Algal Consortiums: A Novel and Integrated Approach for Wastewater Treatment
Abstract
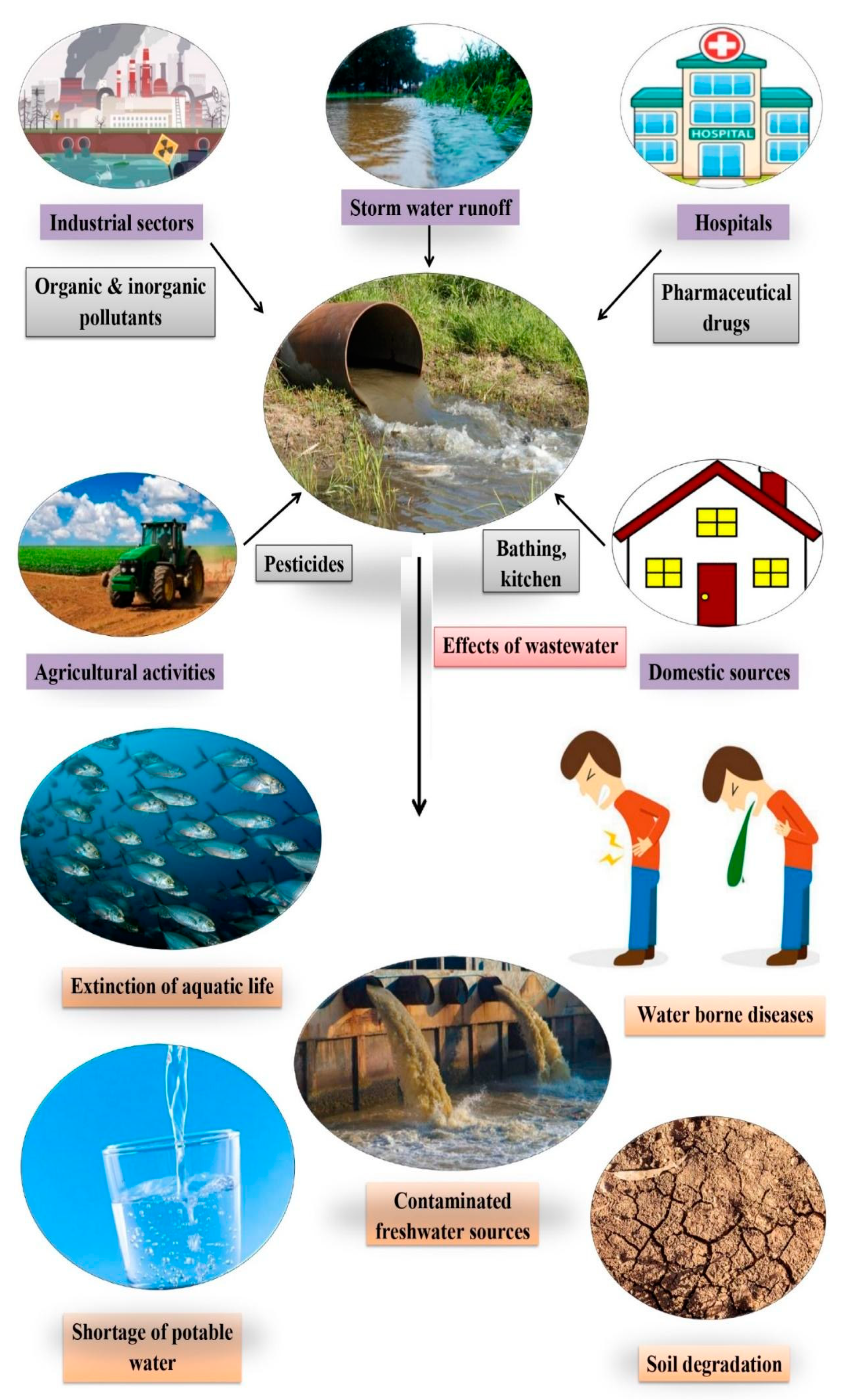
:1. Introduction
2. Wastewater and Associated Conventional Methods for Its Treatment
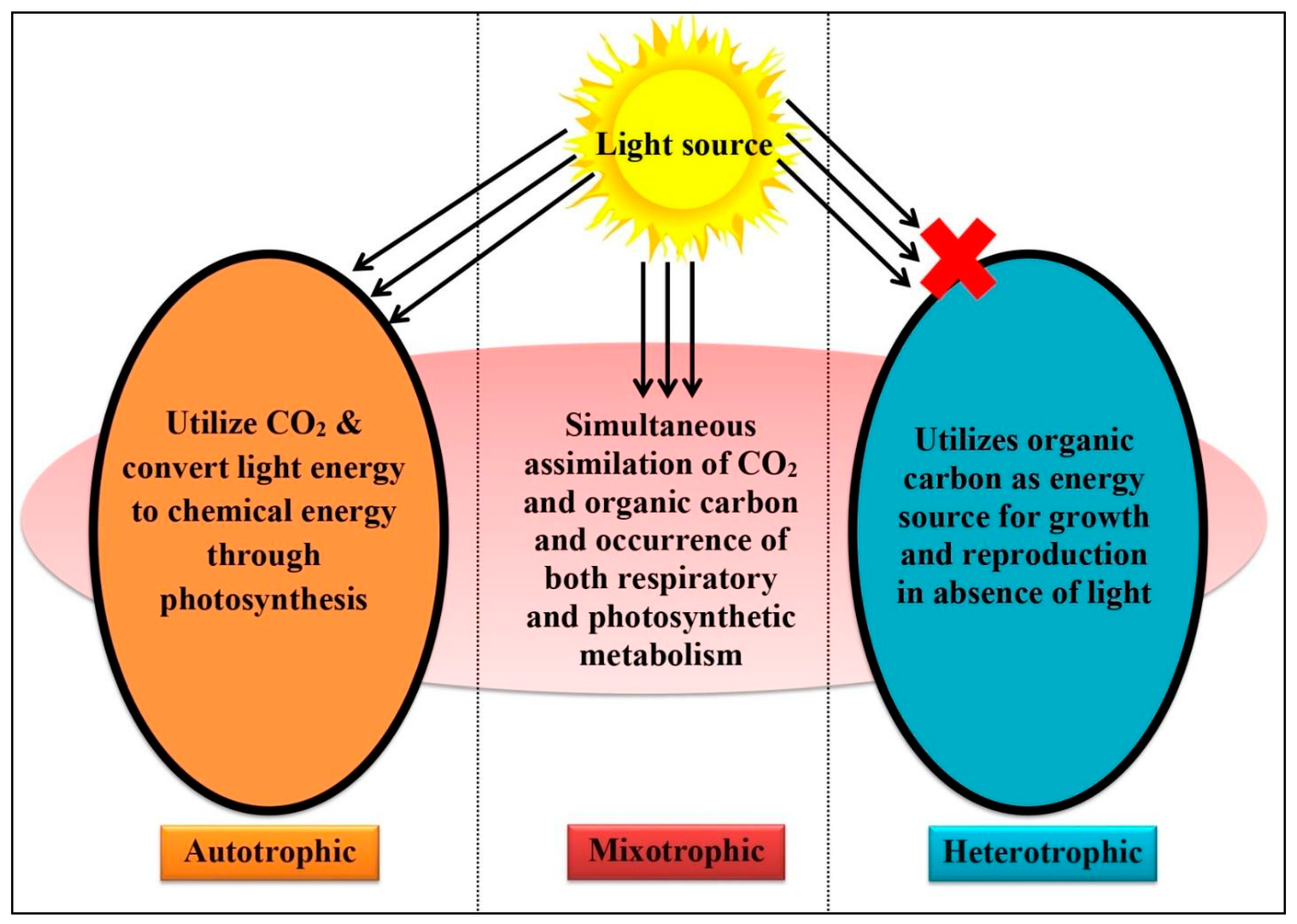
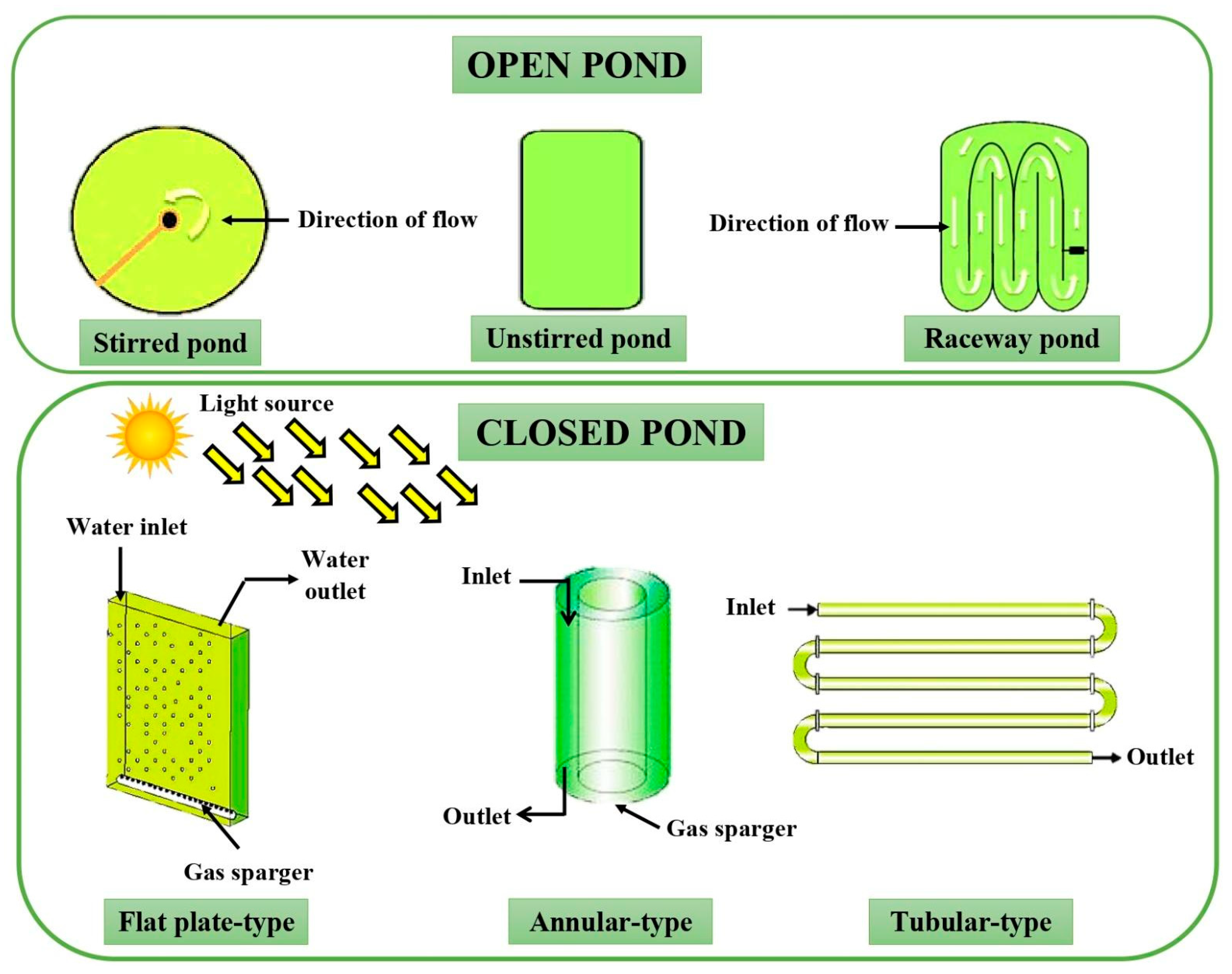
3. Microalgae and Their Cultivation
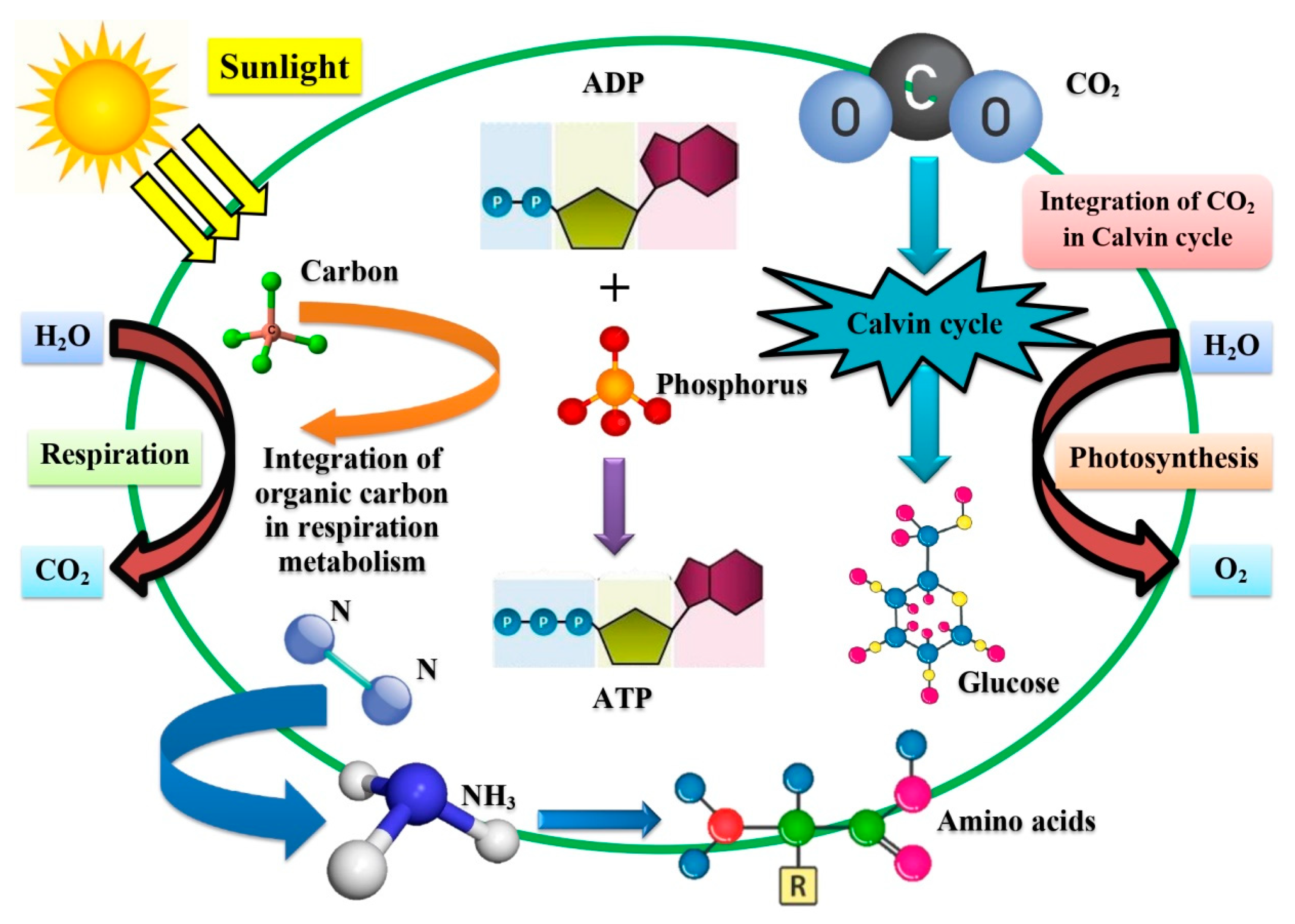
4. Working Action of Algae and Their Different Consortiums for Wastewater Treatment
4.1. Working Action of Algae–Algae Consortiums for Wastewater Treatment
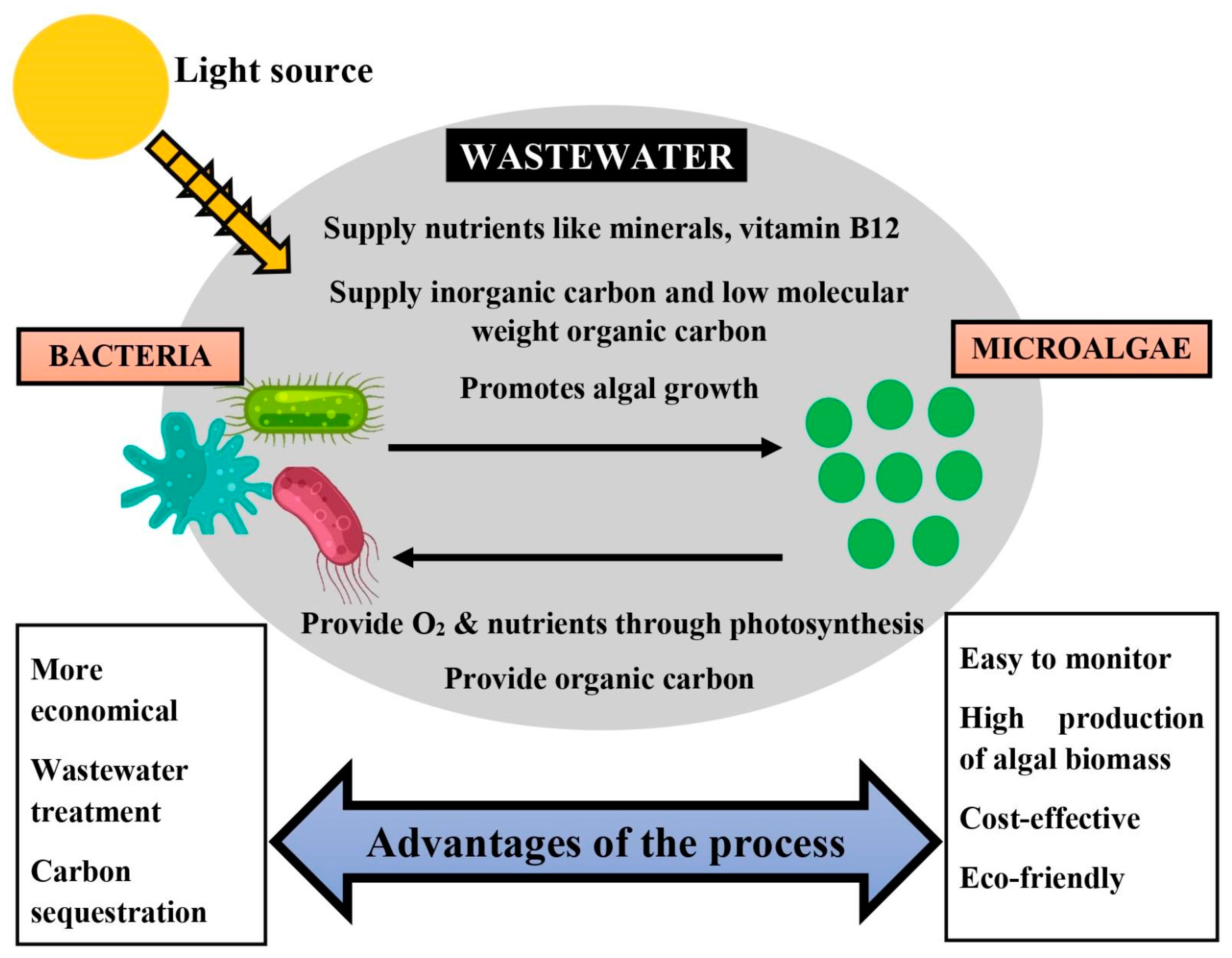
4.2. Working Action of Algal–Bacterial Consortium for Wastewater Treatment
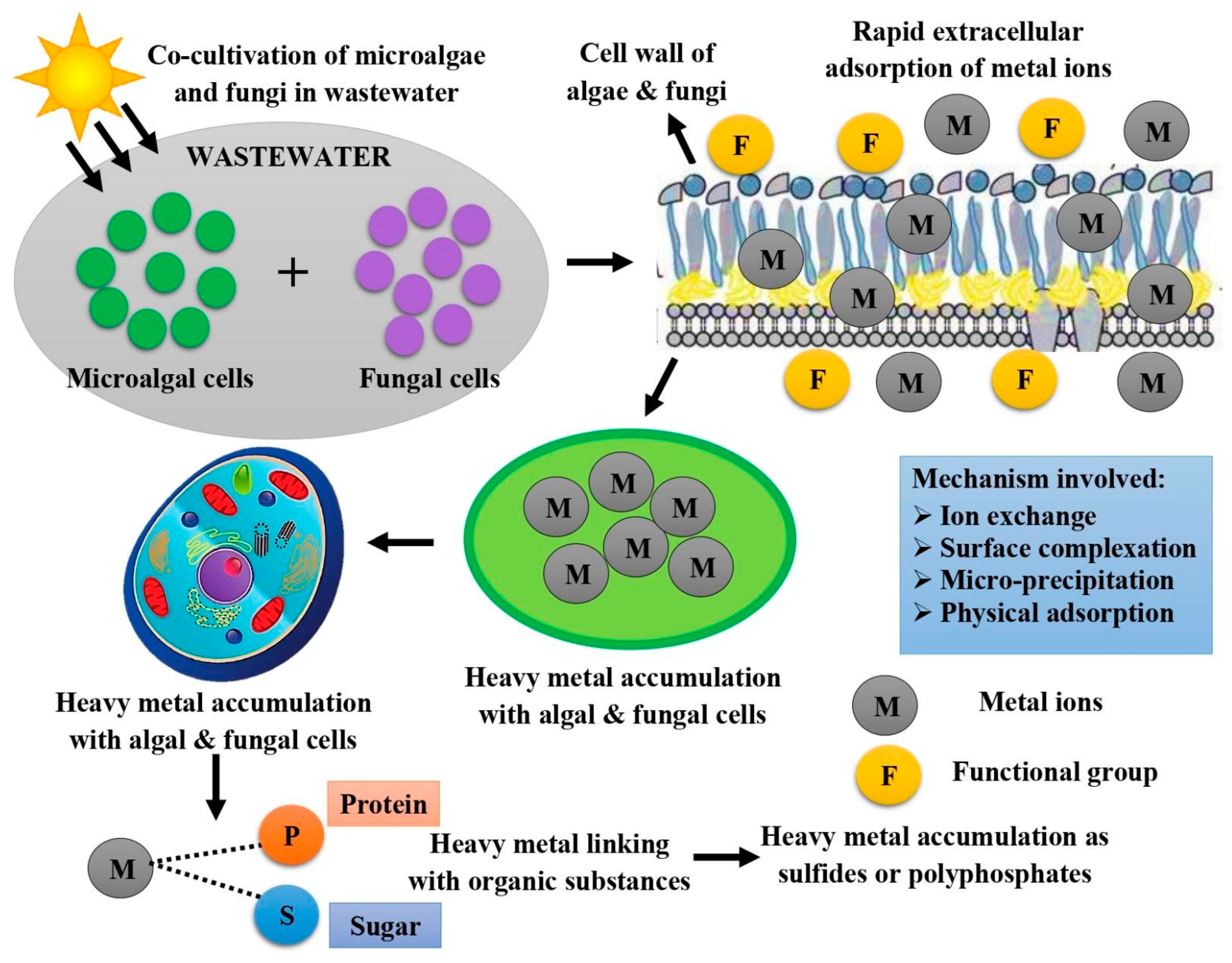
4.3. Working Action of Algal–Fungi Consortium for Wastewater Treatment
5. Utilization of Algae and Its Consortium for Wastewater Treatment
5.1. Utilization of Algae–Algae Consortiums for Wastewater Treatment
5.2. Utilization of Algae–Bacterial Consortiums for Wastewater Treatment
5.3. Utilization of Algae–Fungi Consortiums for Wastewater Treatment
6. Flocculation of Algal Consortiums
7. Factors Affecting Wastewater Treatment by Algal System
7.1. Lighting at Night-Time
7.2. Mixing
7.3. Depth of Algal Tank
7.4. Recycle Ratio of Settled Algal Sludge
8. Observed Yield Coefficient
9. Future Prospects and Challenges
- (i)
- the prolongation of homeostasis;
- (ii)
- maintaining the prolonged potency of the consortium, even at the time of gene transfer;
- (iii)
- the inclusion of stable alterations in the genomes of microbes taking part in the consortium;
- (iv)
- the improved performance of the consortium system.
- (i)
- studying the influence of various environmental conditions, such as nutrient availability, light, temperature and pH, on the behavior of consortium systems;
- (ii)
- experimenting on a mass scale;
- (iii)
- gaining a complete understanding of the associations, such as commensalism, mutualism and parasitism, taking place between the microalgae, the bacteria and the fungi which, to date, have not been well described;
- (iv)
- evolving authentic mathematical models (such as BIO_ALGAE) that accurately represent the consortium behavior: this might be very supportive, for the determination of operational conditions and process design.
- (i)
- degrading the quality of algal biomass through consumption of valuable algal bio-products;
- (ii)
- directly hindering the growth of algae either by nutrient competition or by an allelopathic action;
- (iii)
- increasing the chances of microalgae culture contamination by pathogenic bacteria.
- (i)
- the preferences of microalgae and fungi species, and their co-cultivation conditions, highly affect this process; presently, filamentous fungi are ratified to be efficient in microalgae harvesting: unfortunately, most of them are pathogenic, and therefore do not have any practical application value;
- (ii)
- inadequate co-cultivation conditions result in reduced flocculation efficacy; the impact of different parameters on the flocculation methods of microalgae and fungi are still in an investigative phase; optimized co-cultivation parameters involving agitating, addition of carbon source and illumination demand high cost, thus hindering implementation on a mass scale;
- (iii)
- generally, wastewater from natural sources contains bacteria: however, most of the studies have utilized wastewater after its filtration and sterilization; it is quite difficult to construct a distinct microalgae–fungi system that totally lacks bacteria, but fungi can effectively guard microalgae from bacterial interference.
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Acknowledgments
Conflicts of Interest
References
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Kumar, V.; Joshi, N.C.; Tomar, M.S.; Pathak, B. Cold plasma technology: Advanced and sustainable approach for wastewater treatment. Environ. Sci. Pollut. Res. 2021, 28, 65062–65082. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, L.; Qiang, X.; Gu, W.; Ma, Z.; Wang, G. The promising way to treat wastewater by microalgae: Approaches, mechanisms, applications and challenges. J. Water Process Eng. 2022, 49, 103012. [Google Scholar] [CrossRef]
- China. China Statistical Yearbook; China Statistics Press: Beijing, China, 2022. [Google Scholar]
- Yakamercan, E.; Ari, A.; Aygün, A. Land application of municipal sewage sludge: Human health risk assessment of heavy metals. J. Clean. Prod. 2021, 319, 128568. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Z.; Wu, G.; Wu, Q.; Zhang, F.; Niu, Z.; Hu, H.Y. Characteristics of water quality of municipal wastewater treatment plants in China: Implications for resources utilization and management. J. Clean. Prod. 2016, 131, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of heavy metals from industrial wastewaters: A review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Dutta, D.; Arya, S.; Kumar, S. Industrial wastewater treatment: Current trends, bottlenecks, and best practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Renuka, N.; Sood, A.; Ratha, S.K.; Prasanna, R.; Ahluwalia, A.S. Evaluation of microalgal consortia for treatment of primary treated sewage effluent and biomass production. J. Appl. Phycol. 2013, 25, 1529–1537. [Google Scholar] [CrossRef]
- Arita, C.E.Q.; Peebles, C.; Bradley, T.H. Scalability of combining microalgae-based biofuels with wastewater facilities: A review. Algal Res. 2015, 9, 160–169. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Gururani, P.; Bisht, B.; Kumar, V. Algal Biochar: An Advance and Sustainable Method for Wastewater Treatment. Octa J. Biosci. 2021, 9, 79–85. [Google Scholar]
- Oswald, W.J.; Gotaas, H.B. Photosynthesis in sewage treatment. Trans. Am. Soc. Civ. Eng. 1957, 122, 73–105. [Google Scholar] [CrossRef]
- Leng, L.; Li, J.; Wen, Z.; Zhou, W. Use of microalgae to recycle nutrients in aqueous phase derived from hydrothermal liquefaction process. Bioresour. Technol. 2018, 256, 529–542. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Alam, M.A.; Mehmood, M.A. Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; a review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.Y.; Alvaro, J.; Hyden, B.; Zienkiewicz, K.; Benning, N.; Zienkiewicz, A.; Bonito, G.; Benning, C. Enhancing oil production and harvest by combining the marine alga Nannochloropsis oceanica and the oleaginous fungus Mortierella elongate. Biotechnol. Biofuels 2018, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lutzu, G.A.; Dunford, N.T. Interactions of microalgae and other microorganisms for enhanced production of high-value compounds. Front. Biosci.-Landmark. 2018, 23, 1487–1504. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.R.; Admassu, W. Mixed algae cultures for low cost environmental compensation in cultures grown for lipid production and wastewater remediation. J. Chem. Technol. Biotechnol. 2013, 88, 992–998. [Google Scholar] [CrossRef]
- Boonma, S.; Chaiklangmuang, S.; Chaiwongsar, S.; Pekkoh, J.; Pumas, C.; Ungsethaphand, T.; Tongsiri, S.; Peerapornpisal, Y. Enhanced carbon dioxide fixation and bio-oil production of a microalgal consortium. CLEAN–Soil Air Water 2015, 43, 761–766. [Google Scholar] [CrossRef]
- Fouilland, E. Biodiversity as a tool for waste phycoremediation and biomass production. Rev. Environ. Sci. Biotechnol. 2012, 11, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Chu, R.; Li, S.; Zhu, L.; Yin, Z.; Hu, D.; Liu, C.; Mo, F. A review on co-cultivation of microalgae with filamentous fungi: Efficient harvesting, wastewater treatment and biofuel production. Renew. Sustain. Energy Rev. 2021, 139, 110689. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Jeevanantham, S.; Yaashikaa, P.R.; Thamarai, P.; Abirami, B.; George, C.S. A review on algal-bacterial symbiotic system for effective treatment of wastewater. Chemosphere 2021, 271, 129540. [Google Scholar] [CrossRef] [PubMed]
- Sonune, A.; Ghate, R. Developments in wastewater treatment methods. Desalination 2004, 167, 55–63. [Google Scholar]
- Karimi-Maleh, H.; Shafieizadeh, M.; Taher, M.A.; Opoku, F.; Kiarii, E.M.; Govender, P.P.; Ranjbari, S.; Rezapour, M.; Orooji, Y. The role of magnetite/graphene oxide nano-composite as a high-efficiency adsorbent for removal of phenazopyridine residues from water samples, an experimental/theoretical investigation. J. Mol. Liq. 2020, 298, 112040. [Google Scholar] [CrossRef]
- Leng, L.; Li, W.; Chen, J.; Leng, S.; Chen, J.; Wei, L.; Peng, H.; Li, J.; Zhou, W.; Huang, H. Co-culture of fungi-microalgae consortium for wastewater treatment: A review. Bioresour. Technol. 2021, 330, 125008. [Google Scholar] [CrossRef] [PubMed]
- Veerabadhran, M.; Natesan, S.; MubarakAli, D.; Xu, S.; Yang, F. Using different cultivation strategies and methods for the production of microalgal biomass as a raw material for the generation of bioproducts. Chemosphere 2021, 285, 131436. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Salama, E.S.; Kurade, M.B.; Abou-Shanab, R.A.; El-Dalatony, M.M.; Yang, I.S.; Min, B.; Jeon, B.H. Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew. Sustain. Energy Rev. 2017, 79, 1189–1211. [Google Scholar] [CrossRef]
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.H.; Show, P.L. A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef] [Green Version]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Chong, W.T.; Lam, M.K.; Loh, P.K.; Vellayan, V. Microalgae biofuels as an alternative to fossil fuel for power generation. Renew. Sustain. Energy Rev. 2016, 58, 180–197. [Google Scholar] [CrossRef]
- Furmaniak, M.A.; Misztak, A.E.; Franczuk, M.D.; Wilmotte, A.; Waleron, M.; Waleron, K.F. Edible cyanobacterial genus Arthrospira: Actual state of the art in cultivation methods, genetics, and application in medicine. Front. Microbiol. 2017, 8, 2541. [Google Scholar] [CrossRef] [PubMed]
- Ramaraj, R.; Tsai, D.D.W.; Chen, P.H. Carbon dioxide fixation of freshwater microalgae growth on natural water medium. Ecol. Eng. 2015, 75, 86–92. [Google Scholar] [CrossRef]
- Singh, D.; Yadav, K.; Singh, R.S. Biofixation of carbon dioxide using mixed culture of microalgae. Indian J. Biotechnol. 2015, 14, 228–232. [Google Scholar]
- Zhu, L. Microalgal culture strategies for biofuel production: A review. Biofuel Bioprod. Biorefin. 2015, 9, 801–814. [Google Scholar] [CrossRef]
- Razzak, S.A.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; De Lasa, H. Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renew. Sustain. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: Environmental pollution control and future directions. Bioresour. Technol. 2020, 301, 122804. [Google Scholar] [CrossRef]
- Zhu, J.; Rong, J.; Zong, B. Factors in mass cultivation of microalgae for biodiesel. Chin. J. Catal. 2013, 34, 80–100. [Google Scholar] [CrossRef]
- Nugroho, Y.K.; Zhu, L. An integration of algal biofuel production planning, scheduling, and order-based inventory distribution control systems. Biofuel Bioprod. Biorefin. 2019, 13, 920–935. [Google Scholar] [CrossRef]
- Su, Y.; Jacobsen, C. Treatment of clean in place (CIP) wastewater using microalgae: Nutrient upcycling and value-added byproducts production. Sci. Total Environ. 2021, 785, 147337. [Google Scholar] [CrossRef]
- Michelon, W.; da Silva, M.L.B.; Matthiensen, A.; de Andrade, C.J.; de Andrade, L.M.; Soares, H.M. Amino acids, fatty acids, and peptides in microalgae biomass harvested from phycoremediation of swine wastewaters. Biomass Convers. Biorefin. 2022, 12, 869–880. [Google Scholar] [CrossRef]
- Shi, J.; Podola, B.; Melkonian, M. Application of a prototype-scale Twin-Layer photobioreactor for effective N and P removal from different process stages of municipal wastewater by immobilized microalgae. Bioresour. Technol. 2014, 154, 260–266. [Google Scholar] [CrossRef]
- Muñoz, R.; Guieysse, B. Algal–bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef] [PubMed]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Consortia of cyanobacteria/microalgae and bacteria: Biotechnological potential. Biotechnol. Adv. 2011, 29, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Molinuevo-Salces, B.; García-González, M.C. Nitrogen transformations under different conditions in open ponds by means of microalgae–bacteria consortium treating pig slurry. Bioresour. Technol. 2011, 102, 960–966. [Google Scholar] [CrossRef] [PubMed]
- He, P.J.; Mao, B.; Lü, F.; Shao, L.M.; Lee, D.J.; Chang, J.S. The combined effect of bacteria and Chlorella vulgaris on the treatment of municipal wastewaters. Bioresour. Technol. 2013, 146, 562–568. [Google Scholar] [CrossRef]
- Jagmann, N.; Philipp, B. Design of synthetic microbial communities for biotechnological production processes. J. Biotechnol. 2014, 184, 209–218. [Google Scholar] [CrossRef]
- Li, F.; Wang, W.; Li, C.; Zhu, R.; Ge, F.; Zheng, Y.; Tang, Y. Self-mediated pH changes in culture medium affecting biosorption and biomineralization of Cd2+ by Bacillus cereus Cd01. J. Hazard. Mater. 2018, 358, 178–186. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Y.; Jia, X. Co-cultivation of fungal-microalgal strains in biogas slurry and biogas purification under different initial CO2 concentrations. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Hernández-García, A.; Velásquez-Orta, S.B.; Novelo, E.; Yáñez-Noguez, I.; Monje-Ramírez, I.; Ledesma, M.T.O. Wastewater-leachate treatment by microalgae: Biomass, carbohydrate and lipid production. Ecotoxicol. Environ. Saf. 2019, 174, 435–444. [Google Scholar] [CrossRef]
- Bacellar Mendes, L.B.; Vermelho, A.B. Allelopathy as a potential strategy to improve microalgae cultivation. Biotechnol. Biofuels 2013, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Meneses, Y.E.; Stratton, J.; Wang, B. Acclimation of consortium of micro-algae help removal of organic pollutants from meat processing wastewater. J. Clean. Prod. 2019, 214, 95–102. [Google Scholar] [CrossRef]
- Moreno-García, A.F.; Neri-Torres, E.E.; Mena-Cervantes, V.Y.; Altamirano, R.H.; Pineda-Flores, G.; Luna-Sánchez, R.; García-Solares, M.; Vazquez-Arenas, J.; Suastes-Rivas, J.K. Sustainable biorefinery associated with wastewater treatment of Cr (III) using a native microalgae consortium. Fuel 2021, 290, 119040. [Google Scholar] [CrossRef]
- Hena, S.; Fatimah, S.; Tabassum, S. Cultivation of algae consortium in a dairy farm wastewater for biodiesel production. Water Resour. Indus. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Lee, S.A.; Lee, N.; Oh, H.M.; Ahn, C.Y. Enhanced and balanced microalgal wastewater treatment (COD, N, and P) by interval inoculation of activated sludge. J. Microbiol. Biotechnol. 2019, 29, 1434–1443. [Google Scholar] [CrossRef]
- Solimeno, A.; García, J. Microalgae and bacteria dynamics in high rate algal ponds based on modelling results: Long-term application of BIO_ALGAE model. Sci. Total Environ. 2019, 650, 1818–1831. [Google Scholar] [CrossRef]
- Mu, R.; Jia, Y.; Ma, G.; Liu, L.; Hao, K.; Qi, F.; Shao, Y. Advances in the use of microalgal–bacterial consortia for wastewater treatment: Community structures, interactions, economic resource reclamation, and study techniques. Water Environ. Res. 2021, 93, 1217–1230. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multi-functional options in modern agriculture: Current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef]
- Alam, M.; Vandamme, D.; Chun, W.; Zhao, X.; Foubert, I.; Wang, Z.; Muylaert, K.; Yuan, Z. Bioflocculation as an innovative harvesting strategy for microalgae. Rev. Environ. Sci. Biotechnol. 2016, 15, 573–583. [Google Scholar] [CrossRef]
- Rwehumbiza, V.M.; Harrison, R.; Thomsen, L. Alum-induced flocculation of preconcentrated Nannochloropsis salina: Residual aluminium in the biomass, FAMEs and its effects on microalgae growth upon media recycling. Chem. Eng. J. 2012, 200, 168–175. [Google Scholar] [CrossRef]
- Bounnit, T.; Saadaoui, I.; Rasheed, R.; Schipper, K.; Al Muraikhi, M.; Al Jabri, H. Sustainable production of Nannochloris atomus biomass towards biodiesel production. Sustainability 2020, 12, 2008. [Google Scholar] [CrossRef] [Green Version]
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Jaiswal, K.K.; Kumar, V.; Kumar, S.; Vlaskin, M.S.; Grigorenko, A.V.; Rindin, K.G. Recent advances and viability in sustainable thermochemical conversion of sludge to bio-fuel production. Fuel 2022, 316, 123351. [Google Scholar] [CrossRef]
- Talapatra, N.; Gautam, R.; Mittal, V.; Ghosh, U.K. A comparative study of the growth of microalgae-bacteria symbiotic consortium with the axenic culture of microalgae in dairy wastewater through extraction and quantification of chlorophyll. Mater. Today Proc. 2021, in press. [CrossRef]
- Li, D.; Liu, R.; Cui, X.; He, M.; Zheng, S.; Du, W.; Gao, M.; Wang, C. Co-culture of bacteria and microalgae for treatment of high concentration biogas slurry. J. Water Process Eng. 2021, 41, 102014. [Google Scholar] [CrossRef]
- Sforza, E.; Pastore, M.; Sanchez, S.S.; Bertucco, A. Bioaugmentation as a strategy to enhance nutrient removal: Symbiosis between Chlorella protothecoides and Brevundimonas diminuta. Bioresour. Technol. Rep. 2018, 4, 153–158. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Sun, L.; Sun, Z.; Li, D. Screening of a Chlorella-bacteria consortium and research on piggery wastewater purification. Algal Res. 2020, 47, 101840. [Google Scholar] [CrossRef]
- Unnithan, V.V.; Unc, A.; Smith, G.B. Mini-review: A priori considerations for bacteria–algae interactions in algal biofuel systems receiving municipal wastewaters. Algal Res. 2014, 4, 35–40. [Google Scholar] [CrossRef]
- Rashid, N.; Park, W.K.; Selvaratnam, T. Binary culture of microalgae as an integrated approach for enhanced biomass and metabolites productivity, wastewater treatment, and bioflocculation. Chemosphere 2018, 194, 67–75. [Google Scholar] [CrossRef]
- Kouzuma, A.; Watanabe, K. Exploring the potential of algae/bacteria interactions. Curr. Opin. Biotechnol. 2015, 33, 125–129. [Google Scholar] [CrossRef]
- Padmaperuma, G.; Kapoore, R.V.; Gilmour, D.J.; Vaidyanathan, S. Microbial consortia: A critical look at microalgae co-cultures for enhanced biomanufacturing. Crit. Rev. Biotechnol. 2018, 38, 690–703. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wu, Y.; Wu, C.; Muylaert, K.; Vyverman, W.; Yu, H.Q.; Muñoz, R.; Rittmann, B. Advanced nutrient removal from surface water by a consortium of attached microalgae and bacteria: A review. Bioresour. Technol. 2017, 241, 1127–1137. [Google Scholar] [CrossRef]
- Liu, J.; Lewitus, A.J.; Brown, P.; Wilde, S.B. Growth-promoting effects of a bacterium on raphidophytes and other phytoplankton. Harmful Algae 2008, 7, 1–10. [Google Scholar] [CrossRef]
- Yang, K.; Chen, Q.; Zhang, D.; Zhang, H.; Lei, X.; Chen, Z.; Li, Y.; Hong, Y.; Ma, X.; Zheng, W.; et al. The algicidal mechanism of prodigiosin from Hahella sp. KA22 against Microcystis aeruginosa. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, D.; Riaño, B.; Coca, M.; García-González, M.C. Treatment of agro-industrial wastewater using microalgae–bacteria consortium combined with anaerobic digestion of the produced biomass. Bioresour. Technol. 2013, 135, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Padri, M.; Boontian, N.; Piasai, C.; Tamzil, M.S. Construction of co-culture of microalgae with microorganisms for enhancing biomass production and wastewater treatment: A review. Environ. Earth Sci. 2021, 623, 012024. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Microalgae–bacteria biofilms: A sustainable synergistic approach in remediation of acid mine drainage. Appl. Microbiol. Biotechnol. 2018, 102, 1131–1144. [Google Scholar] [CrossRef]
- Srinuanpan, S.; Chawpraknoi, A.; Chantarit, S.; Cheirsilp, B.; Prasertsan, P. A rapid method for harvesting and immobilization of oleaginous microalgae using pellet-forming filamentous fungi and the application in phytoremediation of secondary effluent. Int. J. Phytoremed. 2018, 20, 1017–1024. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Heubeck, S.; Park, J.; Turnbull, M.H.; Craggs, R.J. Seasonal performance of a full-scale wastewater treatment enhanced pond system. Water Res. 2018, 136, 150–159. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, Y.; Zhao, Y. Biocapture of CO2 by different microalgal-based technologies for biogas upgrading and simultaneous biogas slurry purification under various light intensities and photoperiods. Int. J. Environ. Res. Public Health 2018, 15, 528. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Xie, S.; Pu, Y.; Zhang, R.; Huang, F.; Ragauskas, A.J.; Yuan, J.S. Synergistic enzymatic and microbial lignin conversion. Green Chem. 2016, 18, 1306–1312. [Google Scholar] [CrossRef]
- Ling, J.; Nip, S.; Cheok, W.L.; de Toledo, R.A.; Shim, H. Lipid production by a mixed culture of oleaginous yeast and microalga from distillery and domestic mixed wastewater. Bioresour. Technol. 2014, 173, 132–139. [Google Scholar] [CrossRef]
- Walls, L.E.; Velasquez-Orta, S.B.; Romero-Frasca, E.; Leary, P.; Noguez, I.Y.; Ledesma, M.T.O. Novel fungal pelletization-assisted technology for algae harvesting and wastewater treatment. Appl. Biochem. Biotechnol. 2012, 167, 214–228. [Google Scholar]
- Santos, F.M.; Mazur, L.P.; Mayer, D.A.; Vilar, V.J.; Pires, J.C. Inhibition effect of zinc, cadmium, and nickel ions in microalgal growth and nutrient uptake from water: An experimental approach. Chem. Eng. J. 2019, 366, 358–367. [Google Scholar] [CrossRef]
- Zhang, C.; Tao, Y.; Li, S.; Tian, J.; Ke, T.; Wei, S.; Wang, P.; Chen, L. Simultaneous degradation of trichlorfon and removal of Cd (II) by Aspergillus sydowii strain PA F-2. Environ. Sci. Pollut. Res. 2019, 26, 26844–26854. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Chang, J.S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, C.; Yañez-Mansilla, E.; Jeison, D. Bioremoval of heavy metals from metal mine tailings water using microalgae biomass. Algal Res. 2019, 43, 101659. [Google Scholar] [CrossRef]
- Shen, Y.; Gao, J.; Li, L. Municipal wastewater treatment via co-immobilized microalgal-bacterial symbiosis: Microorganism growth and nutrients removal. Bioresour. Technol. 2017, 243, 905–913. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Mishra, B.B.; Devi, N. Biosorption for removal of hexavalent chromium using microalgae Scenedesmus sp. J. Clean. Prod. 2019, 209, 617–629. [Google Scholar] [CrossRef]
- Shen, L.; Wang, J.; Li, Z.; Fan, L.; Chen, R.; Wu, X.; Li, J.; Zeng, W. A high-efficiency Fe2O3@ Microalgae composite for heavy metal removal from aqueous solution. J. Water Process Eng. 2020, 33, 101026. [Google Scholar] [CrossRef]
- Ibuot, A.; Dean, A.P.; McIntosh, O.A.; Pittman, J.K. Metal bioremediation by CrMTP4 over-expressing Chlamydomonas reinhardtii in comparison to natural wastewater-tolerant microalgae strains. Algal Res. 2017, 24, 89–96. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Ralph, P.J. Microalgal bioremediation of emerging contaminants-Opportunities and challenges. Water Res. 2019, 164, 114921. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, G.; Sun, S.; Hu, C.; Liu, J. Co-pelletization of microalgae and fungi for efficient nutrient purification and biogas upgrading. Bioresour. Technol. 2019, 289, 121656. [Google Scholar] [CrossRef] [PubMed]
- Wrede, D.; Taha, M.; Miranda, A.F.; Kadali, K.; Stevenson, T.; Ball, A.S.; Mouradov, A. Co-cultivation of fungal and microalgal cells as an efficient system for harvesting microalgal cells, lipid production and wastewater treatment. PLoS ONE 2014, 9, e113497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Li, S.; Hu, T.; Nugroho, Y.K.; Yin, Z.; Hu, D.; Chu, R.; Mo, F.; Liu, C.; Hiltunen, E. Effects of nitrogen source heterogeneity on nutrient removal and biodiesel production of mono-and mix-cultured microalgae. Energy Convers. Manag. 2019, 201, 112144. [Google Scholar] [CrossRef]
- Abinandan, S.; Shanthakumar, S. Challenges and opportunities in application of microalgae (Chlorophyta) for wastewater treatment: A review. Renew. Sustain. Energy Rev. 2015, 52, 123–132. [Google Scholar] [CrossRef]
- Peccia, J.; Haznedaroglu, B.; Gutierrez, J.; Zimmerman, J.B. Nitrogen supply is an important driver of sustainable microalgae biofuel production. Trends Biotechnol. 2013, 31, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, P.; Zhang, C.; Zhou, X.; Yin, Z.; Hu, T.; Liu, C.; Zhu, L. Influence of polystyrene microplastics on the growth, photosynthetic efficiency and aggregation of freshwater microalgae Chlamydomonas reinhardtii. Sci. Total Environ. 2020, 714, 136767. [Google Scholar] [CrossRef]
- Foladori, P.; Petrini, S.; Nessenzia, M.; Andreottola, G. Enhanced nitrogen removal and energy saving in a microalgal–bacterial consortium treating real municipal wastewater. Water Sci. Technol. 2018, 78, 174–182. [Google Scholar] [CrossRef]
- Fatima, N.; Kumar, V. Microalgae based hybrid approach for bioenergy generation and bioremediation: A review. Octa J. Biosci. 2020, 8, 113–123. [Google Scholar]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T.; Yang, Z. Immobilizing unicellular microalga on pellet-forming filamentous fungus: Can this provide new insights into the remediation of arsenic from contaminated water? Bioresour. Technol. 2019, 284, 231–239. [Google Scholar] [CrossRef]
- Samorì, G.; Samorì, C.; Guerrini, F.; Pistocchi, R. Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater treatment: Part I. Water Res. 2013, 47, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Koreivienė, J.; Valčiukas, R.; Karosienė, J.; Baltrėnas, P. Testing of Chlorella/Scenedesmus microalgae consortia for remediation of wastewater, CO2 mitigation and algae biomass feasibility for lipid production. J. Environ. Eng. Landsc. Manag. 2014, 22, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K.C. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.C.; Agustini, C.B.; Trierweiler, L.F.; Gutterres, M. Influence of period light on cultivation of microalgae consortium for the treatment of tannery wastewaters from leather finishing stage. J. Clean. Prod. 2020, 263, 121618. [Google Scholar] [CrossRef]
- Arias, D.M.; Rueda, E.; García-Galán, M.J.; Uggetti, E.; García, J. Selection of cyanobacteria over green algae in a photo-sequencing batch bioreactor fed with wastewater. Sci. Total Environ. 2019, 653, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Ambrosano, L.; Graça, S.; Sousa, C.; Marques, P.A.; Ribeiro, B.; Botrel, E.P.; Neto, P.C.; Gouveia, L. Combining urban wastewater treatment with biohydrogen production–an integrated microalgae-based approach. Bioresour. Technol. 2015, 184, 230–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberholster, P.J.; Steyn, M.; Botha, A.M. A comparative study of improvement of phycoremediation using a consortium of microalgae in municipal wastewater treatment pond systems as an alternative solution to Africa’s sanitation challenges. Processes 2021, 9, 1677. [Google Scholar] [CrossRef]
- Kumar, P.; Prajapati, S.K.; Malik, A.; Vijay, V.K. Cultivation of native algal consortium in semi-continuous pilot scale raceway pond for greywater treatment coupled with potential methane production. J. Environ. Chem. Eng. 2017, 5, 5581–5587. [Google Scholar] [CrossRef]
- Mustafa, E.M.; Phang, S.M.; Chu, W.L. Use of an algal consortium of five algae in the treatment of landfill leachate using the high-rate algal pond system. J. Appl. Phycol. 2012, 24, 953–963. [Google Scholar] [CrossRef]
- Bhakta, J.N.; Lahiri, S.; Pittman, J.K.; Jana, B.B. Carbon dioxide sequestration in wastewater by a consortium of elevated carbon dioxide-tolerant microalgae. J. CO2 Util. 2015, 10, 105–112. [Google Scholar] [CrossRef]
- Naaz, F.; Bhattacharya, A.; Pant, K.K.; Malik, A. Investigations on energy efficiency of biomethane/biocrude production from pilot scale wastewater grown algal biomass. Appl. Eng. 2019, 254, 113656. [Google Scholar] [CrossRef]
- Ryu, B.G.; Kim, J.; Han, J.I.; Yang, J.W. Feasibility of using a microalgal-bacterial consortium for treatment of toxic coke wastewater with concomitant production of microbial lipids. Bioresour. Technol. 2017, 225, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Nanda, M.; Kumar, V. Implications of bacterial multi-metal tolerance for mitigation of heavy metal pollutants from wastewater. Octa J. Biosci. 2021, 9, 37–44. [Google Scholar]
- Fang, Y.; Hu, Z.; Zou, Y.; Zhang, J.; Zhu, Z.; Zhang, J.; Nie, L. Improving nitrogen utilization efficiency of aquaponics by introducing algal-bacterial consortia. Bioresour. Technol. 2017, 245, 358–364. [Google Scholar] [CrossRef]
- Tang, C.C.; Tian, Y.; He, Z.W.; Zuo, W.; Zhang, J. Performance and mechanism of a novel algal-bacterial symbiosis system based on sequencing batch suspended biofilm reactor treating domestic wastewater. Bioresour. Technol. 2018, 265, 422–431. [Google Scholar] [CrossRef]
- Katam, K.; Bhattacharyya, D. Simultaneous treatment of domestic wastewater and bio-lipid synthesis using immobilized and suspended cultures of microalgae and activated sludge. J. Ind. Eng. Chem. 2019, 69, 295–303. [Google Scholar] [CrossRef]
- Luo, L.; Lin, X.; Zeng, F.; Luo, S.; Chen, Z.; Tian, G. Performance of a novel photobioreactor for nutrient removal from piggery biogas slurry: Operation parameters, microbial diversity and nutrient recovery potential. Bioresour. Technol. 2019, 272, 421–432. [Google Scholar] [CrossRef]
- Biswas, T.; Bhushan, S.; Prajapati, S.K.; Chaudhuri, S.R. An eco-friendly strategy for dairy wastewater remediation with high lipid microalgae-bacterial biomass production. J. Environ. Mng. 2021, 286, 112196. [Google Scholar] [CrossRef]
- da Silva Rodrigues, D.A.; da Cunha, C.C.R.F.; Freitas, M.G.; de Barros, A.L.C.; e Castro, P.B.N.; Pereira, A.R.; de Queiroz Silva, S.; da Fonseca Santiago, A.; de Cássia Franco Afonsog, R.J. Biodegradation of sulfamethoxazole by microalgae-bacteria consortium in wastewater treatment plant effluents. Sci. Total Environ. 2020, 749, 141441. [Google Scholar] [CrossRef]
- Yang, J.; Gou, Y.; Fang, F.; Guo, J.; Lu, L.; Zhou, Y.; Ma, H. Potential of wastewater treatment using a concentrated and suspended algal-bacterial consortium in a photo membrane bioreactor. Chem. Eng. J. 2018, 335, 154–160. [Google Scholar] [CrossRef]
- Posadas, E.; Bochon, S.; Coca, M.; García-González, M.C.; García-Encina, P.A.; Muñoz, R. Microalgae-based agro-industrial wastewater treatment: A preliminary screening of biodegradability. J. Appl. Phycol. 2014, 26, 2335–2345. [Google Scholar] [CrossRef]
- Maza-Márquez, P.; González-Martínez, A.; Martínez-Toledo, M.V.; Fenice, M.; Lasserrot, A.; González-López, J. Biotreatment of industrial olive washing water by synergetic association of microalgal-bacterial consortia in a photobioreactor. Environ. Sci. Pollut. Res. 2017, 24, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Tighiri, H.O.; Erkurt, E.A. Biotreatment of landfill leachate by microalgae- bacteria consortium in sequencing batch mode and product utilization. Bioresour. Technol. 2019, 286, 121396. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, Y.; Huang, S.; Qiu, D.; Schideman, L.; Chai, X.; Zhao, Y. Characterization of microalgae-bacteria consortium cultured in landfill leachate for carbon fixation and lipid production. Bioresour. Technol. 2014, 156, 322–328. [Google Scholar] [CrossRef]
- Fito, J.; Alemu, K. Microalgae–bacteria consortium treatment technology for municipal wastewater management. Nanotechnol. Environ. Eng. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Sátiro, J.; Cunha, A.; Gomes, A.P.; Simões, R.; Albuquerque, A. Optimization of Microalgae–Bacteria Consortium in the Treatment of Paper Pulp Wastewater. Appl. Sci. 2022, 12, 5799. [Google Scholar] [CrossRef]
- Xu, K.; Zou, X.; Xue, Y.; Qu, Y.; Li, Y. The impact of seasonal variations about temperature and photoperiod on the treatment of municipal wastewater by algae-bacteria system in lab-scale. Algal Res. 2021, 54, 102175. [Google Scholar] [CrossRef]
- Su, Y.; Zhu, X.; Zou, R.; Zhang, Y. The interactions between microalgae and wastewater indigenous bacteria for treatment and valorization of brewery wastewater. Resour. Conserv. Recycl. 2022, 182, 106341. [Google Scholar] [CrossRef]
- Rossi, S.; Pizzera, A.; Bellucci, M.; Marazzi, F.; Mezzanotte, V.; Parati, K.; Ficara, E. Piggery wastewater treatment with algae-bacteria consortia: Pilot-scale validation and techno-economic evaluation at farm level. Bioresour. Technol. 2022, 351, 127051. [Google Scholar] [CrossRef]
- Liu, H.; Lu, Q.; Wang, Q.; Liu, W.; Wei, Q.; Ren, H.; Ming, C.; Min, M.; Chen, P.; Ruan, R. Isolation of a bacterial strain, Acinetobacter sp. from centrate wastewater and study of its cooperation with algae in nutrients removal. Bioresour. Technol. 2017, 235, 59–69. [Google Scholar]
- Huo, S.; Kong, M.; Zhu, F.; Qian, J.; Huang, D.; Chen, P.; Ruan, R. Co-culture of Chlorella and wastewater-borne bacteria in vinegar production wastewater: Enhancement of nutrients removal and influence of algal biomass generation. Algal Res. 2020, 45, 101744. [Google Scholar] [CrossRef]
- Leng, L.; Wei, L.; Xiong, Q.; Xu, S.; Li, W.; Lv, S.; Lu, Q.; Wan, L.; Wen, Z.; Zhou, W. Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere 2020, 238, 124680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, C.; Liu, J.; Sun, S.; Zhao, Y.; Wei, J. Effects of exogenous GR24 on biogas upgrading and nutrient removal by co-culturing microalgae with fungi under mixed LED light wavelengths. Chemosphere 2021, 281, 130791. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Yang, K.X.; Zhu, Y.R.; Zhu, X.Y.; Nie, D.F.; Jiao, N.; Angelidaki, I. One-step co-cultivation and flocculation of microalgae with filamentous fungi to valorize starch wastewater into high-value biomass. Bioresour. Technol. 2022, 361, 127625. [Google Scholar] [CrossRef] [PubMed]
- Walls, L.E.; Velasquez-Orta, S.B.; Romero-Frasca, E.; Leary, P.; Noguez, I.Y.; Ledesma, M.T.O. Non-sterile heterotrophic cultivation of native wastewater yeast and microalgae for integrated municipal wastewater treatment and bioethanol production. Biochem. Eng. J. 2019, 151, 107319. [Google Scholar] [CrossRef]
- Bodin, H.; Daneshvar, A.; Gros, M.; Hultberg, M. Effects of biopellets composed of microalgae and fungi on pharmaceuticals present at environmentally relevant levels in water. Ecol. Eng. 2016, 91, 169–172. [Google Scholar]
- Shen, N.; Chirwa, E.M. Live and lyophilized fungi-algae pellets as novel biosorbents for gold recovery: Critical parameters, isotherm, kinetics and regeneration studies. Bioresour. Technol. 2020, 306, 123041. [Google Scholar] [CrossRef]
- Yang, L.; Li, H.; Wang, Q. A novel one-step method for oil-rich biomass production and harvesting by co-cultivating microalgae with filamentous fungi in molasses wastewater. Bioresour. Technol. 2019, 275, 35–43. [Google Scholar] [CrossRef]
- Cao, W.; Wang, X.; Sun, S.; Hu, C.; Zhao, Y. Simultaneously upgrading biogas and purifying biogas slurry using cocultivation of Chlorella vulgaris and three different fungi under various mixed light wavelength and photoperiods. Bioresour. Technol. 2017, 241, 701–709. [Google Scholar] [CrossRef]
- Guo, G.; Cao, W.; Sun, S.; Zhao, Y.; Hu, C. Nutrient removal and biogas upgrading by integrating fungal–microalgal cultivation with anaerobically digested swine wastewater treatment. J. Appl. Phycol. 2017, 29, 2857–2866. [Google Scholar]
- Jaiswal, K.K.; Kumar, V.; Gururani, P.; Vlaskin, M.S.; Parveen, A.; Nanda, M.; Kurbatova, A.; Gautam, P.; Grigorenko, A.V. Bio-flocculation of oleaginous microalgae integrated with municipal wastewater treatment and its hydrothermal liquefaction for biofuel production. Environ. Technol. Innov. 2022, 26, 102340. [Google Scholar] [CrossRef]
- Kumar, V.; Gururani, P.; Parveen, A.; Verma, M.; Kim, H.; Vlaskin, M.; Grigorenko, A.V.; Rindin, K.G. Dairy Industry wastewater and stormwater energy valorization: Effect of wastewater nutrients on microalgae-yeast biomass. Biomass Convers. Biorefin. 2022, 1–10. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Ferrer, I.; Uggetti, E.; Arnabat, C.; Salvadó, H.; García, J. Settling velocity distribution of microalgal biomass from urban wastewater treatment high rate algal ponds. Algal Res. 2016, 16, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Malik, S.; Khan, F.; Atta, Z.; Habib, N.; Haider, M.N.; Wang, N.; Alam, A.; Jambi, E.J.; Gull, M.; Mehmood, M.A.; et al. Microalgal flocculation: Global research progress and prospects for algal biorefinery. Biotechnol. Appl. Biochem. 2020, 67, 52–60. [Google Scholar] [PubMed]
- Larkum, A.W.; Ross, I.L.; Kruse, O.; Hankamer, B. Selection, breeding and engineering of microalgae for bioenergy and biofuel production. Trends Biotechnol. 2012, 30, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Mennerich, A.; Urban, B. Comparison of nutrient removal capacity and biomass settleability of four high-potential microalgal species. Bioresour. Technol. 2012, 124, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, D.; Muylaert, K.; Fraeye, I.; Foubert, I. Floc characteristics of Chlorella vulgaris: Influence of flocculation mode and presence of organic matter. Bioresour. Technol. 2014, 151, 383–387. [Google Scholar] [CrossRef] [Green Version]
- Mehan, L.; Verma, R.; Kumar, R.; Srivastava, A. Illumination wavelengths effect on Arthrospira platensis production and its process applications in River Yamuna water treatment. J. Water Process Eng. 2018, 23, 91–96. [Google Scholar] [CrossRef]
- Abu-Ghosh, S.; Fixler, D.; Dubinsky, Z.; Iluz, D. Flashing light in microalgae biotechnology. Bioresour. Technol. 2016, 203, 357–363. [Google Scholar] [CrossRef]
- Binnal, P.; Babu, P.N. Optimization of environmental factors affecting tertiary treatment of municipal wastewater by Chlorella protothecoides in a lab scale photobioreactor. J. Water Process Eng. 2017, 17, 290–298. [Google Scholar] [CrossRef]
- Raeisossadati, M.; Moheimani, N.R.; Parlevliet, D. Luminescent solar concentrator panels for increasing the efficiency of mass microalgal production. Renew. Sust. Energ. Rev. 2019, 101, 47–59. [Google Scholar] [CrossRef]
- Martínez, C.; Mairet, F.; Bernard, O. Theory of turbid microalgae cultures. J. Theor. Biol. 2018, 456, 190–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmuskero, A.; Chauton, M.S.; Boström, T. Light and photosynthetic microalgae: A review of cellular-and molecular-scale optical processes. Prog. Oceanogr. 2018, 168, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Ramanna, L.; Rawat, I.; Bux, F. Light enhancement strategies improve microalgal biomass productivity. Renew. Sustain. Energ. Rev. 2017, 80, 765–773. [Google Scholar] [CrossRef]
- Gonzalez-Camejo, J.; Viruela, A.; Ruano, M.V.; Barat, R.; Seco, A.; Ferrer, J. Effect of light intensity, light duration and photoperiods in the performance of an outdoor photobioreactor for urban wastewater treatment. Algal Res. 2019, 40, 101511. [Google Scholar] [CrossRef]
- Habibi, A.; Nematzadeh, G.A.; Amrei, H.D.; Teymouri, A. Effect of light/dark cycle on nitrate and phosphate removal from synthetic wastewater based on BG11 medium by Scenedesmus sp. Biotech 2019, 9, 1–9. [Google Scholar] [CrossRef]
- De Godos, I.; Mendoza, J.L.; Acién, F.G.; Molina, E.; Banks, C.J.; Heaven, S.; Rogalla, F. Evaluation of carbon dioxide mass transfer in raceway reactors for microalgae culture using flue gases. Bioresour. Technol. 2014, 153, 307–314. [Google Scholar] [CrossRef]
- Zerrouki, D.; Henni, A. Outdoor microalgae cultivation for wastewater treatment. In Application of Microalgae in Wastewater Treatment; Springer: Cham, Switzerland, 2019; pp. 81–99. [Google Scholar]
- Kim, B.H.; Choi, J.E.; Cho, K.; Kang, Z.; Ramanan, R.; Moon, D.G.; Kim, H.S. Influence of water depth on microalgal production, biomass harvest, and energy consumption in high rate algal pond using municipal wastewater. J. Microbiol. Biotechnol. 2018, 28, 630–637. [Google Scholar] [CrossRef]
- Craggs, R.; Park, J.; Heubeck, S.; Sutherland, D. High rate algal pond systems for low-energy wastewater treatment, nutrient recovery and energy production. N. Z. J. Bot. 2014, 52, 60–73. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, Z.; Tu, X.; Wu, K.; Chen, G.; Chai, X.S. In-situ determination of the observed yield coefficient of aerobic activated sludge by headspace gas chromatography. J. Chromatogr. A 2020, 1610, 460560. [Google Scholar] [CrossRef]
- Bordel, S.; Guieysse, B.; Munoz, R. Mechanistic model for the reclamation of industrial wastewaters using algal− bacterial photobioreactors. Environ. Sci. Technol. 2009, 43, 3200–3207. [Google Scholar] [CrossRef] [PubMed]
- Berthold, D.E.; Shetty, K.G.; Jayachandran, K.; Laughinghouse, H.D., IV; Gantar, M. Enhancing algal biomass and lipid production through bacterial co-culture. Biomass Bioenerg. 2019, 122, 280–289. [Google Scholar] [CrossRef]
- Kumsiri, B.; Pekkoh, J.; Pathom-aree, W.; Lumyong, S.; Pumas, C. Synergistic effect of co-culture of microalga and actinomycete in diluted chicken manure digestate for lipid production. Algal Res. 2018, 33, 239–247. [Google Scholar] [CrossRef]
- Toyama, T.; Hanaoka, T.; Yamada, K.; Suzuki, K.; Tanaka, Y.; Morikawa, M.; Mori, K. Enhanced production of biomass and lipids by Euglena gracilis via co-culturing with a microalga growth-promoting bacterium, Emticicia sp. EG3. Biotechnol. Biofuels 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Molina, D.; de Carvalho, J.C.; Júnior, A.I.M.; Faulds, C.; Bertrand, E.; Soccol, C.R. Biological contamination and its chemical control in microalgal mass cultures. Appl. Microbiol. Biotechnol. 2019, 103, 9345–9358. [Google Scholar] [CrossRef]
- Ni, Z.Y.; Li, J.Y.; Xiong, Z.Z.; Cheng, L.H.; Xu, X.H. Role of granular activated carbon in the microalgal cultivation from bacteria contamination. Bioresour. Technol. 2018, 247, 36–43. [Google Scholar] [CrossRef]
- Solimeno, A.; Parker, L.; Lundquist, T.; García, J. Integral microalgae-bacteria model (BIO_ALGAE): Application to wastewater high rate algal ponds. Sci. Total Environ. 2017, 601, 646–657. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Li, Y.; Pei, H. Algal–bacterial consortia for bioproduct generation and wastewater treatment. Renew. Sustain. Energy Rev. 2021, 149, 111395. [Google Scholar] [CrossRef]
| S.No. | Algal Species Used | Pre-Culture | Cultivation Conditions | Type of Wastewater | Source of Wastewater | Target Pollutant/Physicochemical Characteristics | Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | Chlorella sorokiniana, Chlorella vulgaris, Scenedesmus obliquus | 0.04 g drybiomss/L | In WPW 27 ± 2 °C with a photoperiod of 16 h light: 8 h dark | Meat processing wastewater | Beef packaging plant in Nebraska, USA | COD | 91 | [53] |
| TN | 67 | |||||||
| TP-PO43− | 69 | |||||||
| 2. | Chlorella saccharophila, Chlamydomonas pseudococcum, Scenedesmus sp., Neochloris oleoabundans | 0.1 g L−1 | In BG-11 at light intensity of 80 mmol m−2 s−1 at 30 °C with 12 h light: 12 h dark for two weeks | Dairy wastewater | Dairy farm in Perlis, Malaysia | BOD | 82.60 to 83.14 | [55] |
| COD | 88.90 to 89.02 | |||||||
| TSS | 86.25 to 76.16 | |||||||
| TDS | 77.23 to 80.40 | |||||||
| TKN | 98.33 to 97.83 | |||||||
| NH4-N | 99.61 to 98.00 | |||||||
| NO3-N | 96.97 to 89.93 | |||||||
| PO4-P | 93.02 to 88.84 | |||||||
| 3. | Tetraselmis sp. | - | 24 h of illumination under constant aeration with a flow of 1 L min−1 | Tannery wastewater | Leather finishing stage in Novo Hamburgo, Brazil | TN | 71.74 | [106] |
| P-PO4 | 97.64 | |||||||
| TOC | 31.35 | |||||||
| COD | 56.70 | |||||||
| BOD | 20.68 | |||||||
| NH3 | 100 | |||||||
| 4. | Coelastrum microporum | 4.0 g/L | Light intensity of 120 µmol m−2 s−1 with 12 h light: 12 h dark at 25 °C in PBR with aeration at 0.2 vvm by globular stone. | Activated sludge (primary influent to the WWTP) | Daejeon Metropolitan City Facilities Management Corporation in Daejeon, Korea | TDN | 97.0 | [56] |
| TDP | 98.3 | |||||||
| SCOD | 77.1 | |||||||
| 5. | Tetradesmus sp., Scenedesmus sp. and Ascomycota sp. | - | In BBM at room temperature, pH 8 with light intensity of 20 µmol m−2 s−1 in PBR | Tannery wastewater | Tannery industry in Mexico | Cr (III) | 99 | [54] |
| 6. | Scenedesmus sp., other species of green algae, Cyanobacteria, diatoms | Mixed Culture (93 ± 2%; 4 ± 1%; 2 ± 1%; 1 ± 0.01%) respectively. | Light intensity of 220 μmol m−2 s−1 at 27 ± 2 °C with a photoperiod of 12 h light: 12 h dark in PBR at Ph 8.5 | Digestate and secondary effluent | Lab-scale microalgae anaerobic digester and secondary settler treating urban wastewater | TN | 58 | [107] |
| TP | 83 | |||||||
| TOC | 85 | |||||||
| 7. | Chlorella, Scenedesmus, Chaetophora and Navicula | - | The microalgae were grown in PBR under natural light and temperature | Urban wastewater | Águas da Figueira (AdF, Figueira da Foz, PT) | NH4+ | 99.5 | [108] |
| P | 100 | |||||||
| COD | 40.64 | |||||||
| 8. | Chlorella vulgaris, Chlorella protothecoides | 40 g/L | In PBR with 1000 L of de-chlorinated tap water and 10 g of synthetic fertilizer at pH 8.8 under 27 to 28 °C | Municipal Wastewater | WWTP in South Africa | TN | 35.4 | [109] |
| TP | 74.4 | |||||||
| TOC | 22.2 | |||||||
| COD | 60.0 | |||||||
| Orthophosphate | 87.0 | |||||||
| 9. | Chlorella sp., Merismopedia sp., Closteriopsis sp., Scenedesmus sp. | 10 mL/100 mL | In BG-11 medium at 25 ± 2 °C with a light intensity of 4.5 Klux | Gray water | Drainage line at IIT Delhi, India | TDP | 98.28 | [110] |
| TAN | 88.23 | |||||||
| NO3-N | 86.55 | |||||||
| COD | 82.45 | |||||||
| 10. | Chlorella vulgaris, Chlorella protothecoides | 40 g/L | In PBR with 1000 L of de-chlorinated tap water and 10 g of synthetic fertilizer at pH 9.1 under 29 to 31 °C | Municipal Wastewater | WWTP in South Africa | TN | 73.1 | [109] |
| TP | 50.0 | |||||||
| TOC | 54.0 | |||||||
| COD | 6.6 | |||||||
| Orthophosphate | 83.0 | |||||||
| 11. | Scenedesmus quadricauda, Euglena gracilis, Chlorella vulgaris, Ankistrodesmus convolutes, Chlorococcum oviforme | 10% from exponential phase | In BBM constituting 0, 25, 50, 75 and 100% of leachate under 42 μmol photons m−2 s−1 of irradiance with a photoperiod of 12 h light: 12 h dark at 25 ± 1 °C | Landfill leachate | Sanitary landfill in Selangor, Malaysia | NH4-N | 92.01 to 98.73 | [111] |
| COD | 69.41 to 90.97 | |||||||
| PO4-P | 44.93 to 85.97 | |||||||
| 12. | Chlorella sp., Scenedesmus sp., Sphaerocystis sp., Spirulina sp. | 105 cells mL−1 equivalent to DBW 0.13 g/L | In 90 mL of CHU 10 medium inoculated with 10 mL of wastewater at 31 ± 1 °C under 16 h light: 8 h dark with 80 mmol m−2 s−1 and 50% (v/v) of CO2 | Domestic wastewater | Facultative pond at domestic WWTP in Kalyani, India | CO2 | 53 to 100 | [112] |
| PO4-P | 59 | |||||||
| NH4-N | 39 | |||||||
| 13. | Phormidium and Chlorella pyrenoidosa | 300 mL | Initially maintained in ACA and then transferred to slants in BG 11 medium under 70 ± 5 µmol m−2 s−1 at 25 ± 2 °C | Municipal wastewater | Drain in IIT Delhi | COD | 53 ± 2 | [113] |
| TAN | 81 ± 3 | |||||||
| TDP | 75 ± 2 | |||||||
| NO3-N | 87 ± 5 |
| S.No. | Algal Strain Used | Bacterial Strain Used | Way of Cultivation | Reactor Type | Cultivation Conditions | Type of Wastewater | Source of Wastewater | Target Pollutant/Physicochemical Characteristics | Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Scenedesmus obliquus and Chlorella vulgaris | Raoultella terrigena and P. agglomerans | Batch | Pilot-scale PBR | 14.5 L of synthetic medium for OWW+ 1.5 L of consortium with 1012 cells mL−1 and 103 CFU mL−1 of microalgae and bacteria at 25 ± 1 °C, 160 rpm rotation with light intensity of 200 µmol m−2 s−2 for 16:8 h light-dark cycle for 48–72 h | Olive-washing water |
Olive oil factory of Spain | TPC | 90.3 ± 11.4 | [124] |
| COD | 80.7 ± 9.7 | |||||||||
| BOD5 | 97.8 ± 12.7 | |||||||||
| Turbidity | 82.9 ± 8.4 | |||||||||
| Color | 83.3 ± 10.4 | |||||||||
| 2. | Tetraselmis indica | Pseudomonas aeruginosa | Batch | 500 mL Erlenmeyer flasks | Light intensity of 130 µmol/(m2s) with a 16 h/8 h light/dark cycle at 28 °C for 10 days | Dairy wastewater | Kwality Ltd., dairy processing plant in Saharanpur, India | COD | 87.49 | [64] |
| TDN | 83.76 | |||||||||
| TDP | 79.83 | |||||||||
| 3. | Microcystis sp., Oscillatoria sp., Chlorella sp., Scenesdesmus sp., Stigeoclonium sp. | Strain was not specified | Batch | 10 L- PBR | Continuous illumination of 76 µmol m−2 s−1 and 5 L loading with 10% (v/v) diluted landfill leachate at 25 ± 1 °C, 5.0–8.0 mg/L of DO with 6.5–8.5 of pH | Landfill leachate | Northern Cyprus leachate storage tank | TN | 99.4 | [125] |
| P-PO43− | 98.88% to 99.39 | |||||||||
| COD | 90.1 to 92.34 | |||||||||
| Phenol | 99.55 | |||||||||
| 4. | Chlorella pyrenoidosa | Strain was not specified | Batch | 500 mL flasks | 400 mL of municipal wastewater spiked with 0%, 5%, 10%, 15%, 20% of leachate inoculated with 0.05 g L−1, at 25 °C, light intensity of 8000 Lux | Municipal wastewater and landfill leachate | Grit chamber at Quyang Wastewater Plant and Laogang Landfill in Shanghai, China. | NH4+-N | 95 | [126] |
| P | <95 | |||||||||
| 5. | Scenedesmus obliquus | Bacillus megaterium | Batch | 500 mL conical flask | Microalgae and bacteria at a concentration of 3 × 105 cells/mL and 1 × 105 cells/mL in 200 mL of biogas slurry at 25 ± 2 °C with light intensity of 45 µmol/m2/s and light:dark cycle of 14 h:10 h | Biogas slurry | Anaerobic tank of a pig farm in Yantai, Shandong province | COD | 85.98 | [65] |
| TP | 81.03 | |||||||||
| NH4+-N | 65.48 | |||||||||
| 6. | Chlorella sp., Chlamydomonas sp. and Scenedesmus sp. | Strain was not specified | Batch | 1 L of bioreactor | Microalgae–bacteria consortium was prepared at a fixed ratio of 18% culture to wastewater by volume with a light intensity of 120 µE/m2s at room temperature | Municipal wastewater | WWTP in Akaki Kality sub city of Addis Ababa, Ethiopia | TKN | 69 | [127] |
| TP | 59 | |||||||||
| PO43−-P | 73 | |||||||||
| COD | 84 | |||||||||
| BOD5 | 85 | |||||||||
| 7. | Scenedesmus sp. | Strain was not specified | Batch | PBR | Microalgae and activated sludge were mixed in the ratio of 1:0; 0:1; 1:1; 1:3; 1:5 and 3:1 with a constant air injection of 2 L/min at 25 ± 2 ℃ with 12 h:12 h of light-dark cycle at 200 µmol/m2·s at a pH of 7.5 ± 0.5 | Paper pulp Wastewater | Paper pulp industry WWTP in Portugal | COD | 85.50 | [128] |
| PO43−-P | 86 | |||||||||
| NH4+–N | 86.81 | |||||||||
| 8. | Chlorella vulgaris and Scenedesmus obliquus | Proteobacteria, Firmicutes, Bacteroidetes and Chloroflexi | Batch | PBR | Algae:sludge inoculation ratio was 1:1 (w/w) with a light intensity of 40 to 50 µmol.m−2 s−1 at 100 rpm with no pH control and aeration at a flow rate of 15 L h−1. Temperature and photoperiod were 31.2 °C (light): 20.5 °C (dark) of a 14.2 h: 9.8 h light/dark cycle and 25.8 °C (light): 16.9 °C (dark) of a 12.4 h: 11.6 h light/dark cycle | Municipal wastewater | Aerated grit chamber in third sewage treatment plant of China | COD | 93.7 ± 0.9 | [129] |
| NH4+ | 100.0 ± 0.0 | |||||||||
| PO43− | 98.4 ± 1.5 | |||||||||
| TSS | 96.3 ± 2.1 | |||||||||
| 9. | Chlorella vulgaris | Exiguobacterium and B. licheniformis | Batch | 1.0 L columnar PBR | Algae:bacteria inoculation ratio were 1:0:0; 1:2:0; 1:0:2; 1:1:1 and the amounts of Chlorella and bacteria were 6.8 × 106 cells mL−1 and 13.6 × 106 CFU mL−1 with a light intensity of 120.0 µmol photons m−2 s−1 at temperature 25.0 ± 1.0 °C with 0.3 L m−1 of ventilation rate | Piggery wastewater | Yantai Longda Breeding Co., Ltd., in Yantai, Shandong | TN | 78.3 | [67] |
| TP | 87.2 | |||||||||
| NH4+-N | 84.4 | |||||||||
| COD | 86.3 | |||||||||
| 10. | Chlamydomonas reinhardtii, Chlorella vulgari and Scenedesmus obliquus | Strain was not specified | Batch | 2 L glass bottles with 3 ports lids (air inlet and outlet ports topped with 0.45 µm filter to avoid contamination and sampling port) | Algal concentration was 0.20–0.25 g/L in 1.8 L of brewery wastewater at 20 °C with light intensity 70 µmol photons m−2s−1 with 12 h light/12 h dark and 100 rpm consistent mixing | Brewery wastewater | __ | COD | >85 | [130] |
| TN | >80 | |||||||||
| TP | >70 | |||||||||
| 11. | Chlorellaceae sp., Scenedesmaceae sp., Chlamydomonadaceae sp. | Strain was not specified | Batch | Outdoor high-rate algal pond (or raceway pond) | Microalgal species at a concentration of 1.106 cells.mL−1; 0.2 × 106 cells.mL−1 and 0.2 × 106 cells mL−1 in 500 L of piggery wastewater and 340 L of tap water | Piggery wastewater | Piggery farm in Cremona Province (Po Valley, Northern Italy) | NH4+-N Orthophosphate COD | 90 90 59 | [131] |
| 12. | Chlorella sp. | Acinetobacter sp. | Batch | Pilot-scale bioreactor | Algal cells with a density of 0.275 ± 0.025 g/L were inoculated in 100 mL of centrate wastewater at a light intensity 120 ± 10 µmol photons m−2s−1 at 25 ± 1 °C with relative humidity 45 ± 3% at 200 rpm | Centrate wastewater | Municipal WWTP in St. Paul (Minnesota, USA) | COD | 93.01 | [132] |
| TP | 98.78 | |||||||||
| 13. | Chlorella sp. | Bacillus firmus and Beijerinckia fluminensis | Batch | 500 mL Erlenmeyer flasks | Concentration of algae and bacteria were 1.0 × 105 cells/mL and 1% (v/v) or 10% (v/v) at 26 °C with light intensity of 50 ± 10 µmol/(m2s) in 200 mL of vinegar fermentation wastewater | Vinegar production wastewater | Hengshun Vinegar Industry Co., Ltd., Zhenjiang, Jiangsu, China | COD | 22.1 | [133] |
| TN | 20.0 | |||||||||
| TP | 18.1 |
| S.No. | Algal Strain Used | Fungal Strain Used | Type of Wastewater | Source of Wastewater | Target Pollutant/Physicochemical Characteristics | Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Chlorella pyrenoidosa | Rhodosporidium toruloides | Rice wine distillery wastewater and domestic wastewater | S1 distillery in Foshan, China and local wastewater treatment plant in Macau, China | SCOD | 95.34 ± 0.07 | [82] |
| TN | 51.18 ± 2.17 | ||||||
| TP | 89.29 ± 4.91 | ||||||
| 2. | Scenedesmus obliquus | Trichoderma reesei | Municipal wastewater | Effluent of a treatment plant in Mexico | Nitrate TAN Orthophosphate | 96 100 93 | [137] |
| 3. | Chlorella vulgaris | Aspergillus sp. | Swine manure wastewater | Umore Park (Rosemount, MN, USA) | Ammonium | 23.23 | [83] |
| TN | 44.68 | ||||||
| TP | 84.70 | ||||||
| COD | 70.34 | ||||||
| 4. | Chlorella vulgaris | Ganoderma lucidum | Biogas slurry | Anaerobic digestion reactor in a livestock WWTP in Jiaxing pig farm, Zhejiang, China | COD | 92.17 ± 5.28 | [93] |
| TN | 89.83 ± 4.36 | ||||||
| TP | 90.31 ± 4.69 | ||||||
| CO2 | 74.26 ± 3.14 | ||||||
| 5. | Chlorella vulgaris | Aspergillus Niger | Artificially prepared wastewater | __ | Ranitidine | 50 ± 19 | [138] |
| 6. | Chlorella vulgaris | Aspergillus oryzae | Artificially prepared wastewater | __ | Arsenic | 51.14 | [102] |
| 7. | Scenedesmus sp. | Trichoderma reesei | Secondary effluent | Seafood processing plant | COD | >74 | [78] |
| TN | >44 | ||||||
| TP | >93 | ||||||
| 8. | Tetradesmus obliquus | Aspergillus niger | Gold mining wastewater | Tailing of Sibanye Stillwater in South Africa | Gold | 97.77 | [139] |
| 9. | Chlorella vulgaris | Aspergillus sp. | Molasses wastewater | Local plant in Guangzhou, China | Color | 69.98 | [140] |
| COD | 70.68 | ||||||
| TP | 88.39 | ||||||
| TN | 67.09 | ||||||
| NH3-N | 94.72 | ||||||
| 10. | Chlorella vulgaris | Ganoderma lucidum | Biogas slurry | __ | COD | 70 | [141] |
| TN | 75 | ||||||
| TP | 78 | ||||||
| 11. | Chlorella vulgaris | Ganoderma lucidum | Biogas slurry | Anaerobic digester in Hongmao Hacienda, Kunshan City, China | COD | 68.29 | [141] |
| TN | 61.75 | ||||||
| TP | 64.21 | ||||||
| CO2 | 64.68 | ||||||
| 12. | Chlorella vulgaris | Ganoderma lucidum | Anaerobically digested swine wastewater | Anaerobic digestion reactor in a livestock WWTP of pig farm in Jiaxing, Zhejiang, China | COD | 79.74 ± 4.87 | [142] |
| TN | 74.28 ± 6.13 | ||||||
| TP | 85.37 ± 6.84 | ||||||
| 13. | Chlorella sorokiniana | Aspergillus niger | Municipal wastewater | Prem Nagar sewer system, Dehradun, Uttarakhand, India | TKN | 95.40 | [143] |
| BOD | 81.78 | ||||||
| COD | 83.67 | ||||||
| TOC | 70.26 | ||||||
| 14. | Scenedesmus abundans | Saccharomyces Cerevisiae | Dairy wastewater | Graphic Era University dairy, Uttarakhand, India | TN | 41.7 | [144] |
| TP | 60.9 | ||||||
| COD | 83 | ||||||
| BOD | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gururani, P.; Bhatnagar, P.; Kumar, V.; Vlaskin, M.S.; Grigorenko, A.V. Algal Consortiums: A Novel and Integrated Approach for Wastewater Treatment. Water 2022, 14, 3784. https://doi.org/10.3390/w14223784
Gururani P, Bhatnagar P, Kumar V, Vlaskin MS, Grigorenko AV. Algal Consortiums: A Novel and Integrated Approach for Wastewater Treatment. Water. 2022; 14(22):3784. https://doi.org/10.3390/w14223784
Chicago/Turabian StyleGururani, Prateek, Pooja Bhatnagar, Vinod Kumar, Mikhail S. Vlaskin, and Anatoly V. Grigorenko. 2022. "Algal Consortiums: A Novel and Integrated Approach for Wastewater Treatment" Water 14, no. 22: 3784. https://doi.org/10.3390/w14223784






