T.; Douay, F.; Proix, N.; Fourrier, H.; Perdrix, E. Assessment of the Contamination of Cultivated Soils by Eighteen Trace Elements Around Smelters in the North of France. Water Air Soil Pollut. 2002, 135, 173–194. [Google Scholar] [CrossRef]
- Wang, J.; Su, J.; Li, Z.; Liu, B.; Cheng, G.; Jiang, Y.; Li, Y.; Zhou, S.; Yuan, W. Source apportionment of heavy metal and their health risks in soil-dustfall-plant system nearby a typical non-ferrous metal mining area of Tongling, Eastern China. Environ. Pollut. 2019, 254, 113089. [Google Scholar] [CrossRef]
- Cai, L.-M.; Xu, Z.-C.; Qi, J.-Y.; Feng, Z.-Z.; Xiang, T.-S. Assessment of exposure to heavy metals and health risks among residents near Tonglushan mine in Hubei, China. Chemosphere 2015, 127, 127–135. [Google Scholar] [CrossRef]
- Qu, L.; Huang, H.; Xia, F.; Liu, Y.; Dahlgren, R.A.; Zhang, M.; Mei, K. Risk analysis of heavy metal concentration in surface waters across the rural-urban interface of the Wen-Rui Tang River, China. Environ. Pollut. 2018, 237, 639–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkham, M.B. Cadmium in plants on polluted soils: Effects of soil factors, hyperaccumulation, and amendments. Geoderma 2006, 137, 19–32. [Google Scholar] [CrossRef]
- Impellitteri, C.A.; Saxe, J.K.; Cochran, M.; Janssen, G.M.C.M.; Allen, H.E. Predicting the bioavailability of copper and zinc in soils: Modeling the partitioning of potentially bioavailable copper and zinc from soil solid to soil solution. Environ. Toxicol. Chem. Int. J. 2010, 22, 1380–1386. [Google Scholar] [CrossRef]
- Sastre, J.; Hernández, E.; Rodrıguez, R.; Alcobé, X.; Vidal, M.; Rauret, G. Use of sorption and extraction tests to predict the dynamics of the interaction of trace elements in agricultural soils contaminated by a mine tailing accident. Sci. Total Environ. 2004, 329, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.; Namiesńik, J. The role of speciation in analytical chemistry. Trends Anal. Chem. 2000, 19, 69–79. [Google Scholar] [CrossRef]
- Rauret, G. Extraction procedures for the determination of heavy metals in contaminated soil and sediment. Talanta 1998, 46, 449–455. [Google Scholar] [CrossRef]
- Karimian, S.; Shekoohiyan, S.; Moussavi, G. Health and ecological risk assessment and simulation of heavy metal-contaminated soil of Tehran landfill. RSC Adv. 2021, 11, 8080–8095. [Google Scholar] [CrossRef]
- Xing, X.Y.; Xin, Y.G.; Li, H.Q.; Nan, S.; Ting, P.X. Spatial Distribution and Pollution Characteristics of Heavy Metals in Soil of Mentougou Mining Area of Beijing City, China. J. Agric. Resour. Environ. 2016, 33, 499–507. [Google Scholar]
- Ohiagu, F.O.; Lele, K.C.; Chikezie, P.C.; Verla, A.W.; Enyoh, C.E. Pollution Profile and Ecological Risk Assessment of Heavy Metals from Dumpsites in Onne, Rivers State, Nigeria. Chem. Afr. 2021, 4, 207–216. [Google Scholar] [CrossRef]
- Ma, Z.X.; Ren, W.X.; Xue, B.; Zhang, L.M.; Geng, Y.; Sun, L.N. Human health risk assessment of heavy metal pollutants in redevelopment brownfield area. Ecol. Sci. 2014, 33, 963–971. [Google Scholar]
- Wang, R.; Deng, H.; Jia, Z.-M.; Wang, J.-B.; Yu, F.; Zeng, Q.-Q. Spatial Distribution Characteristics, Pollution, and Ecological Risk Assessment of Soil Heavy Metals Around Mercury Mining Areas. Environ. Sci. 2021, 42, 3018–3027. [Google Scholar]
- Tan, J.-H.; Duan, J.-C.; Ma, Y.-L.; Yang, F.-M.; Cheng, Y.; He, K.-B.; Yu, Y.-C.; Wang, J.-W. Source of atmospheric heavy metals in winter in Foshan, China. Sci. Total Environ. 2014, 493, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.J.; Liu, Y.; Zhang, A.N. Quantitative contributions of the major sources of heavy metals in soils to ecosystem and human health risks: A case study of Yulin, China. Ecotoxicol. Environ. Saf. 2018, 164, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Muller, G. Index of Geoaccumulation in Sediments of the Rhine River. GeoJournal 1969, 2, 109–118. [Google Scholar]
- Berstel, J.; Perrin, D. Theory of Codes; Academic Press: New York, NY, USA, 1985; pp. 1–433. [Google Scholar]
- Meng, X.-F.; Guo, J.-M.; Yang, J.-X.; Yang, J.; Zheng, G.-D.; Qiao, P.-W.; Bian, J.-L.; Chen, T.-B. Spatial Distribution and Risk Assessment of Heavy Metal Pollution in Farmland Soils Surrounding a Typical Industrial Area of Henan Province. Environ. Sci. 2021, 42, 900–908. [Google Scholar]
- The institution is Ministry of Ecology and Environment. PRC Technical Guidelines for Risk Assessment of Soil Contamination of Land for Construction; China Environment Publishing Group: Beijing, China, 2019; pp. 1–53. [Google Scholar]
- Michael, A. Soil elemental concentrations, geoaccumulation index, non-carcinogenic and carcinogenic risks in functional areas of an informal e-waste recycling area in Accra, Ghana. Chemosphere 2019, 235, 908–917. [Google Scholar]
- Bin, L.Z.; Fu, C.; Yuan, Z.W.; Yan, F.Q.; Liang, Z.S.; Jing, M. Heavy Metal Contamination and Health Risk Assessment in the Soil Surrounding a Secondary Lead Plant. Environ. Sci. Technol. 2018, 41, 197–207. [Google Scholar]
- Lu, S.; Wang, Y.; Teng, Y.; Yu, X. Heavy metal pollution and ecological risk assessment of the paddy soils near a zinc-lead mining area in Hunan. Environ. Monit. Assess. 2015, 187, 627. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, Y.; Ji, H. Distribution, speciation, and risk assessment of selected metals in the gold and iron mine soils of the catchment area of Miyun Reservoir, Beijing, China. Environ. Monit. Assess. 2013, 185, 8525–8545. [Google Scholar] [CrossRef]
- Zhiyuan, W.; Zhang, D.; Tianxiang, X.; Xiaoyang, J. Soil Heavy Metals Contamination of a Typical Calcium Carbide Slag Dump Sites: Source Apportionment and Risk Assessment. Available online: https://www.researchgate.net/publication/349267980_Soil_Heavy_Metals_Contamination_of_a_Typical_Calcium_Carbide_Slag_Dump_Sites_Source_Apportionment_and_Risk_Assessment (accessed on 12 April 2022).
- Edori, O.S.; Iyama, W. Assessment of Heavy Metals pollution in Soils within Hot Mix Asphalt Plants Vicinity in Port Harcourt, Rivers State, Nigeria. Pharm. Chem. J. 2021, 8, 24–32. [Google Scholar]
- Liu, D.; Wang, F. Heavy Metal Pollution in Street Dusts from Different Functional Zones of Luoyang City and Its Potential Ecological Risk. Environ. Sci. 2012, 33, 253–259. [Google Scholar]
- The institution is Ministry of Ecology and Environment. PRC Soil Environmental Quality-Risk Control Standard for Soil Contamination of Development Land; China Environment Publishing Group: Beijing, China, 2018; pp. 1–18. [Google Scholar]
- Tao, H.; Guo, H.; Wang, Y.; He, Y.; Liu, W.; Chen, Y.; Shen, J. Distribution Characteristics and Ecological Risk Assessment of Pb in Soil at a Typical Lead Battery Sites. Environ. Sci. Technol. 2017, 40, 143–148. [Google Scholar]
- Cui, X.; Geng, Y.; Sun, R.; Xie, M.; Feng, X.; Li, X.; Cui, Z. Distribution, speciation and ecological risk assessment of heavy metals in Jinan Iron & Steel Group soils from China. J. Clean. Prod. 2021, 295, 126504. [Google Scholar] [CrossRef]
- Rieuwerts, J.S.; Farago, M.; Cikrt, M.; Bencko, V. Heavy Metal Concentrations in and Around Households Near a Secondary Lead Smelter. Environ. Monit. Assess. 1999, 58, 317–335. [Google Scholar] [CrossRef]
- Burt, R.; Wilson, M.A.; Keck, T.J.; Dougherty, B.D.; Strom, D.E.; Lindahl, J.A. Trace element speciation in selected smelter-contaminated soils in Anaconda and Deer Lodge Valley, Montana, USA. Adv. Environ. Res. 2003, 8, 51–67. [Google Scholar] [CrossRef]
- Kaasalainen, M.; Yli-Halla, M. Use of sequential extraction to assess metal partitioning in soils. Environ. Pollut. 2003, 126, 225–233. [Google Scholar] [CrossRef]
- Rodríguez, L.; Ruiz, E.; Alonso-Azcárate, J.; Rincón, J. Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J. Environ. Manag. 2009, 90, 1106–1116. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Z.; Li, S.; Chen, J. Bioavailability and toxicity of trace metals (Cd, Cr, Cu, Ni, and Zn) in sediment cores from the Shima River, South China. Chemosphere 2018, 192, 31–42. [Google Scholar] [CrossRef]
- Zhang, C.; Shan, B.; Tang, W.; Dong, L.; Zhang, W.; Pei, Y. Heavy metal concentrations and speciation in riverine sediments and the risks posed in three urban belts in the Haihe Basin. Ecotoxicol. Environ. Saf. 2017, 139, 263–271. [Google Scholar] [CrossRef]
- Jahromi, F.A.; Keshavarzi, B.; Moore, F.; Abbasi, S.; Busquets, R.; Hooda, P.S.; Jaafarzadeh, N. Source and risk assessment of heavy metals and microplastics in bivalves and coastal sediments of the Northern Persian Gulf, Hormogzan Province. Environ. Res. 2021, 196, 110963. [Google Scholar] [CrossRef]
- Wu, T.; Li, X.P.; Cai, Y.; Ai, Y.W.; Sun, X.M.; Yu, H.T. Geochemical behavior and risk of heavy metals in different size lead-polluted soil particles. China Environ. Sci. 2017, 37, 4212–4221. [Google Scholar]
- Luo, G.; Han, Z.; Xiong, J.; He, Y.; Liao, J.; Wu, P. Heavy metal pollution and ecological risk assessment of tailings in the Qinglong Dachang antimony mine, China. Environ. Sci. Pollut. Res. 2021, 28, 33491–33504. [Google Scholar] [CrossRef] [PubMed]

| Step | Fraction | Extractant | Experimental Process |
|---|---|---|---|
| F1 | Extractable | 32 mL of 0.11 M CH3COOH | Shaking at room temperature for 16 h |
| F2 | Reducible | 32 mL of 0.5 M NH3OH·HCl (pH 1.5) | Shaking at room temperature for 16 h |
| F3 | Oxidizable | 8 mL of 8.8 M H2O2 (pH 2) | Shaking at room temperature for 1 h and digested at 85 °C for 1 h |
| Add 8 mL of 8.8 M H2O2 (pH 2) again | Shaking at 85 °C for 1 h | ||
| Cool, add 40 mL of 1 M CH3COONH4 (pH 2) | Shaking at room temperature for 16 h | ||
| F4 | Residual | Air-dried, 6 mL of HNO3, 2 mL of HCl, 2 mL of HF | Microwave digestion |
| Igeo Value | Igeo Class | Pollution Level |
|---|---|---|
| Igeo ≤ 0 | 0 | Uncontaminated |
| 0 < Igeo ≤ 1 | 1 | Uncontaminated to moderately contaminated |
| 1 < Igeo ≤ 2 | 2 | Moderately contaminated |
| 2 < Igeo ≤ 3 | 3 | Moderately to heavily contaminated |
| 3 < Igeo ≤ 4 | 4 | Heavily contaminated |
| 4 < Igeo ≤ 5 | 5 | Heavily to extremely contaminated |
| Igeo ≥ 5 | 6 | Extremely contaminated |
| RAC | Class | Risk Assessment Levels |
|---|---|---|
| <1% | 0 | No risk |
| 1–10% | 1 | Low risk |
| 11–30% | 2 | Medium risk |
| 31–50% | 3 | High risk |
| >50% | 4 | Very high risk |
| Parameter | Meaning | Value |
|---|---|---|
| OISERca | Oral ingestion of soil exposure (carcinogenic) | 3.64573 × 10−7 |
| OISERnc | Oral ingestion of soil exposure (non-carcinogenic) | 1.1083 × 10−6 |
| PISERca | Soil exposure to inhaled soil particulate matter (carcinogenic) | 3.42058 × 10−9 |
| PISERnc | Soil exposure to inhaled soil particulate matter (non-carcinogenic) | 1.03986 × 10−8 |
| Csur | Concentration of HMs in surface soil | Present study |
| SFo | Oral ingestion of carcinogenic slope factors | - |
| SFi | Respiratory inhalation carcinogenic slope factor | Cr 51.14483 |
| Cd 7.671724 | ||
| RFDo | Oral reference doses | Cr 1.503 |
| Mn 0.14 | ||
| Cu 0.04 | ||
| Zn 0.3 | ||
| Cd 0.001 | ||
| Pb 0.00185 | ||
| RFDi | Respiratory inhalation reference dose | Cr 2.3463 × 10−5 |
| Cd 2.3463 × 10−6 | ||
| SAF | Soil allocation factor | 0.5 |
| HMs | Cr | Mn | Cu | Zn | Cd | Pb | Reference |
|---|---|---|---|---|---|---|---|
| Min. | 21.01 | 171.98 | 1.52 | 57.78 | 0.11 | 48.75 | This study |
| Max. | 139.88 | 994.17 | 50.97 | 495.63 | 10.67 | 16,184.7 | Id. |
| Mean | 84.65 | 598.21 | 15.16 | 162.29 | 1.8 | 1921.77 | Id. |
| S.D. | 18.3 | 147.17 | 10.41 | 96.22 | 2.39 | 3213.39 | Id. |
| C.V. (%) | 22 | 25 | 69 | 59 | 133 | 167 | Id. |
| Hunan Lead and Zinc Mining | 144.3 | - | 112 | 336 | 1.57 | 92.1 | Data from Ref. [31] |
| Beijing Gold Mine | 215.25 | - | 65.39 | 133.9 | 0.86 | 134.25 | Data from Ref. [32] |
| Typical Calcium Carbide Slag Dumps in Qiqihar | 26.98 | - | 11.72 | 32.57 | 0.03 | 26.41 | Data from Ref. [33] |
| Tehran landfill | 82.23 | 994.66 | 76.73 | 113.04 | 0.36 | 41.16 | Data from Ref. [18] |
| Hot-Mix Asphalt Plants in Nigeria | 83.37 | 11.92 | 32.43 | 90.33 | 4.7 | 39.27 | Data from Ref. [34] |
| Soil Background Values in Henan | 63.2 | 567 | 20 | 62.5 | 0.065 | 22.3 | Data from Ref. [35] |
| Intervention Value | 30 | - | 8000 | - | 47 | 800 | Data from Ref. [36] |
| Element | HQois | HQpis | HI |
|---|---|---|---|
| Cr | 0.004615 | 2.773761 | 2.778376 |
| Mn | 0.350444 | - | 0.350444 |
| Cu | 0.031078 | - | 0.031078 |
| Zn | 0.044366 | - | 0.044366 |
| Cd | 0.147347 | 0.589216 | 0.736563 |
| Pb | 85.19633 | - | 85.19633 |
| Element | CRois | CRpis | CRn |
|---|---|---|---|
| Cr | - | 5.47 × 10−4 | 5.47 × 10−4 |
| Cd | - | 1.74 × 10−6 | 1.74 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, F.; Zhang, J.; Li, Z.; Liu, S. Ecological and Health Risk Assessment of Heavy Metals in Soils from Recycled Lead Smelting Sites. Water 2022, 14, 1445. https://doi.org/10.3390/w14091445
Yu F, Zhang J, Li Z, Liu S. Ecological and Health Risk Assessment of Heavy Metals in Soils from Recycled Lead Smelting Sites. Water. 2022; 14(9):1445. https://doi.org/10.3390/w14091445
Chicago/Turabian StyleYu, Furong, Jianuo Zhang, Zhiping Li, and Songtao Liu. 2022. "Ecological and Health Risk Assessment of Heavy Metals in Soils from Recycled Lead Smelting Sites" Water 14, no. 9: 1445. https://doi.org/10.3390/w14091445
APA StyleYu, F., Zhang, J., Li, Z., & Liu, S. (2022). Ecological and Health Risk Assessment of Heavy Metals in Soils from Recycled Lead Smelting Sites. Water, 14(9), 1445. https://doi.org/10.3390/w14091445






