1. Introduction
As a result of anthropogenic (industrial, agricultural) activities (mining, extracting, use of fertilizers) and (partly) geochemical natural processes (volcanic eruptions), pollution with heavy metals has increased in natural water bodies [
1]. Heavy metal sources from natural and anthropogenic activities are shown in
Figure 1.
Cadmium is a naturally occurring element of the heavy metal family that is present in the Earth’s crust at concentrations of approximately 0–11 mg/kg as a natural constituent of rocks [
3]. Research has estimated that soils that are not directly contaminated by cadmium contamination may contain cadmium at concentrations of 0.06–1.1 mg/kg [
3].
Cadmium pollution is known to be of great concern because of its high bioaccumulation and non-biodegrading properties [
4]. Cadmium in drinking water should not exceed the limit value of 0.003 mg/L as recommended by the World Health Organization [
5]. Moreover, from industrial, mining, and other activities, the discharged concentration should be lower than 2 mg/L [
6]. Exceeding these values poses severe health effects by causing cadmium poisoning and itai-itai disease with bone degradation, which can negatively affect blood pressure and even cause cancer [
7]. Cadmium also has a negative effect on plant development, reducing seed germination, growth and plant biomass, affecting photosynthetic activity, evapotranspiration rate, stomatal conductance, electrolyte leakage and relative water content [
8,
9].
Romania has a long history of mining and non-ferrous metallurgy [
10]. As a result, heavy metals can accumulate in many areas, contaminating water and soil. It is estimated that 18% of Romanian population is at risk of serious pollution [
11]. A study has investigated heavy metal soil contamination in 34 counties of Romania (near schools and kindergartens). Their results showed that cadmium levels in the soil were 0–0.86 mg/kg [
12]. The cadmium content of Romanian soils and water bodies, as well as of various crop plants and fungi, has been studied in recent years [
13,
14,
15,
16,
17,
18].
A survey was carried out near the Baia Mare mining and metallurgical complex, where soil samples and grapes (
Vitis vinifera L.) were collected, and their concentrations of heavy metals determined. Cadmium in soil was detected in two areas, with an average of 15.84 mg/kg Cd in the Baia Mare area and 26.84 mg/kg Cd in the Baia Sprie area. Examining the Cd content of different vine species in these areas, it was found that the roots of
Feteasca regala from Baia Mare and
Feteasca alba from Baia Sprie contained the highest levels of cadmium (7.09 ± 0.83 mg/kg and 3.07 ± 0.12 mg/kg, respectively). The cadmium content of the wine samples analyzed (0.02–0.06 mg/L Cd) exceeds the permitted limit (0.01 mg/L) [
11].
Heavy metal pollution in water, sediment and fish meat was studied in Natura 2000 site “Buhuşi-Bacău-Bereşti”. The maximum concentration for water samples approved by the World Health Organization was exceeded in all studied areas with the measured cadmium concentration, 0.0521 mg/L, exceeding 10.42 times the permitted maximum value. In the case of fish samples, the Cd concentration in gills was 0.911 mg/kg and in the muscle reached 0.522 mg/kg, however, the maximum accepted concentration in the EU is 0.05 mg/kg [
19].
In addition to the study of the cadmium content of certain plant foods and waters, it is essential to investigate the Cd removal from soil and water. Many physical and chemical methods have been used to remove heavy metals from soils and wastewaters [
20,
21,
22,
23,
24,
25,
26,
27].
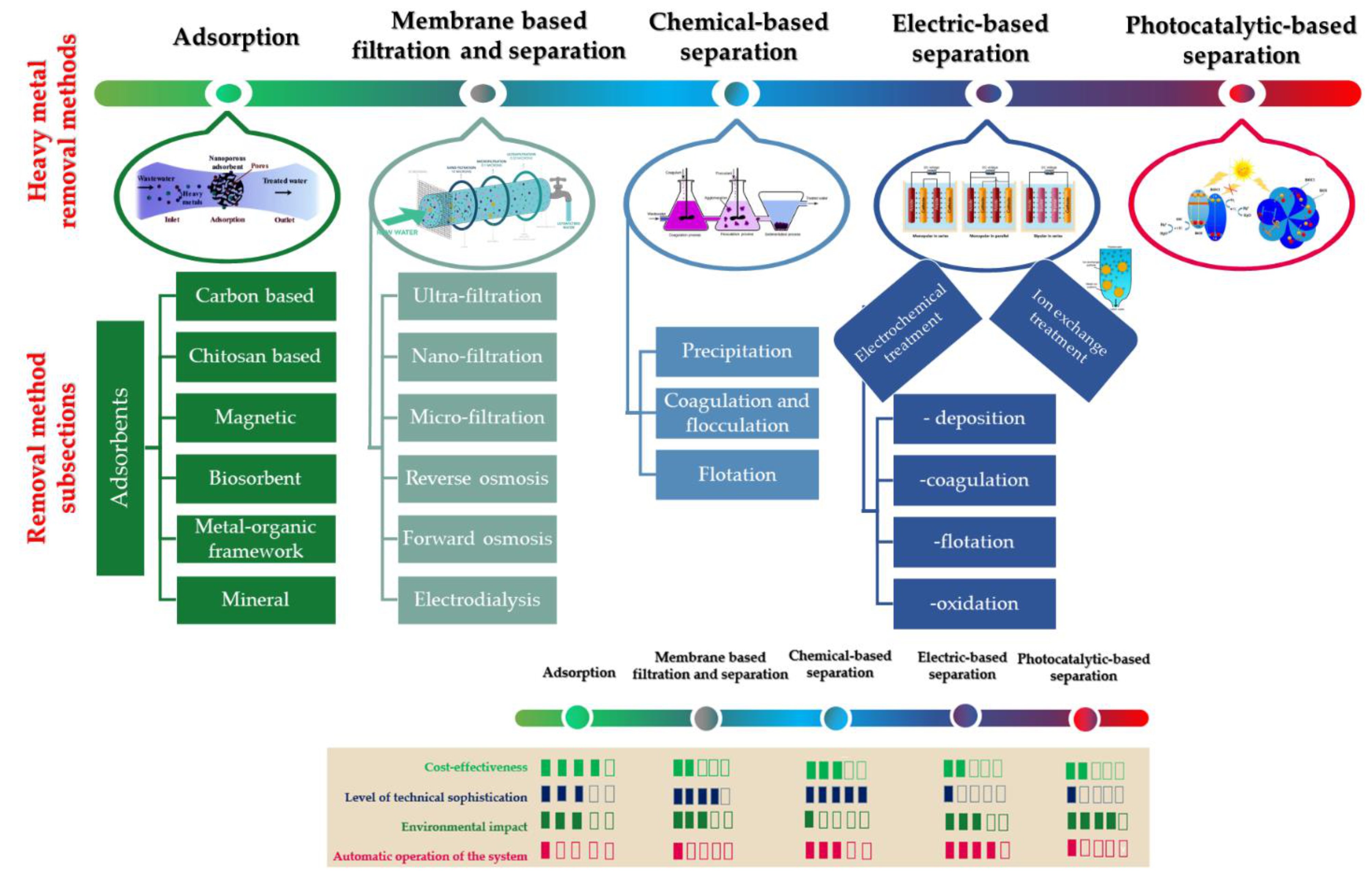
A comprehensive and critical review as its title suggests was written by Naef A.A. Qasem et al., where the authors enlisted a high range of heavy metal removal techniques from wastewaters [
28]. They classify the discussed methods into five types: adsorption-, membrane-, chemical-, electric- and photocatalytic-based remediation treatments (
Figure 2).
Adsorption technology is a widely accepted method because it is both simple to use and effective even at low concentrations [
32]. Adsorption often has advantages over other processes due to its low start-up and installation costs, high selectivity, practicality, flexibility, ease of operation, low environmental risk, resistance to toxic components and significant potential for the removal of hazardous, unsafe contaminants [
33,
34]. Up until now, different materials have been used for the removal of Cd
2+, such as brewery yeast [
35], barley husks [
36], brewed tea waste [
33], nanocomposites [
37,
38], eggshell [
39,
40], sunflower [
41], peanut shell [
42], sugarcane bagasse [
43], different clay materials [
44,
45,
46,
47,
48,
49], and minerals like montmorillonite, kaolinite, illite, zeolite, diatomite, vermiculite [
50].
Clay materials, having a particle size of less than 2 µm, have a high specific surface area. These small particles can clog filters in fixed-bed adsorption. That is why in batch method and in membrane adsorption hybrid systems they can be suitable for heavy metal removal [
51,
52]. The naturally occurring materials are mainly composed of silica, alumina, and water. Furthermore, the surface of clay minerals can contain many exchangeable cations and anions on its surface [
53], but in most cases the surface of the clay is negatively charged. The clay surface can contain Ca
2+, Mg
2+, H
+, K
+, NH
4+, Na
+, SO
4 2−, Cl
−, PO
4 3−, and NO
3−. According to Rajani Srinivasan, these ions can be easily exchanged with other ions while the mineral structure of clay is not affected [
53]. This property also contributes to being an excellent adsorbent for removing metal cations from water [
54]. Compared to other adsorbents, its advantage is that its surface is very porous, resulting in a high attractive force and many active binding sites [
55]. Hence, its binding performance is also higher [
53,
55,
56,
57].
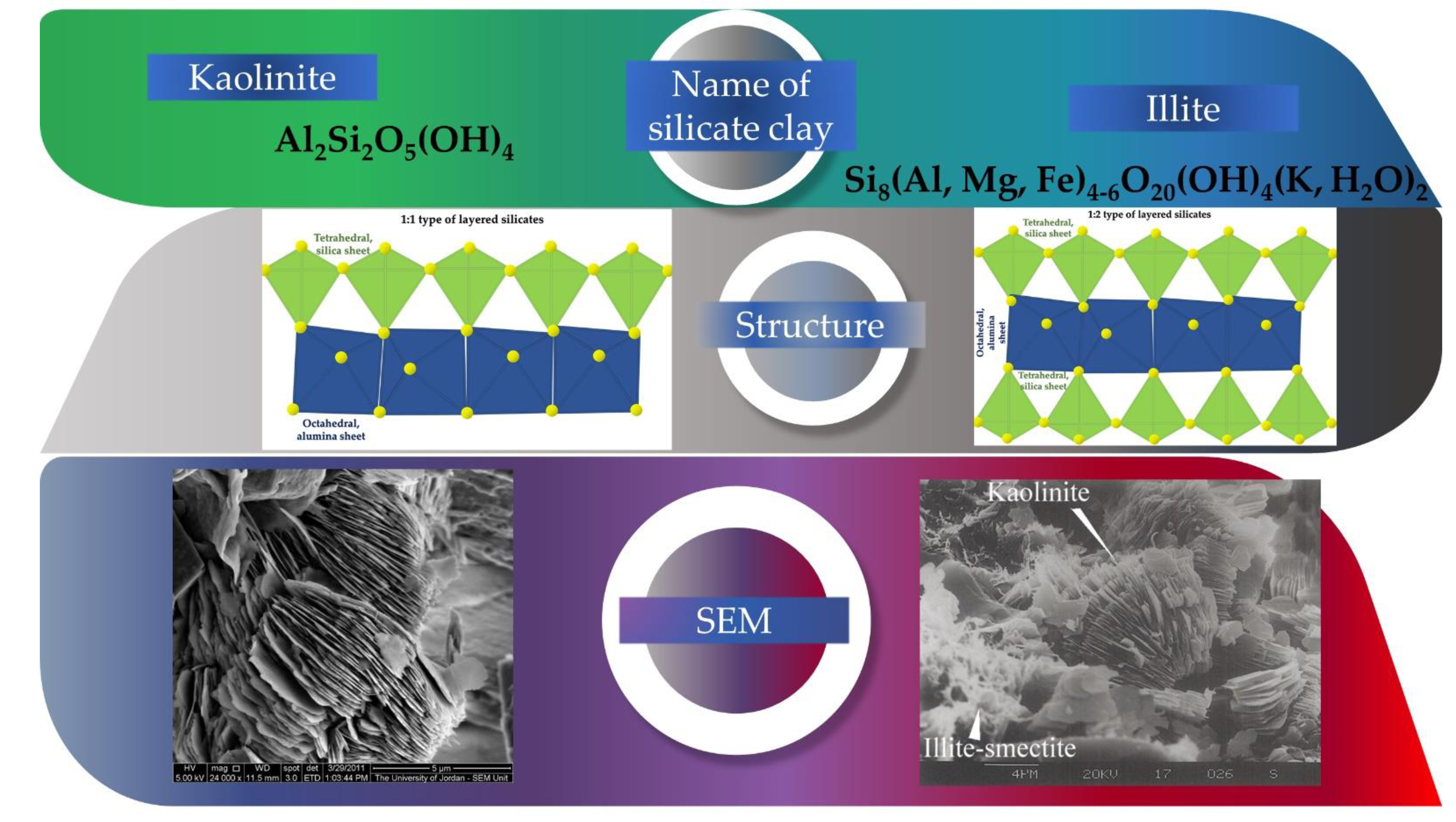
Clay minerals can be classified in various ways; however, these four types can also be divided: layer and chain silicates, sesquioxides, and other inorganic minerals. Layered silicates are the primary constituents of soils [
58]. It consists of a planar octahedral layer, structurally the octahedral layer is attached to a tetrahedral layer both above and below. It is arranged in repeating intervals between the t-o-t layers [
59]. Typical representatives include kaolinite (1:1 layered silicate) and illite (2:1 layered silicate) [
60]. A literature review of the characteristic structure and properties of these two layered silicates is given in
Section 3.1.5.
So far, many studies have investigated natural clay minerals as adsorbents because they are environmentally greener and more economical than conventional adsorbents [
61]. Therefore, the aim of this study is to characterize ASLAVITAL cosmetical clay from the Romanian Pădurea Craiului Mountain (ACC) novel adsorbent and to study the effectiveness of Cd
2+ removal (
Figure 3). As up to our knowledge ACC has not yet been studied as an adsorbent. Batch adsorption studies were carried out, where the effects of initial metal concentration and contact time were investigated as these are the most influencing parameters. Moreover, the motive of this study is to make a morpho-structural characterization of the adsorbent with the help of scanning electron microscopy (SEM), energy dispersive spectroscopy (EDX), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction analysis (XRD) and Raman measurements. We also seek to analyze the equilibrium results using linear (Langmuir, Freundlich, Tempkin and Dubinin–Raduchkevich) and non-linear (two-parameter Langmuir, Freundlich and Temkin, as well as the three-parameter Toth, Khan and Liu) isotherm models and to study the kinetics (pseudo-I and -II order) of the adsorption process and diffusion. After fitting with OriginPro 8.5 software, an error analysis was performed. In addition, we discussed the limitations of ACC novel adsorbent and compared the received adsorption capacity with clays used in previous studies.
2. Materials and Methods
2.1. Details about the Adsorbent
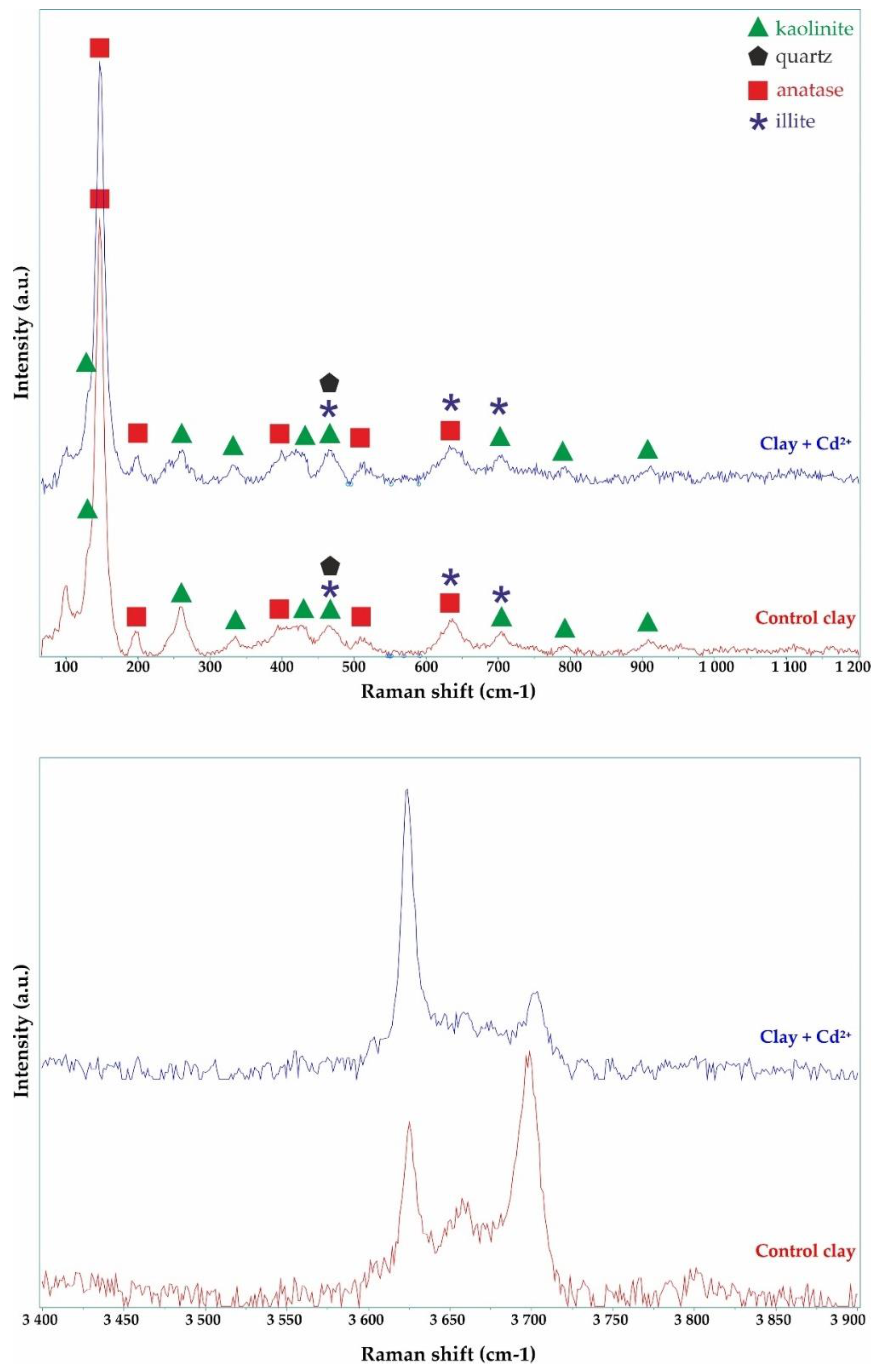
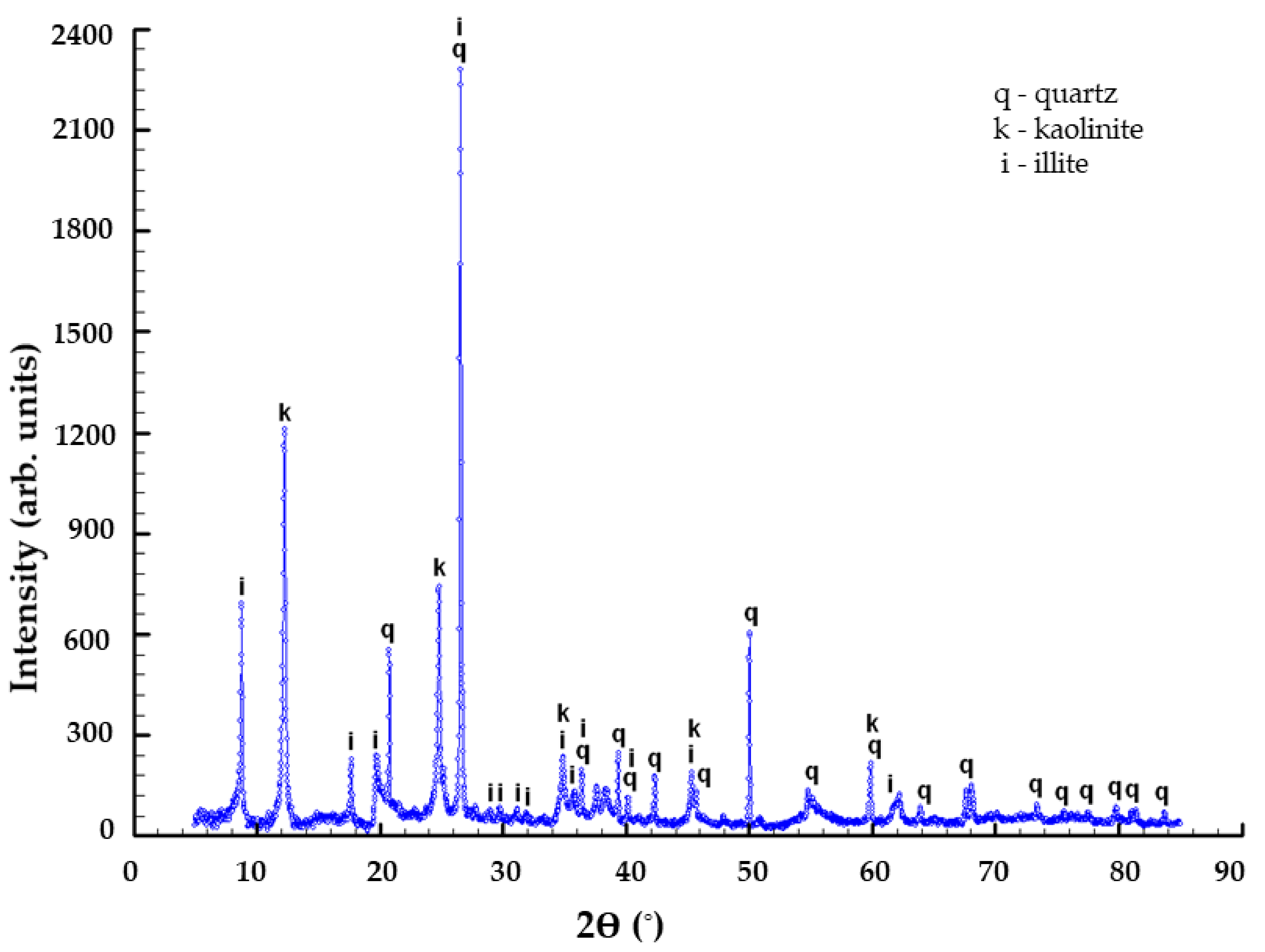
The sorbent (ACC) used in this research is a particular ASLAVITAL Diatomaceous earth (100% natural diatomaceous clay) manufactured and marketed by the Farmec S.A. It comes from the Pădurea Craiului Mountains, therefore, has a well-defined chemical and mineral composition. Moreover, it is not chemically treated. Deposits of Jurassic fire clays are well known within the Pădurea Craiului Mountains. Their compositions are dominated by kaolinite, with minor amounts of illite and quartz [
62]. In the adsorption experiments ACC was used without any physical or chemical alteration.
According to patent 118259/2003 “Composition of a clay-based product and the method of treatment that can be carried out with it”, the mineral composition of the clay is 40–60% kaolinite, 22–30% illite, 4–10% quartz, 1–4% limonite. The chemical composition of the clay is 21–33% Al2O3, 52–59% SiO2, 2–3.8%, Fe2O3, 0.4–1.3% TiO2, 0.4–0.8% CaO, 0.1–1% MgO, 1.7–4% K2O + Na2O.
2.2. Metal Solution Preparation
The synthetic wastewater with different initial concentrations was diluted from 1 g/L stock solutions of the reagent cadmium nitrate tetrahydrate [Cd(NO3)2 ∗ 4H2O] of analytical grade. The as-prepared dilutions were: 10.83 mg/L, 61.67 mg/L, 99.17 mg/L, 120.83 mg/L, 156.57 mg/L.
2.3. Adsorption Experiments
Adsorption of Cd2+ on ASLAVITAL cosmetic clay (ACC) was carried out by the batch equilibrium method. One gram of adsorbent was added to the 100 mL artificial cadmium wastewater of desired concentration in 250 mL Erlenmeyer flasks. The solutions were stirred on a rotary shaker at 300 rpm until equilibrium was reached.
The Cd2+ concentration was investigated with the help of flame atomic absorption spectrophotometer. For this measurement we used SensAA Dual GBS Scientific Equipment, Australia. The unknown solution’s Cd2+ ion concentration was determined by calibration with a standard cadmium solution in the concentration range 0–2.5 mg/L, λ = 228.8 nm.
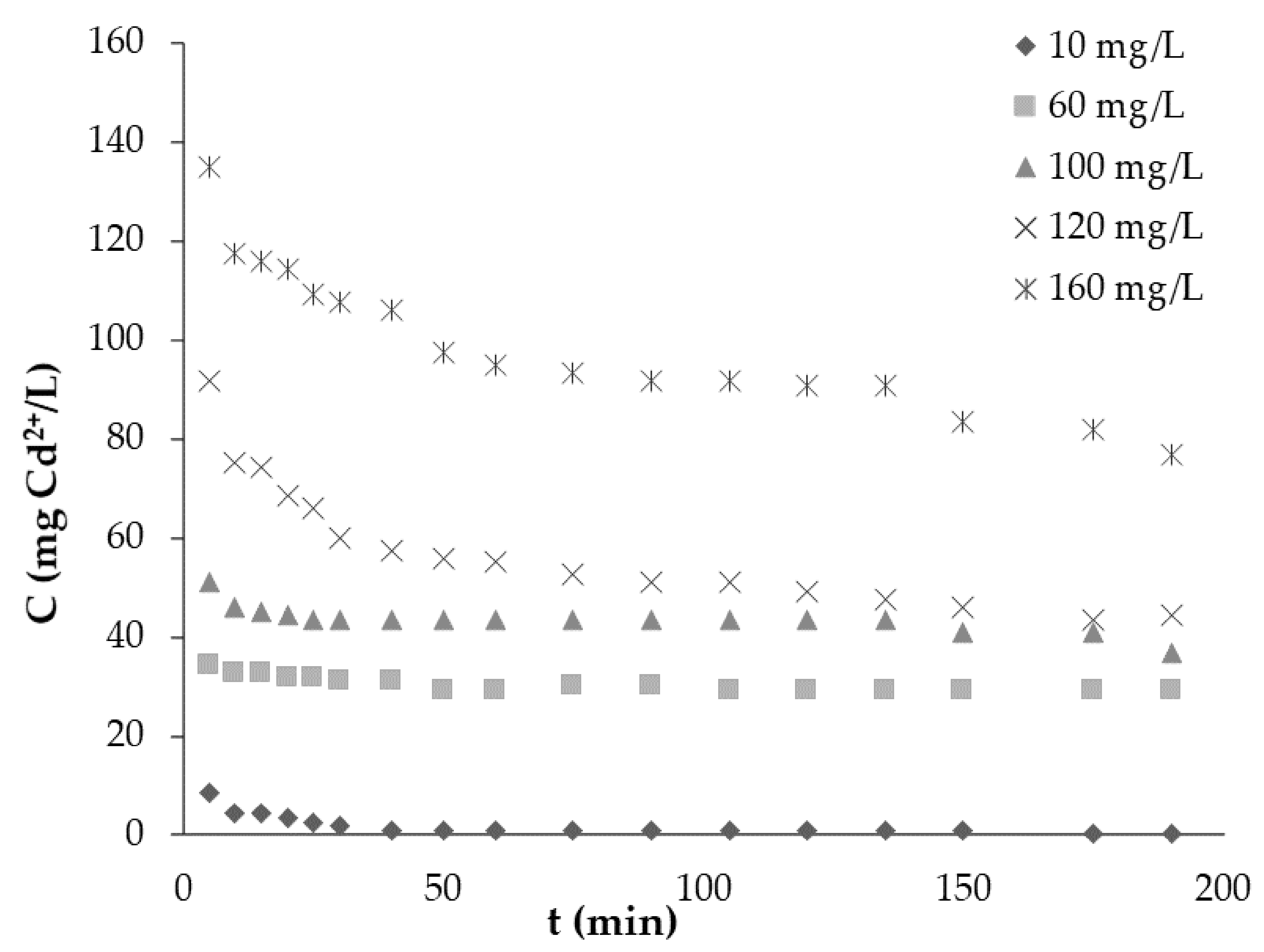
The effect of time on the adsorption of ACC and Cd2+ was determined by analyzing the residual metal ion concentration in the liquid after contact periods of 0, 5, 10, 15, 20, 30, 40, 50, 60, 75, 90, 105, 120, 135, 150, 175 and 190 min.
The effect of initial Cd2+ concentration was tested by varying the Cd2+ concentration in the range of 10–160 mg/L using a magnetic stirrer at room temperature (t = 20 °C) and fixed pH of 7.
From the adsorption equilibrium data, the linearized Langmuir, Freundlich, Tempkin and Dubinin–Radushkevich models were determined (
Figure 4).
Moreover, we studied the adsorption kinetic and diffusion models (
Table 1).
Statistical biases can arise when the isothermal equations are linearized, as the linear fit depends on the method chosen, the data available and the errors of the experiment performed. During the evaluation of experimental data, the linear transformation changes the distribution of the error (either in the positive or negative direction). Data overestimation, data clumping or overweighing can occur during the transformation if the system is sensitive to extreme (too high or too low) experimental values. Often, regardless of the resulting high linear regression coefficient (R
2) values, the model is not representative of the adsorption experimental behavior [
66,
67]. To avoid these limitations, the development of computer programs has made modelling and error analysis possible. Non-linear regression analyses of the two-parameter Langmuir, Freundlich and Temkin as well as the three-parameter Toth [
68], Khan [
65] and Liu [
69] isotherm models were analyzed using OriginPro 8.5 software. We also determined the linear regression coefficient (R
2), chi-square error (χ
2), root mean square error (RMSE), and hybrid fractional error (HYBRID). The equations for these error analyses are given below [
70,
71,
72]:
where n—the number of experiments performed; q
e,exp. (mg/g)—the value of the maximum amount of substance bound in the equilibrium obtained in practice; q
e,calc. (mg/g)—the calculated value of quantity equilibrium; q
e,calc. (mg/g)—average of the calculated quantity equilibrium; p (polynomial model)—the number of parameters included in the isotherm models tested.
2.4. Instrumentation, Analytical Methods
The SEM, EDX, FTIR and XRD studies were carried out at National Institute for Research and Development of Isotopic and Molecular Technologies, INCDTIM Cluj-Napoca, Romania. Raman microspectroscopic measurements were carried out at the Research and Industrial Relations Center (RIRC) at the Faculty of Science, Eötvös Loránd University, Budapest.
2.4.1. SEM and EDX
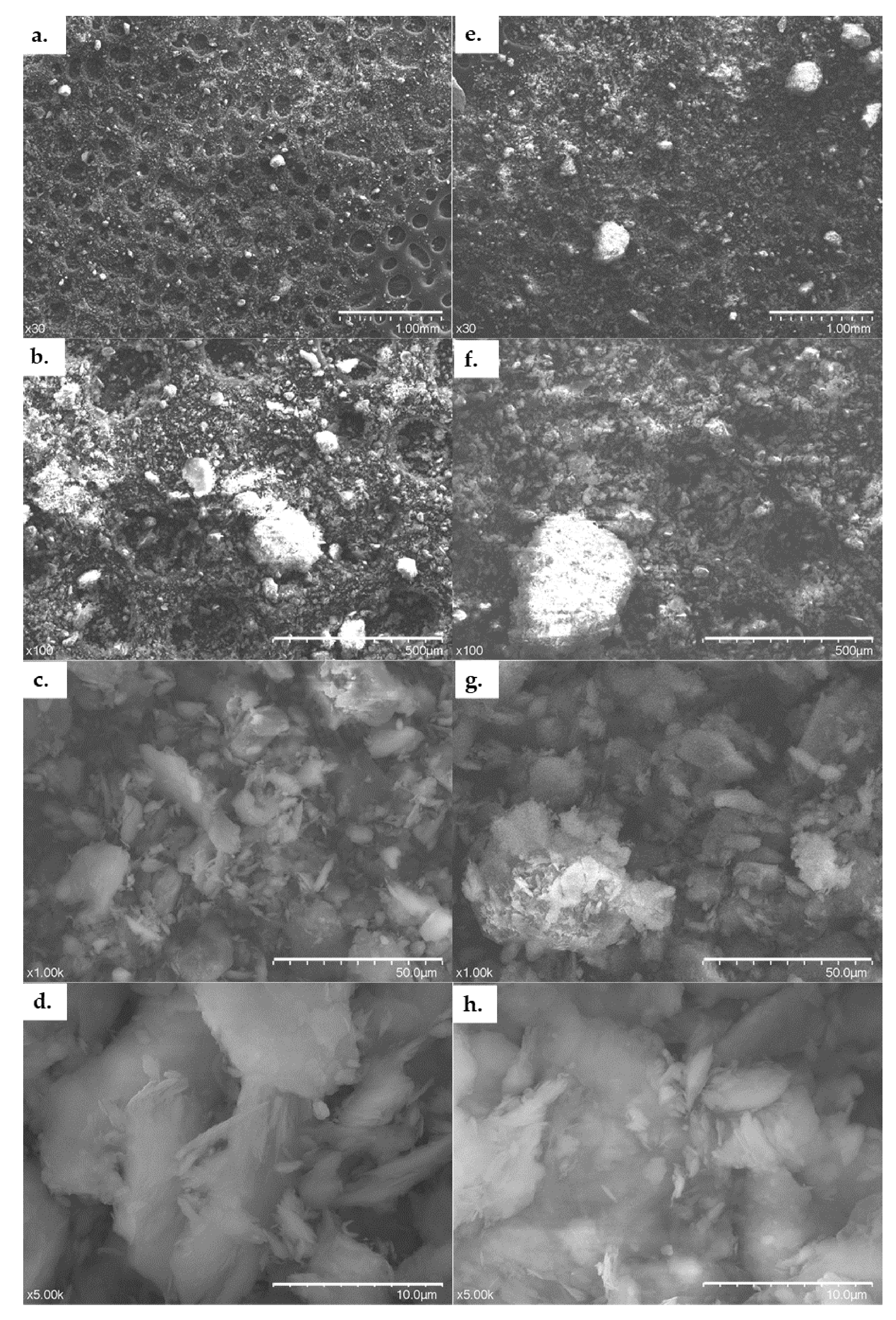
The texture and morphology of ACC before and after Cd2+ adsorption treatment were studied using Scanning electron microscopy (JEOL(USA)JSM5510 LV SEM), while the elemental composition determination was carried out using Energy dispersive spectroscopy.
2.4.2. FTIR
The functional groups of clay were characterized by the FTIR model before and after Cd2+ adsorption, with the help of JASCO 615FTIR at 500–4000 cm–1 wavelength.
2.4.3. XRD
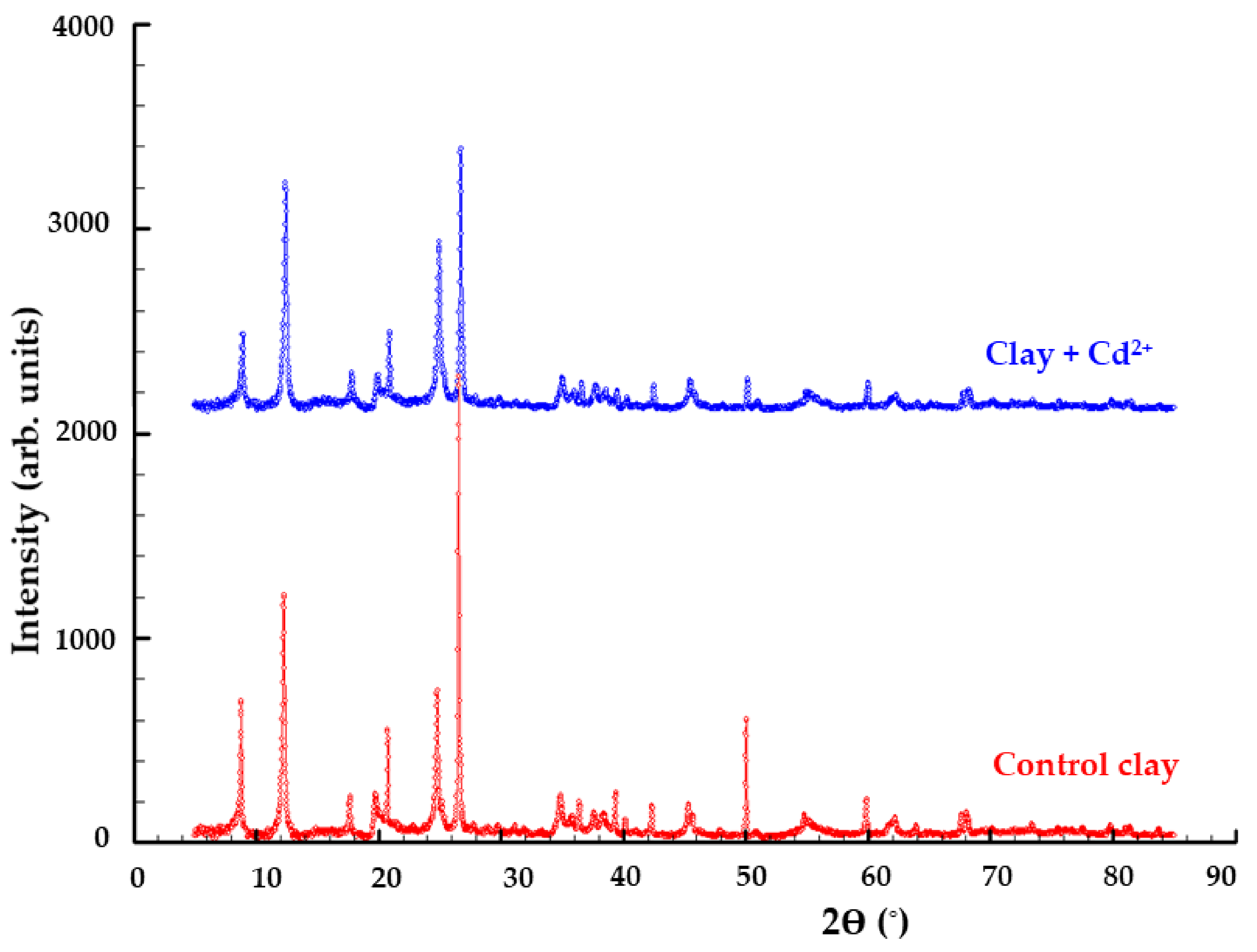
The X-ray powder diffraction patterns were obtained with a Bruker D8 Advance diffractometer using CuKα1 monochromatic radiation (λ = 1.5405980Å) obtained with a germanium (1:1:1) monochromator. The diffractometer is equipped with a LINXEYE detector and X-ray tube operates at 40 kV and 40 mA. DIFFRAC plus XRD Commander Program was used for data acquisition employing a scan rate of 0.05°/s in the angular domain 2θ = 5–85°.
2.4.4. Raman Microspectroscopy
A confocal HORIBA LabRAM HR800 spectrometer with Nd:YAG laser (λ = 532 nm) excitation and 600 grooves/mm optical grating was used. The laser was focused with an Olympus 100× objective (numerical aperture = 0.9) on the sample surface, where the laser power was 25 mW and the laser spot diameter was ~1.5 μm.
4. Summary and Conclusions
In the present work, ACC—ASLAVITAL commercial cosmetic clay—was morphologically and elementally analyzed; moreover, it was successfully used to remove Cd2+ from water.
It was observed that the initial concentration of contaminants decreased with time, as all investigated concentrations exhibited a rapid decrease rate in the first part of the experiment. Then the adsorption rates tended to be flat. Quantity in equilibrium increased from 1–8 mg/g with the increase in initial Cd2+ concentration. Our hypothesis proved that ACC is an excellent adsorbent as 99% Cd2+ removal efficiency was reached within 190 min.
Adsorption equilibrium results were further analyzed with linear (Langmuir, Freundlich, Temkin, Dubinin–Radushkevich) and non-linear (Langmuir, Freundlich, Temkin, Toth, Khan, Liu) isotherm models with OriginPro 8.5 software. The best fit in linear form was obtained for the Langmuir. II model, where R2 = 0.954, while the RL values ranged between 0.037–0.027. The B-Temkin constant was smaller than 20 kJ/mol and the E-Energy was smaller than 8 kJ/mol. Results indicated that physical bonds form during the favorable adsorption. For the non-linear fits, the Liu model proved to be the best R2 = 0.965, χ2 = 1.101. Moreover, the investigations regarding the evaluation of linear regression coefficient values showed that the adsorption system had a higher linear regression coefficient (R2 = 0.988–0.999) value in the case of the pseudo-II-order model. The values of qe(cal) also showed a better fit with the model since they were almost identical with the pseudo-II-order qe(exp) values, (the differences ranged 0.03–0.34).
With the use of wide range of morpho-structural approaches, we studied the structure, texture, morphology and composition of the adsorbent. The morpho-structural investigations revealed that the clay mainly consists of kaolinite and illite in most considerable amounts. The elemental composition of the ACC contained Ca (20.65 ± 0.08%), Si (12.7 ± 1.9%), Al (8.3 ± 0.3%), Ti (0.34 ± 0.01%) and Fe (4.83 ± 0.02%). Using SEM investigations, it was observed that after adsorption, the surface is smoother, and some aggregates appeared on the clay surface.
The presence of clay-bound Cd2+ adsorbate was confirmed by analyzing the elemental contents (EDX) on the surface alongside spectral analysis (FTIR, Raman) and XRD. After adsorption, Wt(%) = 0.2 ± 0.01 Cd2+ was detected in the sample.