Contribution of Spring Snowmelt Water to Soil Water in Northeast China and Its Dynamic Changes
Abstract
:1. Introduction
2. Materials and Methods
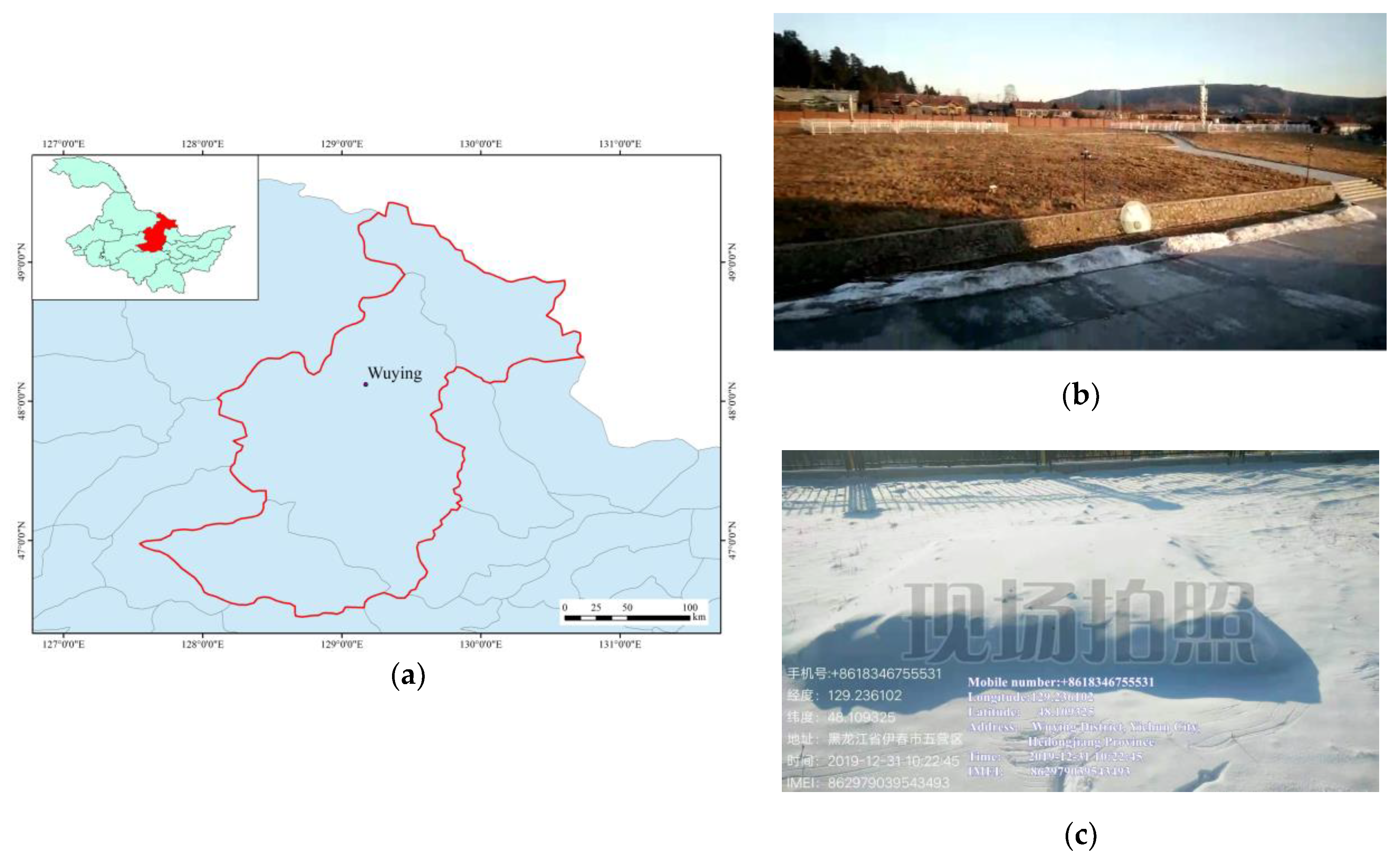
2.1. Experimental Site
2.2. Meteorological Conditions during the Experimental Period
2.3. Experimental Design
2.4. Determination of Hydrogen and Oxygen Isotopes
2.4.1. Isotope Calculation Method and Measurement Standard
2.4.2. Sample Treatment and Analysis
2.5. Binary Mixing Model
Calculation Example
3. Results
3.1. Distribution Characteristics of Isotopes of Snowmelt Water, Soil Water, and Precipitation
3.2. Distribution Characteristics of Isotopes of Soil under Different Snow Depths
3.3. Characteristics of the Contributions of Snow Cover to Soil Water
3.3.1. Distribution Characteristics of Contributions of Different Snow Depths to Soil Water
3.3.2. Change Characteristics of Contributions of Different Snow Depths to Soil Water
3.4. Simulation of the Contributions of Snowmelt Water and Precipitation to Soil Moisture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, Y.R.; Lu, K.X.; Li, P.; Wang, Q.; Zhang, T.G.; Liu, Y. Research on soil water movement based on stable isotopes. Acta Pedol. Sin. 2015, 52, 792–801. [Google Scholar]
- Chen, Y.N.; Li, Z.; Fan, Y.T.; Wang, H.J.; Fang, G.H. Research progress on the impact of climate change on water resources in the arid region of Northwest China. Acta Geogr. Sin. 2014, 69, 1295–1304. [Google Scholar]
- Ma, L.J.; Qing, D.H. Spatial-Temporal Characteristics of Observed Key Parameters for Snow Cover in China during 1957–2009. J. Glaciol. Geocryol. 2012, 34, 1–11. [Google Scholar]
- Jiang, L.; Zhu, F.S. China’s Grain Production Status and Policy Suggestions in Major Grain Producing Areas. Issues Agric. Econ. 2015, 36, 17–24, 110. (In Chinese) [Google Scholar]
- Hu, C.L.; Li, J.; Jiao, M.; Wang, T.; Li, Y.H.; Lin, R.; Wang, Y. Change characteristics of shallow soil humidity and its impact climate factors in Liaoning spring sowing stage. Agric. Res. Arid. Areas 2018, 36, 277–283. [Google Scholar]
- Wang, Q.J.; Lv, J.J.; Li, X.F.; Wang, P.; Ma, G.Z. Distribution Characteristics of Heilongjiang Provincial Agricultural Meteorological Disasters and Effect on Agricultural Production. Heilongjiang Hydraul. Sci. Technol. 2016, 44, 57–61. [Google Scholar]
- Chen, H.; Zhang, L.J.; Li, W.L.; Zhang, J.F.; Gao, Y.H. Study on Risk Assessment and Zoning of Agricultural Arid Disaster in Heilongjiang Province. Chin. Agric. Sci. Bull. 2010, 26, 245–248. [Google Scholar]
- Liang, S. A study on Influence of Snow Cover on Soil Water and Heat in the Farmland in Northeast China. Master Thesis, Jilin University, Jilin, China, 2018. [Google Scholar]
- Sun, S.F. Physics, Biochemistry and Parameterization of Land Surface Model; China Meteorological Press: Beijing, China, 2005. (In Chinese) [Google Scholar]
- Li, Z.X.; Feng, Q.; Wang, Q.J.; Kong, Y.L.; Cheng, A.F.; Song, Y.; Li, Y.G.; Li, J.G.; Guo, X.Y. Contributions of local terrestrial evaporation and transpiration to precipitation using δ18O and D-excess as a proxy in Shiyang inland river basin in China. Glob. Planet. Chang. 2016, 146, 140–151. [Google Scholar]
- Penna, D.; Van Meerveld, H.J.; Zuecco, G.; Dalla Fontana, G.; Borga, M. Hydrological response of an alpine catchment to rainfall and snowmelt events. J. Hydrol. 2016, 537, 382–397. [Google Scholar] [CrossRef]
- Barbieri, M.; Nigro, A.; Petitta, M. Groundwater mixing in the discharge area of San Vittorino Plain (Central Italy): Geochemical characterization and implication for drinking uses. Environ. Earth Sci. 2017, 76, 393. [Google Scholar] [CrossRef]
- Xia, C.C.; Liu, G.D.; Meng, Y.C.; Wang, Z.Y.; Zhang, X.X. Impact of human activities on urban river system and its implication for water-environment risks: An isotope-based investigation in Chengdu, China. Hum. Ecol. Risk Assess. 2021, 27, 1416–1439. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Morsy, S.M.; Zakaria, K.M. Recharge sources and geochemical evolution of groundwater in the Quaternary aquifer at Atfih area, the northeastern Nile Valley, Egypt. J. Afr. Earth Sci. 2018, 142, 82–92. [Google Scholar] [CrossRef]
- Yang, N.; Wang, G.; Shi, Z.; Zhao, D.; Jiang, W.; Guo, L.; Liao, F.; Zhou, P.P. Application of multiple approaches to investigate the hydrochemistry evolution of groundwater in an arid region: Nomhon, Northwestern China. Water 2018, 10, 1667. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.P.; Li, M.; Lu, Y.D. Hydrochemistry and isotope hydrology for groundwater sustainability of the coastal multilayered aquifer system (Zhanjiang, China). Geofluids 2017, 2017, 7080346. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.H.; Song, J.X.; Zhang, Y.; Fu, G.B.; Cheng, D.D.; Zhang, G.T.; Xue, Y. Surface Water-Groundwater Interaction in the Guanzhong Section of the Weihe River Basin, China. Ground Water 2019, 57, 647–660. [Google Scholar] [CrossRef]
- Wen, G.C.; Wang, W.K.; Duan, L.; Gu, X.F.; Li, Y.M.; Zhao, J.H. Quantitatively evaluating exchanging relationship between river water and groundwater in Bayin River Basin of northwest China using hydrochemistry and stable isotopes. Arid. Land Geogr. 2018, 41, 734–743. [Google Scholar]
- Yang, Y.G.; Xiao, H.L.; Wei, Y.P.; Zhao, L.J.; Zou, S.B.; Yin, Z.L.; Yang, Q. Hydrologic processes in the different landscape zones of Mafengou River basin in the alpine cold region during the melting period. J. Hydrol. 2011, 409, 149–156. [Google Scholar] [CrossRef]
- Li, Z.X.; Feng, Q.; Liu, W.; Wang, T.T.; Chen, A.F.; Gao, Y.; Guo, X.Y.; Pan, Y.H.; Li, J.G.; Guo, R.; et al. Study on the contribution of cryosphere to runoff in the cold alpine basin: A case study of Hulugou River Basin in the Qilian Mountains. Glob. Planet. Chang. 2014, 122, 345–361. [Google Scholar]
- Li, Z.X.; Feng, Q.; Liu, W.; Wang, T.T.; Gao, Y.; Wang, Y.M.; Cheng, A.F.; Li, J.G.; Liu, L. Spatial and temporal trend of potential evapotranspiration and related driving forces in Southwestern China, during 1961–2009. Quat. Int. 2014, 336, 127–144. [Google Scholar]
- Li, Z.X.; Feng, Q.; Wang, Q.J.; Song, Y.; Li, J.G.; Li, Y.G. The influence from the shrinking cryosphere and strengthening evopotranspiration on hydrologic process in a cold basin, Qilian Mountains. Glob. Planet. Chang. 2016, 144, 119–128. [Google Scholar]
- Penna, D.; Engel, M.; Mao, L.; Dell’Agnese, A.; Bertoldi, G.; Comiti, F. Tracer-based analysis of spatial and temporal variations of water sources in a glacierized catchment. Hydrol. Earth Syst. Sci. 2014, 18, 5271–5288. [Google Scholar] [CrossRef] [Green Version]
- Turner, K.W.; Wolfe, B.B.; Edwards, T.W. Characterizing the role of hydrological processes on lake water balances in the Old Crow Flats, Yukon Territory, Canada, using water isotope tracers. J. Hydrol. 2010, 386, 103–117. [Google Scholar] [CrossRef]
- Barbieri, M.; Ricolfi, L.; Battistel, M.; Nigro, A.; Garone, A.; Ferranti, F.; Sappa, G. Monitoring wetland deterioration in a coastal protected area in central Italy: Implications for management. Euro-Mediterr. J. Environ. Integr. 2019, 4, 1–10. [Google Scholar] [CrossRef]
- Krishan, G.; Vashisht, R.; Sudarsan, N.; Rao, M.S. Groundwater salinity and isotope characterization: A case study from South-West Punjab, India. Environ. Earth Sci. 2021, 80, 1–11. [Google Scholar] [CrossRef]
- Krishan, G.; Prasad, G.; Anjali, K.C.P.; Patidar, N.; Yadav, B.K.; Kansal, M.L.; Singh, S.; Sharma, L.M.; Bradley, A.; Verma, S.K. Identifying the seasonal variability in source of groundwater salinization using deuterium excess—A case study from Mewat, Haryana, India. J. Hydrol. Reg. Stud. 2020, 31, 100724. [Google Scholar] [CrossRef]
- Dincer, T.; Payne, B.R.; Florkowski, T.; Martinec, J.; Tongiorgi, E. Snowmelt runoff from measurements of tritium and oxygen-18. Water Resour. Res. 1970, 6, 110–124. [Google Scholar] [CrossRef]
- Fritz, P.; Cherry, J.A.; Weyer, K.V.; Sklash, M.G. Storm runoff analyses using environmental isotope and major ions. In Interpretation of Environmental Isotope and Hydrochemical Data in Groundwater Hydrology; I.A.E.A.: Vienna, Austria, 1976; pp. 111–130. [Google Scholar]
- Sklash, M.G. The Role of Groundwater in Storm and Snowmelt Runoff Generation. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 1978. [Google Scholar]
- Gui, J.; Li, Z.; Yuan, R.; Xue, J. Hydrograph Separation and the Influence from Climate Warming on Runoff in the North-Eastern Tibetan Plateau. Quat. Int. 2019, 525, 45–53. [Google Scholar] [CrossRef]
- Li, Z.X.; Gui, J.; Wang, X.F.; Feng, Q.; Zhao, T.T.G.; Ouyang, C.J.; Guo, X.Y.; Zhang, B.J.; Shi, Y. Water resources in inland regions of central Asia: Evidence from stable isotope tracing. J. Hydrol. 2019, 570, 1–16. [Google Scholar] [CrossRef]
- Sklash, M.G.; Farvolden, R.N. The role of groundwater in storm runoff. Dev. Water Sci. 1979, 12, 45–65. [Google Scholar]
- Hooper, R.P.; Christophersen, N.; Peters, N.E. Modelling streamwater chemistry as a mixture of soilwater end-members—An application to the Panola Mountain catchment, Georgia, USA. J. Hydrol. 1990, 116, 321–343. [Google Scholar] [CrossRef]
- Hooper, R.P. Diagnostic tools for mixing models of stream water chemistry. Water Resour. Res. 2003, 39, 1055. [Google Scholar] [CrossRef]
- Yao, T.D.; Valerie, M.V.; Gao, J.; Yu, W.S.; Yang, X.X.; Risi, C. A review of climatic controls on δ18O in precipitation over the Tibetan Plateau: Observations and simulations. Rev. Geophys. 2013, 51, 525–548. [Google Scholar] [CrossRef]
- Yu, W.S.; Yao, T.D.; Lewis, S.; Tian, L.; Ma, Y.M.; Xu, B.Q.; Qu, D.M. Stable oxygen isotope differences between the areas to the north and south of Qinling Mountains in China reveal different moisture sources. Int. J. Climatol. 2014, 34, 1760–1772. [Google Scholar] [CrossRef]
- Jouzel, J.; Souchez, R.A. Melting–refreezing at the glacier sole and the isotopic composition of the ice. J. Glaciol. 1982, 28, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Hren, M.T.; Bookhagen, B.; Blisniuk, P.; Booth, A.L.; Chamberlain, C.P. δ18O and δD of streamwaters across the Himalaya and Tibetan Plateau: Implications for moisture sources and paleoelevation reconstructions. Earth Planet. Sci. Lett. 2009, 288, 20–32. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, W.X.; Zhang, Q.L. The distribution of deuterium and heavy oxygen in snow and ice, in the Jolmo Lungma regions of the southern Tibet. Sci. China B 1973, 4, 430–433. [Google Scholar]
- Pang, H.X.; He, Y.Q.; Theakstone, W.H. Soluble ionic and oxygen isotopic composition of a shallow firn profile, Baishui Glacier No.1, southeastern Tibetan Plateau. Ann. Glaciol. 2007, 46, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Karim, A.; Veizer, J. Water balance of the Indus River Basin and moisture source in the Karakoram and western Himalayas: Implications from hydrogen and oxygen isotopes in river water. J. Geophys. Res. Atmos. 2002, 107, 1–9. [Google Scholar] [CrossRef]
- Quade, J.; Garzione, C.; Eiler, J. Paleoelevation reconstruction using pedogenic carbonates. Rev. Mineral. Geochem. 2007, 6, 53–87. [Google Scholar] [CrossRef]
- Chen, K.; Meng, Y.; Liu, G.; Xia, C. Analysis for effects of monsoon activities on oxygen and hydrogen isotopes variation based on Hong Kong GNIP long-term data. J. Radioanal. Nucl. Chem. 2021, 328, 1055–1068. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Qian, H. Preliminary assessment of hydraulic connectivity between river water and shallow groundwater and estimation of their transfer rate during dry season in the Shidi River, China. Environ. Earth Sci. 2016, 75, 1–16. [Google Scholar] [CrossRef]
- Song, X.F.; Wang, S.Q.; Xiao, G.Q.; Wang, Z.M.; Liu, X. Time Series Analysis of Soil Water Dynamics in the Shallow Groundwater Areas of North China Plain. J. Nat. Resour. 2011, 26, 145–155. [Google Scholar]
- Xu, Y.D.; Wang, J.K.; Gao, X.D.; Zhang, Y.L. Application of Hydrogen and Oxygen Stable Isotope Techniques on Soil Water Research: A Review. J. Soil Water Conserv. 2018, 32, 1–9, 15. [Google Scholar]
- Xiao, D.A.; Wang, S.J. Comments on the progress and direction in soil water research. Ecol. Environ. Sci. 2009, 18, 1182–1188. [Google Scholar]
- Zhang, X.J.; Song, W.F.; Wu, J.K.; Wang, Z.J. Characteristics of Hydrogen and Oxygen Isotopes of Soil Water in the Water Source Area of Yuanyang Terrace. Environ. Sci. 2015, 36, 2102–2108. [Google Scholar]
- Wu, Y.J.; Du, T.S. Stable Hydrogen and Oxygen Isotopic Distributions and Water Movement in the Soil Under Plastic Film-mulching Furrow Irrigation. China Rural. Water Hydropower 2016, 9, 73–76. [Google Scholar]
- Wu, W.; Jiang, Y.J.; Jia, Y.N.; Peng, X.Y.; Duan, S.H.; Liu, J.C.; Wang, Z.X. Temporal and Spatial Distribution of the Soil Water δD and δ18O in a Typical Karst Valley: A Case Study of the Zhongliang Mountain, Chongqing City. Environ. Sci. 2018, 39, 5418–5427. [Google Scholar]
- Robertson, J.A.; Gazis, C.A. An oxygen isotope study of seasonal trends in soil water fluxes at two sites along a climate gradient in Washington state (USA). J. Hydrol. 2006, 328, 375–387. [Google Scholar] [CrossRef]
- Fu, Q.; Hou, R.J.; Wang, Z.L.; Li, T.X. Soil moisture thermal interaction effects under snow cover during freezing and thawing period. Trans. Chin. Soc. Agric. Eng. 2015, 31, 101–107, (In Chinese with English abstract). [Google Scholar]
- Fu, Q.; Hou, R.J.; Liu, D.; Li, T.X.; Wang, Z.L. Spatial distribution of soil moisture content under condition of snowcover. Trans. Chin. Soc. Agric. Eng. 2016, 32, 120–126, (In Chinese with English abstract). [Google Scholar]
- Lu, L.; Liu, J.H.; Ye, R.; Li, W.; Qin, D.Y. Preliminary Study on Relationship between Snow Covers and Soil Moisture in North China. Water Sav. Irrig. 2011, 1, 10–13, 17, (In Chinese with English abstract). [Google Scholar]
- Liang, S.; Li, X.; Zheng, X.; Jiang, T.; Qiao, D. Effects of winter snow cover on spring soil moisture based on remote sensing data product over farmland in northeast China. Remote Sens. 2020, 12, 2716. [Google Scholar] [CrossRef]
- Možný, M.; Hájková, L.; Tolasz, R. Influence of Snow Cover on Soil Moisture and Frost Dynamics in Winter of 2016–2017 in Czech Republic. In Proceedings of the ‘Snow an ecological phenomenon’, Smolenice, Slovakia, 19–21 September 2017. [Google Scholar]
- Sun, N.X. Study on Field Water Transformation Using Isotopes: A Case of Daxing District, Beijing. Master Thesis, China University of Geosciences, Beijing, China, 2015. [Google Scholar]
- Dansgaard, W. The abundance of 18O in atmospheric water and water vapor. Tellus 1953, 5, 461–469. [Google Scholar] [CrossRef]
- Vodila, G.; Palcsu, L.; Futó, I.; Szántó, Z. A 9-year record of stable isotope ratios of precipitation in Eastern Hungary: Implications on isotope hydrology and regional palaeoclimatology. J. Hydrol. 2011, 400, 144–153. [Google Scholar] [CrossRef]
- Negrel, P.; Petelet-Giraud, E.; Millot, R. Tracing water cycle in regulated basin using stable δ18O–δ2H isotopes: The Ebro river basin (Spain). Chem. Geol. 2016, 422, 71–81. [Google Scholar] [CrossRef]
- Ma, J.; Song, W.F.; Wu, J.K.; Wang, Z.J.; Zhang, X.J.; Liu, Z.B. Characteristics of Hydrogen and Oxygen Isotopes of Precipitation and Soil Water in Woodland in Water Source Area of Yuanyang Terrace. J. Soil Water Conserv. 2016, 30, 243–248, 254. [Google Scholar]
- Gazis, C.; Feng, X. A stable isotope study of soil water: Evidence for mixing and preferential flow paths. Geoderma 2004, 119, 97–111. [Google Scholar] [CrossRef]
- Zhang, L.J.; Wang, C.Z.; Li, Y.S.; Huang, Y.T.; Zhang, F.; Pan, T. High-latitude snowfall as a sensitive indicator of climate warming: A case study of Heilongjiang Province, China. Ecol. Indic. 2021, 122, 107249. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Y.F.; Wang, J.J. Dynamic Variation characteristics of Hydrogen and Oxygen Stable Isotope Composition in Soil Water after Salt Water Irrigation in Arid Area. Saf. Environ. Eng. 2020, 27, 32–39. [Google Scholar]
- Panichi, C.; Gonfiantini, R. Environmental isotopes in geothermal studies. Geothermics 1977, 6, 143–161. [Google Scholar] [CrossRef]
- Vimeux, F.; Masson, V.; Jouzel, J.; Petit, J.R.; Steig, E.J.; Stievenard, M.; Vaikmae, R.; White, J.W.C. Holocene hydrological cycle changes in the Southern Hemisphere documented in East Antarctic deuterium excess records. Clim. Dyn. 2001, 17, 503–513. [Google Scholar] [CrossRef]
- Craig, H.; Gordon, L.I.; Horibe, Y. Isotopic exchange effects in the evaporation of water: 1. Low-temperature experimental results. J. Geophys. Res. 1963, 68, 5079–5087. [Google Scholar] [CrossRef]
- Gat, J.R. Isotope composition of air moisture over the Mediterranean Sea: An index of the air-sea interaction pattern. Tellus Ser. B-Chem. Phys. Meteorol. 2003, 55, 953–965. [Google Scholar] [CrossRef]
- Ma, H.Y.; Wang, X.Y.; Zhang, J.; Huang, J.T.; Yin, L.H.; Dong, J.Q. An Experimental Study of The Isotopic Enrichment of Evaporating Water. Water Sav. Irrig. 2014, 36–39, (In Chinese with English abstract). [Google Scholar]
- Ma, H.Y.; Yin, L.H.; Zhang, J.; Huang, J.T.; Wang, X.Y.; Dong, J.Q. The impact of free air humidity on the fractionation in the process of evaporation. Geol. Bull. China 2015, 34, 2087–2091, (In Chinese with English abstract). [Google Scholar]






| Contents | Equipment | |
|---|---|---|
| Experiment time | November 2019–June 2020 | |
| Experiment area | Four sample plots were selected and subjected to four treatments of bare ground, snow thickness 10 cm, snow thickness 30 cm, and snow thickness 50 cm, and repeated three times. A 50 cm plastic membrane was embedded vertically around each test plot, and a one-meter-wide passage was left around the plot. | Artificially piled up |
| Sampling depth of soil | 0–10 cm, 10–20 cm, 20–30 cm | Earth drill |
| Sampling time | Once every three days after the snow cover had totally melted on every plot | - |
| Sampling method | The soil samples taken out were stored in whirl-pak sterile sampling bags, sealed and stored in the refrigerator. A total of 243 soil samples were collected. We used the J10622 rain gauge to collect each precipitation sample, put it into a sterile water sample bag after each collection, sealed it and stored it in the refrigerator. We collected snow samples, store them in whirl-pak sterile sampling bags, sealed them, and stored them in the refrigerator. | - |
| Sample preprocessing | Treatment of soil samples: The soil samples were subjected to vacuum condensation water extraction with a vacuum distillation device. The extracted soil water was filtered through a 0.45 μm organic filter membrane and placed in a 2 mL injection bottle for storage in a sealed and refrigerated manner. Treatment of snow samples and precipitation samples: The snow melt water and precipitation were filtered with a 0.45 μm organic filter membrane, and then placed in a 2 mL injection bottle, sealed and refrigerated. | - |
| Isotope determination | Needle washing of sample, reduction reaction in elemental analyzer EA, mass spectrometer to measure and compare | - |
| Isotope | Snowmelt Water | Soil Water | Precipitation | |||||
|---|---|---|---|---|---|---|---|---|
| 10 cm | 30 cm | 50 cm | 0–10 cm | 10–20 cm | 20–30 cm | Average Rainfall | Average Snowfall | |
| δD | −176.62 | −169.99 | −172.61 | −128.44 | −99.22 | −94.40 | −79.97‰ | −146.55‰ |
| δ18O | −23.54 | −23.77 | −24.15 | −16.57 | −12.49 | −11.43 | −11.14‰ | −19.39‰ |
| Snow Depth | Isotope | 0–10 cm | 10–20 cm | 20–30 cm | Average |
|---|---|---|---|---|---|
| Bare land | δD | −92.93 | −79.28 | −74.14 | −82.12 |
| δ18O | −10.66 | −9.77 | −9.25 | −9.89 | |
| 10 cm | δD | −102.69 | −83.22 | −78.64 | −88.18 |
| δ18O | −12.57 | −10.60 | −10.20 | −11.12 | |
| 30 cm | δD | −99.54 | −85.71 | −79.88 | −88.38 |
| δ18O | −12.51 | −11.03 | −10.48 | −11.34 | |
| 50 cm | δD | −96.04 | −86.28 | −80.75 | −87.69 |
| δ18O | −11.95 | −11.10 | −10.54 | −11.20 |
| Soil Layer | α = 0.05 | α = 0.01 | |||||
|---|---|---|---|---|---|---|---|
| Snow Cover | 0–10 cm | 10–20 cm | 20–30 cm | 0–10 cm | 10–20 cm | 20–30 cm | |
| 0 cm | −10.66 a | −9.77 a | −9.25 a | −10.66 A | −9.77 A | −9.25 A | |
| 10 cm | −12.57 a | −10.60 a | −10.20 a | −12.57 A | −10.60 A | −10.20 A | |
| 30 cm | −12.51 a | −11.03 a | −10.48 a | −12.51 A | −11.03 A | −10.48 A | |
| 50 cm | −11.95 b | −11.10 b | −10.54 b | −11.95 A | −11.10 A | −10.54 A | |
| Snow Cover | α = 0.05 | α = 0.01 | |||||
|---|---|---|---|---|---|---|---|
| Soil Layer | 10 cm | 30 cm | 50 cm | 10 cm | 30 cm | 50 cm | |
| 0–10 cm | −12.57 a | −12.51 a | −11.95 a | −12.57 A | −12.51 A | −11.95 A | |
| 10–20 cm | −10.60 a | −11.03 a | −11.10 a | −10.60 A | −11.03 A | −11.10 A | |
| 20–30 cm | −10.20 b | −10.48 b | −10.54 b | −10.20 A | −10.48 A | −10.54 A | |
| Soil Layer | 0–10 cm | 10–20 cm | 20–30 cm | ||||
|---|---|---|---|---|---|---|---|
| Snow Cover | Duration | Contribution | Duration | Contribution | Duration | Contribution | |
| 10 cm | 71 | 9.14 | 51 | 8.82 | 67 | 6.43 | |
| 30 cm | 71 | 9.25 | 55 | 9.23 | 62 | 7.17 | |
| 50 cm | 64 | 15.87 | 55 | 10.32 | 48 | 7.53 | |
| Average | 69 | 11.42 | 54 | 9.46 | 59 | 7.04 | |
| Snow Cover | 0–10 cm | 10–20 cm | 20–30 cm | |
|---|---|---|---|---|
| Soil Layer | ||||
| 10 cm | y = −0.251x + 16.803 ** R2 = 0.995 | y = −0.392x + 17.952 ** R2 = 0.961 | y = −0.213x + 13.028 ** R2 = 0.987 | |
| 30 cm | y = −0.233x + 15.064 ** R2 = 0.994 | y = −0.346x + 18.817 ** R2 = 1.00 | y = −0.306x + 13.468 ** R2 = 0.982 | |
| 50 cm | y = −0.505x + 29.022 ** R2 = 0.994 | y = −0.346x + 18.817 ** R2 = 0.995 | y = −0.217x + 14.660 ** R2 = 0.999 | |
| Soil Layer | 0–10 cm | 10–20 cm | 20–30 cm | Snow Cover | 10 cm | 20 cm | 30 cm | ||
|---|---|---|---|---|---|---|---|---|---|
| Snow Cover | Soil Layer | ||||||||
| 10 cm | 7.932 a | 7.156 a | 5.973 a | 0–10 cm | 7.932 a | 6.374 a | 10.309 a | ||
| 30 cm | 6.374 a | 7.794 b | 5.303 b | 10–20 cm | 7.156 b | 7.794 b | 6.548 b | ||
| 50 cm | 10.309 b | 6.548 c | 7.014 c | 20–30 cm | 5.973 b | 5.303 c | 7.014 c | ||
| Soil Layer | x1 | x2 | R2 |
|---|---|---|---|
| 0–10 cm | 0.331 | −0.702 | 0.461 ** |
| 10–20 cm | 0.106 | −0.751 | 0.596 ** |
| 20–30 cm | 0.201 | −0.801 | 0.661 ** |
| Three-layer average | 0.148 | −0.656 | 0.435 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Du, C.; Zhang, L.; Tan, Y.; Huang, Y.; Jiang, M. Contribution of Spring Snowmelt Water to Soil Water in Northeast China and Its Dynamic Changes. Water 2022, 14, 1368. https://doi.org/10.3390/w14091368
Zhang W, Du C, Zhang L, Tan Y, Huang Y, Jiang M. Contribution of Spring Snowmelt Water to Soil Water in Northeast China and Its Dynamic Changes. Water. 2022; 14(9):1368. https://doi.org/10.3390/w14091368
Chicago/Turabian StyleZhang, Wenshuai, Chen Du, Lijuan Zhang, Yulong Tan, Yutao Huang, and Meiyi Jiang. 2022. "Contribution of Spring Snowmelt Water to Soil Water in Northeast China and Its Dynamic Changes" Water 14, no. 9: 1368. https://doi.org/10.3390/w14091368
APA StyleZhang, W., Du, C., Zhang, L., Tan, Y., Huang, Y., & Jiang, M. (2022). Contribution of Spring Snowmelt Water to Soil Water in Northeast China and Its Dynamic Changes. Water, 14(9), 1368. https://doi.org/10.3390/w14091368





