Nitrogen Modulates the Effects of Short-Term Heat, Drought and Combined Stresses after Anthesis on Photosynthesis, Nitrogen Metabolism, Yield, and Water and Nitrogen Use Efficiency of Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
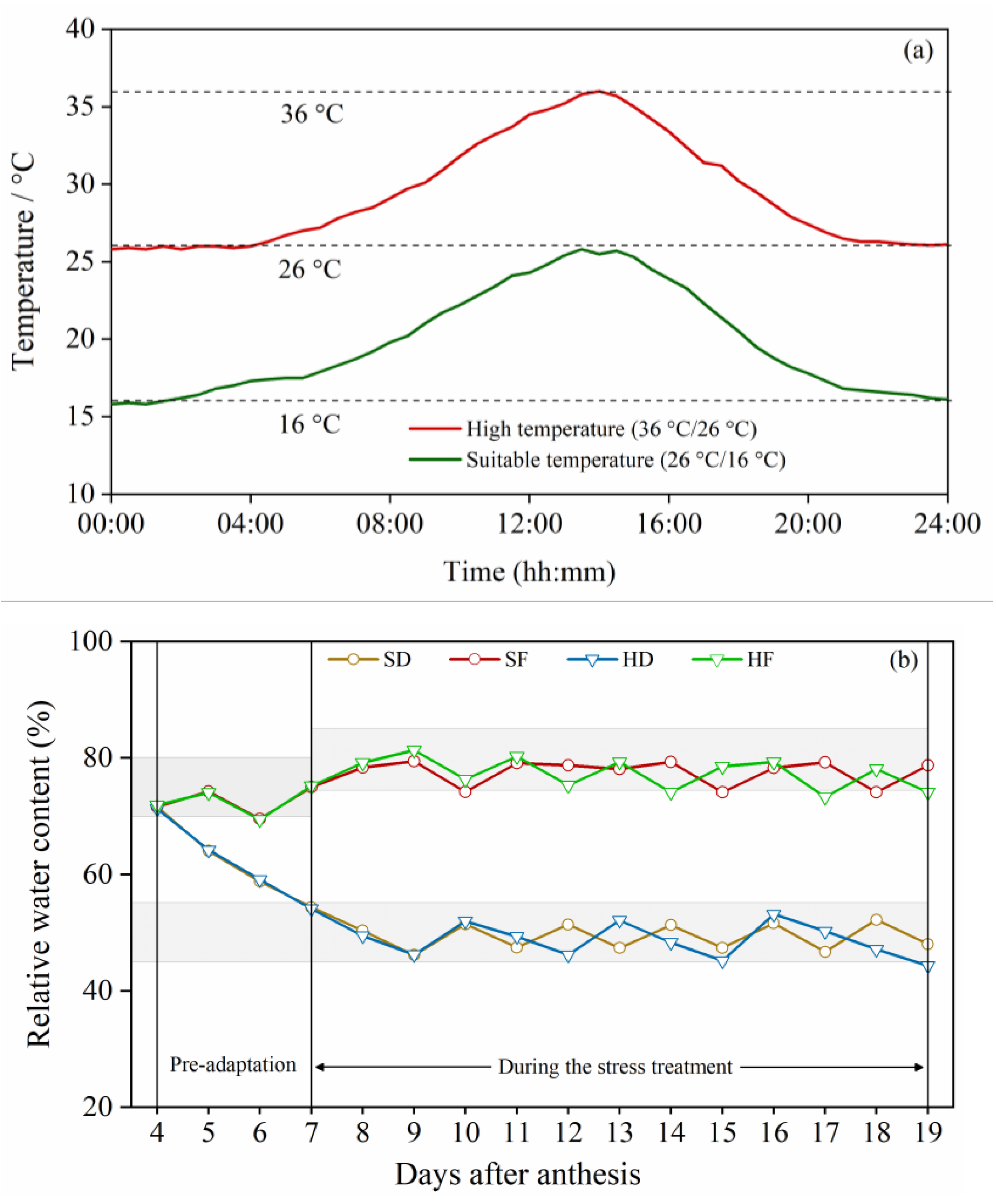
2.2. Experimental Design
2.3. Determination of Physiological Indexes
Leaf Gas Exchange and Chlorophyll Fluorescence
2.4. Determination of Biochemical Indexes
2.4.1. Activities of Nitrate Reductase and Glutamine Synthetase
2.4.2. Plant N and Grain Protein
2.5. Determination of Growth Traits
2.6. Determination of Yield, Water and N Use Efficiency
2.7. Data Analysis
3. Results
3.1. Effect of Nitrogen on Physiological Characteristics under Post-Anthesis Heat, Drought and Combined Stress
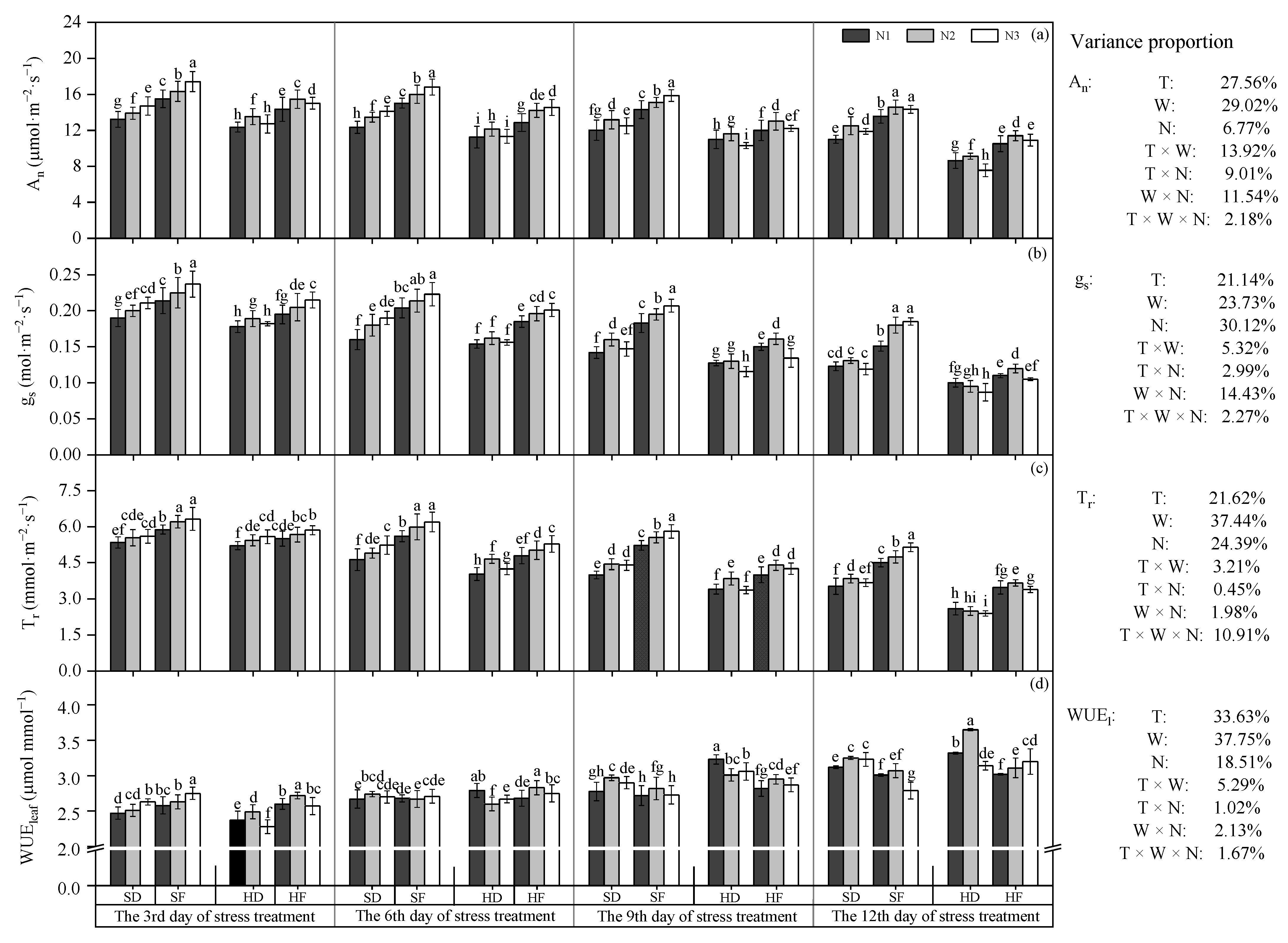
3.1.1. Leaf Photosynthesis Parameters
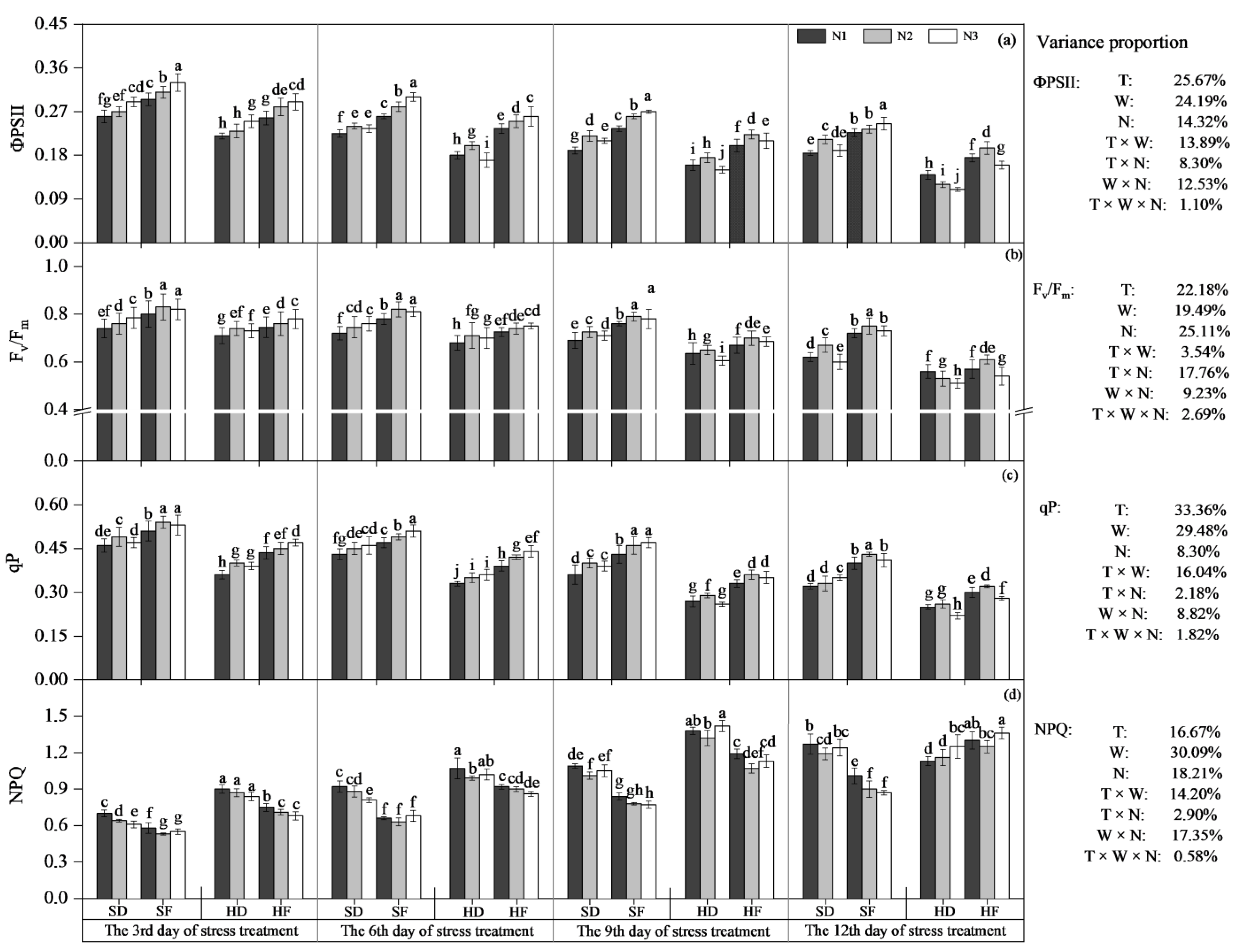
3.1.2. Chlorophyll Fluorescence Parameters
3.2. Effect of Nitrogen on Biochemical Characteristics under Post-Anthesis Heat, Drought and Combined Stress
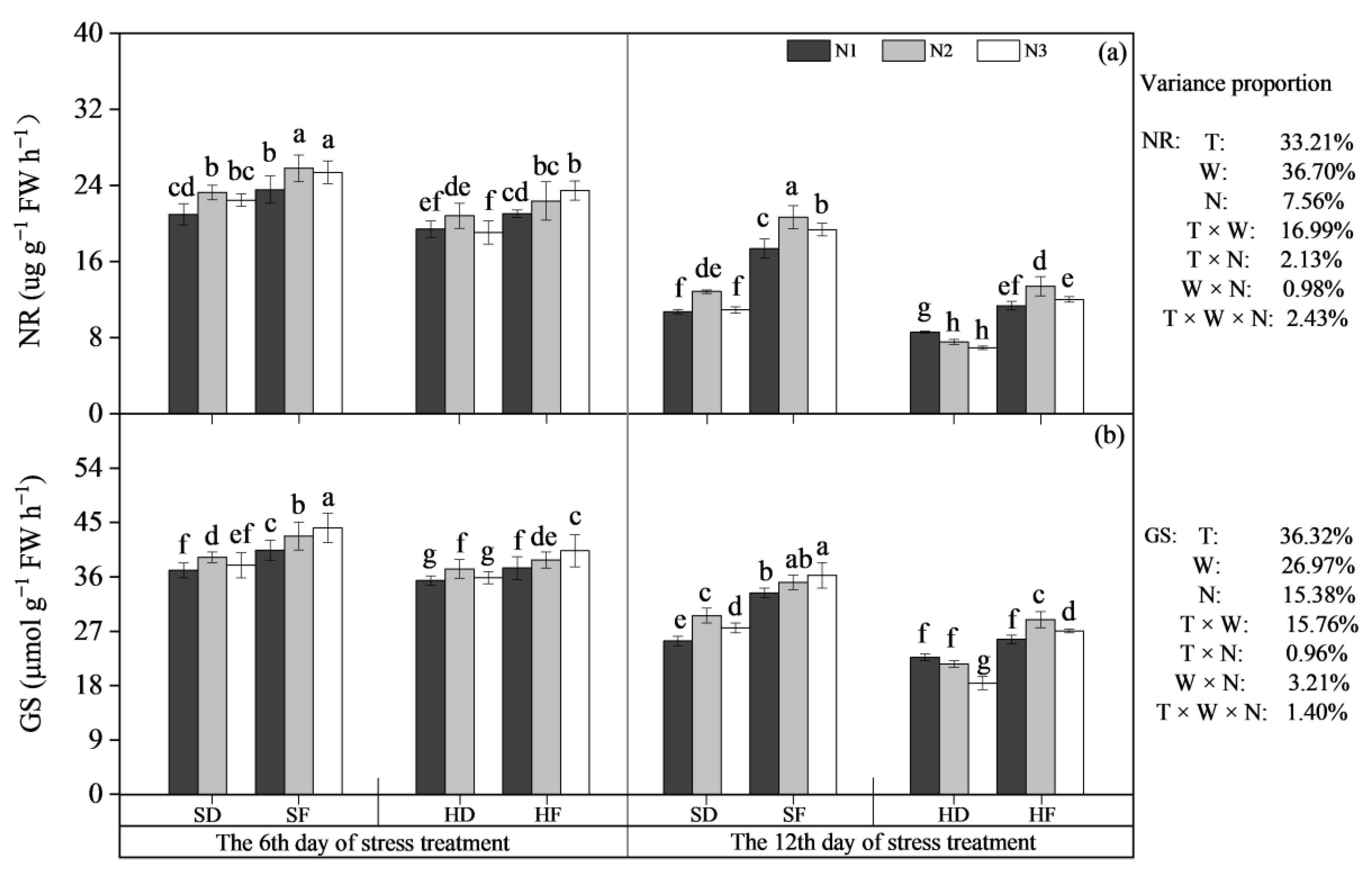
3.2.1. The Activity of N Metabolism-Related Enzymes
3.2.2. N Accumulation, Protein Content and Protein Yield
3.3. Effect of Nitrogen on Wheat Growth under Post-Anthesis Heat, Drought and Combined Stress
3.4. Effect of Nitrogen on Yield, Water and N Use Efficiency under Post-Anthesis Heat, Drought and Combined Stress
3.5. Principal Component Analysis
3.6. The Relationship between Photosynthetic Rate and Biological Factors
4. Discussion
4.1. Appropriate N Application Can Improve Photosynthesis under Short-Term Heat, Drought and Their Combination Stress after Anthesis
4.2. Comparison of Photosynthesis, Chlorophyll Fluorescence Parameters and Yield among Different N Applications under Short-Term Heat, Drought and Their Combination Stress after Anthesis
4.3. Relationships between Photosynthesis and Biological Factors of Leaves
4.4. Appropriate N Application Can Improve Grain Yield by N Metabolism Enzyme Activities, N Accumulation and Growth under Short-Term Heat, Drought and Their Combined Stress after Anthesis
4.5. Appropriate N Supply Can Improve the WUE and NUE of Wheat under Short-Term Heat, Drought and Their Combined Stress after Anthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- IPCC. The physical science basis. Working group I technical support unit. Climate change. In Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stpcker, T.F., Qin, D., Plattner, G.P., Tignor, M.M.B., Allen, A.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Trnka, M.; Olesen, J.E.; Kersebaum, K.C.; Skjelvag, A.O.; Eitzinger, J.; Seguin, B.; Peltonen-Sainio, P.; Rötter, R.; Iglesias, A.; Orlandini, S.; et al. Agroclimatic conditions in Europe under climate change. Glob. Chang. Biol. 2011, 17, 2298–2318. [Google Scholar] [CrossRef] [Green Version]
- Redden, R. New Approaches for Crop Genetic Adaptation to the Abiotic Stresses Predicted with Climate Change. Agronomy 2013, 3, 419–432. [Google Scholar] [CrossRef]
- Reyer, C.P.O.; Leuzinger, S.; Rammig, A.; Wolf, A.; Bartholomeus, R.P.; Bonfante, A.; Lorenzi, F.D.; Dury, M.; Gloning, P.; Jaoudé, R.A.; et al. Plant’s perspective of extremes: Terrestrial plant responses to changing climatic variability. Glob. Chang. Biol. 2013, 19, 75–89. [Google Scholar] [CrossRef]
- Lott, N.; Ross, T.; Smith, A.; Houston, T.; Shein, K. Billion Dollar US Weather Disasters, 1980–2010; National Climatic Data Center: Asheville, NC, USA, 2011. Available online: http://www.ncdc.noaa.gov/oa/reports/billionz.html (accessed on 24 October 2021).
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Miao, L.J.; Jiang, Z.H.; Wang, G.J.; Gnyawali, K.R.; Zhang, J.; Zhang, H.; Fang, K.; He, Y.; Li, C. Projected drought conditions in Northwest China with CMIP6 models under combined SSPs and RCPs for 2015–2099. Adv. Clim. Chang. Res. 2020, 11, 210–217. [Google Scholar] [CrossRef]
- Zheng, Z.; Hoogenboom, G.; Cai, H.J.; Wang, Z.K. Winter wheat production on the Guanzhong Plain of Northwest China under projected future climate with SimCLIM. Agr. Water Manag. 2020, 239, 106233. [Google Scholar] [CrossRef]
- Ju, Y.L.; Min, Z.; Zhang, Y.; Zhang, K.K.; Liu, M.; Fang, Y.L. Transcriptome profiling provide new insights into the molecular mechanism of grapevine response to heat, drought, and combined stress. Sci. Hortic. 2021, 286, 110076. [Google Scholar] [CrossRef]
- Szymańska, R.; Slesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophys. 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Abdelhakim, L.O.A.; Palma, C.F.F.; Zhou, R.; Wollenweber, B.; Ottosen, C.O.; Rosenqvist, E. The effect of individual and combined drought and heat stress under elevated CO2 on physiological responses in spring wheat genotypes. Plant Physiol. Biochem. 2021, 162, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Urban, O.; Hlaváová, M.; Klem, K.; Novotna, K.; Rapantova, B.; Smutna, P.; Horakova, V.; Hlavinka, P.; Skarpa, P.; Trnka, M. Combined effects of drought and high temperature on photosynthetic characteristics in four winter wheat genotypes. Field Crop Res. 2018, 223, 137–149. [Google Scholar] [CrossRef]
- Alvar-Beltranaz, J.; Dao, A.; Dalla Marta, A.; Saturnin, C.; Casini, P.; Orlandini, S. Effect of drought, nitrogen fertilization, temperature, and photoperiodicity on quinoa plant growth and development in the Sahel. Agronomy 2019, 9, 607. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.; Staggenborg, S.; Ristic, Z. Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. In response of crops to limited water: Understanding and modeling water stress effects on plant growth processes. In Advances in Agricultural Systems Modeling Series 1; Ahuja, L.H., Saseendran, S.A., Eds.; ASA-CSSA: Madison, WI, USA, 2008; pp. 301–355. [Google Scholar]
- Xiong, X.; Chang, L.Y.; Muhammad, K.; Zhang, J.J.; Huang, D.F. Alleviation of Drought Stress by Nitrogen Application in Brassica campestris ssp. Chinensis L. Agronomy 2018, 8, 66. [Google Scholar] [CrossRef] [Green Version]
- Tao, Z.Q.; Wang, D.M.; Chang, X.H.; Wang, Y.J.; Yang, Y.S.; Zhao, G.C. Effects of zinc fertilizer and short-term high temperature stress on wheat grain production and wheat flour proteins. J. Integr. Agr. 2018, 17, 1979–1990. [Google Scholar] [CrossRef] [Green Version]
- Zahra, N.; Wahid, A.; Hafeez, M.B.; Ullah, A.; Siddique, K.H.M.; Farooq, M. Grain Development in Wheat under Combined Heat and Drought Stress: Plant Responses and Management. Environ. Exp. Bot. 2021, 188, 104517. [Google Scholar] [CrossRef]
- Mu, Q.; Cai, H.J.; Sun, S.K.; Wen, S.S.; Xu, J.T.; Dong, M.Q.; Saddique, Q. The physiological response of winter wheat under short-term drought conditions and the sensitivity of different indices to soil water changes. Agric. Water Manag. 2021, 243, 106475. [Google Scholar] [CrossRef]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H.M. Heat stress in wheat during reproductive and grain-filling phases. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Ma, T.; Chen, K.W.; He, P.R.; Dai, Y.; Yin, Y.Q.; Peng, S.H.; Ding, J.H.; Yu, S.E.; Huang, J.S. Sunflower Photosynthetic Characteristics, Nitrogen Uptake, and Nitrogen Use Efficiency under Different Soil Salinity and Nitrogen Applications. Water 2022, 14, 982. [Google Scholar] [CrossRef]
- Zhang, F.F.; Gao, S.; Zhao, Y.Y.; Zhao, X.L.; Liu, X.M.; Xiao, K. Growth traits and nitrogen assimilation-associated physiological parameters of wheat (Triticum aestivum L.) under low and high N conditions. J. Integr. Agric. 2015, 14, 1295–1308. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.D.; Niu, W.Q.; Zhang, Q.; Cui, B.J.; Zhang, Z.H.; Wang, Z.; Sun, J. A synthetic analysis of the effect of water and nitrogen inputs on wheat yield and water- and nitrogen-use efficiencies in China. Field Crop Res. 2021, 265, 108105. [Google Scholar] [CrossRef]
- Riley, W.J.; Ortiz-Monasterio, I.; Matso, P.A. Nitrogen leaching and soil nitrate, nitrite, and ammonium levels under irrigated wheat in Northern Mexico. Nutr. Cycl. Agroecosyst. 2001, 61, 223–236. [Google Scholar] [CrossRef]
- Agami, R.A.; Alamri, S.A.M.; Abd El-Mageed, T.A.; Abousekken, M.S.M.; Hashem, M. Role of exogenous nitrogen supply in alleviating the deficit irrigation stress in wheat plants. Agric. Water Manag. 2018, 210, 216–270. [Google Scholar] [CrossRef]
- Brueck, H.; Erdle, K.; Gao, Y.Z.; Giese, M.; Zhao, Y.; Peth, S.; Lin, S. Effects of N and water supply on water use-efficiency of semiarid grassland in Inner Mongolia. Plant Soil. 2010, 328, 495–505. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Halim, A.; Aziz, T. Alleviation of temperature stress by nutrient management in crop plants: A review. J. Soil Sci. Plant Nutr. 2012, 12, 221–244. [Google Scholar] [CrossRef] [Green Version]
- Elía, M.; Slafer, G.A.; Savin, R. Yield and grain weight responses to post-anthesis increases in maximum temperature under field grown wheat as modified by nitrogen supply. Field Crops Res. 2018, 221, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.; Duan, A.W.; Dassanayake, K.; Chen, D.; Gao, Y.; Wang, X.; Shen, X. Effects of regulated deficit irrigation on grain yield and quality traits in winter wheat. Trans. ASABE 2016, 59, 897–907. [Google Scholar]
- Lu, J.S.; Hu, T.T.; Geng, C.M.; Cui, X.L.; Fan, J.L.; Zhang, F.C. Response of yield, yield components and water-nitrogen use efficiency of winter wheat to different drip fertigation regimes in Northwest China. Agric. Water Manag. 2021, 255, 107034. [Google Scholar] [CrossRef]
- Gaudinová, A. The effect of cytokinins on nitrate reductase activity. Biol. Plantarum 1990, 32, 89–96. [Google Scholar] [CrossRef]
- Zhang, C.F.; Peng, S.B.; Peng, X.X.; Chavez, A.Q.; Bennett, J. Response of glutamine synthetase isoforms to nitrogen sources in rice (Oryza sativa L.) roots. Plant Sci. 1997, 125, 163–170. [Google Scholar] [CrossRef]
- Lyu, X.K.; Liu, Y.; Li, N.; Ku, L.B.; Hou, Y.T.; Wen, X.X. Foliar applications of various nitrogen (N) forms to winter wheat affect grain protein accumulation and quality via N metabolism and remobilization. Crop J. 2021. [Google Scholar] [CrossRef]
- Karimi, M.; Siddique, K. Crop growth and relative growth rates of old and modern wheat cultivars. Aust. J. Agric. Res. 1991, 42, 13–20. [Google Scholar] [CrossRef]
- Loka, D.A.; Oosterhuis, D.M.; Baxevanos, D.; Noulas, C.; Hu, W. Single and combined effects of heat and water stress and recovery on cotton (Gossypium hirsutum L.) leaf physiology and sucrose metabolism. Plant Physiol. Biochem. 2020, 148, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Tian, M.Y.; Cai, J.; Jing, D.; Cao, W.X.; Dai, T.B. Effects of low nitrogen supply on relationships between photosynthesis and nitrogen status at different leaf position in wheat seedlings. Plant Growth Regul. 2013, 70, 257–263. [Google Scholar] [CrossRef]
- Zhong, C.; Cao, X.C.; Hu, J.J.; Zhu, L.F.; Zhang, J.H.; Huang, J.L.; Jin, Q.Y. Nitrogen metabolism in adaptation of photosynthesis to water stress in rice grown under different nitrogen levels. Front. Plant Sci. 2017, 8, 1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrell, A.K.; Mullet, J.E.; Georgee Jaeggli, B.; van Oosterom, E.J.; Hammer, G.L.; Klein, P.E.; Jordan, D.R. Drought adaptation of stayegreen sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake. J. Exp. Bot. 2014, 21, 6251–6263. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.Q.; Li, X.N.; Santos, T.M.D.; Rosenqvist, E.; Ottosen, C.O. Combined high light and heat stress induced complex response in tomato with better leaf cooling after heat priming—ScienceDirect. Plant Physiol. Biochem. 2020, 151, 1–9. [Google Scholar] [CrossRef]
- Zivcak, M.; Olsovska, K.; Slamka, P.; Galambosova, J.; Rataj, V.; Shao, H.S.; Brestic, M. Application of chlorophyll fluorescence performance indices to assess the wheat photosynthetic functions influenced by nitrogen deficiency. Plant Soil Environ. 2014, 60, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.B.; Liu, W.Z.; Chang, S.X.; Anyia, A.O. Water-deficit and high temperature affected water use efficiency and arabinoxylan concentration in spring wheat. J. Cereal Sci. 2010, 52, 263–269. [Google Scholar] [CrossRef]
- Makino, A. Photosynthesis, grain yield, and nitrogen utilization in rice andwheat. Plant Physiol. 2011, 155, 125–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, D.B.; Ma, C.R.; Song, Y.; Wu, Q.; Wu, X.Y.; Sun, Y.X.; Zhao, G.; Wan, Y. Post-Anthesis Photosynthetic Properties Provide Insights into Yield Potential of Tartary Buckwheat Cultivars. Agronomy 2019, 9, 149. [Google Scholar] [CrossRef] [Green Version]
- Gaju, O.; Allard, V.; Martre, P.; Snape, J.W.; Heumez, E.; LeGouis, J.; Moreau, D.; Bogard, M.; Griffiths, S.; Orford, S. Identification of traits to improve thenitrogen-use efficiency of wheat genotypes. Field Crop Res. 2011, 123, 139–152. [Google Scholar] [CrossRef]
- Hikosaka, K. Interspecific difference in the photosynthesis-nitrogen relationship: Patterns, physiological causes, and ecological importance. J. Plant Res. 2004, 117, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Bindraban, P.S. Impact of canopy nitrogen profile in wheat on growth. Field Crop Res. 1999, 63, 63–77. [Google Scholar] [CrossRef]
- Tambussi, E.A.; Nogués, S.; Araus, J.L. Ear of durum wheat under waterstress: Water relations and photosynthetic metabolism. Planta 2005, 221, 446–458. [Google Scholar] [CrossRef]
- Cui, G.C.; Zhang, Y.; Zhang, W.J.; Lang, D.Y.; Zhang, X.J.; Li, Z.X.; Zhang, X.H. Response of Carbon and Nitrogen Metabolism and Secondary Metabolites to Drought Stress and Salt Stress in Plants. J. Plant Biol. 2019, 62, 387–399. [Google Scholar] [CrossRef]
- Yang, H.; Huang, T.Q.; Ding, M.Q.; Lu, D.L.; Lu, W.P. High temperature during grain filling impacts on leaf senescence in waxy maize. Agron. J. 2017, 109, 906–916. [Google Scholar] [CrossRef]
- Gao, X.P.; Lukow, O.M.; Grant, C.A. Grain concentrations of protein, iron and zinc and bread making quality in spring wheat as affected by seeding date and nitrogen fertilizer management. J. Geochem. Explor. 2012, 121, 36–44. [Google Scholar] [CrossRef]
- Zhang, M.W.; Ma, D.Y.; Ma, G.; Wang, C.Y.; Xie, X.D.; Kang, G.Z. Responses of glutamine synthetase activity and gene expression to nitrogen levels in winter wheat cultivars with different grain protein content. J. Cereal Sci. 2017, 74, 187–193. [Google Scholar] [CrossRef]
- Goodall, A.J.; Kumar, P.; Tobin, A.K. Identification and expression analyses of cytosolic glutamine synthetase genes in barley (Hordeum vulgare L.). Plant Cell Physiol. 2013, 54, 492–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolic, O.; Zivanovic, T.; Jelic, M.; Djalovic, I. Interrelationships between grain nitrogen content and other indicators of nitrogen accumulation and utilization efficiency in wheat plants. Chil. J. Agric. Res. 2012, 72, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Lobell, D.B.; Hammer, G.L.; Chenu, K.; Zheng, B.; McLean, G.; Chapman, S.C. The shifting influence of drought and heat stress for crops in northeast Australia. Glob Chang. Biol. 2015, 21, 4115–4127. [Google Scholar] [CrossRef] [PubMed]
- Engels, C.; Marschne, H. Plant uptake and utilization of nitrogen. In Nitrogen Fertilization and the Environment; Bacon, P.E., Ed.; Marcel Dekker: New York, NY, USA, 1995; pp. 41–83. [Google Scholar]
- Guilioni, L.; Wery, J.; Lecoeur, J. High temperature and water deficit may reduce seed number in field pea purely by decreasing plant growth rate. Funct. Plant Biol. 2003, 30, 1151–1164. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.J.; Wang, M.H.; Shah, L.; Lu, S.Q.; Wang, X.B.; Ma, C.X. Unraveling field crops sensitivity to heat stress: Mechanisms, approaches, and future prospects. Agronomy 2018, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Lalelou, F.S.; Fateh, M. Effects of water deficit stress and nitrogen fertilizer on wheat varieties. Int. J. Biosci. 2014, 4, 183–189. [Google Scholar]
- Westgate, M.E.; Passioura, J.B.; Munns, R. Water status and ABA content of floral organs in drought-stressed wheat. Aust. J. Plant Physiol. 1996, 23, 763–772. [Google Scholar] [CrossRef]
- Zhou, C.L.; Zhang, H.J.; Li, F.Q.; Wang, Z.Y.; Wang, Y.C. Photosynthetic characteristics and yield response of isatis indigotica to regulated deficit irrigation in a cold and arid environment. Water 2021, 13, 3510. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Sauer, T.J.; Prueger, J.H. Managing soilsto achieve greater water use efficiency: A review. Agron. J. 2001, 93, 271–280. [Google Scholar] [CrossRef]
- He, M.Z.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Wang, G.S.; Hamani, A.K.M.; Amin, A.S.; Sun, W.H.; Zhang, Y.Y.; Liu, Z.D.; Gao, Y. Optimization of Nitrogen Fertilizer Application with Climate-Smart Agriculture in the North China Plain. Water 2021, 13, 3415. [Google Scholar] [CrossRef]
- Shamme, S.K.; Raghavaiah, C.V.; Balemi, T.; Hamza, I. Sorghum (Sorghum bicolor L.) growth, productivity, nitrogen removal, N-use efficiencies and economics in relation to genotypes and nitrogen nutrition in Kellem-Wollega zone of Ethiopia, east Africa. Adv. Crop Sci. Technol. 2016, 4, 218. [Google Scholar] [CrossRef] [Green Version]

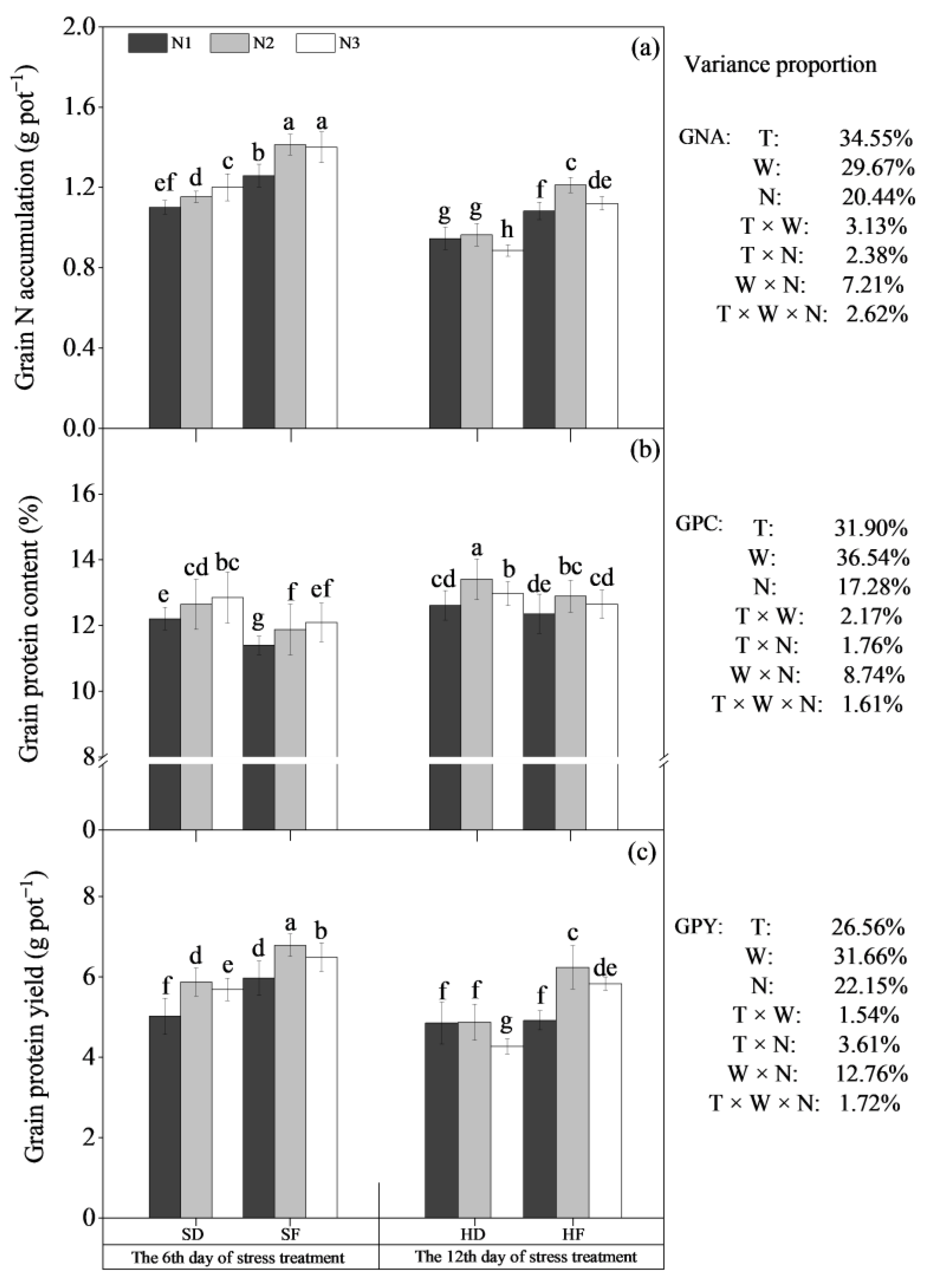
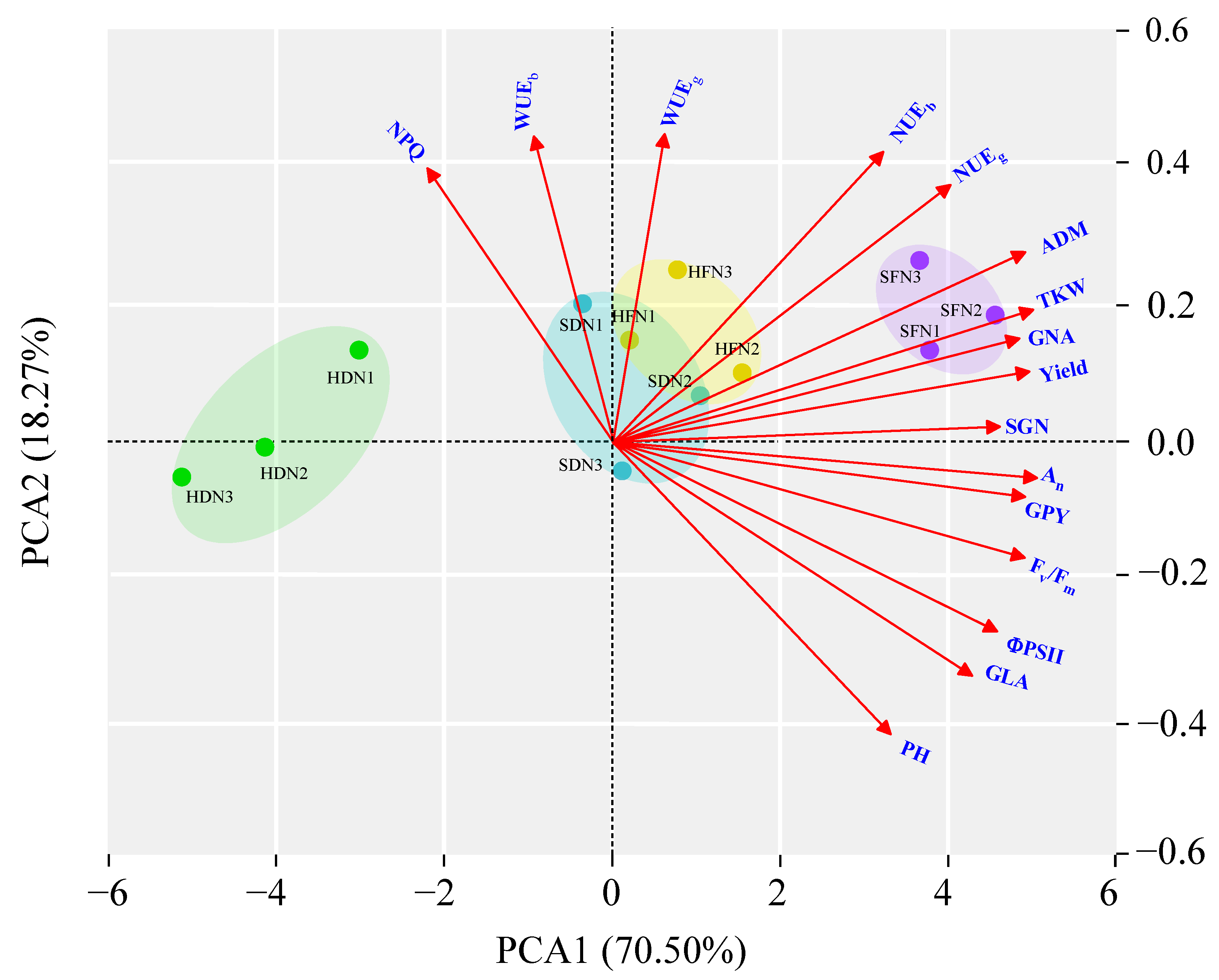
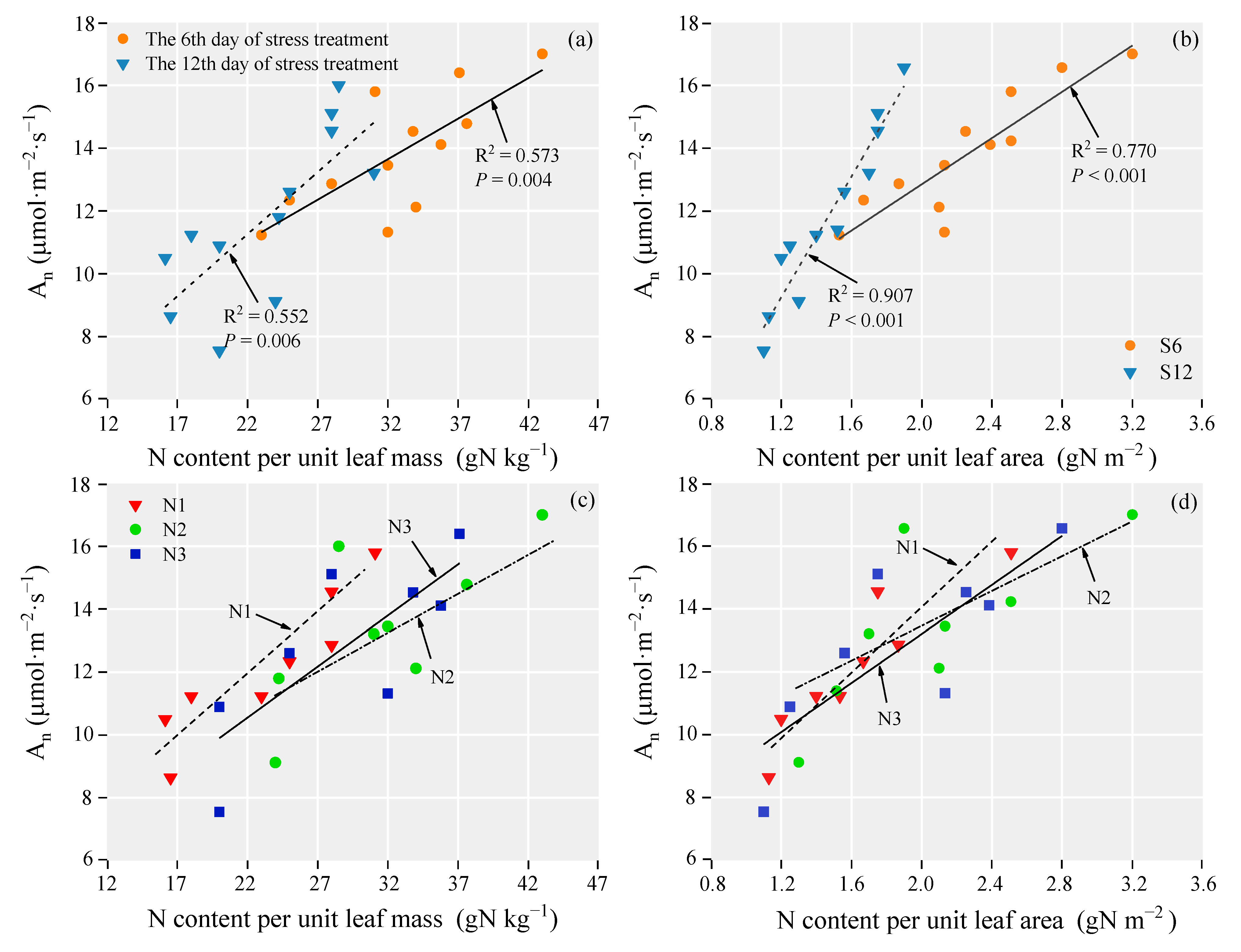
| Treatments | Growth Traits | |||||
|---|---|---|---|---|---|---|
| Temperature/Water/Nitrogen Regimes | Green Leaf Area (cm2/Plant) | Plant Height (cm) | ADM (g/Plant) | RGR (mg·g−1·Day−1) | ||
| S | D | N1 | 56.24 ± 4.21 g | 62.17 ± 1.23 g | 2.90 ± 0.045 g | 21.67 ± 1.21 f |
| N2 | 67.65 ± 4.40 cde | 64.07 ± 0.88 f | 3.10 ± 0.029 e | 23.44 ± 0.87 de | ||
| N3 | 71.02 ± 6.53 c | 64.65 ± 1.23 ef | 2.99 ± 0.077 fg | 22.90 ± 1.45 ef | ||
| F | N1 | 89.55 ± 4.42 a | 66.89 ± 2.10 bc | 3.45 ± 0.045 c | 30.48 ± 0.60 b | |
| N2 | 90.04 ± 6.54 a | 67.67 ± 1.55 b | 3.61 ± 0.120 b | 32.63 ± 2.01 a | ||
| N3 | 82.49 ± 5.89 b | 69.01 ± 0.77 a | 3.72 ± 0.082 a | 33.74 ± 1.32 a | ||
| H | D | N1 | 64.50 ± 4.71 e | 61.80 ± 1.20 g | 2.61 ± 0.039 hi | 15.82 ± 0.41 g |
| N2 | 58.93 ± 2.67 fg | 60.25 ± 1.83 h | 2.69 ± 0.60 h | 15.32 ± 0.29 g | ||
| N3 | 49.44 ± 3.02 h | 58.56 ± 1.35 i | 2.53 ± 0.021 j | 13.08 ± 0.56 h | ||
| F | N1 | 60.37 ± 4.32 f | 63.86 ± 2.43 f | 2.95 ± 0.051 fg | 22.48 ± 1.01 ef | |
| N2 | 68.70 ± 3.23 cd | 65.89 ± 0.67 cd | 3.21 ± 0.101 d | 25.08 ± 0.82 c | ||
| N3 | 65.40 ± 3.45 de | 65.18 ± 1.78 de | 3.02 ± 0.084 ef | 24.65 ± 1.33 cd | ||
| Variance proportion (%) | T | 29.74 | 19.87 | 31.80 | 30.40 | |
| W | 19.10 | 21.30 | 23.44 | 24.73 | ||
| N | 7.52 | 19.55 | 14.32 | 11.76 | ||
| T × W | 21.03 | 16.24 | 12.15 | 10.19 | ||
| T × N | 7.88 | 8.39 | 4.85 | 6.26 | ||
| W × N | 0.75 | 4.48 | 12.38 | 12.65 | ||
| T × W × N | 13.98 | 10.17 | 1.06 | 4.01 | ||
| Treatments | Yield and Its Components | Water and N Efficiency | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature/Water/ Nitrogen Regimes | GN (no.) | TKW (g) | Yield (g pot−1) | WUEg (g kg−1) | WUEb (g kg−1) | NUEg (g g−1 N) | NUEb (g g−1 N) | ||
| S | D | N1 | 31.01 ± 0.45 f | 37.32 ± 0.98 h | 41.10 ± 1.59 f | 2.32 ± 0.05 bc | 4.83 ± 0.12 c | 31.23 ± 0.64 d | 65.48 ± 1.63 ef |
| N2 | 33.28 ± 0.78 d | 40.12 ± 0.77 de | 46.41 ± 2.01 d | 2.46 ± 0.05 a | 5.15 ± 0.21 a | 33.43 ± 0.65 b | 68.35 ± 1.18 b | ||
| N3 | 32.25 ± 0.99 e | 39.12 ± 1.45 f | 44.30 ± 1.87 e | 2.50 ± 0.02 a | 4.96 ± 0.08 b | 30.23 ± 0.81 e | 63.39 ± 1.25 g | ||
| F | N1 | 36.21 ± 0.88 b | 42.32 ± 0.93 d | 52.39 ± 2.44 b | 2.27 ± 0.06 cd | 4.48 ± 0.15 f | 35.73 ± 0.24 a | 70.58 ± 2.56 a | |
| N2 | 38.40 ± 0.56 a | 44.19 ± 1.54 a | 57.19 ± 1.45 a | 2.33 ± 0.05 bc | 4.56 ± 0.17 ef | 36.03 ± 0.55 a | 70.25 ± 1.52 a | ||
| N3 | 36.20 ± 0.65 b | 43.89 ± 1.20 b | 53.63 ± 1.89 b | 2.23 ± 0.03 de | 4.51 ± 0.09 f | 33.58 ± 0.53 b | 67.82 ± 0.89 bc | ||
| H | D | N1 | 29.34 ± 0.87 g | 36.21 ± 1.01 i | 38.50 ± 0.98 g | 2.19 ± 0.06 ef | 4.53 ± 0.13 ef | 32.05 ± 0.78 c | 66.92 ± 1.78 d |
| N2 | 28.05 ± 0.66 h | 35.04 ± 0.78 g | 36.35 ± 2.10 h | 2.14 ± 0.03 f | 4.75 ± 0.09 cd | 29.20 ± 0.55 f | 62.82 ± 2.25 g | ||
| N3 | 26.12 ± 0.77 i | 32.44 ± 1.67 j | 32.89 ± 1.32 i | 1.99 ± 0.08 g | 4.60 ± 0.14 e | 26.21 ± 0.45 g | 60.48 ± 1.46 h | ||
| F | N1 | 32.01 ± 1.05 e | 37.56 ± 1.60 gh | 39.77 ± 2.76 fg | 2.24 ± 0.07 de | 4.71 ± 0.12 d | 31.10 ± 0.77 d | 65.77 ± 2.55 e | |
| N2 | 34.13 ± 0.78 c | 41.01 ± 1.10 ef | 48.40 ± 1.45 c | 2.37 ± 0.04 b | 5.02 ± 0.07 b | 33.26 ± 0.80 b | 67.42 ± 2.09 cd | ||
| N3 | 34.78 ± 0.65 c | 38.34 ± 1.32 c | 46.07 ± 1.01 de | 2.29 ± 0.08 cd | 4.80 ± 0.19 c | 31.77 ± 0.36 cd | 64.77 ± 3.58 f | ||
| Variance proportion (%) | T | 18.19 | 35.66 | 33.33 | 4.51 | 5.91 | 30.76 | 33.18 | |
| W | 16.23 | 33.90 | 29.67 | 18.90 | 33.73 | 28.31 | 17.42 | ||
| N | 19.57 | 7.44 | 10.47 | 19.42 | 9.52 | 13.21 | 15.30 | ||
| T × W | 3.66 | 15.61 | 20.75 | 23.28 | 31.35 | 10.39 | 18.54 | ||
| T × N | 17.23 | 5.28 | 2.46 | 18.18 | 7.44 | 7.73 | 13.01 | ||
| W × N | 15.58 | 1.64 | 1.54 | 14.52 | 9.06 | 8.64 | 0.79 | ||
| T × W × N | 9.54 | 0.47 | 1.78 | 1.19 | 2.99 | 0.96 | 1.76 | ||
| Dependent Variable | Independent Variables | N Application | a | b | R2 | p |
|---|---|---|---|---|---|---|
| An (µmol·m−2·s−1) | N-mass (gN kg−1) | N1 | 0.381 | 3.358 | 0.835 | <0.001 |
| N2 | 0.263 | 5.131 | 0.526 | 0.042 | ||
| N3 | 0.325 | 3.392 | 0.604 | 0.023 | ||
| N-area (gN m−2) | N1 | 5.031 | 3.845 | 0.849 | <0.001 | |
| N2 | 3.017 | 7.206 | 0.575 | 0.029 | ||
| N3 | 3.902 | 5.403 | 0.639 | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ru, C.; Hu, X.; Chen, D.; Song, T.; Wang, W.; Lv, M.; Hansen, N.C. Nitrogen Modulates the Effects of Short-Term Heat, Drought and Combined Stresses after Anthesis on Photosynthesis, Nitrogen Metabolism, Yield, and Water and Nitrogen Use Efficiency of Wheat. Water 2022, 14, 1407. https://doi.org/10.3390/w14091407
Ru C, Hu X, Chen D, Song T, Wang W, Lv M, Hansen NC. Nitrogen Modulates the Effects of Short-Term Heat, Drought and Combined Stresses after Anthesis on Photosynthesis, Nitrogen Metabolism, Yield, and Water and Nitrogen Use Efficiency of Wheat. Water. 2022; 14(9):1407. https://doi.org/10.3390/w14091407
Chicago/Turabian StyleRu, Chen, Xiaotao Hu, Dianyu Chen, Tianyuan Song, Wene Wang, Mengwei Lv, and Neil C. Hansen. 2022. "Nitrogen Modulates the Effects of Short-Term Heat, Drought and Combined Stresses after Anthesis on Photosynthesis, Nitrogen Metabolism, Yield, and Water and Nitrogen Use Efficiency of Wheat" Water 14, no. 9: 1407. https://doi.org/10.3390/w14091407
APA StyleRu, C., Hu, X., Chen, D., Song, T., Wang, W., Lv, M., & Hansen, N. C. (2022). Nitrogen Modulates the Effects of Short-Term Heat, Drought and Combined Stresses after Anthesis on Photosynthesis, Nitrogen Metabolism, Yield, and Water and Nitrogen Use Efficiency of Wheat. Water, 14(9), 1407. https://doi.org/10.3390/w14091407





