Advancement of a Liquid Scintillation Counter and Semiconductor Alpha Spectroscopy Detector to Estimate the Radon Concentration in Groundwater
Abstract
:1. Introduction
2. Materials and Methods
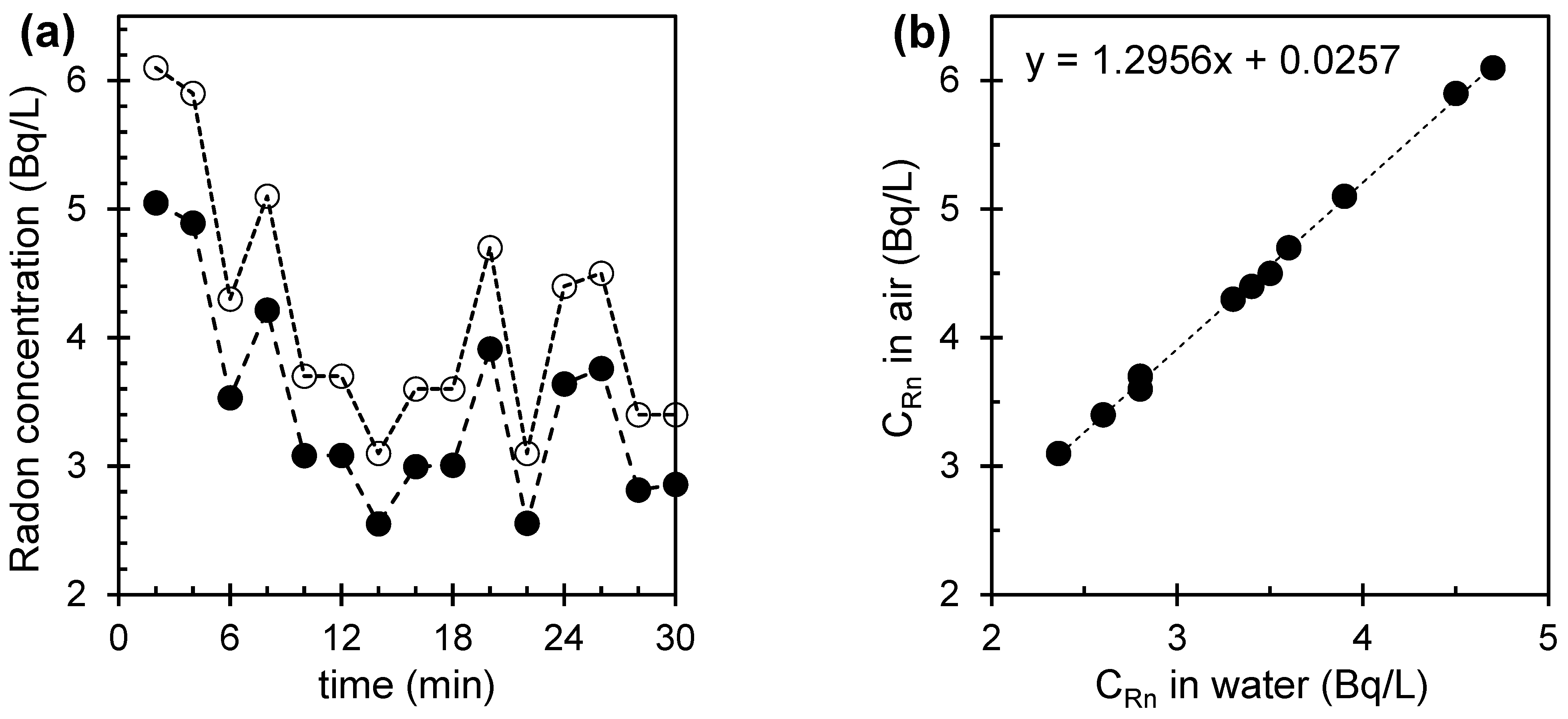
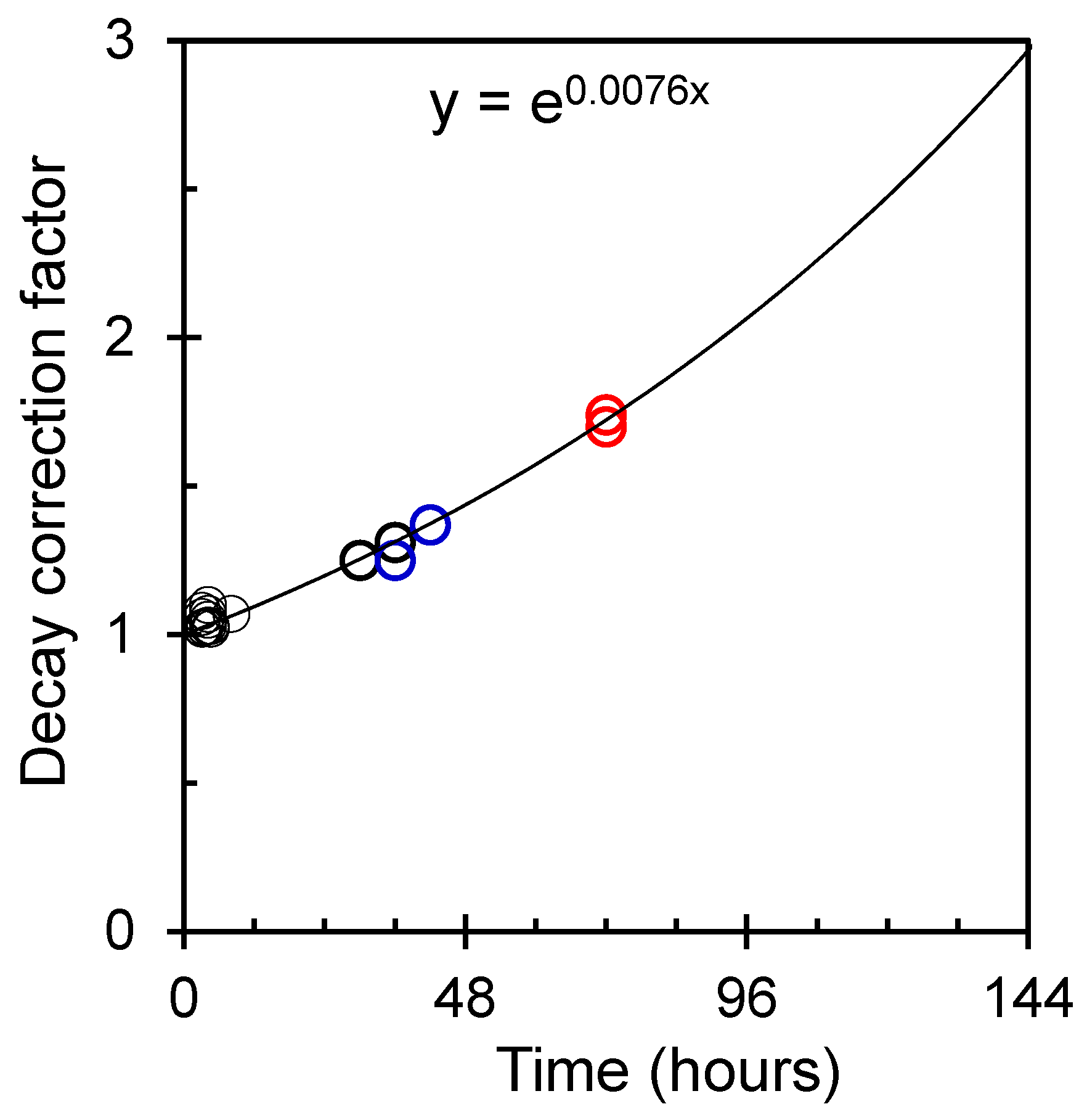
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNSCEAR Sources. Effects and Risks of Ionizing Radiation; United Nations: New York, NY, USA, 1988; p. 199. [Google Scholar]
- Aleissa, K.A.; Alghamdi, A.S.; Almasoud, F.I.; Islam, M.S. Measurement of radon levels in groundwater supplies of Riyadh with liquid scintillation counter and the associated radiation dose. Radiat. Prot. Dosim. 2012, 154, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Przylibski, T.A.; Domin, E.; Gorecka, J.; Kowalska, A. 222Rn Concentration in Groundwaters Circulating in Granitoid Massifs of Poland. Water 2020, 12, 748. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.D. Lung cancer risk and effective dose coefficients for radon: UNSCEAR review and ICRP conclusions. J. Radiol. Prot. 2021, 41, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Petoussi-Henss, N.; Satoh, D.; Endo, A.; Eckerman, K.; Bolch, W.; Hunt, J.; Jansen, J.T.M.; Kim, C.H.; Lee, C.; Saito, K.; et al. ICRP Publication 144: Dose Coefficients for External Exposures to Environmental Sources. Ann. ICRP 2020, 49, 11–145. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.F.I.; Rabaiee, N.A.; Jaafar, M.S. Determination of radon concentration in water using RAD7 with RAD H2accessories. In Proceedings of the National Physics Conference 2014 (PERFIK 2014), Kuala Lumpur, Malaysia, 18–19 November 2014; p. 120005. [Google Scholar]
- Hopke, P.K.; Borak, T.B.; Doull, J.; Cleaver, J.E.; Eckerman, K.F.; Gundersen, L.C.S.; Harley, N.H.; Hess, C.T.; Kinner, N.E.; Kopecky, K.J.; et al. Health Risks Due to Radon in Drinking Water. Environ. Sci. Technol. 2000, 34, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Calin, M.R.; Ion, A.C.; Radulescu, I.; Simion, C.A.; Mincu, M.M.; Ion, I. Analysis of the radon concentrations in natural mineral and tap water using Lucas cells technique. J. Environ. Eng. Landsc. Manag. 2022, 30, 370–379. [Google Scholar] [CrossRef]
- De Simone, G.; Galli, G.; Lucchetti, C.; Tuccimei, P. Calibration of Big Bottle RAD H2O set-up for radon in water using HDPE bottles. Radiat. Meas. 2015, 76, 1–7. [Google Scholar] [CrossRef]
- Alonso, H.; Cruz-Fuentes, T.; Rubiano, J.G.; González-Guerra, J.; Cabrera, M.D.C.; Arnedo, M.A.; Tejera, A.; Rodríguez-Gonzalez, A.; Pérez-Torrado, F.J.; Martel, P. Radon in Groundwater of the Northeastern Gran Canaria Aquifer. Water 2015, 7, 2575–2590. [Google Scholar] [CrossRef] [Green Version]
- Durridge. RAD7 Manual. Durridge Company Inc., USA. Available online: https://durridge.com/documentation/RAD7%20Manual.pdf (accessed on 15 September 2022).
- Cho, B.; Hwang, J.; Lee, B.; Oh, Y.; Choo, C. Radon Concentrations in Raw Water and Treated Water Used for Bottled Water in South Korea. Sustainability 2020, 12, 5313. [Google Scholar] [CrossRef]
- Abuelhia, E. Evaluation of annual effective dose from indoor radon concentration in Eastern Province, Dammam, Saudi Arabia. Radiat. Phys. Chem. 2017, 140, 137–140. [Google Scholar] [CrossRef]
- Abuelhia, E. Assessment of radiation dose from radon ingestion and inhalation in commercially bottled drinking water and its annual effective dose in Eastern Province, Saudi Arabia. Int. J. Environ. Health Res. 2019, 29, 164–172. [Google Scholar] [CrossRef]
- Salih, N.F. Determine the Contaminations of Radon in the Drinking Water Using NTDs (CR-39) and RAD7 Detectors. Arab. J. Sci. Eng. 2021, 46, 6061–6074. [Google Scholar] [CrossRef]
- Vincent, P. Saudi Arabia: An Environmental Overview; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Alsharhan, A.S.; Rizk, Z.A.; Nairn, A.E.M.; Bakhit, D.W.; Alhajari, S.A. Preface. In Hydrogeology of an Arid Region: The Arabian Gulf and Adjoining Areas; Alsharhan, A.S., Rizk, Z.A., Nairn, A.E.M., Bakhit, D.W., Alhajari, S.A., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Durrani, S.A. Radon concentration values in the field: Correlation with underlying geology. Radiat. Meas. 1999, 31, 271–276. [Google Scholar] [CrossRef]
- Arun, B.; Viswanathan, S.; Venkatesan, S.; Jose, M.T.; Balasubramaniam, V. Study of Triple to Double Coincidence Method for Tritium Measurements. Radiochemistry 2021, 63, 221–226. [Google Scholar] [CrossRef]
- Broda, R.; Maletka, K. The TDCR Method as an Important Tool in Radionuclide Metrology; International Atomic Energy Agency: Vienna, Austria, 2003. [Google Scholar]
- Alzurfi, S.; Abojassim, A.; Mraity, H. Monthly Monitoring of Physicochemical and Radiation Properties of Kufa River, Iraq. Pak. J. Sci. Ind. Res. Ser. A Phys. Sci. 2018, 61, 43–50. [Google Scholar] [CrossRef]
- Choppin, G.R.; Liljenzin, J.-O.; Rydberg, J.A.N. (Eds.) CHAPTER 5—Radionuclides in Nature. In Radiochemistry and Nuclear Chemistry, 3rd ed.; Butterworth-Heinemann: Woburn, UK, 2002; pp. 94–122. [Google Scholar] [CrossRef]
- Lucas, L.L.; Unterweger, M.P. Comprehensive Review and Critical Evaluation of the Half-Life of Tritium. J. Res. Natl. Inst. Stand. Technol. 2000, 105, 541–549. [Google Scholar] [CrossRef]
- Aljaloud, K.B.; ElBatouti, M. Statistical analysis of 222Rn concentration in Zamzam and other water sources in the Kingdom of Saudi Arabia. Heliyon 2021, 7, e06057. [Google Scholar] [CrossRef]
- Salvato, C. The role of micro-strategies in the engineering of firm evolution. J. Manag. Stud. 2003, 40, 83–108. [Google Scholar] [CrossRef]
- USEPA. National Primary Drinking Water Regulations; Radionuclides; Final Rule. Fed. Reg. 2000, 65, 76708. [Google Scholar]
- UNSCEAR. The General Assembly with Scientific Annex; United Nations: New York, NY, USA, 2000. [Google Scholar]
- EC. Radon in Workplaces: Implementing the Requirements in Council Directive; Publications Office: Luxembourg, 2019. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; Volume 216, pp. 303–304. [Google Scholar]
- WHO. Management of Radioactivity in Drinking-Water; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Al-Jaseem, Q.K.; Almasoud, F.I.; Ababneh, A.M.; Al-Hobaib, A.S. Radiological assessment of water treatment processes in a water treatment plant in Saudi Arabia: Water and sludge radium content, radon air concentrations and dose rates. Sci. Total Environ. 2016, 563–564, 1030–1036. [Google Scholar] [CrossRef]
- Althoyaib, S.; El-Taher, A. Natural radioactivity measurements in groundwater from Al-Jawa, Saudi Arabia. J. Radioanal. Nucl. Chem. 2015, 304, 547–552. [Google Scholar] [CrossRef]
- El-Taher, A.; Al-Turki, A. Radon activity measurements in irrigation water from Qassim Province by RAD7. J. Environ. Biol. 2016, 37, 1299–1302. [Google Scholar] [PubMed]
- Tayyeb, Z.; Kinsara, A.; Farid, S. A study on the radon concentrations in water in Jeddah (Saudi Arabia) and the associated health effects. J. Environ. Radioact. 1998, 38, 97–104. [Google Scholar] [CrossRef]
- El-Araby, E.H.; Soliman, H.A.; Abo-Elmagd, M. Measurement of radon levels in water and the associated health hazards in Jazan, Saudi Arabia. J. Radiat. Res. Appl. Sci. 2019, 12, 31–36. [Google Scholar] [CrossRef]
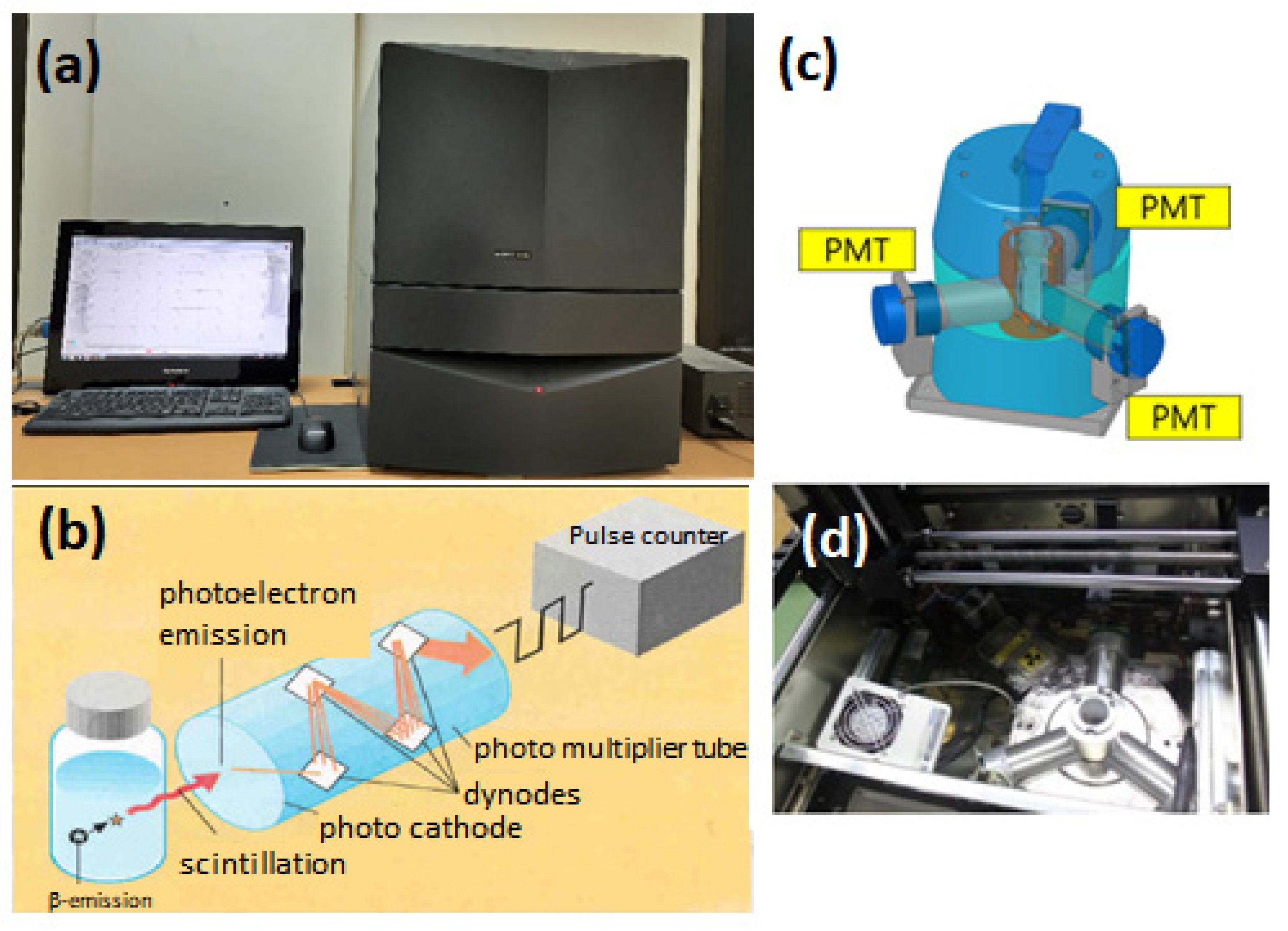
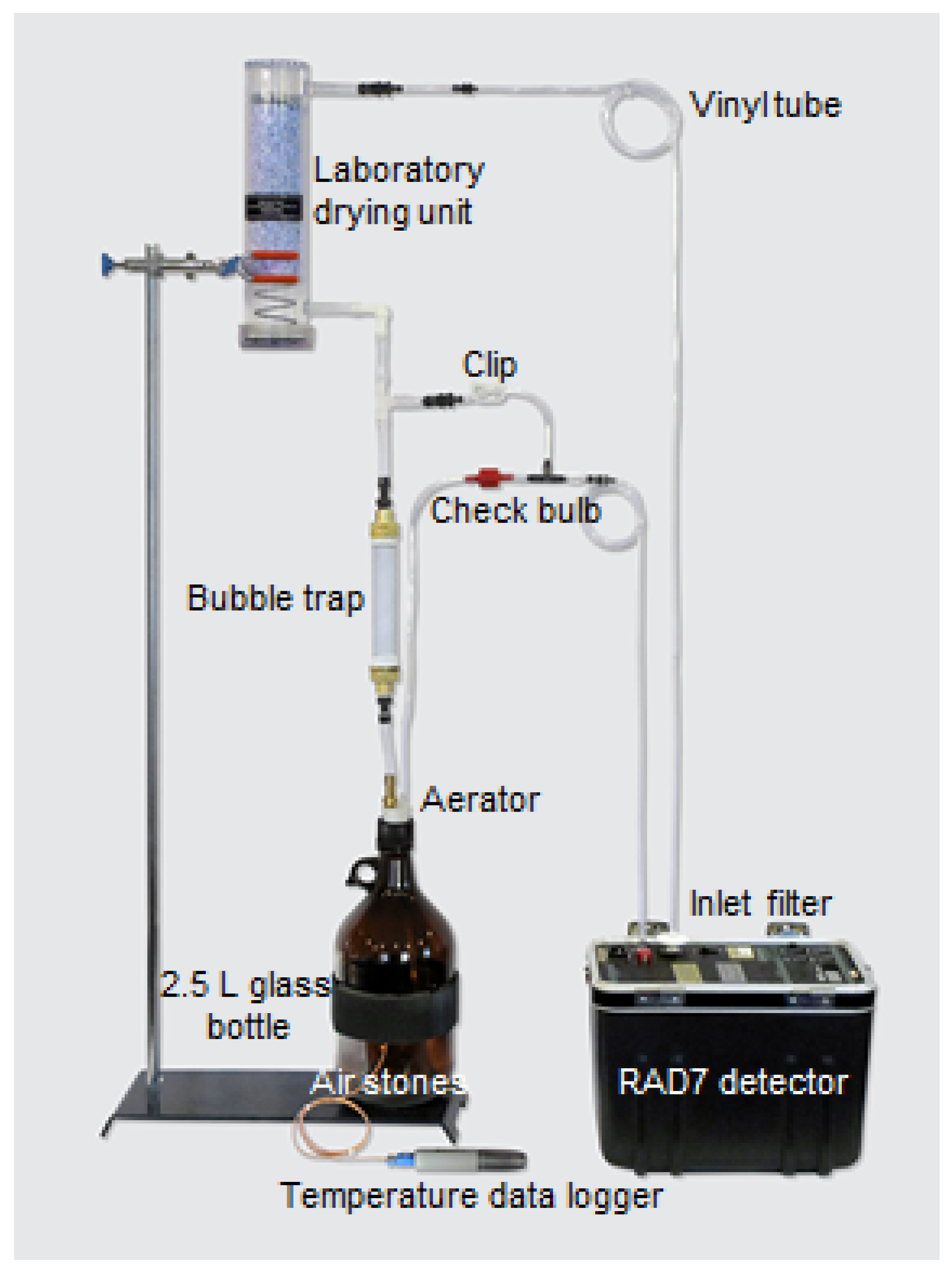
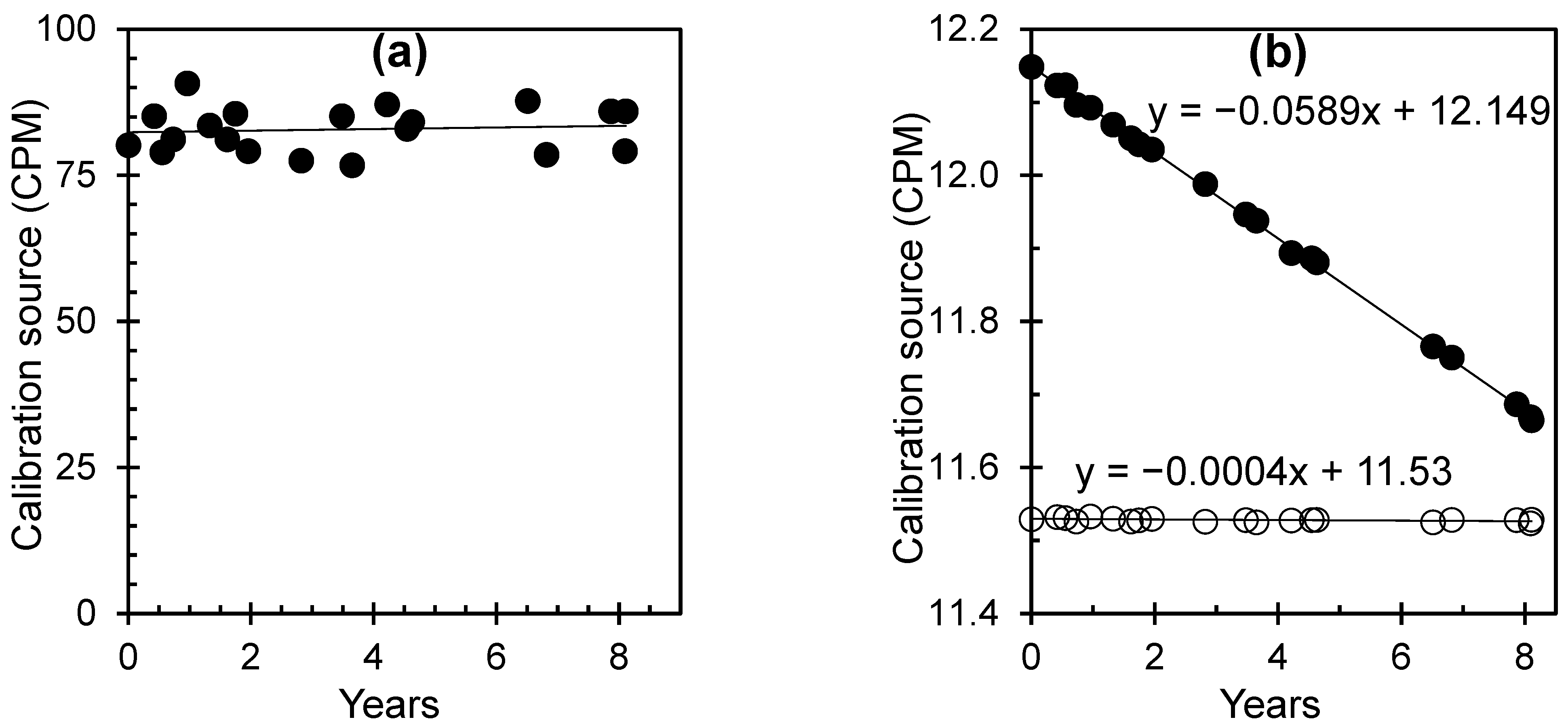
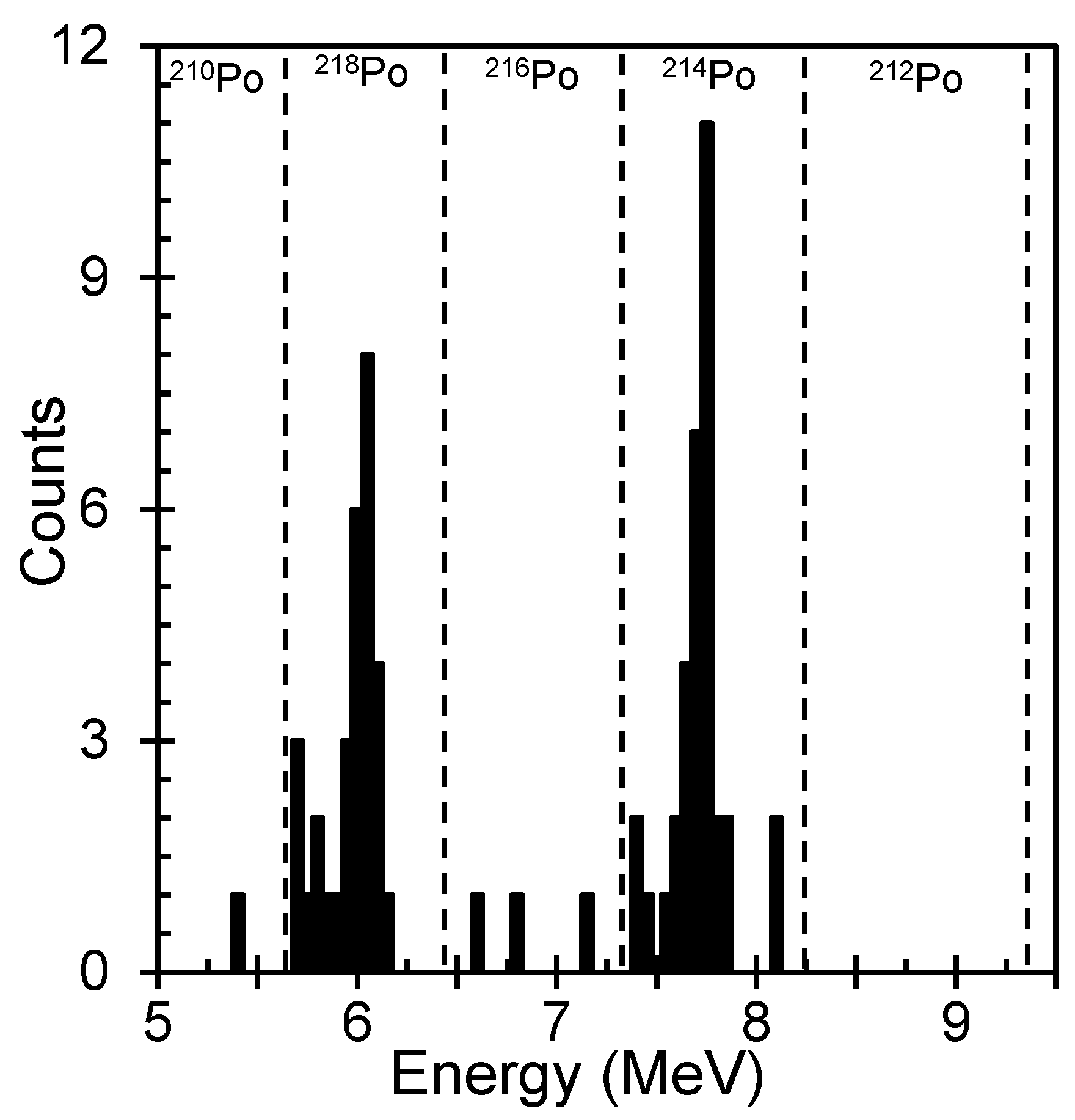
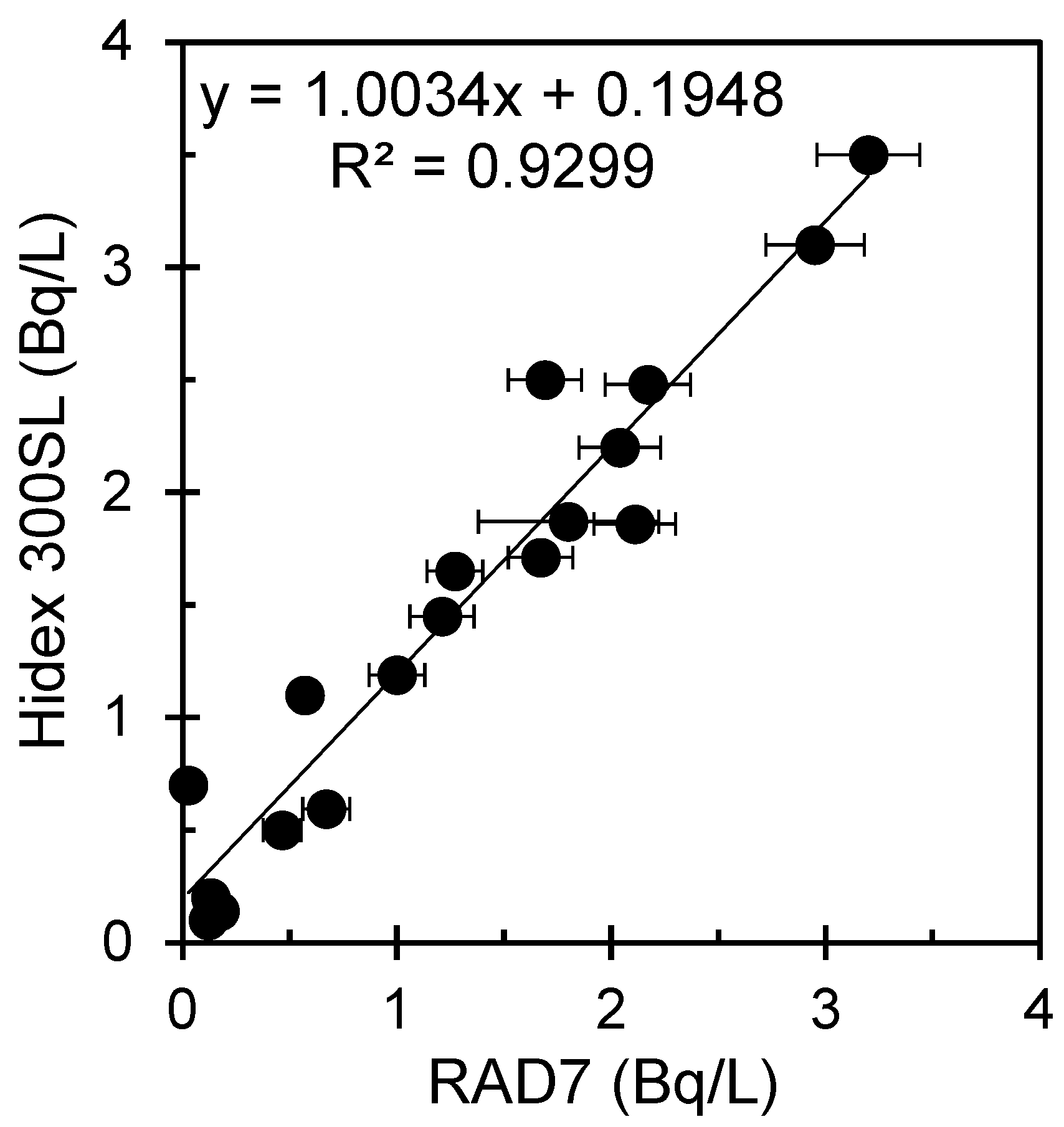
| Serial No | Sample ID | Area | Depth of Wells | Purpose of Use | Location | Hidex 300SL (Bq/L) | RAD7 (Bq/L) | Measurement Uncertainty for RAD7 (Bq/L) |
|---|---|---|---|---|---|---|---|---|
| 1 | 02d01 | Hafr | Shallow | domestic use only | 28.291586, 45.948391 | 0.70 | 0.03 | 0.02 |
| 2 | 03DSt01 | Hafr | Deep | Drinking water treatment plant | 28.463895, 45.983765 | 1.86 | 2.11 | 0.19 |
| 3 | 05DSt03 | Hafr | Deep | Drinking water treatment plant | 28.406443, 45.929505 | 1.19 | 1.00 | 0.13 |
| 4 | 07DSt05 | Hafr | Deep | Drinking water treatment plant | 28.400995, 45.997280 | 0.60 | 0.67 | 0.11 |
| 5 | 09d03 | Hafr | Shallow | domestic and irrigation | 28.322659, 45.900795 | 1.65 | 1.27 | 0.13 |
| 6 | 10d04 | Hafr | Shallow | domestic use only | 28.248143, 45.930633 | 3.50 | 3.20 | 0.24 |
| 7 | 11St06 | Hafr | Deep | Drinking water treatment plant | 28.389733, 46.001613 | 1.71 | 1.67 | 0.15 |
| 8 | 12St07 | Hafr | Deep | Drinking water treatment plant | 28.362908, 45.990894 | 0.14 | 0.17 | 0.05 |
| 9 | 15d07 | Hafr | Shallow | domestic use only | 28.424565, 46.037209 | 0.50 | 0.46 | 0.09 |
| 10 | 19St08 | Hafr | Deep | Drinking water treatment plant | 28.391609, 45.985509 | 3.10 | 2.95 | 0.23 |
| 11 | 20d11 | Hafr | Shallow | domestic use only | 28.372591, 45.998095 | 1.87 | 1.80 | 0.42 |
| 12 | 24d15 | Thybiyah | Shallow | domestic use only | 28.064204, 45.743546 | 2.50 | 1.69 | 0.17 |
| 13 | 25d16 | Thybiyah | Shallow | domestic use only | 28.122389, 45.671439 | 2.48 | 2.17 | 0.20 |
| 14 | 28St11 | Thybiyah | Deep | Drinking water treatment plant | 28.115714, 45.662715 | 2.20 | 2.04 | 0.19 |
| 15 | 32St12 | Thybiyah | Deep | Drinking water treatment plant | 28.109055, 45.663482 | 1.45 | 1.21 | 0.15 |
| 16 | 38d24 | Hafr | Shallow | domestic use only | 28.350853, 45.986374 | 1.10 | 0.57 | 0.04 |
| 17 | 40d26 | Qaisumah | Shallow | domestic use only | 28.294281, 46.095929 | 0.20 | 0.13 | 0.05 |
| 18 | 44d30 | Qaisumah | Shallow | domestic use only | 28.300982, 46.138808 | 0.10 | 0.12 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamun, A.; Alazmi, A.S. Advancement of a Liquid Scintillation Counter and Semiconductor Alpha Spectroscopy Detector to Estimate the Radon Concentration in Groundwater. Water 2022, 14, 3849. https://doi.org/10.3390/w14233849
Mamun A, Alazmi AS. Advancement of a Liquid Scintillation Counter and Semiconductor Alpha Spectroscopy Detector to Estimate the Radon Concentration in Groundwater. Water. 2022; 14(23):3849. https://doi.org/10.3390/w14233849
Chicago/Turabian StyleMamun, Al, and Amira Salman Alazmi. 2022. "Advancement of a Liquid Scintillation Counter and Semiconductor Alpha Spectroscopy Detector to Estimate the Radon Concentration in Groundwater" Water 14, no. 23: 3849. https://doi.org/10.3390/w14233849
APA StyleMamun, A., & Alazmi, A. S. (2022). Advancement of a Liquid Scintillation Counter and Semiconductor Alpha Spectroscopy Detector to Estimate the Radon Concentration in Groundwater. Water, 14(23), 3849. https://doi.org/10.3390/w14233849








