Geochemical Characteristics and Their Environmental Implications for the Water Regime of Hulun Lake, Inner Mongolia, China
Abstract
1. Introduction
2. Materials and Methods
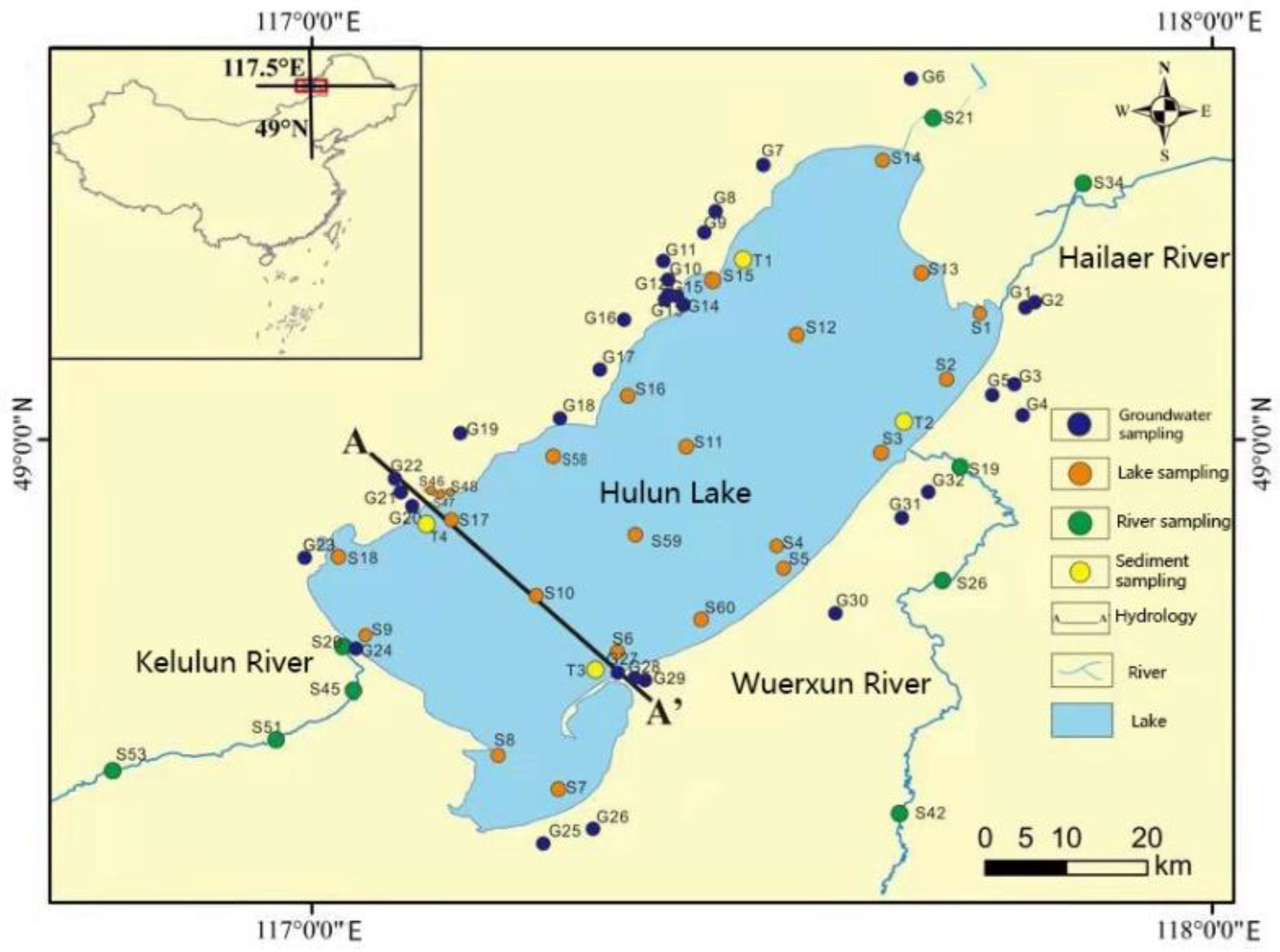
2.1. Sampling
2.2. Sample Treatment and Analysis
2.3. Quality Assurance and Data Analysis
3. Results and Discussion
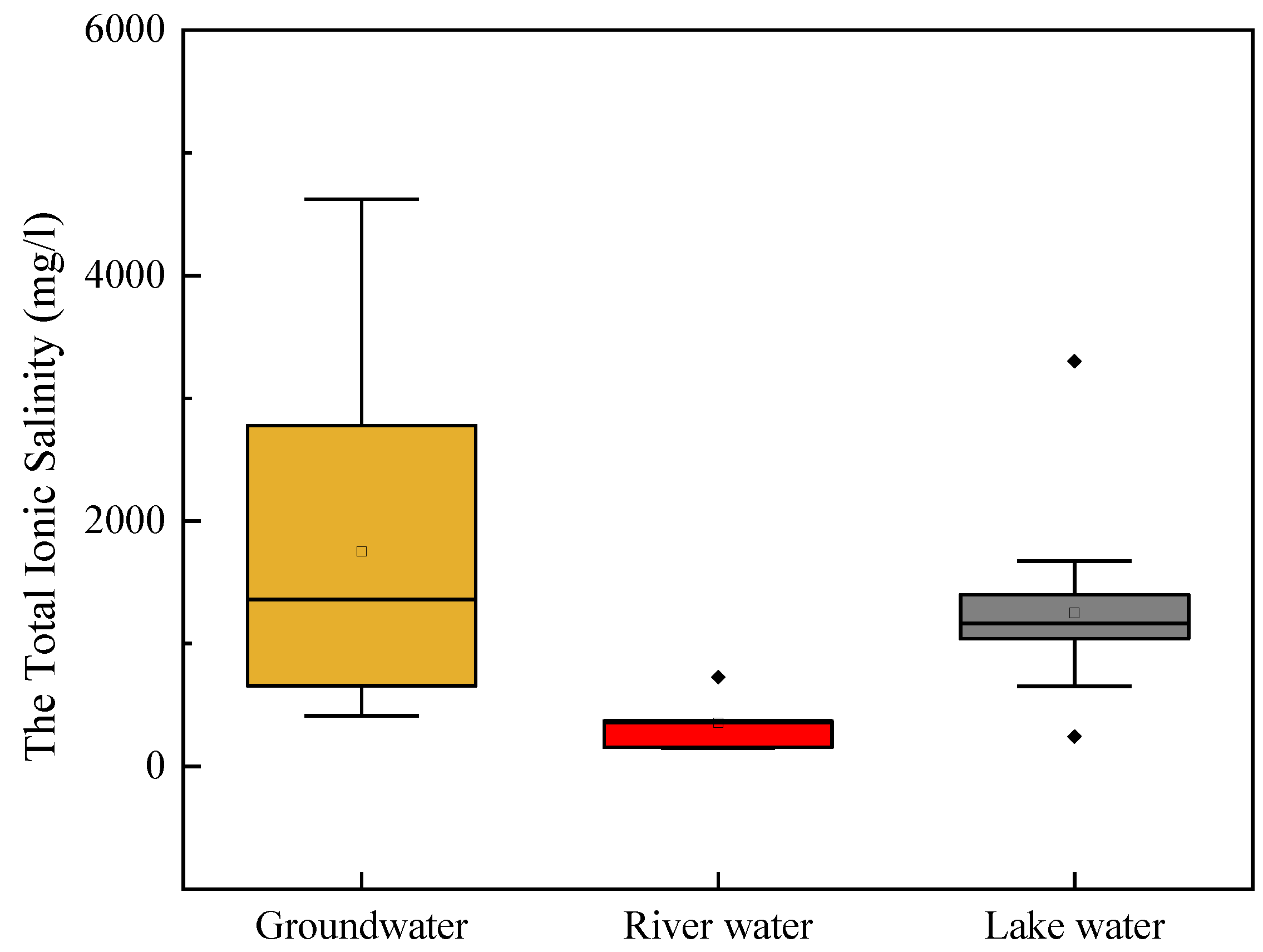
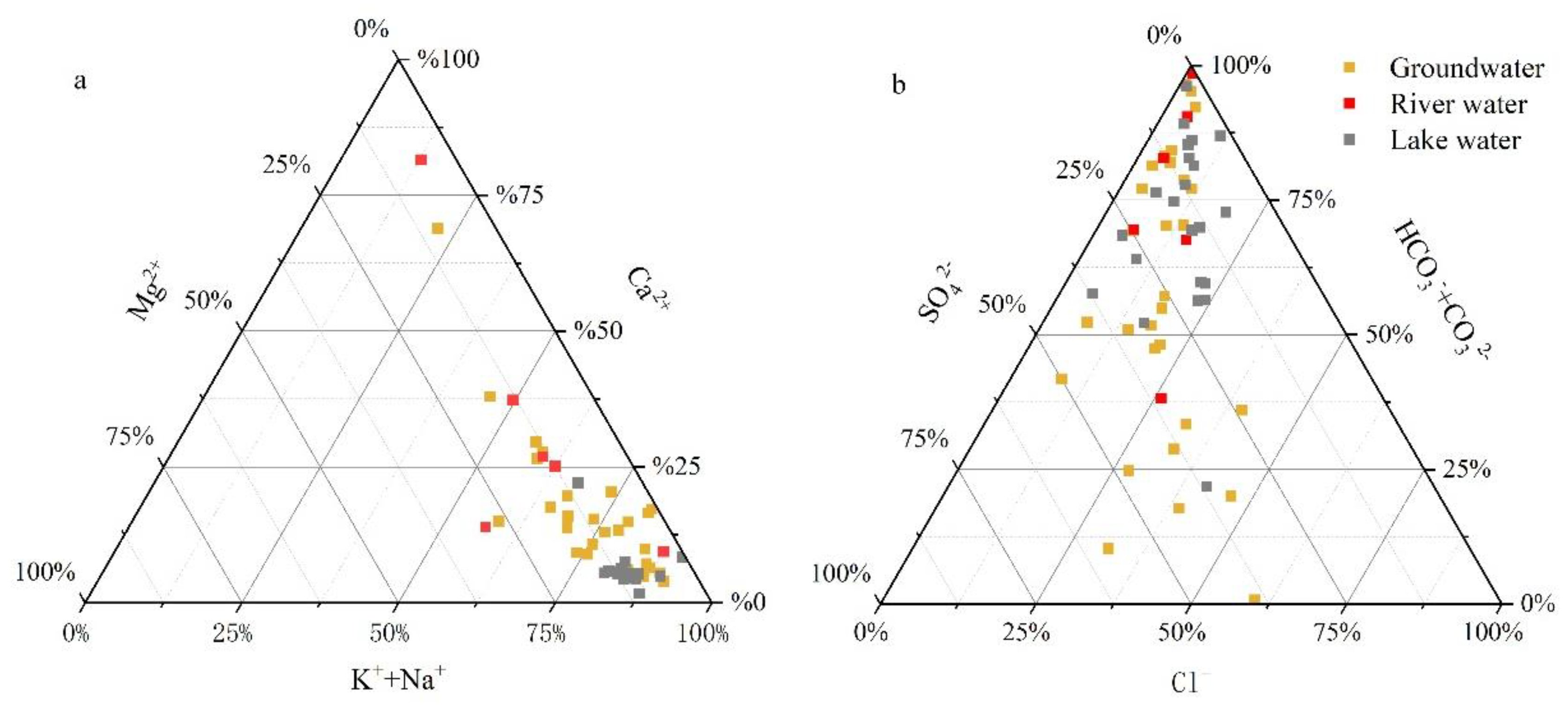
3.1. Water Chemistry
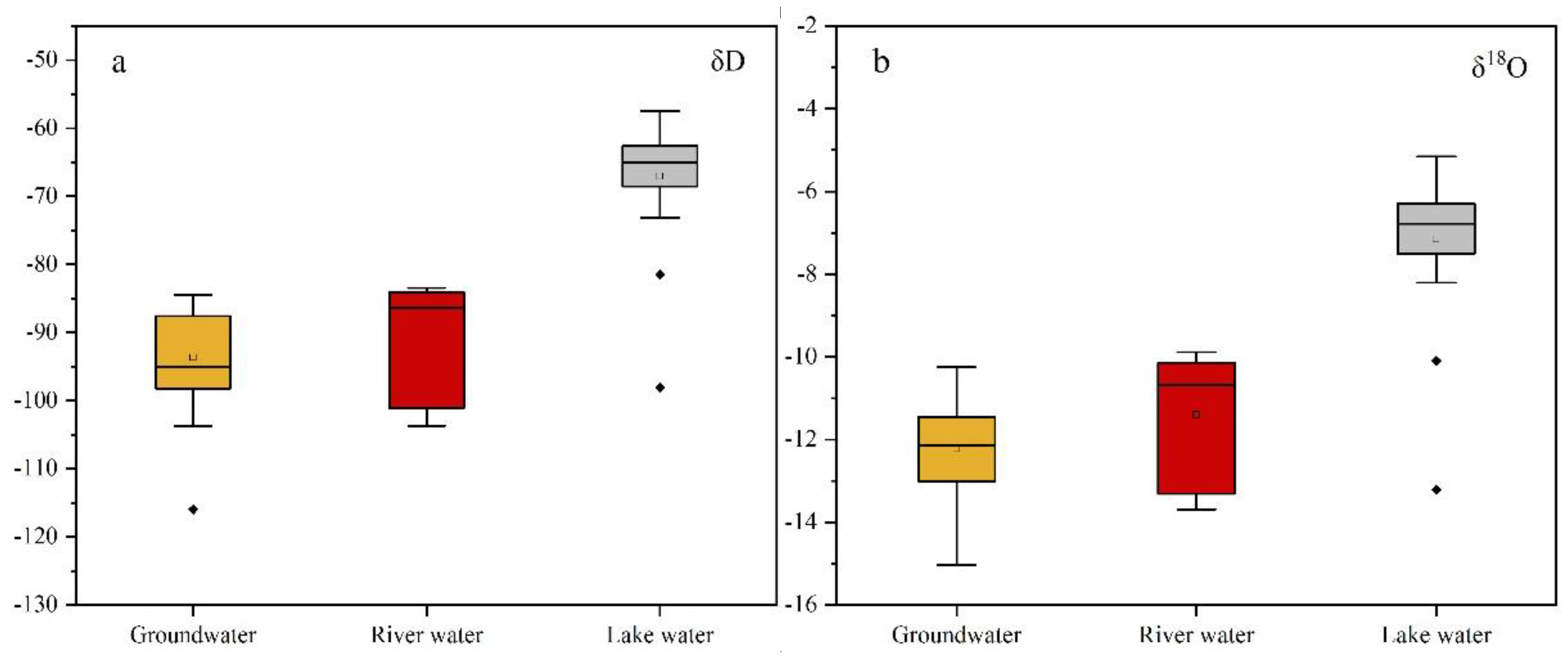
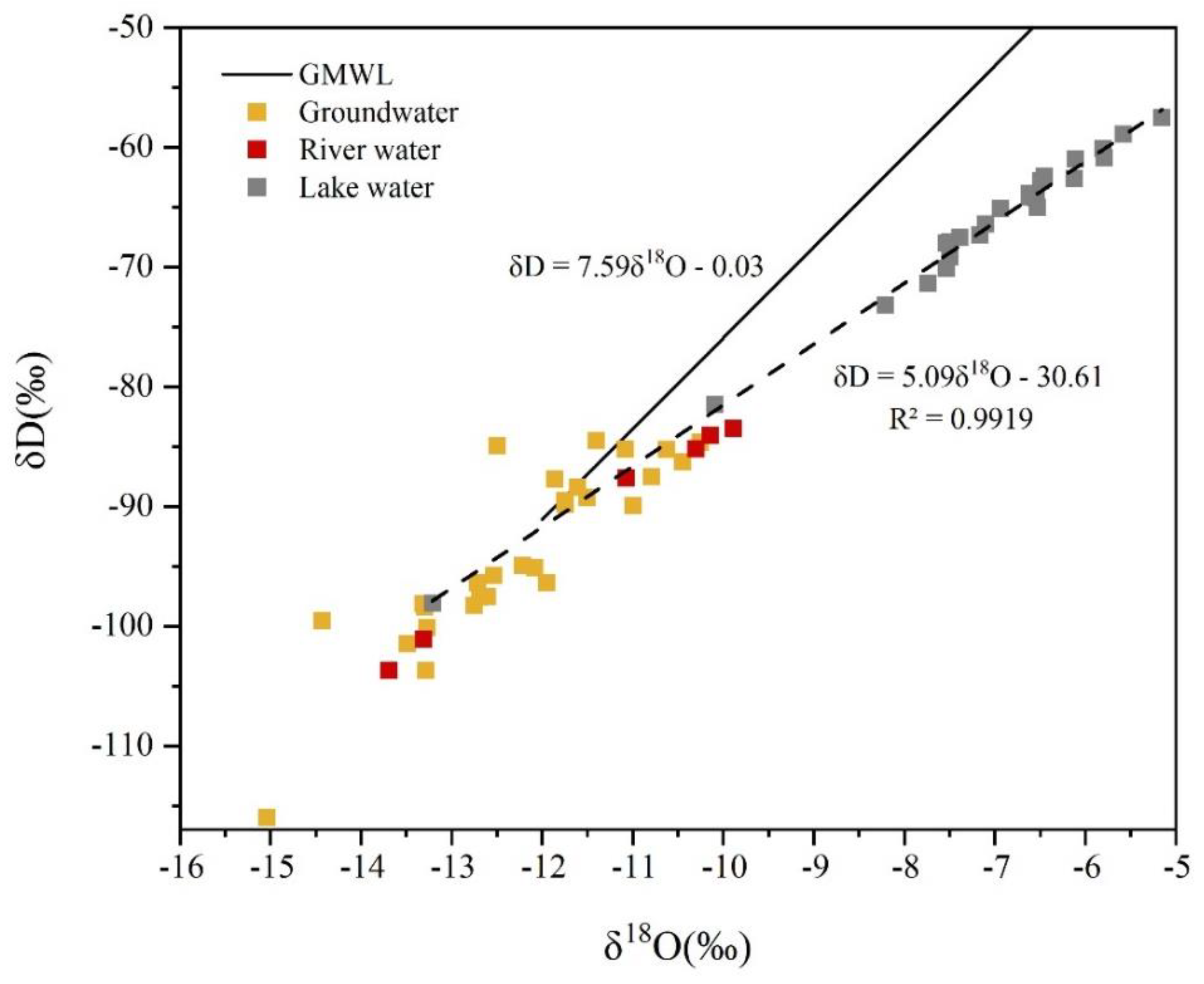
3.2. δD and δ18O
3.3. DOM
3.4. Implications of the Water Geochemstry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, H.; Qian, H.; Zheng, L.; Feng, W.; Wang, H.; Gao, Y. Alterations to groundwater chemistry due to modern water transfer for irrigation over decades. Sci. Total Environ. 2020, 717, 137170. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zou, J.; Liu, J.; Zhang, J.; Zhen, P. Factors controlling groundwater chemical evolution with the impact of reduced exploitation. CATENA 2022, 214, 106261. [Google Scholar] [CrossRef]
- Johansson, K.; Wilander, A.; Fölster, J.; Göransson, E. Synchronous variation in water chemistry for 80 lakes in Southern Sweden. Environ. Monit. Assess. 2005, 102, 389–403. [Google Scholar] [CrossRef]
- Tao, Y.; Yuan, Z.; Fengchang, W.; Wei, M. Six-Decade Change in Water Chemistry of Large Freshwater Lake Taihu, China. Environ. Sci. Technol. 2013, 47, 9093–9101. [Google Scholar] [CrossRef]
- Fuoco, I.; Marini, L.; De Rosa, R.; Figoli, A.; Gabriele, B.; Apollaro, C. Use of reaction path modelling to investigate the evolution of water chemistry in shallow to deep crystalline aquifers with a special focus on fluoride. Sci. Total Environ. 2022, 830, 154566. [Google Scholar] [CrossRef]
- Susheela, A.K.; Mudgal, A.; Keast, G. Fluoride in water: An overview. Water Front 1999, 1, 11–13. [Google Scholar]
- Missi, C.; Atekwana, E.A. Physical, chemical and isotopic characteristics of groundwater and surface water in the Lake Chilwa Basin, Malawi. J. Afr. Earth Sci. 2019, 162, 103737. [Google Scholar] [CrossRef]
- Vaiphei, S.P.; Kurakalva, R.M.; Sahadevan, D.K. Water quality index and GIS-based technique for assessment of groundwater quality in Wanaparthy watershed, Telangana, India. Environ. Sci. Pollut. Res. 2020, 27, 4504–45062. [Google Scholar] [CrossRef]
- Apollaro, C.; Di Curzio, D.; Fuoco, I.; Buccianti, A.; Dinelli, E.; Vespasiano, G.; Castrignanò, A.; Rusi, S.; Barca, D.; Figoli, A.; et al. A multivariate non-parametric approach for estimating probability of exceeding the local natural background level of arsenic in the aquifers of Calabria region (Southern Italy). Sci. Total Environ. 2021, 806, 150345. [Google Scholar] [CrossRef]
- Bertilsson, S.; Tranvik, L.J. Photochemical transformation of dissolved organic matter in lakes. Limnol. Oceanogr. 2000, 45, 753–762. [Google Scholar] [CrossRef]
- Liu, D.; Du, Y.; Yu, S.; Luo, J.; Duan, H. Human activities determine quantity and composition of dissolved organic matter in lakes along the Yangtze River. Water Res. 2019, 168, 115132. [Google Scholar] [CrossRef] [PubMed]
- Al-Kharusi, E.S.; Tenenbaum, D.E.; Abdi, A.M.; Kutser, T.; Karlsson, J.; Bergström, A.-K.; Berggren, M. Large-Scale Retrieval of Coloured Dissolved Organic Matter in Northern Lakes Using Sentinel-2 Data. Remote Sens. 2020, 12, 157. [Google Scholar] [CrossRef]
- A Stedmon, C.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Hudson, N.; Baker, A.; Reynolds, D.M. Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters—A review. River Res. Appl. 2007, 23, 631–649. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, Y.; Wang, X.C.; Wang, Y.; Dzakpasu, M. Characterization and biogeochemical implications of dissolved organic matter in aquatic environments. J. Environ. Manag. 2021, 294, 113041. [Google Scholar] [CrossRef]
- Ye, Z.; Chen, S.; Zhang, Q.; Liu, Y.; Zhou, H. Ecological Water Demand of Taitema Lake in the Lower Reaches of the Tarim River and the Cherchen River. Remote Sens. 2022, 14, 832. [Google Scholar] [CrossRef]
- Ayenew, T.; Gebreegziabher, Y. Application of a spreadsheet hydrological model for computing the long-term water balance of Lake Awassa, Ethiopia. Hydrol. Sci. J. 2006, 51, 418–431. [Google Scholar] [CrossRef]
- Tweed, S.; Leblanc, M.; Cartwright, I.; Favreau, G.; Leduc, C. Arid zone groundwater recharge and salinisation processes; an example from the Lake Eyre Basin, Australia. J. Hydrol. 2011, 408, 257–275. [Google Scholar] [CrossRef]
- Yang, K.; Han, G. Controls over hydrogen and oxygen isotopes of surface water and groundwater in the Mun River catchment, northeast Thailand: Implications for the water cycle. Appl. Hydrogeol. 2020, 28, 1021–1036. [Google Scholar] [CrossRef]
- Che, Q.; Su, X.; Zheng, S.; Li, Y. Interaction between surface water and groundwater in the Alluvial Plain (anqing section) of the lower Yangtze River Basin: Environmental isotope evidence. J. Radioanal. Nucl. Chem. Artic. 2021, 329, 1331–1343. [Google Scholar] [CrossRef]
- Doctor, D.H.; Petrič, M.; Kogovšek, J.; Urbanc, J.; Lojen, S.; Stichler, W. Quantification of karst aquifer discharge components during storm events through end-member mixing analysis using natural chemistry and stable isotopes as tracers. Hydrogeol. J. 2006, 14, 1171–1191. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, E.; Yin, Y.; Van Dijk, M.A.; Feng, L.; Shi, Z.; Liu, M.; Qina, B. Characteristics and sources of chromophoric dissolved organic matter in lakes of the Yungui Plateau, China, differing in trophic state and altitude. Limnol. Oceanogr. 2010, 55, 2645–2659. [Google Scholar] [CrossRef]
- Rasmus, B. PARAFAC. Tutorial and applications. Chemom. Intell. Lab. Syst. 1997, 38, 149–171. [Google Scholar]
- Ishii, S.K.L.; Boyer, T.H. Behavior of Reoccurring PARAFAC Components in Fluorescent Dissolved Organic Matter in Natural and Engineered Systems: A Critical Review. Environ. Sci. Technol. 2012, 46, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef]
- Zhang, J.; Song, F.; Li, T.; Xie, K.; Yao, H.; Xing, B.; Li, Z.; Bai, Y. Simulated photo-degradation of dissolved organic matter in lakes revealed by three-dimensional excitation-emission matrix with regional integration and parallel factor analysis. J. Environ. Sci. 2019, 90, 310–320. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Liu, W.-Q.; Zhao, N.-J.; Li, H.-B.; Zhang, Y.-J.; Si-Ma, W.-C.; Liu, J.-G. Composition analysis of colored dissolved organic matter in Taihu Lake based on three dimension excitation-emission fluorescence matrix and PARAFAC model, and the potential application in water quality monitoring. J. Environ. Sci. 2007, 19, 787–791. [Google Scholar] [CrossRef]
- Gao, H.; Ryan, M.C.; Li, C.; Sun, B. Understanding the Role of Groundwater in a Remote Transboundary Lake. Water 2017, 9, 363. [Google Scholar] [CrossRef]
- Zheng, J.; Ke, C.; Shao, Z.; Li, F. Monitoring changes in the water volume of Hulun Lake by integrating satellite altimetry data and Landsat images between 1992 and 2010. J. Appl. Remote Sens. 2016, 10, 16029. [Google Scholar] [CrossRef]
- El-Dars, F.M.S.E.; Salem, W.A.; Fahim, M.M.; Taha, M.M.N. A Comparative Evaluation of Soil Characteristics at the 10th of Ramadan and El-Gabal El-Asfar, Egypt. Chem. Afr. 2022, 5, 1611–1626. [Google Scholar] [CrossRef]
- Sun, Z.; Soldatova, E.; Guseva, N.; Shvartsev, S. Impact of Human Activity on the Groundwater Chemical Composition of the South Part of the Poyang Lake Basin. IERI Procedia 2014, 8, 113–118. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation–emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Pitta, E.; Zeri, C. The impact of combining data sets of fluorescence excitation—Emission matrices of dissolved organic matter from various aquatic sources on the information retrieved by PARAFAC modeling. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 258, 119800. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.R.; Stedmon, C.A.; Waite, T.D.; Ruiz, G.M. Distinguishing between terrestrial and autochthonous organic matter sources in marine environments using fluorescence spectroscopy. Mar. Chem. 2008, 108, 40–58. [Google Scholar] [CrossRef]
- Du, Y.; Lu, Y.; Roebuck, J.A.; Liu, D.; Chen, F.; Zeng, Q.; Xiao, K.; He, H.; Liu, Z.; Zhang, Y.; et al. Direct versus indirect effects of human activities on dissolved organic matter in highly impacted lakes. Sci. Total Environ. 2020, 752, 141839. [Google Scholar] [CrossRef]
- Yamashita, Y.; Cory, R.M.; Nishioka, J.; Kuma, K.; Tanoue, E.; Jaffé, R. Fluorescence characteristics of dissolved organic matter in the deep waters of the Okhotsk Sea and the northwestern North Pacific Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 1478–1485. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Park, S.E.; Kim, T.-H.; Kim, B.-G.; Kang, D.-J.; Rho, T. Impact of aquaculture on distribution of dissolved organic matter in coastal Jeju Island, Korea, based on absorption and fluorescence spectroscopy. Environ. Sci. Pollut. Res. 2021, 29, 553–563. [Google Scholar] [CrossRef]
- Zhuang, W.-E.; Chen, W.; Cheng, Q.; Yang, L. Assessing the priming effect of dissolved organic matter from typical sources using fluorescence EEMs-PARAFAC. Chemosphere 2020, 264, 128600. [Google Scholar] [CrossRef]
- Jia, F.; Yang, Q.; Liu, X.; Li, X.; Li, B.; Zhang, L.; Peng, Y. Stratification of Extracellular Polymeric Substances (EPS) for Aggregated Anammox Microorganisms. Environ. Sci. Technol. 2017, 51, 3260–3268. [Google Scholar] [CrossRef]
- Garcia, R.D.; Reissig, M.; Queimaliños, C.P.; Garcia, P.E.; Dieguez, M.C. Climate-driven terrestrial inputs in ultraoligotrophic mountain streams of Andean Patagonia revealed through chromophoric and fluorescent dissolved organic matter. Sci. Total Environ. 2015, 521–522, 280–292. [Google Scholar] [CrossRef]
- Soto Cárdenas, C.; Gerea, M.; Garcia, P.E.; Pérez, G.L.; Diéguez, M.C.; Rapacioli, R.; Reissig, M.; Queimaliños, C. Interplay between climate and hydrogeomorphic features and their effect on the seasonal variation of dissolved organic matter in shallow temperate lakes of the Southern Andes (Patagonia, Argentina): A field study based on optical properties. Ecohydrology 2017, 10, e1872. [Google Scholar] [CrossRef]
- Harjung, A.; Sabater, F.; Butturini, A. Hydrological connectivity drives dissolved organic matter processing in an intermittent stream. Limnologica 2018, 68, 71–81. [Google Scholar] [CrossRef]
- Zhang, B.; Song, X.; Zhang, Y.; Han, D.; Yang, L.; Tang, C. Relationship between surface water and groundwater in the second Songhua River basin. Shuikexue Jinzhan/Adv. Water Sci. 2014, 25, 336–347. [Google Scholar]
- Zhan, L.; Chen, J.; Zhang, S.; Li, L.; Huang, D.; Wang, T. Isotopic signatures of precipitation, surface water, and groundwater interactions, Poyang Lake Basin, China. Environ. Earth Sci. 2016, 75, 1307. [Google Scholar] [CrossRef]
- Ratino, S.; Seangmeng, H.; Ratha, D.; Kong, C.; Eam, E.K.; Siev, S.; Thea, S.; Khanal, R.; Yoshimura, C. Groundwater and Surface Water Exchange in the Lake Basin. In Water and Life in Tonle Sap Lake; Yoshimura, C., Khanal, R., Sovannara, U., Eds.; Springer: Singapore, 2022; pp. 81–90. [Google Scholar] [CrossRef]
- Fan, R.; Qian, G.; Li, Y.; Short, M.D.; Schumann, R.C.; Chen, M.; Smart, R.S.C.; Gerson, A.R. Evolution of pyrite oxidation from a 10-year kinetic leach study: Implications for secondary mineralisation in acid mine drainage control. Chem. Geol. 2021, 588, 120653. [Google Scholar] [CrossRef]
- Fuoco, I.; De Rosa, R.; Barca, D.; Figoli, A.; Gabriele, B.; Apollaro, C. Arsenic polluted waters: Application of geochemical modelling as a tool to understand the release and fate of the pollutant in crystalline aquifers. J. Environ. Manag. 2021, 301, 113796. [Google Scholar] [CrossRef]
- Segovia, B.T.; Pereira, D.G.; Bini, L.M.; De Meira, B.R.; Nishida, V.S.; Lansac-Tôha, F.A.; Velho, L.F.M. The Role of Microorganisms in a Planktonic Food Web of a Floodplain Lake. Microb. Ecol. 2014, 69, 225–233. [Google Scholar] [CrossRef]


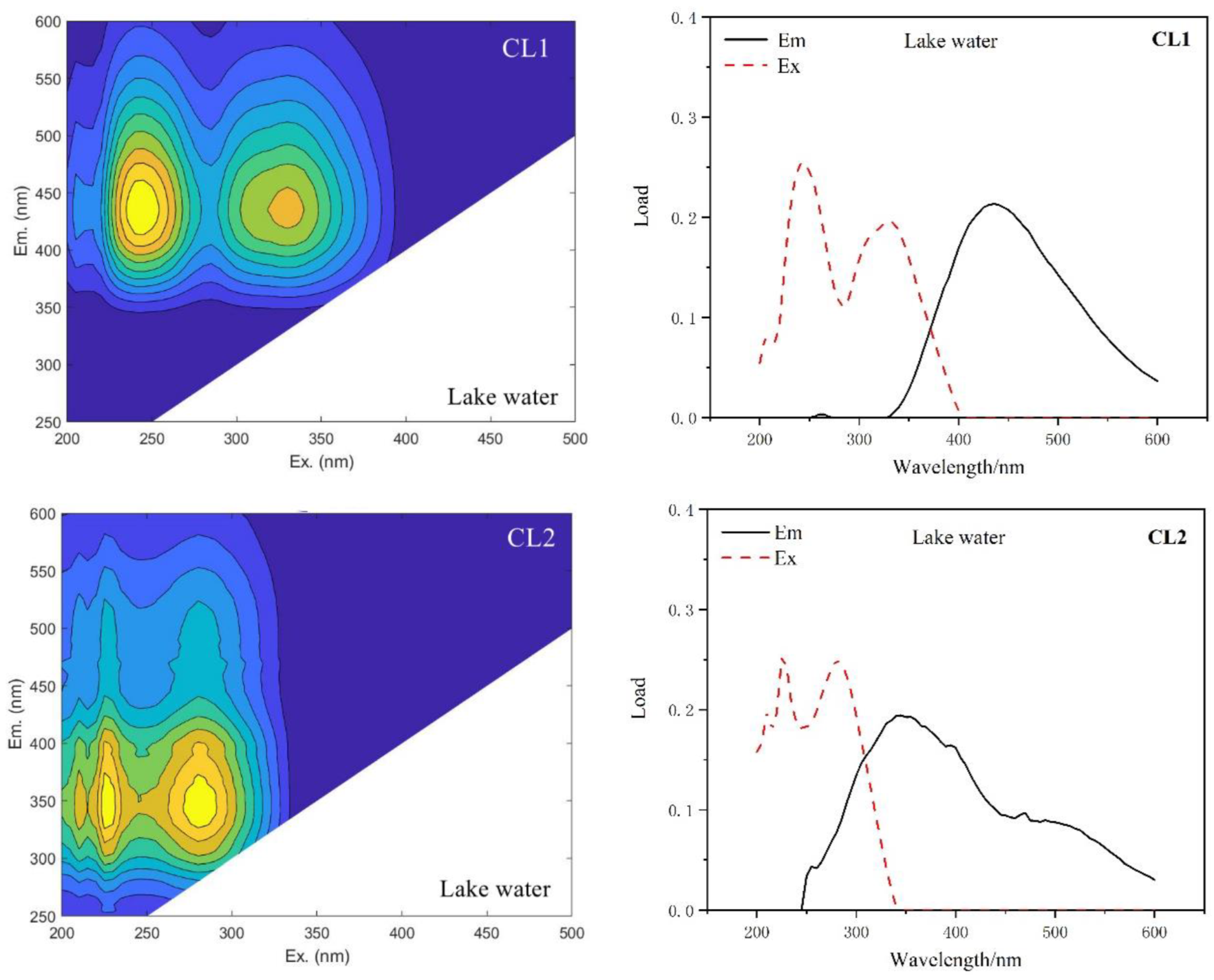
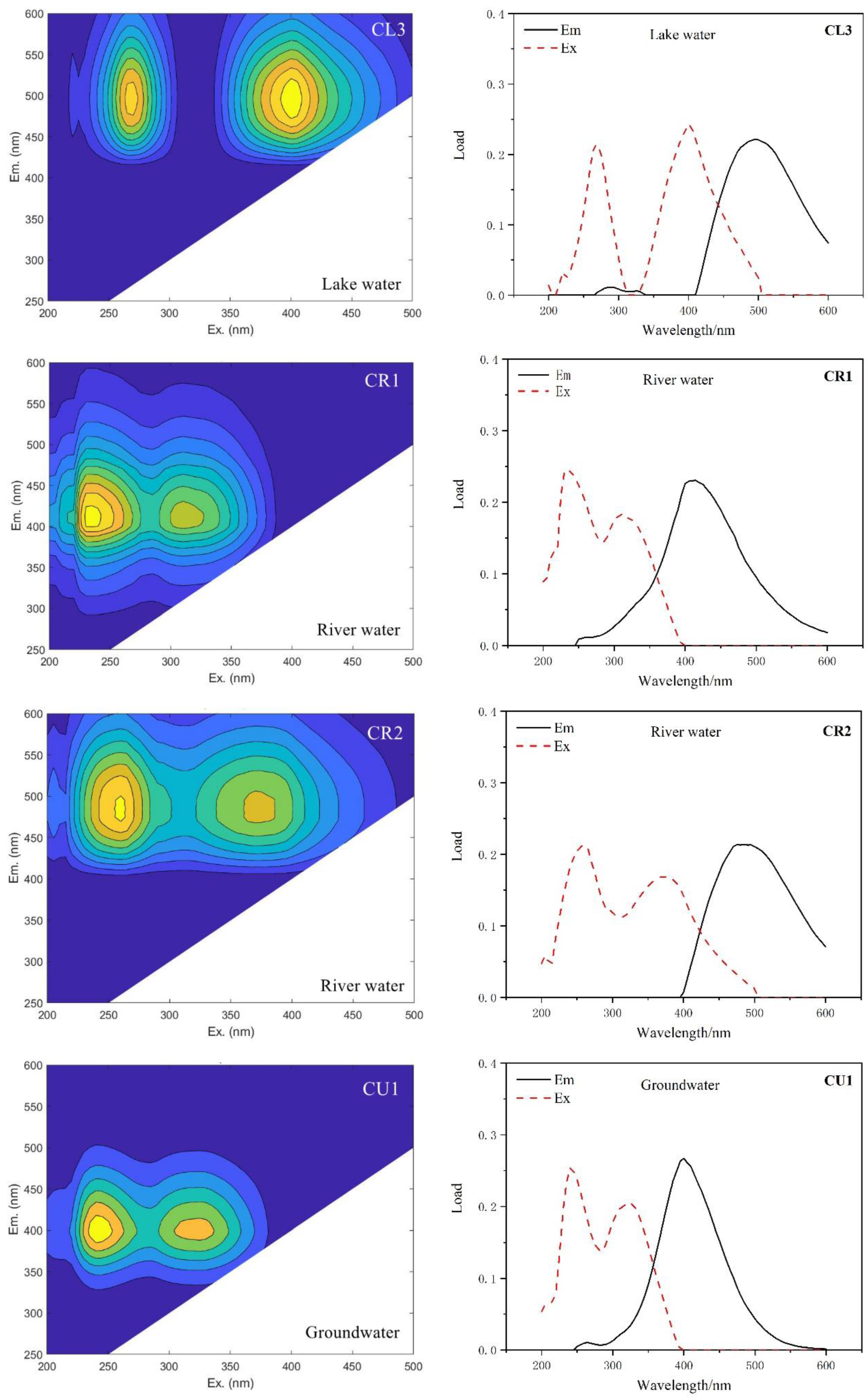

| Type of Water | This Study | Compared with Traditional Peaks | ||
|---|---|---|---|---|
| Component | Ex(max)/Em(max) | Traditional Peaks | Substance Represented | |
| Groundwater | CU1 | 240,325/400 | A, M | Humus-like, aquatic organisms |
| CU2 | 255,365/475 | A, C | Humus-like | |
| River Water | CR1 | 235,310/415 | A, M | Humus-like, aquatic organisms |
| CR2 | 260,370/485 | A, C | Humus-like | |
| Lake Water | CL1 | 250,330/435 | A, C | Humus-like |
| CL2 | 225,280/345 | T | Tryptophan-like, protein-like | |
| CL3 | 270,400/495 | No correspondence | High molecular weight, aromatic group, fulvic acid, etc. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, S.; Xu, W.; Zhang, B.; Yi, L.; Lu, X. Geochemical Characteristics and Their Environmental Implications for the Water Regime of Hulun Lake, Inner Mongolia, China. Water 2022, 14, 3696. https://doi.org/10.3390/w14223696
Zhang Y, Wang S, Xu W, Zhang B, Yi L, Lu X. Geochemical Characteristics and Their Environmental Implications for the Water Regime of Hulun Lake, Inner Mongolia, China. Water. 2022; 14(22):3696. https://doi.org/10.3390/w14223696
Chicago/Turabian StyleZhang, Yan, Shiyu Wang, Weijie Xu, Bo Zhang, Lixin Yi, and Xueqiang Lu. 2022. "Geochemical Characteristics and Their Environmental Implications for the Water Regime of Hulun Lake, Inner Mongolia, China" Water 14, no. 22: 3696. https://doi.org/10.3390/w14223696
APA StyleZhang, Y., Wang, S., Xu, W., Zhang, B., Yi, L., & Lu, X. (2022). Geochemical Characteristics and Their Environmental Implications for the Water Regime of Hulun Lake, Inner Mongolia, China. Water, 14(22), 3696. https://doi.org/10.3390/w14223696







