How Much Recurrent Outbreaks of the Moon Jellyfish May Impact the Dynamics of Bacterial Assemblages in Coastal Lagoons?
Abstract
:1. Introduction
2. Materials and Methods
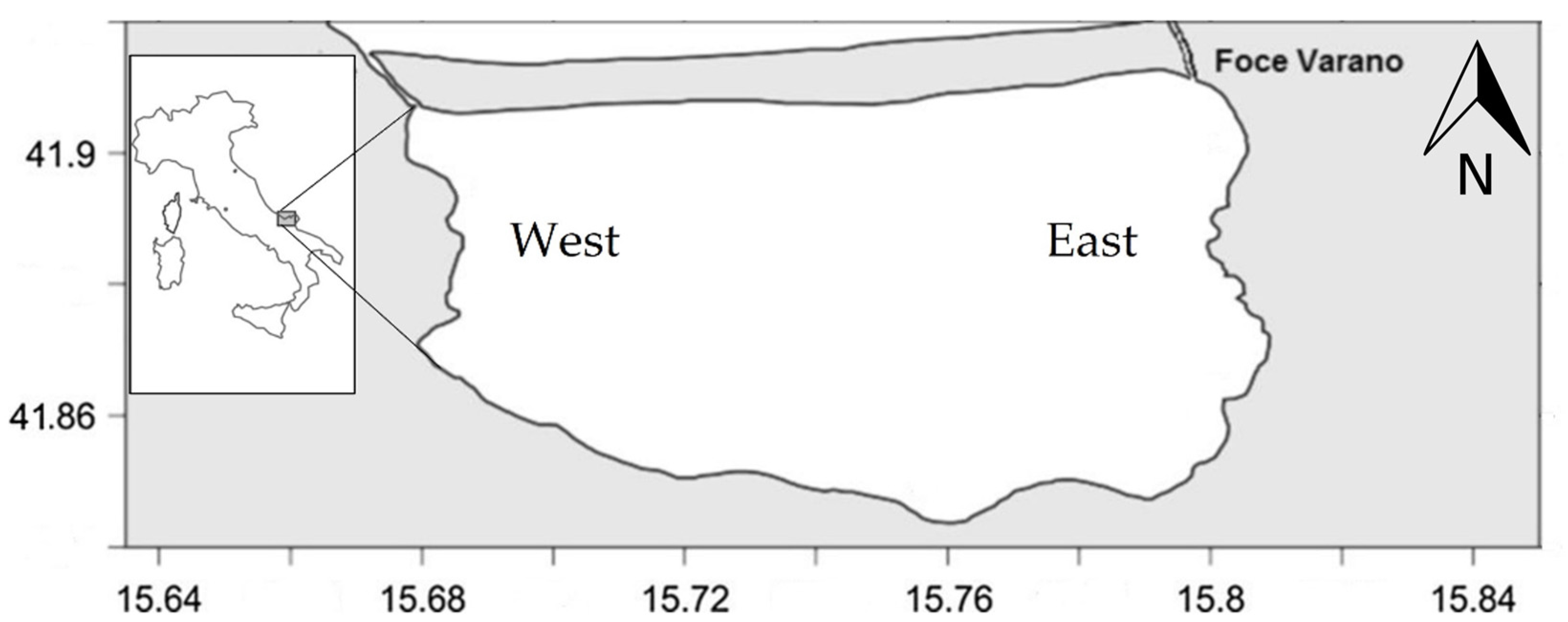
2.1. Sampling Area
2.2. Sample Collection
2.3. Nutrients in the Water Column
2.4. Jellyfish Density
2.5. Total Bacterial Biomass
2.6. Culturable Bacteria
2.6.1. Culturable Vibrios
2.6.2. Culturable Bacteria at 37 °C
2.6.3. Microbial Pollution Indicators
2.7. Statistical Analyses
3. Results
3.1. Abiotic Parameters
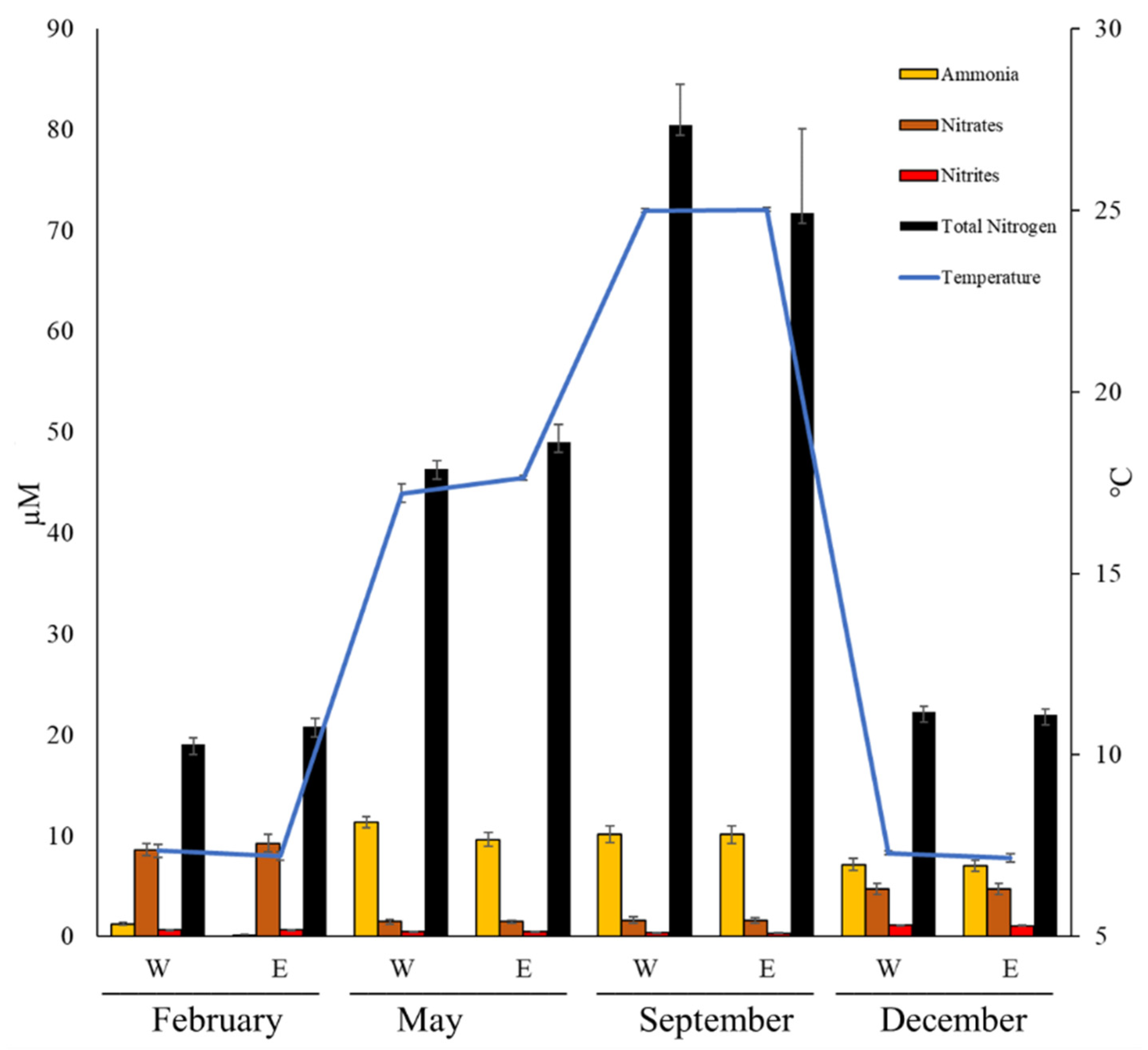
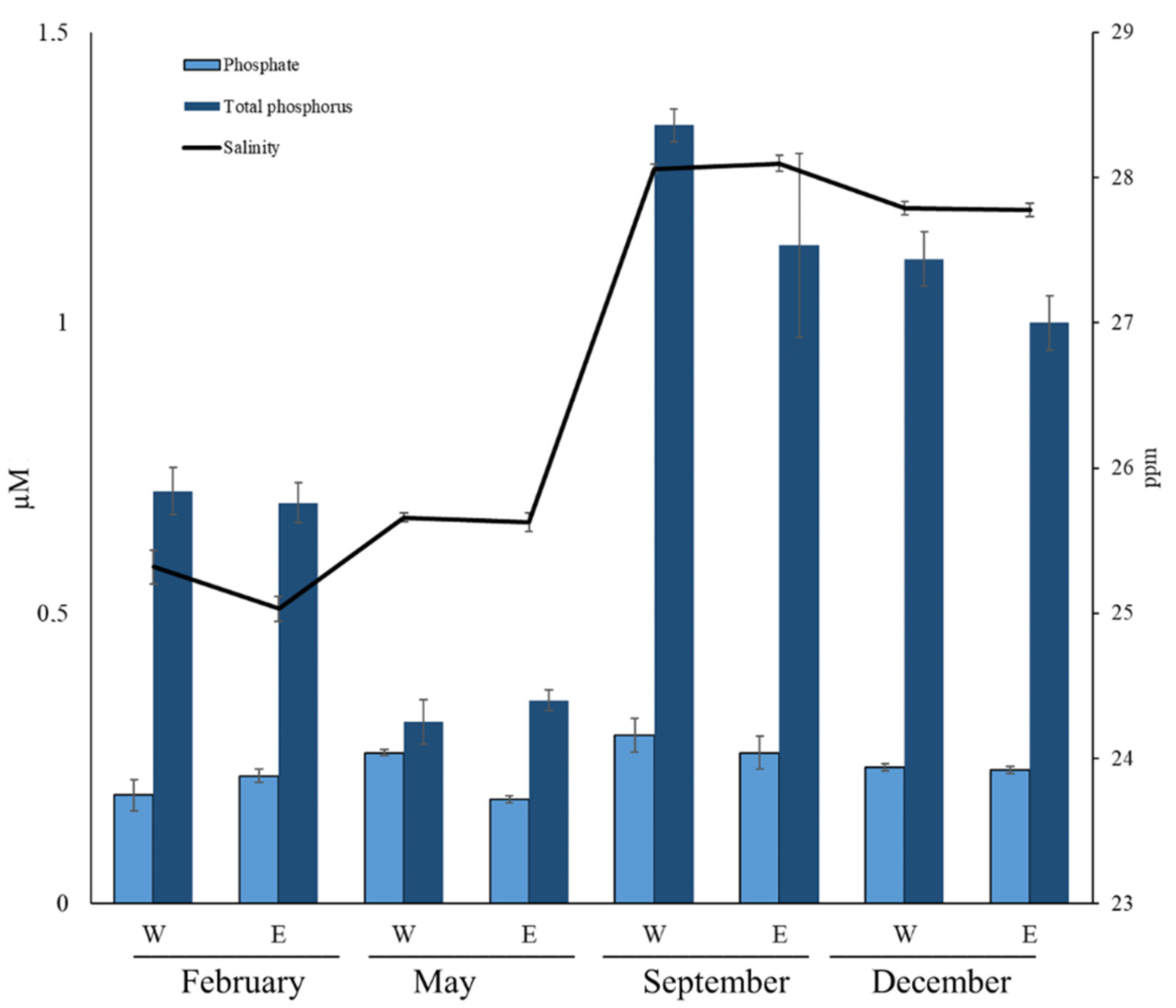
3.2. Inorganic Nutrients
3.3. Jellyfish Density
3.4. Bacterial Biomass
3.5. Culturable Bacteria
3.5.1. Culturable Bacteria at 37 °C
3.5.2. Culturable Vibrios
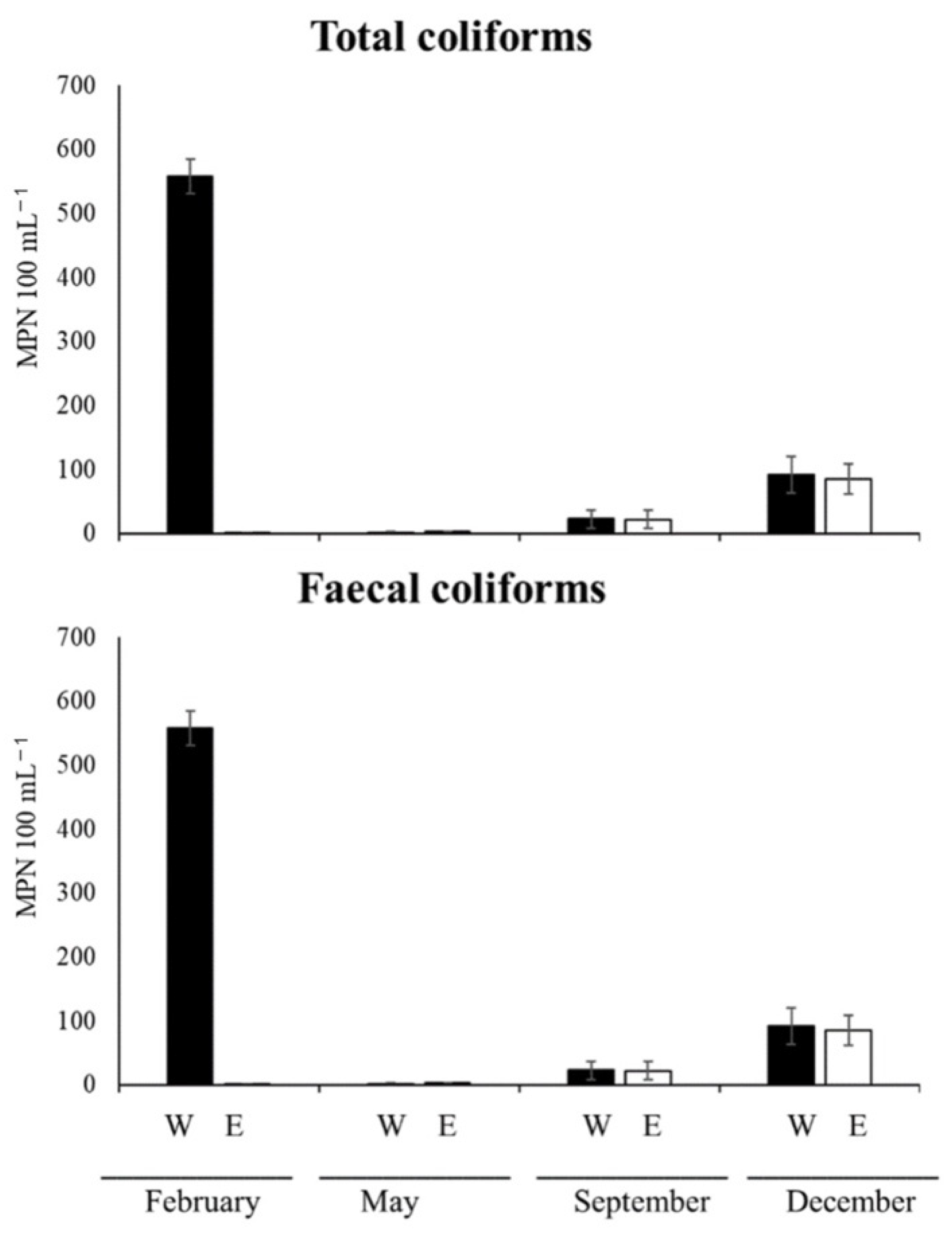
3.5.3. Microbial Pollution Indicators
3.5.4. Total and Faecal Coliforms
3.6. Multivariate Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Purcell, J.E.; Uye, S.; Lo, W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- Condon, R.H.; Graham, W.M.; Duarte, C.M.; Pitt, K.A.; Lucas, C.H.; Haddock, S.H.D.; Sutherland, K.R.; Robinson, K.L.; Dawson, M.N.; Decker, M.B.; et al. Questioning the Rise of Gelatinous Zooplankton in the World’s Oceans. Bioscience 2012, 62, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Arai, M.N. Pelagic coelenterates and eutrophication: A review. Jellyfish Bloom. Ecol. Soc. Importance 2001, 451, 69–87. [Google Scholar]
- Mills, C.E. Jellyfish blooms: Are populations increasing globally in response to changing ocean conditions? Jellyfish Bloom. Ecol. Soc. Importance 2001, 451, 55–68. [Google Scholar]
- Oguz, T. Black Sea Ecosystem Response to Climatic Teleconnections. Oceanography 2005, 18, 122–133. [Google Scholar] [CrossRef]
- Oguz, T. Long-Term Impacts of Anthropogenic Forcing on the Black Sea Ecosystem. Oceanography 2005, 18, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Purcell, J.E. Climate effects on formation of jellyfish and ctenophore blooms: A review. J. Mar. Biol. Assoc. UK 2005, 85, 461–476. [Google Scholar] [CrossRef]
- Hay, S. Marine Ecology: Gelatinous Bells May Ring Change in Marine Ecosystems. Curr. Biol. 2006, 16, R679–R682. [Google Scholar] [CrossRef] [Green Version]
- Graham, W.M.; Bayha, K.M. Biological Invasions by Marine Jellyfish. In Biological Invasions; Springer: Berlin/Heidelberg, Germany, 2007; pp. 239–255. [Google Scholar]
- Boero, F.; Bouillon, J.; Gravili, C.; Miglietta, M.P.; Parsons, T.; Piraino, S. Gelatinous plankton: Irregularities rule the world (sometimes). Mar. Ecol. Prog. Ser. 2008, 356, 299–310. [Google Scholar] [CrossRef]
- Goy, J.; Morand, P.; Etienne, M. Long-term fluctuations of Pelagia noctiluca (Cnidaria, Scyphomedusa) in the western Mediterranean Sea. Prediction by climatic variables. Deep Sea Res. Part A Oceanogr. Res. Pap. 1989, 36, 269–279. [Google Scholar] [CrossRef]
- Brodeur, R.D.; Decker, M.B.; Ciannelli, L.; Purcell, J.E.; Bond, N.A.; Stabeno, P.J.; Acuna, E.; Hunt, G.L. Rise and fall of jellyfish in the eastern Bering Sea in relation to climate regime shifts. Prog. Oceanogr. 2008, 77, 103–111. [Google Scholar] [CrossRef]
- Kogovšek, T.; Bogunović, B.; Malej, A. Recurrence of bloom-forming scyphomedusae: Wavelet analysis of a 200-year time series. In Jellyfish Blooms: New Problems and Solutions; Springer: Dordrecht, The Netherlands, 2010; pp. 81–96. [Google Scholar]
- Daskalov, G.M.; Grishin, A.N.; Rodionov, S.; Mihneva, V. Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. Proc. Natl. Acad. Sci. USA 2007, 104, 10518–10523. [Google Scholar] [CrossRef] [PubMed]
- Basso, L.; Rizzo, L.; Marzano, M.; Intranuovo, M.; Fosso, B.; Pesole, G.; Piraino, S.; Stabili, L. Jellyfish summer outbreaks as bacterial vectors and potential hazards for marine animals and humans health? The case of Rhizostoma pulmo (Scyphozoa, Cnidaria). Sci. Total Environ. 2019, 692, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Rizzo, L.; Basso, L.; Marzano, M.; Fosso, B.; Pesole, G.; Piraino, S. The Microbial Community Associated with Rhizostoma pulmo: Ecological Significance and Potential Consequences for Marine Organisms and Human Health. Mar. Drugs 2020, 18, 437. [Google Scholar] [CrossRef]
- Lebrato, M.; Jones, D.O. Expanding the oceanic carbon cycle: Jellyfish biomass in the biological pump. Biochemist 2011, 33, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Pitt, K.A.; Welsh, D.T.; Condon, R.H. Influence of jellyfish blooms on carbon, nitrogen and phosphorus cycling and plankton production. Hydrobiologia 2009, 616, 133–149. [Google Scholar] [CrossRef]
- Hansson, L.J.; Norrman, B. Release of dissolved organic carbon (DOC) by the scyphozoan jellyfish Aurelia aurita and its potential influence on the production of planktic bacteria. Mar. Biol. 1995, 121, 527–532. [Google Scholar] [CrossRef]
- Carlson, C.A. Production and Removal Processes. Biogeochem. Mar. Dissolved Org. Matter 2002, 91–151. [Google Scholar] [CrossRef]
- Condon, R.H.; Steinberg, D.K. Development, biological regulation, and fate of ctenophore blooms in the York River estuary, Chesapeake Bay. Mar. Ecol. Prog. Ser. 2008, 369, 153–168. [Google Scholar] [CrossRef]
- Riemann, L.; Titelman, J.; Båmstedt, U. Links between jellyfish and microbes in a jellyfish dominated fjord. Mar. Ecol. Prog. Ser. 2006, 325, 29–42. [Google Scholar] [CrossRef]
- Titelman, J.; Riemann, L.; Sørnes, T.A.; Nilsen, T.; Griekspoor, P.; Båmstedt, U. Turnover of dead jellyfish: Stimulation and retardation of microbial activity. Mar. Ecol. Prog. Ser. 2006, 325, 43–58. [Google Scholar] [CrossRef]
- Ducklow, H.W.; Mitchell, R. Composition of mucus released by coral reef coelenterates1. Limnol. Oceanogr. 1979, 24, 706–714. [Google Scholar] [CrossRef]
- Wild, C.; Woyt, H.; Huettel, M. Influence of coral mucus on nutrient fluxes in carbonate sands. Mar. Ecol. Prog. Ser. 2005, 287, 87–98. [Google Scholar] [CrossRef]
- Angilè, F.; Del Coco, L.; Girelli, C.R.; Basso, L.; Rizzo, L.; Piraino, S.; Stabili, L.; Fanizzi, F.P. 1H NMR Metabolic Profile of Scyphomedusa Rhizostoma pulmo (Scyphozoa, Cnidaria) in Female Gonads and Somatic Tissues: Preliminary Results. Molecules 2020, 25, 806. [Google Scholar] [CrossRef] [Green Version]
- Azam, F. Oceanography: Microbial Control of Oceanic Carbon Flux: The Plot Thickens. Science 1998, 280, 694–696. [Google Scholar] [CrossRef] [Green Version]
- Azam, F.; Simmons, F.W.; Mulvaney, R.L. Mineralization of N from plant residues and its interaction with native soil N. Soil Biol. Biochem. 1993, 25, 1787–1792. [Google Scholar] [CrossRef]
- Cho, B.C.; Azam, F. Biogeochemical significance of bacterial biomass in the ocean’s euphotic zone. Mar. Ecol. Prog. Ser. 1990, 63, 253–259. [Google Scholar] [CrossRef]
- Cole, J.J.; Findlay, S.; Pace, M.L. Bacterial production in fresh and saltwater ecosystems: A cross-system overview. Mar. Ecol. Prog. Ser. 1988, 43, 1–10. [Google Scholar] [CrossRef]
- Condon, R.H.; Steinberg, D.K.; del Giorgio, P.A.; Bouvier, T.C.; Bronk, D.A.; Graham, W.M.; Ducklow, H.W. Jellyfish blooms result in a major microbial respiratory sink of carbon in marine systems. Proc. Natl. Acad. Sci. USA 2011, 108, 10225–10230. [Google Scholar] [CrossRef] [Green Version]
- Tinta, T.; Kogovšek, T.; Turk, V.; Shiganova, T.A.; Mikaelyan, A.S.; Malej, A. Microbial transformation of jellyfish organic matter affects the nitrogen cycle in the marine water column—A Black Sea case study. J. Exp. Mar. Bio. Ecol. 2016, 475, 19–30. [Google Scholar] [CrossRef]
- Lebrato, M.; Pitt, K.A.; Sweetman, A.K.; Jones, D.O.B.; Cartes, J.E.; Oschlies, A.; Condon, R.H.; Molinero, J.C.; Adler, L.; Gaillard, C.; et al. Jelly-falls historic and recent observations: A review to drive future research directions. Hydrobiologia 2012, 690, 227–245. [Google Scholar] [CrossRef]
- West, E.J.; Welsh, D.T.; Pitt, K.A. Influence of decomposing jellyfish on the sediment oxygen demand and nutrient dynamics. Hydrobiologia 2009, 616, 151–160. [Google Scholar] [CrossRef]
- Sweetman, A.K.; Chelsky, A.; Pitt, K.A.; Andrade, H.; van Oevelen, D.; Renaud, P.E. Jellyfish decomposition at the seafloor rapidly alters biogeochemical cycling and carbon flow through benthic food-webs. Limnol. Oceanogr. 2016, 61, 1449–1461. [Google Scholar] [CrossRef]
- Tinta, T.; Malej, A.; Kos, M.; Turk, V. Degradation of the Adriatic medusa Aurelia sp. by ambient bacteria. Hydrobiologia 2010, 645, 179–191. [Google Scholar] [CrossRef]
- Blanchet, M.; Pringault, O.; Bouvy, M.; Catala, P.; Oriol, L.; Caparros, J.; Ortega-Retuerta, E.; Intertaglia, L.; West, N.; Agis, M.; et al. Changes in bacterial community metabolism and composition during the degradation of dissolved organic matter from the jellyfish Aurelia aurita in a Mediterranean coastal lagoon. Environ. Sci. Pollut. Res. 2015, 22, 13638–13653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzari, C.; Fosso, B.; Marzano, M.; Annese, A.; Caprioli, R.; D’Erchia, A.M.; Gissi, C.; Intranuovo, M.; Picardi, E.; Santamaria, M.; et al. The influence of invasive jellyfish blooms on the aquatic microbiome in a coastal lagoon (Varano, SE Italy) detected by an Illumina-based deep sequencing strategy. Biol. Invasions 2015, 17, 923–940. [Google Scholar] [CrossRef] [Green Version]
- Tinta, T.; Kogovšek, T.; Malej, A.; Turk, V. Jellyfish modulate bacterial dynamic and community structure. PLoS ONE 2012, 7, e39274. [Google Scholar] [CrossRef]
- Tinta, T.; Klun, K.; Herndl, G.J. The importance of jellyfish–microbe interactions for biogeochemical cycles in the ocean. Limnol. Oceanogr. 2021, 66, 2011–2032. [Google Scholar] [CrossRef]
- Turk, V.; Lučić, D.; Flander-Putrle, V.; Malej, A. Feeding of Aurelia sp. (Scyphozoa) and links to the microbial food web. Mar. Ecol. 2008, 29, 495–505. [Google Scholar] [CrossRef]
- Tinta, T.; Zhao, Z.; Escobar, A.; Klun, K.; Bayer, B.; Amano, C.; Bamonti, L.; Herndl, G.J. Microbial Processing of Jellyfish Detritus in the Ocean. Front. Microbiol. 2020, 11, 590995. [Google Scholar] [CrossRef]
- Scorrano, S.; Aglieri, G.; Boero, F.; Dawson, M.N.; Piraino, S. Unmasking Aurelia species in the Mediterranean Sea: An integrative morphometric and molecular approach. Zool. J. Linn. Soc. 2017, 180, 243–267. [Google Scholar] [CrossRef]
- Hansen, H.P.; Koroleff, F. Determination of nutrients. In Methods of Seawater Analysis, 3rd ed.; Grasshoff, K., Kremling, K., Ehrhardt, M., Eds.; Wiley-VCH: Weinheim, Germany, 1999; pp. 159–228. Available online: https://gradocienciasdelmar.files.wordpress.com/2012/09/methods-of-seawater-analysis.pdf (accessed on 23 November 2022).
- Porter, K.G.; Feig, Y.S. The use of DAPI for identifying and counting aquatic microflora1. Limnol. Oceanogr. 1980, 25, 943–948. [Google Scholar] [CrossRef]
- Danovaro, R.; Marrale, D.; Croce, N.D.; Dell’Anno, A.; Fabiano, M. Heterotrophic Nanoflagellates, Bacteria, and Labile Organic Compounds in Continental Shelf and Deep-Sea Sediments of the Eastern Mediterranean. Microb. Ecol. 1998, 35, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Fry, J.C. Direct methods and biomass estimation. Methods Microbiol. 1990, 22, 41–85. [Google Scholar]
- Høi, L.; Larsen, J.L.; Dalsgaard, I.; Dalsgaard, A. Occurrence of Vibrio vulnificus Biotypes in Danish Marine Environments. Appl. Environ. Microbiol. 1998, 64, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- APHA Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005.
- ISO 9308-3:1998; Water Quality. Detection and Enumeration of Escherichia Coli and Coliform Bacteria in Surface and Waste Water. ISO: Geneva, Switzerland, 1998.
- ISO 7899-1:1998; Water Quality. Detection and Enumeration of Intestinal Enterococci in Surface and Waste Water. ISO: Geneva, Switzerland, 1998.
- Anderson, M.; Braak, C. Ter Permutation tests for multi-factorial analysis of variance. J. Stat. Comput. Simul. 2003, 73, 85–113. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER V6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Specchiulli, A.; Focardi, S.; Renzi, M.; Scirocco, T.; Cilenti, L.; Breber, P.; Bastianoni, S. Environmental heterogeneity patterns and assessment of trophic levels in two Mediterranean lagoons: Orbetello and Varano, Italy. Sci. Total Environ. 2008, 402, 285–298. [Google Scholar] [CrossRef]
- Guélorget, O.; Perthuisot, J.P. Le domaine paralique. Expressions géologiques, biologiques et économiques du confinement. Trav. Lab. Géologie Ecole Norm. Sup. Paris 1983, 16, 136. [Google Scholar]
- Marolla, V. Nota Preliminare Su Alcune Caratteristiche Chimico-Fisiche Della Laguna Di Varano Durante L’Anno 1970; Technical Report of National Research Council-Institute of Marine Science-Department of Lesina; CNR: Lesina, Italy, 1980; 17p. [Google Scholar]
- Caroppo, C. The contribution of picophytoplankton to community structure in a Mediterranean brackish environment. J. Plankton Res. 2000, 22, 381–397. [Google Scholar] [CrossRef]
- Duyl, F.C.; van Ko, F.C.; van Kopp, A.J. Seasonal patterns of bacterial production and biomass in intertidal sediments of the western Dutch Wadden Sea. Mar. Ecol. Prog. Ser. 1990, 59, 249–261. [Google Scholar] [CrossRef]
- Zaccone, R.; Caruso, G. Microbial enzymes in the Mediterranean Sea: Relationship with climate changes. AIMS Microbiol. 2019, 5, 251–271. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.; Almeida, A.; Coelho, F.J.R.C.; Gomes, N.C.M.; Oliveira, V.; Santos, A.L. Bacterial Extracellular Enzymatic Activity in Globally Changing Aquatic Ecosystems. In Current Research, Technology and Education, Topics in Applied Microbiology and Microbial Biotechnology; Mendez-Vilas, A., Ed.; FORMATEX Research Center: Badajoz, Spain, 2010; pp. 124–135. [Google Scholar]
- Sarmento, H.; Montoya, J.M.; Vázquez-Domínguez, E.; Vaqué, D.; Gasol, J.M. Warming effects on marine microbial food web processes: How far can we go when it comes to predictions? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2137–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavuta, G.; Di Matteo, D. Landscapes protection and eco-development: The case study of Gargano National Park, Italy. GeoJournal Tour. Geosites 2016, 17, 95–111. [Google Scholar]
- Lucas, C.H.; Jones, D.O.B.; Hollyhead, C.J.; Condon, R.H.; Duarte, C.M.; Graham, W.M.; Robinson, K.L.; Pitt, K.A.; Schildhauer, M.; Regetz, J. Gelatinous zooplankton biomass in the global oceans: Geographic variation and environmental drivers. Glob. Ecol. Biogeogr. 2014, 23, 701–714. [Google Scholar] [CrossRef]
- Condon, R.H.; Steinberg, D.K.; Bronk, D.A. Production of dissolved organic matter and inorganic nutrients by gelatinous zooplankton in the York River estuary, Chesapeake Bay. J. Plankton Res. 2010, 32, 153–170. [Google Scholar] [CrossRef]
- Dinasquet, J.; Kragh, T.; Schrøter, M.-L.; Søndergaard, M.; Riemann, L. Functional and compositional succession of bacterioplankton in response to a gradient in bioavailable dissolved organic carbon. Environ. Microbiol. 2013, 15, 2616–2628. [Google Scholar] [CrossRef]
- Tinta, T.; Vojvoda, J.; Mozetič, P.; Talaber, I.; Vodopivec, M.; Malfatti, F.; Turk, V. Bacterial community shift is induced by dynamic environmental parameters in a changing coastal ecosystem (northern Adriatic, northeastern Mediterranean Sea)–a2-year time-series study. Environ. Microbiol. 2015, 17, 3581–3596. [Google Scholar] [CrossRef]
- Kolmakova, A.A.; Kolmakov, V.I. Amino Acid Composition of Green Microalgae and Diatoms, Cyanobacteria, and Zooplankton (Review). Inl. Water Biol. 2019, 12, 452–461. [Google Scholar] [CrossRef]
- Stabili, L.; Cardone, F.; Alifano, P.; Tredici, S.M.; Piraino, S.; Corriero, G.; Gaino, E. Epidemic Mortality of the Sponge Ircinia variabilis (Schmidt, 1862) Associated to Proliferation of a Vibrio Bacterium. Microb. Ecol. 2012, 64, 802–813. [Google Scholar] [CrossRef]
- Rizzo, L.; Fraschetti, S.; Alifano, P.; Tredici, M.S.; Stabili, L. Association of Vibrio community with the Atlantic Mediterranean invasive alga Caulerpa cylindracea. J. Exp. Mar. Biol. Ecol. 2016, 475, 129–136. [Google Scholar] [CrossRef]
- Rizzo, L.; Fraschetti, S.; Alifano, P.; Pizzolante, G.; Stabili, L. The alien species Caulerpa cylindracea and its associated bacteria in the Mediterranean Sea. Mar. biol. 2016, 163, 1–12. [Google Scholar] [CrossRef]
- Sarjito, S.; Radjasa, O.K.; Sabdono, A.; Prayitno, S.B.; Hutabarat, S. Phylogenetic diversity of the causative agents of vibriosis associated with groupers fish from Karimunjawa Islands, Indonesia. Curr. Res. Bacteriol. 2009, 2, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Farmer, J.J., III; Janda, M.; Brenner, F.W.; Cameron, D.N.; Birkhead, K.M.; Genus, I. Vibrio Pacini 1854, 411AL. In Bergey’s Manual of Systematic Bacteriology. The Proteobacteria. Part B. The Gammaproteobacteria, 2nd ed.; Brenner, D.J., Krieg, N.R., Staley, J.T., Eds.; Springer: New York, NY, USA, 2005; Volume 2, pp. 494–546. [Google Scholar]
- Vezzulli, L.; Colwell, R.R.; Pruzzo, C. Ocean Warming and Spread of Pathogenic Vibrios in the Aquatic Environment. Microb. Ecol. 2013, 65, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, L.; Grande, C.; Reid, P.C.; Hélaouët, P.; Edwards, M.; Höfle, M.G.; Brettar, I.; Colwell, R.R.; Pruzzo, C. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc. Natl. Acad. Sci. USA 2016, 113, E5062–E5071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, J.C.; Herbst, S.; Rechenburg, A.; Suk, J.E.; Höser, C.; Schreiber, C.; Kistemann, T. Climate Change Impact Assessment of Food- and Waterborne Diseases. Crit. Rev. Environ. Sci. Technol. 2012, 42, 857–890. [Google Scholar] [CrossRef]
- Tinta, T.; Kogovšek, T.; Klun, K.; Malej, A.; Herndl, G.J.; Turk, V. Jellyfish-associated microbiome in the marine environment: Exploring its biotechnological potential. Mar. Drugs 2019, 17, 94. [Google Scholar] [CrossRef] [Green Version]
- Caroppo, C. Variability and interactions of phytoplankton and bacterioplankton in Varano lagoon (Adriatic Sea). J. Plankton Res. 2002, 24, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Tolomio, C.; Andreoli, C.; Montanari, M. Etude sur l’hydrologie et le phytoplancton du lac de Varano (Mer Adriatique). Mai 1985–Avril 1986. Algol. Stud. 1990, 58, 57–86. [Google Scholar]
- Spagnoli, F.; Specchiulli, A.; Scirocco, T.; Carapella, G.; Villani, P.; Casolino, G.; Schiavone, P.; Franchi, M. The Lago di Varano: Hydrologic Characteristics and Sediment Composition. Mar. Ecol. 2002, 23, 384–394. [Google Scholar] [CrossRef]
- Holst, S.; Jarms, G. Substrate choice and settlement preferences of planula larvae of five Scyphozoa (Cnidaria) from German Bight, North Sea. Mar. Biol. 2007, 151, 863–871. [Google Scholar] [CrossRef]
- Lo, W.-T.; Purcell, J.E.; Hung, J.-J.; Su, H.-M.; Hsu, P.-K. Enhancement of jellyfish (Aurelia aurita) populations by extensive aquaculture rafts in a coastal lagoon in Taiwan. ICES J. Mar. Sci. 2008, 65, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Rakow, K.C.; Graham, W.M. Orientation and swimming mechanics by the scyphomedusa Aurelia sp. in shear flow. Limnol. Oceanogr. 2006, 51, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Billett, D.S.M.; Bett, B.J.; Jacobs, C.L.; Rouse, I.P.; Wigham, B.D. Mass deposition of jellyfish in the deep Arabian Sea. Limnol. Oceanogr. 2006, 51, 2077–2083. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, J.; Hirose, M.; Ohtani, T.; Sugimoto, K.; Hirase, K.; Shimamoto, N.; Shimura, T.; Honda, N.; Fujimori, Y.; Mukai, T. Transportation of organic matter to the sea floor by carrion falls of the giant jellyfish Nemopilema nomurai in the Sea of Japan. Mar. Biol. 2008, 153, 311–317. [Google Scholar] [CrossRef]
- Hollibaugh, J.T.; Azam, F. Microbial degradation of dissolved proteins in seawater. Limnol. Oceanogr. 1983, 28, 1104–1116. [Google Scholar] [CrossRef]
- Hoppe, H.-G.; Kim, S.-J.; Gocke, K. Microbial Decomposition in Aquatic Environments: Combined Process of Extracellular Enzyme Activity and Substrate Uptake. Appl. Environ. Microbiol. 1988, 54, 784–790. [Google Scholar] [CrossRef]
- Keil, R.G.; Kirchman, D.L. Dissolved combined amino acids: Chemical form and utilization by marine bacteria. Limnol. Oceanogr. 1993, 38, 1256–1270. [Google Scholar] [CrossRef]
- Kremer, P. Respiration and excretion by the ctenophore Mnepiopsis leidyi. Mar. Biol. 1977, 44, 43–50. [Google Scholar] [CrossRef]
- Mozetič, P.; Solidoro, C.; Cossarini, G.; Socal, G.; Precali, R.; Francé, J.; Bianchi, F.; De Vittor, C.; Smodlaka, N.; Fonda Umani, S. Recent Trends Towards Oligotrophication of the Northern Adriatic: Evidence from Chlorophyll a Time Series. Estuaries Coasts 2010, 33, 362–375. [Google Scholar] [CrossRef] [Green Version]
- Klun, K.; Falnoga, I.; Mazej, D.; Šket, P.; Faganeli, J. Colloidal Organic Matter and Metal(loid)s in Coastal Waters (Gulf of Trieste, Northern Adriatic Sea). Aquat. Geochem. 2019, 25, 179–194. [Google Scholar] [CrossRef]

| Temperature | Salinity | Jellyfish Density | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | df | MS | Pseudo-F | p | MS | Pseudo-F | p | MS | Pseudo-F | p |
| T | 3 | 446.67 | 8847.70 | *** | 13.03 | 1004.20 | *** | 258.47 | 1391.10 | *** |
| Z | 1 | 0.01 | 0.10 | ns | 0.03 | 1.07 | ns | 60.34 | 1.07 | ns |
| T × Z | 3 | 0.11 | 2.09 | ns | 0.03 | 2.41 | ns | 56.33 | 303.17 | *** |
| Res | 16 | 0.05 | 0.01 | 0.19 | ||||||
| Tot | 23 | |||||||||
| Ammonia | Nitrites | Nitrates | Total Nitrogen | ||||||||||
| Source | df | MS | Pseudo-F | p | MS | Pseudo-F | p | MS | Pseudo-F | p | MS | Pseudo-F | p |
| T | 3 | 123 | 109.46 | *** | 0.66 | 221.69 | *** | 73.27 | 98.52 | *** | 4141.60 | 119.38 | *** |
| Z | 1 | 3.34 | 3.40 | ns | 4.59 × 10−4 | 0.20 | ns | 0.12 | 0.16 | ns | 8.17 | 0.20 | ns |
| T × Z | 3 | 0.98 | 0.88 | ns | 2.32 × 10−3 | 0.78 | ns | 0.17 | 0.22 | ns | 40.56 | 1.17 | ns |
| Res | 16 | 1.12 | 2.97 × 10−3 | 0.74 | 34.69 | ||||||||
| Tot | 23 | ||||||||||||
| Phosphates | Total Phosphorus | Silicates | |||||||||||
| Source | df | MS | Pseudo-F | p | MS | Pseudo-F | p | MS | Pseudo-F | p | |||
| T | 3 | 5.63 × 10−3 | 5.60 | ** | 0.96 | 73.355 | *** | 2235.30 | 2780.00 | *** | |||
| Z | 1 | 2.50 × 10−3 | 0.74 | ns | 0.03 | 1.9821 | ns | 2.09 | 4.28 | ns | |||
| T × Z | 3 | 3.38 × 10−3 | 3.37 | * | 0.02 | 1.2978 | ns | 0.49 | 0.61 | ns | |||
| Res | 16 | 1.00 × 10−3 | 0.01 | 0.80 | |||||||||
| Tot | 23 | ||||||||||||
| Temperature | Salinity | Jellyfish Density | Ammonia | Nitrates | Nitrites | Total Nitrogen | Phosphates | Total Phosphorus | Silicates | Culturable Bacteria at 37 °C | Vibrio spp. | Enterococci | Total Coliforms | Faecal Coliforms | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salinity | 0.38 | ||||||||||||||
| Jellyfish Density | 0.86 | 0.40 | |||||||||||||
| Ammonia | 0.73 | 0.50 | 0.65 | ||||||||||||
| Nitrates | −0.78 | −0.47 | −0.65 | −0.91 | |||||||||||
| Nitrites | −0.82 | 0.15 | −0.70 | −0.35 | 0.48 | ||||||||||
| Total Nitrogen | 0.97 | 0.44 | 0.85 | 0.69 | −0.74 | −0.76 | |||||||||
| Phosphates | 0.47 | 0.51 | 0.67 | 0.50 | −0.34 | −0.17 | 0.49 | ||||||||
| Total Phosphorus | 0.20 | 0.81 | 0.26 | 0.10 | −0.01 | 0.20 | 0.28 | 0.52 | |||||||
| Silicates | 0.09 | 0.57 | 0.20 | −0.27 | 0.27 | 0.08 | 0.18 | 0.34 | 0.86 | ||||||
| Culturable bacteria at 37 °C | 0.13 | 0.89 | 0.15 | 0.34 | −0.24 | 0.40 | 0.21 | 0.45 | 0.81 | 0.54 | |||||
| Vibrio spp. | 0.84 | 0.59 | 0.80 | 0.43 | −0.53 | −0.63 | 0.87 | 0.44 | 0.53 | 0.54 | 0.34 | ||||
| Enterococci | −0.52 | 0.50 | −0.42 | 0.00 | 0.08 | 0.88 | −0.46 | 0.02 | 0.36 | 0.12 | 0.66 | −0.34 | |||
| Total coliforms | −0.43 | −0.27 | −0.35 | −0.54 | 0.54 | 0.17 | −0.43 | −0.40 | −0.02 | 0.18 | −0.25 | −0.27 | −0.02 | ||
| Faecal coliforms | −0.43 | −0.28 | −0.35 | −0.54 | 0.54 | 0.17 | −0.43 | −0.40 | −0.02 | 0.18 | −0.25 | −0.27 | −0.02 | 1.00 | |
| Bacterial biomass | 0.86 | 0.54 | 0.83 | 0.44 | −0.47 | −0.66 | 0.87 | 0.58 | 0.58 | 0.58 | 0.33 | 0.94 | −0.41 | −0.22 | −0.22 |
| Bacterial Biomass | Culturable Bacteria at 37 °C | Vibrio spp. | ||||||||
| Source | df | MS | Pseudo-F | p | MS | Pseudo-F | p | MS | Pseudo-F | p |
| T | 3 | 2.50 × 106 | 1364.60 | *** | 2.66 × 107 | 34.96 | *** | 3.73 × 105 | 68.48 | *** |
| Z | 1 | 7203.10 | 1.83 | ns | 1.48 × 105 | 1.73 | ns | 620.17 | 2.26 | ns |
| T × Z | 3 | 3.93 × 103 | 2.14 | ns | 85698.00 | 0.11 | ns | 274.35 | 0.05 | ns |
| Res | 16 | 1835 | 7.62 × 105 | 5441.60 | ||||||
| Tot | 23 | |||||||||
| Faecal Enterococci | Total Coliforms | Faecal Coliforms | ||||||||
| Source | df | MS | Pseudo-F | p | MS | Pseudo-F | p | MS | Pseudo-F | p |
| T | 3 | 4575.20 | 115.58 | *** | 95795 | 101.75 | *** | 94661 | 112.43 | *** |
| Z | 1 | 1.50 | 27.00 | ns | 1.19 × 105 | 1.03 | ns | 1.17 × 105 | 1.03 | ns |
| T × Z | 3 | 0.06 | 1.40 × 10−3 | ns | 1.16 × 105 | 123.05 | *** | 1.14 × 105 | 135.35 | *** |
| Res | 16 | 217.08 | 941.46 | 841.92 | ||||||
| Tot | 23 | |||||||||
| Nutrient Composition | ||||
|---|---|---|---|---|
| Source | df | MS | Pseudo-F | p |
| T | 3 | 46.62 | 55.63 | *** |
| Z | 1 | 1.74 | 0.87 | ns |
| T × Z | 3 | 2.00 | 2.39 | ns |
| Res | 16 | 0.84 | ||
| Tot | 23 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stabili, L.; Rizzo, L.; Caprioli, R.; Alabiso, G.; Piraino, S. How Much Recurrent Outbreaks of the Moon Jellyfish May Impact the Dynamics of Bacterial Assemblages in Coastal Lagoons? Water 2022, 14, 3908. https://doi.org/10.3390/w14233908
Stabili L, Rizzo L, Caprioli R, Alabiso G, Piraino S. How Much Recurrent Outbreaks of the Moon Jellyfish May Impact the Dynamics of Bacterial Assemblages in Coastal Lagoons? Water. 2022; 14(23):3908. https://doi.org/10.3390/w14233908
Chicago/Turabian StyleStabili, Loredana, Lucia Rizzo, Rosa Caprioli, Giorgio Alabiso, and Stefano Piraino. 2022. "How Much Recurrent Outbreaks of the Moon Jellyfish May Impact the Dynamics of Bacterial Assemblages in Coastal Lagoons?" Water 14, no. 23: 3908. https://doi.org/10.3390/w14233908









