Abstract
Biological activated carbon (BAC) biofilter coupling ultrafiltration (UF) is a promising process for the treatment of river water contaminated by pharmaceutical and personal care products (PPCPs). However, the pilot-scale study should be conducted to reveal the long-term removal performance and the respective contributions of BAC and UF. In this study, a BAC-UF system with treatment capacity of 0.16 m3 h−1 was operated for 130 days. The water quality was analyzed in terms of CODMn, UV254, NH4+-N, and PPCPs. The results showed that both BAC and UF were related to the removal of organic matter (CODMn and UV254), achieving the removals of 56.00% and 55.25%, respectively. Similarly, BAC and UF were both relevant to the removal effects of ammonia nitrogen, nitrite, and nitrate. Moreover, the BAC-UF process was featured with a high efficiency in the removal of PPCPs, and the average removal of total PPCPs reached 47.84%, especially anhydroerythromycin, sulfachloropyridazine, sulfadiazine, trimethoprim, and caffeine. Besides, it was found that the BAC unit played a key role in PPCPs removal and the UF unit also degraded them by the biomass on UF membranes. Therefore, this study proved the removal performance of BAC-UF for treating popular pollutants from river water, and the BAC-UF process in this work can be considered as a feasible method of producing clean drinking water.
1. Introduction
Over the last few decades, the risks of pharmaceutical and personal care products (PPCPs) in the aquatic environment have become a worldwide environmental issue [1,2]. The PPCPs catalog includes a variety of chemicals, such as human and veterinary drugs, fragrances, and disinfectants used in personal care products (e.g., soaps, lotions, sunscreens, and body cleaning products), and household chemicals [3,4]. These PPCPs usually appear in waters at trace concentrations, ranging from several ng L−1 to thousands of μg L−1 [5]. Since discharge of PPCPs degrades water quality, the contaminated water cannot be directly used for potable water and industrial applications [6,7,8]. However, the concentrations and diversities complicate the associated detection and even create challenges for water purification processes. Generally, many PPCPs flow through conventional water treatment processes (the coagulation–sedimentation–sand filter) with little degradation due to the PPCPs with persistency or/and the continuous introduction [9].
Ultrafiltration (UF) as an emerging alternative technology to conventional water treatment processes has been widely used to remove pollutants such as particles, colloids, bacteria, and viruses, thus reducing the risk of water-borne diseases [10]. Size exclusion is considered the primary removal mechanism for the UF. However, in the case of the PPCPs with a small molecular weight (typically < 600 Da), UF membranes also cannot effectively reject these PPCPs, but nanofiltration (NF) and reverse osmosis are able to remove these PPCPs based on the thin-film composite [11,12]. Nevertheless, they play an unnecessary desalination role and are operated with a much higher energy consumption than UF [13]. The combined process with UF may be another promising choice as an alternative to nanofiltration for removing PPCPs in rural areas.
The oxidation process and the adsorption process have been applied to dealing with the risk of PPCPs pollution [14]. The oxidation method exhibited a fast reaction speed and high removal efficiency [15,16]. Borikar et al. [17] investigated the pilot-scale advanced oxidation processes (AOPs) for removing various PPCPs. The results showed the superior removal rates of over 95% involving carbamazepine, fluoxetine, naproxen, gemfibrozil, and ibuprofen. Yang et al. [14] found that UV/chlorine showed a superior removal performance of PPCPs, but disinfection byproducts (DBPs) were formed after chlorination. Actually, the adsorption using powder activated carbon was generally carried out for short-term emergency treatment when PPCPs suddenly increased [18]. Biological activated carbon (BAC) combined the adsorption and biologic degradation and consumed low power energy and chemicals without concern of DBPs production and with no need of frequent updates for activated carbon media. BAC sandwiching slow sand filters can remove more than 93.2% of diethyltoluamide, paracetamol, caffeine, and triclosan [19]. To further reveal the contribution of adsorption and biotransformation in PPCPs removal, Paredes et al. [20] compared BAC biofilters with biological activity and that without biological activity. The biotransformation was critically important to improve the degradation of PPCPs and extend the lifespan of the BAC media. In the BAC biofilters, the biotransformation and adsorption both contributed to the PPCPs removal. The activated carbon adsorbed PPCPs to the surface and interior, where microorganisms were suitable for growth. Under the long-term effect of high-concentration PPCPs, the dominant microorganisms in the biofilter were selected to survive. These microorganisms mostly transformed PPCPs into many segments and even directly mineralized them to CO2 [3,14]. However, the BAC biofilters were colonized by invertebrates and thereby caused microbial leakage out from the conventional purification process, which decreased the microbiological and chemical safety of drinking water quality [21]. Studies have shown that the number of bacteria will be increased significantly along with fine activated carbon particles in the effluent of the BAC process [22]. Besides, these particles can protect bacteria against disinfection [23]. During practical operation, the surviving invertebrates including Rotifers, Copepods, Cladocerans, Oligochaetes, and Nematodes threaten human health as pathogen hosts [24]. As for the operation of BAC biofilters, it needs assistance from other processes to build a barrier for the invertebrates and fine BAC particles.
BAC biofilter coupling UF is a promising process for the treatment of river water contaminated by PPCPs. The BAC biofilter can remove the PPCPs, and then the following UF can reject microorganisms and particles flowing out from the biofilter to ensure the quality of drinking water. The above coupling process makes up for the defects concerning respective operations of the BAC biofilter and UF. However, the current BAC-UF system research focuses on the removal performance with the lab scale. Shanmuganathan et al. [25] confirmed the microfiltration–BAC hybrid system for wastewater reclamation, achieving removal of 45–80% hydrophobic organics and 50–80% hydrophilic organics. The removal of various PPCPs (e.g., diclofenac, trimethoprim, caffeine) ranged from 33% to 92%. Snyder et al. [26] investigated the role of pilot-scale membranes and activated carbon in the advanced treatment of municipal wastewater effluents. Additionally, BAC improved the threshold flux values to a great extent for the UF membranes by reducing the reversible fouling resistance [27]. Although many pilot-scale setups were used to treat secondary wastewater effluent for water reclamation, this type of raw water quality was different from the river water, causing the different potential of biofilm growing. As far as we investigated, the lack of enough attention to long-term pilot study exists. Therefore, in this study, a BAC-UF system was carried out for several months with pilot scale to access the long-term removal performances and the respective contributions of BAC and UF. Besides, the water quality was analyzed from the aspects of CODMn, NH4+-N, and PPCPs.
2. Materials and Methods
2.1. Characteristics of Raw Water
The experimental study was carried out in a rural area. The raw water was withdrawn from a natural river (Foshan, China), which exhibited the typical characteristics of high-level ammonia nitrogen (NH4+-N, mg·L−1), chemical oxygen demand (CODMn, mg·L−1), and PPCP (mg·L−1) contamination. In addition, the quality of raw water is summarized in Table 1.

Table 1.
Raw water quality parameters of river water (significant value, 0.05; sample size (n), 44).
2.2. Pilot-Scale System
The experiment was performed in the pilot scale, which consisted of a BAC column and a gravity-driven UF system that was operated automatically by a programmable logic controller (PLC). A schematic diagram of the treatment process is shown in Figure 1. The BAC column with 200 mm in effective diameter and 3 m in total effective height, contained 1.6 m of granular activated carbon particles (1.7–4.0 mm, prepared from coconut shell), and 0.3 m of cobble supporting layer. Apart from that, the commercial hollow-fiber modules of the polyvinyl chloride (PVC) UF membrane were provided by Litree Purifying Technology Company (Suzhou, China), and they contained membrane fibers with an effective area of 10 m2 and a molecular weight cutoff of 100,000 Da. Furthermore, all pipes for water flow were made of PVC in the pilot-scale system.
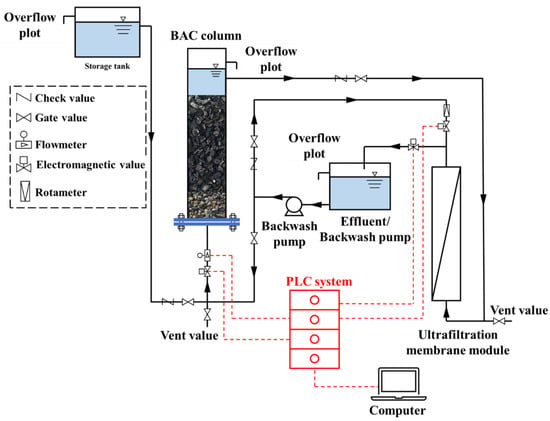
Figure 1.
Schematic diagram of the experimental set-up.
2.3. Experimental Protocols
The natural river water was continuously pumped from a nearby river to a storage tank (Figure 1). The BAC system was fed using the raw water in the storage tank, while the BAC column was operated in an up-flow mode, which maintained 25% expansion of the carbon bed using the flow rate of m3 h−1. In order to reduce the clogging of BAC filter media, a backwash procedure was followed every 7 days, and the details were shown below: the gas scrubbing was firstly performed with the backwash time and intensity of 2 min and 10 L m−2 h−1, respectively, using an air compressor; then, the hydraulic backwashing was adopted with a duration of 1 min and an intensity of 14 L m−2 h−1, accordingly. Other than that, the UF system was carried out in the gravity-driven mode at a constant transmembrane pressure (6.5 kPa) provided by the water level difference between the BAC column and the effluent tank, while the UF process was operated continuously for 40 min and then applied with forwarding flush and backwash both for 1 min separately [26]. Here, it should be noted that the BAC-UF system was operated for 130 days, from spring to summer.
2.4. Analytical Method
Characteristics of the raw water, the BAC column effluent, and the UF permeate were analyzed in this study. Prior to analysis, 0.45-µm cellulose acetate microfiltration membranes (Xingya Purification Material Co., Shanghai, China) were washed by nano-pure water (18 MΩ.cm) and then used to filter water samples. DO concentration and pH values were measured using a multiple handheld meter (Multi 3420, WTW, Munich, Germany). CODMn and UV254 were employed to evaluate removal performance. UV254, representing the unsaturated organic matter such as humic substances, was measured using a UV-visible spectrophotometer (UV-2450, SHIMDZU, Tokyo, Japan). Moreover, the concentrations of NH4+-N and nitrite nitrogen (NO2−-N) were determined using a spectrophotometer (722G, JK Co., Shanghai, China) with Nessler’s reagent and diazonium coupling reaction, respectively, whereas the total organic carbon (TOC) and dissolved organic carbon (DOC) concentrations were measured using an automatic total organic carbon analyzer (TOC-L, SHIMDZU, Tokyo, Japan). Before the TOC determination, the samples were mixed using a magnetic stirrer. Besides, the data were evaluated by conducting the IBM SPSS 26 one sample t-test and independent sample t-test.
Target PPCPs were extracted using solid-phase extraction (SPE) and analyzed by high-performance liquid chromatography coupled to triple quadrupole tandem mass spectrometry (HPLC-MS/MS). Before the extraction, all the water samples were vacuum filtered through 0.22-µm glass fiber filters and then spiked with corresponding internal standard solutions. In addition, target PPCPs were determined using HPLC-MS/MS (6410B, Agilent Technologies, Santa Clara, CA, USA).
Potassium permanganate, H2SO4, potassium sodium tartrate tetrahydrate, Nessler’s reagent, and NaOH were purchased from a commercial company and certified as AR purity (Guangzhou Chemical Reagent Factory, Guangzhou, China), while PPCP standards were provided by three companies. Sulfamethoxazole (SMX), sulfametoxydiazine (SMD), sulfachloropyridazine (SCP), sulfamethoxypyridazine (SMP), erythromycin (EM), and anhydroerythromycin (EA) were obtained from the Dr. Ehrenstorfer Company in Germany. Furthermore, sulfadimidine (SM2), sulfadiazine (SDZ), and trimethoprim (TMP) were bought from the National Institute of Metrology in China, whereas caffeine (CF) was acquired from the Toronto Research Chemicals Company in Canada.
3. Results and Discussion
3.1. Removal of Organic Matter
Figure 2 shows the DO consumption and the removal performance of the BAC and the BAC-UF system on organic matter. The DO concentrations (Figure 2a) in raw water varied with approximately 4.35–7.46 mg L−1. When the operation time was prolonged, the DO concentrations in the BAC effluent and UF permeate gradually decreased (Sig (2-tailed) < 0.001), which indicated the increase of biomass in this pilot-scale system. Sometimes, the periodic backwash (7 days) of BAC caused the dissolved oxygen concentration of BAC-effluent higher than that of raw water. After the gas scrubbing and the hydraulic backwashing, the dissolved oxygen detection of the effluent was carried out, resulting in the above results for dissolved oxygen. Besides, the t-test was used and proved the significant difference between BAC-Effluent and BAC/UF-effluent for the DO concentration. As seen in Figure 2b, the CODMn of the raw water was 1.88–13.79 mg L−1. As the concentration of organic matter in the raw water varied, the CODMn concentrations also waved in the BAC effluent. However, the UF membrane (Sig (2-tailed) < 0.001) controlled the CODMn concentration in permeate stably to a great extent, keeping the removal rate mainly above 20%. This enhancement was related to the bio-fouling layer on the UF membranes [28]. The average removal rate of CODMn was 35.45% for BAC, while the removal rate of BAC-UF increased to 56.00%, which implied that both BAC and UF could effectively remove the organic matter. The results might also support the evidence of PPCP removals. Similar to CODMn, the UV254 value in the raw water was pulsed between 0.06 and 0.49 cm−1 due to the pollution from the upstream of the river. While the UV254 values were increased in the raw water, the removal rates of BAC and UF were also improved. Averagely, the removal rate of BAC reached about 34.98%, and that of the BAC-UF system achieved 55.25% (Sig (2-tailed) < 0.001), which showed that the further process after BAC using the UF membrane was necessary to secure the cleanness of potable water.
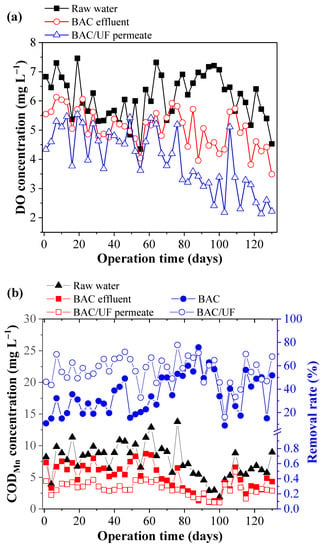
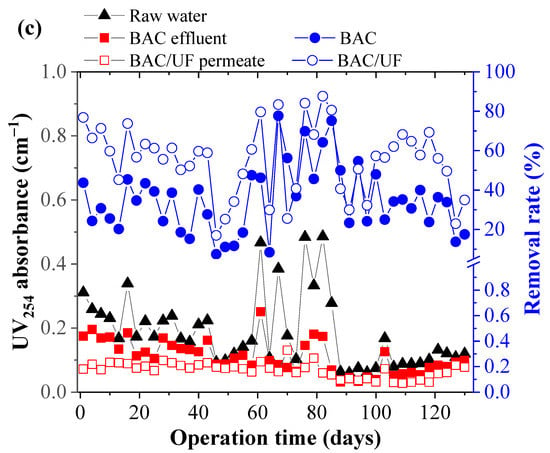
Figure 2.
The removal performance of CODMn and UV254 by BAC-UF system: (a) DO, (b) CODMn, (c) UV254.
The same trend (Figure 2) was mainly due to the stable removal ability of BAC and UF for organics, causing the removal restriction.
In order to confirm the removal mechanism of organic substances, the fluorescent substances were analyzed at the end of the operation. Four peaks are illustrated in Figure 3: Peak A (Ex/Em = 230.0/330.0 nm), Peak B (Ex/Em = 270.0/298.0 nm), Peak C (Ex/Em = 260.0/418.0 nm), and Peak D (Ex/Em= 320.0/410.0 nm) represented aromatic protein, soluble microbial production (similar to the protein), fulvic acid-like substances, and humic acid-like substances, respectively [29]. As seen from Figure 3, after the BAC treatment, the intensities of four peaks obviously decreased. This system showed a similar trend of fluorescent substance removal to previous studies where the synergistic behaviors of adsorption and microbial d degradation removed various pollutants in an effective way [30]. However, after treated by UF, the intensities of Peak C and Peak D were increased, which might be caused by the conversation from proteins to humic substances. The decomposition process of microorganisms including fungi on the surface of the UF membrane could contribute to such conversation [31]. Therefore, the above results proved the effective bio-degradation of organic matter in the BAC-UF system.
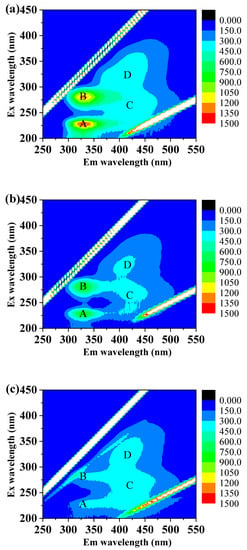
Figure 3.
The removal performance of fluorescent substances by BAC-UF system: (a) raw water, (b) effluent of BAC, (c) BAC-UF permeate.
3.2. Removal of Nitrogen
Figure 4 shows removal of NH4+-N, NO2−-N, and NO3−-N via the BAC-UF system. As for NH4+-N concentration in the first 76 days (Figure 4a), it was averagely higher than 3.10 mg L−1 in the raw water. Due to the limitation of dissolved oxygen concentration (Figure 2a), the BAC removed ammonia nitrogen with a low efficiency (15.14%). After that, while the ammonia nitrogen concentration decreased below 2 mg L−1, the removal rate was increased to 50.19% in the BAC. Overall, in the operation, the trends of removal performance were displayed in Figure 2a in parallel for the BAC and the BAC-UF, indicating the BAC dominated the ammonia nitrogen removal. Specifically, the average removal rate on NH4+-N was 29.48% for the BAC, and UF further enhanced the performance up to 44.38% (Sig (2-tailed) < 0.05). Thus, the gravity-driven UF was able to remove ammonia nitrogen, which was consistent with the previous study [28]. In terms of (NO2−-N), the concentration in raw water stabilized among 0.15–0.54 mg L−1. As the operation time increased, its removal performance was improved for the BAC-UF system, which may be due to two reasons: (a) The reduction of NH4+-N concentration weakened the competitive behavior of functional microbiome and promoted the oxidation of nitrite; and (b) the biomass gradually increased over time and their removal performance was increased. Meanwhile, it can be seen from Figure 4c that gravity-driven UF also showed a significant removal effect on nitrite and enhanced the removal efficiency of BAC-UF. With regard to nitrate, the process also reflected the above phenomenon in removal trend, and both BAC and UF were related to the removal effects.
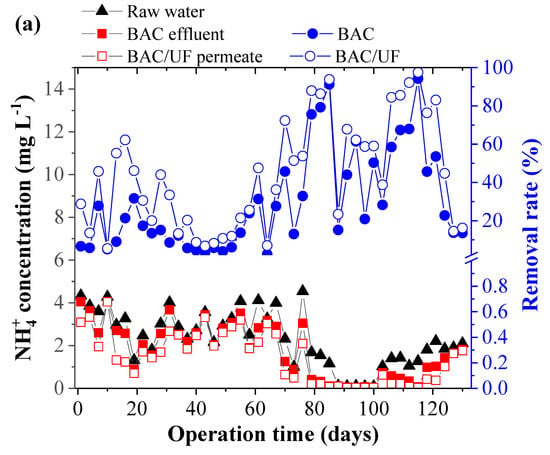
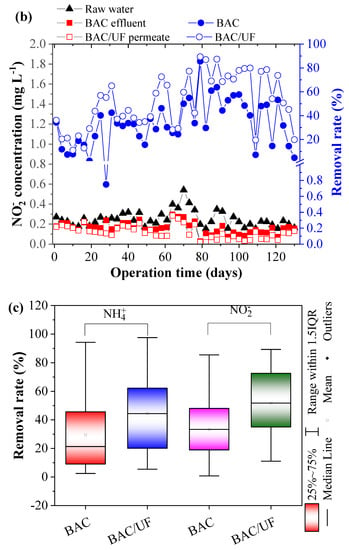
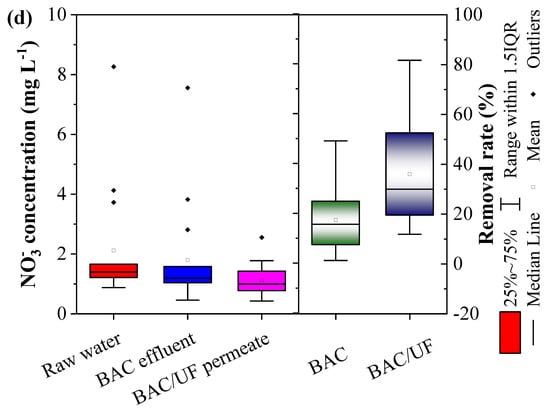
Figure 4.
The removal performance of NH4+-N, NO2−-N, and NO3−-N by BAC-UF system: (a) NH4+-N, (b) NO2−-N, (c) comparison of BAC and BAC-UF for removals of NH4+-N and NO2−-N, (d) NO3−-N.
3.3. Removal of PPCPs
In Table 2, the concentrations of the dominant PPCPs in the raw water are summarized. The poultry on factory farms was routinely fed with these ten PPCPs, causing such river water pollution. EM as a macrolide antibiotic reagent was widely used in industrial poultry breeding, which contributed to the high concentrations (500.56–3994.07 ng L−1) in the raw water. Other five PPCPs, including SM2, SMP, SMD, SDZ, and CF, existed in the raw water with the maximum concentrations of above 100 ng L−1. Besides, EA, SMX, SCP, and TMP were often detected despite relatively low concentration.

Table 2.
Concentrations of typical antibiotics in the raw water (significant value, 0.05; sample size (n), 15).
Figure 5 depicts the removal of ten typical PPCPs using the BAC-UF system. This work was carried out during the rainy season in South China, and the rainfall played an important role in influencing the PPCPs concentration due to the dilution effect. Thus, the PPCPs concentration in the raw water showed a decreasing trend from March to July. From Figure 5b,i, the concentrations of EA, SCP, and TMP were sometimes lower than the limitation of HPLC-MS/MS spectrometry due to the rainfall dilution. In most measurements, various PPCPs were detected by the HPLC-MS/MS instrument, especially the EM. Overall, the BAC-UF process showed high efficiency in the removal of PPCPs micropollutants and the average removal of total PPCPs reached 47.84%.
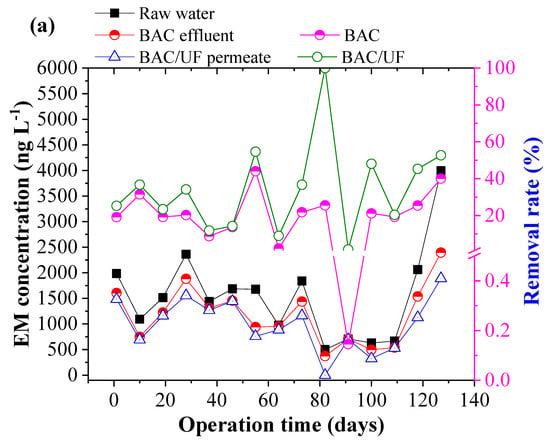
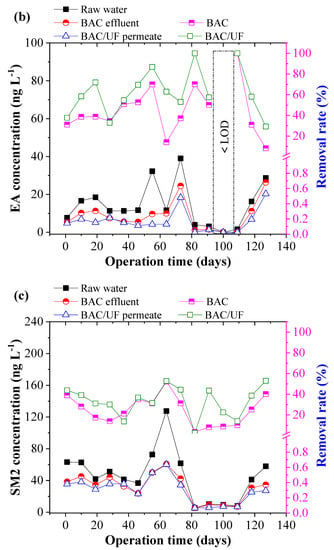
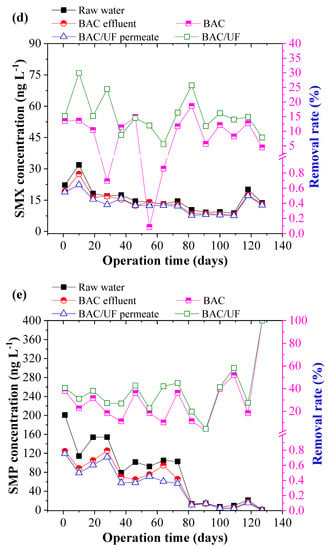
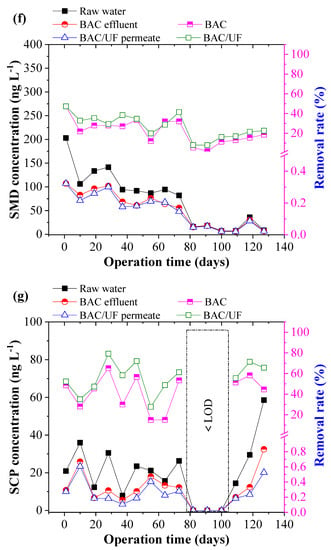
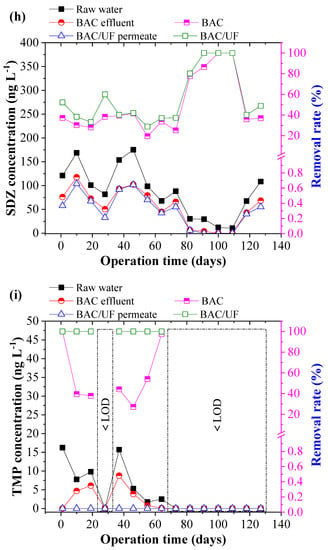
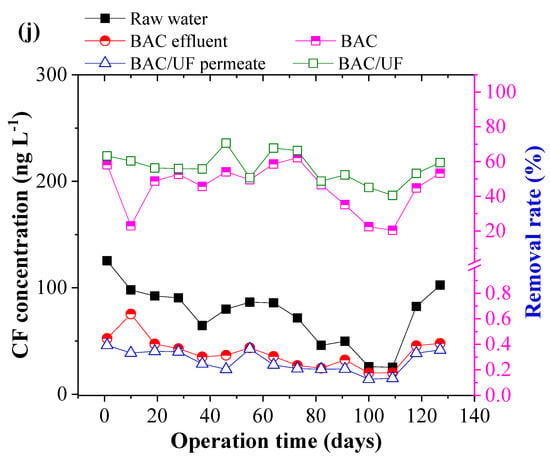
Figure 5.
Removal performance of typical PPCPs by BAC-UF system: (a) EM, (b) EA, (c) SM2, (d) SMX, (e) SMP, (f) SMD, (g) SCP, (h) SDZ, (i) TMP, (j) CF.
Specifically, the BAC effectively removed EM, EA, SM2, SMX, SMP, SMD, SCP, SDZ, TMP, and CF, reaching removal rates of 20.81%, 44.84%, 24.40%, 9.28%, 29.84%, 21.85%, 42.64%, 48.69%, 57.17%, and 45.02%, accordingly, whereas the gravity-driven UF improved the removal performance to 34.27%, 62.35%, 33.20%, 15.48%, 37.84%, 26.77%, 56.21%, 55.88%, 100.00%, and 56.42%, respectively. Therefore, the BAC-UF system showed significant removal rates for EA, SCP, SDZ, TMP, and CF, all of which are above 50%, while the system was characterized with a limited effect on the removal rate of SMX (15.48%). This removal difference may be related to the chemical structure, which favored adsorption and bioprocessing [32]. Besides, the SM2, SMP, and SMD might convert to the similar structure such as the SMX by the BAC system, causing the low removal rate [33].
Figure 6 shows the PPCP removal distribution by the BAC and UF. BAC played the predominant role in the removal of the PPCPs, whereas UF showed a limited effect. During 130-day operation, the contribution of BAC to removal of EM, EA, SM2, SMX, SMP, SMD, SCP, SDZ, TMP, and CF was 60.72%, 71.91%, 73.50%, 59.92%, 78.84%, 81.65%, 75.85%, 87.13%, 57.17%, and 79.80%, respectively. Compared with the UF process, the BAC process demonstrated a higher contribution to the PPCP removal that in the BAC process was mainly attributed to adsorption and biodegradation, whereas the UF process mainly resulted from biomass on the UF membrane. The results showed that the biodegradation functioned importantly in the enhancement of PPCP removal in the BAC-UF system.
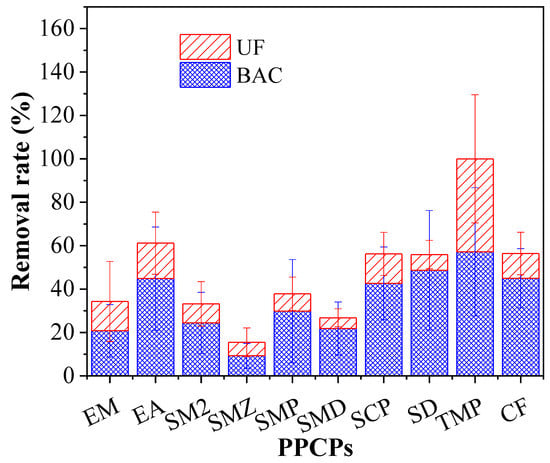
Figure 6.
Typical antibiotics removal contribution by BAC and UF.
3.4. Relevance for Decentralized Drinking Water Treatment
PPCPs risks have been posing severe challenges to the safety of drinking water supply in rural areas due to the absence of the process with simple operation and maintenance as well as reliable performance. In this study, BAC coupling gravity-driven UF was performed continuously, and the rejection performance of membrane filtration and BAC filtration both showed barriers for the conventional pollutants and PPCPs. Furthermore, this study indicated the respective contributions of BAC and UF, showing the role of the two-stage biofilm. Previous works involving BAC generally combined the ozonation with the BAC filter for treating the contaminants of emerging concern, eliminating a majority of PPCPs by more than 90% [34]. However, the regulation and maintenance of machines for ozone products are complicated, and the disinfection by-products will be newly generated in the effluent, which is inconvenient to use in rural areas [35]. In general, coagulation, filtration, and single BAC units worked inefficiently and removed the detected PPCPs by less than 50%, as they were not hydrophobic [34,36]. Hybrid membrane processes such as inline dosing of powdered activated carbon (PAC) prior to UF have already shown promising potential for the abatement of PPCPs; however, the inline dosing PAC is infeasible in rural areas [37]. In this study, the BAC prior to UF enhanced the biological activity by forming a two-stage biofilm system. Therefore, the integrated BAC-UF process can be considered as an economically and technically feasible approach to the decentralized and emergency drinking water treatment.
4. Conclusions
This research presents a pilot-scale BAC-UF system with the long period of backwashing (7 days) in Southern China from spring to summer for the purpose of (i) reducing concentrations of conventional pollutants and (ii) removing PPCP pollutants. The results showed that the BAC reduced 35.45% of organic matter (CODMn), while the removal rate of BAC-UF increased to 56.00%. Besides, the BAC-UF membrane controlled the indexes of CODMn and UV254 in permeate more stable than BAC, while the further process after BAC using the UF membrane was necessary to safeguard the purity of potable water. Despite the fact that the removal rate of UF was lower than that of the BAC, both BAC and UF were related to the removal effects of NH4+-N, NO2−-N, and NO3−-N. Meanwhile, the BAC-UF process showed high efficiency in the removal of PPCPs micropollutants and the average removal of total PPCPs (including 10 pollutants) reached 47.84%. In particular, the removal rates of anhydroerythromycin, sulfachloropyridazine, sulfadiazine, trimethoprim, and caffeine were above 50%. Here, it should be mentioned that the BAC played the key role in PPCP removal and the UF was mainly attributed to biomass on the UF membrane, synergistically degrading various PPCP pollutants.
To sum up, the results show that the proposed BAC-UF system can be effective in the treatment of river water polluted by PPCPs, conventional organic pollutants and ammonia nitrogen. The two-stage biofilms located in the activated carbon column and on the UF membrane synergistically can be conducive to the removal performances. Besides, the results of this analysis can have significant implications for the conventional UF operation procedure and the ozone-activated carbon process, providing a simple decentralized approach to drinking water treatment for the areas where source water is contaminated with PPCPs. However, the mechanisms of the two-stage biofilm, such as bacterial and metazoan communities, membrane fouling, and dissolved oxygen transfer, should be further investigated to enhance the removal efficiency and stability of this system.
Author Contributions
Conceptualization, J.W. and F.W.; methodology, J.C.; software, Q.W.; validation, X.T.; formal analysis, Q.W. and W.Z.; investigation, Q.W.; resources, J.W.; data curation, J.C. and W.Z.; writing—original draft preparation, Q.W.; writing—review and editing, Q.W. and J.W.; visualization, W.G.; supervision, G.L.; project administration, H.L. and X.T.; funding acquisition, X.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was jointly supported by the National Key Research and Development Program of China (2019YFD1100104), National Natural Science Foundation of China (52000049), State Key Laboratory of Urban Water Resource and Environment (2020DX04), and China Postdoctoral Science Foundation (2019M651290) and (220T130153).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this study:
| AOPs | Advanced oxidation processes | PAC | Powdered activated carbon |
| BAC | Biological activated carbon | PLC | Programmable logic controller |
| CF | Caffeine | PPCPs | Pharmaceutical and personal care products |
| CODMn | Chemical oxygen demand | PVC | Polyvinyl chloride |
| DBPs | Disinfection byproducts | SCP | Sulfachloropyridazine |
| DCF | Diclofenac sodium | SDZ | Sulfadiazine |
| DOC | Dissolved organic carbon | SM2 | Sulfadimidine |
| EA | Anhydroerythromycin | SMD | Sulfametoxydiazine |
| EM | Erythromycin | SMP | Sulfamethoxypyridazine |
| HPLC-MS/MS | Triple quadrupole tandem mass spectrometry | SMX | Sulfamethoxazole |
| NF | Nanofiltration | SPE | Solid-phase extraction |
| NH4+-N | Ammonia nitrogen | TMP | Trimethoprim |
| NO2−-N | Nitrite nitrogen | TOC | Total organic carbon |
| NO3−-N | Nitrate nitrogen | UF | Ultrafiltration |
References
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sui, Q.; Lyu, S.; Zhao, W.; Liu, J.; Cai, Z.; Yu, G.; Barcelo, D. Municipal Solid Waste Landfills: An Underestimated Source of Pharmaceutical and Personal Care Products in the Water Environment. Environ. Sci. Technol. 2020, 54, 9757–9768. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Huang, H.; Li, N.; Li, F.; Wang, D.; Luo, Q. Occurrence and ecological risk of pharmaceuticals and personal care products (PPCPs) and pesticides in typical surface watersheds, China. Ecotoxicol. Environ. Saf. 2019, 175, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.A.A.; Mohd-Nasir, H.; Ahmad, A.; Mohd Setapar, S.H.; Peng, W.L.; Chuo, S.C.; Khatoon, A.; Umar, K.; Yaqoob, A.A.; Mohamad Ibrahim, M.N. Role of Nanotechnology for Design and Development of Cosmeceutical: Application in Makeup and Skin Care. Front. Chem. 2019, 7, 739. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, J.; Zhang, M.; Chen, S.; He, B.; Zhao, H.; Li, Q.; Zhang, S.; Wang, T. Which type of pollutants need to be controlled with priority in wastewater treatment plants: Traditional or emerging pollutants? Environ. Int. 2019, 131, 104982. [Google Scholar] [CrossRef]
- Panagopoulos, A. Energetic, economic and environmental assessment of zero liquid discharge (ZLD) brackish water and seawater desalination systems. Energy Convers. Manag. 2021, 235, 113957. [Google Scholar] [CrossRef]
- Panagopoulos, A. Techno-economic assessment of minimal liquid discharge (MLD) treatment systems for saline wastewater (brine) management and treatment. Process Saf. Environ. Prot. 2021, 146, 656–669. [Google Scholar] [CrossRef]
- Panagopoulos, A. Study and evaluation of the characteristics of saline wastewater (brine) produced by desalination and industrial plants. Environ. Sci. Pollut. Res. 2021, 1–14. [Google Scholar] [CrossRef]
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef]
- Al Aani, S.; Mustafa, T.N.; Hilal, N. Ultrafiltration membranes for wastewater and water process engineering: A comprehensive statistical review over the past decade. J. Water Process Eng. 2020, 35, 101241. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Hu, Z. Selective removal of pharmaceuticals and personal care products from water by titanium incorporated hierarchical diatoms in the presence of natural organic matter. Water Res. 2021, 189, 116628. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Liu, Y.; Bossa, N.; Ding, A.; Ren, N.; Li, G.; Liang, H.; Wiesner, M.R. Incorporation of Cellulose Nanocrystals (CNCs) into the Polyamide Layer of Thin-Film Composite (TFC) Nanofiltration Membranes for Enhanced Separation Performance and Antifouling Properties. Environ. Sci. Technol. 2018, 52, 11178–11187. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, X.; Cheng, X.; Xing, J.; Wang, H.; Li, G.; Liang, H. In-situ crystallization generated by CEM electrolysis for NF concentrate softening along with the alleviation of ceramic membrane fouling. Desalination 2021, 516, 115243. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.-H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Yaqoob, A.A.; Noor, M.; Serrà, A.; Mohamad Ibrahim, M.N. Advances and Challenges in Developing Efficient Graphene Oxide-Based ZnO Photocatalysts for Dye Photo-Oxidation. Nanomaterials 2020, 10, 932. [Google Scholar] [CrossRef]
- Borikar, D.; Mohseni, M.; Jasim, S. Evaluations of conventional, ozone and UV/H2O2 for removal of emerging contaminants and THM-FPs. Water Qual. Res. J. 2014, 50, 140–151. [Google Scholar] [CrossRef]
- Yu, P.; Li, X.; Zhang, X.; Zhou, H.; Xu, Y.; Sun, Y.; Zheng, H. Insights into the glyphosate removal efficiency by using magnetic powder activated carbon composite. Sep. Purif. Technol. 2021, 254, 117662. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Campos, L.C. The application of GAC sandwich slow sand filtration to remove pharmaceutical and personal care products. Sci. Total Environ. 2018, 635, 1182–1190. [Google Scholar] [CrossRef]
- Paredes, L.; Fernandez-Fontaina, E.; Lema, J.M.; Omil, F.; Carballa, M. Understanding the fate of organic micropollutants in sand and granular activated carbon biofiltration systems. Sci. Total Environ. 2016, 551–552, 640–648. [Google Scholar] [CrossRef]
- Tang, X.; Pronk, W.; Ding, A.; Cheng, X.; Wang, J.; Xie, B.; Li, G.; Liang, H. Coupling GAC to ultra-low-pressure filtration to modify the biofouling layer and bio-community: Flux enhancement and water quality improvement. Chem. Eng. J. 2018, 333, 289–299. [Google Scholar] [CrossRef]
- Han, L.; Liu, W.; Chen, M.; Zhang, M.; Liu, S.; Sun, R.; Fei, X. Comparison of NOM removal and microbial properties in up-flow/down-flow BAC filter. Water Res. 2013, 47, 4861–4868. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Chen, W.; Wang, L. Particle properties in granular activated carbon filter during drinking water treatment. J. Environ. Sci. 2010, 22, 681–688. [Google Scholar] [CrossRef]
- Wang, Q.; You, W.; Li, X.; Yang, Y.; Liu, L. Seasonal changes in the invertebrate community of granular activated carbon filters and control technologies. Water Res. 2014, 51, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, S.; Johir, M.A.H.; Nguyen, T.V.; Kandasamy, J.; Vigneswaran, S. Experimental evaluation of microfiltration–granular activated carbon (MF-GAC)/nano filter hybrid system in high quality water reuse. J. Membr. Sci. 2015, 476, 1–9. [Google Scholar] [CrossRef]
- Snyder, S.A.; Adham, S.; Redding, A.M.; Cannon, F.S.; DeCarolis, J.; Oppenheimer, J.; Wert, E.C.; Yoon, Y. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 2007, 202, 156–181. [Google Scholar] [CrossRef]
- Wu, B.; Zamani, F.; Lim, W.; Liao, D.; Wang, Y.; Liu, Y.; Chew, J.W.; Fane, A.G. Effect of mechanical scouring by granular activated carbon (GAC) on membrane fouling mitigation. Desalination 2017, 403, 80–87. [Google Scholar] [CrossRef]
- Tang, X.; Pronk, W.; Traber, J.; Liang, H.; Li, G.; Morgenroth, E. Integrating granular activated carbon (GAC) to gravity-driven membrane (GDM) to improve its flux stabilization: Respective roles of adsorption and biodegradation by GAC. Sci. Total Environ. 2021, 768, 144758. [Google Scholar] [CrossRef]
- Xing, J.; Wang, H.; Cheng, X.; Tang, X.; Luo, X.; Wang, J.; Wang, T.; Li, G.; Liang, H. Application of low-dosage UV/chlorine pre-oxidation for mitigating ultrafiltration (UF) membrane fouling in natural surface water treatment. Chem. Eng. J. 2018, 344, 62–70. [Google Scholar] [CrossRef]
- Moona, N.; Murphy, K.R.; Bondelind, M.; Bergstedt, O.; Pettersson, T.J.R. Partial renewal of granular activated carbon biofilters for improved drinking water treatment. Environ. Sci. Water Res. Technol. 2018, 4, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tang, X.; Liu, Y.; Xie, B.; Li, G.; Liang, H. Self-sustained ultrafiltration coupling vermifiltration for decentralized domestic wastewater treatment: Microbial community and mechanism. Resour. Conserv. Recycl. 2022, 177, 106008. [Google Scholar] [CrossRef]
- Jia, Y.; Khanal, S.K.; Zhang, H.; Chen, G.-H.; Lu, H. Sulfamethoxazole degradation in anaerobic sulfate-reducing bacteria sludge system. Water Res. 2017, 119, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Li, F.; Zhou, Q. Degradation mechanisms of sulfamethoxazole and its induction of bacterial community changes and antibiotic resistance genes in a microbial fuel cell. Bioresour. Technol. 2019, 289, 121632. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Fu, J.; Li, X.; Li, B.; Wang, X. Occurrence and fate of PPCPs in typical drinking water treatment plants in China. Environ. Geochem. Health 2019, 41, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Lin, T.; Chen, W.; Tao, H.; Tan, Y.; Ma, B. Removal of precursors of typical nitrogenous disinfection byproducts in ozonation integrated with biological activated carbon (O3/BAC). Chemosphere 2018, 209, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Qu, Y.; Liu, L.; He, Y.; Li, W.; Huang, J.; Yang, H.; Yu, G. PPCPs in a drinking water treatment plant in the Yangtze River Delta of China: Occurrence, removal and risk assessment. Front. Environ. Sci. Eng. 2019, 13, 27. [Google Scholar] [CrossRef]
- Schwaller, C.; Hoffmann, G.; Hiller, C.X.; Helmreich, B.; Drewes, J.E. Inline dosing of powdered activated carbon and coagulant prior to ultrafiltration at pilot-scale—Effects on trace organic chemical removal and operational stability. Chem. Eng. J. 2021, 414, 128801. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).