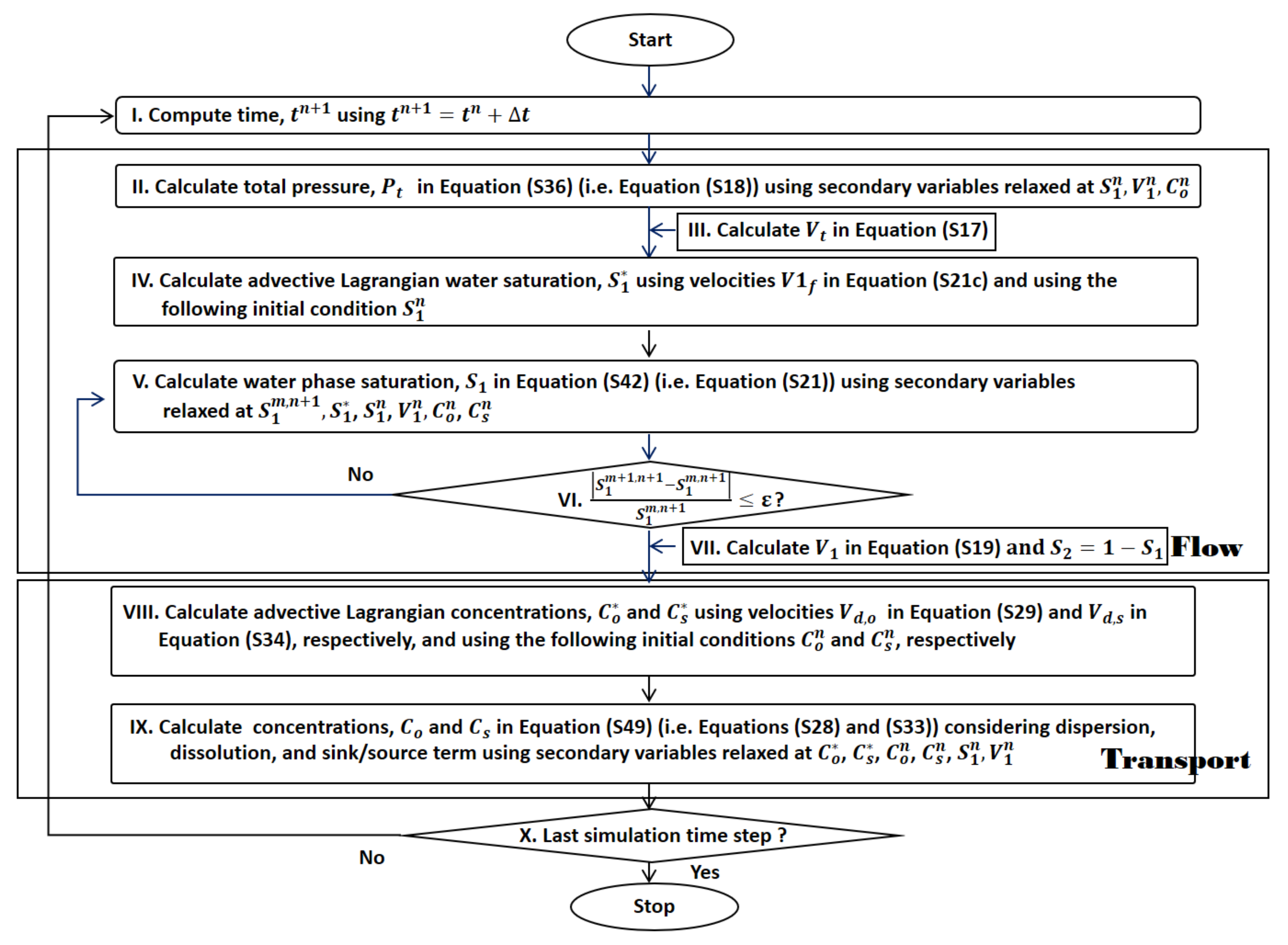
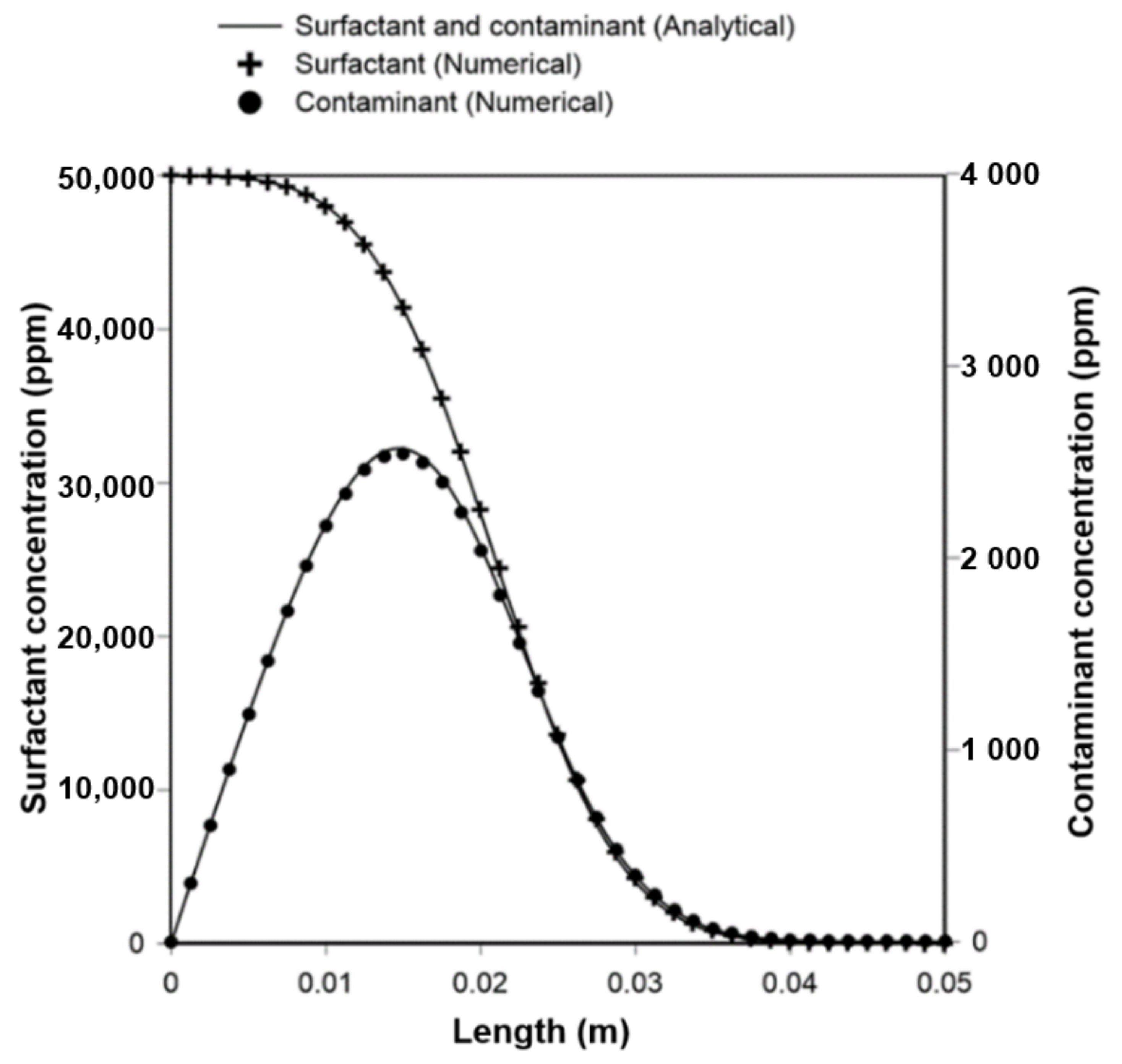
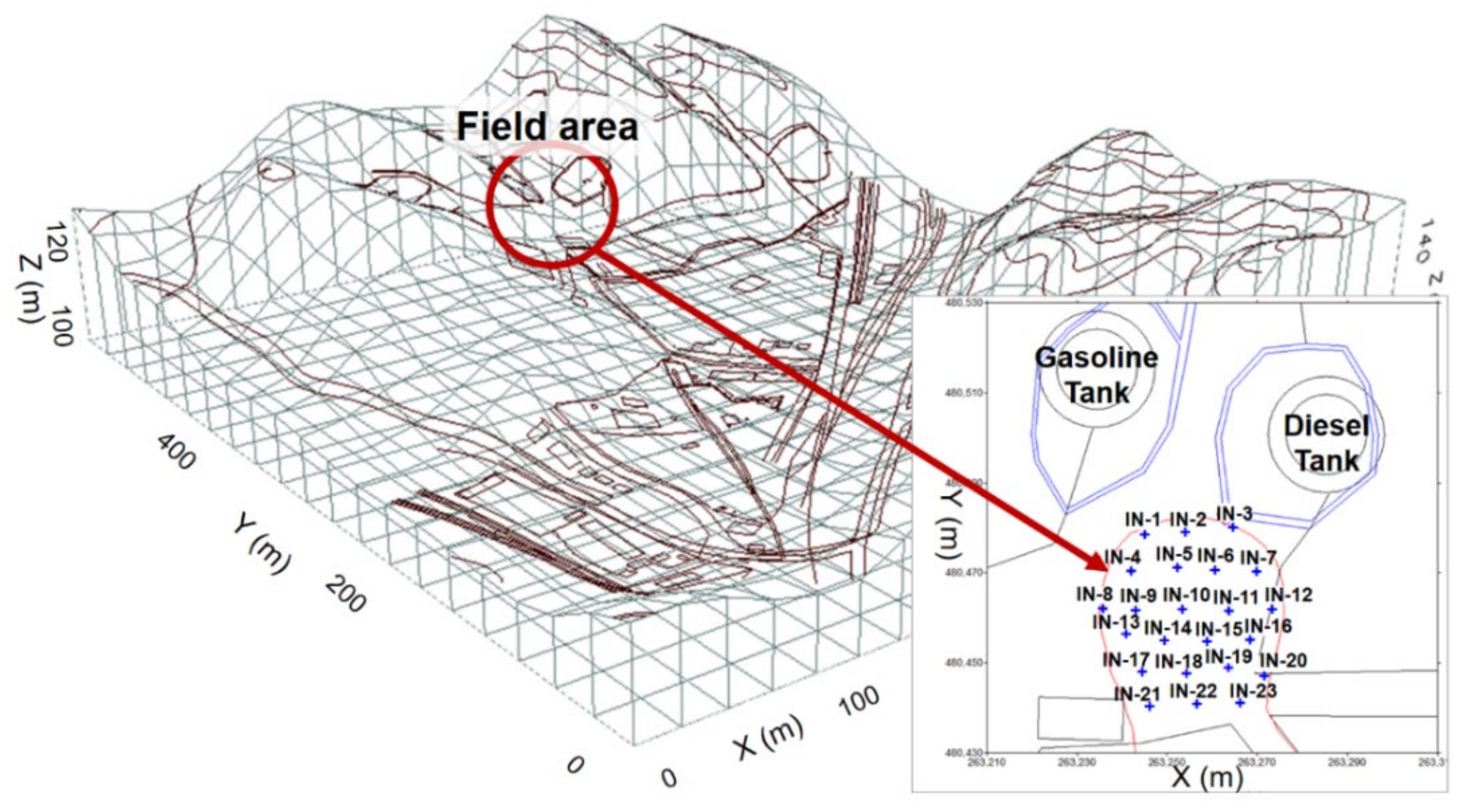
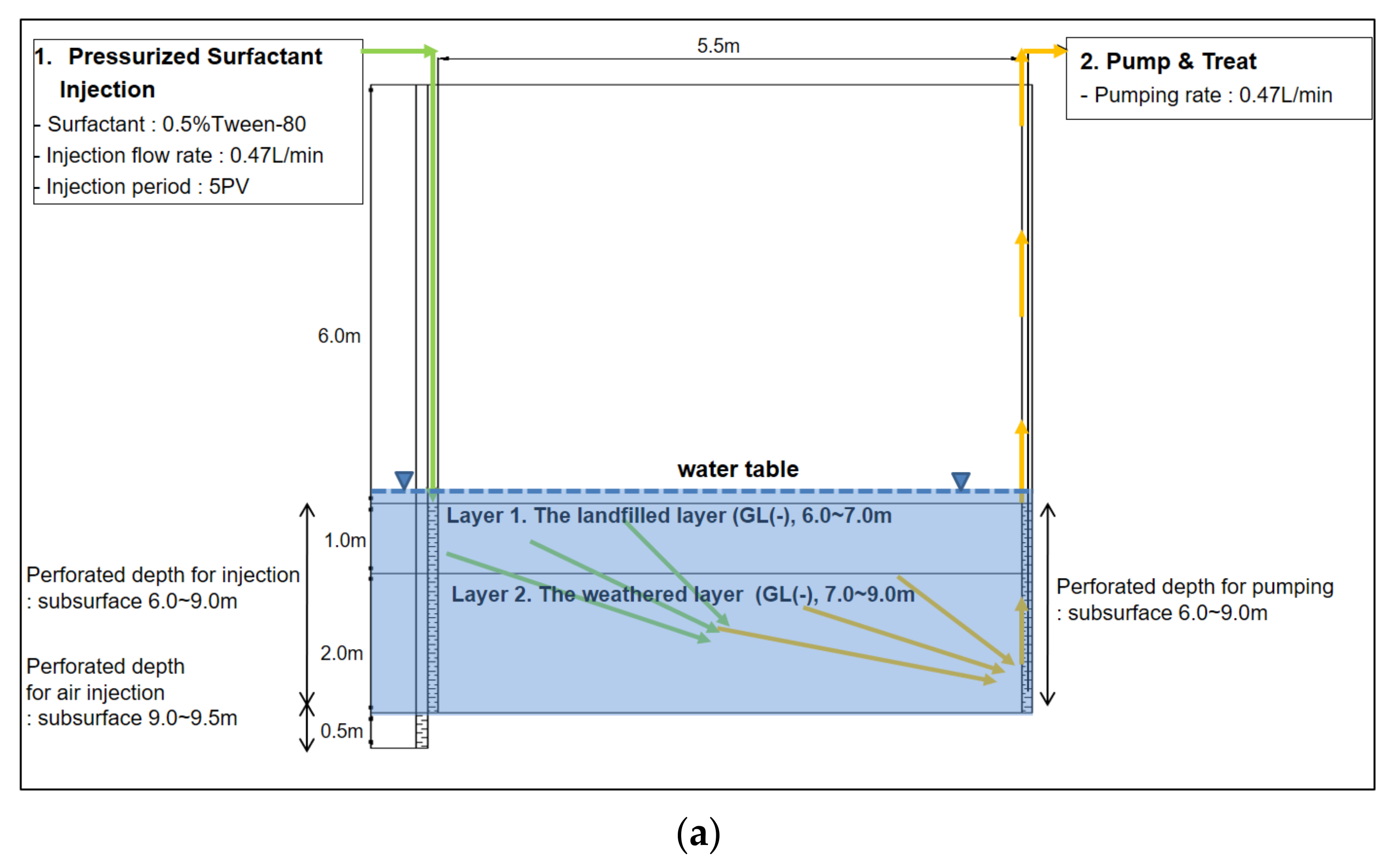
1. Introduction
The duration and cost of in situ treatment in an unconfined aquifer increases with increasing heterogeneity of the soil, and such treatment has limited applicability in layered subsurface structure. Additionally, it is regarded as a difficult situation for non-aqueous phase liquid (NAPL) remediation when solutes are released from the low-permeability zone due to back diffusion after primary sources are removed.
Because of the inefficiency of the conventional pumps and treatment technologies used to remediate aquifers, including the low permeability mentioned above, which are contaminated with non-aqueous phase liquid, considerable effort has been expended to develop alternative remediation technologies, such as surfactant-enhanced aquifer remediation. Shen et al. [
1] measured the relative permeability of sandstone and showed that the reduction in interfacial tension associated with the addition of a surfactant caused the relative permeability of both the aqueous and oil phases to increase in a water–oil system. Moreover, Torabzadeh and Handy [
2] showed experimentally that, at a certain temperature, the relative permeability of both the oil and water phases increases with decreasing interfacial tension at a given water saturation in a water–oil system. In addition to relative permeability, interfacial tension changes induced by the addition of surfactant affect the capillary pressure-saturation relationship in unsaturated flow. Desai et al. [
3] evaluated the influence of surfactant on the capillary pressure-saturation relationship and reported that lower surface tension enhanced drainage from unsaturated soil.
Surface tension reduction has been exploited to allow air sparing to be used as a subsurface remediation technique, referred to as the “surfactant-enhanced air sparging” method [
4,
5,
6]. As the surface tension reduction induced by the addition of surfactant decreased the water-holding capacity of sand, the ability of air to displace groundwater could be enhanced, thereby increasing the area of air sparging compared to that without surfactant and increasing the contact between air and NAPL. Furthermore, the flow of gas and surfactant solution through porous media results in foam generation in situ, which leads to increased viscosity and has the tendency to preferentially block channels and high-permeability regions in porous media, thus diverting gas to lower-permeability regions [
7,
8,
9].
A few studies have used numerical modeling to investigate the mechanism of enhanced remediation associated with various physical and chemical processes involved in surfactant flushing combined with air injection [
10,
11]. Similarly, with the aim of enhancing oil recovery, numerical simulations have been conducted to investigate the effectiveness of SAG injection for increasing displacement efficiency; these studies have considered surfactant foam transport in terms of the gas mobility reduction and relative permeability changes associated with a reduction of interfacial tension [
12,
13,
14,
15,
16,
17,
18,
19]. However, most of these works considered only some, and not all, of the processes that improve remediation when surfactant flushing is combined with air injection. For example, Lee et al. [
10] simulated foam flow process using method of characteristics (MOC)-based three-phase fractional flow theory (water, NAPL, foam), in which gas mobility reduction factor was calculated. However, they did not consider surfactant transport and interphase mass transfer of residual NAPL into the aqueous phase, such as dissolution, and they only considered a one-dimensional problem. However, Lee and Kam [
11] performed MOC-based modelling and simulation of foam-enhanced oil recovery processes (EOR) in a multi-layered system. Lee and Kam [
11] considered the surfactant transport advection effect but not the dispersion process. Zoeir et al. [
14] simulated the foam EOR process using commercial software such as CMG STARS. The CMG STARS explicitly solves three-phase saturation equations and phase-pressure equations to account for capillary, viscous and gravitational forces affecting multiphase flow. However, in their study, surfactant transport was not explicitly considered even in homogenous porous media. Majidaie et al. [
13] conducted numerical simulation using CMG STARS to investigate the potential of chemically enhanced water alternating gas injection as a new, enhanced oil-recovery method. They used alkaline, surfactant and polymer additives as a chemical slug, which is injected during the water alternating gas process to reduce the interfacial tension and simultaneously improve the mobility ratio. Additionally, due to immiscible carbon dioxide dissolution, oil viscosity reduction was simulated. However, Majidale et al. [
13] did not explicitly consider surfactant transport. Hosseini-Nasab et al. [
12] applied a local equilibrium with an implicit texture foam model for numerical modelling of foam flow in the sandstone rock in the presence of oleic phase. This model assumes that a local steady state of foam dynamics is reached instantaneously wherever gas and surfactant coexist in porous media. Additionally, this model calculated reduction of gas mobility due to the presence of a foaming agent and gas-relative permeability reduction, but surfactant concentration was assumed to be constant across simulation domain. In other words, surfactant transport was not considered. Commonly, all of above works simulated foam transport, interfacial tension reduction, relative permeability change, gas-phase mobility reduction, and viscosity change in their works, but did not explicitly simulate surfactant transport, or interphase mass transfer of residual NAPL phase to the aqueous phase, such as surfactant enhanced dissolution.
Although a comprehensive and sophisticated model considering all relevant processes, including foam transport, interfacial tension reduction, relative permeability change, gas-phase mobility reduction, viscosity change, surfactant-enhanced mass transfer, dissolved NAPL transport, and the capillary effect, is desirable, this would require numerous physical parameters that are not easily determined for field applications, making numerical simulations impractical to perform.
In the present study, we developed a numerical model and used it to investigate the effects of various physical and chemical processes associated with surfactant flushing and air injection on removal efficiency in a field-scale test. Here, to improve the practical application of numerical modeling, foam flooding and the increased aqueous phase relative permeability due to interfacial tension were not explicitly modeled but instead considered in the context of the increased hydraulic conductivity, which was determined simply through an aquifer test performed at the field site during the process of surfactant flushing combined with air injection. Meanwhile, the aqueous contaminant concentration and degree of contaminant removal from contaminant sites were simulated over time.
The objectives of this study were to: (1) develop a numerical model for simulating enhanced flushing to overcome difficult-to-treat contamination caused by a layered subsurface structure, (2) comparatively evaluate the results of field-scale tests and predictive models to clarify the characteristics of the remediation method, and (3) compare the results of the conventional and enhanced flushing methods to establish the superiority of the enhanced flushing method over the conventional flushing method.
4. Numerical Experiment
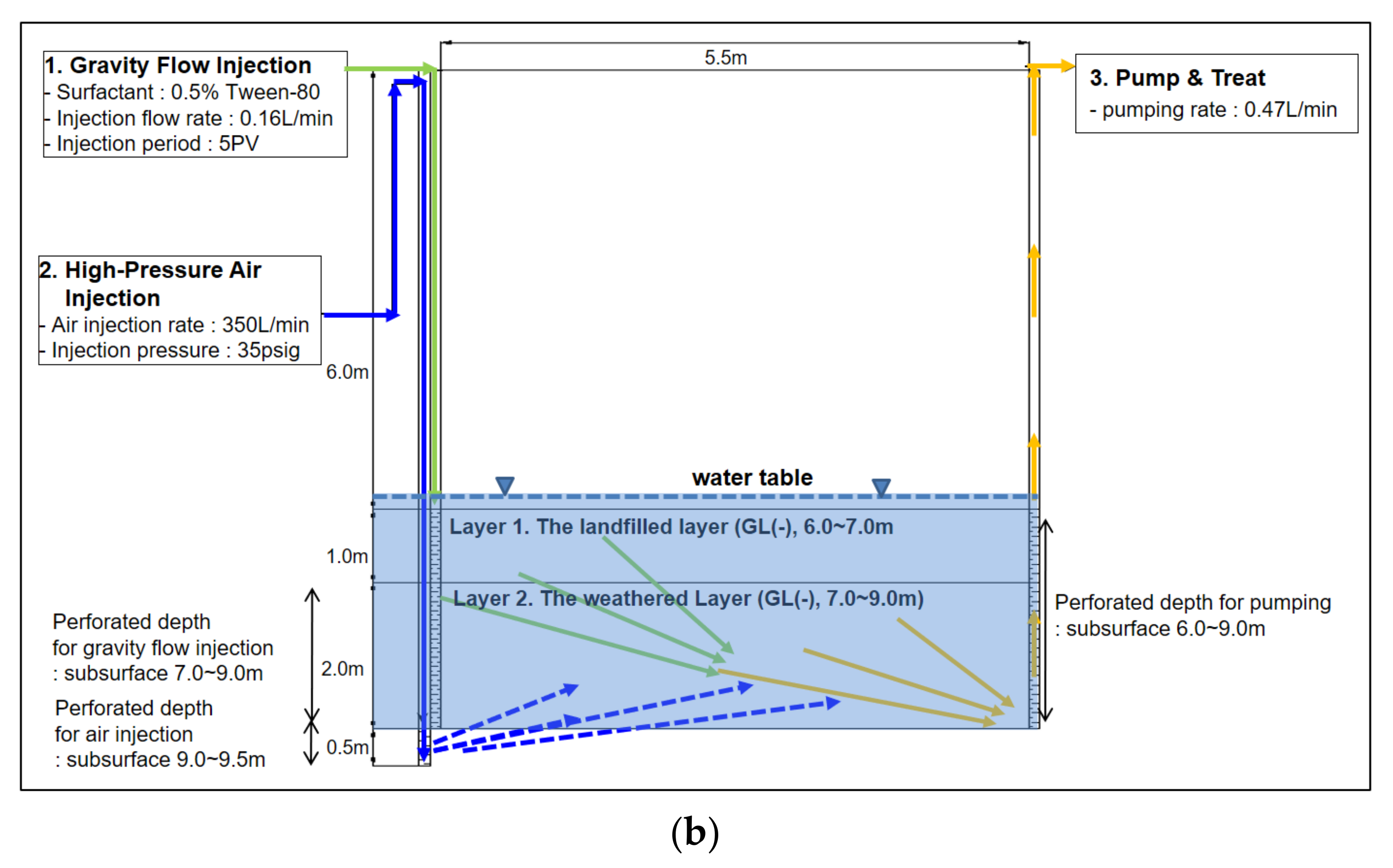
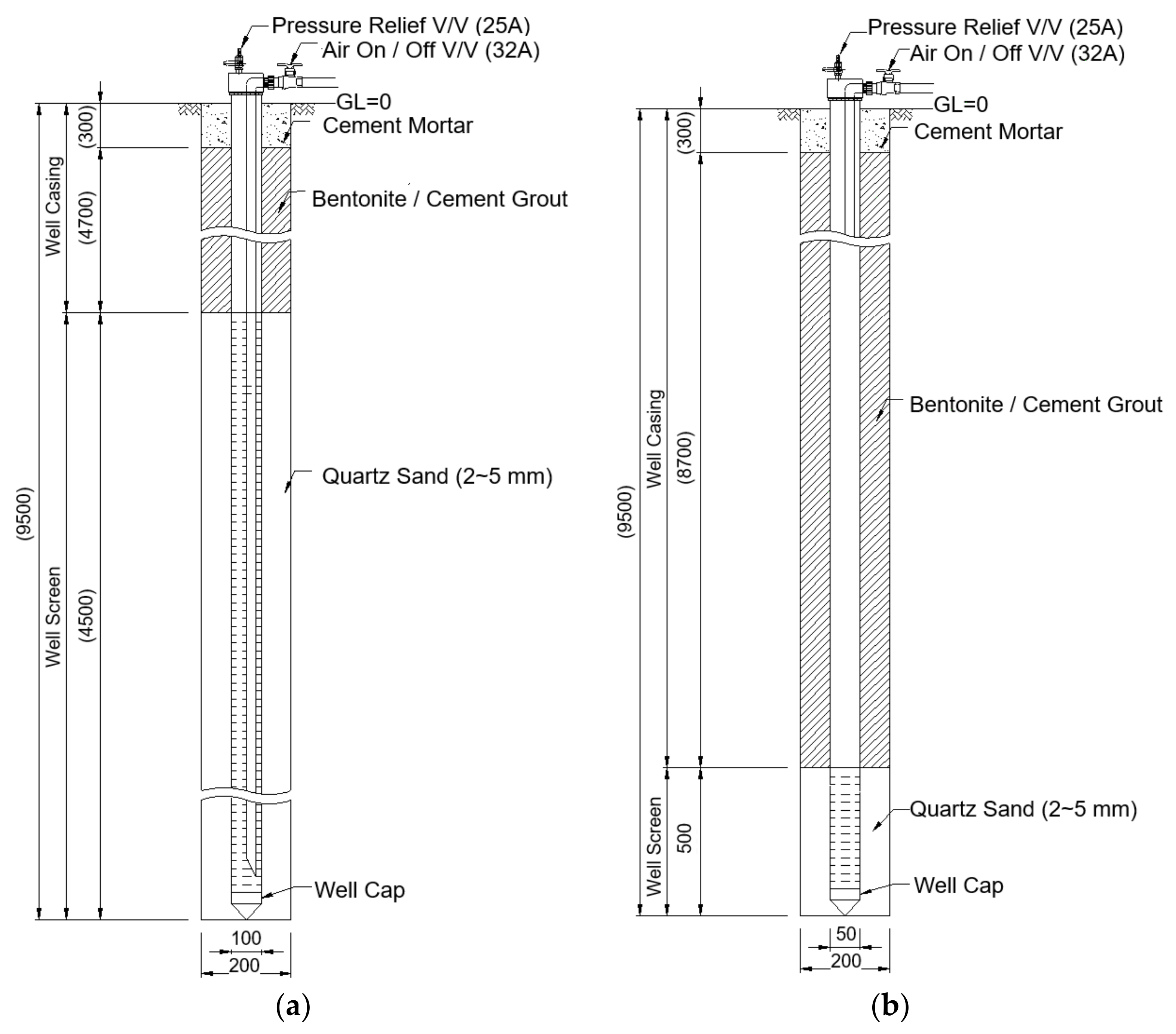
To explore the effects of high-pressure air injection during the enhanced flushing operation on remediation efficiency in the field, enhanced flushing and conventional flushing scenarios were simulated using the newly developed model to obtain concentration distributions of surfactant and dissolved NAPL over time, as well as residual NAPL saturation over time; the numerical results were compared. To reflect the aquifer and pollution distribution characteristics of the study site, the thickness of the upper layer (layer 1) was assumed to be 1 m and that of the lower layer (layer 2) to be 2 m, while the residual NAPL saturation of the upper and lower layers was set to 0.0062 and 0.0131, respectively, as shown in
Figure 8. In addition, to simulate conventional and enhanced flushing under the same conditions encountered in the field, a high rate of surfactant injection (i.e., 0.47 m/day for 5 pore volumes) was assumed for an injection well at the left boundary for conventional flushing, whereas a low rate of surfactant injection (i.e., 0.16 m/day for 5 pore volumes) was assumed for an injection well at the left boundary in the enhanced flushing scenario. The input parameters associated with the aquifer properties and field characteristics of the contaminated site used for simulating the enhanced and conventional flushing scenarios are listed in
Table 3. The surfactant-enhanced dissolution fitting parameters controlling non-equilibrium interphase mass transfer are provided in
Table 4.
To simulate the effect of air injection during enhanced flushing on remediation efficiency, we assumed that the hydraulic conductivity of layer 2 in the enhanced flushing scenario was increased 5-fold relative to that of layer 2 in the conventional flushing scenario since delivery of surfactant into layer 2 at a lower hydraulic conductivity would be facilitated by flooding with foam formed from surfactant and air. This foam flooding would reduce interfacial tension as the surfactant in layer 2 would decrease the capillary pressure, in turn leading to increased hydraulic conductivity in layer 2 (consistent with previous research). Moreover, it has been reported that in the study area, the average hydrological conductivity was increased about 4–5-fold relative to the level prior to enhanced flushing, based on slug tests carried out in three monitoring wells after the enhanced flushing operation [
31]. Further evidence of this hydraulic conductivity increase is provided by the results of a field hydraulic test, in which the zone of influence of air injection increased about 5-fold after the enhanced flushing operation [
31]. Accordingly, all input parameters in
Table 3 used the same values in both the conventional and enhanced flushing scenarios, except the hydraulic conductivity of layer 2.
5. Simulation Results
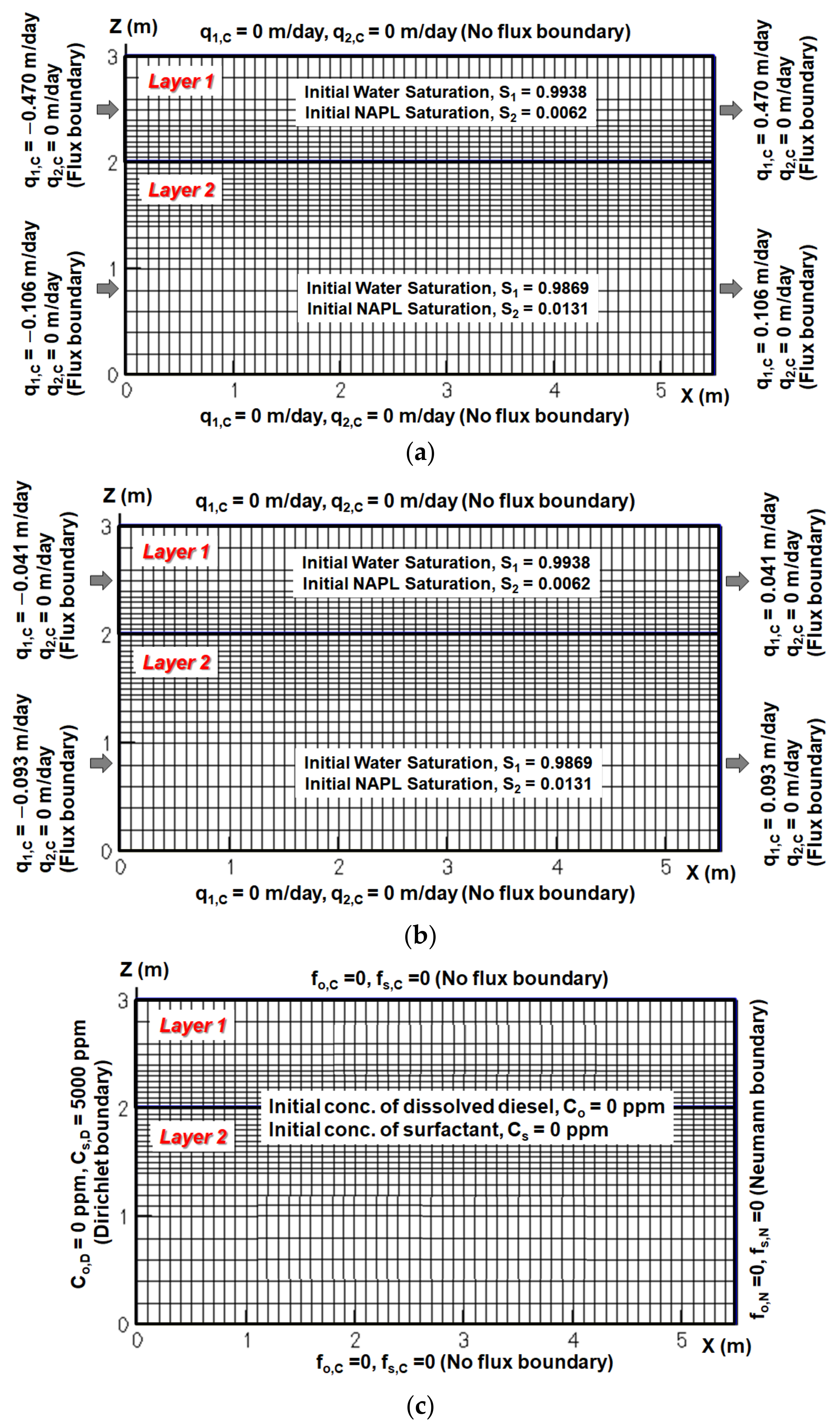
In this section, the NAPL saturation and the concentrations of the dissolved contaminant and surfactant are calculated. For this simulation, initial and boundary conditions for flow simulation of conventional flushing are presented in
Figure 8a, for flow simulation of enhanced flushing in
Figure 8b, and for solution transport of dissolved contaminant and surfactant in
Figure 8c. Here, the main dominant flow of surfactant and dissolved NAPL species would be directed from the injection well to the pumping well through two horizontal layers, so the lateral migration of the chemical species for the x-z cross section was estimated to be relatively small compared to the migration along the direction aligned with the injection and pumping wells. In addition, since the purpose of this modeling was to compare the relative difference in remedial efficiency between the conventional flushing approach and the proposed improved flushing approach, we inferred that two-dimensional simulation did not cause significant errors in investigating the difference between the two flushing approaches compared to three-dimensional simulation. Accordingly, we employed two-dimensional simulation scenarios in this study without great loss of confidence.
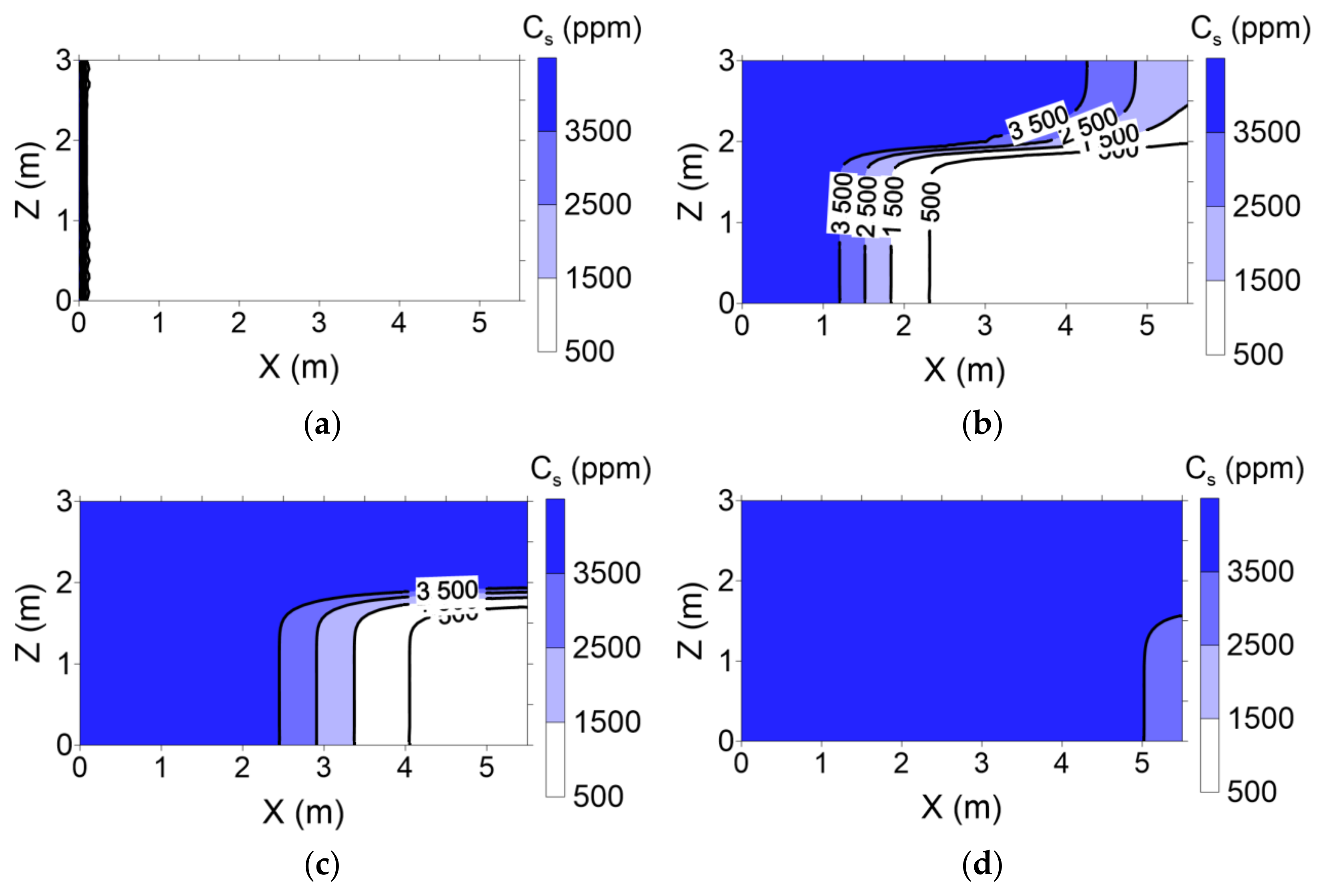
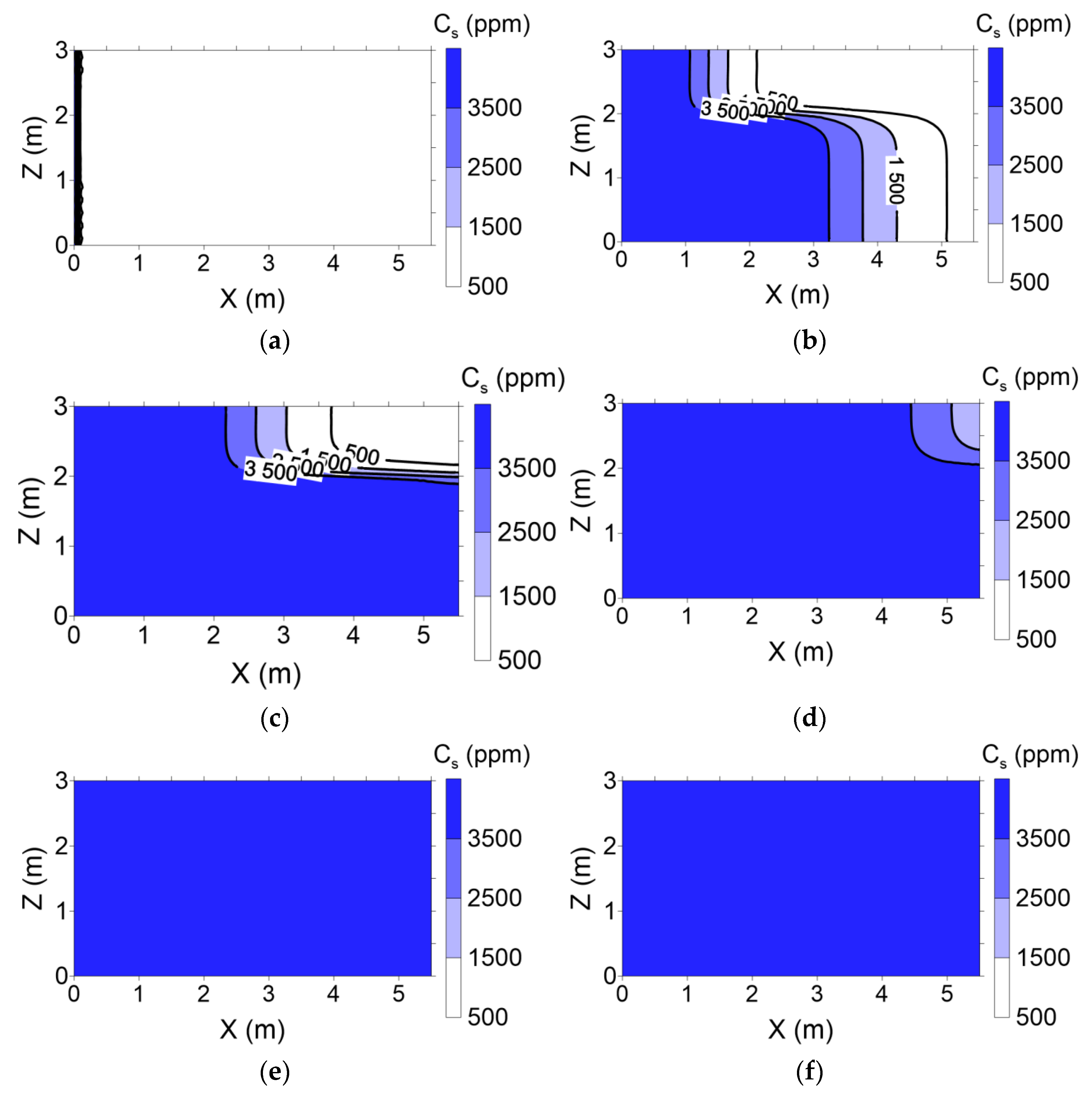
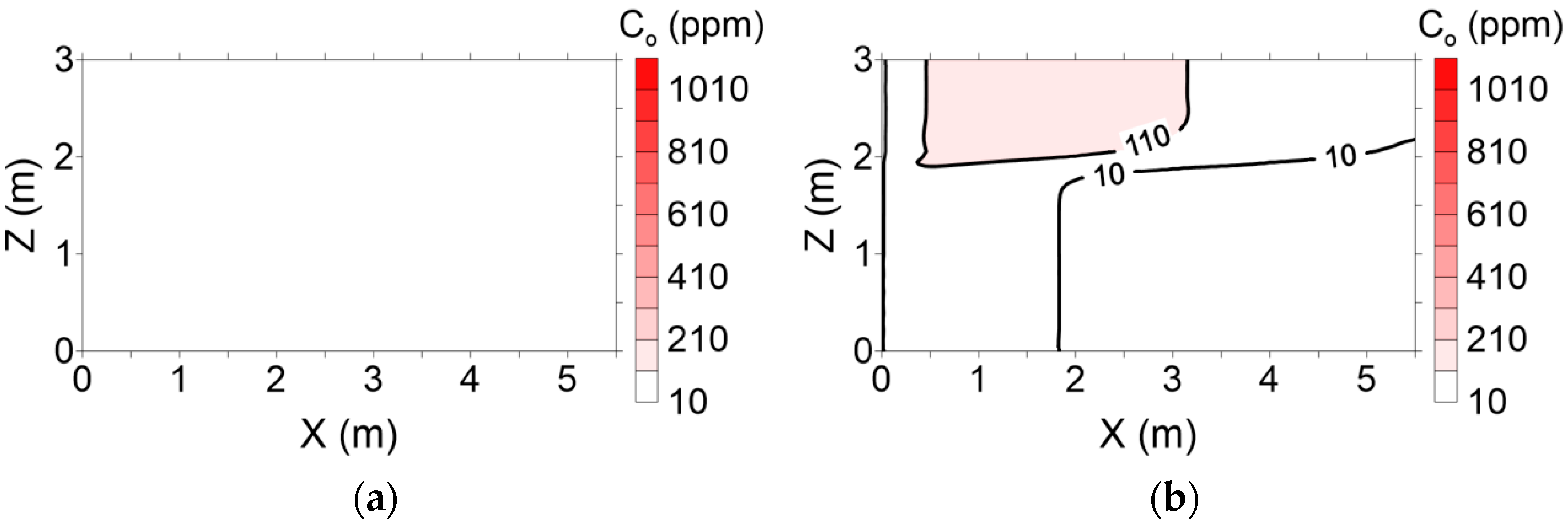
Surfactant concentration profiles with various pore volumes for conventional and enhanced flushing are shown in
Figure 9 and
Figure 10, respectively. In the conventional flushing scenarios, the hydraulic conductivity of layer 1 was 3-fold higher than that of layer 2 (
Table 3), indicating that the surfactant injected into layer 1 moved more rapidly than that injected into layer 2. The surfactant was completely distributed through layers 1 and 2 at approximately 2 pore volumes (
Figure 9). In contrast, the hydraulic conductivity of layer 1 was lower than that of layer 2 due to air injection in layer 2 in the enhanced flushing scenarios, resulting in rapid movement of the surfactant in layer 2 (
Figure 10).
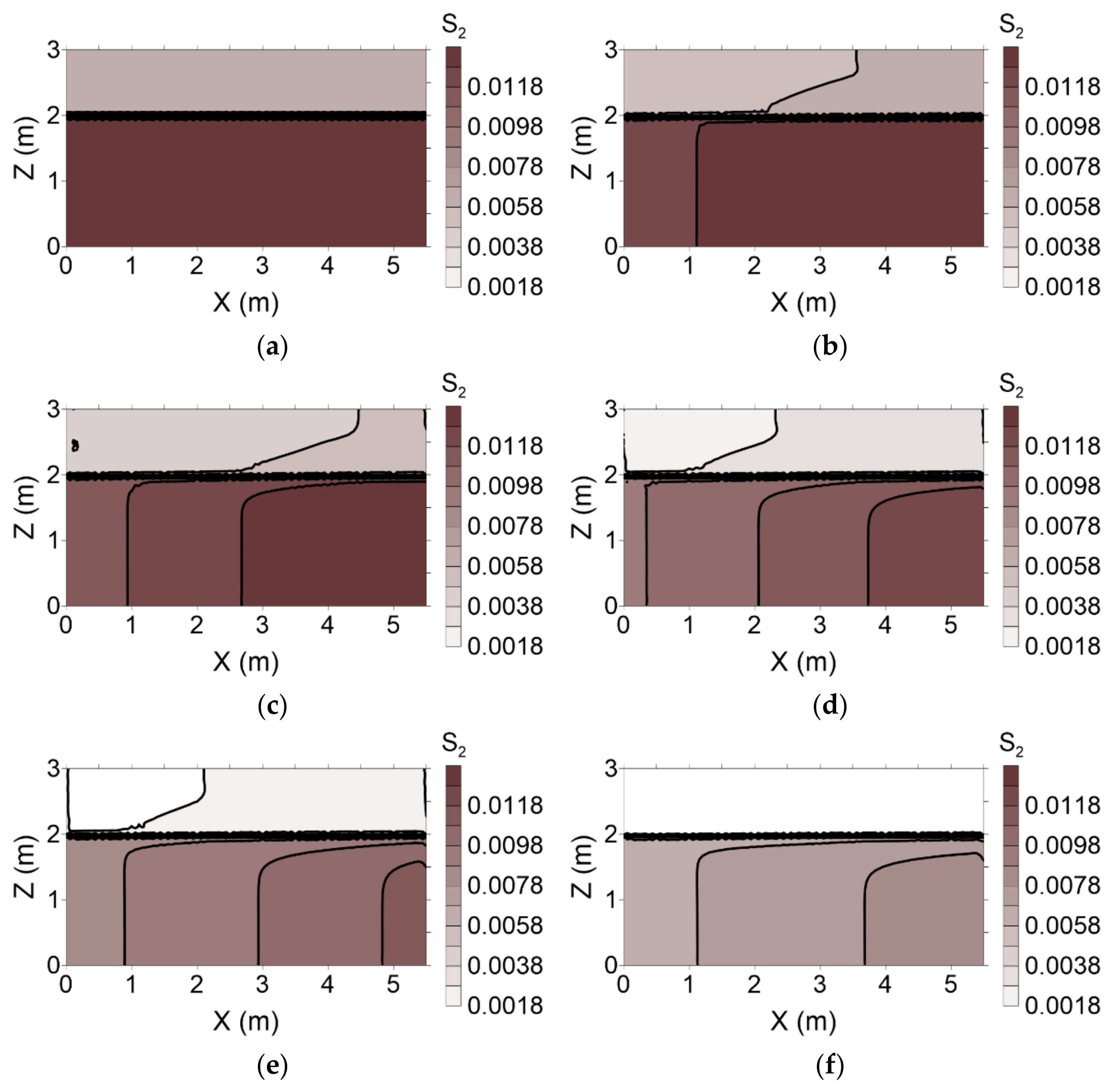
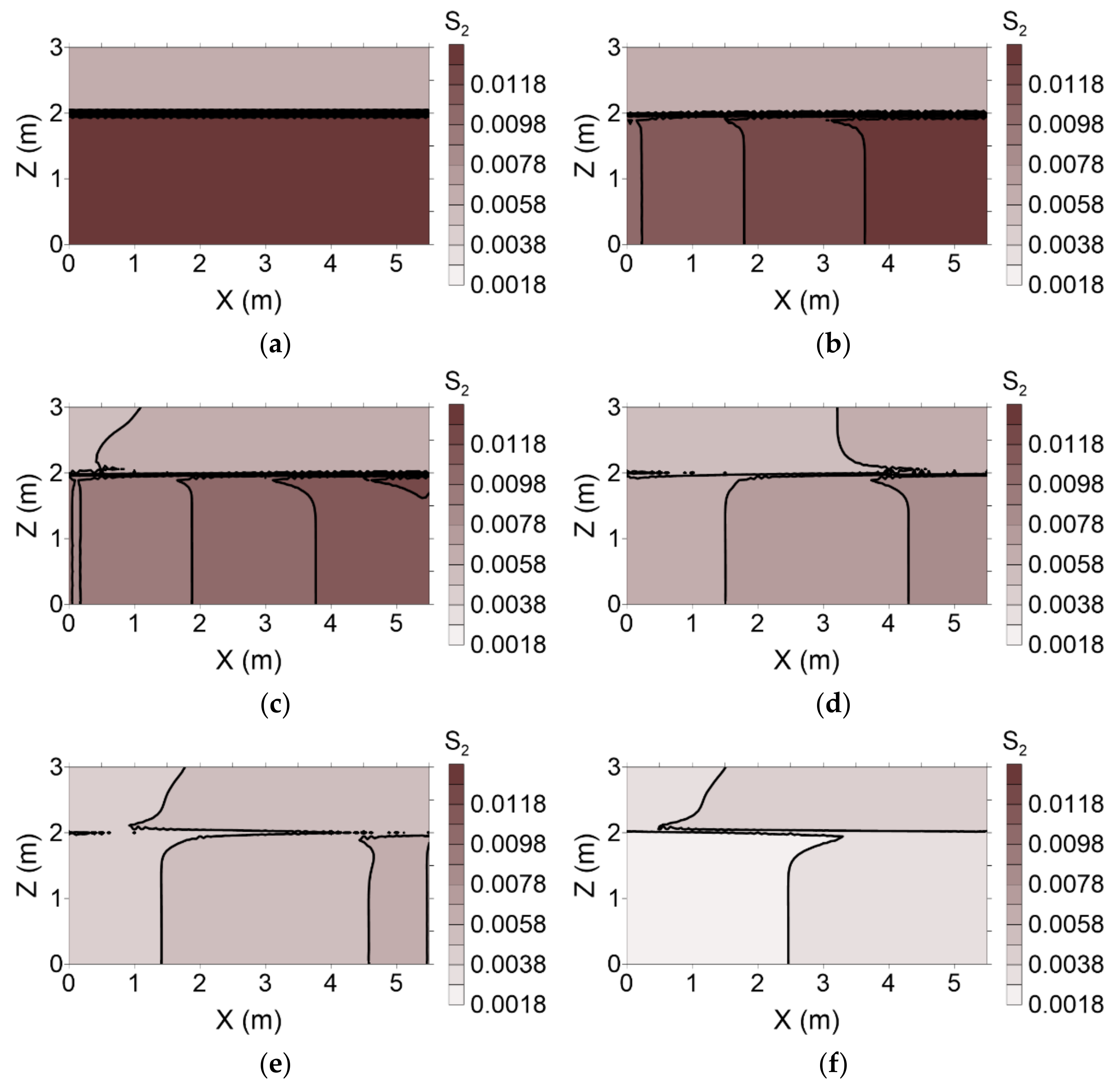
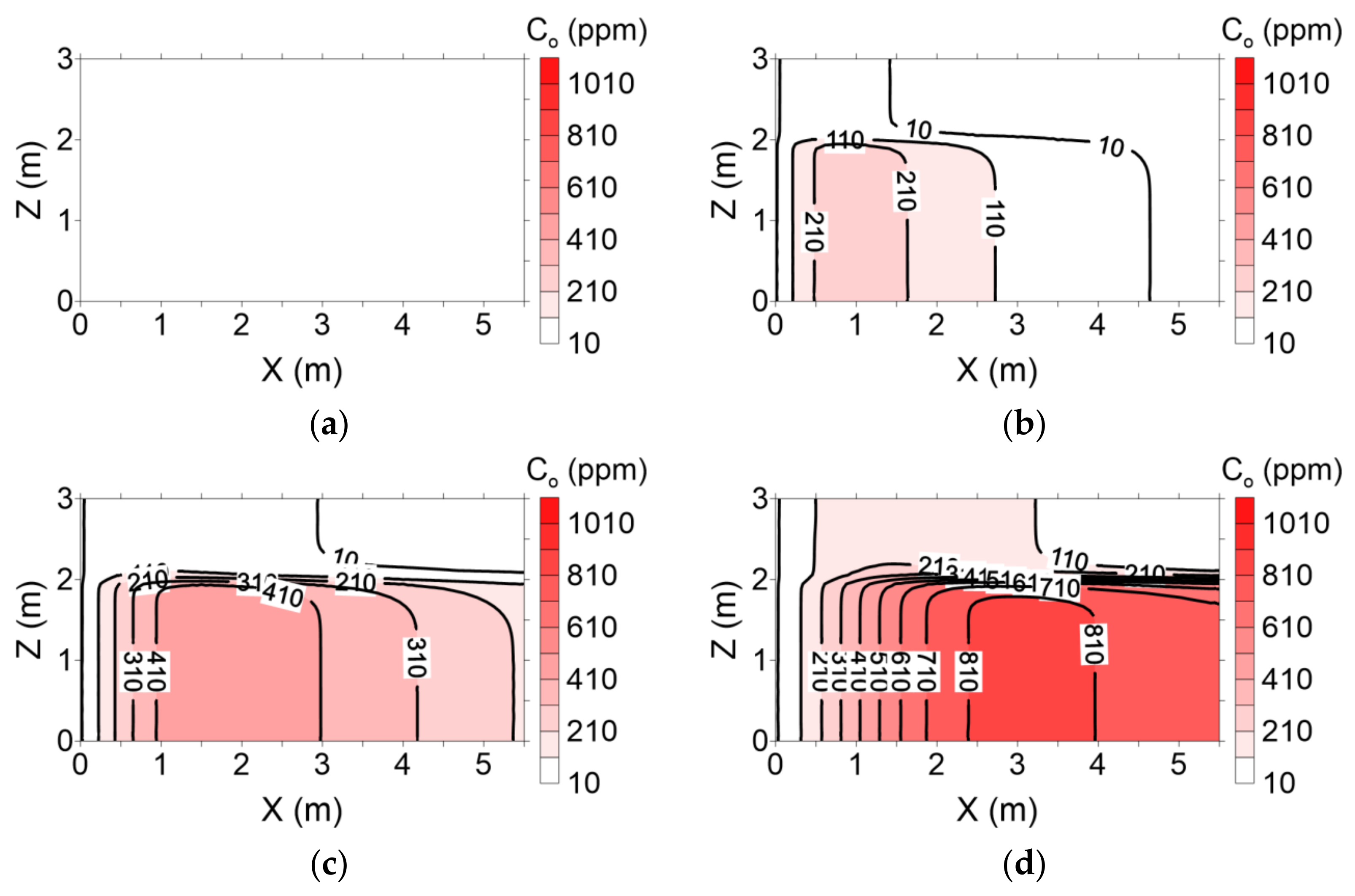
The distributions of residual NAPL saturation following surfactant injection during both flushing operations are presented in
Figure 11 and
Figure 12, respectively. With conventional flushing, surfactant injected into layer 1 moved more rapidly than that injected into layer 2. This result indicates that the surfactant facilitated dissolution of the residual NAPL. Therefore, residual NAPL in layer 1 was remediated more rapidly than that in layer 2 (
Figure 11). However, with enhanced flushing, the residual NAPL in layer 2 was remediated more rapidly than that in layer 1 because the surfactant in layer 2 moved more rapidly than its counterpart in layer 1 (
Figure 12).
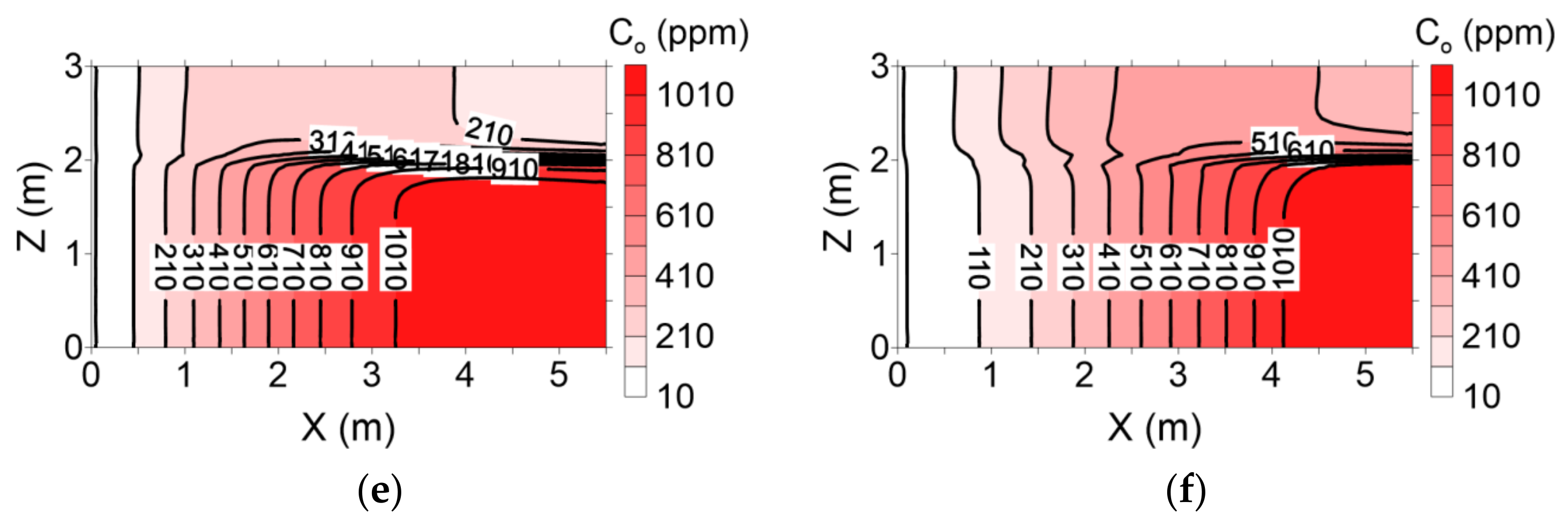
The dissolved contaminant concentrations in groundwater (i.e., the aqueous phase) following the two surfactant injection processes are shown in
Figure 13 and
Figure 14. A higher dissolved contaminant concentration was observed in layer 1 in conventional flushing scenario before the injection rate of three pore volumes was reached (
Figure 13). Subsequently, the concentrations of dissolved contaminants increased gradually and were greater in layer 2 than in layer 1. Presumably, this difference arose because the residual NAPL in layer 1 was entirely remediated, whereas a large quantity of residual NAPL remained in layer 2 beyond 3 pore volumes (
Figure 11). The dissolved contaminant concentrations of the groundwater in layers 1 and 2 were fairly high for all pore volumes tested in the enhanced flushing scenario (
Figure 14). This similarity is presumably due to the presence of residual NAPL in both layers (
Figure 12).
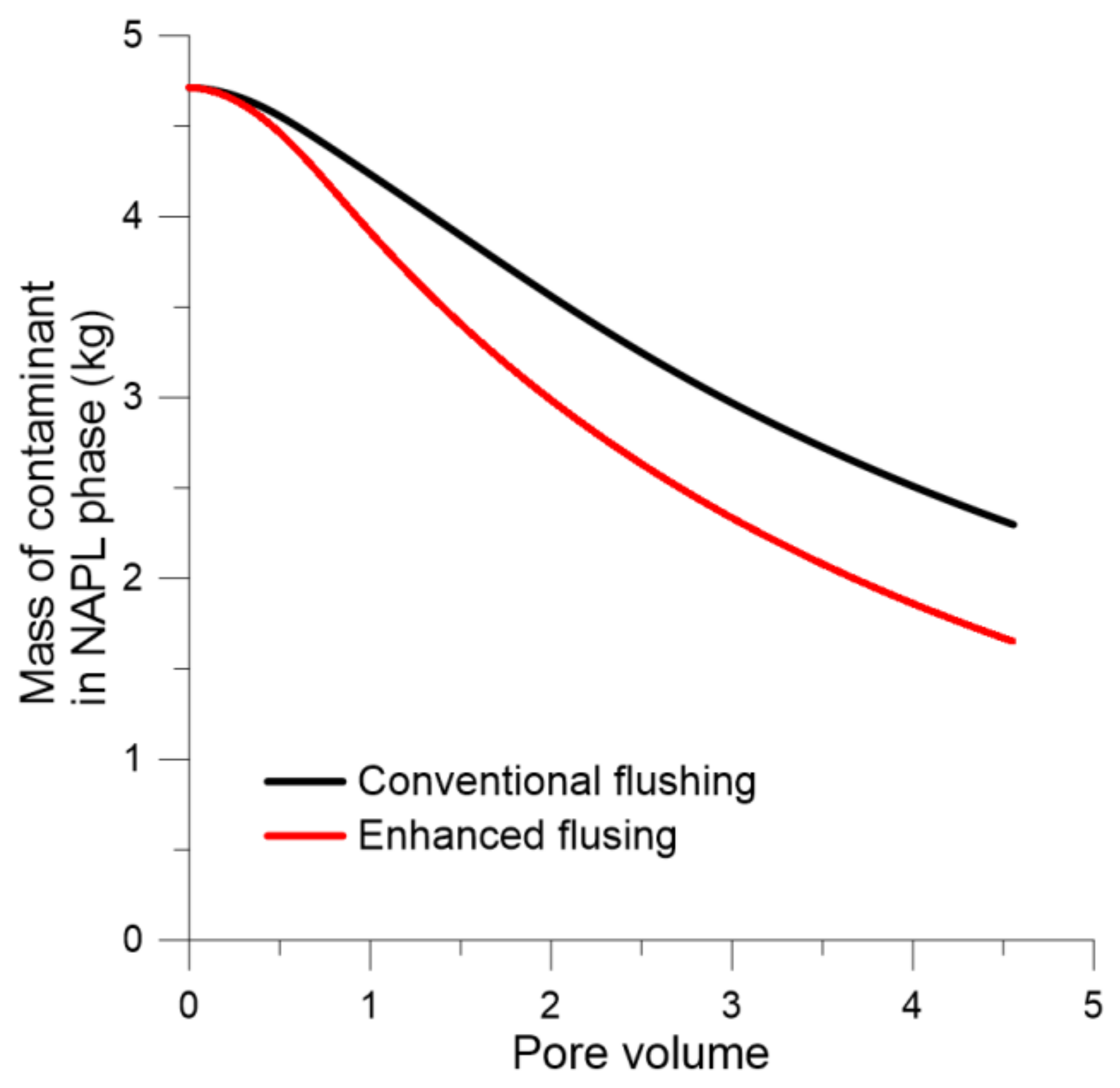
Figure 15 shows the total mass of residual NAPL in soil according to pore volume in the conventional and enhanced flushing scenarios. The removal efficiency of residual NAPL was approximately 12% greater with enhanced flushing than with conventional flushing. The difference is because the hydraulic conductivity in layer 2 with greater residual NAPL was enhanced 5-fold, as determined by field hydraulic testing. Therefore, the residual NAPL in layer 2, which had lower permeability, was remediated more efficiently.
The total mass of NAPL dissolved in the aqueous phase within the pores is shown in
Figure 16. In conventional flushing, the total mass of NAPL dissolved in the aqueous phase rapidly increased up to 2 pore volumes and slowly increased thereafter. As the hydraulic conductivity was higher in layer 1 than layer 2, resulting in more rapid movement of surfactant and dissolution of residual NAPL in layer 1, the level of residual NAPL in layer 1 decreased rapidly up to 2 pore volumes, as shown in
Figure 11. The contaminant concentration in the aqueous phase of layer 1 increased rapidly (
Figure 13). Moreover, most residual NAPL in layer 2 was removed following the nearly complete removal of residual NAPL from layer 1 at 2 pore volumes. Because the surfactant moved slowly in layer 2, the residual NAPL in layer 2 was remediated slowly; the contaminant concentration in the aqueous phase of layer 2 also increased slowly. In enhanced flushing, the total mass of NAPL in the aqueous phase decreased after reaching a maximum at 3–4 pore volumes (
Figure 14). The hydraulic conductivity of layer 2, with a residual NAPL content of 68%, was enhanced 5-fold, which accelerated surfactant transport in layer 2 and facilitated the dissolution of residual NAPL in that layer through enhanced flushing. As shown in
Figure 14, the contaminant concentration in the aqueous phase of layer 2 was very high at 3 pore volumes. Consequently, the total NAPL mass in the aqueous phase reached its maximum value at 3–4 pore volumes and then decreased during enhanced flushing (
Figure 16).
6. Field Applications of the Enhanced Flushing and Numerical Modeling
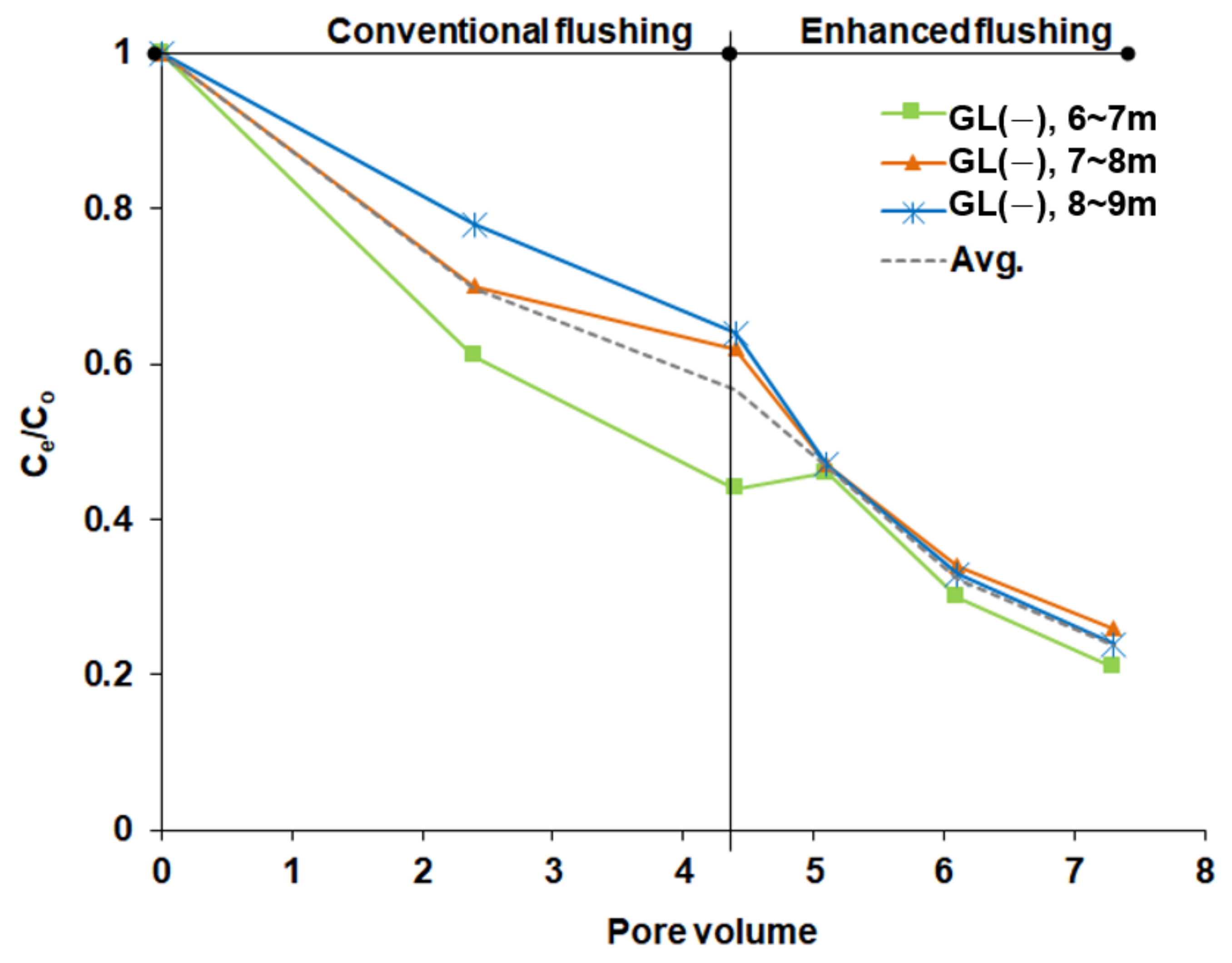
To clean up a contaminated military site in Korea to meet the Korean regulatory standard, a conventional flushing operation was performed until 4.4 pore volumes were injected at a high-flux surfactant injection rate of 0.47 L/min. Then, enhanced flushing via low-flux surfactant injection and high-pressure air injection was applied from 4.4 to 7.3 pore volumes. To compare TPH removal rates between the enhanced and conventional flushing operations at the full field scale, variations in TPH removal rates were visualized as a function of the pore volume of surfactant injected, as shown in
Figure 17, when the flushing methods were applied successively. At 4.4 pore volumes, i.e., immediately after the conventional flushing operation, the TPH removal rates were 55.8%, 39.6% and 38.1% at subsurface depths of 6.0–7.0, 7.0–8.0 and 8.0–9.0 m, respectively, as shown in
Figure 17. However, after 4.4 pore volumes, when enhanced flushing started, significant reductions in the TPH concentration occurred at all depths in the heterogeneous aquifer relative to conventional flushing. The TPH removal rates were 52.8%, 57.4% and 61.8% for subsurface depths of 6.0–7.0, 7.0–8.0 and 8.0–9.0 m, respectively.
In particular, a significantly enhanced removal rate was observed in layer 2 at a depth of 7–9 m, as shown in
Figure 17. In conventional flushing, the lowest removal rate occurred at that depth, as surfactant solution preferentially flowed through the high-permeability layer (layer 1) rather than the low-permeability layer (layer 2).
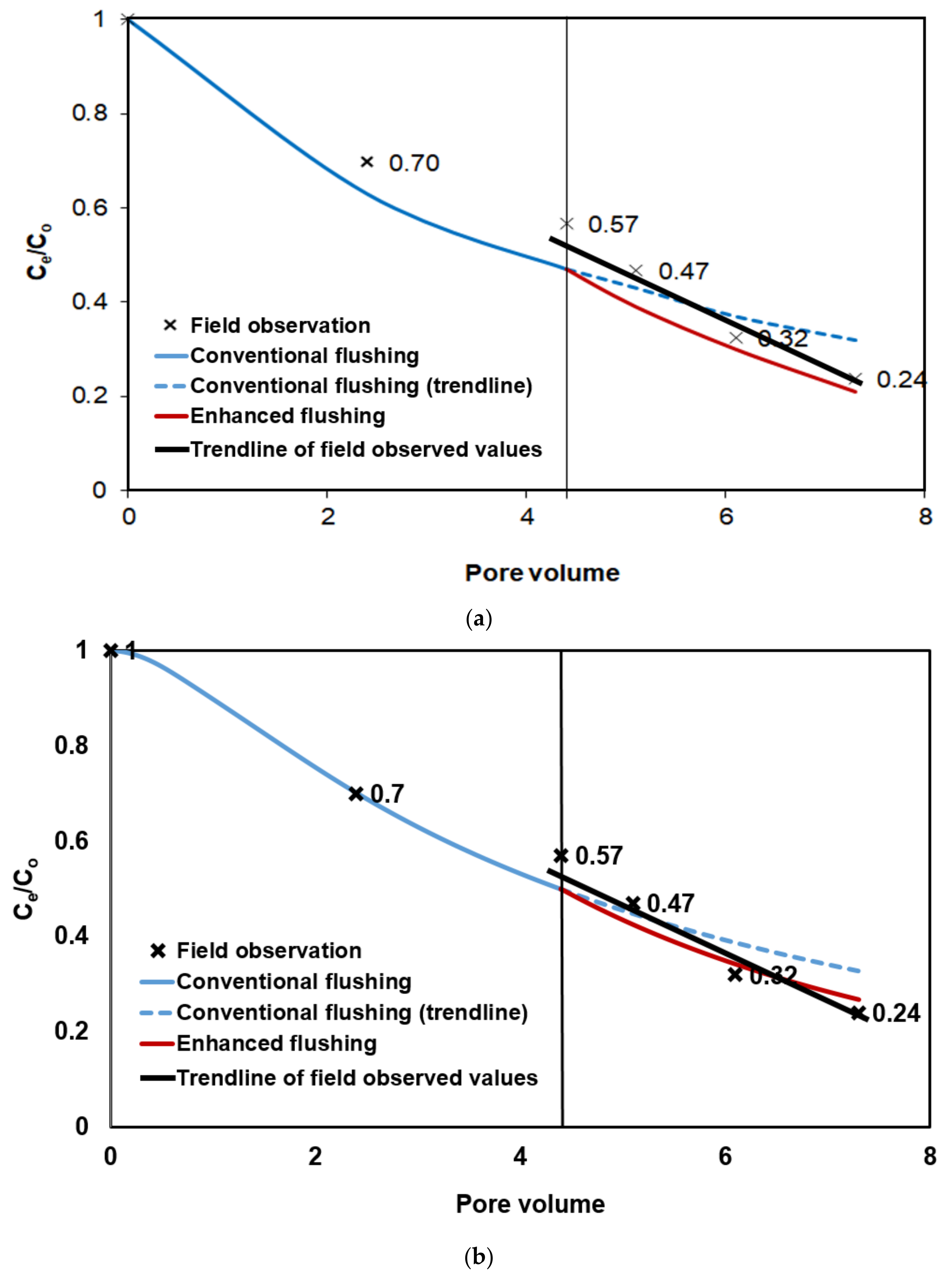
To investigate the improved removal efficiency of enhanced flushing relative to conventional flushing, we simulated successive soil flushing operations, i.e., an initial period of conventional flushing until 4.4 pore volumes followed by enhanced flushing from 4.4 to 7.3 pore volumes, and compared the simulation results against field data, as shown in
Figure 18. In this simulation, the hydraulic conductivity of layer 2 during the enhanced flushing period was set to a value 5 times larger than that used with conventional flushing, to account for the interaction between air and surfactant. The effluent to initial TPH concentration ratio (C
e/C
o) was determined through 7.3 pore volumes. As shown in
Figure 18a, the overall simulation results showed good concordance with the field data, except at the initial time points. Initially, the simulation results showed slight underestimation compared to the field data, suggesting that the numerical simulation slightly overestimated the removal of contaminants compared to real field conditions. We had guessed that this discrepancy was produced from simply using the isotropy of hydraulic conductivity in the numerical simulation and had tried to reduce this discrepancy by considering the anisotropy. The numerical simulation was performed taking into account anisotropic cases where the vertical hydraulic conductivity of
s in both layers is 10 times smaller than the horizontal hydraulic conductivity of
s, respectively. However, due to top and bottom no-flux boundary conditions, we found out that there were negligible differences in vertical velocity distributions between isotropic and anisotropic cases, and in turn contaminant removal between isotropic and anisotropic cases was almost same. In addition, the top boundary condition was changed from the no-flux boundary condition to the recharge boundary condition, so that the vertical velocity distribution was changed according to the anisotropic situation. Despite the change in the top boundary conditions, the simulated contaminant removal between the isotropic and anisotropic cases in this study showed little difference. Therefore, the reason for the discrepancy between the simulation results and the observed values in this study could not be explained by the anisotropic effect alone.
Accordingly, the parameter of the mean particle size
associated with dissolution under field conditions has been calibrated to correct a slight overestimation of numerical contaminant removal compared to actual field observations, as shown in
Figure 18a. The landfill layer in this area consists of 3.6% granules, 94.1% sand and 2.3% silt/clay, while the weathering layer consists of 1.3% granules, 89.7% sand and 8.9% silt/clay (
Table 2). According to Krumbein [
47], granules, coarse sand and silt/clay have particle sizes of 1–5 mm, 1–2 mm and 0.002–0.1 mm, respectively. In this study,
was calculated by multiplying each of the mean particle sizes of the soils by the percentages of each soil composition and then adding them. The
range was estimated at 1.1–1.9 mm and 1.0–1.8 mm in landfill and weathered layers, respectively.
In order to investigate effect of the mean particle size on the dissolution, the relationship between mass exchange rate
and mean particle size
can be written from Equations (22) and (23) as follows:
where
[
25,
27]. From Equation (26), we found out that mass exchange rate is inversely proportional to mean particle size. Therefore, in order to reduce the discrepancy between the simulated overestimated mass removal and the observed values, as shown in
Figure 18a, we performed numerical calibration by gradually increasing the mean particle sizes of the landfill layer and of the weathered layer from 1.5 to 1.9 mm and from 1.4 to 1.8 mm, respectively. Finally, the numerical solutions matched best with the observations when the
s of the landfill layer and of the weathered layer were 1.875 mm and 1.75 mm, respectively (
Figure 18b). In particular, at least before 4.4 PV, the numerical solution exactly matched with the observed values. Even after 4.4 PV, the numerical simulation results of enhanced flushing were in overall good agreement with the observed values. Compared to previous smaller mean particle sizes, calibrated simulation result obtained using the larger mean particle sizes was better matched with observed values, as shown in
Figure 18b. Although exact match between enhanced flushing and field observations cannot be obtained after 4.4 PV, we figured out from numerical simulation that contaminant removal rate after enhanced flushing operation was accelerated compared to conventional flushing operation before 4.4 PV, which was consistent with field observation. Therefore, in
Figure 18b, slope of line in case of enhanced flushing is better matched with that of field observation trendline than slope of line in case of conventional flushing. Lastly, the small discrepancies between enhanced flushing and field observation may be still attributed to various causes such as failures to consider various wettability of geological material (contact angle), explicit surfactant foam transport, solute diffusion through immobile water films sandwiched between NAPL blobs and soil grains, and physical bypassing of mobile phases around contaminated regions.
In conclusion, the interplay between air injection and surfactant flushing during enhanced flushing gives rise to various complex chemical and physical processes that affect remediation efficiency, including surfactant foam transport, diversion of surfactant to the low-permeability layer by foam, interfacial tension reduction due to surfactant in the low-permeability layer, an increase in relative permeability, and surfactant-enhanced drainage following a reduction of capillary action and interfacial tension. To simulate all of these processes accurately, a complex, comprehensive and sophisticated numerical model would be needed, including many parameters that are not easy to measure and thus generally not suitable for field applications. However, considering only one parameter that is related to all of these processes, i.e., hydraulic conductivity, allows for the application of a simple numerical simulation to field conditions. For broader practical application of numerical modeling, further investigation of the empirical and experimental relationships of enhanced hydraulic conductivity with various processes that occur during enhanced flushing is needed.
7. Conclusions
Numerical modeling was performed using a newly developed model to predict the efficiency of contaminant removal and to elucidate the characteristics of the enhanced flushing system through comparison with other flushing methods. An enhanced flushing operation was conducted at the field scale to overcome the difficult treatment conditions associated with contamination in a heterogeneous geological setting.
To investigate the effects of high-pressure air injection during enhanced flushing on remediation efficiency in the field, enhanced and conventional flushing scenarios were simulated using the developed model to obtain concentration distributions of surfactant and dissolved NAPL over time, as well as residual NAPL saturation over time; the numerical results were compared to the field data. In the numerical experiment, the removal efficiency of residual NAPL was approximately 12% greater with enhanced flushing than with conventional flushing at 5 pore volumes because the hydraulic conductivity of layer 2, which had a residual NAPL content of 68%, was enhanced 5-fold, thus accelerating surfactant transport in layer 2 and facilitating the dissolution of residual NAPL through enhanced flushing.
At a contaminated military site in Korea, where successive soil flushing operations were performed to investigate the improved removal efficiency of enhanced flushing relative to conventional flushing, we simulated successive soil flushing operations using our newly developed model and compared the simulation results with field data. During the early stage of the conventional flushing operation, TPH removal rates at 4.4 pore volumes were 55.8%, 39.6% and 38.1% at subsurface depths of 6.0–7.0, 7.0–8.0 and 8.0–9.0 m, respectively. However, with enhanced flushing from 4.4 to 7.3 pore volumes, the TPH removal rates were 52.8%, 57.4% and 61.8% at subsurface depths of 6.0–7.0, 7.0–8.0 and 8.0–9.0 m, respectively. A significant reduction in contaminant concentration was observed regardless of depth within the heterogeneous aquifer. This enhanced removal could be simulated using the model developed in this study, as shown in
Figure 18a,b. The simulations slightly overestimated contaminant removal compared to the field-scale test in
Figure 18a. By performing the numerical calibration of the mean particle size and by using the larger mean particle sizes, we obtained the calibrated solutions results that accurately matched the observations in
Figure 18b. This result indicated that successful simulation results can be obtained using only one parameter, i.e., enhanced hydraulic conductivity, which was identified based on the effects of various chemical and physical processes involved in the interplay between air injection and surfactant flushing, seen during enhanced flushing.
In conclusion, our field-scale test showed that low-flux injection of a surfactant, combined with high-pressure air sparging, is an effective novel method for improving the solubility and mobility of contaminants through enhancement of hydraulic conductivity. Moreover, we found that a simple numerical model considering only enhanced hydraulic conductivity rather than all of the chemical and physical processes involved in the interplay of air injection with surfactant flushing, facilitating assessment of the remediation performance of enhanced flushing technology.