Lebanese Cannabis: Agronomic and Essential Oil Characteristics as Affected by Sowing Date and Irrigation Practice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Climatic Data
2.2. Management of Crop and Experimental Design
2.3. Collection of Data and Analysis
2.3.1. Field Measurements and Sample Collection
2.3.2. Hydrodistillation for Essential Oil Extraction
2.3.3. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
- Chromatographic conditions
- 2.
- Mass spectrometric conditions
- 3.
- Identification of constituents
2.3.4. Seed Composition
- The dry matter and ash content
- 2.
- The crude protein
- 3.
- The crude fat
- 4.
- The Crude fiber
2.4. Statistical Analysis
3. Results
3.1. Biometric, Productive Parameters and WUE
3.2. Essential Oil Composition of the Inflorescence
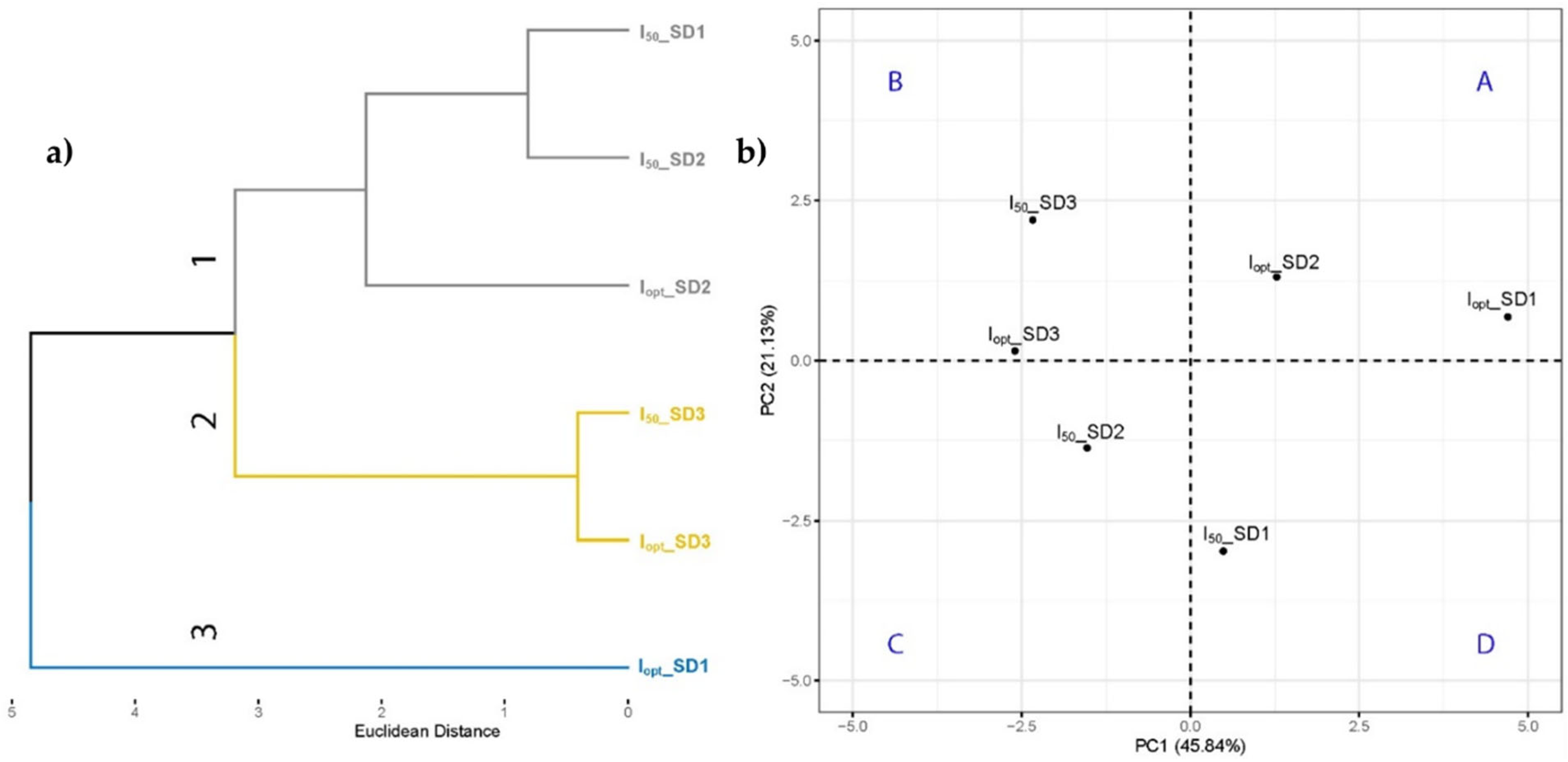
3.3. Multivariate Statistical Analysis
3.4. Seed Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carus, M.; Sarmento, L. The European Hemp Industry: Cultivation, Processing and Applications for Fibres, Shivs, Seeds and Flowers; European Industrial Hemp Association: Brussels, Belgium, 2016; pp. 1–9. [Google Scholar]
- Finnan, J.; Styles, D. Hemp: A more sustainable annual energy crop for climate and energy policy. Energy Policy 2013, 58, 152–162. [Google Scholar] [CrossRef]
- Bennett, S.J.; Snell, R.; Wright, D. Effect of Variety, Seed Rate and Time of Cutting on Fibre Yield of Dew-Retted Hemp. Ind. Crops Prod. 2006, 24, 79–86. [Google Scholar] [CrossRef]
- Salentijn, E.M.J.; Zhang, Q.; Amaducci, S.; Yang, M.; Trindade, L.M. New developments in fiber hemp (Cannabis sativa L.) breeding. Ind. Crops Prod. 2015, 68, 32–41. [Google Scholar] [CrossRef]
- Russo, E.B. The pharmacological history of Cannabis. In Handbook of Cannabinoids; Pertwee, R., Ed.; Oxford University Press: Oxford, UK, 2014; pp. 23–43. [Google Scholar]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Hillig, K.W. Genetic evidence for speciation in Cannabis (Cannabaceae). Genet. Resour. Crop Evol. 2005, 53, 161–180. [Google Scholar] [CrossRef]
- De Meijer, E.P.M.; Giuseppe, M.; Bagatta, M.; Carboni, A.; Ranalli, P. The inheritance of chemical phenotype in Cannabis sativa L. Genetics 2003, 163, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Aizpurua-olaizola, O.; Soydaner, U.; Ekin, O.; Schibano, D.; Simsir, Y. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod 2016, 79, 324–331. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Bernstein, N.; Gorelick, J.; Koch, S. Interplay between Chemistry and Morphology in Medical Cannabis (Cannabis sativa, L.). Ind. Crops Prod. 2019, 129, 185–194. [Google Scholar] [CrossRef]
- Sawler, J.; Stout, J.M.; Gardner, K.M.; Hudson, D.; Vidmar, J.; Butler, L.; Page, J.E.; Myles, S. The Genetic Structure of Marijuana and Hemp. PLoS ONE 2015, 10, e0133292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsohly, M.A.; Slade, D. Chemical Constituents of Marijuana: The Complex Mixture of Natural Cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa—From Plant Genome to Humans. Plant Sci. J. 2019, 284, 67–72. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential Cannabis Synergy and Phytocannabinoid-Terpenoid Entourage Effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Richins, R.D.; Rodriguez-Uribe, L.; Lowe, K.; Ferral, R.; O’Connell, M.A. Accumulation of Bioactive Metabolites in Cultivated Medical Cannabis. PLoS ONE 2018, 13, 0201119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namdar, D.; Voet, H.; Ajjampura, V.; Nadarajan, S.; Mayzlish-Gati, E.; Mazuz, M.; Shalev, N.; Koltai, H. Terpenoids and Phytocannabinoids Co-Produced in Cannabis sativa Strains Show Specific Interaction for Ell Cytotoxic Activity. Molecules 2019, 24, 3031. [Google Scholar] [CrossRef] [Green Version]
- Struik, P.C.; Amaducci, S.; Bullard, M.J.; Stutterheim, N.C.; Venturi, G.; Cromack, H.T.H. Agronomy of Fibre Hemp (Cannabis sativa, L.) in Europe. Ind. Crops Prod. 2000, 11, 107–118. [Google Scholar] [CrossRef]
- Amaducci, S.; Zatta, A.; Pelatti, F.; Venturi, G. Influence of Agronomic Factors on Yield and Quality of Hemp (Cannabis sativa, L.) Fibre and Implication for an Innovative Production System. Field Crops Res. 2008, 107, 161–169. [Google Scholar] [CrossRef]
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key Cultivation Techniques for Hemp in Europe and China. Ind. Crops Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Amaducci, S.; Colauzzi, M.; Zatta, A.; Venturi, G. Flowering Dynamics in Monoecious and Dioecious Hemp Genotypes. J. Ind. Hemp. 2008, 13, 5–19. [Google Scholar] [CrossRef]
- Huaran, H.; Hao, L.; Feihu, L. Seed Germination of Hemp (Cannabis sativa, L.) Cultivars Respond to the Stress of Salt Type and Concentration. Ind. Crops Prod. 2018, 123, 254–261. [Google Scholar]
- Bertoli, A.; Tozzi, S.; Pistelli, L.; Angelini, L.G. Fibre Hemp Inflorescences: From Crop-Residues to Essential Oil Production. Ind. Crops Prod. 2010, 32, 329–337. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Geccarini, L.; Tavarini, S.; Flamini, G.; Angelini, L.G. Valorisation of Hemp inflorescence after Seed Harvest: Cultivation Site and Harvest Time influence Agronomic Characteristics and Essential Oil Yield and Composition. Ind. Crops Prod. 2019, 139, 111541. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Testa, G.; Scordia, D.; Copani, V. Sowing Time and Prediction of Flowering of Different Hemp (Cannabis sativa, L.) Genotypes in Southern Europe. Ind. Crops Prod. 2012, 37, 20–33. [Google Scholar] [CrossRef]
- García-Tejero, L.F.; Durán-Zuazo, V.H.; Pérez-Álvarez, R.; Hernández, A.; Casano, S.; Morón, M.; Muriel-Fernández, J.L. Impact of plant density and irrigation on yield of hemp (Cannabis sativa L.) in a Mediterranean semi-arid environment. J. Agric. Sci. Technol. 2014, 16, 887–895. [Google Scholar]
- Campiglia, E.; Radicetti, E.; Mancinelli, R. Plant Density and Nitrogen Fertilization Affect Agronomic Performance of Industrial Hemp (Cannabis sativa, L.) in Mediterranean Environment. Ind. Crops Prod. 2017, 100, 246–254. [Google Scholar] [CrossRef]
- Tang, K.; Struik, P.C.; Yinb, X.; Calzolaria, D.; Musioa, S.; Thouminotc, C.; Bjelkovád, M.; Stramkalee, V.; Magagninif, G.; Amaducci, S. Comprehensive Study of Planting Density and Nitrogen Fertilization Effect on Dual-Purpose Hemp (Cannabis sativa, L.) Cultivation. Ind. Crops Prod. 2017, 107, 427–438. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Durán Zuazob, V.H.; Sánchez-Carneneroc, C.; Hernándeza, A.; Ferreiro-Verac, C.; Casano, S. Seeking Suitable Agronomical Practices for Industrial Hemp (Cannabis sativa, L.) Cultivation for Biomedical Applications. Ind. Crops Prod. 2019, 139, 111524. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Riggi, E.; Testa, G.; Scordia, D.; Copani, V. Evaluation of European Developed Fiber Hemp Genotypes (Cannabis sativa, L.) in Semi-Arid Mediterranean Environment. Ind. Crops Prod. 2013, 50, 312–324. [Google Scholar] [CrossRef]
- Babaei, M.; Ajdanian, A. Screening of Different Iranian Ecotypes of Cannabis under Water Deficit Stress. Sci. Hortic. 2019, 260, 108904. [Google Scholar] [CrossRef]
- Mirniyam, G.; Rahimmalek, M.; Arzani, A.; Matkowski, A.; Gharibi, S.; Szumny, A. Changes in Essential Oil Composition, Polyphenolic Compounds and Antioxidant Capacity of Ajowan (Trachyspermum ammi L.) Populations in Response to Water Deficit. Foods 2022, 11, 3084. [Google Scholar] [CrossRef]
- Khodadadi, F.; Shahriari Ahmadi, F.; Talebi, M.; Moshtaghi, N.; Matkowski, A.; Szumny, A.; Rahimmalek, M. Essential oil composition, physiological and morphological variation in Salvia abrotanoides and S. yangii under drought stress and chitosan treatments. Ind. Crops Prod. 2022, 187, 115429. [Google Scholar] [CrossRef]
- Afshari, M.; Rahimmalek, M.; Sabzalian, M.; Bielecka, M.; Matkowski, A.; Talebi, M. Changes in physiological, phytochemical traits and gene expression of two Perovskia species in response to water deficit. Sci. Hortic. 2022, 293, 110747. [Google Scholar] [CrossRef]
- Shebaby, W.; Saliba, J.; Faour, W.H.; Ismail, J.; El Hage, M.; Daher, C.F.; Taleb, R.I.; Nehmeh, B.; Dagher, C.; Chrabieh, C.; et al. In Vivo and in Vitro Anti-Inflammatory Activity Evaluation of Lebanese Cannabis sativa, L. Ssp. Indica (Lam.). J. Ethnopharmacol. 2021, 270, 113743. [Google Scholar] [CrossRef] [PubMed]
- Bercht, C.L.; Lousberg, R.J.C.; Küppers, F.J.; Salemink, C.A. Cannabicitran: A new naturally occurring tetracyclic diether from Lebanese Cannabis sativa. Phytochemistry 1974, 13, 619–621. [Google Scholar] [CrossRef]
- McDonald, P.A.; Gough, T.A. Determination of the distribution of Cannabinoids in Cannabis resin from the Lebanon using HPLC. Part III. J. Chromatogr. Sci. 1984, 22, 282–284. [Google Scholar] [CrossRef]
- Ohlsson, A.; Abou-Chaar, C.; Agurell, S.; Nilsson, I.; Olofsson, K.; Sandberg, F. Cannabinoid constituents of male and female Cannabis sativa. UN Bull. Narc. 1971, 23, 29–32. [Google Scholar]
- Valle, J.; Lapa, A.; Barros, G.G. Pharmacological activity of Cannabis according to the sex of the plant. J. Pharm. Pharmacol. 1968, 20, 798–799. [Google Scholar] [CrossRef]
- Todorovic, M. An Excel-based tool for real time irrigation management at field scale. In Proceedings of the International Symposium on “Water and Land Management for Sustainable Irrigated Agriculture”, Adana, Turkey, 4–8 April 2006. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration. In Guidelines for Computing Crop Water Requirements; Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Pejic, B.; Sikora, V.; Milic, S.; Mackic, K.; Koren, A.; Bajic, I. Effect of drip irrigation on yield and evapotranspiration of hemp. Ratar. Povrt. 2018, 55, 130–134. [Google Scholar] [CrossRef]
- Guenther, E. The Essential Oils; D. Van Nostrand Company Inc.: New York, NY, USA, 1948; Volume I. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Husson, F.; Josse, J.L.S.; Mazet, J. Facto Mine R: Multivariate Exploratory Data Analysis and Data Mining with R. R Package Version 1.25. 2013, pp. 102–123. Available online: http://CRAN.R-project.org/package=FactoMineR (accessed on 1 October 2020).
- R Core Team. R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Datta, S. Methods for evaluating clustering algorithms for gene expression data using a reference set of functional classes. BMC Bioinform. 2006, 7, 397. [Google Scholar] [CrossRef]
- Abdollahi, M.; Sefidkon, F.; Calagari, M.; Mousavi, A.; Mahomoodally, M.F. Impact of four hemp (Cannabis sativa L.) varieties and stage of plant growth on yield and composition of essential oils. Ind. Crops Prod. 2020, 155, 112793. [Google Scholar] [CrossRef]
- Brenneisen, R. Chemistry and analysis of phytocannabinoids and other cannabis constituents. In Marijuana and the Cannabinoids. Forensic Science and Medicine; ElSohly, M., Ed.; Humana Press: New York, NY, USA, 2007; pp. 17–49. [Google Scholar]
- Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H.; Verpoorte, R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 2010, 71, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Kleine, S.; Müller, C. Drought stress and leaf herbivory affect root terpenoid concentrations and growth of Tanaecetum vulgare. J. Chem. Ecol. 2014, 40, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zha, F.; Peckrul, A.; Hanson, B.; Johnson, B.; Rao, J. Genotype x Environmental Effects on Yielding Ability and Seed Chemical Composition of Industrial Hemp (Cannabis sativa, L.) Varieties Grown in North Dakota, USA. J. Am. Oil Chem. Soc. 2019, 96, 1417–1425. [Google Scholar]


| Source of Variation | Aboveground | Aboveground | Plant Height (cm) | Essential Oil Yield (%) | WUE- Fresh Biomass (Kg/m3) | WUE- Dry Biomass (Kg/m3) | WUE-Essential Oil Extracted From Flowers (Kg/m3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh Biomass | Dry Biomass | ||||||||||||||
| (t.ha−1) | (t.ha−1) | ||||||||||||||
| Pr. | Means | Pr. | Means | Pr. | Means | Pr. | Means | Pr. | Means | Pr. | Means | Pr. | Means | ||
| Water regime (Wr) | *** | * | ** | ns | *** | * | ns | ||||||||
| I50 | 34.22 ± 11.37 b | 19.02 ± 8.41 b | 92.78 ± 17.70 b | 0.27 ± 0.09 | 10.67 ± 3.85 a | 5.95 ± 2.81 a | 0.59 ± 0.37 | ||||||||
| Iopt | 53.07 ± 10.05 a | 30.36 ± 7.48 a | 132.78 ± 22.65 a | 0.21 ± 0.05 | 8.23 ± 1.84 b | 4.72 ± 1.31 b | 0.68 ± 0.20 | ||||||||
| Sowing date (SD) | **** | *** | **** | ns | **** | **** | ** | ||||||||
| SD1 | 53.60 ± 10.92 a | 33.07 ± 5.15 a | 129.17 ± 26.16 a | 0.25 ± 0.10 | 12.19 ± 2.59 a | 7.63 ± 1.9 a | 0.90 ± 0.24 a | ||||||||
| SD2 | 46.63 ± 9.70 b | 24.93 ± 8.38 b | 120.83 ± 24.38 a | 0.24 ± 0.09 | 10.19 ± 1.83 b | 5.25 ± 0.83 b | 0.61 ± 0.19 b | ||||||||
| SD3 | 30.70 ± 11.99 c | 16.07 ± 6.93 c | 88.33 ± 19.15 b | 0.22 ± 0.04 | 5.98 ± 0.56 c | 3.12 ± 0.51 c | 0.39 ± 0.22 b | ||||||||
| Wr × SD | ns | ns | ns | * | * | * | ns | ||||||||
| Source of Variation | Water Regime (Wr) | Sowing Date (SD) | Wr × SD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I50 | Iopt | SD1 | SD2 | SD3 | ||||||
| Monoterpenes | β-pinene % | Pr. | 0.023 * | 0.032 * | <0.0001 **** | |||||
| Means | 14.32 ± 2.03 a | 8.87 ± 1.88 b | 11.62 ± 4.93 ab | 12.19 ± 3.21 a | 10.99 ± 1.86 b | |||||
| β-myrcene % | Pr. | 0.053 | 0.006 ** | 0.020 * | ||||||
| Means | 14.81 ± 2.97 | 9.96 ± 2.58 | 14.83 ± 3.94 a | 10.9 ± 4.18 b | 11.42 ± 1.44 b | |||||
| D-Limonene % | Pr. | 0.532 | 0.024 * | 0.034 * | ||||||
| Means | 9.69 ± 1.5 | 8.99 ± 2.49 | 8.46 ± 1.35 b | 10.86 ± 0.99 a | 8.71 ± 2.64 b | |||||
| β-ocimene % | Pr. | 0.721 | 0.015 * | 0.414 | ||||||
| Means | 6.33 ± 1.65 | 6.44 ± 1.35 | 4.94 ± 0.48 b | 6.77 ± 1.07 a | 7.45 ± 1.36 a | |||||
| δ-3-carene % | Pr. | 0.273 | 0.224 | 0.197 | ||||||
| Means | 3.13 ± 0.76 | 2.88 ± 0.33 | 2.72 ± 0.45 | 2.99 ± 0.56 | 3.32 ± 0.65 | |||||
| 4-Carene % | Pr. | 0.729 | 0.164 | 0.406 | ||||||
| Means | 4.98 ± 1.46 | 5.05 ± 1.42 | 5.52 ± 1.46 | 3.97 ± 1.06 | 5.55 ± 1.21 | |||||
| α-phelandrene % | Pr. | 0.566 | 0.005 ** | 0.151 | ||||||
| Means | 2.49 ± 0.73 | 2.09 ± 0.97 | 2.69 ± 0.38 a | 2.65 ± 0.71 a | 1.52 ± 0.88 b | |||||
| α-thujene % | Pr. | 0.864 | 0.511 | 0.315 | ||||||
| Means | 1.7 ± 0.53 | 1.75 ± 0.45 | 1.89 ± 0.47 | 1.73 ± 0.55 | 1.56 ± 0.44 | |||||
| Borneol % | Pr. | 0.164 | 0.022 * | 0.161 | ||||||
| Means | 1.42 ± 0.30 | 1.63 ± 0.26 | 1.65 ± 0.23 a | 1.64 ± 0.37 a | 1.29 ± 0.05 b | |||||
| α-fenchol | Pr. | 0.463 | 0.343 | 0.260 | ||||||
| Means | 1.5 ± 0.40 | 1.73 ± 0.51 | 1.84 ± 0.48 | 1.55 ± 0.59 | 1.46 ± 0.18 | |||||
| Camphene % | Pr. | 0.475 | 0.189 | 0.290 | ||||||
| Means | 1.36 ± 0.54 | 1.73 ± 0.44 | 1.80 ± 0.47 | 1.46 ± 0.48 | 1.38 ± 0.57 | |||||
| γ-terpinene % | Pr. | 0.104 | 0.001 ** | 0.147 | ||||||
| Means | 1.46 ± 0.25 | 1.3 ± 0.22 | 1.16 ± 0.05 c | 1.38 ± 0.21 b | 1.60 ± 0.20 a | |||||
| Terpinolene % | Pr. | 0.245 | 0.142 | 0.383 | ||||||
| Means | 0.48 ± 0.27 | 0.6 ± 0.28 | 0.74 ± 0.29 | 0.47 ± 0.27 | 0.42 ± 0.15 | |||||
| 1.8-cineol % | Pr. | 0.012 | 0.003 | 0.477 | ||||||
| Means | 0.32 ± 0.19 b | 0.52 ± 0.33 a | 0.59 ± 0.33 a | 0.48 ± 0.23 a | 0.19 ± 0.05 b | |||||
| Sesquiterpenes | Β-Caryophyllene % | Pr. | 0.010 * | 0.002 ** | 0.001 ** | |||||
| Means | 9.53 ± 1.26 a | 19.01 ± 3.76 b | 13.07 ± 5.44 b | 16.45 ± 7.67 a | 13.3 ± 2.99 b | |||||
| α-Caryophyllene % | Pr. | 0.221 | 0.0001 *** | 0.007 ** | ||||||
| Means | 9.86 ± 1.65 | 10.88 ± 3.61 | 9.84 ± 1.34 b | 8.01 ± 1.37 c | 13.25 ± 2.35 a | |||||
| Caryophyllene oxide % | Pr. | 0.425 | 0.073 | 0.0004 *** | ||||||
| Means | 5.25 ± 1.23 | 4.42 ± 1.38 | 4.57 ± 1.45 | 4.6 ± 1.27 | 5.32 ± 1.4 | |||||
| Aromadandrene % | Pr. | 0.535 | 0.920 | 0.071 | ||||||
| Means | 2.44 ± 0.46 | 2.27 ± 0.55 | 2.41 ± 0.46 | 2.31 ± 0.69 | 2.34 ± 0.41 | |||||
| α-farnescene % | Pr. | 0.567 | 0.516 | 0.625 | ||||||
| Means | 1.80 ± 0.42 | 1.86 ± 0.52 | 2.05 ± 0.4 | 1.74 ± 0.49 | 1.69 ± 0.48 | |||||
| α-bisabolol % | Pr. | 0.938 | 0.952 | 0.095 | ||||||
| Means | 1.5 ± 0.23 | 1.48 ± 0.3 | 1.47 ± 0.27 | 1.51 ± 0.27 | 1.49 ± 0.30 | |||||
| β-bisabolene % | Pr. | 0.383 | 0.585 | 0.322 | ||||||
| Means | 1.54 ± 0.42 | 1.64 ± 0.35 | 1.46 ± 0.36 | 1.61 ± 0.39 | 1.7 ± 0.40 | |||||
| Guaiol % | Pr. | 0.417 | 0.166 | 0.324 | ||||||
| Means | 1.19 ± 0.57 | 1.41 ± 0.25 | 1.23 ± 0.48 | 1.57 ± 0.24 | 1.11 ± 0.49 | |||||
| γ-selinene % | Pr. | 0.417 | 0.407 | 0.446 | ||||||
| Means | 1.41 ± 0.26 | 1.27 ± 0.13 | 1.26 ± 0.19 | 1.34 ± 0.29 | 1.42 ± 0.12 | |||||
| δ-guaiene % | Pr. | 0.219 | 0.411 | 0.027 * | ||||||
| Means | 0.67 ± 0.4 | 0.98 ± 0.41 | 0.94 ± 0.56 | 0.68 ± 0.19 | 0.85 ± 0.47 | |||||
| cis-α-bergamotene % | Pr. | 0.022 * | 0.571 | 0.225 | ||||||
| Means | 0.37 ± 0.15 b | 0.61 ± 0.24 a | 0.51 ± 0.19 | 0.55 ± 0.28 | 0.42 ± 0.24 | |||||
| γ-cadinene % | Pr. | 0.055 | <0.0001 **** | <0.0001 **** | ||||||
| Means | 0.48 ± 0.1 | 0.54 ± 0.26 | 0.70 ± 0.15 a | 0.54 ± 0.03 b | 0.30 ± 0.06 c | |||||
| Principal Components | PC1 | PC2 | PC3 |
|---|---|---|---|
| Eigenvalue | 6.42 | 2.96 | 1.99 |
| Relative variance (%) | 45.84 | 21.13 | 14.28 |
| Cumulative variance (%) | 45.84 | 66.97 | 81.25 |
| Eigenvectors | |||
| β-myrcene % | −0.114 | −0.830 | −0.297 |
| D-Limonene % | 0.037 | 0.393 | −0.801 |
| δ-3-carene % | −0.424 | 0.702 | −0.031 |
| 4-Carene % | 0.237 | 0.415 | −0.789 |
| α-phelandrene % | 0.558 | -0.399 | 0.367 |
| α-thujene % | 0.823 | 0.298 | 0.102 |
| α-fenchol | 0.908 | 0.328 | −0.161 |
| Camphene % | 0.834 | 0.165 | −0.044 |
| γ-terpinene % | −0.835 | 0.355 | −0.125 |
| β-pinene % | −0.483 | −0.695 | 0.210 |
| β-ocimene % | −0.662 | 0.675 | 0.273 |
| borneol % | 0.787 | −0.035 | 0.484 |
| 1,8-cineol % | 0.902 | 0.026 | 0.096 |
| terpinolene % | 0.926 | 0.048 | −0.309 |
| Principal Components | PC1 | PC2 | PC3 |
|---|---|---|---|
| Eigenvalue | 4.40 | 2.96 | 2.16 |
| Relative variance (%) | 36.70 | 24.65 | 17.99 |
| Cumulative variance (%) | 36.70 | 61.35 | 79.34 |
| Eigenvectors | |||
| Aromadandrene % | 0.739 | 0.662 | −0.013 |
| α-farnescene % | −0.107 | 0.923 | 0.075 |
| γ-cadinene % | −0.512 | 0.756 | −0.122 |
| α-bisabolol % | 0.708 | 0.482 | −0.290 |
| β-bisabolene % | −0.524 | −0.296 | 0.712 |
| β-Caryophyllene % | −0.833 | −0.048 | −0.154 |
| α-Caryophyllene % | 0.502 | −0.096 | 0.295 |
| Caryophyllene oxide % | 0.842 | −0.345 | −0.393 |
| cis-α-bergamotene % | −0.642 | −0.229 | −0.657 |
| Guaiol % | −0.182 | 0.582 | −0.342 |
| γ-selinene % | 0.678 | 0.013 | 0.624 |
| δ-guaiene % | −0.499 | 0.504 | 0.603 |
| Source of Variation | Crude Fat (%) | Ash (%) | Crude Protein (%) | Dry Matter (%) | Crude Fiber (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pr. | Means | Pr. | Means | Pr. | Means | Pr. | Means | Pr. | Means | ||
| Water regime (Wr) | * | ns | * | ns | ns | ||||||
| I50 | 5.04 ± 2.0 b | 12.76 ± 2.77 | 20.54 ± 1.59 b | 92.00 ± 1.24 | 27.51 ± 2.82 | ||||||
| Iopt | 7.03 ± 1.25 a | 13.75 ± 2.55 | 24.63 ± 1.49 a | 92.26 ± 1.71 | 28.55 ± 1.16 | ||||||
| Sowing date (SD) | *** | ns | ns | ns | ns | ||||||
| SD1 | 7.18 ± 0.73 a | 14.77 ± 2.33 | 22.93 ± 3.07 | 91.38 ± 1.73 | 28.43 ± 3.01 | ||||||
| SD2 | 6.85 ± 1.21 a | 13.64 ± 2.88 | 23.02 ± 1.88 | 92.58 ± 0.62 | 27.48 ± 2.21 | ||||||
| SD3 | 4.07 ± 1.85 b | 11.36 ± 1.6 | 21.81 ± 2.93 | 92.42 ± 1.68 | 28.18 ± 1.17 | ||||||
| Wr × SD | ns | ns | ns | ns | ns | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sleiman, R.; Abi Saab, M.T.; Gerard, J.A.; Fahed, S.; Chehade, A.; Elhajj, A.K.; Jammoul, A.; Mansour, G.; Sellami, M.H.; Todorovic, M.; et al. Lebanese Cannabis: Agronomic and Essential Oil Characteristics as Affected by Sowing Date and Irrigation Practice. Water 2022, 14, 3842. https://doi.org/10.3390/w14233842
Sleiman R, Abi Saab MT, Gerard JA, Fahed S, Chehade A, Elhajj AK, Jammoul A, Mansour G, Sellami MH, Todorovic M, et al. Lebanese Cannabis: Agronomic and Essential Oil Characteristics as Affected by Sowing Date and Irrigation Practice. Water. 2022; 14(23):3842. https://doi.org/10.3390/w14233842
Chicago/Turabian StyleSleiman, Rhend, Marie Therese Abi Saab, Jocelyne Adjizian Gerard, Salim Fahed, Ali Chehade, Abdel Kader Elhajj, Adla Jammoul, Georges Mansour, Mohamed Houssemeddine Sellami, Mladen Todorovic, and et al. 2022. "Lebanese Cannabis: Agronomic and Essential Oil Characteristics as Affected by Sowing Date and Irrigation Practice" Water 14, no. 23: 3842. https://doi.org/10.3390/w14233842









