Applications of Computational and Statistical Models for Optimizing the Electrochemical Removal of Cephalexin Antibiotic from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Electrocoagulation Reactor
2.3. Research Roadmap
2.4. Optimization and Modeling the Experiments Using the Machine Learning Model
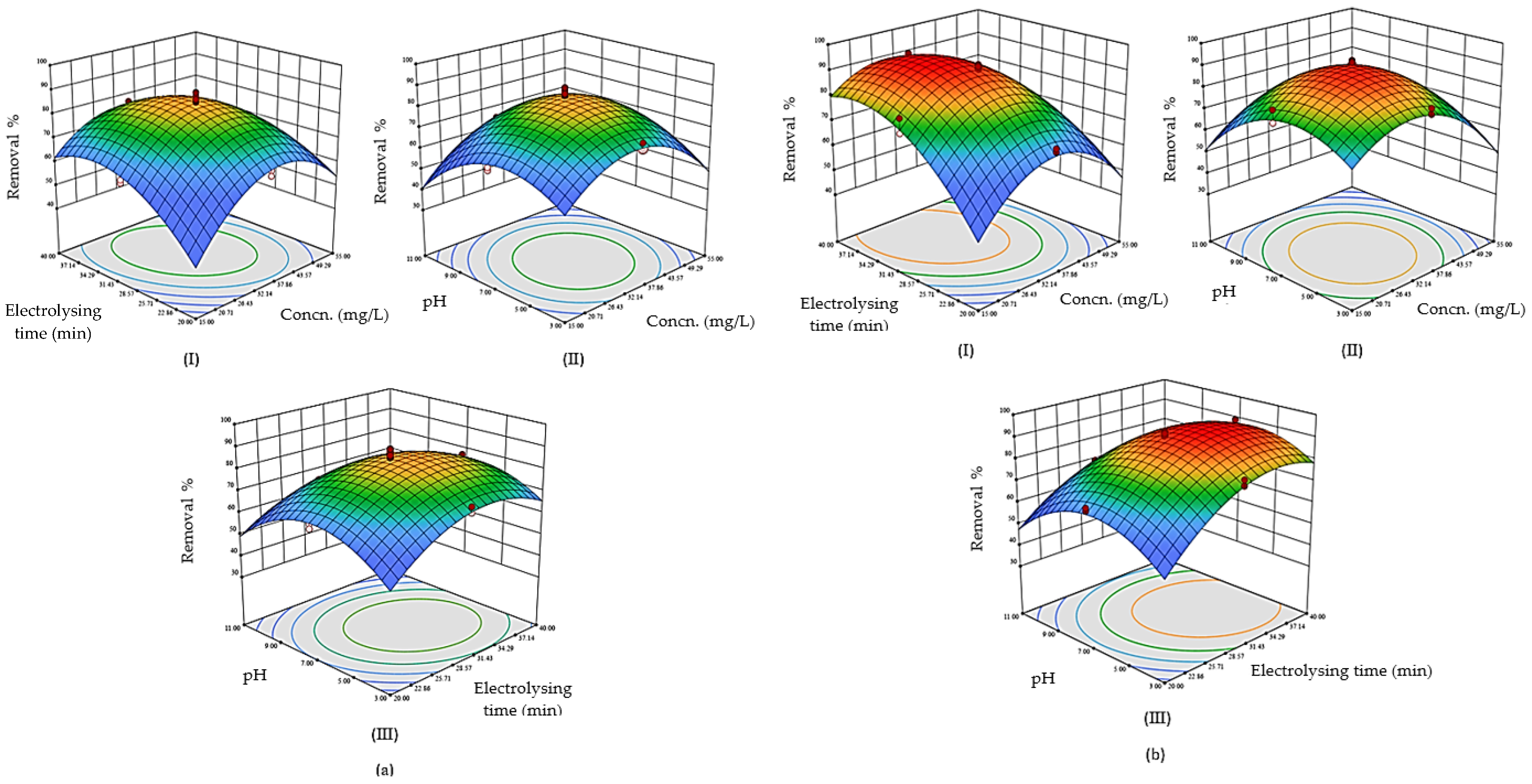
2.4.1. Experimental Design and Optimization
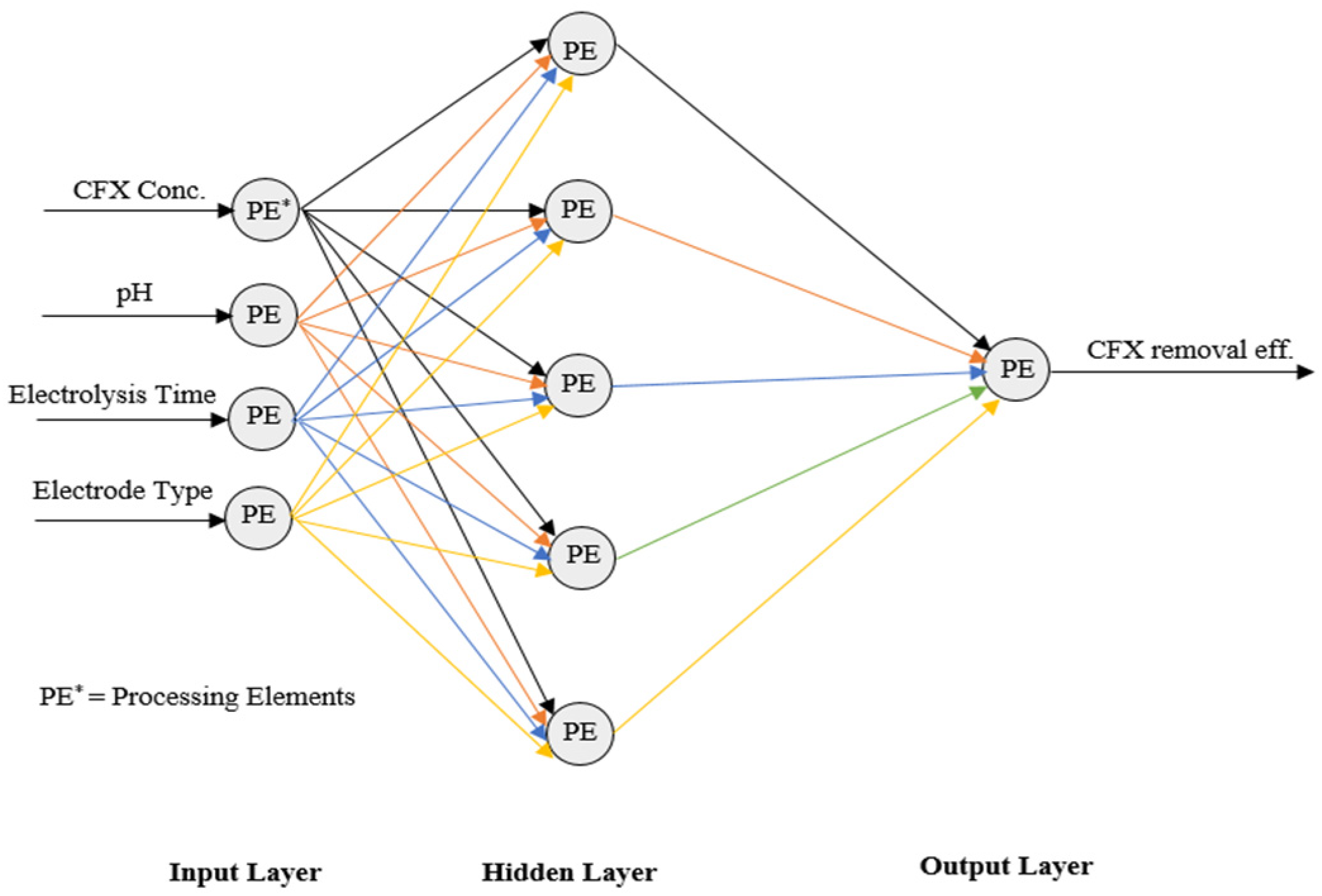
2.4.2. Modeling an Artificial Neural Network
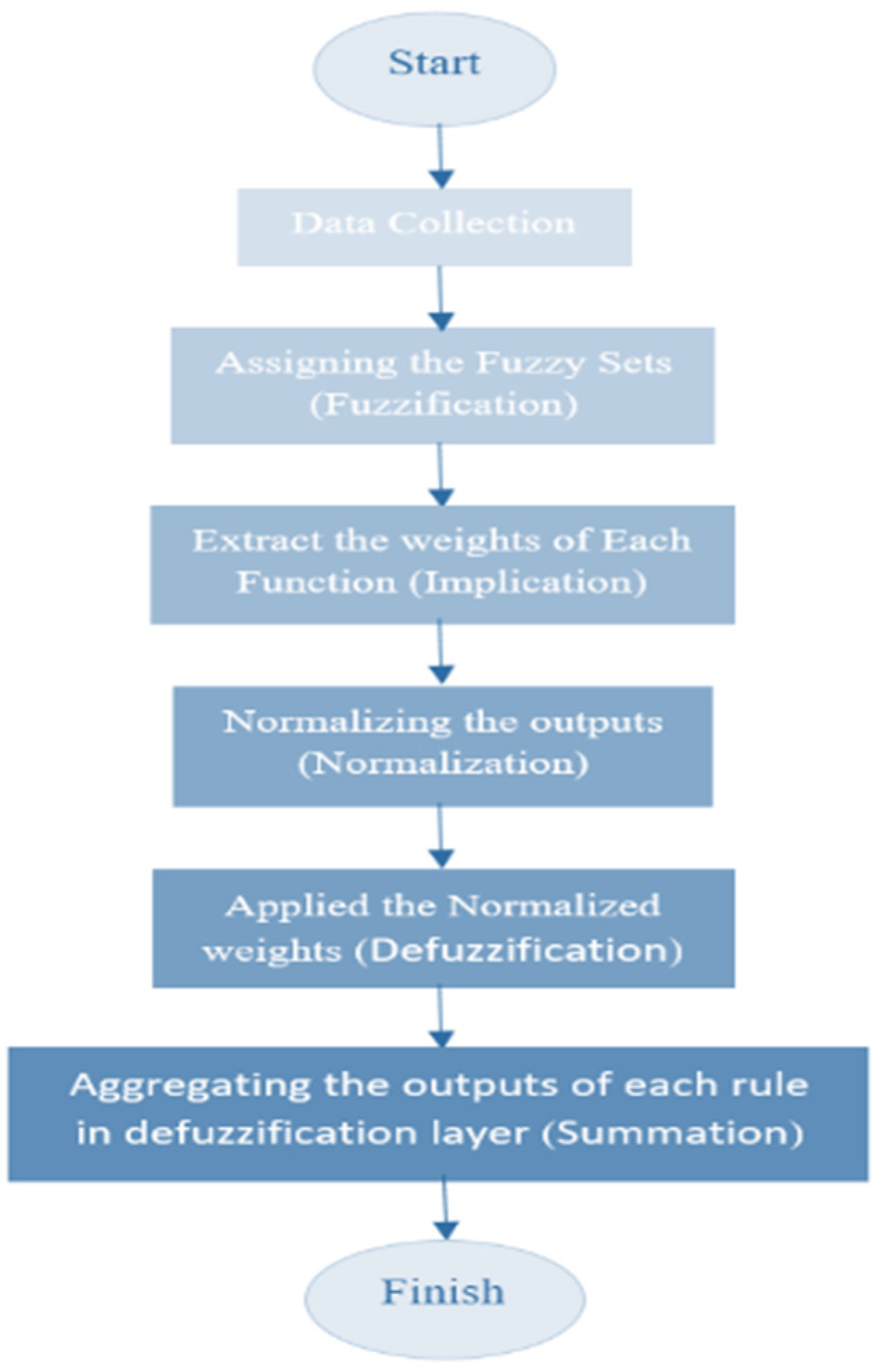
2.4.3. ANFIS
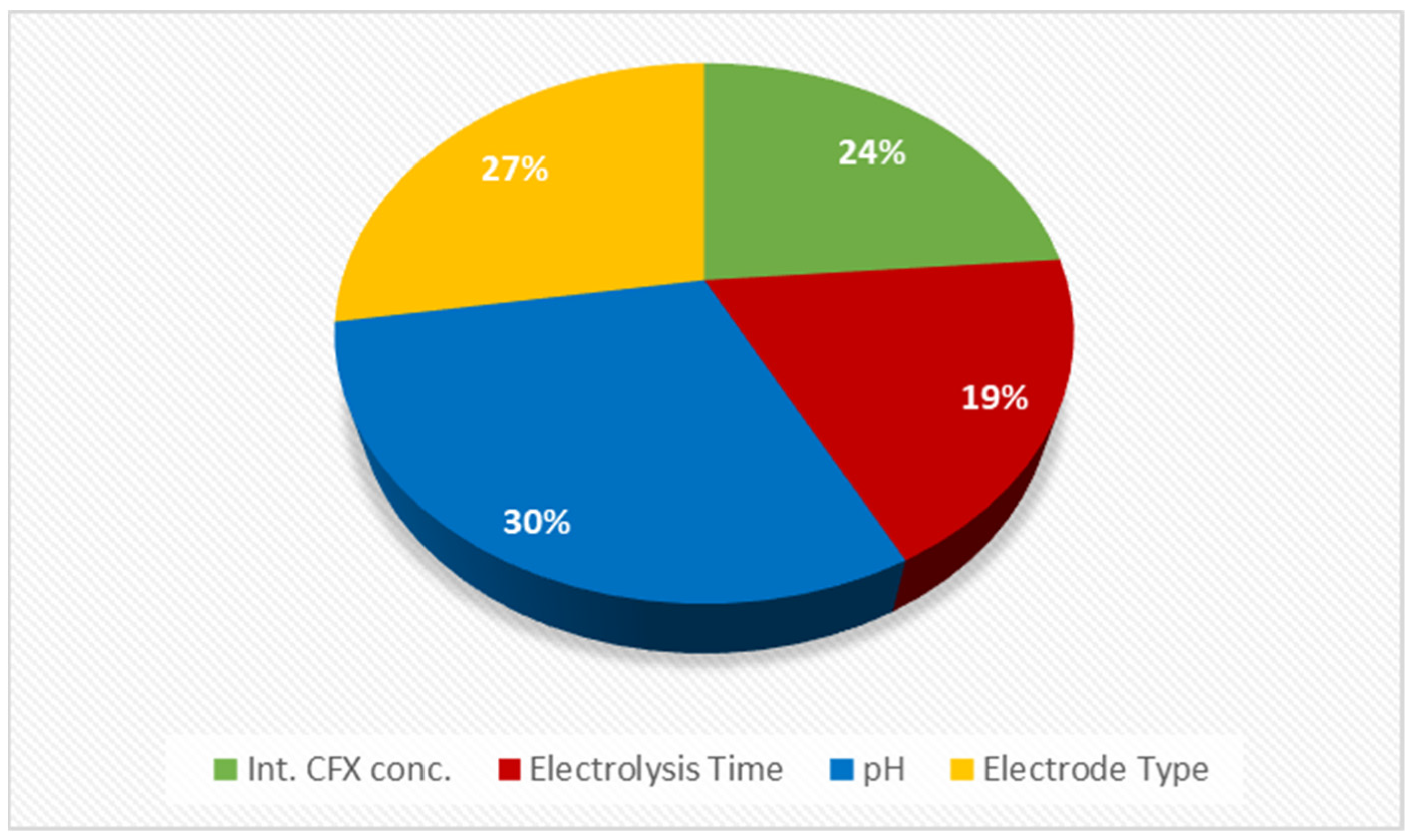
2.4.4. Sensitivity Analysis
- Formation of a matrix containing the input-hidden and hidden-output neurons’ weight.
- Calculation of the effect of input neurons on the network output through each of the hidden neurons (AH1I1). Therefore, at first, it is necessary to determine the hidden-input layers’ weight (WH1I1) and the output-hidden layers’ weight (WO1H1) (Equation (5)).
- Calculation of the relative effect of each input neurons on the output signal for latent neurons (RH1I1) and determination of the total R for the input neurons (SI1)
- Calculation of the relative importance of each input variables (II1)
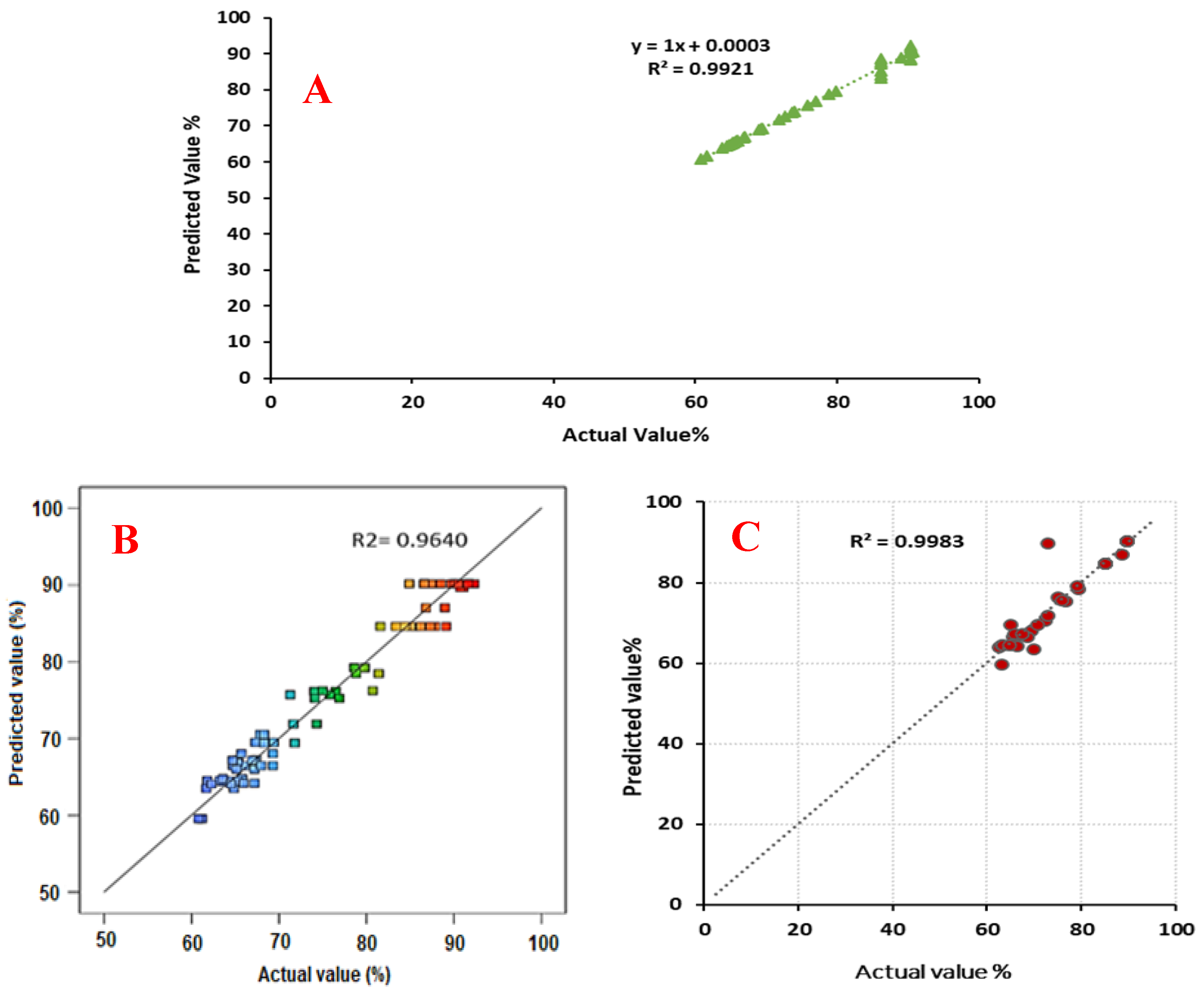
3. Results and Discussion
4. Conclusions
- The CCD-RSM, ANN, and ANFIS algorithms have an acceptable accuracy in simulating the removal of CEX.
- The ANFIS model has the best performance in predicting the removal of CEX by the electrocoagulation method.
- According to the CCD-RSM and ANOVA results, the electrolysis time is the most important parameter in removing CEX.
- The best removal of CEX was 93.87% achieved at a CEX concentration of 30.16 mg/L, electrolysis time of 34.62 min, pH of 6.14 and application of insulated electrodes.
Author Contributions
Funding
Conflicts of Interest
References
- Lieberman, J.M. Appropriate antibiotic use and why it is important: The challenges of bacterial resistance. Pediatric Infect. Dis. J. 2003, 22, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Vieno, N.M.; Tuhkanen, T.; Kronberg, L. Analysis of neutral and basic pharmaceuticals in sewage treatment plants and in recipient rivers using solid phase extraction and liquid chromatography–tandem mass spectrometry detection. J. Chromatogr. A 2006, 1134, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.; Theydan, S.K. Adsorption of cephalexin onto activated carbons from Albizia lebbeck seed pods by microwave-induced KOH and K2CO3 activations. Chem. Eng. J. 2012, 211, 200–207. [Google Scholar] [CrossRef]
- Nazari, G.; Abolghasemi, H.; Esmaieli, M. Batch adsorption of cephalexin antibiotic from aqueous solution by walnut shell-based activated carbon. J. Taiwan Inst. Chem. Eng. 2016, 58, 357–365. [Google Scholar] [CrossRef]
- Rodayan, A.; Segura, P.A.; Yargeau, V. Ozonation of wastewater: Removal and transformation products of drugs of abuse. Sci. Total Environ. 2014, 487, 763–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziylan, A.; Ince, N.H. The occurrence and fate of anti-inflammatory and analgesic pharmaceuticals in sewage and fresh water: Treatability by conventional and non-conventional processes. J. Hazard. Mater. 2011, 187, 24–36. [Google Scholar] [CrossRef]
- Gabet-Giraud, V.; Miege, C.; Choubert, J.; Ruel, S.M.; Coquery, M. Occurrence and removal of estrogens and beta blockers by various processes in wastewater treatment plants. Sci. Total Environ. 2010, 408, 4257–4269. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.; Alothman, Z.A.; Badjah, A.Y. Kinetics, thermodynamics, and modeling of amido black dye photodegradation in water using Co/TiO2 nanoparticles. Photochem. Photobiol. 2018, 94, 935–941. [Google Scholar] [CrossRef]
- Basheer, A.A. Advances in the smart materials applications in the aerospace industries. Aircr. Eng. Aerosp. Technol. 2020, 92, 1027–1035. [Google Scholar] [CrossRef]
- Leili, M.; Fazlzadeh, M.; Bhatnagar, A. Green synthesis of nano-zero-valent iron from Nettle and Thyme leaf extracts and their application for the removal of cephalexin antibiotic from aqueous solutions. Environ. Technol. 2018, 39, 1158–1172. [Google Scholar] [CrossRef]
- Shirani, Z.; Song, H.; Bhatnagar, A. Efficient removal of diclofenac and cephalexin from aqueous solution using Anthriscus sylvestris-derived activated biochar. Sci. Total Environ. 2020, 745, 140789. [Google Scholar] [CrossRef] [PubMed]
- Alwash, R.S.M. Treatment of Highly Polluted Water with Phosphate using BAPPP-Nanoparticles. Master’s Thesis, University of Technology, Baghdad, Iraq, 2017. [Google Scholar]
- Abdulhadi, B.A.; Kot, P.; Hashim, K.S.; Shaw, A.; Khaddar, R.A. Influence of current density and electrodes spacing on reactive red 120 dye removal from dyed water using electrocoagulation/electroflotation (EC/EF) process. In Proceedings of the First International Conference on Civil and Environmental Engineering Technologies (ICCEET), Najaf, Iraq, 23–24 April 2019; pp. 12–22. [Google Scholar]
- Abdulhadi, B.; Kot, P.; Hashim, K.; Shaw, A.; Muradov, M.; Al-Khaddar, R. Continuous-flow electrocoagulation (EC) process for iron removal from water: Experimental, statistical and economic study. Sci. Total Environ. 2021, 760, 143417. [Google Scholar] [CrossRef] [PubMed]
- Hashim, K.S.; Shaw, A.; AlKhaddar, R.; Kot, P.; Al-Shamma’a, A. Water purification from metal ions in the presence of organic matter using electromagnetic radiation-assisted treatment. J. Clean. Prod. 2021, 280, 124427. [Google Scholar] [CrossRef]
- Coimbra, E.C.L.; Mounteer, A.H.; do Carmo, A.L.V.; Michielsen, M.J.F.; Tótola, L.A.; Guerino, J.P.F.; Gonçalves, J.G.A.N.; da Silva, P.R. Electrocoagulation of kraft pulp bleaching filtrates to improve biotreatability. Process Saf. Environ. Prot. 2021, 147, 346–355. [Google Scholar] [CrossRef]
- Wagle, D.; Lin, C.-J.; Nawaz, T.; Shipley, H.J. Evaluation and optimization of electrocoagulation for treating Kraft paper mill wastewater. J. Environ. Chem. Eng. 2020, 8, 103595. [Google Scholar] [CrossRef]
- Gondudey, S.; Kumar, C.P.; Dharmadhikari, S.; Singh, T.R. Treatment of sugar industry effluent using electrocoagulation process: Process optimization using response surface methodology. J. Serb. Chem. Soc. 2020, 85, 1357–1369. [Google Scholar] [CrossRef]
- Gönder, Z.B.; Balcıoğlu, G.; Vergili, I.; Kaya, Y. An integrated electrocoagulation–nanofiltration process for carwash wastewater reuse. Chemosphere 2020, 253, 126713. [Google Scholar] [CrossRef]
- Morales-Rivera, J.; Sulbarán-Rangel, B.; Gurubel-Tun, K.J.; del Real-Olvera, J.; Zúñiga-Grajeda, V. Modeling and Optimization of COD Removal from Cold Meat Industry Wastewater by Electrocoagulation Using Computational Techniques. Processes 2020, 8, 1139. [Google Scholar] [CrossRef]
- Sharifi, S.; Nabizadeh, R.; Akbarpour, B.; Azari, A.; Ghaffari, H.R.; Nazmara, S.; Mahmoudi, B.; Shiri, L.; Yousefi, M. Modeling and optimizing parameters affecting hexavalent chromium adsorption from aqueous solutions using Ti-XAD7 nanocomposite: RSM-CCD approach, kinetic, and isotherm studies. J. Environ. Health Sci. Eng. 2019, 17, 873–888. [Google Scholar] [CrossRef]
- Xu, L.; Xu, X.; Cao, G.; Liu, S.; Duan, Z.; Song, S.; Song, M.; Zhang, M. Optimization and assessment of Fe–electrocoagulation for the removal of potentially toxic metals from real smelting wastewater. J. Environ. Manag. 2018, 218, 129–138. [Google Scholar] [CrossRef]
- Witek-Krowiak, A.; Chojnacka, K.; Podstawczyk, D.; Dawiec, A.; Pokomeda, K. Application of response surface methodology and artificial neural network methods in modelling and optimization of biosorption process. Bioresour. Technol. 2014, 160, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Sabour, M.R.; Amiri, A. Comparative study of ANN and RSM for simultaneous optimization of multiple targets in Fenton treatment of landfill leachate. Waste Manag. 2017, 65, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Gadekar, M.R.; Ahammed, M.M. Modelling dye removal by adsorption onto water treatment residuals using combined response surface methodology-artificial neural network approach. J. Environ. Manag. 2019, 231, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Valente, G.; Mendonça, R.; Pereira, J.; Felix, L. Artificial neural network prediction of chemical oxygen demand in dairy industry effluent treated by electrocoagulation. Sep. Purif. Technol. 2014, 132, 627–633. [Google Scholar] [CrossRef]
- Belkacem, S.; Bouafia, S.; Chabani, M. Study of oxytetracycline degradation by means of anodic oxidation process using platinized titanium (Ti/Pt) anode and modeling by artificial neural networks. Process Saf. Environ. Prot. 2017, 111, 170–179. [Google Scholar] [CrossRef]
- Viana, D.F.; Salazar-Banda, G.R.; Leite, M.S. Electrochemical degradation of Reactive Black 5 with surface response and artificial neural networks optimization models. Sep. Sci. Technol. 2018, 53, 2647–2661. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, W.; Qiu, J.; Jin, H.; Ma, H.; Li, Z.; Cang, D. Modeling and optimization study on sulfamethoxazole degradation by electrochemically activated persulfate process. J. Clean. Prod. 2018, 197, 297–305. [Google Scholar] [CrossRef]
- Zarei, M.; Khataee, A.; Ordikhani-Seyedlar, R.; Fathinia, M. Photoelectro-Fenton combined with photocatalytic process for degradation of an azo dye using supported TiO2 nanoparticles and carbon nanotube cathode: Neural network modeling. Electrochim. Acta 2010, 55, 7259–7265. [Google Scholar] [CrossRef]
- Salari, D.; Niaei, A.; Khataee, A.; Zarei, M. Electrochemical treatment of dye solution containing CI Basic Yellow 2 by the peroxi-coagulation method and modeling of experimental results by artificial neural networks. J. Electroanal. Chem. 2009, 629, 117–125. [Google Scholar] [CrossRef]
- da Silva Ribeiro, T.; Grossi, C.D.; Merma, A.G.; dos Santos, B.F.; Torem, M.L. Removal of boron from mining wastewaters by electrocoagulation method: Modelling experimental data using artificial neural networks. Miner. Eng. 2019, 131, 8–13. [Google Scholar] [CrossRef]
- Khan, S.U.; Khan, H.; Anwar, S.; Khan, S.; Zanoni, M.V.B.; Hussain, S. Computational and statistical modeling for parameters optimization of electrochemical decontamination of synozol red dye wastewater. Chemosphere 2020, 253, 126673. [Google Scholar] [CrossRef] [PubMed]
- Walczak, S. Artificial neural networks. In Encyclopedia of Information Science and Technology, 4th ed.; IGI Global: Hershey, PA, USA, 2018; pp. 120–131. [Google Scholar]
- Elazzouzi, M.; El Kasmi, A.; Haboubi, K.; Elyoubi, M. A novel electrocoagulation process using insulated edges of Al electrodes for enhancement of urban wastewater treatment: Techno-economic study. Process Saf. Environ. Prot. 2018, 116, 506–515. [Google Scholar] [CrossRef]
- Kayri, M. Predictive abilities of bayesian regularization and Levenberg–Marquardt algorithms in artificial neural networks: A comparative empirical study on social data. Math. Comput. Appl. 2016, 21, 20. [Google Scholar] [CrossRef]
- Taheri, M.; Moghaddam, M.R.A.; Arami, M. Improvement of the/Taguchi/design optimization using artificial intelligence in three acid azo dyes removal by electrocoagulation. Environ. Prog. Sustain. Energy 2015, 34, 1568–1575. [Google Scholar] [CrossRef]
- Nasr, M.; Ateia, M.; Hassan, K. Artificial intelligence for greywater treatment using electrocoagulation process. Sep. Sci. Technol. 2016, 51, 96–105. [Google Scholar] [CrossRef]
- Maleki, A.; Daraei, H.; Shahmoradi, B.; Razee, S.; Ghobadi, N. Electrocoagulation efficiency and energy consumption probing by artificial intelligent approaches. Desalination Water Treat. 2014, 52, 2400–2411. [Google Scholar] [CrossRef]


| Factors | Experimental Field | |||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| CEX Initial Conc. (mg/L) | 15 | 23.1 | 35 | 46.89 | 55 | |
| Electrolysis Time (min) | 20 | 24.05 | 30 | 35.94 | 40 | |
| Initial pH | 3 | 4.62 | 7 | 9.38 | 11 | |
| Electrode Type | Non-Insulated | Insulated | ||||
| Statistical Indicator | Formulation | Parameters | Description |
|---|---|---|---|
| R2 | n: Number of data yi,cal: observed values, yi,exp: predicted values, yavg,exp: predicted average values. | Coefficient of Determination. The average squared difference between the estimated values and the actual value. | |
| MSE | n: Number of data, yi,cal: observed values, yi,exp: Predicted values. | Mean Absolute Error The average of all absolute errors or a measure of prediction accuracy of a forecasting method | |
| RMSE | n: Number of data, yi,cal: observed values, yi,exp: Predicted values. | Root Mean Square error. The difference between the values predicted by the model and the recorded data |
| Factor | Sum of Squares | Mean Square | F-Ratio | p-Value (Prob > F) |
|---|---|---|---|---|
| Model | 8053.85 | 671.15 | 147.27 | <0.0001 |
| Init. CEX Conc. | 169.21 | 169.21 | 37.13 | <0.0001 |
| B- electrolysis time | 990.47 | 990.47 | 217.33 | <0.0001 |
| C- pH | 427.54 | 427.54 | 93.81 | <0.0001 |
| D- electrode type | 615.33 | 615.33 | 135.02 | <0.0001 |
| AB | 177.57 | 177.57 | 38.96 | <0.0001 |
| AC | 34.36 | 34.36 | 7.54 | 0.0077 |
| AD | 181.83 | 181.83 | 39.90 | <0.0001 |
| BC | 37.84 | 37.84 | 8.30 | 0.0053 |
| BD | 188.08 | 188.08 | 41.27 | <0.0001 |
| CD | 0.0201 | 0.0201 | 0.0066 | 0.6360 |
| A2 | 2867.87 | 2867.87 | 629.28 | <0.0001 |
| B2 | 1294.09 | 1294.09 | 283.95 | <0.0001 |
| C2 | 2057.40 | 2057.40 | 451.44 | <0.0001 |
| Residual | 305.34 | 4.56 | - | - |
| Lack of fit | 111.22 | 6.54 | 1.69 | 0.0777 |
| Pure error | 194.12 | 3.88 |
| No. of Neurons in the Hidden Layer | No. of Layers | |||||
|---|---|---|---|---|---|---|
| 1 Hidden Layer | 2 Hidden Layer | |||||
| R2 | MSE | RMSE | R2 | MSE | RMSE | |
| 5 | 0.91853 | 10.43 | 3.2295 | 0.96575 | 10.7753 | 3.28257 |
| 6 | 0.98587 | 4.063 | 2.00157 | 0.9831 | 4.269 | 2.06615 |
| 7 | 0.94192 | 25.9804 | 5.09709 | 0.93291 | 4.2733 | 2.06719 |
| 8 | 0.94804 | 19.2963 | 4.3927 | 0.96753 | 15.5505 | 3.94341 |
| 9 | 0.97112 | 2.0534 | 1.43296 | 0.98615 | 5.3168 | 2.30581 |
| 10 | 0.98466 | 7.9797 | 2.82483 | 0.98322 | 7.6628 | 2.76817 |
| 11 | 0.9816 | 3.4759 | 1.86437 | 0.9571 | 10.1992 | 3.19361 |
| 12 | 0.93707 | 12.0046 | 3.46476 | 0.98172 | 4.3321 | 2.08136 |
| 13 | 0.86697 | 16.0169 | 4.00211 | 0.98494 | 2.8548 | 1.68961 |
| 14 | 0.9841 | 3.2313 | 1.79758 | 0.98444 | 3.8091 | 1.95169 |
| 15 | 0.9791 | 4.4144 | 2.10104 | 0.96624 | 4.0183 | 2.00456 |
| 16 | 0.93155 | 5.6492 | 2.376804 | 095561 | 15.2422 | 3.90412 |
| 17 | 0.94337 | 17.8711 | 4.22742 | 0.95468 | 4.2971 | 2.07294 |
| 18 | 0.95113 | 5.4189 | 2.32785 | 0.98337 | 5.7874 | 2.40570 |
| 19 | 0.98186 | 7.0144 | 2.64847 | 0.98597 | 4.9296 | 2.22027 |
| 20 | 0.9697 | 6.7675 | 2.60144 | 0.98524 | 4.0565 | 2.01407 |
| Input layer to Hidden Layer Weights | |||||
|---|---|---|---|---|---|
| W1 (Input 1) | W1 (Input 2) | W1 (Input 3) | W1 (Input 4) | Bias | |
| PE1 | −0.021 | −0.965 | 4.373 | 2.237 | −4.117 |
| PE2 | −7.369 | −3.284 | 0.686 | 7.401 | −4.524 |
| PE3 | −9.214 | 4.764 | 21.949 | 13.686 | 8.786 |
| PE4 | 2.778 | 2.277 | 1.178 | 3.869 | −1.082 |
| PE5 | 1.645 | −1.559 | −1.661 | −0.131 | −1.134 |
| Hidden Layer to Output Layer Weights | |||||
| W2PE1 | W2PE2 | W2PE3 | W2PE4 | W2PE5 | Bias |
| −0.719 | 0.249 | −0.676 | 0.660 | −0.786 | −0.737 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arab, M.; Faramarz, M.G.; Hashim, K. Applications of Computational and Statistical Models for Optimizing the Electrochemical Removal of Cephalexin Antibiotic from Water. Water 2022, 14, 344. https://doi.org/10.3390/w14030344
Arab M, Faramarz MG, Hashim K. Applications of Computational and Statistical Models for Optimizing the Electrochemical Removal of Cephalexin Antibiotic from Water. Water. 2022; 14(3):344. https://doi.org/10.3390/w14030344
Chicago/Turabian StyleArab, Maliheh, Mahdieh Ghiyasi Faramarz, and Khalid Hashim. 2022. "Applications of Computational and Statistical Models for Optimizing the Electrochemical Removal of Cephalexin Antibiotic from Water" Water 14, no. 3: 344. https://doi.org/10.3390/w14030344
APA StyleArab, M., Faramarz, M. G., & Hashim, K. (2022). Applications of Computational and Statistical Models for Optimizing the Electrochemical Removal of Cephalexin Antibiotic from Water. Water, 14(3), 344. https://doi.org/10.3390/w14030344







