The Role of Habitat Protection in Maintaining the Diversity of Aquatic Fauna in Rural and Industrial Areas
Abstract
:1. Introduction
2. Material and Methods
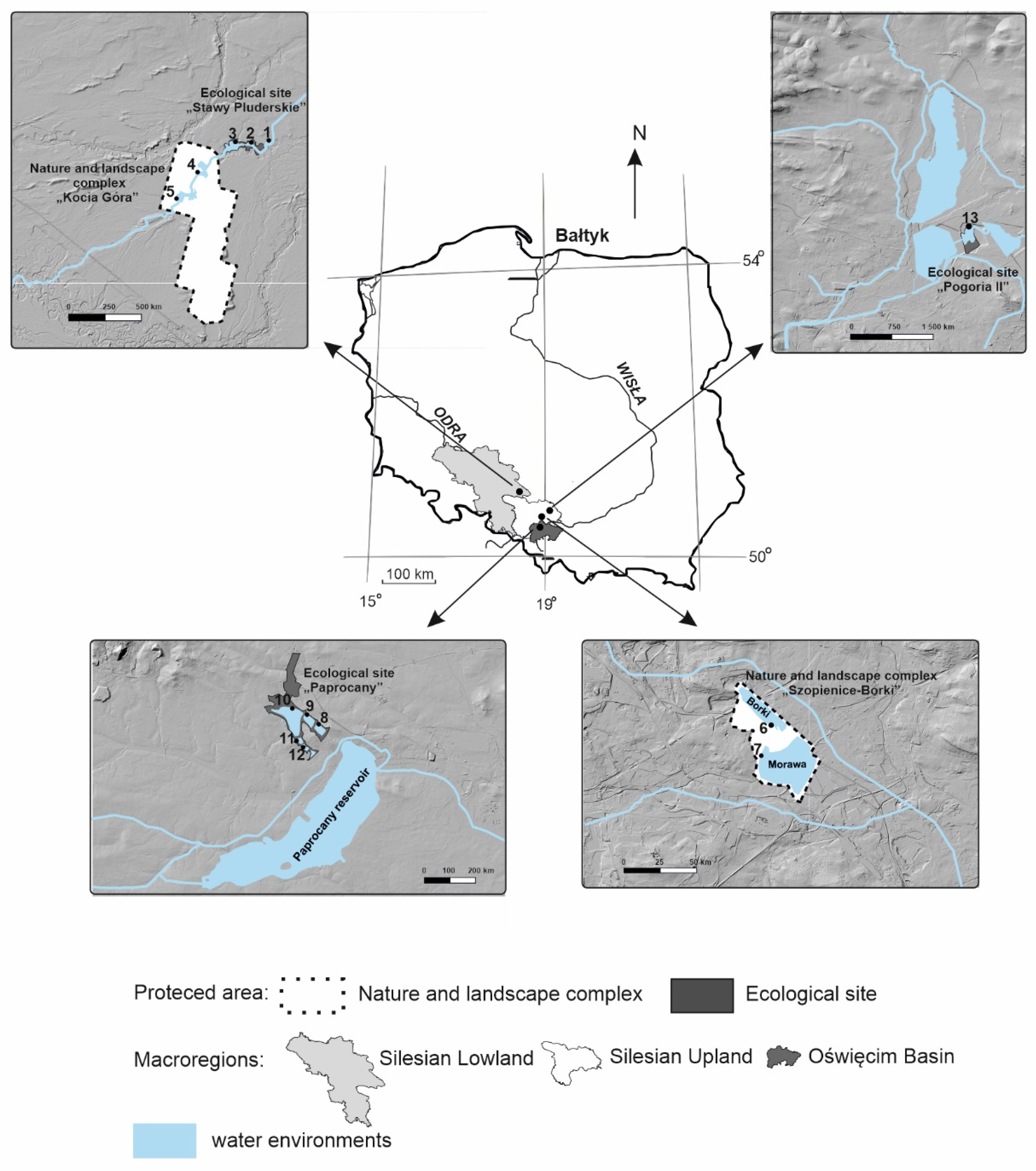
2.1. Study Area
2.2. Sampling Procedure
2.3. Zoocenological Methods and Data Analysis
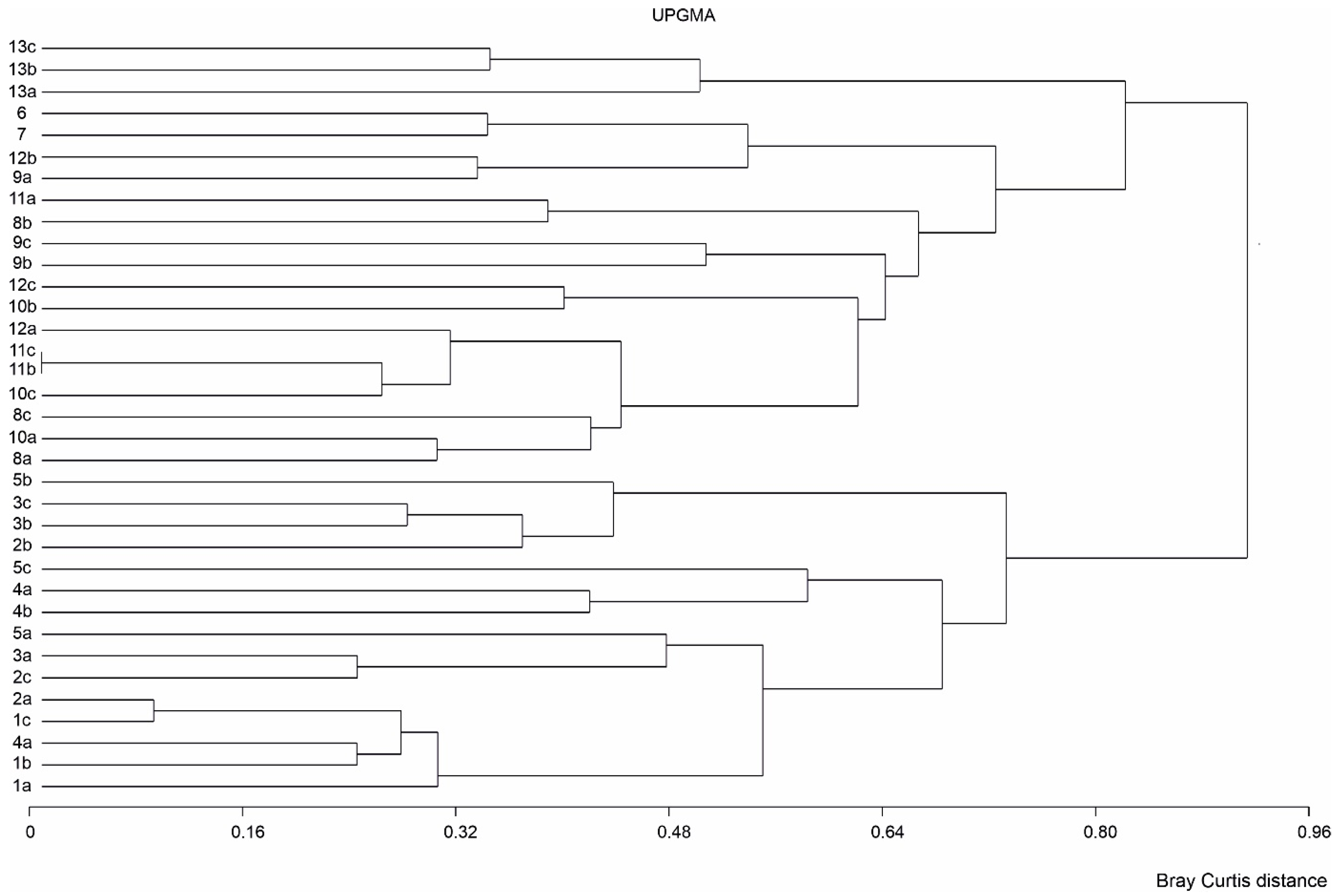
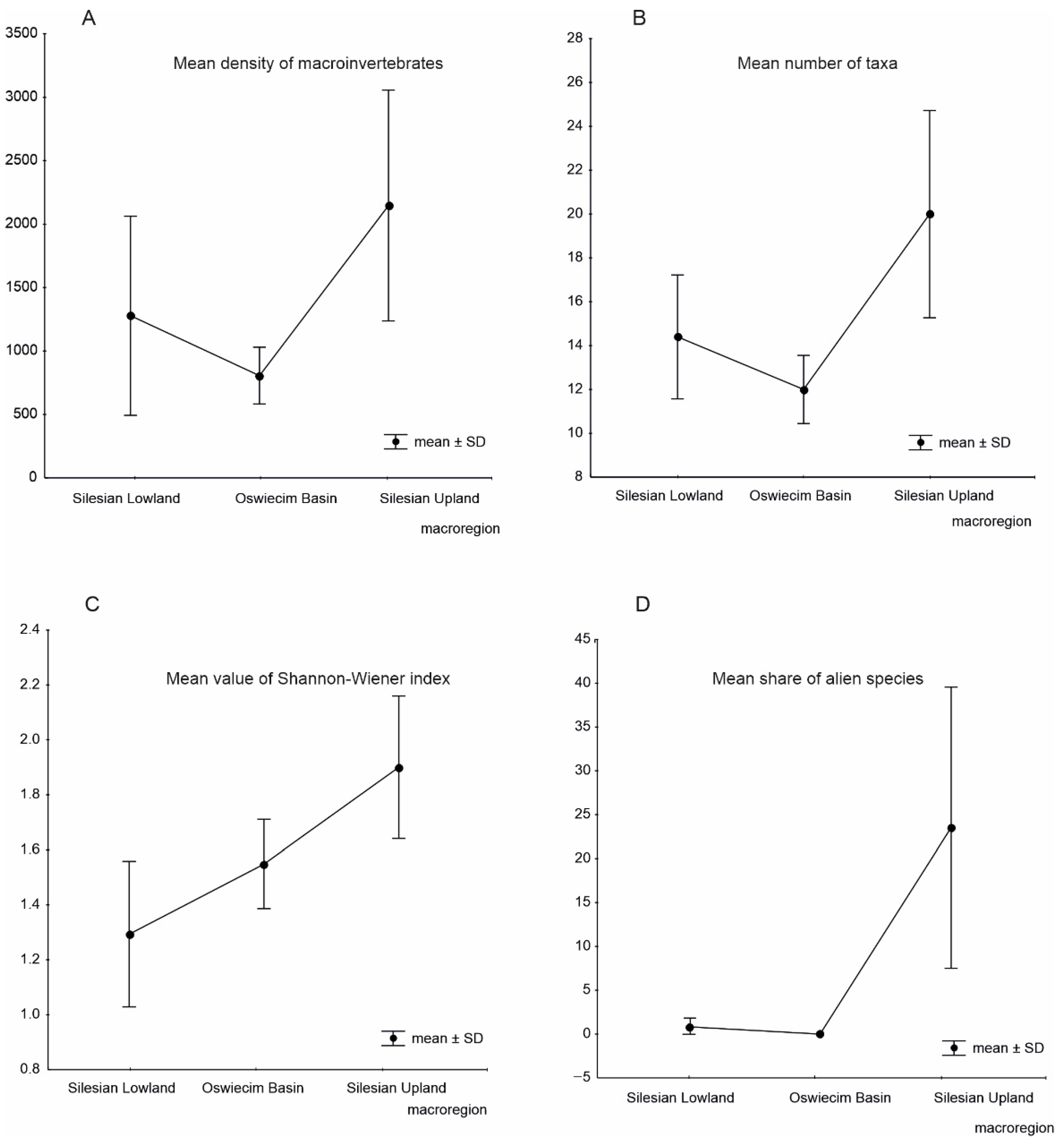
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groves, C.R. Drafting a Conservation Blueprint: A Practitioner’s Guide to Planning for Biodiversity; Island Press: Washington, DC, USA, 2003. [Google Scholar]
- Gray, C.L.; Hill, S.L.; Newbold, T.; Hudson, L.N.; Börger, L.; Contu, S.; Hoskins, A.J.; Ferrier, S.; Purvis, A.; Scharlemann, J.P. Local biodiversity is higher inside than outside terrestrial protected areas worldwide. Nat. Commun. 2016, 7, 12306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaston, K.J.; Jackson, S.F.; Cantú-Salazar, L.; Cruz-Piñón, G. The ecological performance of protected areas. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 93–113. [Google Scholar] [CrossRef] [Green Version]
- IUCN. Guidelines for Protected Area Management Categories: International Union for the Conservation of Nature; IUCN: Gland, Switzerland, 1994; Available online: https://portals.iucn.org/library/sites/library/files/documents/pag-021.pdf (accessed on 5 November 2022).
- UNESCO. Man and the Biosphere (MAB) Programme. 2019. Available online: https://en.unesco.org/mab (accessed on 5 November 2022).
- Brooks, T.M.; Bakarr, M.I.; Boucher, T.; Da Fonseca, G.A.; Hilton-Taylor, C.; Hoekstra, J.M.; Moritz, T.; Olivieri, S.; Parrish, J.; Pressey, R.L.; et al. Coverage provided by the global protected-area system: Is it enough? BioScience 2004, 54, 1081–1091. [Google Scholar] [CrossRef]
- Dz. U. z 2022 r. poz. 916. Official Journal of the Laws. 16 April 2004. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20040920880 (accessed on 5 November 2022).
- Dz. U. z 2015 r. poz. 469. Official Journal of the Laws. 18 July 2001. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20150000469 (accessed on 5 November 2022).
- Juśkiewicz, W.; Gierszewski, P. Toxic metal pollution of aquatic ecosystems of European Union nature protection areas in a region of intensive agriculture (Lake Gopło, Poland). Aquat. Sci. 2022, 84, 52. [Google Scholar] [CrossRef]
- Špulerová, J.; Izakovičová, Z.; Vlachovičová, M.; Černecký, J. Natural or Semi-natural Landscape Features as Indicator of Biocultural Value: Observations from Slovakia. Hum. Ecol. 2022, 50, 531–543. [Google Scholar] [CrossRef]
- Spyra, A.; Cieplok, A. Structure and dynamics of gastropod communities in highly transformed aquatic environments colonized and uncolonized by globally invasive Potamopyrgus antipodarum (Gray, 1843). Aquat. Invasions 2022, 17, 431–452. [Google Scholar] [CrossRef]
- Izakovičová, Z.; Špulerová, J.; Kozelová, I. The Approach to Typology of the Biocultural Landscape in Slovakia. Environ. Manag. 2022, 70, 746–762. [Google Scholar] [CrossRef]
- Dz. U. 12.10.1991 r., nr 114 poz. 492 z późn. zm. Official Journal of the Laws. 16 October 1991. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20150000469 (accessed on 15 November 2022).
- Convention on Biological Diversity. Decision VII/30. Strategic Plan: Future Evaluation of Progress; UNEP: Kuala Lumpur, Malaysia, 2004. [Google Scholar]
- Margules, C.R.; Pressey, R.L. Systematic conservation planning. Nature 2000, 405, 243–253. [Google Scholar] [CrossRef]
- Nogueira, J.G.; Teixeira, A.; Varandas, S.; Lopes-Lima, M.; Sousa, R. Assessment of a terrestrial protected area for the conservation of freshwater biodiversity. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 520–530. [Google Scholar] [CrossRef]
- Hutahaean, A.A.; Rustam, A.; Daulat, A.; Rahayu, Y.P.; Suryono, D.D.; Salim, H.L.; Kusumaningtyas, M.A. Biodiversity and Aquatic Vegetation Succession in Biawak Island Marine Protected Area, Indramayu-West Java. J. Segara 2022, 18, 37–46. [Google Scholar] [CrossRef]
- Caro-Borrero, A.; Carmona-Jiménez, J.; Rivera-Ramírez, K.; Bieber, K. The effects of urbanization on aquatic ecosystems in peri-urban protected areas of Mexico City: The contradictory discourse of conservation amid expansion of informal settlements. Land Use Policy 2021, 102, 105226. [Google Scholar] [CrossRef]
- Rolón, E.; Ondarza, P.M.; Miglioranza, K.S.; Rosso, J.J.; Mabragaña, E.; Volpedo, A.V.; Avigliano, E. Multi-matrix approach reveals the distribution of pesticides in a multipurpose protected area from the Atlantic Rainforest: Potential risk for aquatic biota and human health? Environ. Sci. Pollut. Res. 2021, 28, 34386–34399. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.; Lutyńska, S. The influence of anthropopression on the water environment in the area of Landscape-Nature Protected Complex “Żabie Doły” (Upper Silesia). Arch. Waste Manag. Environ. Prot. 2015, 17, 11–18. [Google Scholar]
- Ledwoń, K.; Imielski, M.; Koj, A. The characteristics of nature landscape complex Żabie Doły. Aura 1999, 3, 30–31. (In Polish) [Google Scholar]
- De Wit, H.A.; Lepistö, A.; Marttila, H.; Wenng, H.; Bechmann, M.; Blicher-Mathiesen, G.; Eklöf, K.; Futter, M.N.; Kortelainen, P.; Kronvang, B.; et al. Land-use dominates climate controls on nitrogen and phosphorus export from managed and natural Nordic headwater catchments. Hydrol. Process. 2020, 34, 4831–4850. [Google Scholar] [CrossRef]
- Heino, J.; Ilmonen, J.; Kotanen, J.; Mykrä, H.; Paasivirta, L.; Soininen, J.; Virtanen, R. Surveying biodiversity in protected and managed areas: Algae, macrophytes and macroinvertebrates in boreal forest streams. Ecol. Indic. 2009, 9, 1179–1187. [Google Scholar] [CrossRef]
- Covich, A.P.; Palmer, M.A.; Crowl, T.A. The role of benthic invertebrate species in freshwater ecosystems: Zoobenthic species influence energy flows and nutrient cycling. BioScience 1999, 49, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Noor-Ul-Islam, H.; Khan, K.; Zia, S.A.; Naeem, M.; Shams, W.A. Heavy metals accumulation in dragonflies (Odonata) and their habitats in district Swabi, Khyber Pakhtunkhwa, Pakistan: Assessing dragonfly bionomics in the region. Bull. Environ. Contam. Toxicol. 2021, 107, 838–847. [Google Scholar] [CrossRef]
- Schwantes, D.; Junior, A.C.G.; Manfrin, J.; Campagnolo, M.A.; Zimmermann, J.; Junior, E.C.; Bertoldo, D.C. Distribution of heavy metals in sediments and their bioaccumulation on benthic macroinvertebrates in a tropical Brazilian watershed. Ecol. Eng. 2021, 163, 106194. [Google Scholar] [CrossRef]
- Rzętała, M. Funkcjonowanie Zbiorników Wodnych Oraz Przebieg Procesów Limnicznych w Warunkach Zróżnicowanej Antropopresji na Przykładzie Regionu Górnośląskiego; Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2008. [Google Scholar]
- Nixdorf, B.; Lessmann, D.; Deneke, R. Mining lakes in a disturbed landscape: Application of the EC Water Framework Directive and future management strategies. Ecol. Eng. 2005, 24, 67–73. [Google Scholar] [CrossRef]
- Vandysh, O.I. Zooplankton as an indicator of the state of lake ecosystems polluted with mining wastewater in the Kola Peninsula. Russ. J. Ecol. 2004, 35, 110–116. [Google Scholar] [CrossRef]
- Antolak, M.; Pochodyła, E. Ścieżki widokowe na obszarach chronionych na przykładzie użytku ekologicznego Rozlewisko Morąskie. Acta Sci. Pol. Adm. Locorum 2019, 18, 19–31. [Google Scholar] [CrossRef]
- Kozak, A. Obszary chronione możliwością rozwoju turystyki edukacyjnej na przykładzie ścieżek przyrodniczo-edukacyjnych “Bużny Most” i “Nadbużańskie Łęgi”. Probl. Ekol. Kraj. 2012, 34, 111–116. [Google Scholar]
- Acreman, M.; Hughes, K.A.; Arthington, A.H.; Tickner, D.; Dueñas, M.A. Protected areas and freshwater biodiversity: A novel systematic review distils eight lessons for effective conservation. Conserv. Lett. 2019, 13, e12684. [Google Scholar] [CrossRef]
- Spałek, K.; Badora, K.; Czuban, R.; Kuńka, A.; Nowak, A. Projekt Docelowej Gminnej Sieci Obszarów Chronionych Wraz z Aktualizacją Inwentaryzacji i Waloryzacji Przyrodniczej Gminy Kolonowskie; BIO_PLAN: Krasiejów, Poland, 2006. [Google Scholar]
- Franczak, U.; Wójcicka-Rosińska, A. Plan Urządzenia Lasu dla Nadleśnictwa Zawadzkie Regionalna Dyrekcja Lasów Państwowych w Katowicach na Okres od 01 Stycznia 2013 r. do 31 Grudnia 2022 r; Program Ochrony Przyrody: Brzeg, Poland, 2013.
- Tokarska-Guzik, B.; Rostański, A.; Kupka, R. Katowice. Przyroda Miasta; Kubajak: Krzeszowice, Poland, 2002; p. 128. [Google Scholar]
- Puchalski, W. Poeksploatacyjne zbiorniki wodne–wstęp do charakterystyki ekologicznej. Wiadomości Ekol. 1985, 31, 2–20. [Google Scholar]
- Tończyk, G.; Siciński, J.; Grabowski, M.; Jabłońska, A.; Jażdżewski, K.; Jurasz, W. Klucz do Oznaczania Makrobezkręgowców Bentosowych dla Potrzeb Oceny Stanu Ekologicznego wód Powierzchniowych; Główny Inspektorat Ochrony Środowiska: Warszawa, Poland, 2013. (In Polish)
- Piechocki, A.; Wawrzyniak-Wydrowska, B. Guide to Freshwater and Marine Mollusca of Poland; Bogucki Wydawnictwo Naukowe: Poznan, Poland, 2016. [Google Scholar]
- Wright, I.A.; Ryan, M. Impact of mining and industrial pollution on stream macroinvertebrates: Importance of taxonomic resolution, water geochemistry and EPT indices for impact detection. Hydrobiologia 2016, 772, 103–115. [Google Scholar] [CrossRef]
- Szafer, W.; Kulczyński, S.; Pawłowski, B. Rośliny Polskie, cz. I i II; PWN: Warszawa, Poland, 1988; pp. 30–198. [Google Scholar]
- Myślińska, E. Organic Soils and Their Laboratory Methods; Wydawnictwo PWN: Warszawa, Poland, 2001; p. 208. (In Polish) [Google Scholar]
- Hermanowicz, W.; Dojlido, J.; Dożańska, W.; Koziorowski, B.; Zerbe, J. Fizyczno-Chemicznebadanie Wody i Ścieków; Wydawnictwo Arkady: Warszawa, Poland, 1999; p. 555. [Google Scholar]
- Cowx, I.G. Analysis of threats to freshwater fish conservation: Past and present challenges. In Conservation of Freshwater Fishes: Options for the Future; Collares-Pereira, M.J., Cowx, I.G., Coelho, M.M., Eds.; Blackwell Scientific Press: Oxford, UK, 2002; pp. 201–220. [Google Scholar]
- Suski, C.D.; Cooke, S.J. Conservation of aquatic resources through the use of freshwater protected areas: Opportunities and challenges. Biodivers. Conserv. 2007, 16, 2015–2029. [Google Scholar] [CrossRef]
- Türkmen, G.; Kazanci, N. Applications of various biodiversity indices to benthic macroinvertebrate assemblages in streams of a national park in Turkey. Rev. Hydrobiol. 2010, 3, 111–125. [Google Scholar]
- Hudak, M. Odrzański turystyczny szlak wodny a obszary Natura 2000 i inne obszary chronione w województwie lubuskim. Zesz. Nauk. Inż. Śr./Uniw. Zielonogór. 2010, 140, 74–82. [Google Scholar]
- Dudley, N. (Ed.) Guidelines for Applying Protected Area Management Categories; IUCN: Gland, Switzerland, 2008. [Google Scholar]
- Santoul, F.; Gaujard, A.; Angélibert, S.; Mastrorillo, S.; Céréghino, R. Gravel pits support waterbird diversity in an urban landscape. In Pond Conservation in Europe; Springer: Dordrecht, The Netherlands, 2009; pp. 263–270. [Google Scholar]
- Frochot, B.; Godreau, V.; Roche, J. L’expansion récente des oiseaux d’eau. Alauda 2008, 76, 279–286. [Google Scholar]
- Mancini, L.; Formichetti, P.; Anselmo, A.; Tancioni, L.; Marchini, S.; Sorace, A. Biological quality of running waters in protected areas: The influence of size and land use. Biodivers. Conserv. 2005, 14, 351–364. [Google Scholar] [CrossRef]
- Chessman, B.C. Do protected areas benefit freshwater species? A broad-scale assessment for fish in Australia’s Murray–Darling Basin. J. Appl. Ecol. 2013, 50, 969–976. [Google Scholar] [CrossRef]
- Czachorowski, S.; Moroz, M.D. Caddisflies (Trichoptera) of the protected territories of Belarus. Entomol. Rev. 2007, 87, 980–987. [Google Scholar] [CrossRef]
- Cieplok, A.; Spyra, A. The roles of spatial and environmental variables in the appearance of a globally invasive Physa acuta in water bodies created due to human activity. Sci. Total Environ. 2020, 744, 140928. [Google Scholar] [CrossRef]
- Strayer, D.L. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 2010, 55, 152–174. [Google Scholar] [CrossRef]
- Dudgeon, D. Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R960–R967. [Google Scholar] [CrossRef] [PubMed]
- Maceda-Veiga, A.; Baselga, A.; Sousa, R.; Vilà, M.; Doadrio, I.; De Sostoa, A. Fine-scale determinants of conservation value of river reaches in a hotspot of native and non-native species diversity. Sci. Total Environ. 2017, 574, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Cinner, J.E.; Maina, J.; Graham, N.A.J.; Daw, T.M.; Stead, S.M.; Wamukota, A.; Brown, K.; Ateweberhan, M.; Venus, V.; et al. Conservation action in a changing climate. Conserv. Lett. 2008, 1, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, R.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
| Name | “Kocia Góra” Natural-Landscape Complex, NATURA 2000 “Stawy Pluderskie” Ecological Site | “Szopienice-Borki” Natural Landscape Complex | Water Bodies in Ecological Site Paprocany | Ecological Site Pogoria II |
|---|---|---|---|---|
| Geographical location | Silesian Lowland | Silesian Upland | Oświecim Basin | Silesian Upland |
| Landscape location | rural | industrial | industrial | industrial |
| Year of origin | 1880–1893 | 1960 | nd | 1976–1978 |
| Water body area [ha] | 3.85–13.57 | 12–34.70 | 4–6.5 ha | 26 |
| Depth | 1.1–1.85 | 4.0–4.3 | 0.8–2 | 2.6 |
| Water supply | Canal supplies water from the river Lubliniec, atmospheric precipitation | Ground water, atmospheric precipitation | Ground water, surface run-off, atmospheric precipitation | Ground water, surface run-off, atmospheric precipitation |
| Management | Fish stocking | Fish stocking, recreation | Fish stocking | Angling, recreation |
| Avifauna | Tachybaptus ruficollis, Anas crecca, Fulica atra, Gallinula chloropus, Grus grus, Acrocephalus arundinaceus, Anas platyrhynos | Cygnus olor, Anas platyrhynos, Aythya ferina, Aythya, fuligula, Fulica atra, Rallus aquaticus, Gallinula chloropus, Podiceps cristatus, Tachybaptus ruficollis, Acrocephalus scirpaeus, Acrocephalus arundinaceus, Acrocephalus achoenobaenus, Emberiza schoeniclus, Larus ridibundus | Turdus merula, Turdus philomelos, Oriolus oriolus, Anthus trivalis, Sylvia borin, Parus caeruleus, Fringilla coelebs | Alcedo atthis, Ixobrychus minutus, Actitis hypolucos, Remiz pendulinus, Acrocephalus arundinaceus, Acrocephalus palustris |
| Ichthyofauna | Cyprinus carpio, Ctenopharyngodon idella, Tinca tinca, Esox lucius, Perca fluviatilis, Rutilus rutilus, Scardinius erythrophthalmus, Abramis brama | Rutilus rutilus, Scardinus erythrophthalmatus, Perca fluviatilis, Esox anguilla, Abramis brama, Carassius carassius, Leucaspius delineatus | Tinca tinca, Carassius carassius, Esox lucius, Rutilus rutilus, Scardinus erythrophthalmus, Misgurnus fossilis, Perca fluviatilis | Ctenopharyngodon idella, Tinca tinca, Cyprinus carpio, Gasterosteus aculeatus, Gobio gobio, Leucaspius delineatus, Perca fluviatilis, Sander lucioperca, Esox lucius, Anguilla anguilla, Abramis brama, Rutilus rutilus |
| Aquatic plants | Nuphar lutea, Nymphaea alba, Sparganium erectum, Lycopus europaeus, Iris pseudacorus, Glyceria aquatica, Mentha aquatica, Schoenoplectus lacustris, Hottonia palustris, Typha latifolia, T. latifolia, Utricularia vulgaris, Eleocharis palustris, E. acicularis, Solanum dulcamara, Lemna minor, Scirpus sylvaticus, Acorus calamus, Lysimachia nummularia, Phragmites australis, Carex sp., Eriophorum angustifolium, Myriophyllum spicatum, M. verticilatum, Alisma plantago aquatica, C. cyperoides, C. cyperoides, Eleocharis ovata, Lycopodium calvatum, Arctostaphyllos uva-ursi | Phragmites australis, Glyceria aquatica, Hydrocharis morsus-ranae, Juncuss effuses, Lemna minor, Carex vesicaria, Fontinalis antypyretica, Typha latifolia, T. angustifolia, Eleocharis palustris, Lycopus europaeus, Alisma plantago-aquatica, Scirpus silvaticus, Myriophyllum spicatum, Elodea canadensis, Utricularia vulgaris, Ceratophyllum demersum, Potamogeton filiformis, Najas marina, Nuphar lutea | Caltha palustris, Lysimachia vulgaris, L. thyrsiflora, Iris pseudoacorus, Phalaris arundinacea, Equisetum sylvaticum, Eriophorum angustifolium, Viola palustris, Carex echinate, Menyanthes trifoliata, Typha latifolia, Glyceria aquatica, Sparganium erectus, S. angustifolium, Phragmites australis, Acorus calamus, Equisetum fluviatile, Alisma plantago-aquatica, Juncuss effusus, Sagittaria sagittifolia, Lemna minor, Peucedanum palustre, Nymphaea alba, Muphar lutea, Solanum dulcamara, Rumex hydrolapathum, Myosotis palustris, Scutellaria galericulata, Utricularia vulgaris | Nuphar lutea, Nymphaea alba, Iris pseudoacorus, Typha latifolia, T. angustifolia, Carex acutiformis, C. pseudocyperus, Rorippa amphibia, C. riparia, C. vesicaria, Myriophyllum spicatum, M. verticilatum, Alisma plantago-aquatica, Rumex hydrolapathum, Potamogeton natans, Phragmites australis, Phalaris arundinacea |
| Parameters | Water Body Complexes | ||||
|---|---|---|---|---|---|
| “Kocia Góra” Natural-Landscape Complex | “Stawy Pluderskie” Ecological Site | “Szopienice-Borki” Natural-Landscape Complex | Ecological Site Paprocany | Ecological Site Pogoria II | |
| Temperature (°C) | 19.1–25.8 | 17.9–26.0 | 16.5–24.3 | 7.8–24.1 | 14.8–25.1 |
| pH | 6.5–7.4 | 6.2–7.6 | 7.6–8.1 | 6.0–7.0 | 7.6–7.9 |
| EC (µS cm−1) | 180–270 | 180–290 | 360–450 | 100–380 | 580–610 |
| TDS (mg L−1) | 80–120 | 80–130 | 170–215 | 40–180 | 270–290 |
| Oxygen (mg O2 L−1) | 6.8–10.5 | 3.4–10.7 | nd | 1.43–7.46 | nd |
| Total hardness (mg CaCO3 L−1) | 110–152 | 110–155 | 115–120 | 52–78 | 130–143 |
| Alkalinity (mg CaCO3 L−1) | 50–125 | 55–100 | 110–120 | 25–45 | 135–160 |
| Chlorides (mg Cl L−1) | 18–38 | 20–50 | 16–31 | 14–36 | 38–46 |
| Calcium (mg Ca L−1) | 30–78 | 30–85 | 32–39 | 18–24 | 58–73 |
| Ammonia (mg NH3 L−1) | 0.46–1.03 | 0.53–1.24 | 0.11–0.13 | 0.38–2.04 | 0.1–0.2 |
| Nitrates (mg NO3 L−1) | 1.32–17.6 | 0–4.43 | 7.46–8.02 | 1.33–8.86 | 4.21–5.32 |
| Nitrites (mg NO2 L−1) | 0–0.006 | 0–0.02 | 0.05–0.06 | 0.03–0.20 | 0.06–0.09 |
| Phosphates (mg PO4 L−1) | 0–1.33 | 0–2.50 | 0.01–0.13 | 0.05–0.22 | 0.10–0.26 |
| Iron (mg Fe L−1) | 0.07–0.63 | 0.86–2.83 | 0.09–0.10 | 0.21–3.68 | 0.08–0.09 |
| Organic matter (%) | 0.7–43.2 | 2.4–34.8 | 3.2–4.9 | 0.4–42.2 | 16.2–21.0 |
| Taxa | “Stawy Pluderskie” Ecological Site | “Kocia Góra” Natural Landscape Complex | “Szopienice-Borki” Natural Landscape Complex | Ecological Site Paprocany | Ecological Site Pogoria II | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code of water body | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Oligochaeta | 5.4 | 2.3 | 3.5 | 17.2 | 39.2 | 2.1 | 1.2 | 4.5 | 20.0 | 10.5 | 7.3 | 18.0 | 0.0 |
| Asellidae | 0.0 | 0.4 | 0.0 | 0.2 | 0.2 | 3.2 | 0.4 | 55.1 | 13.2 | 26.3 | 36.1 | 12.8 | 0.0 |
| Nematoda | 0.0 | 0.0 | 0.0 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Glossiphoniidae | 2.1 | 0.1 | 0.3 | 1.8 | 0.7 | 0.8 | 1.3 | 3.1 | 1.9 | 5.7 | 0.4 | 0.0 | 3.4 |
| Erpobdellidae | 8.8 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 1.7 | 0.9 | 4.3 | 0.9 | 0.0 | 0.9 |
| Haemopidae | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Piscicolidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Coenagrionidae | 0.0 | 1.1 | 0.3 | 1.2 | 3.5 | 9.3 | 0.8 | 0.3 | 0.4 | 0.4 | 0.5 | 0.0 | 1.8 |
| Calopterygidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Lestidae | 0.9 | 1.5 | 4.2 | 0.5 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Platycnemididae | 0.0 | 0.7 | 0.0 | 1.1 | 0.6 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 |
| Libellulidae | 0.3 | 0.0 | 0.3 | 0.2 | 0.4 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | 0.0 |
| Aeshnidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 | 0.6 | 0.0 |
| Cordulegastridae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Corduliidae | 0.0 | 0.1 | 0.0 | 0.7 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.2 | 0.2 | 0.6 | 1.3 |
| Baetidae | 0.0 | 2.1 | 1.0 | 5.7 | 3.7 | 2.1 | 1.5 | 0.0 | 3.8 | 0.0 | 4.3 | 1.7 | 0.7 |
| Siphlonuridae | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Caenidae | 1.4 | 0.9 | 2.3 | 10.1 | 9.8 | 6.8 | 7.3 | 0.2 | 0.0 | 6.8 | 0.0 | 0.6 | 2.9 |
| Leptoceridae | 0.7 | 2.0 | 0.3 | 4.0 | 1.4 | 4.6 | 0.6 | 6.1 | 0.4 | 1.8 | 3.8 | 0.7 | 0.0 |
| Ecnomidae | 0.3 | 2.8 | 0.0 | 0.0 | 1.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Polycentropodidae | 0.0 | 0.0 | 0.0 | 0.5 | 1.1 | 0.0 | 1.6 | 0.0 | 0.6 | 0.0 | 0.2 | 0.0 | 0.5 |
| Phryganeidae | 0.0 | 0.0 | 0.0 | 0.3 | 0.6 | 0.0 | 0.0 | 0.1 | 0.0 | 1.0 | 0.4 | 0.0 | 0.1 |
| Hydropsychidae | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Limnephilidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Psychomyidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Philopotamidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 |
| Beraeidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Glossosomatidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Molannidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 |
| Odontoceridae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Hydroptilidae | 0.0 | 0.1 | 1.0 | 0.4 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Tabanidae | 0.6 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.2 | 0.1 | 0.2 | 0.4 | 0.2 | 0.0 |
| Tipulidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Ceratopogonidae | 0.2 | 0.5 | 1.0 | 0.3 | 0.1 | 2.4 | 1.5 | 1.9 | 0.1 | 2.3 | 0.4 | 0.0 | 0.0 |
| Chironomidae | 78.3 | 80.2 | 64.5 | 51.6 | 20.8 | 15.5 | 11.4 | 23.8 | 19.2 | 36.6 | 36.3 | 35.9 | 20.7 |
| Dixidae | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Limoniidae | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Sciomyzidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Stratiomyidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Chaoboridae | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Haliplidae | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 1.8 | 0.0 | 1.0 | 0.0 | 0.4 | 0.2 | 0.0 |
| Hydrophilidae | 0.0 | 0.0 | 0.3 | 0.3 | 0.0 | 0.1 | 0.1 | 0.2 | 2.4 | 0.0 | 0.0 | 0.7 | 0.4 |
| Dytiscidae | 0.0 | 0.4 | 6.5 | 0.0 | 0.5 | 0.4 | 0.9 | 0.3 | 4.7 | 0.0 | 0.2 | 0.7 | 0.9 |
| Elmidae | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Helodidae | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.8 | 0.0 | 0.0 | 0.2 | 3.1 |
| Donaciidae | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Hygrobiidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 |
| Naucoridae | 0.0 | 0.0 | 1.0 | 0.2 | 0.2 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 |
| Nepidae | 0.0 | 0.1 | 1.3 | 0.0 | 0.8 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Pleidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Corixidae | 0.0 | 0.0 | 1.3 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mesoveliidae | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Notonectidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.6 | 0.0 |
| Veliidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.2 | 0.0 |
| Sialidae | 0.0 | 0.0 | 0.3 | 0.1 | 0.0 | 0.0 | 0.2 | 0.1 | 0.0 | 0.0 | 0.9 | 0.2 | 0.4 |
| Planipenia | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Lymnaeidae | 0.0 | 0.7 | 0.0 | 0.3 | 0.6 | 0.2 | 0.4 | 0.0 | 0.0 | 0.0 | 0.4 | 0.0 | 2.5 |
| Tateidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 28.4 |
| Planorbidae | 0.3 | 3.2 | 8.7 | 2.3 | 12.4 | 36.9 | 58.2 | 1.3 | 28.4 | 3.7 | 6.3 | 25.8 | 8.6 |
| Physidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 14.3 | 9.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Sphaeriidae | 0.2 | 0.0 | 1.3 | 0.2 | 0.0 | 0.0 | 1.0 | 0.2 | 0.3 | 0.0 | 0.1 | 0.0 | 18.4 |
| Dreissenidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.1 |
| Unionidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.9 |
| Mean density of inverebrates (ind./m−2) | 1235 | 752 | 310 | 3223 | 855 | 2078 | 3199 | 1143 | 901 | 684 | 746 | 538 | 1828 |
| Taxa number | 16 | 24 | 21 | 30 | 24 | 24 | 22 | 19 | 21 | 14 | 21 | 19 | 28 |
| Shannon-Wiener index | 0.917 | 1.013 | 1.505 | 1.72 | 1.929 | 2.005 | 1.599 | 1.437 | 2 | 1.817 | 1.685 | 1.665 | 2.191 |
| Share of alien species (%) | 0.0 | 0.1 | 1.3 | 0.3 | 3.6 | 14.3 | 8.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 29.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieplok, A.; Krodkiewska, M.; Franiel, I.; Starzak, R.; Sowa, M.; Spyra, A. The Role of Habitat Protection in Maintaining the Diversity of Aquatic Fauna in Rural and Industrial Areas. Water 2022, 14, 3983. https://doi.org/10.3390/w14233983
Cieplok A, Krodkiewska M, Franiel I, Starzak R, Sowa M, Spyra A. The Role of Habitat Protection in Maintaining the Diversity of Aquatic Fauna in Rural and Industrial Areas. Water. 2022; 14(23):3983. https://doi.org/10.3390/w14233983
Chicago/Turabian StyleCieplok, Anna, Mariola Krodkiewska, Izabella Franiel, Rafał Starzak, Martina Sowa, and Aneta Spyra. 2022. "The Role of Habitat Protection in Maintaining the Diversity of Aquatic Fauna in Rural and Industrial Areas" Water 14, no. 23: 3983. https://doi.org/10.3390/w14233983
APA StyleCieplok, A., Krodkiewska, M., Franiel, I., Starzak, R., Sowa, M., & Spyra, A. (2022). The Role of Habitat Protection in Maintaining the Diversity of Aquatic Fauna in Rural and Industrial Areas. Water, 14(23), 3983. https://doi.org/10.3390/w14233983








