History of Colonization of Jeju Island (Republic of Korea) by the Water Fleas (Crustacea: Cladocera) Is Reflected by the Seasonal Changes in Their Fauna and Species Associations
Abstract
1. Introduction
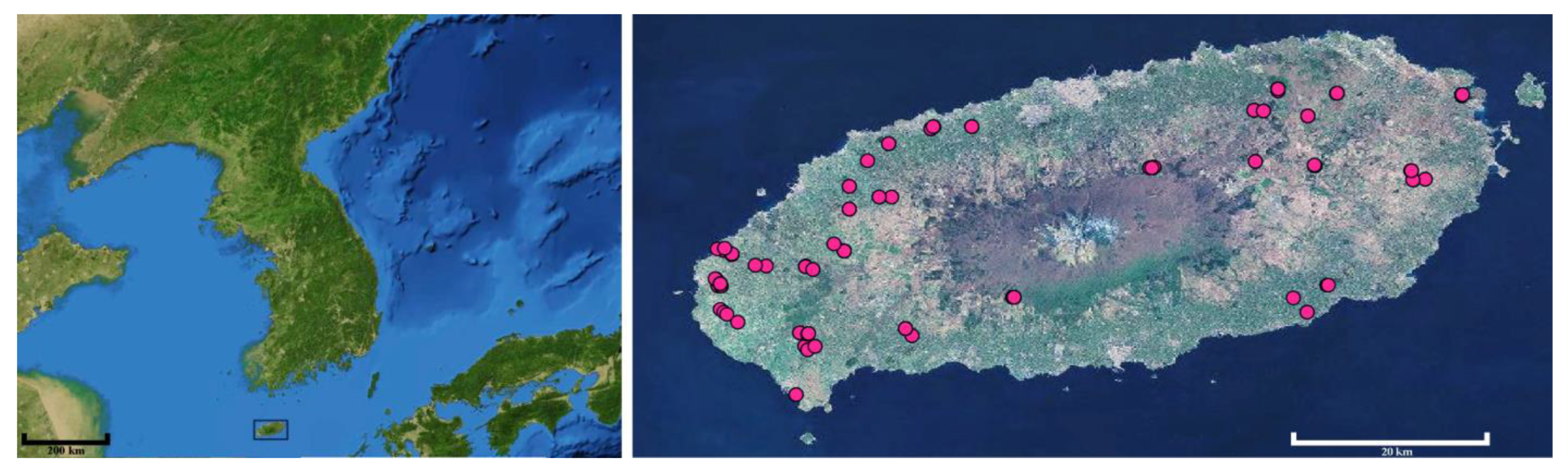
2. Materials and Methods
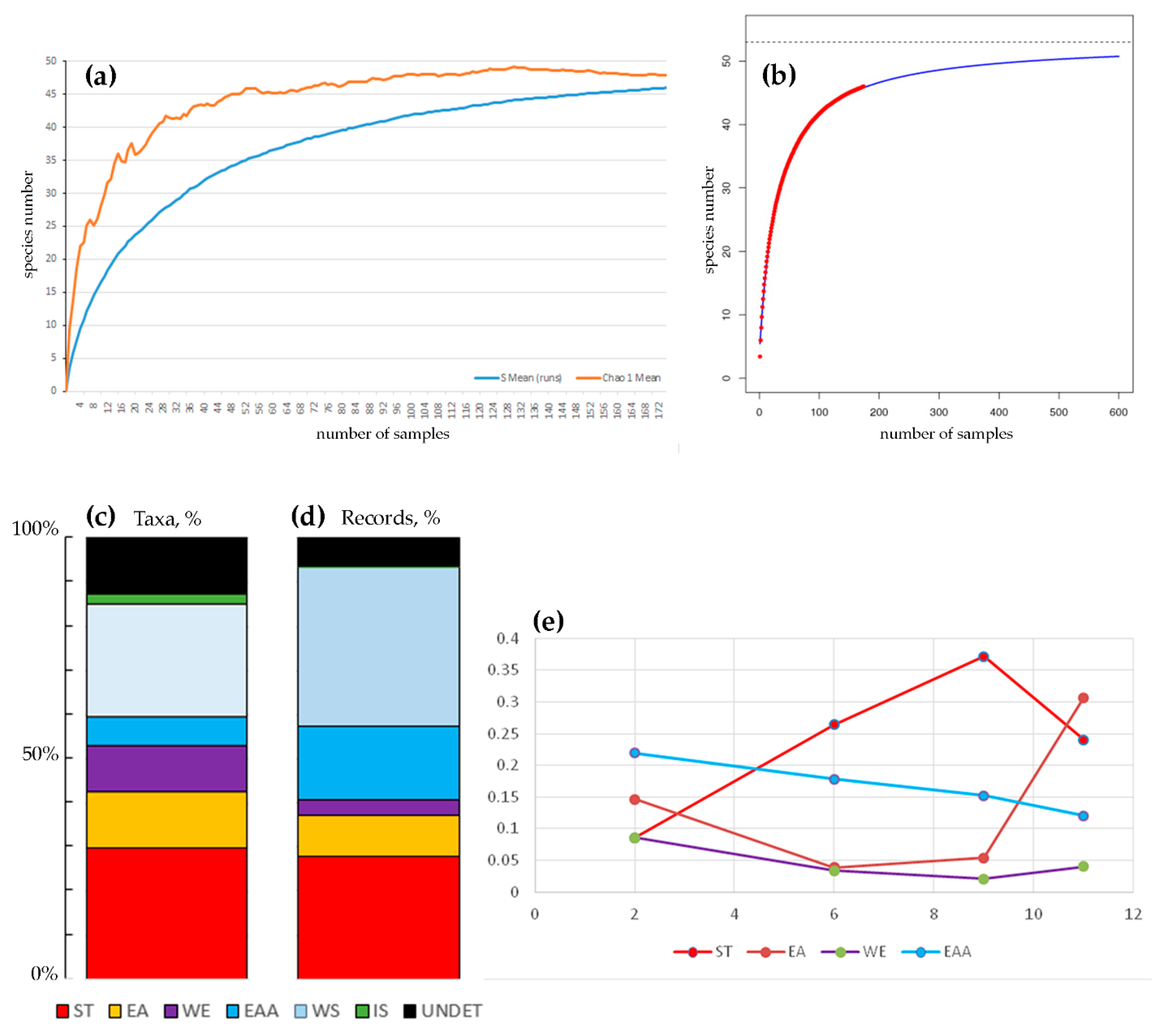
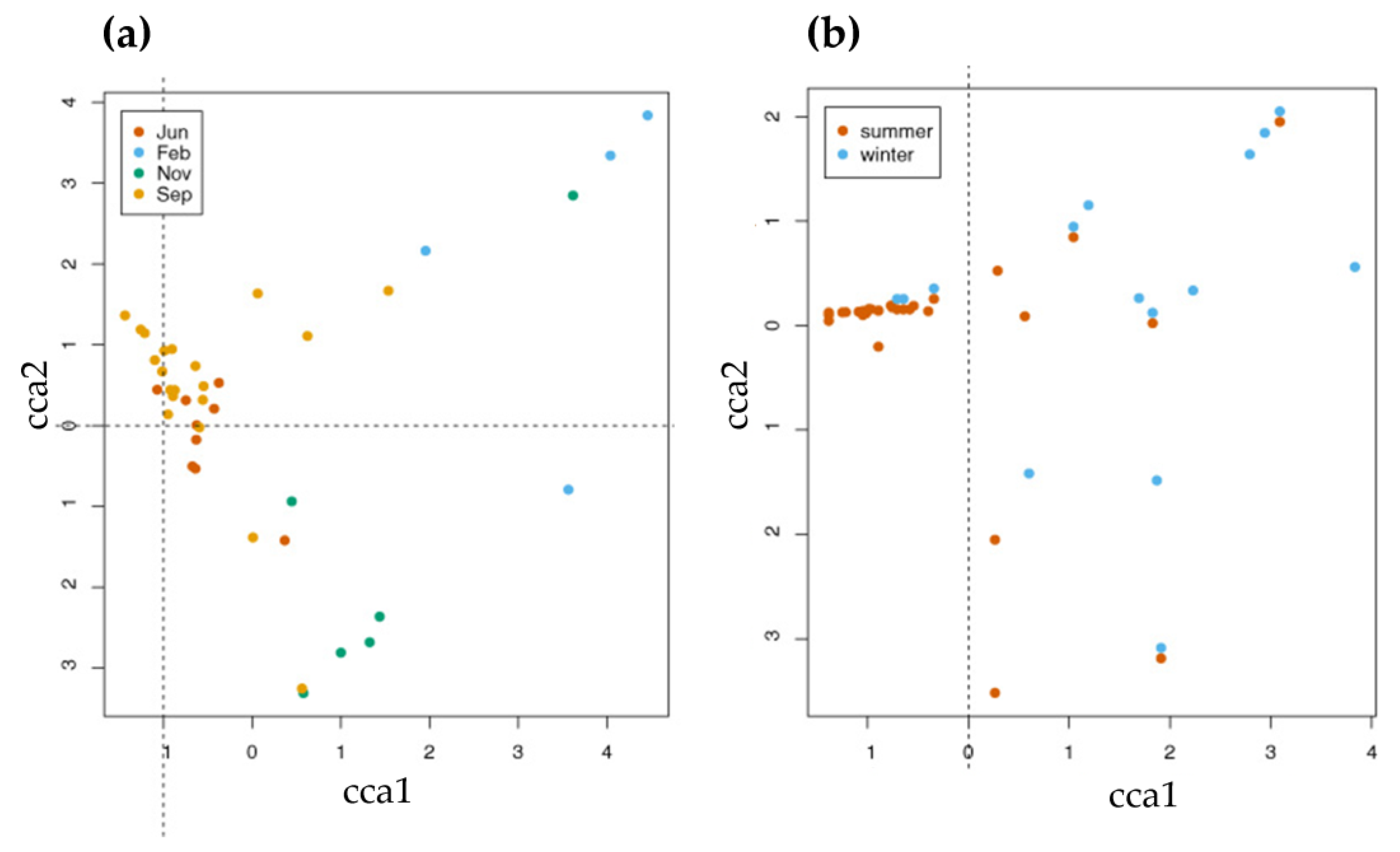
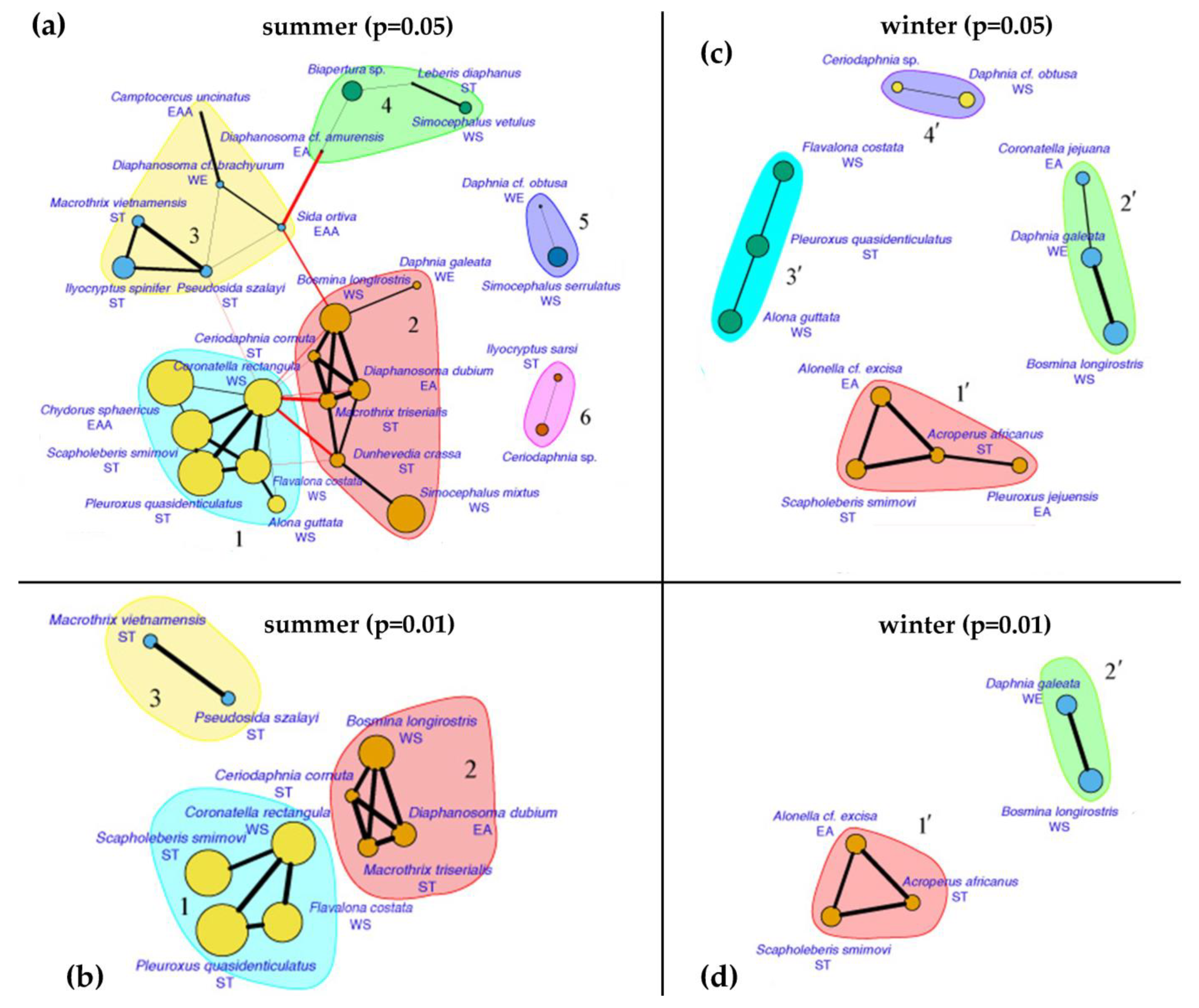
3. Results
4. Discussion
4.1. Impoverished Island Cladoceran Fauna
4.2. Notes on Faunistic Structure
4.3. Seasonal Changes in Fauna and Species Associations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.Q. Contributing to the progress of descriptive taxonomy. Zootaxa 2008, 1968, 65–68. [Google Scholar] [CrossRef]
- Agnarsson, I.; Kuntner, M. Taxonomy in a changing world: Seeking solutions for a science in crisis. Syst. Biol. 2007, 56, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Â.P.; Mejdalani, G.; Mounce, R.; Silveira, L.F.; Marinoni, L.; Rafael, J.A. Are publications on zoological taxonomy under attack? R. Soc. Open Sci. 2021, 8, 201617. [Google Scholar] [CrossRef] [PubMed]
- Kotov, A.A.; Gololobova, M.A. Traditional taxonomy: Quo vadis? Integr. Zool. 2016, 11, 500–505. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Ratnasingham, S.; deWaard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270 (Suppl. S1), S96–S99. [Google Scholar] [CrossRef]
- Taberlet, P.; Coissac, E.; Pompanon, F.; Brochmann, C.; Willerslev, E. Towards next-generation biodiversity assessment using DNA metabarcoding. Mol. Ecol. 2012, 21, 2045–2050. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X. eDNA metabarcoding in zooplankton improves the ecological status assessment of aquatic ecosystems. Environ. Int. 2020, 134, 105230. [Google Scholar] [CrossRef]
- Pentinsaari, M.; Ratnasingham, S.; Miller, S.E.; Hebert, P.D.N. BOLD and GenBank revisited—Do identification errors arise in the lab or in the sequence libraries? PLoS ONE 2020, 15, e0231814. [Google Scholar] [CrossRef]
- Jin, S.; Kim, K.Y.; Kim, M.-S.; Park, C. An assessment of the taxonomic reliability of DNA barcode sequences in publicly available databases. Algae 2020, 35, 293–301. [Google Scholar] [CrossRef]
- Garibian, P.G.; Neretina, A.N.; Taylor, D.J.; Kotov, A.A. Partial revision of the neustonic genus Scapholeberis Schoedler, 1858 (Crustacea: Cladocera): Decoding of the barcoding results. PeerJ 2020, 8, e10410. [Google Scholar] [CrossRef]
- Lampert, W. Daphnia: Development of a Model Organism in Ecology and Evolution; International Ecology Institute: Oldendorf/Luhe, Germany, 2011; ISBN 978-3-946729-21-1. [Google Scholar]
- Shaw, J.R.; Pfrender, M.E.; Eads, B.D.; Klaper, R.; Callaghan, A.; Sibly, R.M.; Colson, I.; Jansen, B.; Gilbert, D.; Colbourne, J.K. Daphnia as an emerging model for toxicological genomics. In Comparative Toxicogenomics; Elsevier: Amsterdam, The Netherlands, 2008; pp. 165–328. ISBN 9780444532749. [Google Scholar]
- Korovchinsky, N.M. How many species of Cladocera are there? Hydrobiologia 1996, 321, 191–204. [Google Scholar] [CrossRef]
- Elías-Gutiérrez, M.; Valdez-Moreno, M. A new cryptic species of Leberis Smirnov, 1989 (Crustacea, Cladocera, Chydoridae) from the Mexican semi-desert region, highlighted by DNA barcoding. Hidrobiologica 2008, 18, 63–74. [Google Scholar]
- Martínez-Caballero, A.L.; Morales-Gutiérrez, S.; Elías-Gutiérrez, M. First record of the genus Bunops Birge, 1893 (Cladocera: Macrothricidae) in the Neotropical highlands of Mexico with a detailed study of morphology and DNA barcodes. Zootaxa 2017, 4300, 589. [Google Scholar] [CrossRef]
- Elmoor-Loureiro, L. Brazilian cladoceran studies: Where do we stand. Nauplius 2000, 8, 117–131. [Google Scholar]
- Sousa, F.D.R.; Elmoor-Loureiro, L.M.A. Cladocera from the Upper Xingu River Basin with the description of a new genus of the Chydoridae (Crustacea: Branchiopoda: Anomopoda). Zootaxa 2018, 4418, 545–561. [Google Scholar] [CrossRef]
- Sousa, F.D.R.; Elmoor-Loureiro, L.M.A. Cladóceros fitófilos (Crustacea, Branchiopoda) do Parque Nacional das Emas, estado de Goiás. Biota Neotrop. 2008, 8, 159–166. [Google Scholar] [CrossRef]
- Fuentes-Reines, J.M.; Elmoor-Loureiro, L.M.A. Annotated checklist and new records of Cladocera from the Ciénaga El Convento, Atlántico-Colombia. Pan-Am. J. Aquat. Sci. 2015, 10, 189–202. [Google Scholar]
- Fuentes-Reinés, J.M.; Eslava-Eljaiek, P.; Elmoor-Loureiro, L.M. Cladocera (Crustacea, Branchiopoda) of a temporary shallow pond from northern Colombia. Rev. Peruana Biol. 2019, 26, 351–366. [Google Scholar] [CrossRef][Green Version]
- Andrade-Sossa, C.; Buitron-Caicedo, L.; Elías-Gutiérrez, M. A new species of Scapholeberis Schoedler, 1858 (Anomopoda: Daphniidae: Scapholeberinae) from the Colombian Amazon basin highlighted by DNA barcodes and morphology. PeerJ 2020, 8, e9989. [Google Scholar] [CrossRef]
- López, C.; Mosquera, P.; Hampel, H.; Neretina, A.N.; Alonso, M.; Van Damme, K.; Kotov, A.A. An annotated checklist of the freshwater cladocerans (Crustacea: Branchiopoda: Cladocera) of Ecuador and the Galápagos Islands. Invert. Zool. 2018, 15, 277–291. [Google Scholar] [CrossRef]
- Chalkley, A.; Robson, H.; The Cladocera Interest group. Available online: http://www.boxvalley.co.uk/nature/cladocera/newsletter2.asp (accessed on 10 August 2022).
- Petrusek, A.; Hobaek, A.; Nilssen, J.P.; Skage, M.; Černý, M.; Brede, N.; Schwenk, K. A taxonomic reappraisal of the European Daphnia longispina complex (Crustacea, Cladocera, Anomopoda). Zool. Scripta 2008, 37, 507–519. [Google Scholar] [CrossRef]
- Petrusek, A.; Tollrian, R.; Schwenk, K.; Haas, A.; Laforsch, C. A “crown of thorns” is an inducible defense that protects Daphnia against an ancient predator. Proc. Natl. Acad. Sci. USA 2009, 106, 2248–2252. [Google Scholar] [CrossRef] [PubMed]
- Thielsch, A.; Knell, A.; Mohammadyari, A.; Petrusek, A.; Schwenk, K. Divergent clades or cryptic species? Mito-nuclear discordance in a Daphnia species complex. BMC Evol. Biol. 2017, 17, 227. [Google Scholar] [CrossRef]
- Ye, Z.; Williams, E.; Zhao, C.; Burns, C.W.; Lynch, M. The rapid, mass invasion of New Zealand by North American Daphnia “pulex”. Limnol. Oceanogr. 2021, 66, 2672–2683. [Google Scholar] [CrossRef]
- Alonso, M. Crustacea: Branchiopoda, Fauna Iberica; Consejo Superior de Investigaciones Cientificas: Madrid, Spain, 1996; ISBN 978-8400075712. [Google Scholar]
- Alonso, M.; Neretina, A.N.; Ventura, M. Ceriodaphnia smirnovi (Crustacea: Cladocera), a new species from the Mediterranean Region, and a phylogenetic analysis of the commonest species. Zootaxa 2021, 4974, 146. [Google Scholar] [CrossRef] [PubMed]
- Korovchinsky, N.M. The genus Leptodora Lilljeborg (Crustacea: Branchiopoda: Cladocera) is not monotypic: Description of a new species from the Amur River basin (Far East of Russia). Zootaxa 2009, 2120, 39–52. [Google Scholar] [CrossRef]
- Kotov, A.A.; Sinev, A.Y. Cladocera (Crustacea, Branchiopoda) of the Zeya basin (Amurskaya Area, Russian Federation). 2. Descriptions of new taxa. Zool. Zh. 2011, 90, 272–284. [Google Scholar]
- Kotov, A.A.; Sinev, A.Y.; Korovchinsky, N.M.; Smirnov, N.N.; Bekker, E.I.; Sheveleva, N.G. Cladocera (Crustacea, Branchiopoda) of the Zeya basin (Amurskaya Area, Russian Federation). 1. New taxa for fauna of Russia. Zool. Zh. 2011, 90, 131–142. [Google Scholar]
- Garibian, P.G. Nicsmirnovius eximius (Kiser 1948) (Cladocera, Chydoridae) from the Primorsky Territory: The first record of the genus from Russia. Zool. Zh. 2017, 96, 1359–1363. [Google Scholar] [CrossRef]
- Kotov, A.A.; Garibian, P.G.; Bekker, E.I.; Taylor, D.J.; Karabanov, D.P. A new species group from the Daphnia curvirostris species complex (Cladocera: Anomopoda) from the eastern Palaearctic: Taxonomy, phylogeny and phylogeography. Zool. J. Linn. Soc. 2021, 191, 772–822. [Google Scholar] [CrossRef]
- Taylor, D.J.; Connelly, S.J.; Kotov, A.A. The Intercontinental phylogeography of neustonic daphniids. Sci. Rep. 2020, 10, 1818. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.-F.; Ji, G.-H.; Chen, S.-Z.; Yu, G.-L.; Xu, L.; Han, B.-P.; Kotov, A.A.; Dumont, H.J. Annotated Checklist of Chinese Cladocera (Crustacea: Branchiopoda). Part I. Haplopoda, Ctenopoda, Onychopoda and Anomopoda (families Daphniidae, Moinidae, Bosminidae, Ilyocryptidae). Zootaxa 2015, 3904, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.-H.; Xiang, X.-F.; Chen, S.-Z.; Yu, G.-L.; Kotov, A.A.; Dumont, H.J. Annotated checklist of Chinese Cladocera (Crustacea: Branchiopoda). Part II. Order Anomopoda (families Macrotrichidae, Eurycercidae and Chydoridae). Zootaxa 2015, 4044, 241–269. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Petrusek, A.; Wolinska, J.; Gieβler, S.; Zhong, Y.; Yang, Z.; Hu, W.; Yin, M. Diversity of the Daphnia longispina species complex in Chinese lakes: A DNA taxonomy approach. J. Plankton Res. 2015, 37, 56–65. [Google Scholar] [CrossRef][Green Version]
- Pajk, F.; Zhang, J.; Han, B.-P.; Dumont, H.J. Thermal reaction norms of a subtropical and a tropical species of Diaphanosoma (Cladocera) explain their distribution. Limnol. Oceanogr. 2018, 63, 1204–1220. [Google Scholar] [CrossRef]
- Wang, J.; Ni, Y.; Hu, W.; Yin, M. Lineage diversity and gene introgression in freshwater cladoceran crustaceans of the Chydorus sphaericus species complex. Limnol. Oceanogr. 2021, 66, 95–107. [Google Scholar] [CrossRef]
- Yoon, S.M.; Kim, H.S. A systematic study on the freshwater Cladocera from Korea. Korean J. Syst. Zool. 1987, 3, 175–207. [Google Scholar]
- Yoon, S.M. Arthropoda: Branchiopoda: Anostraca, Notostraca, Spinicaudata, Laevicaudata, Ctenopoda, Anomopoda, Haplopoda Branchiopods. Invertebr. Fauna Korea 2010, 21, 1–156. [Google Scholar]
- Korovchinsky, N.M. A new species of the genus Diaphanosoma Fischer, 1850 (Crustacea: Cladocera: Sididae) from Japan. Limnology 2013, 14, 13–18. [Google Scholar] [CrossRef]
- Lakatos, C.; Urabe, J.; Makino, W. Cryptic diversity of Japanese Diaphanosoma (Crustacea: Cladocera) revealed by morphological and molecular assessments. Inland Waters 2015, 5, 253–262. [Google Scholar] [CrossRef]
- Makino, W.; Maruoka, N.; Nakagawa, M.; Takamura, N. DNA barcoding of freshwater zooplankton in Lake Kasumigaura, Japan. Ecol. Res. 2017, 32, 481–493. [Google Scholar] [CrossRef]
- Makino, W.; Machida, R.J.; Okitsu, J.; Usio, N. Underestimated species diversity and hidden habitat preference in Moina (Crustacea, Cladocera) revealed by integrative taxonomy. Hydrobiologia 2020, 847, 857–878. [Google Scholar] [CrossRef]
- Kotov, A.A. Faunistic complexes of the Cladocera (Crustacea, Branchiopoda) of Eastern Siberia and the Far East of Russia. Zool. Zh. 2016, 95, 748–768. [Google Scholar] [CrossRef]
- Nikolslky, G.V. On biological specifics of the faunistic complexes and significance of its analysis for the biogeography. Zool. Zh. 1947, 26, 221–231. [Google Scholar]
- Shtegman, B.K. Basics of the ornitographic subdivision of Palaearctic: Fauna SSSR. Novaya Seria №19. Birds; AN SSSR: Moscow, Russia, 1938. [Google Scholar]
- Garibian, P.G.; Neretina, A.N.; Korovchinsky, N.M.; Sinev, A.Y.; Tchabovsky, A.V.; Kotov, A.A.; Smirnov, N.N. The Southern part of Russian Far East and Korean Peninsula as a transition zone between the boreal and tropical faunas of the waterfleas (Cladocera, Crustacea). Zool. Zh. 2020, 99, 1094–1109. [Google Scholar] [CrossRef]
- Korovchinsky, N.M. The Cladocera (Crustacea: Branchiopoda) as a relict group. Zool. J. Linn. Soc. 2006, 147, 109–124. [Google Scholar] [CrossRef][Green Version]
- Neretina, A.N.; Karabanov, D.P.; Sacherova, V.; Kotov, A.A. Unexpected mitochondrial lineage diversity within the genus Alonella Sars, 1862 (Crustacea: Cladocera) across the Northern Hemisphere. PeerJ 2021, 9, e10804. [Google Scholar] [CrossRef]
- Song, J.H.; Han, Y.D.; Lee, W.K.; Kim, I.H.; Paik, S.G.; Lee, J.; Yong, S.H.; Lee, W.; Jung, J.; Kim, S.H.; et al. Unrecorded species of Korean metazoans discovered through the project of “Discovery of Korean Indigenous Species” (2006–2010). J. Spec. Res. 2017, 6, 164–171. [Google Scholar] [CrossRef]
- Kotov, A.A.; Jeong, H.G.I.; Lee, W. Cladocera (Crustacea: Branchiopoda) of the south-east of the Korean Peninsula, with twenty new records for Korea. Zootaxa 2012, 3368, 50–90. [Google Scholar] [CrossRef]
- Jeong, H.G.; Kotov, A.A.; Lee, W. A new species of the genus Pleuroxus Baird (Cladocera: Anomopoda: Chydoridae) from Jeju Island, South Korea. Zootaxa 2013, 3666, 31–40. [Google Scholar] [CrossRef][Green Version]
- Jeong, H.; Kotov, A.A.; Lee, W. Checklist of the freshwater Cladocera (Crustacea: Branchiopoda) of South Korea. Proc. Biol. Soc. Wash. 2014, 127, 216–228. [Google Scholar] [CrossRef]
- Jeong, H.G.; Sinev, A.Y.; Brancelj, A.; Chang, K.-H.; Kotov, A.A. A new blind groundwater-dwelling genus of the Cladocera (Crustacea: Branchiopoda) from the Korean Peninsula. Zootaxa 2017, 4341, 451–474. [Google Scholar] [CrossRef] [PubMed]
- Sinev, A.Y.; Lee, W.; Kotov, A.A. A new species of Coronatella Dybowski & Grochowski, 1984 (Cladocera: Chydoridae) endemic to Jeju Island (Republic of Korea). Zootaxa 2022, 5159, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Cho, D.L.; Kim, Y.B.; Kim, J.C.; Cho, B.W.; Jang, Y.N.; Lee, B.J.; Lee, S.R.; Son, B.K.; Cheon, H.Y.; et al. Geological Report on the Segwipo-Hanyori Sheet. Scale 1;50.000; Jeju Provincial Government: Jeju City, Korea, 2000. [Google Scholar]
- Woo, K.S. Jeju Island Geopark: A Volcanic Wonder of Korea, 1st ed.; Springer: New York, NY, USA, 2013; ISBN 978-3-642-20563-7. [Google Scholar]
- Jeju Special Self-Government Province. Jeju Green Environment. Mid-Term Basic Plan for Environment Conservation of Jeju Special Self-Government Province. Available online: https://www.jri.re.kr (accessed on 10 August 2022).
- Colwell, R.K.; Elsensohn, J.E. EstimateS turns 20: Statistical estimation of species richness and shared species from samples, with non-parametric extrapolation. Ecography 2014, 37, 609–613. [Google Scholar] [CrossRef]
- Basualdo, C.V. Choosing the best non-parametric richness estimator for benthic macroinvertebrates databases. Rev. Soc. Entomológ. Argent. 2011, 70, 27–38. [Google Scholar]
- Schoener, T.W. The species-area relation within archipelagos: Models and evidence from island land birds. In Proceedings of the 16th International Ornithological Conference, Canberra, Australia, 12–17 August 1976; Volume 1, pp. 629–642. [Google Scholar]
- Ratkowsky, D.A. Handbook of Nonlinear Regression Models; Marcel Dekker: New York, NY, USA, 1990. [Google Scholar]
- Kotov, A.A.; Karabanov, D.P.; Van Damme, K. Non-Indigenous Cladocera (Crustacea: Branchiopoda): From a Few Notorious Cases to a Potential Global Faunal Mixing in Aquatic Ecosystems. Water 2022, 14, 2806. [Google Scholar] [CrossRef]
- Kotov, A.A.; Sinev, A.Y.; Garibian, P.G.; Neretina, A.N.; Jeong, H.G.; Lee, W.; Chae, K.S.; Min, G.S. Recent progress in studies of the Cladocera (Crustacea: Branchiopoda) of South Korea with seven new records for the Korean Peninsula. J. Spec. Res. 2017, 6, 227–246. [Google Scholar]
- Korovchinsky, N.M.; Kotov, A.A. (Eds.) Water Fleas (Crustacea: Cladocera) of North Eurasia; KMK Press: Moscow, Russia, 2021; Volume 2. [Google Scholar]
- Karabanov, D.P.; Bekker, E.I.; Garibian, P.G.; Shiel, R.J.; Kobayashi, T.; Taylor, D.J.; Kotov, A.A. Multiple Recent Colonizations of the Australian Region by the Chydorus sphaericus Group (Crustacea: Cladocera). Water 2022, 14, 594. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Tukey’s honestly significant difference (HSD) test. Encycl. Res. Des. 2010, 3, 1–5. [Google Scholar]
- Ter Braak, C.J.F. Canonical Correspondence Analysis: A New Eigenvector Technique for Multivariate Direct Gradient Analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. Available online: https://www.R-project.org/ (accessed on 10 August 2022).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5–6. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 August 2022).
- Frossard, J.; Renaud, O. Permutation Tests for Regression, ANOVA, and Comparison of Signals: The permuco Package. J. Stat. Soft. 2021, 99, 1–32. [Google Scholar] [CrossRef]
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. InterJournal Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Kwon, T.-S. Ants foraging on grasses in South Korea: High diversity in Jeju Island and negative correlation with aphids. J. Asia-Pac. Biodivers. 2017, 10, 465–471. [Google Scholar] [CrossRef]
- Chae, H.S.; Kil, H.J.; Seo, J.E. Taxonomic study of freshwater bryozoans from Jeju Island, Korea. J. Spec. Res. 2017, 6, 129–134. [Google Scholar] [CrossRef]
- Hortal, J.; Triantis, K.A.; Meiri, S.; Thébault, E.; Sfenthourakis, S. Island species richness increases with habitat diversity. Am. Nat. 2009, 174, E205–E217. [Google Scholar] [CrossRef]
- Horsák, M.; Hájek, M.; Spitale, D.; Hájková, P.; Díte, D.; Nekola, J.C. The age of island-like habitats impacts habitat specialist species richness. Ecology 2012, 93, 1106–1114. [Google Scholar] [CrossRef]
- Sinev, A.Y.; Gu, Y.; Han, B.-P. Cladocera of Hainan Island, China. Zootaxa 2015, 4006, 569–585. [Google Scholar] [CrossRef]
- Ueno, M. Order Branchiopoda (Class Crustacea). Fauna Nippon. 1937, 9, 1–135. [Google Scholar]
- Novichkova, A.A.; Chertoprud, E.S.; Azovsky, A.I. Composition, characteristics and long-term variability of the freshwater microcrustacean fauna of the Faroe Islands. J. Nat. Hist. 2019, 53, 2449–2465. [Google Scholar] [CrossRef]
- Makrushin, A.V. Anhydrobiosis in Aquatic Invertebrates; Nauka Academic Publishers: Leningrad, Russia, 1985. [Google Scholar]
- Xu, L.; Han, B.-P.; van Damme, K.; Vierstraete, A.; Vanfleteren, J.R.; Dumont, H.J. Biogeography and evolution of the Holarctic zooplankton genus Leptodora (Crustacea: Branchiopoda: Haplopoda). J. Biogeogr. 2011, 38, 359–370. [Google Scholar] [CrossRef]
- Yong, D.L.; Heim, W.; Chowdhury, S.U.; Choi, C.-Y.; Ktitorov, P.; Kulikova, O.; Kondratyev, A.; Round, P.D.; Allen, D.; Trainor, C.R.; et al. The State of Migratory Landbirds in the East Asian Flyway: Distributions, Threats, and Conservation Needs. Front. Ecol. Evol. 2021, 9, 613172. [Google Scholar] [CrossRef]
- Takekawa, J.Y.; Newman, S.H.; Xiao, X.; Prosser, D.J.; Spragens, K.A.; Palm, E.C.; Yan, B.; Li, T.; Lei, F.; Zhao, D.; et al. Migration of waterfowl in the East Asian flyway and spatial relationship to HPAI H5N1 outbreaks. Avian Dis. 2010, 54, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Figuerola, J.; Green, A.J. Dispersal of aquatic organisms by waterbirds: A review of past research and priorities for future studies. Freshw. Biol. 2002, 47, 483–494. [Google Scholar] [CrossRef]
- Frisch, D.; Green, A.J.; Figuerola, J. High dispersal capacity of a broad spectrum of aquatic invertebrates via waterbirds. Aquat. Sci. 2007, 69, 568–574. [Google Scholar] [CrossRef]
- Nanzyo, M.; Dahlgren, R.; Shoji, S. Chapter 6 Chemical Characteristics of Volcanic Ash Soils. Volcanic Ash Soils—Genesis, Properties and Utilization; Elsevier: Amsterdam, The Netherlands, 1993; pp. 145–187. ISBN 9780444897992. [Google Scholar]
- Strawn, D.; Bohn, H.L.; O’Connor, G.A. (Eds.) Soil Chemistry, 4th ed.; Wiley-Blackwell: Chichester, UK, 2015; ISBN 978-1-118-62925-3. [Google Scholar]
- Lee, S.H.; Lee, Y.I.; Yoon, H.I.; Yoo, K.-C. East Asian monsoon variation and climate changes in Jeju Island, Korea, during the latest Pleistocene to early Holocene. Quat. Res. 2008, 70, 265–274. [Google Scholar] [CrossRef]
- Marsden, R.C.; Danišík, M.; Ahn, U.S.; Friedrichs, B.; Schmitt, A.K.; Kirkland, C.L.; McDonald, B.J.; Evans, N.J. Zircon double-dating of Quaternary eruptions on Jeju Island, South Korea. J. Volcan. Geotherm. Res. 2021, 410, 107171. [Google Scholar] [CrossRef]
- Kier, G.; Kreft, H.; Lee, T.M.; Jetz, W.; Ibisch, P.L.; Nowicki, C.; Mutke, J.; Barthlott, W. A global assessment of endemism and species richness across island and mainland regions. Proc. Natl. Acad. Sci. USA 2009, 106, 9322–9327. [Google Scholar] [CrossRef]
- Hong, H.-J.; Kim, C.-K.; Lee, H.-W.; Lee, W.-K. Conservation, Restoration, and Sustainable Use of Biodiversity Based on Habitat Quality Monitoring: A Case Study on Jeju Island, South Korea (1989–2019). Land 2021, 10, 774. [Google Scholar] [CrossRef]
- Lee, W.-O.; Kim, D.-H.; Kim, D.-H.; Baek, J.-M.; Lee, S.-W.; Yang, G.-C. Distribution characteristics of freshwater fish in Jeju-do, Korea. J. Asia-Pac. Biodivers. 2015, 8, 83–94. [Google Scholar] [CrossRef][Green Version]
- Lohman, D.J.; Ingram, K.K.; Prawiradilaga, D.M.; Winker, K.; Sheldon, F.H.; Moyle, R.G.; Ng, P.K.; Ong, P.S.; Wang, L.K.; Braile, T.M.; et al. Cryptic genetic diversity in “widespread” Southeast Asian bird species suggests that Philippine avian endemism is gravely underestimated. Biol. Conserv. 2010, 143, 1885–1890. [Google Scholar] [CrossRef]
- Bekker, E.I.; Karabanov, D.P.; Galimov, Y.R.; Haag, C.R.; Neretina, T.V.; Kotov, A.A. Phylogeography of Daphnia magna Straus (Crustacea: Cladocera) in Northern Eurasia: Evidence for a deep longitudinal split between mitochondrial lineages. PLoS ONE 2018, 13, e0194045. [Google Scholar] [CrossRef] [PubMed]
- Kotov, A.A.; Karabanov, D.P.; Bekker, E.I.; Neretina, T.V.; Taylor, D.J. Phylogeography of the Chydorus sphaericus group (Cladocera: Chydoridae) in the Northern Palearctic. PLoS ONE 2016, 11, e0168711. [Google Scholar] [CrossRef] [PubMed]
- Zuykova, E.I.; Bochkarev, N.A.; Taylor, D.J.; Kotov, A.A. Unexpected endemism in the Daphnia longispina complex (Crustacea: Cladocera) in Southern Siberia. PLoS ONE 2019, 14, e0221527. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Isozaki, Y.; Kimura, G.; Terabayashi, M. Paleogeographic maps of the Japanese Islands: Plate tectonic synthesis from 750 Ma to the present. Island Arc 1997, 6, 121–142. [Google Scholar] [CrossRef]
- Taira, A. Tectonic Evolution of the Japanese Island Arc System. Annu. Rev. Earth Planet. Sci. 2001, 29, 109–134. [Google Scholar] [CrossRef]
- Kimura, M. Paleogeography of the Ryukyu Islands. Tropics 2000, 10, 5–24. [Google Scholar] [CrossRef]
- De Meester, L.; Gómez, A.; Okamura, B.; Schwenk, K. The Monopolization Hypothesis and the dispersal-gene flow paradox in aquatic organisms. Acta Oecol. 2002, 23, 121–135. [Google Scholar] [CrossRef]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W.; et al. Freshwater Ecoregions of the World: A New Map of Biogeographic Units for Freshwater Biodiversity Conservation. Bioscience 2008, 58, 403–414. [Google Scholar] [CrossRef]


| No. | Taxon | 2 | 6 | 9 | 11 | Total Number of Records | Faunistic Complex | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Acroperus africanus Neretina & Kotov, 2015 | + | 5 | ST | [47] | |||
| 2 | Alona guttata Sars, 1862 | + | + | + | + | 16 | WS | [47] |
| 3 | Alonella cf. excisa (Fischer, 1854) | + | + | 16 | EA | [52] | ||
| 4 | Biapertura herricki (Sinev, 2013) | + | + | 2 | IS | [67] | ||
| 5 | Biapertura sp. | + | + | + | 7 | |||
| 6 | Bosmina longirostris (O. F. Müller, 1776) | + | + | + | + | 29 | WS | [47] |
| 7 | Camptocercus uncinatus Smirnov, 1971 | + | + | 2 | EAA | [47] | ||
| 8 | Ceriodaphnia cf. quadrangula (O.F. Müller, 1785) | + | + | 5 | WS | [47] | ||
| 9 | Ceriodaphnia cornuta Sars, 1885 | + | 3 | ST | [47] | |||
| 10 | Ceriodaphnia reticulata (Jurine, 1820) | + | + | + | 37 | WS | [47] | |
| 11 | Ceriodaphnia sp. | + | + | 6 | ||||
| 12 | Chydorus cf. sphaericus (O.F. Müller, 1776) (clade A3) | + | + | + | + | 97 | EAA | [69] |
| 13 | Coronatella jejuana Sinev, Lee & Kotov, 2022 | + | + | 7 | EA | This study | ||
| 14 | Coronatella rectangula (Sars, 1862) | + | + | + | + | 36 | WS | [47] |
| 15 | Daphnia cf. obtusa Kurz, 1875 emend. Scourfield, 1942 | + | + | 6 | WS | [47] | ||
| 16 | Daphnia galeata Sars, 1864 | + | + | 10 | WE | [47] | ||
| 17 | Daphnia jejuana Kotov et al., 2021 | + | + | + | 19 | EA | [34] | |
| 18 | Daphnia sinensis Gu, Xu, Li, Dumont, Han, 2013 | + | 6 | WE | [47] | |||
| 19 | Daphnia (Daphnia) sp. | + | + | 16 | ||||
| 20 | Diaphanosoma cf. amurensis Korovchinsky & Sheveleva, 2009 | + | 1 | EA | [47] | |||
| 21 | Diaphanosoma cf. brachyurum (Liévin, 1848) | + | + | 2 | WE | [47] | ||
| 22 | Diaphanosoma dubium Manujlova, 1964 | + | 7 | EA | [47] | |||
| 23 | Diaphanosoma sarsi Richard, 1894 | + | 3 | ST | This study | |||
| 24 | Diaphanosoma sp. | + | 3 | |||||
| 25 | Disparalona ikarus Kotov & Sinev, 2011 | + | 1 | ST | [68] | |||
| 26 | Dunhevedia crassa King, 1853 | + | 4 | ST | [47] | |||
| 27 | Flavalona costata (Sars, 1862) | + | + | + | + | 33 | WS | [47] |
| 28 | Ilyocryptus cf. raridentatus Smirnov, 1989 | + | 2 | ST | [47] | |||
| 29 | Ilyocryptus spinifer Herrick, 1882 | + | + | + | 10 | ST | [47] | |
| 30 | Leberis diaphanus (King, 1853) | + | 1 | ST | This study | |||
| 31 | Leydigia ciliata (Gauthier, 1939) | + | 1 | ST | [47] | |||
| 32 | Macrothrix rosea (Jurine, 1820) | + | 2 | WE | [47] | |||
| 33 | Macrothrix triserialis Brady, 1886 | + | 5 | ST | [47] | |||
| 34 | Macrothrix vietnamensis Silva-Briano, Dieu & Dumont, 1999 | + | 3 | ST | [47] | |||
| 35 | Moina cf. macrocopa (Straus, 1820) | + | 2 | WE | [47] | |||
| 36 | Moina cf. micrura Kurz, 1875 | + | + | 6 | WS | [47] | ||
| 37 | Moina sp. | + | 1 | |||||
| 38 | Pleuroxus quasidenticulatus (Smirnov, 1996) | + | + | + | + | 77 | ST | [47] |
| 39 | Pleuroxus jejuensis Jeong, Kotov & Lee, 2013 | + | + | 6 | EA | [47] | ||
| 40 | Pseudosida szalayi (Daday, 1898) | + | 3 | ST | [47] | |||
| 41 | Scapholeberis smirnovi Garibian, Neretina, Taylor & Kotov, 2020 | + | + | + | 52 | ST | [50] | |
| 42 | Sida ortiva Korovchinsky, 1979 | + | 2 | EAA | [47] | |||
| 43 | Simocephalus congener (Koch, 1841) | + | 1 | WS | [47] | |||
| 44 | Simocephalus mixtus Sars, 1903 | + | + | + | 34 | WS | [47] | |
| 45 | Simocephalus serrulatus (Koch, 1841) | + | + | 8 | WS | [47] | ||
| 46 | Simocephalus vetulus (O.F. Müller, 1776) | + | + | 7 | WS | [47] | ||
| 47 | Simocephalus sp. | + | + | 5 | ||||
| Total number of taxa | 19 | 26 | 31 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotov, A.A.; Seleznev, D.G.; Garibian, P.G.; Korovchnsky, N.M.; Neretina, A.N.; Sinev, A.Y.; Jeong, H.-G.; Yang, H.-M.; Lee, W. History of Colonization of Jeju Island (Republic of Korea) by the Water Fleas (Crustacea: Cladocera) Is Reflected by the Seasonal Changes in Their Fauna and Species Associations. Water 2022, 14, 3394. https://doi.org/10.3390/w14213394
Kotov AA, Seleznev DG, Garibian PG, Korovchnsky NM, Neretina AN, Sinev AY, Jeong H-G, Yang H-M, Lee W. History of Colonization of Jeju Island (Republic of Korea) by the Water Fleas (Crustacea: Cladocera) Is Reflected by the Seasonal Changes in Their Fauna and Species Associations. Water. 2022; 14(21):3394. https://doi.org/10.3390/w14213394
Chicago/Turabian StyleKotov, Alexey A., Dmitry G. Seleznev, Petr G. Garibian, Nikolai M. Korovchnsky, Anna N. Neretina, Artem Y. Sinev, Hyun-Gi Jeong, Hee-Min Yang, and Wonchoel Lee. 2022. "History of Colonization of Jeju Island (Republic of Korea) by the Water Fleas (Crustacea: Cladocera) Is Reflected by the Seasonal Changes in Their Fauna and Species Associations" Water 14, no. 21: 3394. https://doi.org/10.3390/w14213394
APA StyleKotov, A. A., Seleznev, D. G., Garibian, P. G., Korovchnsky, N. M., Neretina, A. N., Sinev, A. Y., Jeong, H.-G., Yang, H.-M., & Lee, W. (2022). History of Colonization of Jeju Island (Republic of Korea) by the Water Fleas (Crustacea: Cladocera) Is Reflected by the Seasonal Changes in Their Fauna and Species Associations. Water, 14(21), 3394. https://doi.org/10.3390/w14213394








