Copper Bioremediation Ability of Ciliate Paramecium multimicronucleatum Isolated from Industrial Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling of Wastewater
2.2. Purification and Isolation of Paramecium sp.
2.3. Selection of Copper-Resistant Paramecium sp.
2.4. Identification of Copper-Resistant Paramecium sp. Using Eukaryotic 18S rRNA Gene Primers
2.5. Growth Assay under Copper Stress
2.6. Optimization of Temperature and pH for Paramecium Growth
2.7. Estimation of Copper Accumulation and Uptake Ability
2.8. Assessment of Glutathione Levels as Antioxidant
2.9. Estimation of Other Non-Protein Thiols from Copper-Resistant Paramecium Culture
2.10. Estimation of Total Protein Contents from Copper-Resistant Paramecium Culture
2.11. Statistical Analysis
3. Results
3.1. Copper-Resistant Paramecium Species
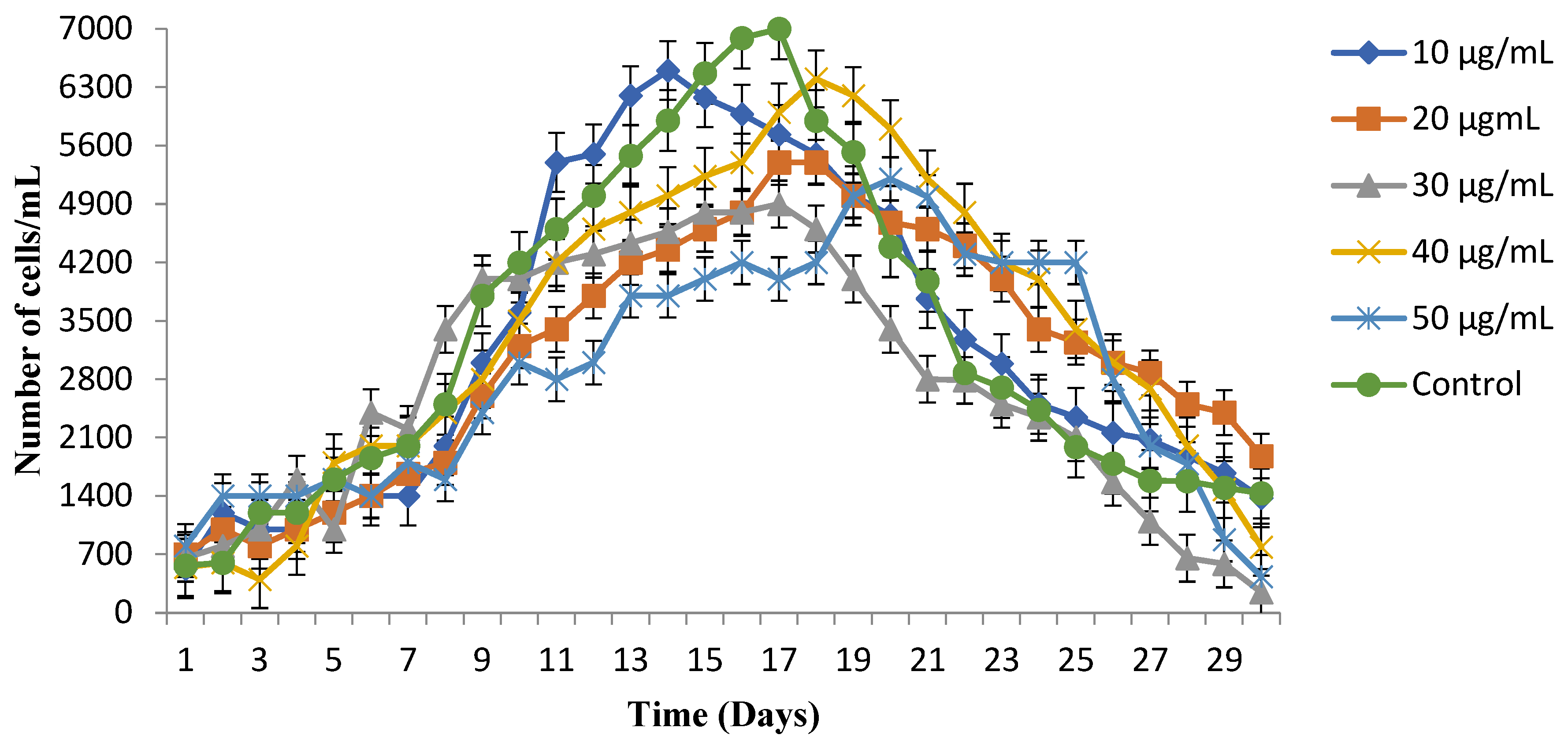
3.2. Growth Observations under Copper Stress
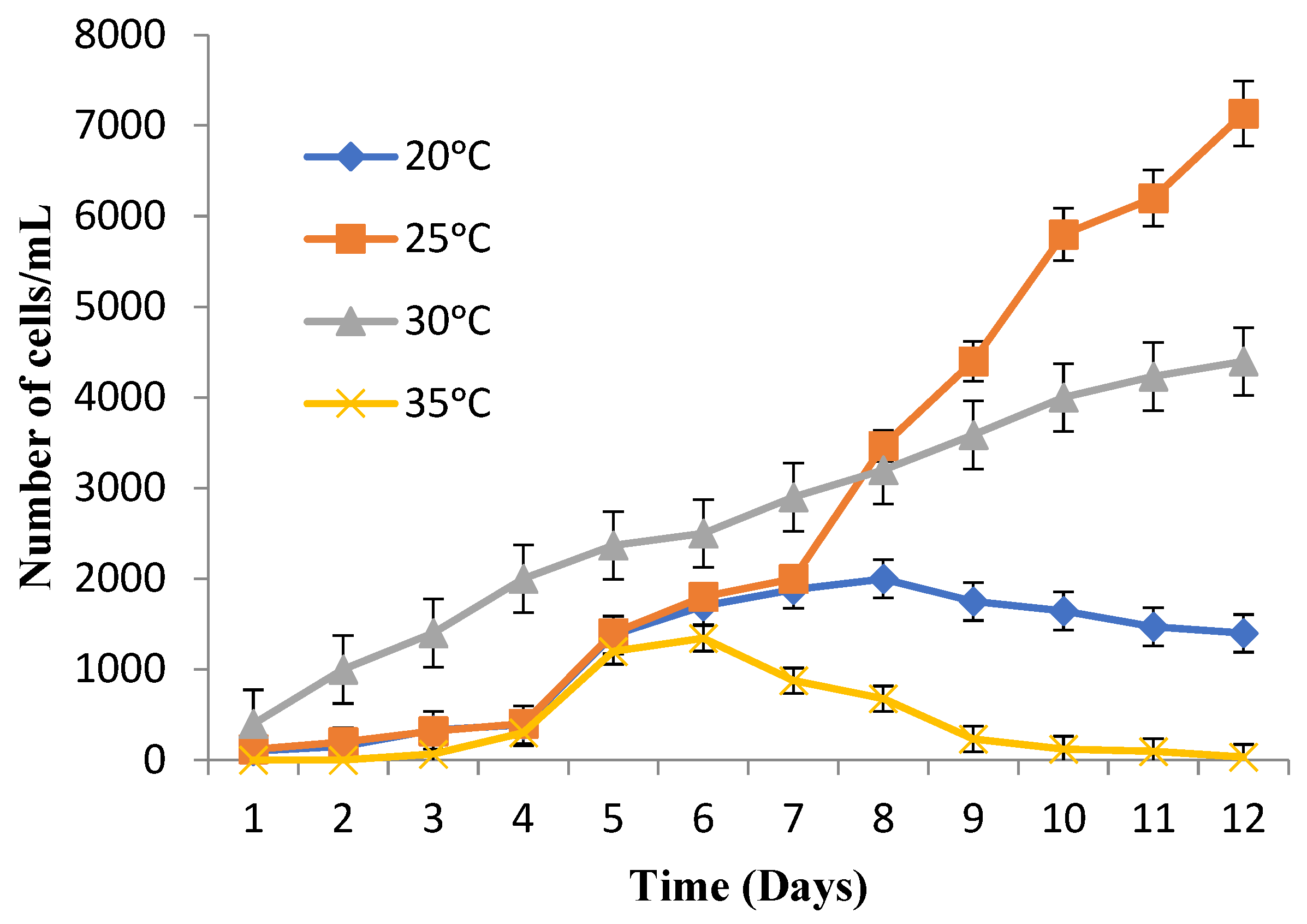
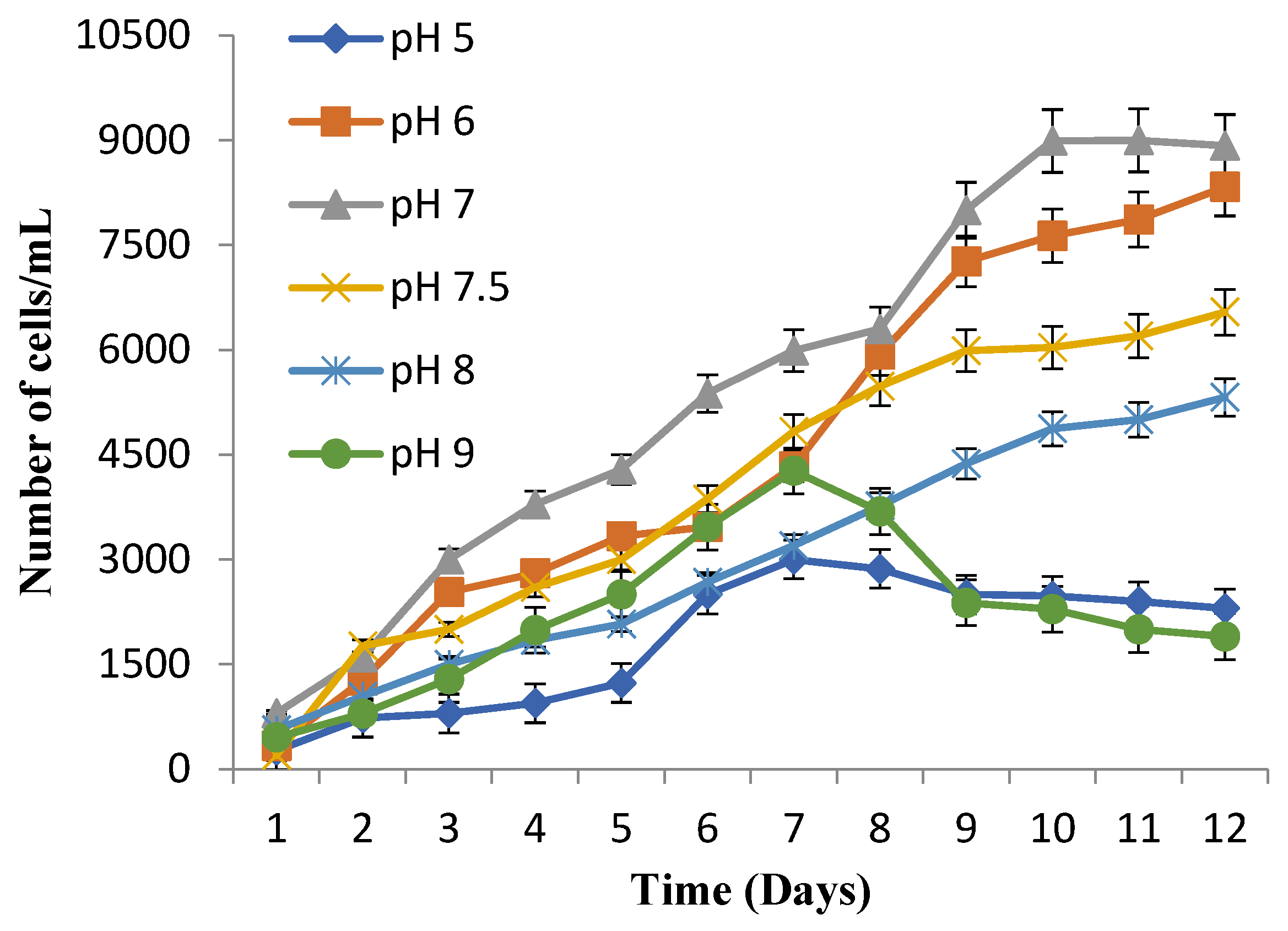
3.3. Suitable Temperature and pH for Maximum Cell Growth
3.4. Copper Accumulation and Uptake Ability of Paramecium Cells
3.5. GSH Response and Non-Protein Thiols
3.6. Protein Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Rehman, A.; Shakoori, F.R.; Shakoori, A.R. Heavy metal resistant Distigma proteus (Euglenophyta) isolated from industrial effluents and its possible role in bioremediation of contaminated wastewaters. World J. Microbiol. Biotechnol. 2007, 23, 753–758. [Google Scholar] [CrossRef]
- Rehman, A.; Shakoori, F.R.; Shakoori, A.R. Heavy metal resistant freshwater ciliate, Euplotes mutabilis, isolated from industrial effluents has potential to decontaminate wastewater of toxic metals. Bioresour. Technol. 2008, 99, 3890–3895. [Google Scholar] [CrossRef] [PubMed]
- Boldrin, F.; Santovito, G.; Formigari, A.; Bisharyan, Y.; Cassidy-Hanley, D.; Clark, T.G.; Piccinni, E. MTT2, a copper-inducible metallothionein gene from Tetrahymena thermophile. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 147, 232–240. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, S.P.; Parakh, S.K.; Tong, Y.W. Health hazards of hexavalent chromium (Cr (VI)) and its microbial reduction. Bioengineered 2022, 13, 4923–4938. [Google Scholar] [CrossRef]
- Rehman, A.; Shakoori, A.R. Heavy metal resistant Chlorella spp., isolated from tannery effluents, and their role in remediation of hexavalent chromium in industrial wastewater. Bull. Environ. Contam. Toxicol. 2001, 66, 542–547. [Google Scholar] [CrossRef]
- Pavasant, P.; Apiratikul, R.; Sungkhum, V.; Suthiparinyanont, P.; Wattanachira, S.; Marhaba, T.F. Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Bioresour. Technol. 2006, 97, 2321–2329. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Balasubramanian, R.; Iyer, C.S.P. Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu (II) from aqueous solutions. Bioresour. Technol. 2007, 98, 452–455. [Google Scholar] [CrossRef]
- Selonen, S.; Setala, H. Nutrient leaching, soil pH and changes in microbial community increase with time in lead-contaminated boreal forest soil at a shooting range area. Environ. Sci. Pollut. Res. Int. 2017, 24, 5415–5425. [Google Scholar] [CrossRef]
- Madoni, P. Protozoa in wastewater treatment processes: A minireview. Ital. J. Zool. 2011, 78, 3–11. [Google Scholar] [CrossRef]
- Zahid, M.T.; Shakoori, F.R.; Zulfiqar, S.; Al-Ghanim, K.A.; Shakoori, A.R. Growth characteristics, metal uptake and expression analysis of copper metallothionein in a newly reported ciliate, Tetrahymena farahensis 2018. Pak. J. Zool. 2018, 50, 1171–1181. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pizzorno, J. Glutathione! Integr. Med. 2014, 13, 8–12. [Google Scholar]
- Kamika, I.; Momba, M.N.B. Effect of nickel on nutrient removal by selected indigenous protozoan species in wastewater systems. Saudi J. Biol. Sci. 2015, 22, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Rehman, A.; Shakoori, F.R.; Shakoori, A.R. Multiple metal resistance and uptake by a ciliate, Stylonychia mytilus, isolated from industrial effluents and its possible use in wastewater treatment. Bull. Environ. Contam. Toxicol. 2007, 79, 410–414. [Google Scholar] [CrossRef]
- Shakoori, A.R.; Rehman, A.; Haq, R.U. Multiple metal resistance in the ciliate protozoan, Vorticella microstoma, isolated from industrial effluents and its potential in bioremediation of toxic wastes. Bull. Environ. Contam. Toxicol. 2004, 72, 1046–1051. [Google Scholar] [CrossRef]
- Regensbogenova, M.; Pristas, P.; Javorsky, P.; Moon-van der Stay, S.Y.; Van der Stay, G.W.M.; Hackstein, J.H.P.; Newbold, C.J.; McEwan, N.R. Assessment of ciliates in the sheep rumen by DGGE. Lett. Appl. Microbiol. 2004, 39, 144–147. [Google Scholar] [CrossRef]
- Moumeni, O.; Berrebbah, H.; Azzouz, Z.; Amamra, R.; Otmani, H.; Alayat, A.; Benosmane, S.; Djebar, M.R. Effects of cycloxydim on population growth, phagocytosis, contractile vacuole activity and antioxidant responses in the freshwater ciliates (Paramecium tetraurelia). Res. J. Environ. Toxicol. 2016, 10, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Israr, M.; Sahi, S.V.J.; Jain, J. Cadmium accumulation and antioxidant responses in the Sesbania drummondii callus. Arch. Environ. Contam. Toxicol. 2006, 50, 121. [Google Scholar] [CrossRef]
- Boldrin, F.; Santovito, G.; Irato, P.; Piccinni, E. Metal interaction and regulation of Tetrahymena pigmentosa metallothionein genes. Protist 2002, 153, 283–291. [Google Scholar] [CrossRef]
- Vieira, R.H.S.F.; Volesky, B. Biosorption: A solution to pollution? Int. Microbiol. 2000, 3, 17–24. [Google Scholar]
- Rehman, A.; Ashraf, S.; Qazi, J.I.; Shakoori, A.R. Uptake of lead by a ciliate, Stylonychia mytilus, isolated from industrial effluents: Potential use in bioremediation of wastewater. Bull. Environ. Contam. Toxicol. 2005, 75, 290–296. [Google Scholar] [CrossRef]
- Gadd, G.M. Heavy metal accumulation by bacteria and other microorganisms. Experientia 1990, 13, 273–280. [Google Scholar] [CrossRef]
- Francisco, R.; Alpoim, M.C.; Morais, P.V. Diversity of chromium resistant and reducing bacteria in a chromium-contaminated activated sludge. J. Appl. Microbiol. 2002, 92, 837–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, J.C.; Martín-González, A.; Díaz, S.; Ortega, R. Ciliates as a potential source of cellular and molecular biomarkers/biosensors for heavy metal pollution. Eur. J. Protistol. 2003, 39, 461–467. [Google Scholar] [CrossRef]
- Haq, R.U.; Rehman, A.; Shakoori, A.R. Effect of dichromate on population and growth of various protozoa isolated from industrial effluents. Folia Microbiol. 2000, 45, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.C.; Martin-Gonzalez, A.; Diaz, S.; Amaro, F.; Ortega, R.; Gallego, A.; De Lucas, M.P. Ciliates as cellular tools to study the eukaryotic cell–heavy metal interactions. In Heavy Metal Pollution; Brown, S.E., Welton, W.C., Eds.; Nova Science: New York, NY, USA, 2008; pp. 1–44. [Google Scholar]
- Rehman, A.; Shakoori, F.R.; Shakoori, A.R. Multiple heavy metal tolerant ciliates, Oxytricha fallax and Paramecium caudatum, isolated fom industrial effluents and their potential use in wastewater treatment. Pak. J. Zool. 2010, 42, 301–309. [Google Scholar]
- Chaudhry, R.; Chaudhry, M.T.; Al-Ghanim, K.; Meboob, S.; Shakoori, A.R. Structure modeling of small subunit ribosomal RNA (SSU rRNA) of copper resistant ciliates. Pak. J. Zool. 2014, 46, 1625–1632. [Google Scholar]
- Shakoori, F.R.; Zafar, M.F.; Fatehullah, A.; Rehman, A.; Shakoori, A.R. Response of glutathione level in a protozoan ciliate, Stylonychia mytilus, to increasing uptake of and tolerance to nickel and zinc in the medium. Pak. J. Zool. 2011, 43, 569–574. [Google Scholar]
- Madoni, P.; Romeo, M.G. Acute toxicity of heavy metals towards freshwater ciliated protists. Environ. Pollut. 2006, 141, 1–7. [Google Scholar] [CrossRef]
- Martin-Gonzalez, A.; Dias, S.; Borniquel, S.; Gallego, A.; Gutierrez, J.C. Cytotoxicity and bioaccumulation of heavy metals by ciliated protozoa isolated from urban wastewater treatment plants. Res. Microbiol. 2006, 157, 108–118. [Google Scholar] [CrossRef]
- Chaudhry, R.; Shakoori, A.R. Identification of a new copper resistant protist isolate Tetrahymena RT-1 subsp. nov. Pak. J. Zool. 2011, 43, 781–788. [Google Scholar]
- Cuypers, A.; Smeets, K.; Ruytinx, J.; Opdenakker, K.; Keunen, E.; Remans, T.; Horemans, N.; Vanhoudt, N.; Van Sanden, S.; Van Belleghem, F.; et al. The cellular redox state as a modulator in cadmium and copper responses in Arabidopsis thaliana seedlings. J. Plant Physiol. 2011, 168, 309–331. [Google Scholar] [CrossRef]


| 24-h | 48-h | |||
|---|---|---|---|---|
| P. multimicronucleatum (Control) | P. multimicronucleatum (Cu Exposed) | P. multimicronucleatum (Control) | P. multimicronucleatum (Cu Exposed) | |
| GSH (mM/g) | 21.99 ± 0.6 | 22.86 ± 0.4 | 27.14 ± 0.9 | 37.54 ± 0.8 ** |
| GSSG (mM/g) | 9.43 ± 1.0 | 19.83 ± 0.7 * | 13.35 ± 0.9 | 36.75 ± 0.3 ** |
| GSH + GSSG (mM/g) | 31.43 ± 0.5 | 42.70 ± 0.3 * | 40.50 ± 0.2 | 74.30 ± 0.6 ** |
| GSH/GSSG ratio | 2.43 ± 0.3 | 1.16 ± 0.06 | 2.08 ± 0.2 | 1.02 ± 0.03 |
| Non-protein thiols | 16.75 ± 0.1 | 21.75 ± 0.5 * | 15.59 ± 0.1 | 22.20 ± 0.2 ** |
| Protein Content (µg/mL) | |
|---|---|
| Control | 35.43 ± 0.4 |
| Cu Exposed | 77.04 ± 6.0 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liaqat, A.; Zahra, I.; Abbas, S.Z.; Wabaidur, S.M.; Eldesoky, G.E.; Islam, M.A.; Rafatullah, M.; Shakoori, F.R.; Shakoori, A.R. Copper Bioremediation Ability of Ciliate Paramecium multimicronucleatum Isolated from Industrial Wastewater. Water 2022, 14, 1419. https://doi.org/10.3390/w14091419
Liaqat A, Zahra I, Abbas SZ, Wabaidur SM, Eldesoky GE, Islam MA, Rafatullah M, Shakoori FR, Shakoori AR. Copper Bioremediation Ability of Ciliate Paramecium multimicronucleatum Isolated from Industrial Wastewater. Water. 2022; 14(9):1419. https://doi.org/10.3390/w14091419
Chicago/Turabian StyleLiaqat, Ayesha, Itrat Zahra, Syed Zaghum Abbas, Saikh Mohammad Wabaidur, Gaber E. Eldesoky, Md Ataul Islam, Mohd Rafatullah, Farah R. Shakoori, and Abdul R. Shakoori. 2022. "Copper Bioremediation Ability of Ciliate Paramecium multimicronucleatum Isolated from Industrial Wastewater" Water 14, no. 9: 1419. https://doi.org/10.3390/w14091419
APA StyleLiaqat, A., Zahra, I., Abbas, S. Z., Wabaidur, S. M., Eldesoky, G. E., Islam, M. A., Rafatullah, M., Shakoori, F. R., & Shakoori, A. R. (2022). Copper Bioremediation Ability of Ciliate Paramecium multimicronucleatum Isolated from Industrial Wastewater. Water, 14(9), 1419. https://doi.org/10.3390/w14091419









