Phytoextraction and Antioxidant Defense of Mangrove Seedling (Kandelia obovata) to Inorganic Arsenate Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sampling
2.2. Experimental Design
2.3. Sampling and Analysis
2.3.1. Determination of Seedling Height and Biomass
2.3.2. Determination of the Malondialdehyde in Plant
2.3.3. Determination of Antioxidant Enzyme Activity in Plant
2.3.4. Determination of the Total As Concentration in Plants
2.4. Data Analysis
3. Results
3.1. The Effect of As Exposure on the Growth of Mangrove Seedlings
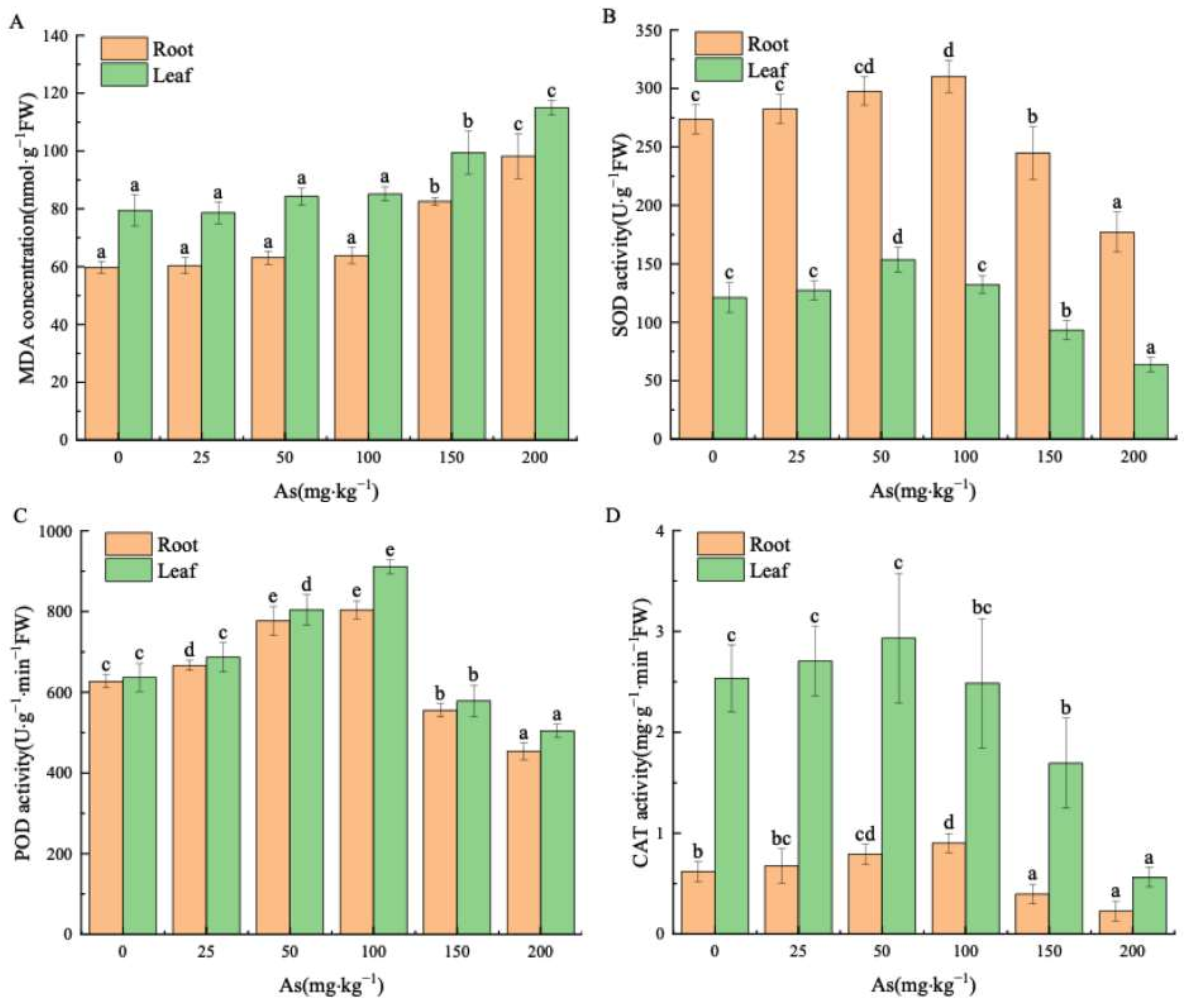
3.2. Effects of As Exposure on MDA and Antioxidant Enzymes of Mangrove Seedlings
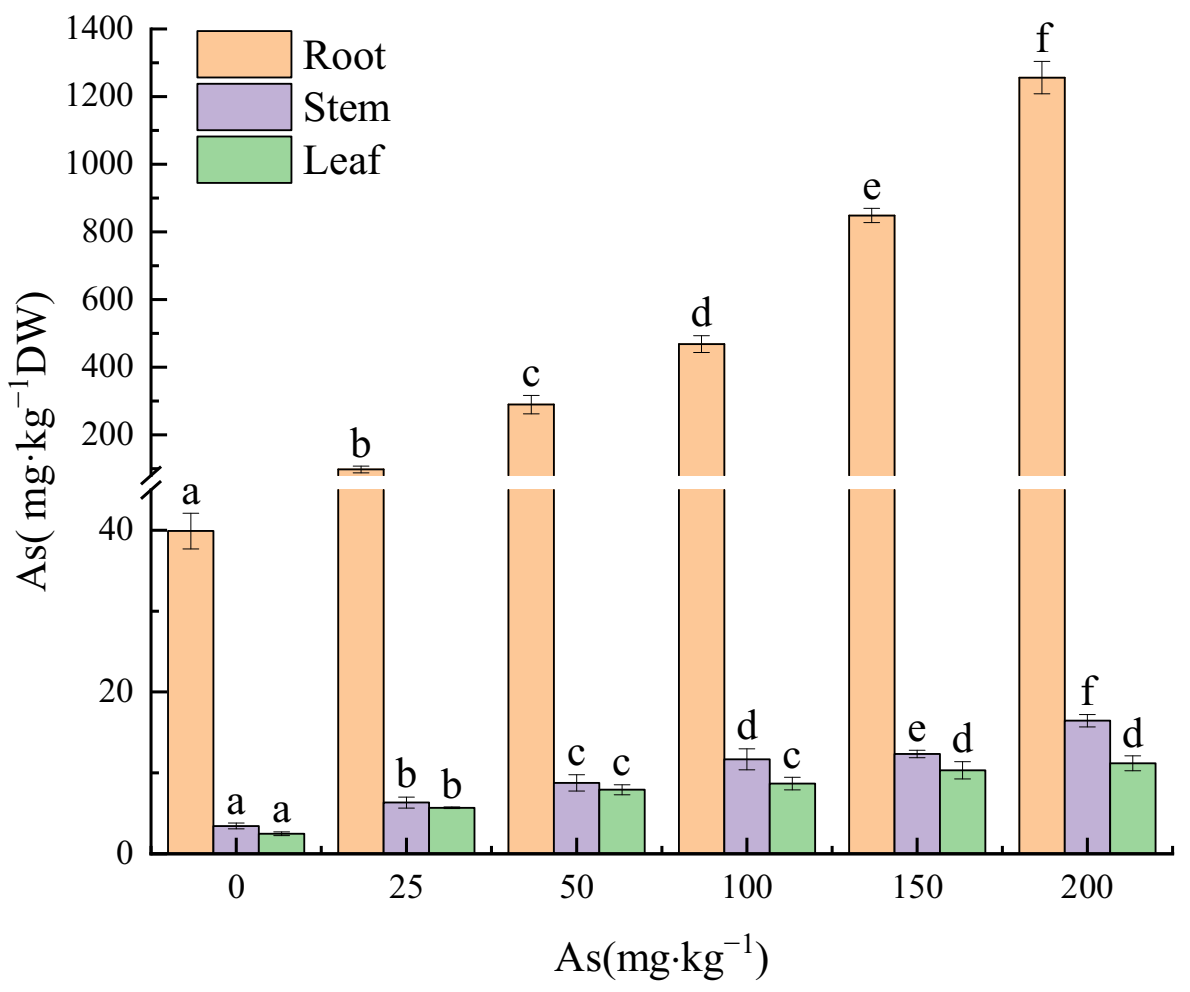
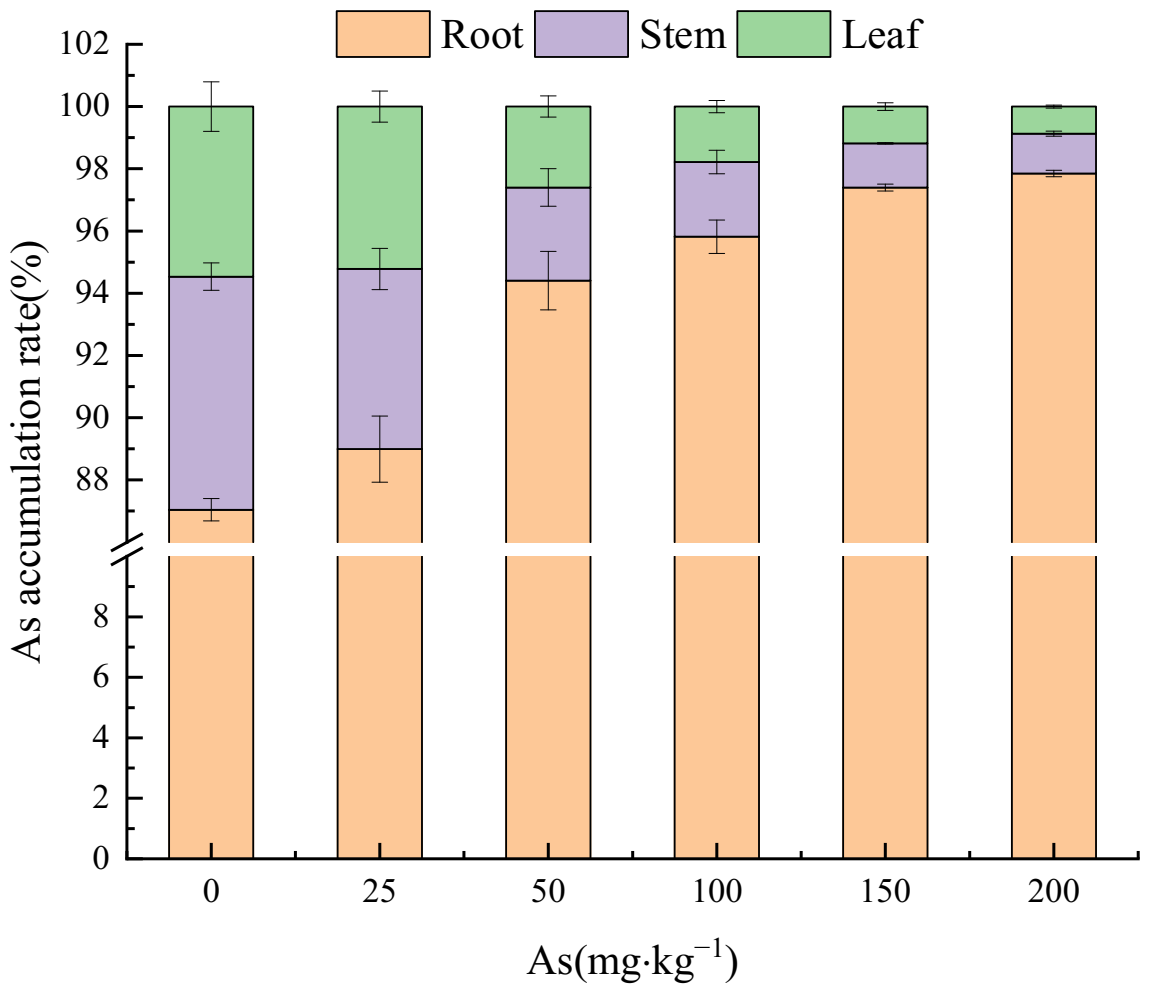
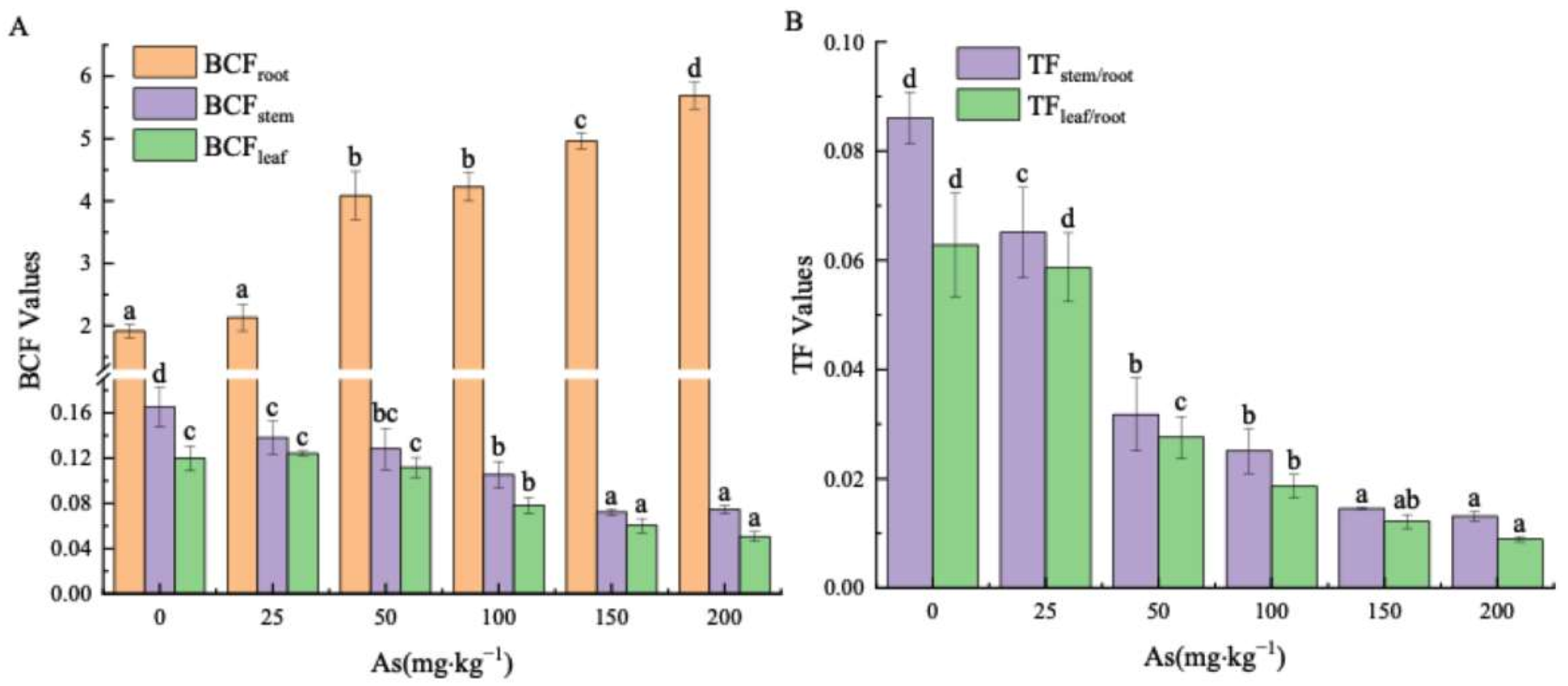
3.3. Effects of As Exposure on As Accumulation and Translocation in Plant Tissues
3.3.1. As Accumulation in Plant Tissues
3.3.2. Arsenic Transfer in the Plant Parts of the Mangrove Seedlings
4. Discussion
4.1. Effects of As Exposure on the Growth of the Seedlings
4.2. Effects of As Exposure on MDA and Antioxidant Enzymes
4.3. The Accumulation and Translocation of As in the Plant
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, P. Mangrove Ecosystem in China; Science Press: Beijing, China, 1997. (In Chinese) [Google Scholar]
- Barbier, E.B. Valuing ecosystems as productive inputs. Econ. Policy 2007, 22, 177–229. [Google Scholar] [CrossRef]
- Das, S.; Crépin, A.S. Mangroves can provide protection against wind damage during storms. Estuar. Coast. Shelf Sci. 2013, 134, 98–107. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Xu, X.R.; Sun, Y.X.; Yu, S.; Chen, Y.S.; Peng, J.X. Heavy metal and organic contaminants in mangrove ecosystems of China: A review. Environ. Sci. Pollut. Res. Int. 2014, 20, 11938–11950. [Google Scholar] [CrossRef]
- Mei, K.; Wu, G.R.; Liu, J.C.; Wu, J.J.; Hong, H.L.; Lu, H.L.; Yan, C.L. Dynamics of low-molecular-weight organic acids for the extraction and sequestration of arsenic species and heavy metals using mangrove sediments. Chemosphere 2022, 286, 131820. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.; Pryor, R.; Wilking, L. Fate and effects of anthropogenic chemicals in mangrove ecosystems: A review. Environ. Pollut. 2011, 159, 2328–2346. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.; Deobagkar, D.; Zinjarde, S. Metals in mangrove ecosystems and associated biota: A global perspective. Ecotoxicol. Environ. Saf. 2018, 153, 215–228. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.C.; Yan, C.L.; Du, D.L.; Lu, H.L. Alleviation effect of iron on cadmium phytotoxicity in mangrove Avicennia marina (Forsk.) Vierh. Chemosphere 2019, 226, 413–420. [Google Scholar]
- Niu, A.Y.; Gao, Y.F.; Xu, S.J. Effects of heavy metal pollution on the carbon content of surface sediments of mangroves in the Pearl River Estuary. Acta Ecol. Sin. 2020, 40, 8549–8558. (In Chinese) [Google Scholar]
- Li, R.; Chai, M.; Li, R.Y.; Xu, H.L.; Qiu, G.Y. Influence of introduced Sonneratia apetala on nutrients and heavy metals in intertidal sediments, South China. Environ. Sci. Pollut. Res. Int. 2017, 24, 2914–2927. [Google Scholar] [CrossRef]
- Mirlean, N.; Medeanic, S.; Garcia, F.A.; Travassos, M.P.; Baisch, P. Arsenic enrichment in shelf and coastal sediment of the Brazilian subtropics. Contin. Shelf Res. 2012, 35, 129–136. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Nath, B.; Birch, G. Accumulation of trace metals in grey mangrove Avicennia marina fine nutritive roots: The role of rhizosphere processes. Mar. Pollut. Bull. 2014, 79, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Zhang, Y.H.; Yang, Z.W. Protection and Restoration of Mangroves along the Coast of Xiamen. J. Xiamen Univ. (Nat. Sci.) 2005, 44, 1–6. (In Chinese) [Google Scholar]
- Yang, S.; Lu, X.; Liu, X.; Chen, Q.X.; Wang, J.W.; Guo, J.M. Experiment of Kandelia obovata Seedlings in Different Estuaries in Zhejiang. J. Zhejiang Sci Techol. 2021, 41, 60–66. (In Chinese) [Google Scholar]
- Zhang, F.Q.; Wang, Y.S.; Lou., Z.P.; Dong, J.D. Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 2007, 67, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Wang, Y.S.; Inyang, A.I. Eco-physiological differences between five mangrove seedlings under heavy metal stress. Mar. Pollut. Bull. 2021, 172, 112900. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tang, J.; Zheng, W.J. Growth and physiological characteristics of Kandelia obovata seedlings under Cu2+ stress. Mar. Sci. 2016, 40, 65–72. (In Chinese) [Google Scholar]
- Shen, X.X.; Li, R.L.; Chai, M.W.; Cheng, S.S.; Niu, Z.Y.; Qiu, G.Y. Interactive effects of single, binary and trinary trace metals (lead, zinc and copper) on the physiological responses of Kandelia obovata seedlings. Environ. Geochem. Health 2019, 41, 135–148. [Google Scholar] [CrossRef]
- Wu, Y.; Leng, Z.; Li, J.; Yan, C.L.; Wang, X.; Jia, H.; Chen, L.; Zhang, S.; Zheng, X.; Du, D. Sulfur mediated heavy metal biogeochemical cycles in coastal wetlands: From sediments, rhizosphere to vegetation. Front. Environ. Sci. Eng. 2022, 16, 102. [Google Scholar] [CrossRef]
- Liu, C.W.; Chen, Y.Y.; Kao, Y.H.; Maji, S.K. Bioaccumulation and translocation of arsenic in the ecosystem of the Guandu Wetland, Taiwan. Wetlands 2014, 34, 129–140. [Google Scholar] [CrossRef]
- Bakshi, M.; Ghosh, S.; Chakraborty, D.; Hazra, S.; Chaudhuri, P. Assessment of potentially toxic metal (PTM) pollution in mangrove habitats using biochemical markers: A case study on Avicennia officinalis L. in and around Sundarban, India. Mar. Pollu. Bull. 2018, 133, 157–172. [Google Scholar] [CrossRef]
- Wang, F.Z.; Wang, Y.S. Effects of Cu2+ and Pb2+ stress on soluble protein content and activities of antioxidant enzymes in Kandelia obovata seedings. Ecol. Sci. 2020, 39, 10–18. (In Chinese) [Google Scholar]
- Yadu, B.; Chandrakar, V.; Tamboli, R.; Keshavkant, S. Dimethylthiourea antagonizes oxidative responses by up-regulating expressions of pyrroline-5-carboxylate synthetase and antioxidant genes under arsenic stress. Int. J. Environ. Sci. Technol. 2019, 16, 8401–8410. [Google Scholar] [CrossRef]
- Kumar, S.; Khare, R.; Trivedi, P.K. Arsenic-responsive high-affinity rice sulphate transporter, OsSultr1;1, provides abiotic stress tolerance under limiting sulphur condition. J. Hazard. Mater. 2019, 373, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Li, H.S. Principles and Techniques of Plant Physiological Biochemical Experiment; Higher Education Press: Beijing, China, 2004. (In Chinese) [Google Scholar]
- Shaibur, M.R.; Kawai, S. Effect of arsenic on visible symptom and arsenic concertation in hydroponic Japanese mustard spinach. Environ. Exp. Bot. 2009, 67, 65–70. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Mechanisms to cope with arsenic or cadmium excess in plant. Curr. Opin. Plant Biol. 2009, 12, 364–372. [Google Scholar] [CrossRef]
- Ren, W.; Yang, G.Y.; Liu, Y.G.; Liu, X.; Li, M.Y.; Zhao, R.; Wang, Y. Effect of arsenic stress on the growth of Typha angustiflia L. in plateau wetland and its arsenic tolerance. J. Yunnan Univ. Naural Sci. Edi. 2021, 43, 164–173. (In Chinese) [Google Scholar]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutase (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Apel, K.; Hitr, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.Y.; Weng, B.S.; Zhao, S.Z.; Yan, C.L. Effects of arbuscular mycorrhizal inoculation and Cd stress on the growth and antioxidant enzyme system of Kandelia obovata. J. Xiamen Univ. (Natural Sci.) 2013, 52, 244–253. (In Chinese) [Google Scholar]
- Sawidis, T.; Breuste, J.; Mitrovic, M.; Tsigaridas, K. Trees as bioindicator of heavy metal pollution in three European cities. Environ. Pollu. 2011, 159, 3560–3570. [Google Scholar] [CrossRef]
- Rodringuez-Bocanegra, J.; Roca, N.; Febrero, A. Assessment of heavy metal tolerance in two plant species growing in experimental disturbed polluted urban soil. J. Soils Sediments 2018, 18, 2305–2317. [Google Scholar] [CrossRef]
- Chen, T.B.; Wei, C.Y.; Huang, Z.C.; Huang, Q.F.; Lu, Q.G.; Fan, Z.L. Arsenic accumulation characteristics of Arsenic hyperaccumulator Pteris Vittata L. Chin. Sci. Bull. 2002, 47, 207–210. (In Chinese) [Google Scholar]
- Zhang, J.L.; Huang, Y.; Wu, L.F.; Gong, Y.H. Liu, Y.G.; Wang, Y.; Yang, S.L. As subcellular distribution and physiological response of Typha angustifolia L. to as exposure. Ecol. Environ. Sci. 2021, 30, 1042–1050. (In Chinese) [Google Scholar]
- Wu, G.R.; Hong, H.L.; Yan, C.L. Arsenic accumulation and translocation in mangrove (Aegiceras corniculatum L.) grown in arsenic contaminated soils. Int. J. Environ. Res. Public Health 2015, 12, 7244–7253. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yu, J.; Yan, C.L.; Du, D.L.; Lu, H.L. Distribution correlations of cadmium to calcium, phosphorus, sodium and chloridion in mangrove Aegiceras corniculatum root tissues. Mar. Pollut. Bull. 2018, 126, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, J.; Du, D.L.; Liu, J.C.; Lu, H.L.; Yan, C.L. Analysis of anatomical changes and cadmium distribution in Aegiceras corniculatum (L.) Blanco roots under cadmium stress. Mar. Pollut. Bull. 2019, 149, 110536. [Google Scholar] [CrossRef] [PubMed]
- Blute, N.K.; Brabander, D.J.; Hemond, H.F.; Sutton, S.R.; Rivers, M.L. Arsenic sequestration by ferric iron plaque on cattail roots. Environ. Sci. Technol. 2004, 38, 6074–6077. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, F.B.; Liu, C.P.; Wu, W.J. The diversity and abundance of As(III) oxidizers on root iron plaque is critical for arsenic bioavailability to rice. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Wu, G.R.; Hong, H.L.; Yan, C.L. Influence of sulfur supply on thiols in Aegiceras corniculatum(L.) Blanco under As stress. J. Xiamen Univ. (Natural Sci.) 2016, 55, 55–59. (In Chinese) [Google Scholar]
- Mei, K.; Liu, J.; Fan, J.; Guo, X.; Wu, J.; Zhou, Y.; Lu, H.; Yan, C. Low-level arsenite boosts rhizospheric exudation of low-molecular-weight organic acids from mangrove seedlings (Avicennia marina): Arsenic phytoextraction, removal, and detoxification. Sci. Total Environ. 2021, 775, 145685. [Google Scholar] [CrossRef] [PubMed]
| Total As (mg kg−1) | Organic Matter (mg kg−1) | Available N (mg kg−1) | Available P (mg kg−1) | Available K (mg kg−1) | Cation Exchange Capacity (μmol kg−1) | pH |
|---|---|---|---|---|---|---|
| 20.81 | 3.08 | 98.42 | 27.43 | 617.93 | 17.57 | 7.11 |
| As (mg kg−1) | Stem High (cm) | Root Biomass (g DW) | Stem Biomass (g DW) | Leaf Biomass (g DW) | Survival Rate (%) |
|---|---|---|---|---|---|
| CK | 42.50 ± 2.58 c | 11.14 ± 1.45 c | 5.85 ± 0.35 b | 3.99 ± 0.83 c | 100 |
| 25 | 43.22 ± 2.25 c | 10.67 ± 1.81 c | 5.47 ± 0.55 b | 4.90 ± 0.83 c | 100 |
| 50 | 39.60 ± 5.16 bc | 10.39 ± 1.10 bc | 5.19 ± 0.24 b | 4.01 ± 0.53 c | 100 |
| 100 | 39.52 ± 3.14 bc | 9.27 ± 1.13 bc | 5.19 ± 0.18 b | 2.93 ± 0.37 bc | 100 |
| 150 | 35.77 ± 2.91 ab | 8.16 ± 1.63 b | 3.91 ± 0.28 a | 2.42 ± 0.56 b | 100 |
| 200 | 32.29 ± 3.09 a | 5.02 ± 0.71 a | 3.71 ± 0.36 a | 0.98 ± 0.16 a | 80 |
| Parameter | Root SOD | Root POD | Root CAT | Leaf SOD | Leaf POD | Leaf CAT |
|---|---|---|---|---|---|---|
| Root SOD | 1.000 | |||||
| Root POD | 0.806 * | 1.000 | ||||
| Root CAT | 0.851 * | 0.664 * | 1.000 | |||
| Leaf SOD | 0.898 * | 0.824 * | 0.814 * | 1.000 | ||
| Leaf POD | 0.890 * | 0.951 * | 0.760 * | 0.887 * | 1.0 | |
| Leaf CAT | 0.841 * | 0.855 * | 0.707 * | 0.899 * | 0.878 * | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, G.; Mei, K.; He, C.; Wang, S.; Jiang, L. Phytoextraction and Antioxidant Defense of Mangrove Seedling (Kandelia obovata) to Inorganic Arsenate Exposure. Water 2022, 14, 643. https://doi.org/10.3390/w14040643
Wu G, Mei K, He C, Wang S, Jiang L. Phytoextraction and Antioxidant Defense of Mangrove Seedling (Kandelia obovata) to Inorganic Arsenate Exposure. Water. 2022; 14(4):643. https://doi.org/10.3390/w14040643
Chicago/Turabian StyleWu, Guirong, Kang Mei, Caimei He, Sujuan Wang, and Liling Jiang. 2022. "Phytoextraction and Antioxidant Defense of Mangrove Seedling (Kandelia obovata) to Inorganic Arsenate Exposure" Water 14, no. 4: 643. https://doi.org/10.3390/w14040643
APA StyleWu, G., Mei, K., He, C., Wang, S., & Jiang, L. (2022). Phytoextraction and Antioxidant Defense of Mangrove Seedling (Kandelia obovata) to Inorganic Arsenate Exposure. Water, 14(4), 643. https://doi.org/10.3390/w14040643





