Monitoring of SARS-CoV-2 Variants by Wastewater-Based Surveillance as a Sustainable and Pragmatic Approach—A Case Study of Jaipur (India)
Abstract
:1. Introduction
2. Methodology
2.1. Sample Collection and Transportation
2.2. Sample Preparation for SARS-CoV-2 Detection Using RT-qPCR
2.3. SARS-CoV-2 Quantitative and Qualitative Detection
2.4. Sample Pre-Processing for NGS RNA Extraction: Virus Filtration and Concentration
2.5. Total RNA Extraction
2.6. Sample Pooling for NGS
2.7. Qualitative and Quantitative Analysis of RNA Samples
2.8. Preparation of 2 × 150 NextSeq500 ARTIC Library
2.9. Quantity and Quality Check (QC) of Library on Agilent 4200 Tape Station
2.10. Cluster Generation and Sequencing
2.11. Bioinformatic Analysis
3. Results
3.1. SARS-CoV-2 Gene Concentrations in Wastewater and the Quality and Depth of the Data Obtained
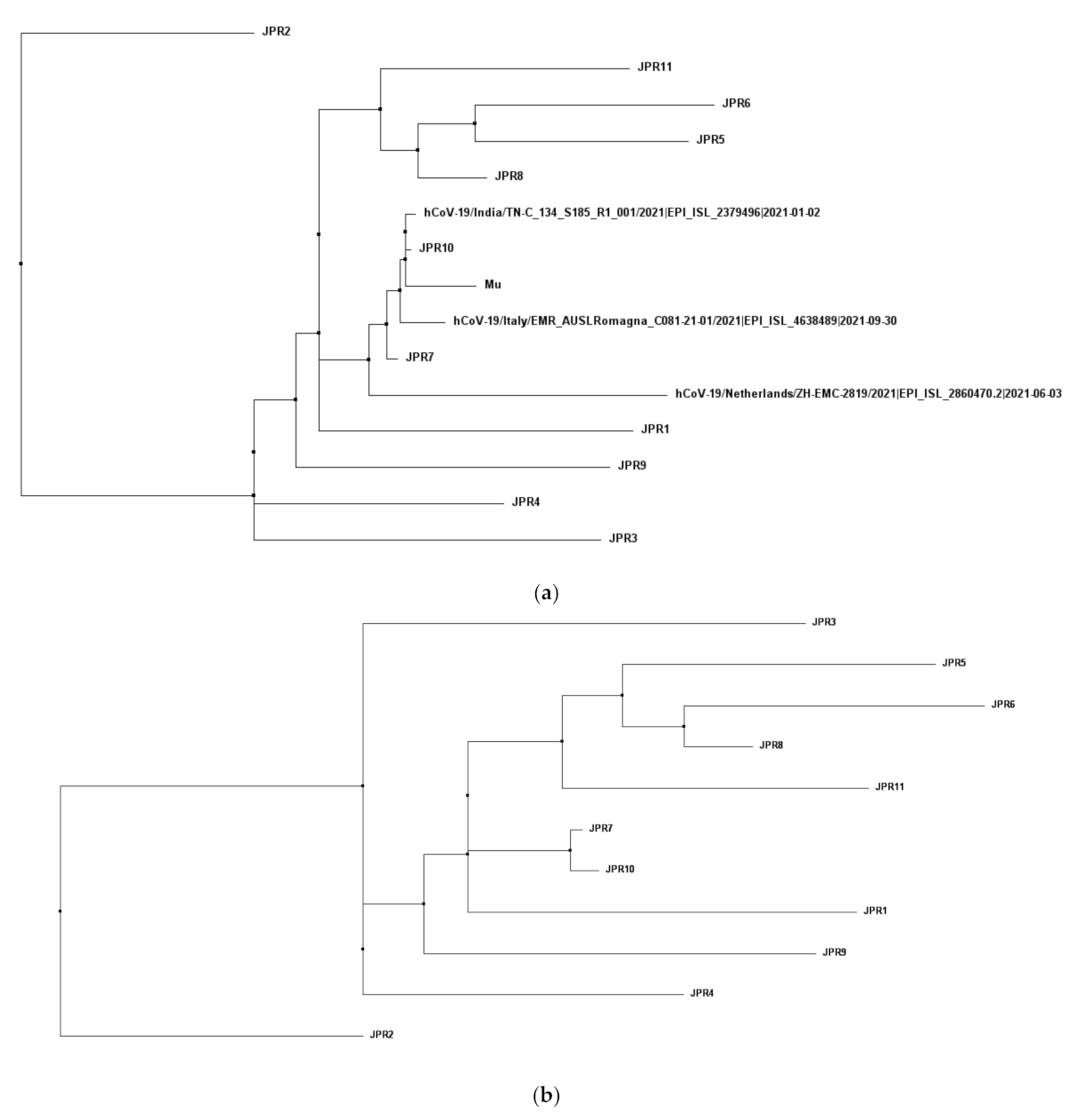
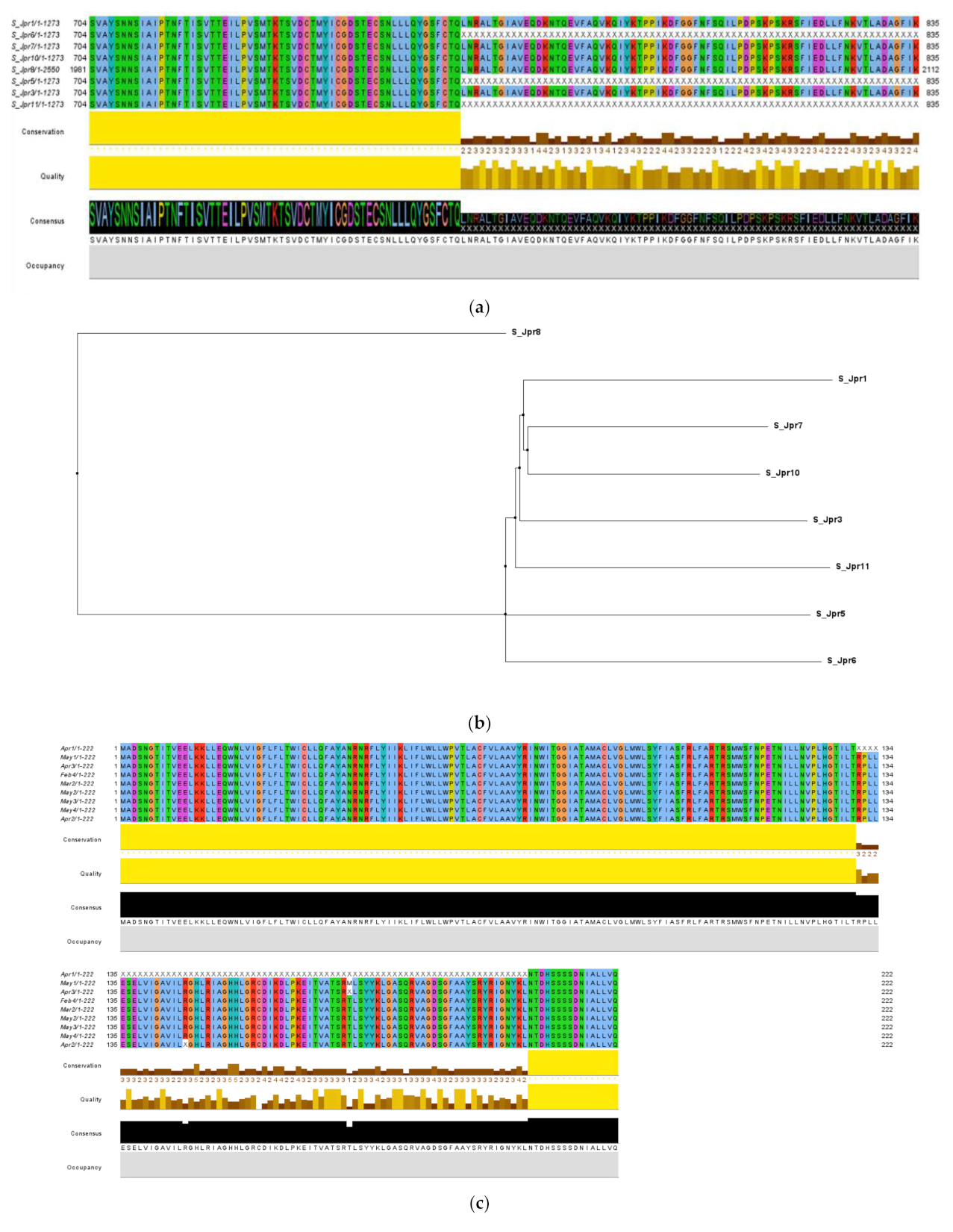
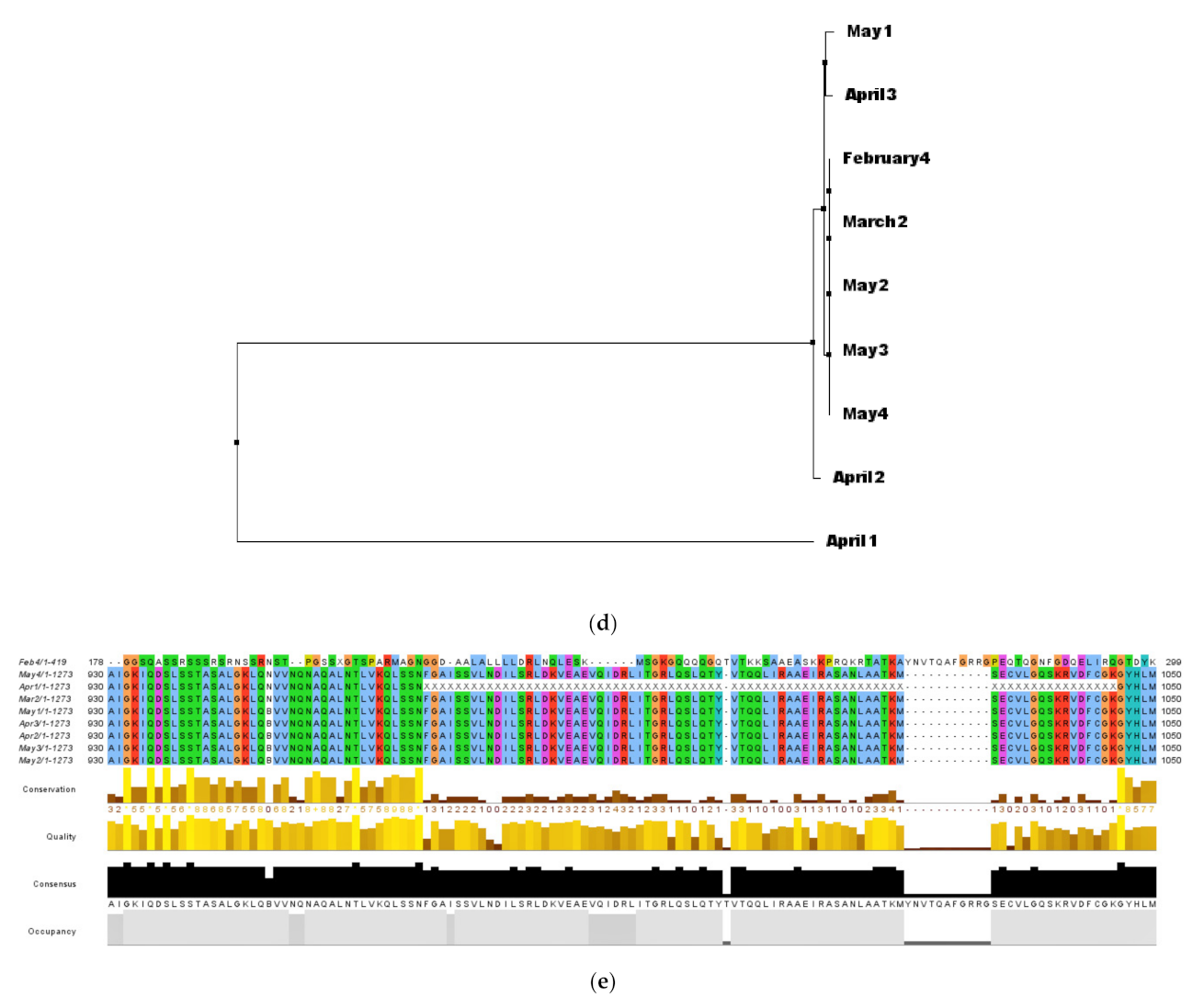
3.2. Lineage Identification and Multiple Sequence Analysis with Other Reference Sequences
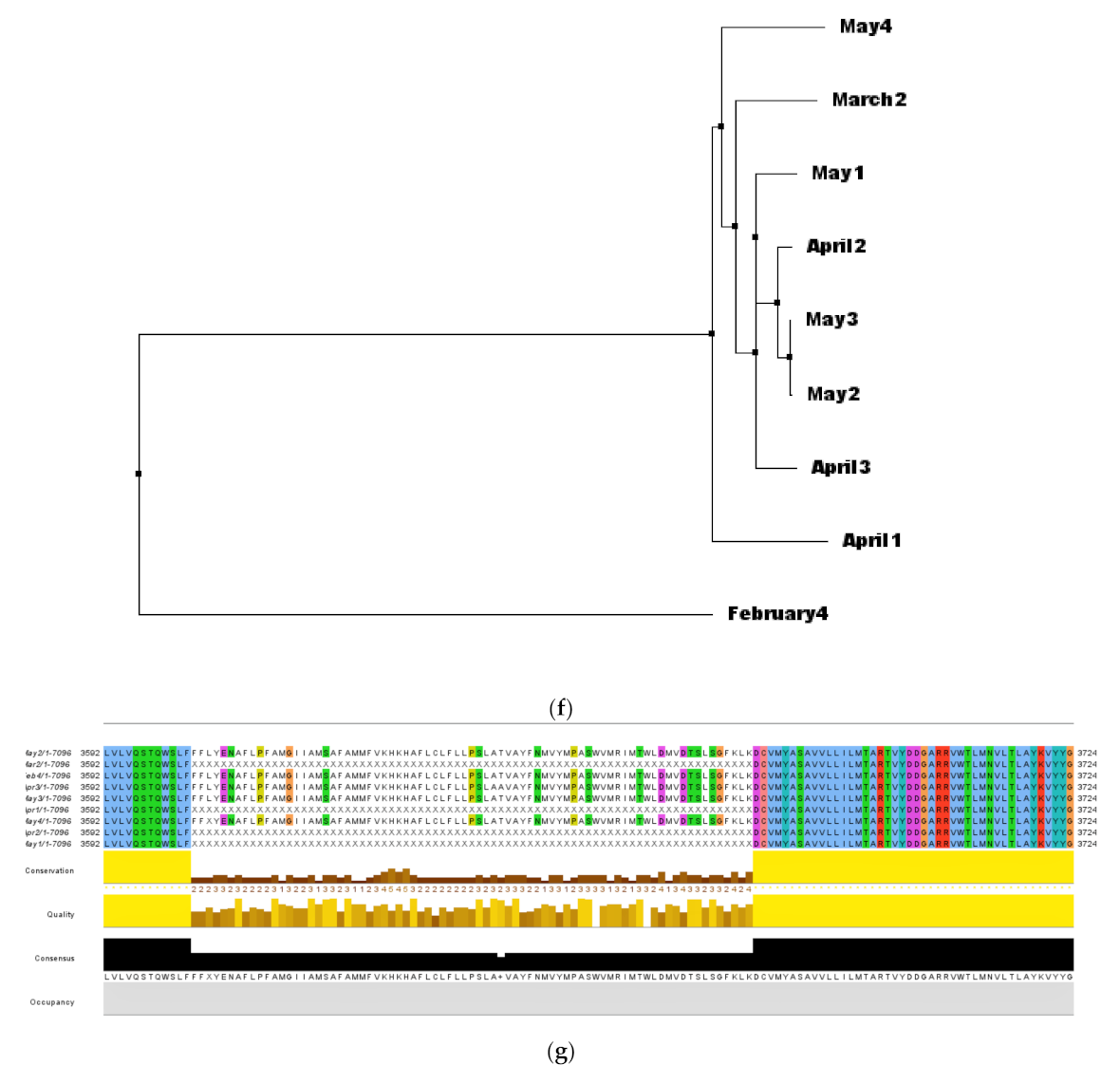
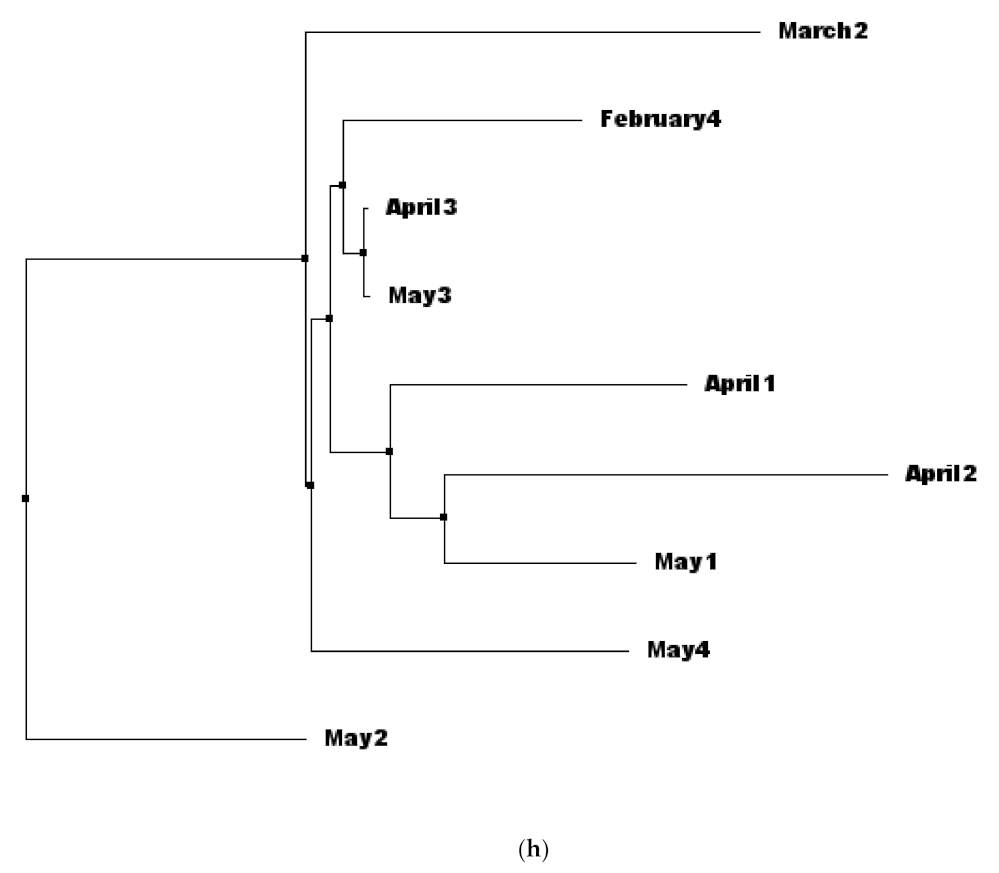
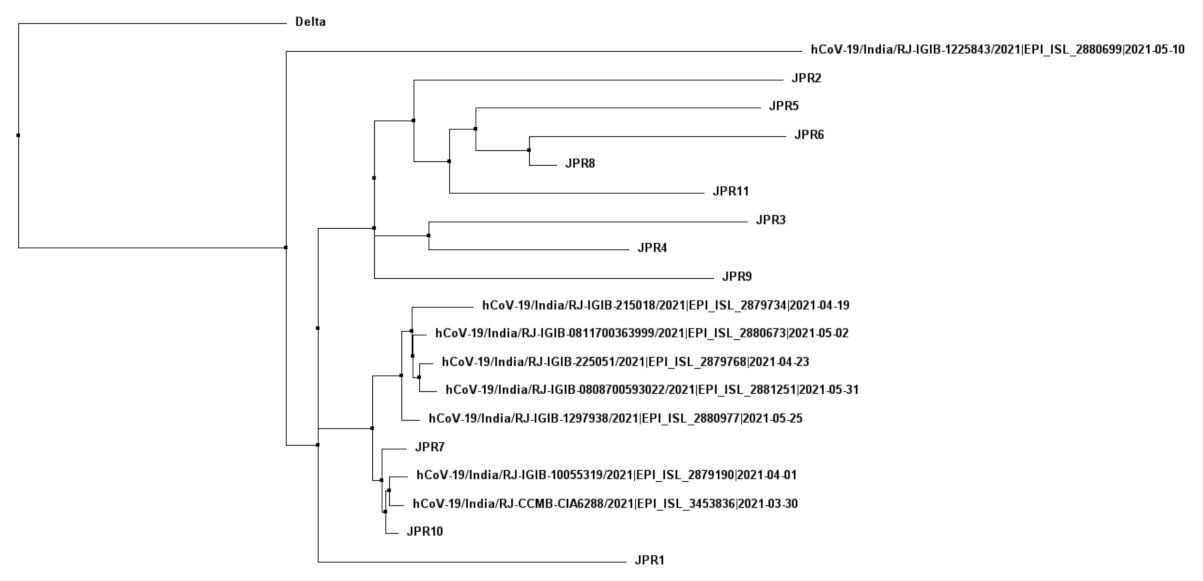
3.3. Analysis of Phylogeny and SNPs in the Samples
3.4. Comparisons of SNPs with the Delta and Delta Plus Variants
3.5. Variations in Various Genes Identified in the WBE Samples
3.6. Comparing the Sequences Obtained in Context of Patient Samples of the City Obtained during the Same Time Frame
3.7. Sequences on Public Databases
4. Discussion
5. Significance of the Work and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, H.; Wang, H.; Li, X.; Zheng, W.; Ye, S.; Zhang, S.; Zhou, J.; Pennington, M. Economic burden of COVID-19, China, January–March, 2020: A cost-of-illness study. Bull. World Health Organ. 2021, 99, 112. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/news/item/28-11-2021-update-on-omicron (accessed on 14 December 2021).
- Bakhshandeh, B.; Jahanafrooz, Z.; Abbasi, A.; Goli, M.B.; Sadeghi, M.; Mottaqi, M.S.; Zamani, M. Mutations in SARS-CoV-2; Consequences in structure, function, and pathogenicity of the virus. Microb. Pathog. 2021, 154, 104831. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Amereh, F.; Negahban-Azar, M.; Isazadeh, S.; Dabiri, H.; Masihi, N.; Jahangiri-Rad, M.; Rafiee, M. Sewage systems surveillance for SARS-CoV-2: Identification of knowledge gaps, emerging threats, and future research needs. Pathogens 2021, 10, 946. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 14 December 2021).
- Nemudryi, A.; Nemudraia, A.; Wiegand, T.; Surya, K.; Buyukyoruk, M.; Cicha, C.; Vanderwood, K.K.; Wilkinson, R.; Wiedenheft, B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Rep. Med. 2020, 1, 100098. [Google Scholar] [CrossRef] [PubMed]
- Di Marcantonio, C.; Chiavola, A.; Gioia, V.; Frugis, A.; Cecchini, G.; Ceci, C.; Spizzirri, M.; Boni, M.R. Impact of COVID19 restrictions on organic micropollutants in wastewater treatment plants and human consumption rates. Sci. Total Environ. 2021, 811, 152327. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 14 December 2021).
- Altmann, D.M.; Boyton, R.J.; Beale, R. Immunity to SARS-CoV-2 variants of concern. Science 2021, 371, 1103–1104. [Google Scholar] [CrossRef]
- Challen, R.; Brooks-Pollock, E.; Read, J.M.; Dyson, L.; Tsaneva-Atanasova, K.; Danon, L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: Matched cohort study. BMJ 2021, 372, n579. [Google Scholar] [CrossRef]
- Grint, D.J.; Wing, K.; Williamson, E.; McDonald, H.I.; Bhaskaran, K.; Evans, D.; Evans, S.J.; Walker, A.J.; Hickman, G.; Nightingale, E.; et al. Case fatality risk of the SARS-CoV-2 variant of concern B. 1.1. 7 in England, 16 November to 5 February. Eurosurveillance 2021, 26, 2100256. [Google Scholar] [CrossRef]
- Davies, N.G.; Jarvis, C.I.; Edmunds, W.J.; Jewell, N.P.; Diaz-Ordaz, K.; Keogh, R.H. Increased mortality in community-tested cases of SARS-CoV-2 lineage B. 1.1. 7. Nature 2021, 593, 270–274. [Google Scholar] [CrossRef]
- Oran, D.P.; Topol, E.J. The Proportion of SARS-CoV-2 Infections That Are Asymptomatic: A Systematic Review. Ann. Intern. Med. 2021, 174, 655–662. [Google Scholar] [CrossRef]
- Crits-Christoph, A.; Kantor, R.S.; Olm, M.R.; Whitney, O.N.; Al-Shayeb, B.; Lou, Y.C.; Flamholz, A.; Kennedy, L.C.; Greenwald, H.; Hinkle, A.; et al. Genome sequencing of sewage detects regionally prevalent SARS-CoV-2 variants. MBio 2021, 12, e02703-20. [Google Scholar] [CrossRef]
- Otero, M.C.B.; Murao, L.A.E.; Limen, M.A.G.; Gaite, P.L.A.; Bacus, M.G.; Acaso, J.T.; Corazo, K.; Knot, I.E.; Sajonia, H.; Francis, L.; et al. Wastewater-Based Epidemiology and Whole-Genome Sequencing for Community-Level Surveillance of SARS-CoV-2 in Selected Urban Communities of Davao City, Philippines: A Pilot Study. medRxiv 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.08.27.21262450v1 (accessed on 14 December 2021).
- Rothman, J.A.; Loveless, T.B.; Kapcia, J., III.; Adams, E.D.; Steele, J.A.; Zimmer-Faust, A.G.; Langlois, K.; Wanless, D.; Griffith, M.; Mao, L.; et al. RNA Viromics of Southern California Wastewater and Detection of SARS-CoV-2 Single-Nucleotide Variants. Appl. Environ. Microbiol. 2021, 87, e01448-21. [Google Scholar] [CrossRef]
- Jahn, K.; Dreifuss, D.; Topolsky, I.; Kull, A.; Ganesanandamoorthy, P.; Fernandez-Cassi, X.; Bänziger, C.; Stachler, E.; Fuhrmann, L.; Jablonski, K.P.; et al. Detection of SARS-CoV-2 variants in Switzerland by genomic analysis of wastewater samples. medRxiv 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.01.08.21249379v1.full.pdf (accessed on 14 December 2021).
- Wilton, T.; Bujaki, E.; Klapsa, D.; Majumdar, M.; Zambon, M.; Fritzsche, M.; Mate, R.; Martin, J. Rapid increase of SARS-CoV-2 variant B. 1.1. 7 detected in sewage samples from England between October 2020 and January 2021. Msystems 2021, 6, e00353-21. [Google Scholar] [CrossRef]
- Landgraff, C.; Wang, L.Y.; Buchanan, C.; Wells, M.; Schonfeld, J.; Bessonov, K.; Ali, J.; Robert, E.; Nadon, C. Metagenomic sequencing of municipal wastewater provides a near-complete SARS-CoV-2 genome sequence identified as the B. 1.1. 7 variant of concern from a Canadian municipality concurrent with an outbreak. medRxiv 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.03.11.21253409v1.full.pdf (accessed on 14 December 2021).
- Dharmadhikari, T.; Rajput, V.; Yadav, R.; Boargaonkar, R.; Panse, D.; Kamble, S.; Dastager, S. High throughput sequencing based detection of SARS-CoV-2 prevailing in wastewater of Pune, West India. medRxiv 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.06.08.21258563v1.full.pdf (accessed on 14 December 2021).
- Medema, G.; Been, F.; Heijnen, L.; Petterson, S. Implementation of environmental surveillance for SARS-CoV-2 virus to support public health decisions: Opportunities and challenges. Curr. Opin. Environ. Sci. Health 2020, 17, 49–71. [Google Scholar] [CrossRef]
- Medema, G.; Heijnen, L.; Elsinga, G.; Italiaander, R.; Brouwer, A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020, 7, 511–516. [Google Scholar] [CrossRef]
- Bivins, A.; North, D.; Ahmad, A.; Ahmed, W.; Alm, E.; Been, F.; Bhattacharya, P.; Bijlsma, L.; Boehm, A.B.; Brown, J.; et al. Wastewater-based epidemiology: Global collaborative to maximize contributions in the fight against COVID-19. Environ. Sci. Technol. 2020, 54, 7754–7757. [Google Scholar] [CrossRef]
- Arora, S.; Nag, A.; Sethi, J.; Rajvanshi, J.; Saxena, S.; Shrivastava, S.K.; Gupta, A.B. Sewage surveillance for the presence of SARS-CoV-2 genome as a useful wastewater based epidemiology (WBE) tracking tool in India. Water Sci. Technol. 2020, 82, 2823–2836. [Google Scholar] [CrossRef]
- Arora, S.; Nag, A.; Rajpal, A.; Tyagi, V.K.; Tiwari, S.B.; Sethi, J.; Sutaria, D.; Rajvanshi, J.; Saxena, S.; Shrivastava, S.K.; et al. Imprints of lockdown and treatment processes on the wastewater surveillance of SARS-CoV-2: A curious case of fourteen plants in northern India. Water 2021, 13, 2265. [Google Scholar] [CrossRef]
- Arora, S.; Nag, A.; Kalra, A.; Sinha, V.; Meena, E.; Saxena, S.; Sutaria, D.; Kaur, M.; Pamnani, T.; Sharma, K.; et al. Successful Application of Wastewater-Based Epidemiology in Prediction and Monitoring of the Second Wave of COVID-19 in India with Fragmented Sewerage Systems-A Case Study of Jaipur (India). medRxiv 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.09.11.21263417v1.full.pdf (accessed on 14 December 2021).
- Available online: https://www.cdc.gov/ (accessed on 8 January 2022).
- Available online: www.covid19india.org (accessed on 8 January 2022).
- Sievers, F.; Higgins, D.G. Clustal Omega, accurate alignment of very large numbers of sequences. In Multiple Sequence Alignment Methods; Humana Press: Totowa, NJ, USA, 2014; pp. 105–116. [Google Scholar]
- Available online: https://www.ncbi.nlm.nih.gov/nuccore/OK090988 (accessed on 14 December 2021).
- Available online: https://www.ncbi.nlm.nih.gov/nuccore/OK342100 (accessed on 14 December 2021).
- Available online: https://www.ncbi.nlm.nih.gov/nuccore/OK342107 (accessed on 14 December 2021).
- Kannan, S.R.; Spratt, A.N.; Cohen, A.R.; Naqvi, S.H.; Chand, H.S.; Quinn, T.P.; Lorson, C.L.; Byrareddy, S.N.; Singh, K. Evolutionary analysis of the Delta and Delta Plus variants of the SARS-CoV-2 viruses. J. Autoimmun. 2021, 124, 102715. [Google Scholar] [CrossRef]
- Emergence of SARS-CoV-2 B.1.617 Variants in India and Situation in the EU/EEA. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Emergence-of-SARS-CoV-2-B.1.617-variants-in-India-and-situation-in-the-EUEEA_0 (accessed on 14 December 2021).
- Assessment, R.R. Coronavirus Disease 2019 (COVID-19) in the EU/EEA and the UK–Ninth Update; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2020. [Google Scholar]
- Available online: https://indianexpress.com/article/explained/explained-who-classifies-india-variant-as-being-of-global-concern-what-does-it-mean-7310999/ (accessed on 14 December 2021).
- Volz, E.; Hill, V.; McCrone, J.T.; Price, A.; Jorgensen, D.; O’Toole, Á.; Southgate, J.; Johnson, R.; Jackson, B.; Nascimento, F.F.; et al. Evaluating the effects of SARS-CoV-2 spike mutation D614G on transmissibility and pathogenicity. Cell 2021, 184, 64–75. [Google Scholar] [CrossRef]
| WHO Label | Pango Lineage | GISAID Clade | Additional Amino Acid Changes Monitored | Earliest Documented Samples | Date of Designation |
|---|---|---|---|---|---|
| Alpha | B.1.1.7 | GRY | +S: 484K +S: 452R | United Kingdom, Sep-2020 | 18 December 2020 |
| Beta | B.1.351 | GH/501Y.V2 | +S: L18F | South Africa, May-2020 | 18 December 2020 |
| Gamma | P.1 | GR/501Y.V3 | +S: 681H | Brazil, Nov-2020 | 11 January 2021 |
| Delta | B.1.617.2 | G/478K.V1 | +S: 417N +S: 484K | India, Oct-2020 | VOI: 4 April 2021 VOC: 11 May 2021 |
| Omicron | B.1.1.529 | GRA | +S: R346K | Multiple countries, Nov-2021 | VUM: 24 November 2021 VOC: 26 November 2021 |
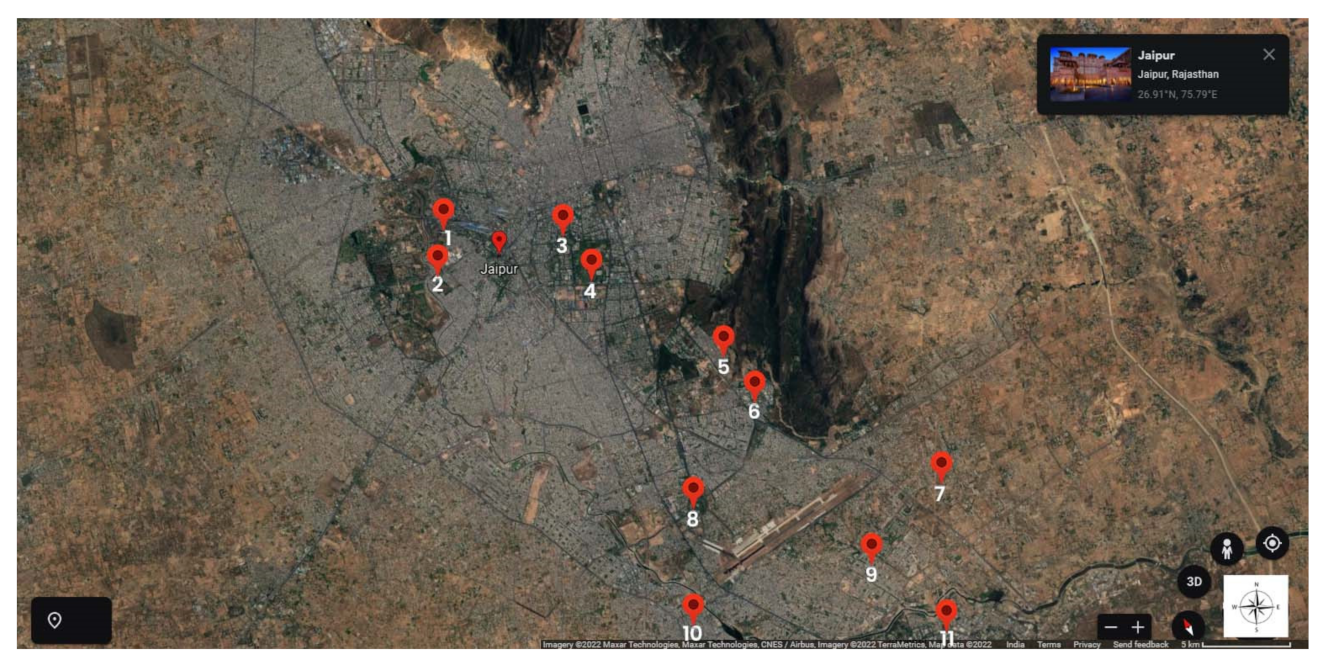
| Site No. | Sampling Location | Type of Secondary Treatment Technology | Type of Tertiary Treatment | Dosage and Contact Time of Tertiary Treatment | Design Capacity (m3/Day) | Flow Rate (Average. MLD) | Number of Connected Residents (Approximately) |
|---|---|---|---|---|---|---|---|
| Site 1 | Brahmpuri, Jaipur 26.9373° N, 75.8250° E | SBR | No treatment | NA | 27,000 m3/day | ~8 | >59,000 |
| Site 2 | Central Park Garden, Jaipur 26.9048° N, 75.8073° E | SBR | Cl2 (Bleach Powder) | 4 ppm by dropping system | 1000 m3/day | ~1 | >7000 |
| Site 3 | Ramniwas Garden, Jaipur 26.8963° N, 75.8100° E | MBBR | UV | NA | 1000 m3/day | ~1 | >7000 |
| Site 4 | MNIT, Jaipur 26.8640° N, 75.8108° E | MBBR | Cl2 (Hypochlorite) | 2.5–3 ppm, 30 min | 1000 m3/day | ~1 | >2000 |
| Site 5 | Jawahar Circle Garden, Jaipur 26.5029 0N, 75.4800 E | MBBR | UV | NA | 1000 m3/day | ~1 | >7000 |
| Site 6 | Dravyavati River, Jaipur 26.7980° N, 75.8039° E | SBR | Cl2 (Hypochlorite) | 3–5 ppm, 30 min | 65,000 m3/day | ~65 | >480,000 |
| Site 7 | Dhelawas, Jaipur 27.3735° N, 75.8926° E | ASP | No treatment | 3 ppm, 30 min | 65,000 m3/day | ~62.5 | >480,000 |
| Site 8 | Paldi Meena, Jaipur 26.8759° N, 75.8945° E | SBR | No treatment | NA | 3000 m3/day | 0.6–0.7 | ~5000 |
| Site 9 | Ralawata, Jaipur 26.76873° N, 75.93092° E | ASP | Cl2 (Hypochlorite) | 10 kg per hour | 30,000 m3/day | 20–22 | ~170,370 |
| Site 10 | Kho Nagorian, Jaipur 26.84063° N, 75.88546° E | SBR | No treatment | NA | 50,000 m3/day | ~45.5 | >480,000 |
| Site 11 | Dr. B. Lal Institute of Biotechnology Institute’s Campus WTWP 26.85697° N, 75.82749° E | BiokubeTM | No treatment | NA | 7.5 m3/day | 7.5 KLD | ~500 |
| Sample No. | Sample Coding | Sample Collection Date | Type of Sample (Total No. of Samples) | Sample Pooled from WWTPs | Total Number PE of Reads |
|---|---|---|---|---|---|
| 1 | JPR 1 | 27 February 2021 | Individual RNA | 3 | 1,409,611 |
| 2 | JPR 2 | 20 February 2021 | Pooled RNA (2) | 9 and 2 | 1,552,223 |
| 3 | JPR 3 | 12 March 2021 | Pooled RNA (2) | 5 and 7 | 1,570,305 |
| 4 | JPR 4 | 19 March 2021 | Pooled RNA (3) | 7, 4, 5 | 1,579,011 |
| 5 | JPR 5 | 1 April 2021 | Pooled RNA (7) | 4, 2, 3, 1, 5, 8, 4 | 1,385,609 |
| 6 | JPR 6 | 9 April 2021 | Pooled RNA (7) | 5, 2, 8, 6,4, 1, 3 | 1,507,711 |
| 7 | JPR 7 | 20 April 2021 | Pooled RNA (6) | 5, 8, 4, 2, 3, 1 | 1,352,690 |
| 8 | JPR 8 | 1 May 2021 | Pooled RNA (5) | 5, 4, 1, 6, 8 | 1,588,702 |
| 9 | JPR 9 | 8 May 2021 | Pooled RNA (5) | 4, 6, 5, 11, 1 | 1,606,125 |
| 10 | JPR 10 | 15 May 2021 | Pooled RNA (6) | 2, 7, 5, 4, 6, 8 | 1,414,505 |
| 11 | JPR 11 | 24 May 2021 | Pooled RNA (6) | 9, 7, 10, 6 | 1,194,455 |
| Sample | Lineage |
|---|---|
| JPR 1 | B.1 |
| JPR 2 | B.1 |
| JPR 3 | B.1.617.2 |
| JPR 4 | B.1.617.2 |
| JPR 5 | B.1.617.2 |
| JPR 6 | B.1.617.2 |
| JPR 7 | B.1.617.2 |
| JPR 8 | B.1.617.2 |
| JPR 9 | B.1.617.2 |
| JPR 10 | B.1.617.2 |
| JPR 11 | B.1.617.2 |
| Sample | Number of SNPs Detected | Number of Genic SNPs Detected |
|---|---|---|
| JPR 1 | 39 | 35 |
| JPR 2 | 44 | 41 |
| JPR 3 | 40 | 36 |
| JPR 4 | 51 | 47 |
| JPR 5 | 39 | 36 |
| JPR 6 | 41 | 37 |
| JPR 7 | 42 | 39 |
| JPR 8 | 45 | 42 |
| JPR 9 | 40 | 36 |
| JPR 10 | 44 | 40 |
| JPR 11 | 37 | 34 |
| Total | 462 | 423 |
| Genes | SNPs Overlapping with Delta and Delta Plus | SNPs Which Were Absent in Jaipur Samples | Total Types of Non-Unique Detected SNPs | Total Unique SNPs Detected |
|---|---|---|---|---|
| ORF1ab | Thr3646Ala | Ala1146Thr | 30 | 61 |
| Thr3255Ile | Ala3209Val | |||
| Val3718Ala | Val2930Leu | |||
| Pro2046Leu | Pro2287Ser | |||
| Pro1640Leu | Ala1306Ser | |||
| Pro2046Leu | ||||
| Thr3750Ile | ||||
| ORF3a | Ser26Leu | 2 | 1 | |
| M | Ile82Thr | 1 | 4 | |
| N | Arg203Met | Gly215Cys | 4 | 8 |
| Asp63Gly | ||||
| Asp377Tyr | ||||
| ORF7A | Thr120Ile | Thr40Ile | 3 | 0 |
| Val82Ala |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nag, A.; Arora, S.; Sinha, V.; Meena, E.; Sutaria, D.; Gupta, A.B.; Medicherla, K.M. Monitoring of SARS-CoV-2 Variants by Wastewater-Based Surveillance as a Sustainable and Pragmatic Approach—A Case Study of Jaipur (India). Water 2022, 14, 297. https://doi.org/10.3390/w14030297
Nag A, Arora S, Sinha V, Meena E, Sutaria D, Gupta AB, Medicherla KM. Monitoring of SARS-CoV-2 Variants by Wastewater-Based Surveillance as a Sustainable and Pragmatic Approach—A Case Study of Jaipur (India). Water. 2022; 14(3):297. https://doi.org/10.3390/w14030297
Chicago/Turabian StyleNag, Aditi, Sudipti Arora, Vikky Sinha, Ekta Meena, Devanshi Sutaria, Akhilendra Bhushan Gupta, and Krishna Mohan Medicherla. 2022. "Monitoring of SARS-CoV-2 Variants by Wastewater-Based Surveillance as a Sustainable and Pragmatic Approach—A Case Study of Jaipur (India)" Water 14, no. 3: 297. https://doi.org/10.3390/w14030297
APA StyleNag, A., Arora, S., Sinha, V., Meena, E., Sutaria, D., Gupta, A. B., & Medicherla, K. M. (2022). Monitoring of SARS-CoV-2 Variants by Wastewater-Based Surveillance as a Sustainable and Pragmatic Approach—A Case Study of Jaipur (India). Water, 14(3), 297. https://doi.org/10.3390/w14030297







