Dynamic Adsorption Characteristics of Phosphorus Using MBCQ
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Instruments and Materials
2.2. Preparation of an Adsorbent
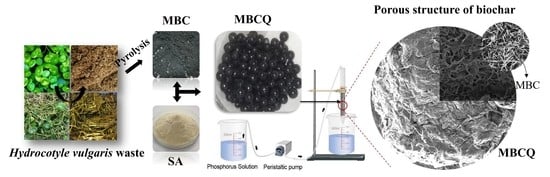
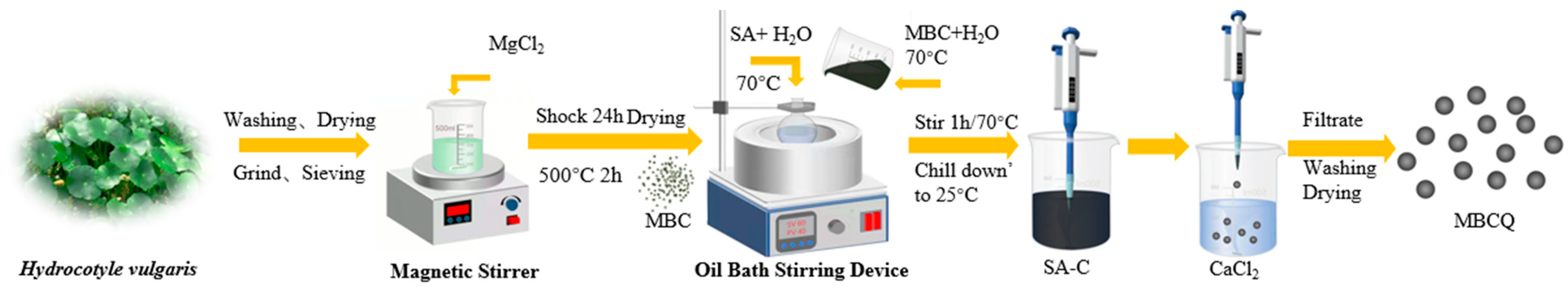
2.2.1. Preparation of MBC
2.2.2. Preparation of Biochar Particle (MBCQ)
2.3. Static Adsorption Experiment
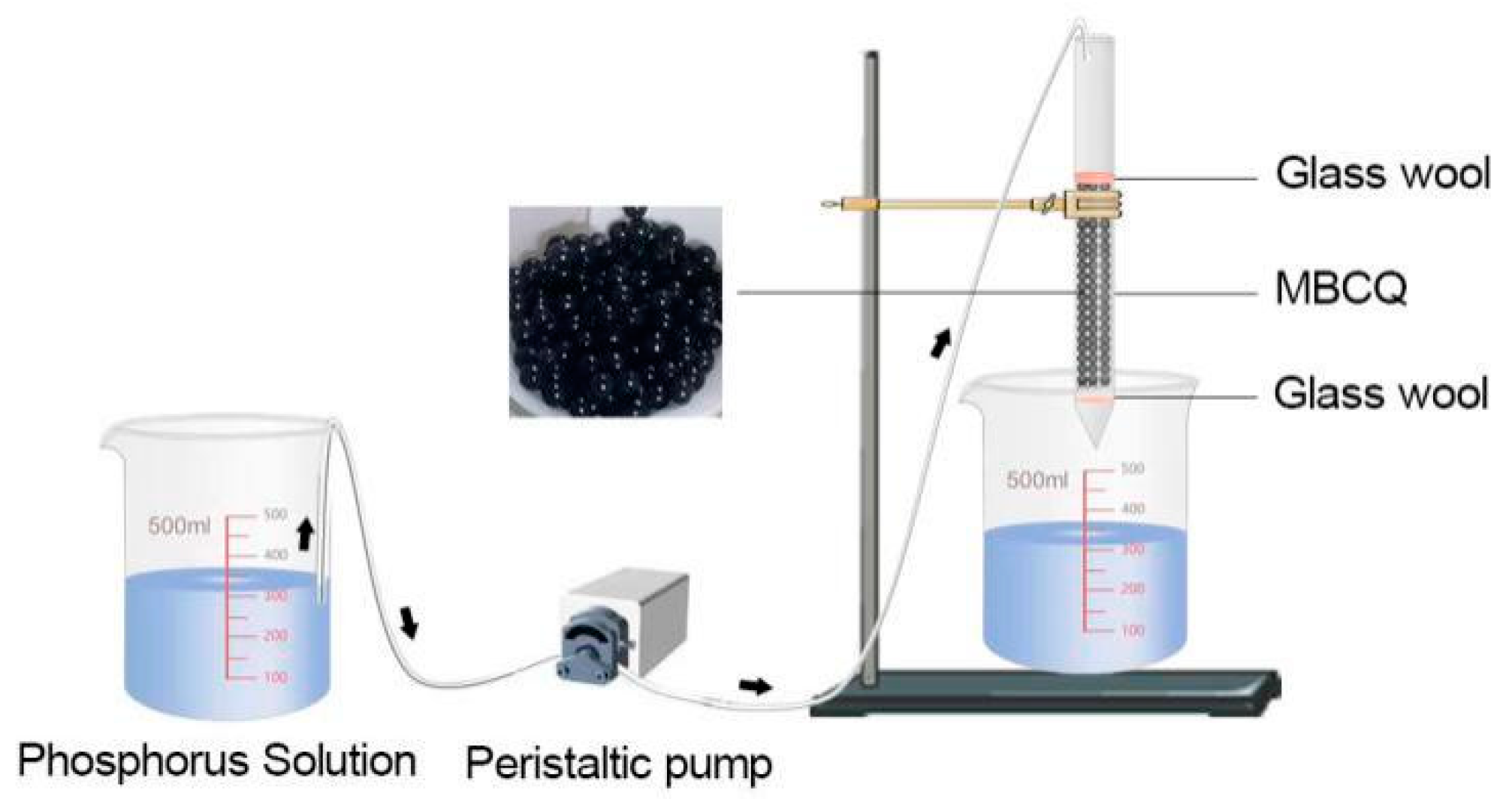
2.4. Adsorption Column Experiment
2.5. Calculation of Adsorption Column Parameters
2.6. Adsorption Model Fitting
2.6.1. Adsorption Isotherm Equation
2.6.2. The Bed Depth Service Time Model
2.6.3. The Tomas Model
2.7. Analytical Methods and Instruments
3. Results
3.1. MBC Phosphorus Removal Performance Analysis
3.2. Static Adsorption Performance of MBCQ
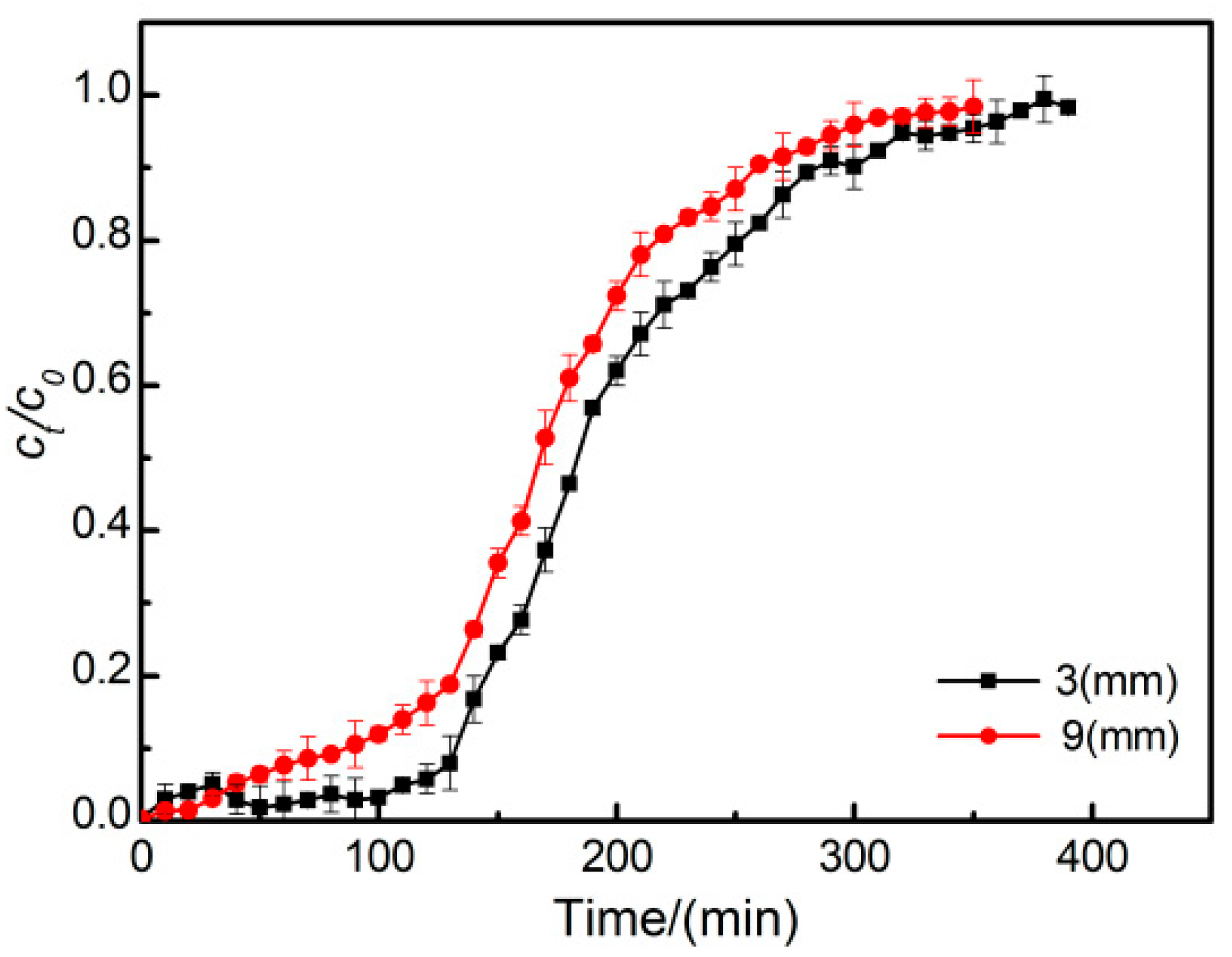
3.3. Effect of Particle Size on the Breakthrough Curve
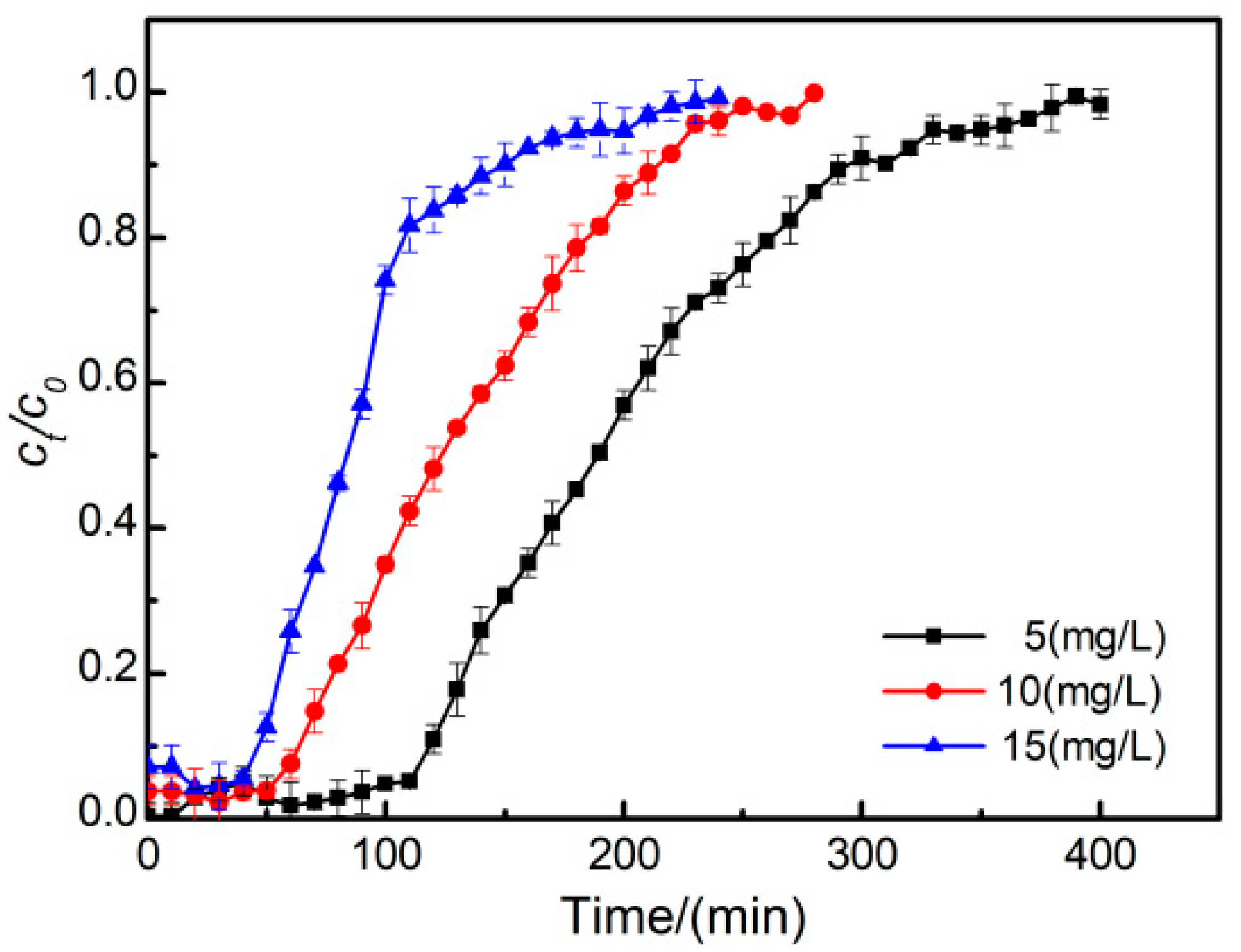
3.4. Effect of Initial Phosphate Concentration on the Penetration Curve
3.5. Effect of Flow Rate on the Breakthrough Curve
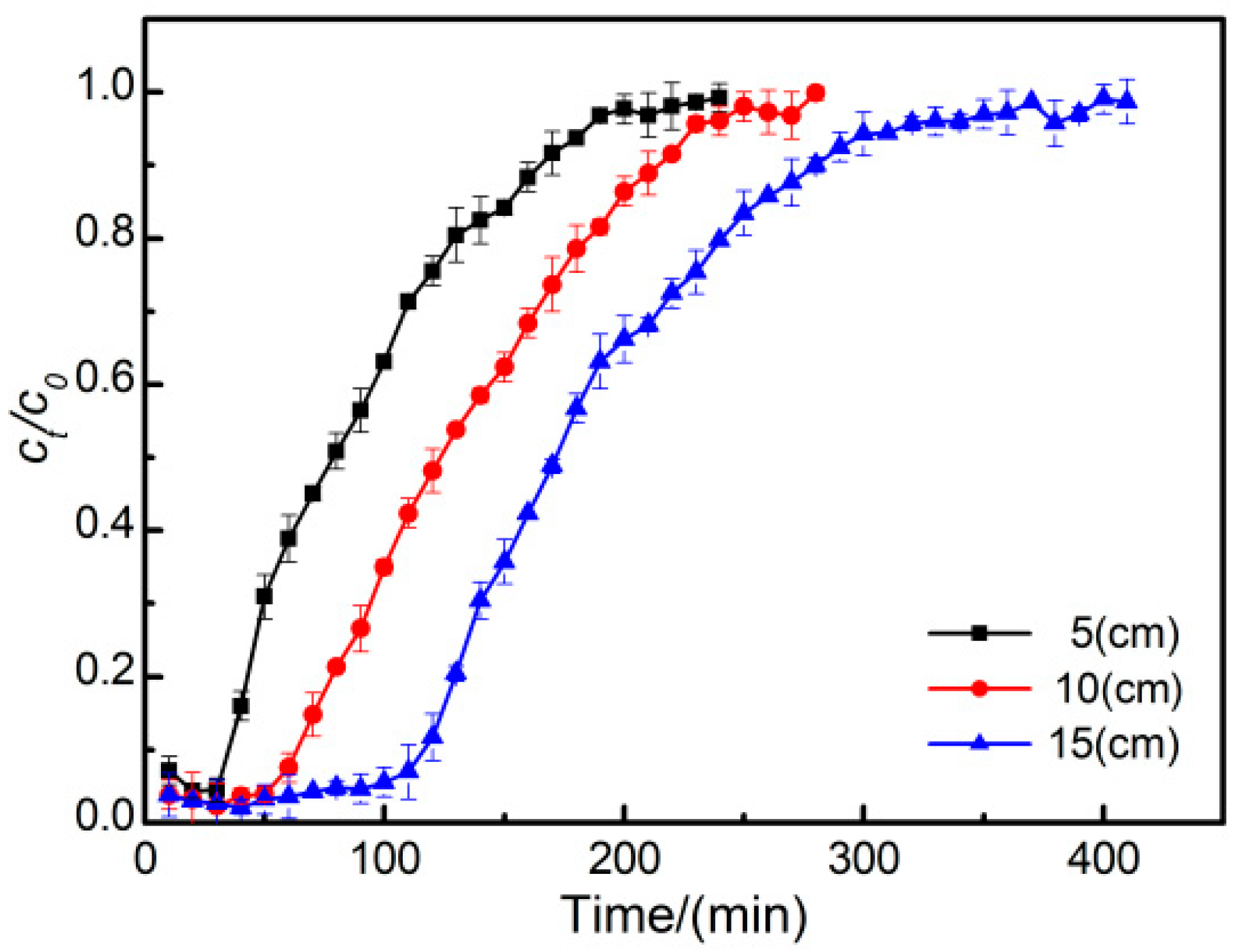
3.6. Effect of Column Height on the Breakthrough Curve
3.7. Analysis of the Influence of Different Factors on the Breakthrough Curve
3.8. Fit of the BDST Model to the Breakthrough Curve
3.9. Fitting of the Thomas Model Breakthrough Curve
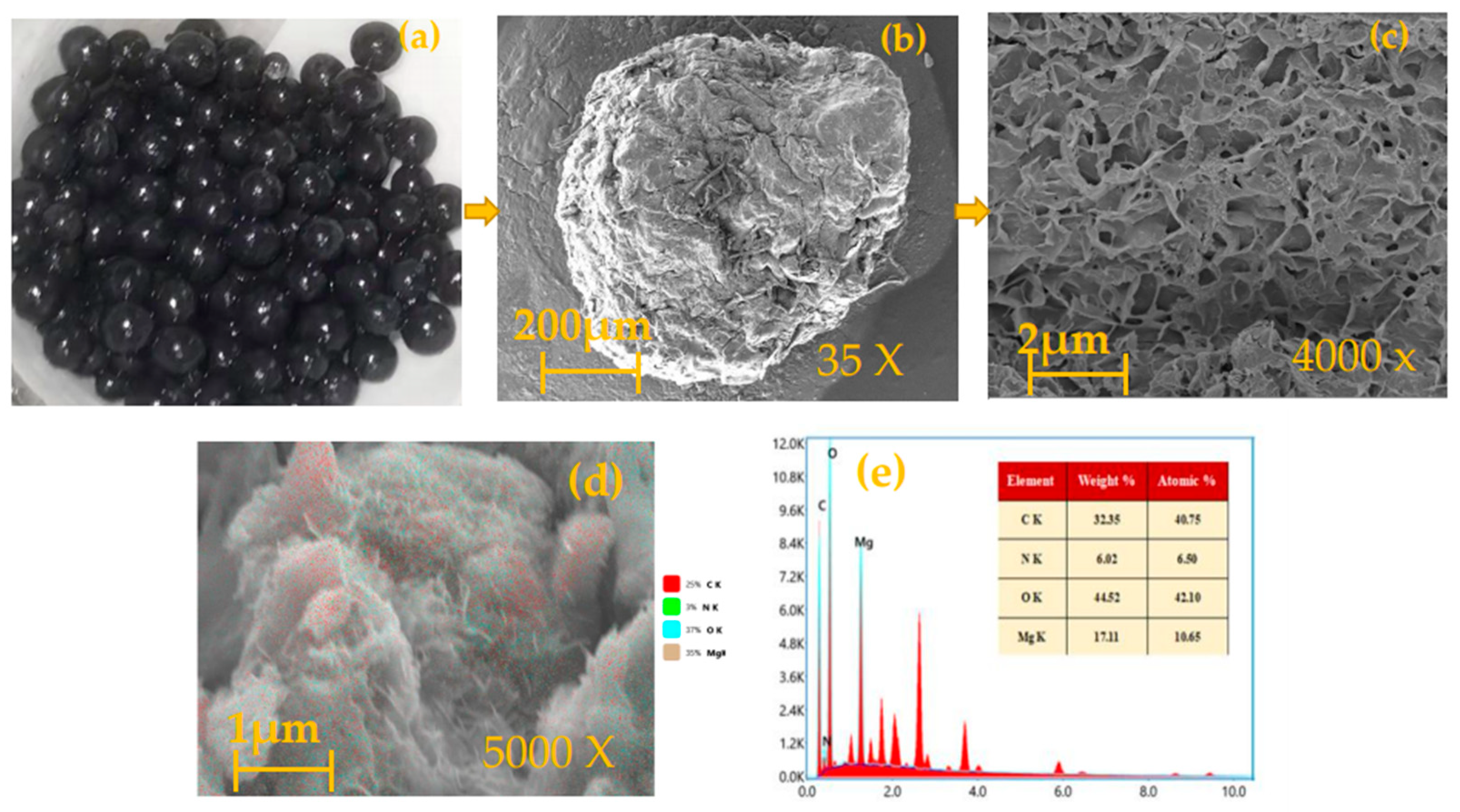
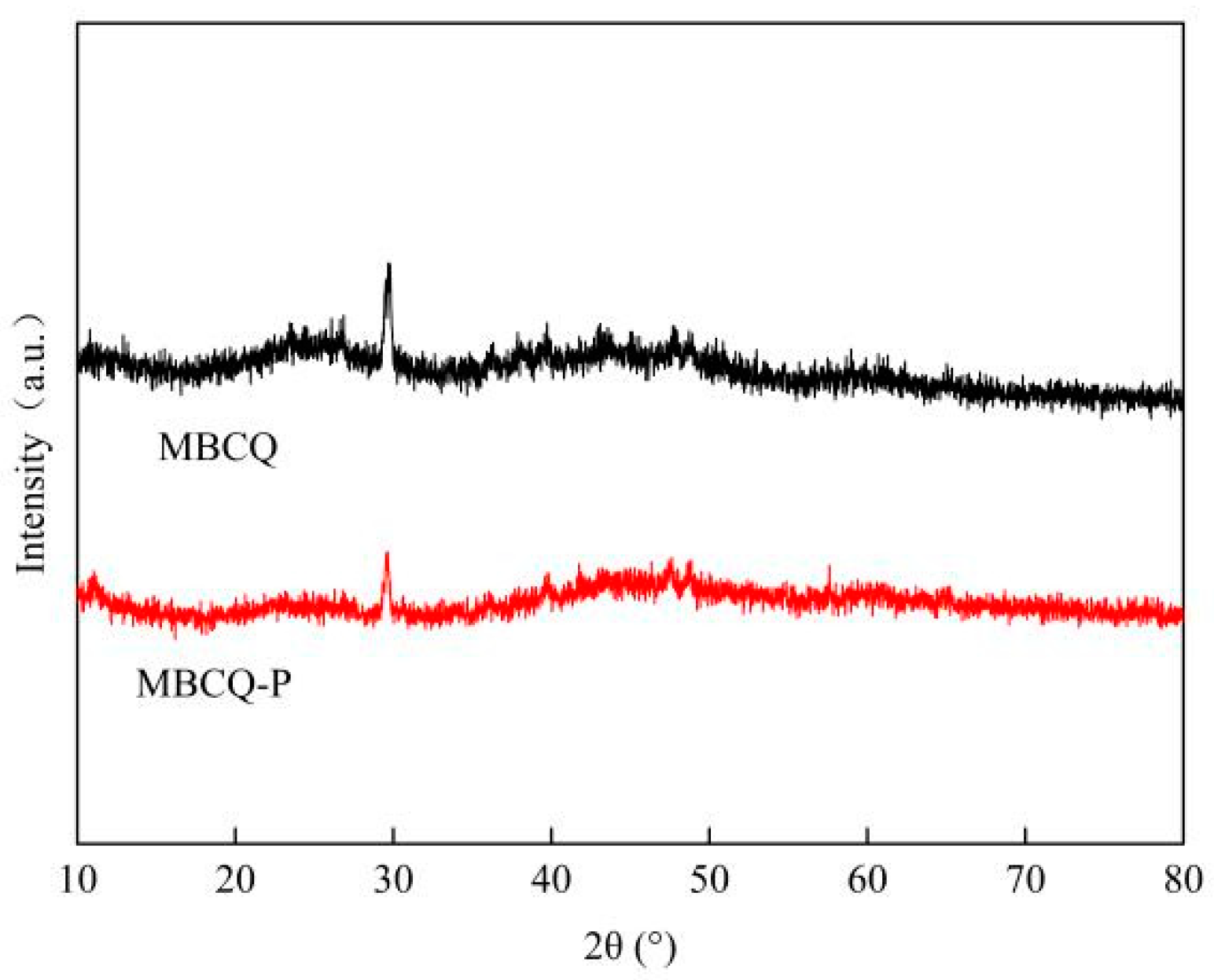
3.10. Characterization of the MBCQ
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sun, Q.; Yue, F.; Chen, J.; Wang, J.; Li, Y.; Li, X.; Bhat, M.A.; Liu, J.; Li, S. Nitrogen and Phosphorus Diffusion Fluxes: Insight from High-Resolution Technology and Hydrodynamic Modeling. Water 2021, 13, 3232. [Google Scholar] [CrossRef]
- Wang, X.; Daigger, G.; de Vries, W.; Kroeze, C.; Yang, M.; Ren, N.-Q.; Liu, J.; Butler, D. Impact hotspots of reduced nutrient discharge shift across the globe with population and dietary changes. Nat. Commun. 2019, 10, 2627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nir, O.; Sengpiel, R.; Wessling, M. Closing the cycle: Phosphorus removal and recovery from diluted effluents using acid resistive membranes. Chem. Eng. J. 2018, 346, 640–648. [Google Scholar] [CrossRef]
- Liu, R.; Hao, X.; Chen, Q.; Li, J. Research advances of Tetrasphaera in enhanced biological phosphorus removal: A review. Water Res. 2019, 166, 115003. [Google Scholar] [CrossRef]
- Bacelo, H.; Pintor, A.M.; Santos, S.C.; Boaventura, R.A.; Botelho, C.M. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 2020, 381, 122566. [Google Scholar] [CrossRef]
- Huang, Q.; Song, S.; Chen, Z.; Hu, B.; Chen, J.; Wang, X. Biochar-based materials and their applications in removal of organic contaminants from wastewater: State-of-the-art review. Biochar 2019, 1, 45–73. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, D.; Li, Y.; Pan, Q.; Wang, J.; Xue, L.; Howard, A. Phosphorus and Nitrogen Adsorption Capacities of Biochars Derived from Feedstocks at Different Pyrolysis Temperatures. Water 2019, 11, 1559. [Google Scholar] [CrossRef] [Green Version]
- Vikrant, K.; Kim, K.-H.; Ok, Y.S.; Tsang, D.C.; Tsang, Y.F.; Giri, B.S.; Singh, R.S. Engineered/designer biochar for the removal of phosphate in water and wastewater. Sci. Total Environ. 2018, 616–617, 1242–1260. [Google Scholar] [CrossRef]
- Srivatsav, P.; Bhargav, B.S.; Shanmugasundaram, V.; Arun, J.; Gopinath, K.P.; Bhatnagar, A. Biochar as an Eco-Friendly and Economical Adsorbent for the Removal of Colorants (Dyes) from Aqueous Environment: A Review. Water 2020, 12, 3561. [Google Scholar] [CrossRef]
- Tang, Q.; Shi, C.; Shi, W.; Huang, X.; Ye, Y.; Jiang, W.; Kang, J.; Liu, D.; Ren, Y.; Li, D. Preferable phosphate removal by nano-La(III) hydroxides modified mesoporous rice husk biochars: Role of the host pore structure and point of zero charge. Sci. Total Environ. 2019, 662, 511–520. [Google Scholar] [CrossRef]
- Yin, Q.; Ren, H.; Wang, R.; Zhao, Z. Evaluation of nitrate and phosphate adsorption on Al-modified biochar: Influence of Al content. Sci. Total Environ. 2018, 631–632, 895–903. [Google Scholar] [CrossRef]
- Wu, K.; Wu, C.; Jiang, X.; Xue, R.; Pan, W.; Li, W.-C.; Luo, X.; Xue, S. Remediation of arsenic-contaminated paddy field by a new iron oxidizing strain (Ochrobactrum sp.) and iron-modified biochar. J. Environ. Sci. 2022, 115, 411–421. [Google Scholar] [CrossRef]
- Deng, Y.; Li, X.; Ni, F.; Liu, Q.; Yang, Y.; Wang, M.; Ao, T.; Chen, W. Synthesis of Magnesium Modified Biochar for Removing Copper, Lead and Cadmium in Single and Binary Systems from Aqueous Solutions: Adsorption Mechanism. Water 2021, 13, 599. [Google Scholar] [CrossRef]
- Siwek, H.; Bartkowiak, A.; Włodarczyk, M. Adsorption of phosphates from aqueous solutions on alginate/goethite hydrogel composite. Water 2019, 11, 633. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Bal, M.; Behera, S.K.; Sen, T.K.; Meikap, B.C. Process Optimization Study of Zn2+ Adsorption on Biochar-Alginate Composite Adsorbent by Response Surface Methodology (RSM). Water 2019, 11, 325. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Wu, X.; Zhou, S.; Chen, Y.; Chen, M.; Chen, R. A Constructed Wetland System for Rural Household Sewage Treatment in Subtropical Regions. Water 2018, 10, 716. [Google Scholar] [CrossRef] [Green Version]
- Chunjian, Y.; Liu, R.; Li, X.; Song, Y.; Gao, H. Degradation of dissolved organic matter in effluent of municipal wastewater plant by a combined tidal and subsurface flow constructed wetland. J. Environ. Sci. 2021, 106, 171–181. [Google Scholar]
- Vymazal, J.; Zhao, Y.; Mander, U. Recent research challenges in constructed wetlands for wastewater treatment: A review. Ecol. Eng. 2021, 169, 106318. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, J.; Shang, D.; Zhang, Y.; Zhang, J.; Xie, H.; Kong, Q.; Wang, Q. Application of constructed wetlands in the PAH remediation of surface water: A review. Sci. Total Environ. 2021, 780, 146605. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, C.; Wu, H. Sustainable utilization of wetland biomass for activated carbon production: A review on recent advances in modification and activation methods. Sci. Total Environ. 2021, 790, 148214. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Tang, S.; Zhang, Y.; Zhang, J.; Li, Y.; Xiong, L. Toxicity, uptake and transport mechanisms of dual-modal polymer dots in penny grass (Hydrocotyle vulgaris L.). Environ. Pollut. 2020, 265, 114877. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liang, X.; Li, C.; Wang, L.; He, X.; Qin, R.; Li, Y.; Yu, F. Hydrocotyle vulgaris L.: A new cadmium-tolerant landscape species and its physiological responses to cadmium exposure. Environ. Sci. Pollut. Res. 2021, 28, 26045–26054. [Google Scholar] [CrossRef]
- Tang, L.; Mo, K.; Chen, Q.; Zhang, J.; Xia, J.; Lin, Y. An experimental study on potential changes in plant community evapotranspiration due to the invasion of Hydrocotyle vulgaris. J. Hydro-Environ. Res. 2020, 30, 63–70. [Google Scholar] [CrossRef]
- Boni, M.; Marzeddu, S.; Tatti, F.; Raboni, M.; Mancini, G.; Luciano, A.; Viotti, P. Experimental and Numerical Study of Biochar Fixed Bed Column for the Adsorption of Arsenic from Aqueous Solutions. Water 2021, 13, 915. [Google Scholar] [CrossRef]
- Ye, J.; Yang, M.; Ding, X.; Tan, W.; Li, G.; Fang, S.; Wang, H. Fixed-bed column dynamics of ultrasound and Na-functionalized diatomite to remove phosphate from water. Environ. Sci. Pollut. Res. 2021, 29, 12441–12449. [Google Scholar] [CrossRef]
- Shin, H.; Tiwari, D.; Kim, D.-J. Phosphate adsorption/desorption kinetics and P bioavailability of Mg-biochar from ground coffee waste. J. Water Process Eng. 2020, 37, 101484. [Google Scholar] [CrossRef]
- Frišták, V.; Pipíška, M.; Turčan, V.; Bell, S.M.; Laughinghouse, H.D.; Ďuriška, L.; Soja, G. Preparation and Characterization of Novel Magnesium Composite/Walnut Shells-Derived Biochar for As and P Sorption from Aqueous Solutions. Agriculture 2021, 11, 714. [Google Scholar] [CrossRef]
- Nardis, B.O.; Carneiro, J.S.D.S.; De Souza, I.M.G.; De Barros, R.G.; Melo, L.C.A. Phosphorus recovery using magnesium-enriched biochar and its potential use as fertilizer. Arch. Agron. Soil Sci. 2020, 68, 1017–1033. [Google Scholar] [CrossRef]
- Haddad, K.; Jellali, S.; Jeguirim, M.; Ben Hassen Trabelsi, A.; Limousy, L. Investigations on phosphorus recovery from aqueous solutions by biochars derived from magnesi-um-pretreated cypress sawdust. J. Environ. Manag. 2018, 216, 305–314. [Google Scholar] [CrossRef]
- Takaya, C.; Fletcher, L.; Singh, S.; Anyikude, K.; Ross, A. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef]
- Cui, X.; Dai, X.; Khan, K.Y.; Li, T.; Yang, X.; He, Z. Removal of phosphate from aqueous solution using magnesium-alginate/chitosan modified biochar micro-spheres derived from Thalia dealbata. Bioresour. Technol. 2016, 218, 1123–1132. [Google Scholar] [CrossRef]
- Villanueva, M.E.; del Rosario-Diez, A.M.; Gonzales, J.A.; Lazaro-Martinez, J.M.; Campo Dall’Orto, V.; Copello, G.J. Phosphorus adsorption by a modified polyampholyte-diatomaceous earth material containing im-idazole and carboxylic acid moieties: Batch and dynamic studies. New J. Chem. 2017, 41, 7667–7673. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Fu, Y.; Jiang, W.; Ye, Y.; Kang, J.; Liu, D.; Ren, Y.; Li, D.; Luo, C.; Xu, Z. Enhanced phosphate removal by zeolite loaded with Mg–Al–La ternary (hydr)oxides from aqueous solutions: Performance and mechanism. Chem. Eng. J. 2019, 357, 33–44. [Google Scholar] [CrossRef]
- Lu, S.; Huang, X.; Tang, M.; Peng, Y.; Wang, S.; Makwarimba, C.P. Synthesis of N-doped hierarchical porous carbon with excellent toluene adsorption properties and its activation mechanism. Environ. Pollut. 2021, 284, 117113. [Google Scholar] [CrossRef] [PubMed]
- Conidi, D.; Parker, W.J.; Smith, S. Effect of solids residence time on dynamic responses in chemical P removal. Water Environ. Res. 2019, 91, 250–258. [Google Scholar] [CrossRef]
- Ha, S.-H.; Younis, S.A.; Vikrant, K.; Szulejko, J.E.; Kim, K.-H. Evidence of the dominant role of particle size in controlling the dynamic adsorption breakthrough behavior of gaseous benzene in a microporous carbon bed system. Chem. Eng. J. 2022, 427, 130977. [Google Scholar] [CrossRef]
- He, Z.J.; Lu, Y.Q.; Zhu, L.; Qiao, F.M. Phosphorus Dynamic Adsorption from Wastewater by Iron-Oxide-Coated-Zeolite. Appl. Mech. Mater. 2014, 522–524, 660–664. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, N.; Wang, D.; Tian, C.; Li, M.; Feng, C. Adsorption of phosphorus based on Hangjin clay granular ceramic from aqueous solution and sewage: Fixed-bed column study. Environ. Prog. Sustain. Energy 2017, 36, 1323–1332. [Google Scholar] [CrossRef]
- Xi, H.; Li, Q.; Yang, Y.; Zhang, J.; Guo, F.; Wang, X.; Xu, S.; Ruan, S. Highly effective removal of phosphate from complex water environment with porous Zr-bentonite alginate hydrogel beads: Facile synthesis and adsorption behavior study. Appl. Clay Sci. 2020, 201, 105919. [Google Scholar] [CrossRef]
- Sankararamakrishnan, N.; Sanghi, R. Preparation and characterization of a novel xanthated chitosan. Carbohydr. Polym. 2006, 66, 160–167. [Google Scholar] [CrossRef]




| Feedstock | Modifying Agent | Specific Surface/(m2/g) | Adsorption Capacity/(mg/g) | Ref. |
|---|---|---|---|---|
| Hydrocotyle vulgar (MBC) | MgCl2·6H2O | 931.1 | 223.4 | This study |
| Ground coffee waste | MgCl2·6H2O | 36.4 | 56.0 | [28] |
| Walnut shells | MgCl2·6H2O | / | 2.6 | [29] |
| Sewage sludge | MgCl2·6H2O | / | 28.1 | [30] |
| Raw cypress sawdust | MgCl2·6H2O | / | 66.7 | [31] |
| Holm Oak | MgCl2·6H2O | / | 64.6 | [32] |
| Thalia dealbata | MgCl2-alginate | 46.2 | 46.6 | [33] |
| MBCQ Particle Size/(mm) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm | KL | R2 | KF | 1/ n | R2 | |
| 2 | 12.193 | 0.203 | 0.983 | 5.701 | 0.233 | 0.962 |
| 3 | 14.752 | 0.294 | 0.973 | 6.634 | 0.296 | 0.984 |
| 4 | 11.235 | 0.192 | 0.972 | 5.654 | 0.225 | 0.959 |
| 5 | 10.908 | 0.093 | 0.981 | 5.062 | 0.215 | 0.973 |
| 6 | 10.889 | 0.086 | 0.973 | 4.973 | 0.203 | 0.959 |
| 7 | 11.324 | 0.205 | 0.956 | 5.467 | 0.229 | 0.962 |
| 8 | 10.735 | 0.077 | 0.987 | 5.748 | 0.195 | 0.961 |
| 9 | 13.946 | 0.260 | 0.984 | 5.934 | 0.249 | 0.953 |
| 10 | 12.849 | 0.237 | 0.963 | 8.786 | 0.230 | 0.966 |
| Particle Size /(mm) | Tb /(min) | Te /(min) | Mad /(mg) | Qexp /(mg/g) | H /(cm) | Δtb /(min) | Δte /(min) | ΔMad /(mg) | Δqexp /(mg/g) | ΔH/ (cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 135 | 345 | 9.374 | 3.901 | 9.942 | 40 | 50 | 0.788 | 0.274 | −1.814 |
| 9 | 95 | 295 | 8.586 | 3.627 | 11.756 |
| Factors | x | H (cm) | tb (min) | Δx | ΔH/(cm) | Δtb/(min) | |Δtb/Δx| | |ΔH/Δx| |
|---|---|---|---|---|---|---|---|---|
| Initial concentration /(mL/L) | 5 | 9.942 | 130 | 10 | 16.110 | −85 | 8.500 | 1.611 |
| 10 | 17.861 | 65 | ||||||
| 15 | 26.052 | 45 | ||||||
| Flow rate /(mL/min) | 1.5 | 8.874 | 110 | 3 | 14.699 | −70 | 23.333 | 4.900 |
| 3 | 17.861 | 65 | ||||||
| 4.5 | 23.573 | 40 | ||||||
| Adsorption column height /(cm) | 5 | 19.062 | 35 | 10 | −3.487 | 80 | 8.000 | 0.349 |
| 10 | 17.861 | 65 | ||||||
| 15 | 15.749 | 115 | ||||||
| Particle size /(mm) | 3 | 9.942 | 130 | 6 | 1.814 | −35 | 5.833 | 0.302 |
| 9 | 11.756 | 95 |
| ct/c0 | Q /(mL/min) | C0 /(mg/L) | a | a′ | b | b′ | Projections t/(min) | Actual Measurement t/(min) | Relative Error/(%) |
|---|---|---|---|---|---|---|---|---|---|
| 0.3 | 1.5 | 10 | 8.52 | 17.05 | 6.24 | 6.24 | 176.70 | 170 | 3.94 |
| 0.3 | 4.5 | 10 | 8.52 | 5.68 | 6.24 | 6.24 | 63.06 | 55 | 14.65 |
| 0.3 | 3 | 5 | 8.52 | 17.05 | 6.24 | 3.12 | 173.58 | 165 | 5.20 |
| 0.3 | 3 | 10 | 8.52 | 8.52 | 6.24 | 6.24 | 91.47 | 85 | 7.61 |
| 0.3 | 3 | 15 | 8.52 | 4.26 | 6.24 | 12.47 | 55.10 | 65 | 15.24 |
| 0.6 | 1.5 | 10 | 9.50 | 19.01 | 23.13 | 23.13 | 213.19 | 205 | 4.00 |
| 0.6 | 4.5 | 10 | 9.50 | 6.34 | 23.13 | 23.13 | 86.48 | 80 | 8.10 |
| 0.6 | 3 | 5 | 9.50 | 19.01 | 23.13 | 11.57 | 201.63 | 200 | 0.81 |
| 0.6 | 3 | 10 | 9.50 | 9.50 | 23.13 | 23.13 | 118.16 | 125 | 5.47 |
| 0.6 | 3 | 15 | 9.50 | 4.25 | 23.13 | 46.26 | 88.78 | 95 | 6.54 |
| 0.8 | 1.5 | 10 | 12.96 | 25.93 | 31.97 | 31.97 | 291.23 | 265 | 9.90 |
| 0.8 | 4.5 | 10 | 12.96 | 8.64 | 31.97 | 31.97 | 118.39 | 100 | 18.39 |
| 0.8 | 3 | 5 | 12.96 | 25.93 | 31.97 | 15.99 | 275.25 | 260 | 5.86 |
| 0.8 | 3 | 10 | 12.96 | 12.96 | 31.97 | 31.97 | 161.60 | 170 | 4.94 |
| 0.8 | 3 | 15 | 12.96 | 6.48 | 31.97 | 63.94 | 128.76 | 110 | 17.05 |
| Project | Initial Concentration/(mg/L) | Flow Rate/(mL/min) | Column Height/(cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 1.5 | 3 | 4.5 | 5 | 10 | 15 | |
| Initial concentration/(mg/L) | — | — | — | 10 | 10 | 10 | 10 | 10 | 10 |
| Flow Rate/(mL/min) | 3 | 3 | 3 | — | — | — | 3 | 3 | 3 |
| Column height/(cm) | 10 | 10 | 10 | 10 | 10 | 10 | — | — | — |
| Kth/[mL/(min·mg)] | 1.054 | 0.853 | 0.613 | 0.695 | 0.853 | 1.232 | 0.719 | 0.853 | 0.921 |
| qcal/(mg/g) | 5.083 | 7.146 | 8.143 | 8.084 | 7.146 | 6.135 | 6.465 | 7.146 | 9.125 |
| qexp/(mg/g) | 3.901 | 5.581 | 5.972 | 7.743 | 5.581 | 4.561 | 4.337 | 5.581 | 7.947 |
| R2 | 0.988 | 0.979 | 0.974 | 0.973 | 0.979 | 0.954 | 0.963 | 0.979 | 0.958 |
| Material | Specific Surface Area/(m2/g) | Mean Pore Size/(nm) | Total Pore Volume/(cm3/g) | Microporous Pore Volume/(cm3/g) | Micropore Specific Area/(m3/g) | External Surface Area/(m2/g) |
|---|---|---|---|---|---|---|
| MBCQ | 266.423 | 7.956 | 0.614 | 0.0131 | 70.423 | 196.233 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Q.; Fu, X.; Wang, P.; Li, X.; Zheng, P. Dynamic Adsorption Characteristics of Phosphorus Using MBCQ. Water 2022, 14, 508. https://doi.org/10.3390/w14030508
Liang Q, Fu X, Wang P, Li X, Zheng P. Dynamic Adsorption Characteristics of Phosphorus Using MBCQ. Water. 2022; 14(3):508. https://doi.org/10.3390/w14030508
Chicago/Turabian StyleLiang, Qihui, Xinxi Fu, Ping Wang, Xinxian Li, and Peiyuan Zheng. 2022. "Dynamic Adsorption Characteristics of Phosphorus Using MBCQ" Water 14, no. 3: 508. https://doi.org/10.3390/w14030508
APA StyleLiang, Q., Fu, X., Wang, P., Li, X., & Zheng, P. (2022). Dynamic Adsorption Characteristics of Phosphorus Using MBCQ. Water, 14(3), 508. https://doi.org/10.3390/w14030508