Granular Natural Zeolites: Cost-Effective Adsorbents for the Removal of Ammonium from Drinking Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zeolite Characteristics
2.2. Batch-Set-Up
2.2.1. Influence of Contact Time and Ammonium Load
2.2.2. Influence of pH
2.2.3. Point of Zero Charge Determination
2.2.4. Influence of Competing Cations and Anions
2.2.5. Single and Multiple Regeneration
2.2.6. Influence of Natural Matrices
2.3. Analytical Methods
3. Results and Discussion
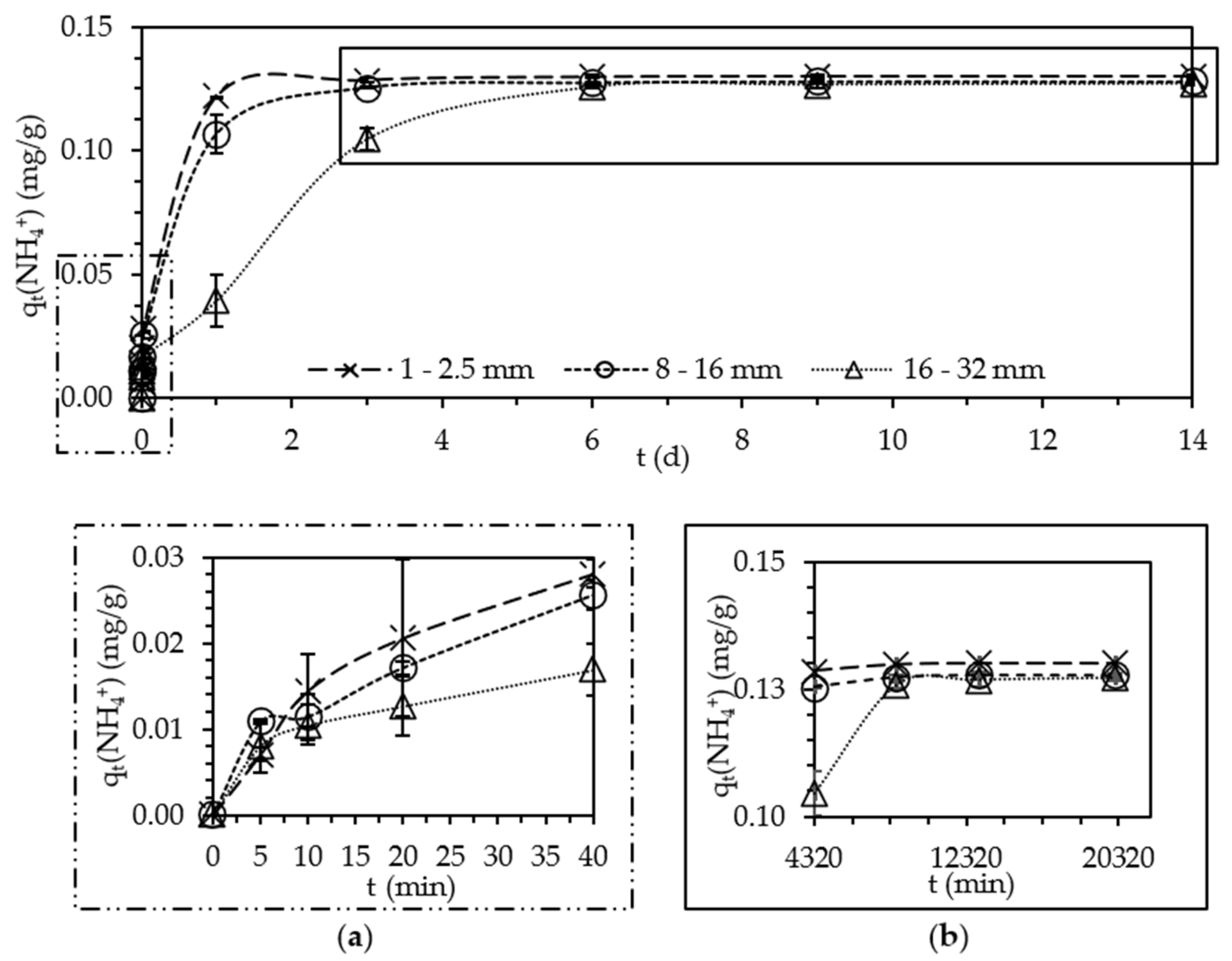
3.1. Comparison of Different Zeolite Grain Sizes
3.1.1. Kinetic Evaluation
3.1.2. Application of Different Reaction Kinetic Models
3.1.3. Zeolite Sorption Capacities under Equilibrium Conditions
3.2. Application of Isothermal Models
Alternative Adsorbents for Ammonium Removal
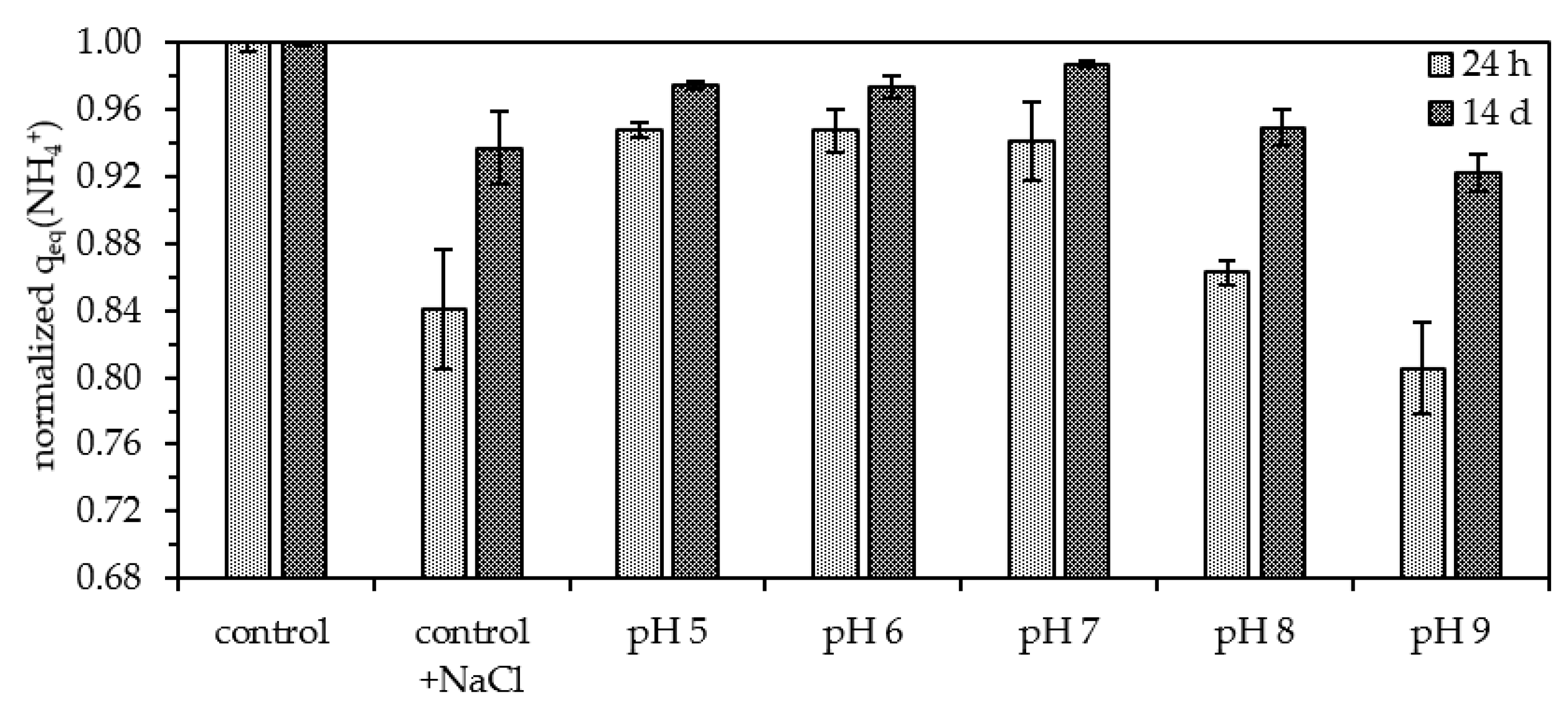
3.3. Influence of pH
3.3.1. Point of Zero Charge
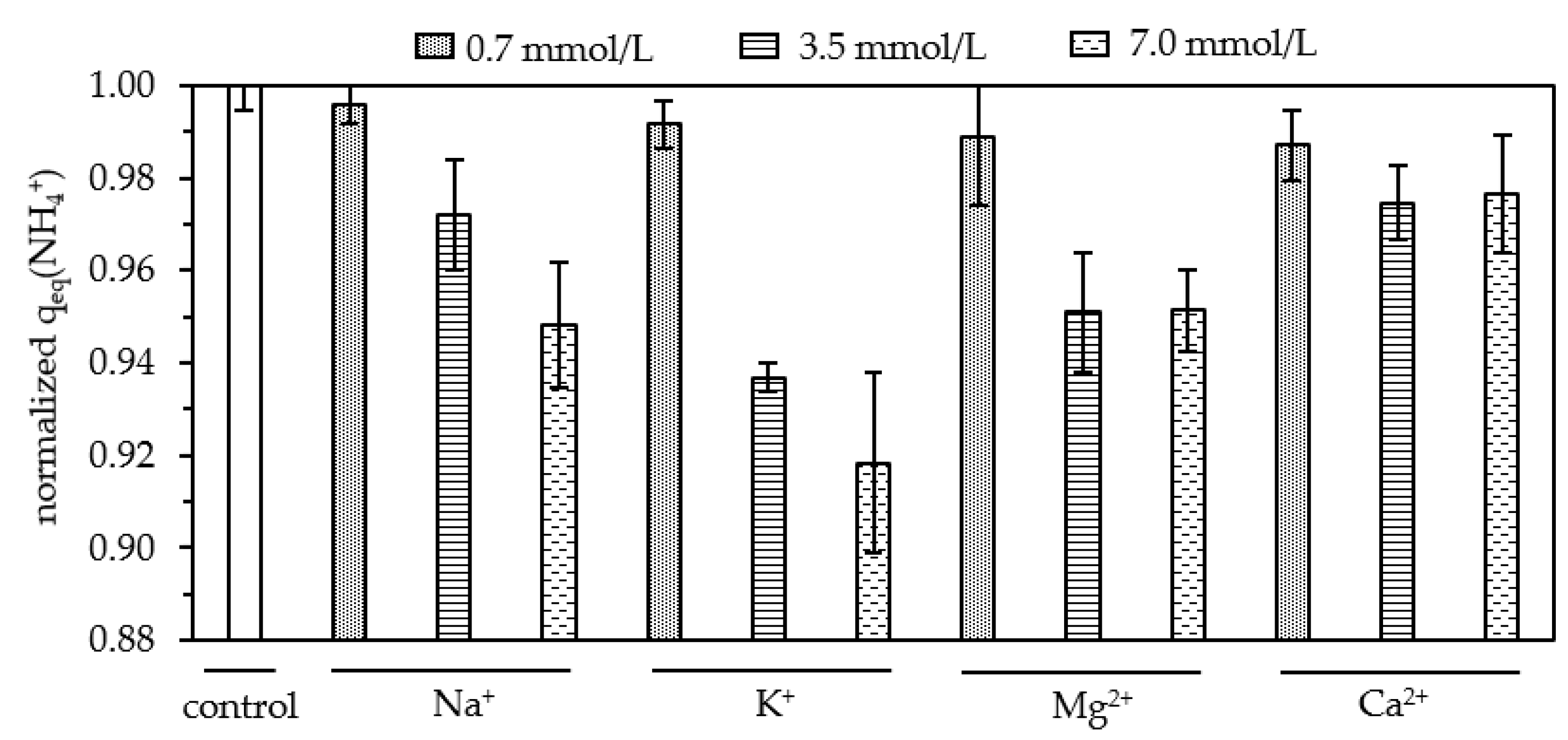
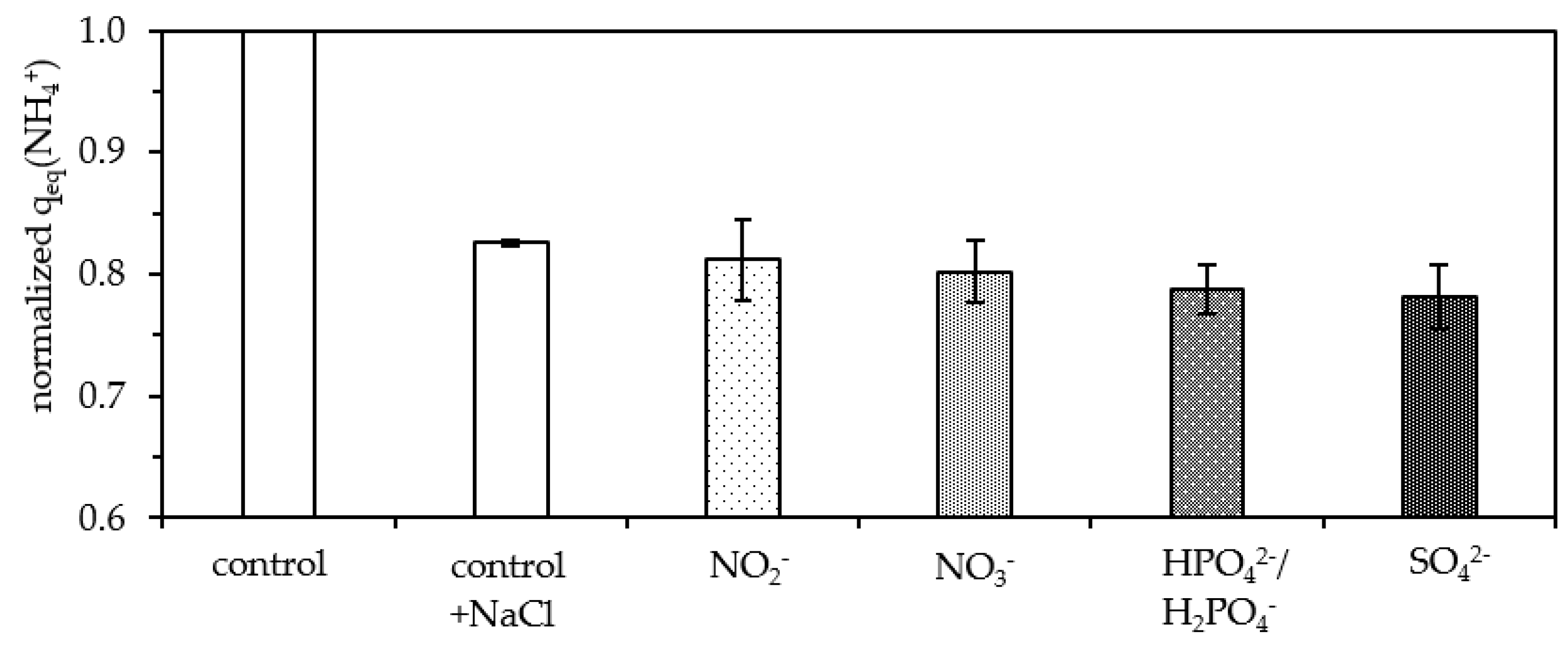
3.4. Competition from Cations and Anions
3.4.1. Cations
3.4.2. Anions
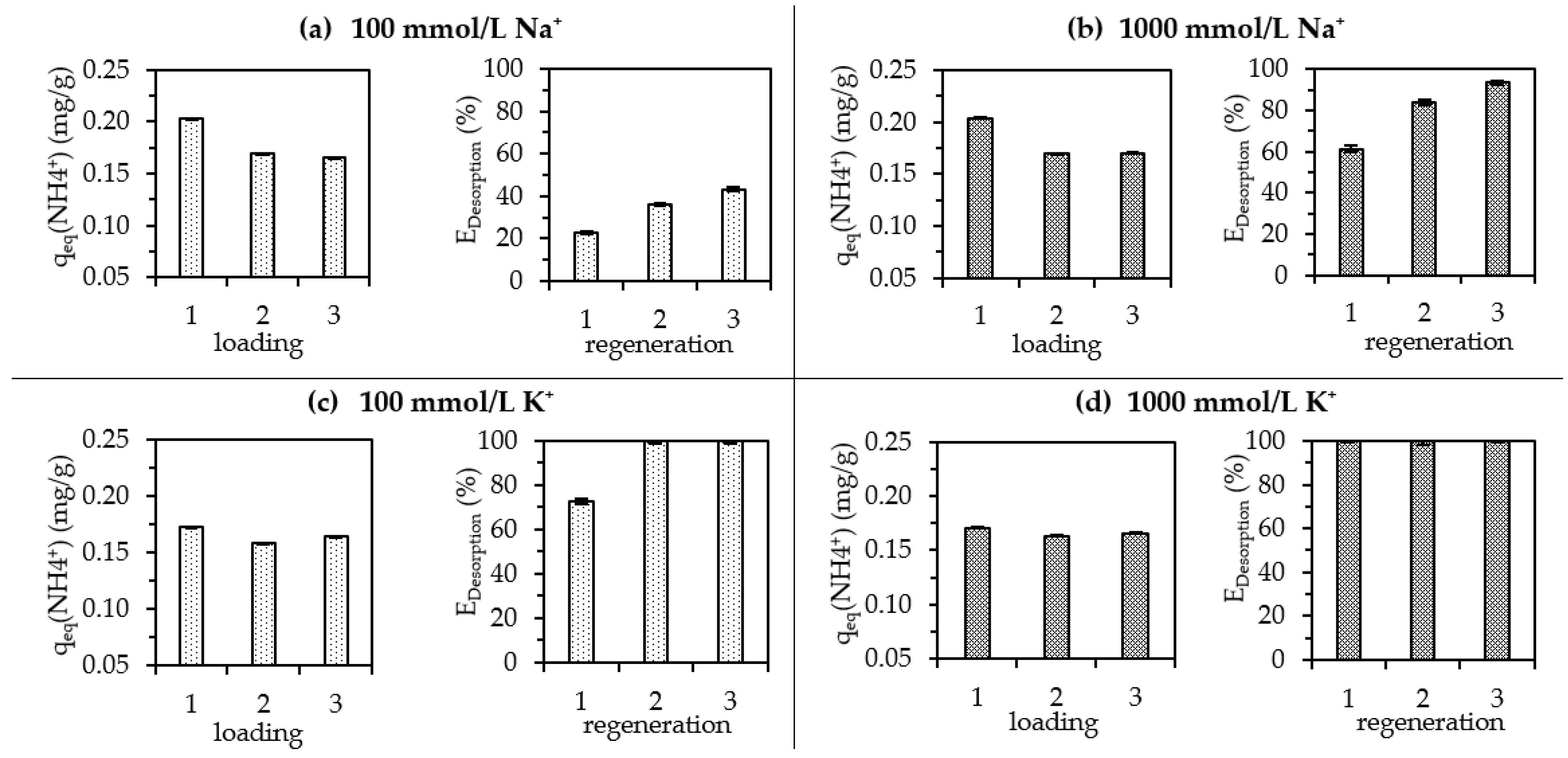
3.5. Regeneration
3.5.1. Single Regeneration
3.5.2. Multiple Regeneration
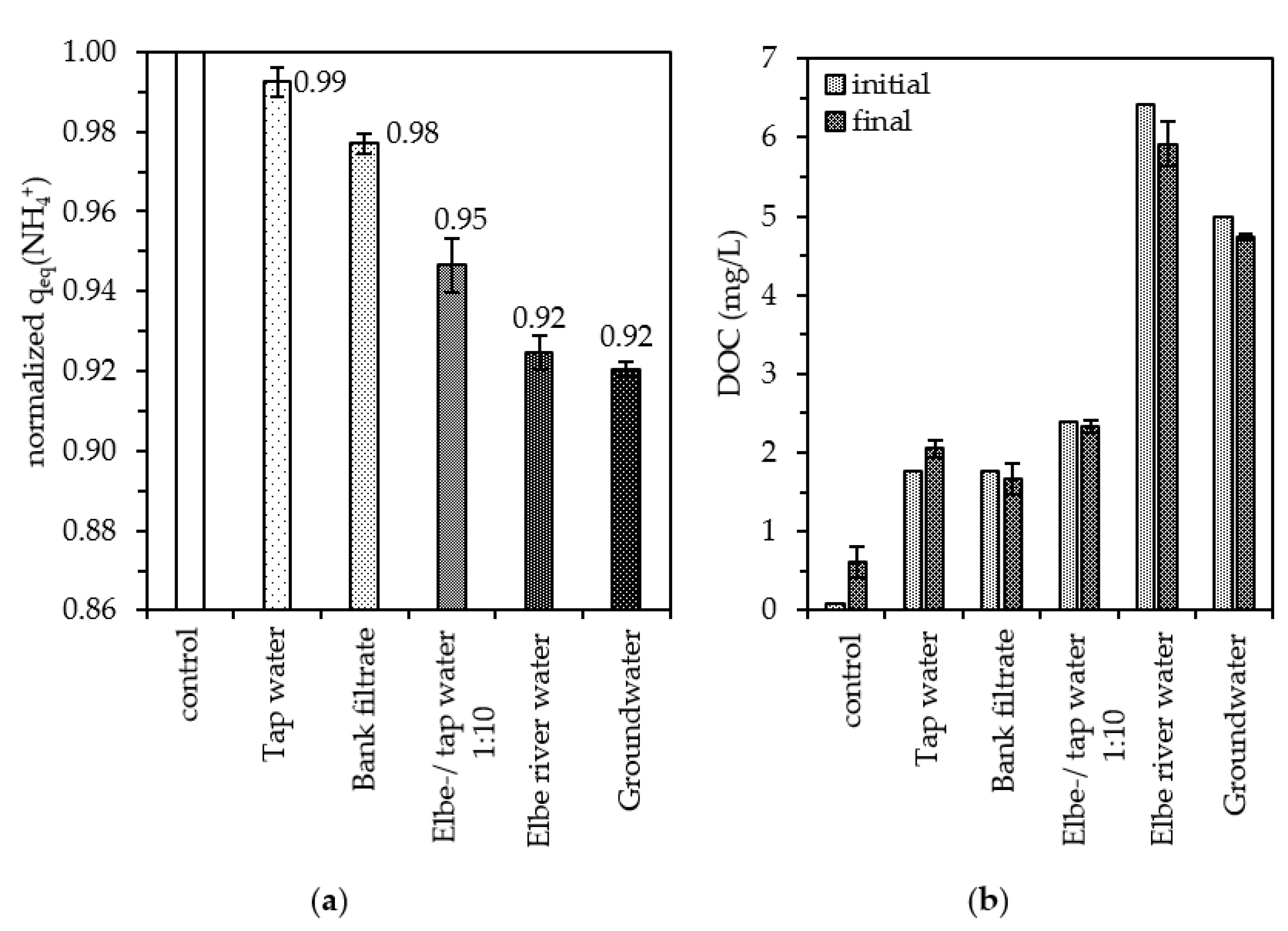
3.6. Influence of Natural Water Matrices
4. Conclusions
- Similar sorption kinetics and capacities were exhibited by 1–2.5 mm and 8–16 mm zeolite grain sizes. For this reason, 8–16 mm granular zeolites have the potential for applications in the field, will reduce milling costs, and thus can be used as cost-effective adsorbents;
- The Langmuir sorption model described the experimental data the most accurately, and ion exchange was revealed to be the primary mechanism behind the sorption of NH4+ at concentrations relevant to drinking water treatment;
- A maximum sorption efficiency was identified at pH 7 and the point of zero charge was determined to be in the range of pH 6.24 to pH 6.47. A reduction in zeolite’s efficiency is not expected in the relevant pH range for drinking water applications;
- K+ concentrations have to be considered when applying granular zeolites to the treatment of drinking water as K+ ions were the most effective competitor for NH4+ sorption to natural zeolites. Accordingly, potassium ions can be used to regenerate NH4+-loaded zeolites cost-effectively;
- Different anions exhibited minor effects on the sorption capacity of granular zeolites and can be neglected in applications in the field;
- The natural water matrices investigated decreased the sorption capacity of granular zeolites by up to 8%. Natural zeolites were also shown to act as adsorbents for organic matter and we showed that the DOC-concentration in a water matrix affects NH4+ sorption efficiency.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jong, H.D. Living Standards in a Modernizing World—A Long-Run Perspective on Material Wellbeing and Human Development. In Global Handbook of Quality of Life; Glatzer, W., Camfield, L., Møller, V., Rojas, M., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 45–74. ISBN 978-94-017-9177-9. [Google Scholar]
- Roser, M. Future Population Growth. Available online: https://ourworldindata.org/future-population-growth#global-population-growth (accessed on 11 November 2021).
- United Nations. World Population Prospects—Population Division—United Nations. Available online: https://population.un.org/wpp/Graphs/ (accessed on 16 July 2021).
- Bohnet, M.; Ullmann, F. Ullmann’s Encyclopedia of Industrial Chemistry, 6th compl. rev. ed.; Wiley-VCH: Weinheim, Germany, 2003; ISBN 9783527303854. [Google Scholar]
- Roser, M.; Ritchie, H. Nitrogen Fertilizers. Available online: https://ourworldindata.org/fertilizers#nitrogen-fertilizer-production (accessed on 14 November 2021).
- Shakya, B.M.; Nakamura, T.; Kamei, T.; Shrestha, S.D.; Nishida, K. Seasonal Groundwater Quality Status and Nitrogen Contamination in the Shallow Aquifer System of the Kathmandu Valley, Nepal. Water 2019, 11, 2184. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Li, P.; Xue, L.; Dong, Z.; Li, D. Solute geochemistry and groundwater quality for drinking and irrigation purposes: A case study in Xinle City, North China. Geochemistry 2020, 80, 125609. [Google Scholar] [CrossRef]
- Rusydi, A.F. Estimation of ammonium sources in Indonesian coastal alluvial groundwater using Cl− and GIS. GEOMATE 2019, 17, 53–58. [Google Scholar] [CrossRef]
- Rusydi, A.F.; Onodera, S.-I.; Saito, M.; Hyodo, F.; Maeda, M.; Sugianti, K.; Wibawa, S. Potential Sources of Ammonium-Nitrogen in the Coastal Groundwater Determined from a Combined Analysis of Nitrogen Isotope, Biological and Geological Parameters, and Land Use. Water 2021, 13, 25. [Google Scholar] [CrossRef]
- Fitts, C.R. Groundwater Science, 2nd ed.; Academic: Oxford, UK, 2013; ISBN 0123847052. [Google Scholar]
- Elshorbagy, W. (Ed.) Water Treatment; InTech: London, UK, 2013; ISBN 978-953-51-0928-0. [Google Scholar]
- McCarty, P.L. What is the Best Biological Process for Nitrogen Removal: When and Why? Environ. Sci. Technol. 2018, 52, 3835–3841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Zhou, X.; Wang, D.; Sun, J.; Wang, Q. Free ammonia pre-treatment of secondary sludge significantly increases anaerobic methane production. Water Res. 2017, 118, 12–19. [Google Scholar] [CrossRef]
- Carrera, J.; Baeza, J.A.; Vicent, T.; Lafuente, J. Biological nitrogen removal of high-strength ammonium industrial wastewater with two-sludge system. Water Res. 2003, 37, 4211–4221. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organisation: Geneva, Switzerland, 2017. [Google Scholar]
- Du, Y.; Ma, T.; Deng, Y.; Shen, S.; Lu, Z. Sources and fate of high levels of ammonium in surface water and shallow groundwater of the Jianghan Plain, Central China. Environ. Sci. Process. Impacts 2017, 19, 161–172. [Google Scholar] [CrossRef] [PubMed]
- de Vet, W.W.J.M.; van Genuchten, C.C.A.; van Loosdrecht, M.C.M.; van Dijk, J.C. Water quality and treatment of river bank filtrate. Drink. Water Eng. Sci. 2010, 3, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Berg, M.; Tran, H.C.; Nguyen, T.C.; Pham, H.V.; Schertenleib, R.; Giger, W. Arsenic contamination of groundwater and drinking water in Vietnam: A human health threat. Environ. Sci. Technol. 2001, 35, 2621–2626. [Google Scholar] [CrossRef] [Green Version]
- Norrman, J.; Sparrenbom, C.J.; Berg, M.; Dang, D.N.; Jacks, G.; Harms-Ringdahl, P.; Pham, Q.N.; Rosqvist, H. Tracing sources of ammonium in reducing groundwater in a well field in Hanoi (Vietnam) by means of stable nitrogen isotope (δ15N) values. Appl. Geochem. 2015, 61, 248–258. [Google Scholar] [CrossRef]
- Jiao, J.J.; Wang, Y.; Cherry, J.A.; Wang, X.; Zhi, B.; Du, H.; Wen, D. Abnormally high ammonium of natural origin in a coastal aquifer-aquitard system in the Pearl River Delta, China. Environ. Sci. Technol. 2010, 44, 7470–7475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiao, C.; Adeyeye, O.; Yang, W.; Liang, X. Source and Mobilization Mechanism of Iron, Manganese and Arsenic in Groundwater of Shuangliao City, Northeast China. Water 2020, 12, 534. [Google Scholar] [CrossRef] [Green Version]
- Verordnung über die Qualität von Wasser für den Menschlichen Gebrauch (Trinkwasserverordnung—TrinkwV). Available online: http://www.gesetze-im-internet.de/trinkwv_2001/BJNR095910001.html#BJNR095910001BJNG000201310 (accessed on 3 August 2021).
- Gupta, V.K.; Sadegh, H.; Yari, M.; Shahryari Ghoshekandi, R.; Maazinejad, B.; Chahardori, M. Removal of ammonium ions from wastewater: A short review in development of efficient methods. Glob. J. Environ. Sci. Manag. 2015, 1, 149–158. [Google Scholar] [CrossRef]
- Uhlmann, W.; Kreuziger, Y.; Kruspe, R.; Neumann, J.; Berg, T. Untersuchungen zu Ammonium in Ostsächsischen Bergbaufolgeseen. Available online: https://www.wasser.sachsen.de/download/Abschlussbericht_TP_05_online.pdf (accessed on 4 August 2021).
- Taddeo, R.; Prajapati, S.; Lepistö, R. Optimizing ammonium adsorption on natural zeolite for wastewaters with high loads of ammonium and solids. J. Porous Mater. 2017, 24, 1545–1554. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Armijos, C.; Cortina, J.L. Simultaneous phosphate and ammonium removal from aqueous solution by a hydrated aluminum oxide modified natural zeolite. Chem. Eng. J. 2015, 271, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Karadag, D.; Tok, S.; Akgul, E.; Turan, M.; Ozturk, M.; Demir, A. Ammonium removal from sanitary landfill leachate using natural Gördes clinoptilolite. J. Hazardous Mater. 2008, 153, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Liu, S.; Cao, Z.; Wang, Y. Ammonia removal from aqueous solution using natural Chinese clinoptilolite. Sep. Purif. Technol. 2005, 44, 229–234. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2021. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021.pdf (accessed on 2 October 2021).
- Virta, R. Minerals Yearbook 2011. Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/zeolites/myb1-2011-zeoli.pdf (accessed on 12 December 2021).
- Zeolite Products. Home—Zeolite Products. Available online: https://www.zeolite-products.com/ (accessed on 15 March 2022).
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Morante-Carballo, F.; Montalván-Burbano, N.; Carrión-Mero, P.; Jácome-Francis, K. Worldwide Research Analysis on Natural Zeolites as Environmental Remediation Materials. Sustainability 2021, 13, 6378. [Google Scholar] [CrossRef]
- Huang, J.; Kankanamge, N.R.; Chow, C.; Welsh, D.T.; Li, T.; Teasdale, P.R. Removing ammonium from water and wastewater using cost-effective adsorbents: A review. J. Environ. Sci. 2018, 63, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Weatherley, L.R.; Miladinovic, N.D. Comparison of the ion exchange uptake of ammonium ion onto New Zealand clinoptilolite and mordenite. Water Res. 2004, 38, 4305–4312. [Google Scholar] [CrossRef] [PubMed]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef]
- Belviso, C. Zeolite for Potential Toxic Metal Uptake from Contaminated Soil: A Brief Review. Processes 2020, 8, 820. [Google Scholar] [CrossRef]
- Kwakye-Awuah, B.; Sefa-Ntiri, B.; Von-Kiti, E.; Nkrumah, I.; Williams, C. Adsorptive Removal of Iron and Manganese from Groundwater Samples in Ghana by Zeolite Y Synthesized from Bauxite and Kaolin. Water 2019, 11, 1912. [Google Scholar] [CrossRef] [Green Version]
- Kotoulas, A.; Agathou, D.; Triantaphyllidou, I.; Tatoulis, T.; Akratos, C.; Tekerlekopoulou, A.; Vayenas, D. Zeolite as a Potential Medium for Ammonium Recovery and Second Cheese Whey Treatment. Water 2019, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Collison, R.; Grismer, M. Upscaling the Zeolite-Anammox Process: Treatment of Anaerobic Digester Filtrate. Water 2018, 10, 1553. [Google Scholar] [CrossRef] [Green Version]
- Collison, R.S.; Grismer, M.E. Upscaling the Zeolite-Anammox Process: Treatment of Secondary Effluent. Water 2018, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Yapsakli, K.; Aktan, C.K.; Mertoglu, B. Anammox-zeolite system acting as buffer to achieve stable effluent nitrogen values. Biodegradation 2017, 28, 69–79. [Google Scholar] [CrossRef]
- Kodewitz, P. Datasheet CLP85+ Natural Zeolite; Zeolite: Berlin, Germany; Available online: https://www.zeolith-gmbh.com/produkte/klinoptilolith-zeolith (accessed on 16 February 2022).
- Worch, E. Adsorption Technology in Water Treatment: Fundamentals, Processes, and Modeling, 2nd ed.; De Gruyter: Berlin, Germany, 2021; ISBN 9783110715507. [Google Scholar]
- William Kajjumba, G.; Emik, S.; Öngen, A.; Kurtulus Özcan, H.; Aydın, S. (Eds.) Modelling of Adsorption Kinetic Processes—Errors, Theory and Application; IntechOpen: London, UK, 2019; ISBN 978-1-78984-819-9. [Google Scholar]
- Edebali, S. (Ed.) Advanced Sorption Process Applications; IntechOpen: London, UK, 2019; ISBN 978-1-78984-818-2. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I. Determination of sorbent point zero charge: Usefulness in sorption studies. Environ. Chem. Lett. 2009, 7, 79–84. [Google Scholar] [CrossRef]
- Tang, H.; Xu, X.; Wang, B.; Lv, C.; Shi, D. Removal of Ammonium from Swine Wastewater Using Synthesized Zeolite from Fly Ash. Sustainability 2020, 12, 3423. [Google Scholar] [CrossRef] [Green Version]
- Mazloomi, F.; Jalali, M. Ammonium removal from aqueous solutions by natural Iranian zeolite in the presence of organic acids, cations and anions. J. Environ. Chem. Eng. 2016, 4, 1664–1673. [Google Scholar] [CrossRef]
- Singh, A.K.; Bhagowati, S.; Das, T.K.; Yubbe, D.; Rahmann, B.; Nath, M.; Obing, P.; Singh, W.S.K.; Renthlei, C.Z.; Pachuau, L.; et al. Thakur. Assessment of Arsenic, Fluoride, Iron, Nitrate and Heavy Metals in Drinking Water of Northeastern India. Himal. Ecol. 2008, 1–7. [Google Scholar]
- Schierano, M.C.; Maine, M.A.; Panigatti, M.C. Dairy farm wastewater treatment using horizontal subsurface flow wetlands with Typha domingensis and different substrates. Environ. Technol. 2017, 38, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Stefanakis, A.I.; Tsihrintzis, V.A. Use of zeolite and bauxite as filter media treating the effluent of Vertical Flow Constructed Wetlands. Microporous Mesoporous Mater. 2012, 155, 106–116. [Google Scholar] [CrossRef]
- Huo, H.; Lin, H.; Dong, Y.; Cheng, H.; Wang, H.; Cao, L. Ammonia-nitrogen and phosphates sorption from simulated reclaimed waters by modified clinoptilolite. J. Hazard. Mater. 2012, 229-230, 292–297. [Google Scholar] [CrossRef]
- Lin, L.; Lei, Z.; Wang, L.; Liu, X.; Zhang, Y.; Wan, C.; Lee, D.-J.; Tay, J.H. Adsorption mechanisms of high-levels of ammonium onto natural and NaCl-modified zeolites. Sep. Purif. Technol. 2013, 103, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.; Karsheva, M.; Koumanova, B. Adsorption of ammonium ions onto natural zeolite. J. Univ. Chem. Technol. Metall. 2010, 45, 295–301. [Google Scholar]
- Cheng, Z.; Ding, W. Ammonium removal from water by natural and modified zeolite: Kinetic, equilibrium, and thermodynamic studies. Desalin. Water Treat. 2015, 55, 978–985. [Google Scholar] [CrossRef]
- Moussavi, G.; Talebi, S.; Farrokhi, M.; Sabouti, R.M. The investigation of mechanism, kinetic and isotherm of ammonia and humic acid co-adsorption onto natural zeolite. Chem. Eng. J. 2011, 171, 1159–1169. [Google Scholar] [CrossRef]
- Chen, H.-F.; Lin, Y.-J.; Chen, B.-H.; Yoshiyuki, I.; Liou, S.; Huang, R.-T. A Further Investigation of NH4+ Removal Mechanisms by Using Natural and Synthetic Zeolites in Different Concentrations and Temperatures. Minerals 2018, 8, 499. [Google Scholar] [CrossRef] [Green Version]
- Senchenya, I.N.; Kazanskii, V.B.; Beran, S. Quantum chemical study of the effect of the structural characteristics of zeolites on the properties of their bridging hydroxyl groups. Part 2. J. Phys. Chem. 1968, 90, 4857–4859. [Google Scholar] [CrossRef]
- Katada, N.; Suzuki, K.; Noda, T.; Sastre, G.; Niwa, M. Correlation between Brønsted Acid Strength and Local Structure in Zeolites. J. Phys. Chem. C 2009, 113, 19208–19217. [Google Scholar] [CrossRef]
- Noda, T.; Suzuki, K.; Katada, N.; Niwa, M. Combined study of IRMS-TPD measurement and DFT calculation on Brønsted acidity and catalytic cracking activity of cation-exchanged Y zeolites. J. Catal. 2008, 259, 203–210. [Google Scholar] [CrossRef]
- Sastre, G.; Katada, N.; Niwa, M. Computational Study of Brønsted Acidity of Mordenite. Effect of the Electric Field on the Infrared OH Stretching Frequencies. J. Phys. Chem. C 2010, 114, 15424–15431. [Google Scholar] [CrossRef]
- Herron, N.; Corbin, D.R. (Eds.) Inclusion Chemistry with Zeolites: Nanoscale Materials by Design; Springer: Dordrecht, The Netherlands, 1995; ISBN 9789401101196. [Google Scholar]
- Van Santen, R.A. Molecular Heterogeneous Catalysis: A Conceptual and Computational Approach; Wiley-VCH: Weinheim, Germany, 2006; ISBN 3527610847. [Google Scholar]
- Zare, K.; Sadegh, H.; Shahryari-ghoshekandi, R.; Asif, M.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Equilibrium and kinetic study of ammonium ion adsorption by Fe3O4 nanoparticles from aqueous solutions. J. Mol. Liq. 2016, 213, 345–350. [Google Scholar] [CrossRef]
- Thornton, A.; Pearce, P.; Parsons, S.A. Ammonium removal from digested sludge liquors using ion exchange. Water Res. 2007, 41, 433–439. [Google Scholar] [CrossRef]
- Otal, E.; Vilches, L.F.; Luna, Y.; Poblete, R.; García-Maya, J.M.; Fernández-Pereira, C. Ammonium Ion Adsorption and Settleability Improvement Achieved in a Synthetic Zeolite-Amended Activated Sludge. Chin. J. Chem. Eng. 2013, 21, 1062–1068. [Google Scholar] [CrossRef]
- Sica, M.; Duta, A.; Teodosiu, C.; Draghici, C. Thermodynamic and kinetic study on ammonium removal from a synthetic water solution using ion exchange resin. Clean. Technol. Environ. Policy 2014, 16, 351–359. [Google Scholar] [CrossRef]
- Jorgensen, T.C.; Weatherley, L.R. Ammonia removal from wastewater by ion exchange in the presence of organic contaminants. Water Res. 2003, 37, 1723–1728. [Google Scholar] [CrossRef]
- Liao, Z.-L.; Chen, H.; Zhu, B.-R.; Li, H.-Z. Combination of powdered activated carbon and powdered zeolite for enhancing ammonium removal in micro-polluted raw water. Chemosphere 2015, 134, 127–132. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kipkemoi Kirui, W.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Juan, R.; Hernández, S.; Andrés, J.M.; Ruiz, C. Ion exchange uptake of ammonium in wastewater from a Sewage Treatment Plant by zeolitic materials from fly ash. J. Hazard. Mater. 2009, 161, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Niu, Y.; Hu, X.; Xi, B.; Peng, X.; Liu, W.; Guan, W.; Wang, L. Removal of ammonium ions from aqueous solutions using zeolite synthesized from red mud. Desalin. Water Treat. 2015, 57, 4720–4731. [Google Scholar] [CrossRef]
- Yu, X.; Wan, C.; Lei, Z.; Liu, X.; Zhang, Y.; Lee, D.-J.; Tay, J.-H. Adsorption of ammonium by aerobic granules under high ammonium levels. J. Taiwan Inst. Chem. Eng. 2014, 45, 202–206. [Google Scholar] [CrossRef]
- Ismail, Z.Z.; Tezel, U.; Pavlostathis, S.G. Sorption of quaternary ammonium compounds to municipal sludge. Water Res. 2010, 44, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Wasielewski, S.; Rott, E.; Minke, R.; Steinmetz, H. Evaluation of Different Clinoptilolite Zeolites as Adsorbent for Ammonium Removal from Highly Concentrated Synthetic Wastewater. Water 2018, 10, 584. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhang, H.; Xu, D.; Han, L.; Niu, D.; Tian, B.; Zhang, J.; Zhang, L.; Wu, W. Removal of ammonium from aqueous solutions using zeolite synthesized from fly ash by a fusion method. Desalination 2011, 271, 111–121. [Google Scholar] [CrossRef]
- Murayama, N.; Yoshida, S.; Takami, Y.; Yamamoto, H.; Shibata, J. Simultaneous Removal of NH4+ and PO43− in Aqueous Solution and Its Mechanism by Using Zeolite Synthesized from Coal Fly Ash. Sep. Sci. Technol. 2003, 38, 113–130. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Aguilar-Armenta, G.; Gonzalez-Gutierrez, L.V.; Guerrero-Coronado, R.M.; Mendoza-Barron, J. Ammonia exchange on clinoptilolite from mineral deposits located in Mexico. J. Chem. Technol. Biotechnol. 2004, 79, 651–657. [Google Scholar] [CrossRef]
- Abatal, M.; Córdova Quiroz, A.V.; Olguín, M.T.; Vázquez-Olmos, A.R.; Vargas, J.; Anguebes-Franseschi, F.; Giácoman-Vallejos, G. Sorption of Pb(II) from Aqueous Solutions by Acid-Modified Clinoptilolite-Rich Tuffs with Different Si/Al Ratios. Appl. Sci. 2019, 9, 2415. [Google Scholar] [CrossRef] [Green Version]
- Ngeno, E.C.; Shikuku, V.O.; Orata, F.; Baraza, L.D.; Kimosop, S.J. Caffeine and Ciprofloxacin Adsorption from Water onto Clinoptilolite: Linear Isotherms, Kinetics, Thermodynamic and Mechanistic Studies. S. Afr. J. Chem. 2019, 72, 136–142. [Google Scholar] [CrossRef]
- Sljivic, M.; Smiciklas, I.; Pejanovic, S.; Plecas, I. Comparative study of Cu2+ adsorption on a zeolite, a clay and a diatomite from Serbia. Appl. Clay Sci. 2009, 43, 33–40. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Mikelionienė, A.; Baltušnikas, A.; Kantautas, A.; Radzevičius, A. Removal of ammonium ion from aqueous solutions by using unmodified and H2O2-modified zeolitic waste. Sci. Rep. 2020, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Saddique, M.T.; Naeem, A.; Westerhoff, P.; Mustafa, S.; Alum, A. Comparison of Different Methods for the Point of Zero Charge Determination of NiO. Ind. Eng. Chem. Res. 2011, 50, 10017–10023. [Google Scholar] [CrossRef]
- Liu, X.; Mäki-Arvela, P.; Aho, A.; Vajglova, Z.; Gun’ko, V.M.; Heinmaa, I.; Kumar, N.; Eränen, K.; Salmi, T.; Murzin, D.Y. Zeta Potential of Beta Zeolites: Influence of Structure, Acidity, pH, Temperature and Concentration. Molecules 2018, 23, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inglezakis, V.J.; Zorpas, A. (Eds.) Handbook of Natural Zeolites: Natural Zeolites Structure and Porosity; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; ISBN 978-1-60805-446-6. [Google Scholar]
- Margeta, K.; Zabukovec, N.; Siljeg, M.; Farkas, A. Natural Zeolites in Water Treatment—How Effective is Their Use. In Water Treatment; Elshorbagy, W., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-0928-0. [Google Scholar]
- Sidey, V. On the effective ionic radii for ammonium. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 626–633. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Nightingale, E.R., Jr. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Akcay, M.U.; Avdan, Z.Y.; Inan, H. Effect of biofiltration process on the control of THMs and HAAs in drinking water. Desalin. Water Treat. 2016, 57, 2546–2554. [Google Scholar] [CrossRef]



| Composition | Value (%) | Characteristics | |
|---|---|---|---|
| SiO2 | 65.00–71.30 | Exchange capacity | 1.2–1.5 mol/kg |
| Al2O3 | 11.50–13.10 | Selectivity | NH4+ > K+ > Na+ > Ca2+ > Mg2+ |
| CaO | 2.70–5.20 | Mean pore diameter | 0.4 nm |
| K2O | 2.20–3.40 | Specific surface | 30–60 m2/g |
| Fe2O3 | 0.70–1.90 | Si/Al | 4.80–5.40 (−) |
| MgO | 0.60–1.20 | Grain sizes | 1–2.5 mm |
| Na2O | 0.20–1.30 | 8–16 mm | |
| TiO2 | 0.10–0.30 | 16–32 mm | |
| Buffer | Chemical A | Chemical B | A (mol/L) | B (mol/L) |
|---|---|---|---|---|
| pH 5 | C2H4O2 | C2H3NaO2 | 0.03 | 0.07 |
| pH 6 | Na2HPO4 | NaH2PO4·H2O | 0.01 | 0.09 |
| pH 7 | Na2HPO4 | NaH2PO4·H2O | 0.06 | 0.04 |
| pH 8 | Na2B4O7·10H2O | HCl | 0.1 | x |
| pH 9 | NaH2PO4·H2O | HCl | 0.1 | x |
| Zeolite | Grain Size (mm) | Si/Al (−) | c0(NH4+) (mg/L) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|---|---|
| KL (L/mg) | qmax (mg/L) | R2 (−) | KF (L/g) | n (−) | R2 (−) | ||||
| CLP85+ (a) | 8–16 | 4.8–5.4 | 1–6890 | 0.004 | 21.3 | 0.992 | 1.21 | 0.33 | 0.975 |
| Slovakia (b) | <0.2 | 5.6 | 10–5000 | 0.006 | 33 | 0.99 | 1.84 | 0.36 | 0.97 |
| China (c) | 0.8–1.43 | 3.38 | 10–4000 | 0.009 | 14.3 | 0.993 | 0.98 | 0.36 | 0.973 |
| Alternative Adsorbent | Product Name | Grain Size (mm) | c0(NH4+) (mg/L) | Capacity (mgNH4+/g) | Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|---|
| Clinoptilolite | CLP85+ | 8–16 | 2–6890 | 0.02–20.29 | 99–30 | This study |
| Other zeolites | Mesolite | NA x | 50 | 55 | 95 | [67] |
| Synthetic zeolite | NA x | 18 | 13 | 70 | [68] | |
| Polymeric ion exchanger | Purolite C 150 H | NA x | 32–193 | 36 | >65 | [69] |
| Dowex 50w-x8 and Purolite MN500 | ~0.18 | 32–257 | 51 | NA x | [70] | |
| Carbon-based adsorbents | Combination of PAC xx and PZ xxx | 0.50–0.85 | 23 | NAx | 49 | [71] |
| Rice husk biochar | 0.25–0.50 | 322–1800 | 51 | 84 | [72] | |
| Industrial wastes | Fly ash (thermally activated) | <0.80 | 67 | 7 | 84 | [73] |
| Red mud | ~0.15 | 6–644 | 22 | >50 | [74] | |
| Nanoparticles | Fe3O4 | NA x | 103–180 | 171 | NA x | [66] |
| Biosorbents | Aerobic granules | NA x | 300 | 24 | NA x | [75] |
| Municipal sludge | NA x | 300 | NA x | 89 | [76] |
| Channel Dimension (nm) | Ring Type | ||
|---|---|---|---|
| Inglezakis and Zorpas [87] | Margeta et al. [88] | Number | Arrangement |
| 0.75·0.31 | 0.72·0.44 | 10 | Horizontal |
| 0.47·0.28 | 0.55·0.40 | 8 | Vertical |
| 0.46·0.36 | 0.47·0.41 | 8 | Horizontal |
| Cation | Charge Density (C/mm3) | Ionic Radius (nm) | Hydrated Radius (nm) |
|---|---|---|---|
| NH4+ | 12 | 0.148 (a) | 0.331 (c) |
| K+ | 14 | 0.138 (b) | 0.331 (c) |
| Na+ | 37 | 0.102 (b) | 0.358 (c) |
| Ca2+ | 76 | 0.100 (b) | 0.412 (c) |
| Mg2+ | 205 | 0.072 (b) | 0.428 (c) |
| Water Matrices | Cations (mmol/L) | DOC(mg/L) | |||
|---|---|---|---|---|---|
| K+ | Na+ | Mg2+ | Ca2+ | ||
| Ulrapure water (control) | <CR x | <CR x | <CR x | <CR x | 0.09 |
| Tap water | 0.04 | 0.38 | 0.30 | 0.41 | 1.77 |
| Bank filtrate | 0.25 | 0.91 | 1.72 | 0.78 | 1.77 |
| Elbe-/tap water (1:10) | 0.05 | 0.46 | 0.36 | 0.44 | 2.39 |
| Groundwater | 1.75 | 1.17 | 1.13 | 0.91 | 5.00 |
| Elbe river water | 0.11 | 0.15 | 0.94 | 0.56 | 6.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eberle, S.; Börnick, H.; Stolte, S. Granular Natural Zeolites: Cost-Effective Adsorbents for the Removal of Ammonium from Drinking Water. Water 2022, 14, 939. https://doi.org/10.3390/w14060939
Eberle S, Börnick H, Stolte S. Granular Natural Zeolites: Cost-Effective Adsorbents for the Removal of Ammonium from Drinking Water. Water. 2022; 14(6):939. https://doi.org/10.3390/w14060939
Chicago/Turabian StyleEberle, Stephan, Hilmar Börnick, and Stefan Stolte. 2022. "Granular Natural Zeolites: Cost-Effective Adsorbents for the Removal of Ammonium from Drinking Water" Water 14, no. 6: 939. https://doi.org/10.3390/w14060939
APA StyleEberle, S., Börnick, H., & Stolte, S. (2022). Granular Natural Zeolites: Cost-Effective Adsorbents for the Removal of Ammonium from Drinking Water. Water, 14(6), 939. https://doi.org/10.3390/w14060939





