Abstract
Sugarcane bagasse as a biomass solid waste has good prospects as a carbon source for biological wastewater treatment. However, it has rarely been investigated for aerobic denitrification (AD). Herein, we investigated its carbon release performance and constructed a bagasse-loaded sequencing batch biofilm reactor (SBBR) to investigate the feasibility of sugarcane bagasse as a carbon source and filler for AD bacteria to treat wastewater with low carbon–nitrogen ratios (C/N). Results showed that chemical oxygen demand (COD) leaching from sugarcane bagasse was 418.07 ± 17.05 mg/g; high-temperature and high-pressure operation had no significant effect on the carbon release performance of the bagasse. A shake-flask experiment revealed that adding sugarcane bagasse significantly enhanced the AD flora denitrification effect in low C/N wastewater; the removal process of NO3−–N by AD flora followed first-order kinetics. In the SBBR inoculated with AD flora, biofilm formation on the bagasse surface was evident; the NO3−–N removal rate reached 20.20 ± 4.27% after 28 operation cycles, which was significantly higher than that of the control sequencing batch reactor during the same period, and the effluent COD stabilized below 50 mg/L. The results provide a reference for AD application in the practical wastewater treatment and biomass resource utilization of sugarcane bagasse.
1. Introduction
Aerobic denitrification (AD) is the process of reducing nitrate and nitrite to gaseous forms of nitrogen, mostly nitrogen (N2) and nitrous oxide (N2O), by certain bacteria in the presence of oxygen, with organic matter as an electron donor and nitrate–nitrogen (NO3−–N) as an electron acceptor [1]. Some advantages of AD bacteria are a fast growth rate, high adaptability, and high denitrification efficiency; consequently, they are of great interest in the field of biological denitrification of wastewater [2]. However, N removal by AD bacteria depends on carbon sources and high carbon–nitrogen ratio (C/N) conditions [3,4,5]. To address this problem, researchers have found that the addition of small-molecule carbon sources (e.g., methanol, ethanol, glucose, and acetate) promotes AD activity [6,7]. However, these carbon sources have limitations in their application because of their high costs and the requirement of repeated dosing. Consequently, researchers have attempted to use non-toxic biomass carbon sources, such as wood chips, sawdust, cotton, corn cobs, and bark, as well as polymeric synthetic materials (polyvinyl alcohol [PVA], polycaprolactone [PCL], polylactic acid [PLA], poly (3-hydroxybutyrate-co-3-hydroxyvalerate) [PHBV], and polylactic acid [PLA]) as slow-release carbon sources, instead of small-molecule carbon sources, for solid-phase denitrification. However, previous studies have mainly focused on anoxic denitrification [8,9]. The hydrophilic surface of slow-release carbon sources can attach to microorganisms to form a biofilm, which can immobilize bacteria and stably provide a carbon source to microbes for a long time.
The worldwide production of agricultural solid waste is immense, and the resource utilization of crop residues is consistent with the goal of sustainable global development. Using biomass as a carbon source for wastewater treatment can both realize the resource recovery of agricultural solid waste and reduce the cost of carbon sources used in the wastewater treatment process. Biomass carbon sources for AD flora, such as corn cob, loofah sponge, straw, rice husk, grapefruit peel, and durian peel, can be used as an additional carbon source to promote the AD process [10,11]. These natural biomasses are characterized by a high cellulose content and high porosity. Sugarcane bagasse, the largest solid waste after sugarcane crushing, produces approximately 25–30% of the total sugarcane crushing volume [12]. It not only has high porosity and is mainly composed of cellulose and hemicellulose, but it also contains approximately 3% sucrose, which is easily utilized by microorganisms; it has good prospects for use as a biomass carbon source in the field of wastewater treatment [13]. Therefore, it is theoretically feasible to apply bagasse as a solid carbon source and filler to wastewater treatment by AD. However, there is a lack of relevant research.
Studies have been performed on the use of sugarcane bagasse as an additional carbon source and filler for sulfate-reducing and anoxic denitrifying bacteria for treating mine and aquaculture wastewater, as well as for remediating groundwater. Sugarcane bagasse has shown good carbon-release and biofilm-hanging properties [14]. Therefore, in this study, sugarcane bagasse was loaded into a wastewater treatment reactor to explore its feasibility as a carbon source and filler for AD flora to treat wastewater with a low C/N ratios.
2. Materials and Methods
2.1. Materials
2.1.1. Activated Sludge
Activated sludge used in this experiment was obtained from a thickening sludge tank in a domestic wastewater treatment plant (WWTP) in Sichuan, China. This plant adopted the A2/O process as the core process, and the sludge moisture content was 91.44 ± 2.07%.
2.1.2. Culture Media
The aerobic denitrification medium (synthetic wastewater containing nitrate) was prepared using 1.95 g/L or 0.26 g/L of C4H4Na2O4·6H2O (C/N 15 or 2, respectively); 0.36 g/L of KNO3; 0.1 g/L of K2HPO4·3H2O; 0.1 g/L of Na2HPO4·12H2O; 0.1 g/L of MgSO4·7H2O; 1 mL/L of trace element solution, and 1 mL/L of (NH4)2Fe(SO4)2·6H2O solution.
The trace element solution was prepared using 2.5 g/L of EDTA; 1.1 g/L of ZnSO4·7H2O; 0.28 g/L of CaCl2; 0.26 g/L of MnCl2·4H2O; 0.06 g/L of (NH4)2MoO4·4H2O; 0.08 g/L of CuSO4·5H2O; and 0.08 g/L of CoCl2·6H2O; at a pH of 7 in a (NH4)2Fe(SO4)2·6H2O solution (0.35 g/L of (NH4)2Fe(SO4)2·6H2O and 10 mL/L of H2SO4).
2.1.3. Sugarcane Bagasse
After peeling, cutting, and juicing, the bagasse was laid flat on a white porcelain tray, placed in an oven, dried at 65 °C to constant weight, cooled, placed in a vacuum bag, and stored at 5 °C. After sieving the bagasse powder, 50 bagasse segments were randomly taken for measurement, and the specific dimensions are shown in Table 1. The average density of the bagasse was 0.0916 g/cm3.

Table 1.
Basic parameters of sugarcane bagasse used to evaluate its feasibility as a carbon source in aerobic denitrification of wastewater.
2.1.4. Reactors
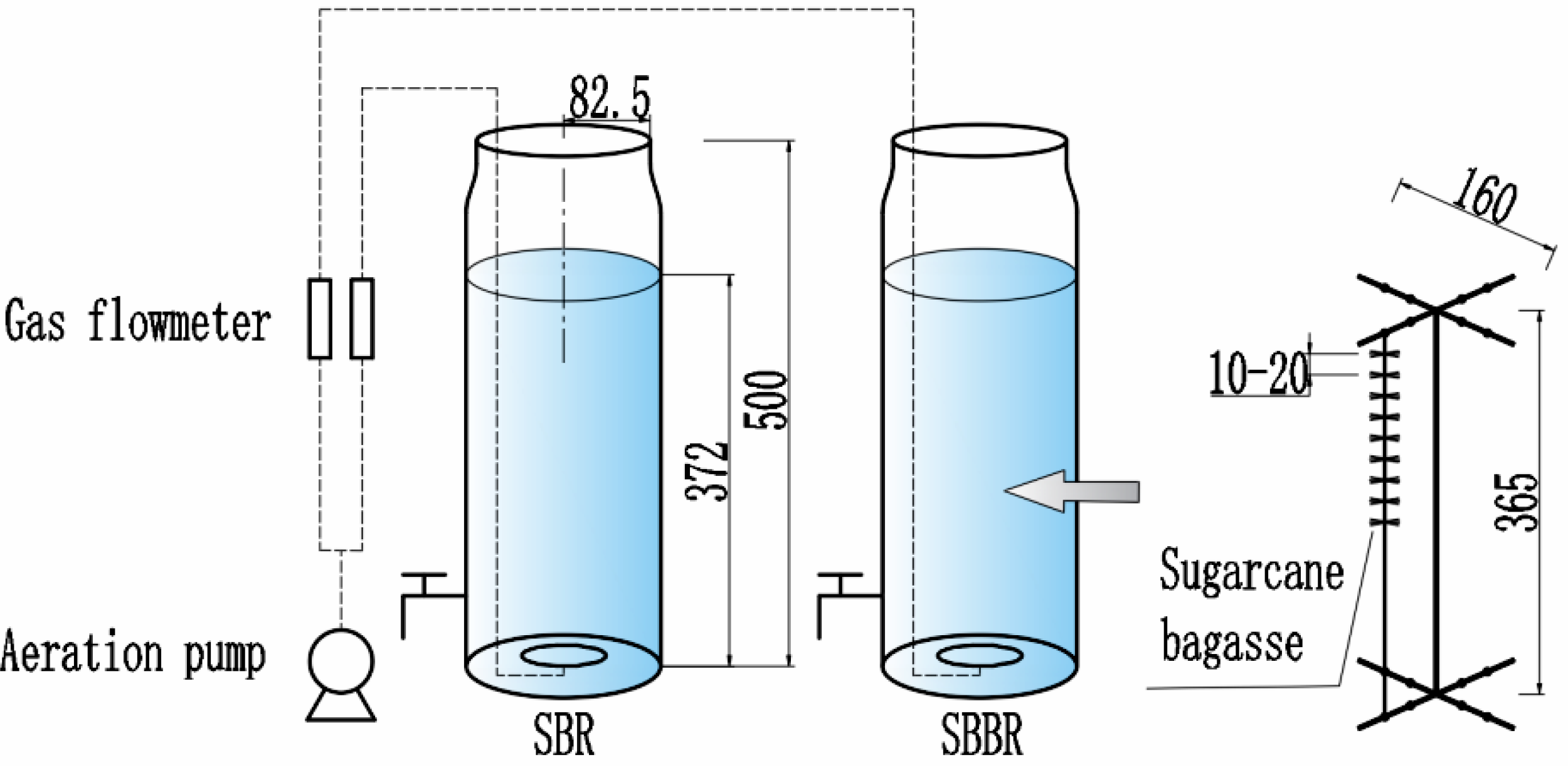
The simulated sequencing batch reactor (SBR) was made of glass (Φ185 × 500 mm) and assembled by members of our laboratory. The end result was a batch reactor with an effective volume of 10 L, equipped with an aeration device (Figure 1).
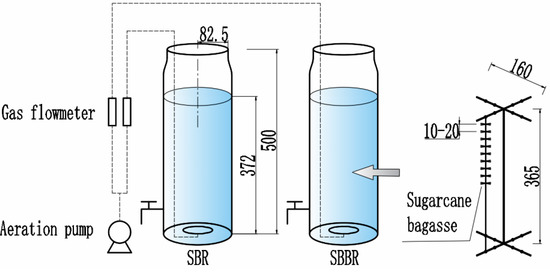
Figure 1.
Schematic of sequencing batch reactor (SBR) and sequencing batch bioreactor (SBBR) loaded with sugarcane bagasse to evaluate the suitability of bagasse for aerobic denitrification. Note: The unit of size in this figure is mm.
The sequencing batch biofilm reactor (SBBR) was loaded with sugarcane bagasse in the SBR at a dose of 0.5%. The bagasse segments were tied onto eight 500-mm cotton threads with an interval of 10–20 mm, and the bagasse threads were tied onto a stainless-steel frame (Figure 1). Finally, the stainless-steel frame was installed inside the SBR.
2.2. Methods
2.2.1. AD Flora Culture
The activated sludge, as described in Section 2.1.1, was incubated in a 10 L SBR, and the mixed-liquid-suspended solid (MLSS) was adjusted to approximately 3000 mg/L. The aerobic denitrification medium with a C/N ratio of 15 (described in Section 2.1.2) was used for the culture. After aeration at an airflow rate of 4 L/min for 2 h, the mixture was sampled and centrifuged at 0 and 2 h, and the supernatant was tested for NO3−–N. After 30 min of settling, the supernatant was drained at 80% of the effective volume and replaced with aerobic denitrification medium to continue the next cycle of incubation.
2.2.2. Carbon-Release Performance Examination of Sugarcane Bagasse
- (1)
- Shake-flask experiment on soaking carbon release
To investigate the effect of autoclaving on the carbon release performance of the bagasse, two groups of 1.0, 2.0, 3.0, 4.0, and 5.0 g of bagasse were added into 250 mL conical flasks. In the sterilization group, tin foil was used to seal the top of the conical flasks, and this group was autoclaved for 30 min at 121 °C. We added 200 mL of sterile water to each autoclaved conical flask, so that the bagasse dosing ratios (W/V) were 0.5, 1, 1.5, 2, and 2.5%, and the flasks were placed in a shaking incubator at 160 rpm. Samples were collected after 1, 3, 6, 9, 21, 24, 30, and 45 h of shaking, then filtered and tested for chemical oxygen demand (COD).
- (2)
- Shake-flask experiment of batch water replacement
Sterile water (200 mL) was added to 1.0, 2.0, 3.0, 4.0, and 5.0 g of bagasse in 250 mL conical flasks to obtain bagasse dosing ratios (W/V) of 0.5, 1, 1.5, 2, and 2.5%, respectively. The flasks were then placed on the shaking incubator and set to 160 r/min. The supernatant samples were collected after 3 h of shaking, and filtered to detect COD. Subsequently, the liquid inside the conical flasks was drained and replaced with an equal volume of sterile water, and the experiment was continued in the next cycle.
2.2.3. Enhanced Treatment of Low C/N Wastewater with Sugarcane Bagasse by AD Flora
- (1)
- Decarbonization of AD flora
The culture of the AD flora described in Section 2.2.1 was enriched with aerobic denitrification culture medium (C/N of 15) with a high organic matter content for a long time. Previous studies have shown that heterotrophic microflora can convert excessive carbon sources into endogenous carbon sources, such as glycogen and PHA, exhibiting storage of organic matter in the environment [7,15]. To avoid the effect of endogenous carbon sources, the AD flora were decarbonized before the enhancement experiment; that is, two cycles of culture were conducted on the AD flora using an aerobic denitrification medium with a C/N ratio of zero. The culture procedure was the same as that described in Section 2.2.1.
- (2)
- Enhancement of denitrification by AD flora in sugarcane bagasse
Sterilized water was used to adjust the MLSS of the decarbonized AD flora to 3000 mg/L. After settling for 30 min, the aerobic denitrification medium with a C/N ratio of two was used to replace 80% of the supernatant. Then, 100 mL of the mixture were added to a 250 mL conical flask, and bagasse was added at 0, 0.5, 1, 1.5, 2, and 2.5%. The conical flask was placed in an oscillating incubator set at 160 rpm; samples were taken every 10 min for the first 1 h, and every 30 min for the second hour, and NO3−–N was detected after centrifugation.
2.2.4. Small-Scale Treatment of Low C/N Wastewater by Bagasse-Loaded SBBR
The AD flora (obtained as described in Section 2.2.1) were inoculated in the SBR (control group) and the SBBR loaded with bagasse (as shown in Section 2.1.4), and the MLSS was adjusted to 3000 mg/L. Synthetic wastewater containing nitrate (as described in Section 2.1.2) with a C/N ratio of two was the influent, and aeration was performed at an airflow rate of 4 L/min for 2 h. The supernatant samples were taken at 0 and 2 h for NO3−–N detection and at 2 h for COD detection. After 30 min of settling, the culture medium was replaced with 80% of the medium for the next cycle, and a total of 60 cycles were performed.
2.3. Parameter Analysis
The UV spectrophotometric method (HJ/T 346-2007) was used to detect NO3−–N; a UV spectrophotometer (DR6000, HACH, Loveland, CO, USA) was used, and the dual-wavelength isoabsorbance spectrophotometric method, based on the potassium dichromate method (GB 11914-89), was used to detect COD [16]. The MLSS was detected using the weight method; a 100 mL mixture was filtered by quantitative filter paper and dried in an oven at 105 °C to constant weight. The mass before and after drying was weighed.
2.4. Data Analysis
Normality and homoscedasticity were examined, and one-way analysis of variance (ANOVA) was used to compare significant differences between groups. The final data of the parallel samples were expressed as the mean ± standard deviation. A two-sample t-test was used to compare significant differences between the two groups. The Pearson correlation coefficient (R-value) was adopted for correlation analysis, and the significance level (p-value) was set at 0.05. Data analysis and plotting of this experiment were performed using OriginPro (version 2021, OriginLab, Northampton, MA, USA) and Excel (version 2020, Microsoft, Redmond, WA, USA).
Kinetic fitting of the NO3−–N removal process was performed using the integrated equations for first-order reaction kinetics (Equation (1)) and second-order reaction kinetics (Equation (2)):
where C0 is the initial NO3−–N concentration (mg/L), C is the NO3−–N concentration at time t (mg/L), t is the time (min), k1 is the first-order reaction rate constant (min−1), and k2 is the second-order reaction rate constant (L·mg−1·min−1).
3. Results
3.1. Culture of AD Flora
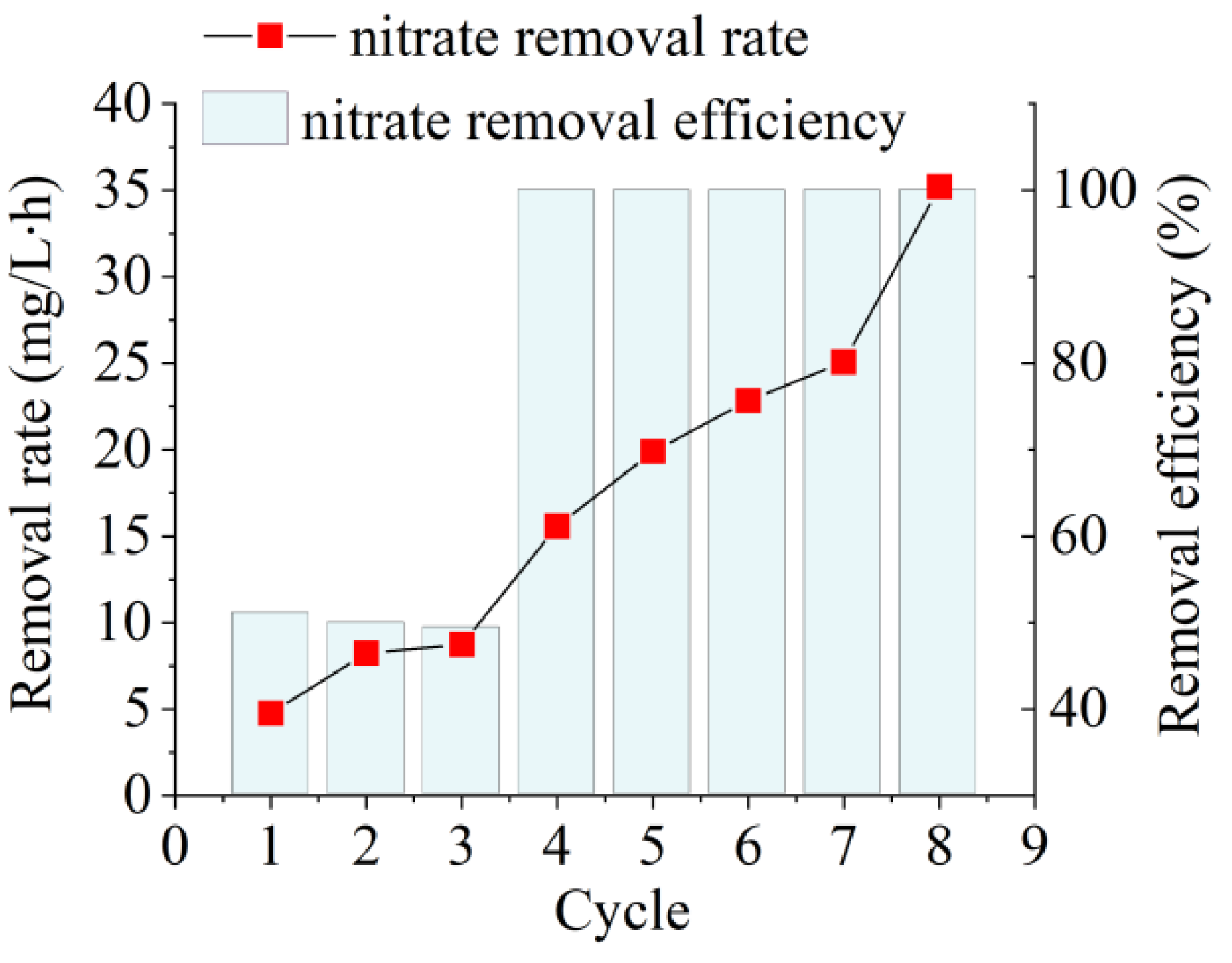
The NO3−–N removal rate of the raw activated sludge was 4.76 mg/L·h (51%) on the first day of incubation (Figure 2). This indicated that the denitrifying bacteria in the activated sludge from a mainstream municipal WWTP using A2/O as the core process were still traditional anoxic denitrifying bacteria. These bacteria use membrane-bound nitrate reductase (NAR) to reduce NO3−–N under anoxic conditions. However, NAR is intolerant to high dissolved oxygen levels, and an increase in oxygen inhibits denitrification [17]. During the AD flora enrichment, high dissolved oxygen was used as a stress condition to reduce the abundance of traditional anoxic denitrifying flora utilizing NAR and enhance the abundance of AD flora utilizing periplasmic nitrate reductase (NAP). The NO3−–N removal rate was enhanced by 4.09 mg/L h (y = 4.0932 × −0.8792, R2 = 0.9597) for each incubation cycle during the incubation, suggesting a rapid enrichment in AD flora. After nine cycles of enrichment culture, the NO3−–N removal rate of the resulting AD flora reached 35.20 mg/L·h, which was 7.40 times higher than that of raw activated sludge.
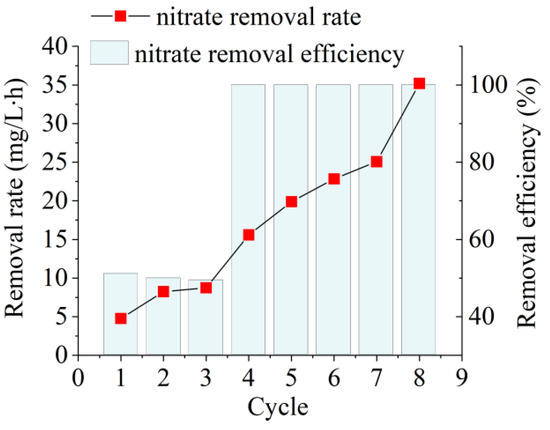
Figure 2.
Nitrate removal performance of aerobic denitrification (AD) flora during enrichment of raw activated sludge obtained from a traditional water treatment processing plant.
3.2. Carbon Source Release from Sugarcane Bagasse
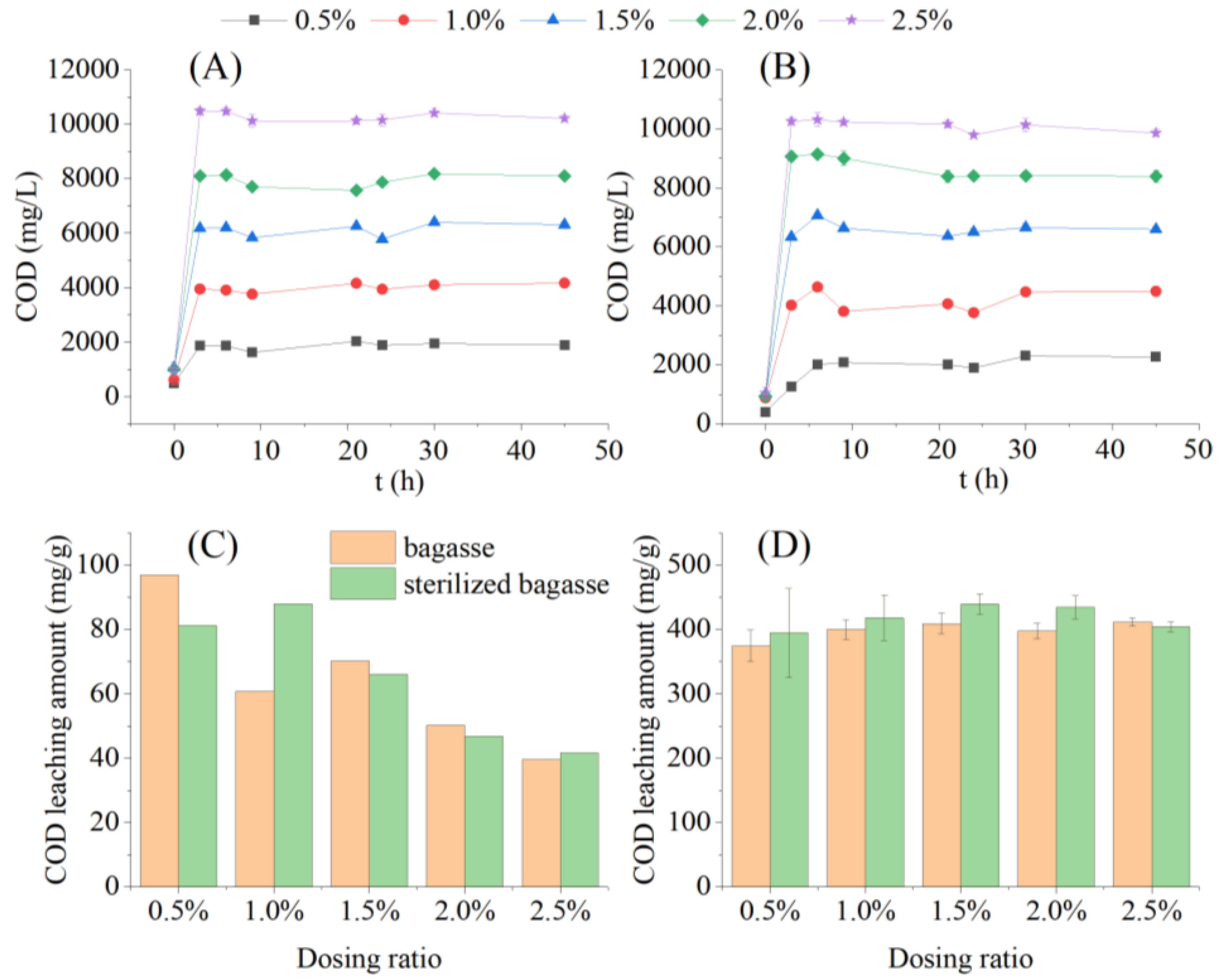
After adding the bagasse and sterilized bagasse at 0.5 to 2.5% to sterilized water for one min, the COD concentration in the liquid phase reached 406.00 ± 18.55 to 1052.92 ± 51.87 mg/L, which showed the rapid dissolution of the soluble organic matter from the bagasse (Figure 3A). The COD leaching amount decreased from 96.95 and 81.20 mg/g to 39.62 and 41.67 mg/g, respectively, as the dosing ratio increased (Figure 3C). The dosing ratio was significantly negatively correlated with the COD leaching amount of sugarcane bagasse (R = −0.9034, p = 0.0355 < 0.05) and sterilized bagasse (R = −0.9313, p = 0.0214 < 0.05), indicating that a relatively lower dosing ratio was beneficial for the rapid leaching of soluble carbon sources from bagasse in a short time.
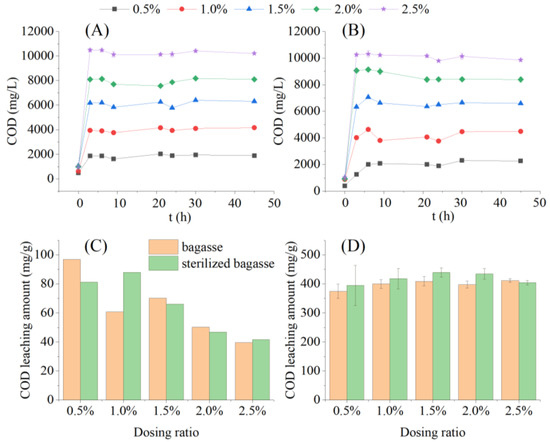
Figure 3.
Chemical oxygen demand (COD) release from sugarcane bagasse and sterilized bagasse under different dosing ratios. Note: Error bars in (D) indicate the standard deviation of COD leaching from 3 to 45 h. (A) COD leaching concentration of bagasse; (B) COD leaching concentration of sterilized bagasse; (C) COD leaching amount within 3 h; (D) COD leaching amount within 3–45 h.
The concentration of dissolved COD from the bagasse and sterilized bagasse reached a maximum after 3 h of oscillation, and remained stable for the next 42 h (Figure 3B). The COD concentration in the liquid phase increased with a higher dosing ratio. The average COD concentration in the liquid phase increased from 1872.52 ± 114.42 to 10287.36 ± 155.02 mg/L within 3–42 h in the sugarcane bagasse and sterilized bagasse groups at dosing ratios of 0.5 to 2.5%. This corroborated the findings of Di et al. [18] during an investigation on the influence of pH conditions on the carbon release effect of bagasse, who observed that the carbon source was released quickly in a short period and stabilized in the later period. As shown in Figure 3D, one-way ANOVA showed that, during the stable period of COD leaching (3 to 42 h), the dosing ratio had no significant effect on the average COD leaching amount of the bagasse and sterilized bagasse groups (F = 1.4359; n = 10; homogeneity of variance; p < 0.05). The average COD leaching amount of bagasse was 418.07 ± 17.05 mg/g, which is comparable to that of corn cob when used as a carbon source (500 mg COD/g corn cob) during nitrogen removal in sewage treatment [19]. Sterilization had no significant effect on the carbon source release of the bagasse (t = 0.2220, n = 160).
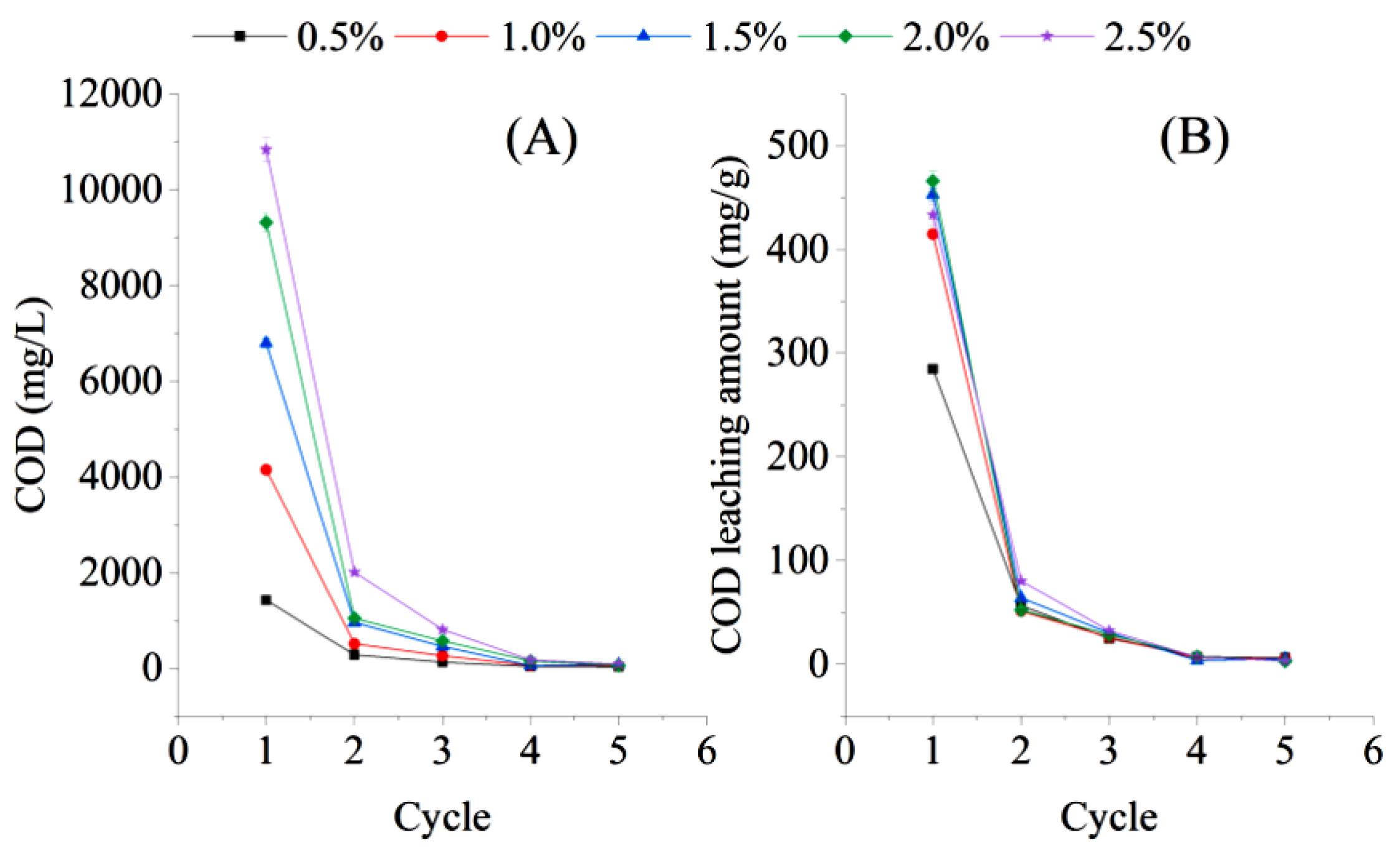
The COD leaching concentration and the amount of bagasse under batch water replacement conditions decreased rapidly with increasing water exchange batches (Figure 4). After the first cycle of water replacement, the COD concentration in cycle 2 under different dosing ratio conditions significantly decreased to 11.22–19.84% of that in cycle 1 (t = 3.2024, n = 10). Then, the COD concentration in cycle 3 decreased to 124.92–812.83 mg/L, which was only 6.16–8.77% of that in cycle 1. The COD concentrations of the liquid phase in cycles 5 and 6 were only 31.25–171.25 mg/L, which is not a sufficient carbon source for wastewater treatment. The release of carbon from the bagasse could only be maintained for three cycles under the batch water replacement conditions. However, because the time of each cycle was set at three hours, the effect of the long-term release of the carbon source from sugarcane bagasse after multiple water changes still needs to be further explored.
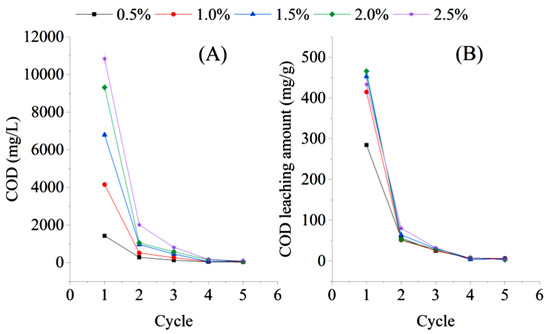
Figure 4.
Chemical oxygen demand (COD) leaching concentration (A) and COD leaching amount (B) of bagasse under batch water replacement conditions during an experiment on the feasibility of using sugarcane bagasse as a carbon source in aerobic denitrification.
3.3. Enhanced Treatment of Low C/N Wastewater with Sugarcane Bagasse by AD Flora
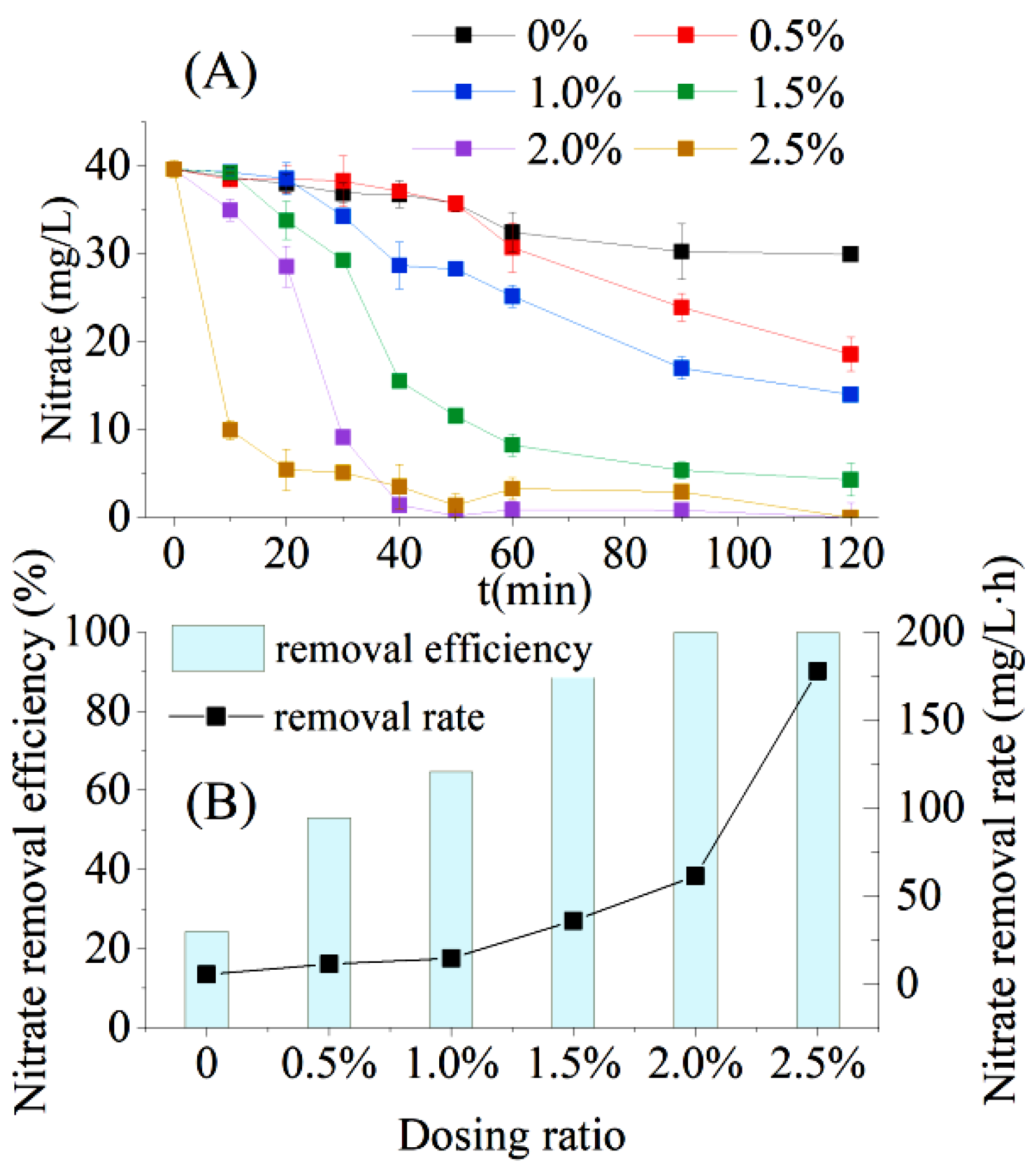
Without bagasse dosing, the NO3−–N removal efficiency of the AD flora was 24.40%, and the NO3−–N removal rate was 5.40 mg/L·h for synthetic wastewater containing nitrate (C/N = 2) within 2 h (Figure 5). The NO3−–N removal efficiency and NO3−–N removal rate increased significantly with increasing bagasse dosage (Figure 5B). The NO3−–N removal efficiency (R = 0.9636, p = 0.02 < 0.05) and NO3−–N removal rate (R = 0.8444, p = 0.03 < 0.05) of the AD flora were significantly positively correlated with the bagasse dosing ratio. Furthermore, the denitrification of the AD flora was strongly dependent on the availability of carbon sources, which corresponded to the findings reported in the existing literature on AD bacteria, where small-molecule carbon sources and high C/N ratios were more favorable for the denitrification process of AD bacteria [20,21].
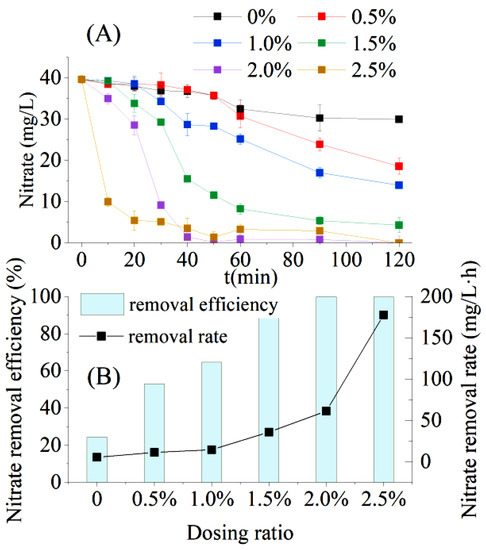
Figure 5.
Performance of bagasse-enhanced aerobic denitrification (AD) flora in low C/N wastewater treatment. (A) Changes in nitrate concentration under different dosing ratios; (B) nitrate removal efficiency and nitrate removal rate under different additive ratios.
Kinetic parameters were obtained from the first- and second-order kinetic fittings of NO3−–N removal (Figure 5A), and for the AD flora under different dosing ratio conditions (Table 2). The R2-values of the first-order kinetic fitting of NO3−–N removal under all dosing ratios were higher than those obtained by the second-order, except for the bagasse dosing ratio of 0.5%, indicating that the removal of NO3−–N by AD flora was best fit by the first-order kinetic equation. Jun et al. [22] and Parangya et al. [23] performed kinetic studies on nitrogen removal by pure AD bacteria, using a first-order kinetic equation to fit the removal of NO3−–N by the AD bacteria Acinetobacter sp. SYF26 and Bacillus cereus GS-5, and obtained an R2-value higher than 0.90. For the first-order kinetic parameters (Table 2), the R2-value gradually decreased as the bagasse dosing ratio increased, indicating that the NO3−–N removal rate was less affected by the NO3−–N substrate concentration when the carbon source was abundantly available to the AD flora.

Table 2.
Kinetic parameters of nitrate removal by aerobic denitrification flora under different bagasse dosing ratios.
Without sugarcane bagasse dosing, the NO3−–N concentration slowly decreased and remained stable between 1 and 2 h. Therefore, the requirement of COD per unit of nitrate removal (△COD/△NO3−–N) of AD flora was 8.28 mg/L, which could be considered as the minimum C/N requirement for the AD flora to completely remove NO3−–N. When the bagasse dosing ratio was 0.5%, approximately 400 mg/L of COD could be dissolved in a short time (Section 3.2). Together with approximately 100 mg/L of COD in the nitrate-containing synthetic wastewater (C/N = 2), the actual C/N in the final system reached ten in the group with a dosing ratio of 0.5%, which is higher than the aforementioned minimum C/N requirement for complete nitrate removal by AD flora. Therefore, in actual wastewater treatment, considering the costs, the amount of bagasse added can be selected as 0.5%.
3.4. Treatment of Low C/N Wastewater by AD Flora in Bagasse-Loaded SBBR
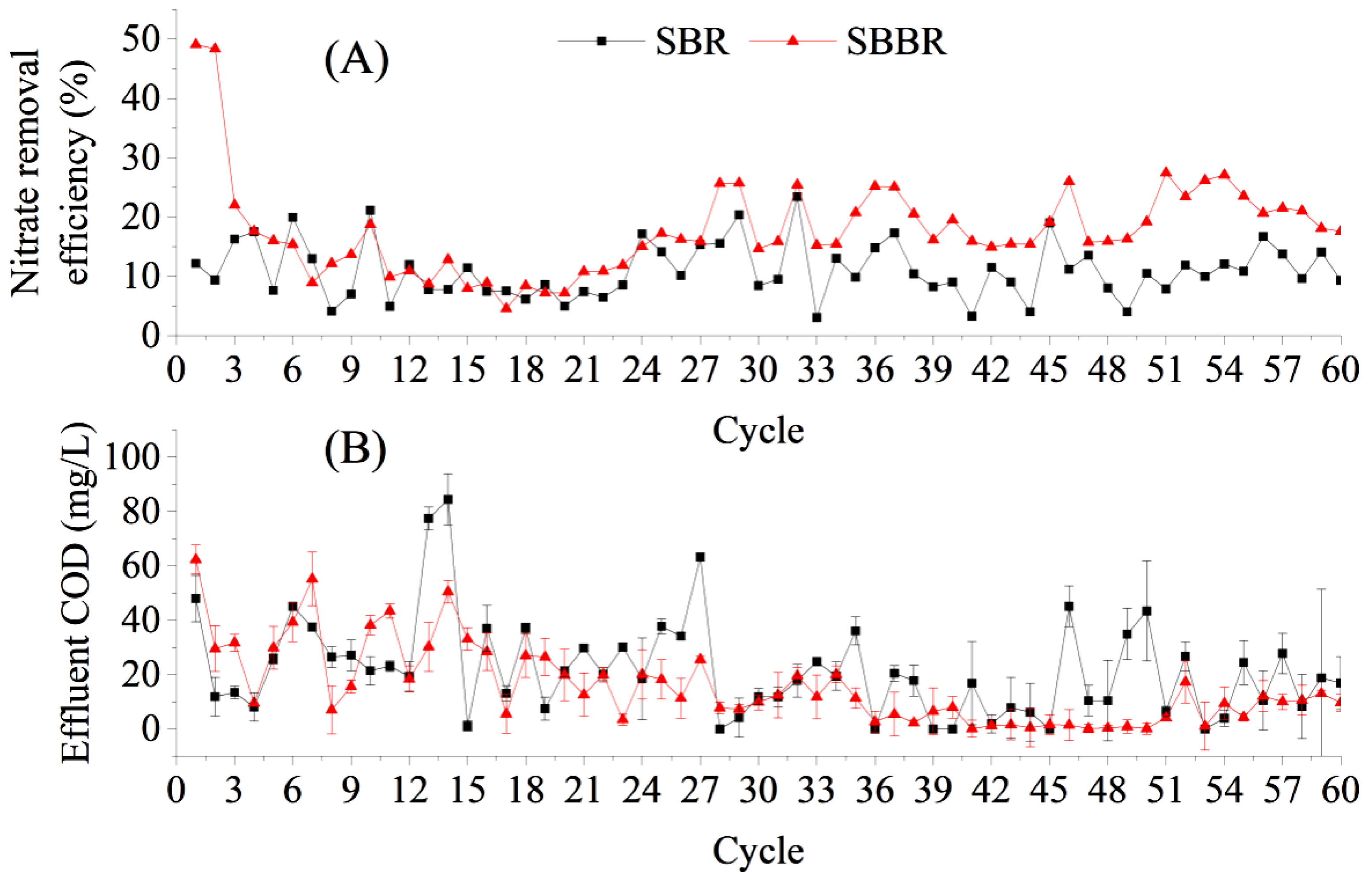
In the first cycle of operation, the NO3−–N removal rate of the SBBR loaded with bagasse was 49.09%, while the NO3−–N removal rate of the SBR (the control group) was only 12.20%, which was related to the rapid release of carbon from the sugarcane bagasse in the SBBR. In the second cycle of operation, the NO3−–N removal rate was maintained at a high level of 48.36%, although the leached COD concentration of the sugarcane bagasse decreased significantly at this time compared to that in the first cycle. Heterotrophic denitrifying microorganisms can convert carbon sources into intracellular carbon sources, such as glycogen and PHA, for storage under conditions with sufficient carbon sources, and these endogenous carbon sources can provide electron donors for denitrification under conditions of carbon source deficiency [24,25]. With an insufficient carbon source supply, the NO3−–N removal rate of the SBBR decreased to 22.02% by the third cycle of operation, which was only 5.74% higher than that of the control.
The NO3−–N removal rate of the SBBR was 11.39 ± 3.91% during the 4th to 19th cycles of operation, which was not significantly different from that of the control (t = 0.6865, n = 16). The carbon release of the bagasse decreased sharply with an increase in water exchange, indicating that the SBBR did not have an advantage over the control during this period (Figure 4). However, the NO3−–N removal rate of the SBBR started to increase gradually from the 20th cycle and reached 20.20 ± 4.27% after 28 cycles, which was significantly higher than that of the SBR (11.33 ± 4.64%) within the same phase (t = 7.9568, n = 33). This may be related to the release of small-molecule carbon sources from the decomposition of cellulose and lignin during long-term liquid-phase immersion and microbial exposure to the sugarcane bagasse [26,27]. However, the sampling time set in the carbon source release experiment (Section 3.2) in this study was too short, and this hypothesis needs to be further verified. In addition, biofilms gradually formed on the surface of the sugarcane bagasse, which acted as a filler in the SBBR. Attached-grown activated sludge systems have been found to have stronger impact resistances and usually exhibit better denitrification than suspended-grown activated sludge systems [28,29].
There was no significant difference in the SBBR effluent COD concentration compared to that of the SBR during the 60 cycles (t = −2.0385; n = 60; Figure 6B). During the first 36 cycles, the effluent COD of both the SBBR and SBR decreased. Overall, the COD removal effect of both reactors was stable, and the COD concentrations were below 50 mg/L in 95% of all effluent samples, which reached the Class I and A standards of the Discharge Standard for Pollutants from Urban Wastewater Treatment Plants (GB 18918-2002).
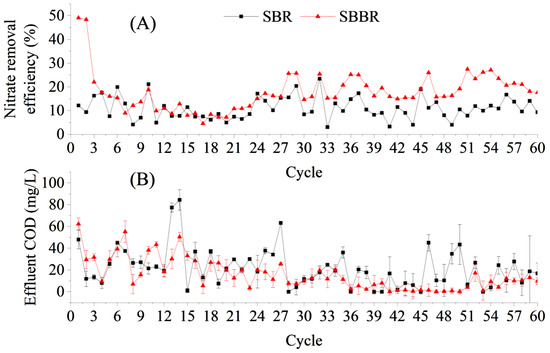
Figure 6.
Performance of aerobic denitrification flora for NO3−–N and chemical oxygen demand (COD) removal of bagasse-loaded sequencing batch biofilm reactor (SBBR) for treating low C/N wastewater. (A) Nitrate removal efficiency in SBR and SBBR; (B) Effluent COD in SBR and SBBR.
4. Discussion
Sugarcane bagasse has a high cellulose content, contains a small molecular carbon source, and has high porosity. Various natural biomasses have been shown to provide carbon sources and fillers for AD bacteria [10,30,31]; however, there is a lack of relevant reports on the use of sugarcane bagasse. Therefore, this study explored the feasibility of sugarcane bagasse as a carbon source and filler for AD flora, and found that the carbon source released from sugarcane bagasse could be applied for the denitrification of AD flora; the construction of an SBBR with sugarcane bagasse as a carbon source and filler could promote NO3−–N removal by AD flora.
The potential of sugarcane bagasse as a microbial solid carbon source has been demonstrated previously. For example, Zhang [32] examined the carbon release capacity of ten agricultural wastes and found that sugarcane bagasse had the highest total organic carbon leaching capacity of 38.66 mg/g, followed by soybean straw, rice straw, corn straw, rice husk, and poplar branches. Zhong et al. [33] studied the carbon leaching capacity of five plant materials, sugarcane bagasse, windmill grass, bamboo reed, plantain, and rice straw, and sugarcane bagasse showed carbon release advantages over the other materials. The COD leaching of sugarcane bagasse exceeded 400 mg/g in this experiment (Figure 3), indicating excellent carbon release. However, the leaching capacity of the sugarcane bagasse decreased rapidly after batch water exchange (Figure 4), similar to the results obtained by Wang et al. [34]. Therefore, despite the high carbon release capacity of sugarcane bagasse in a short time, the carbon release capacity decreased rapidly during the late stage of batch water exchange and was not suitable for wastewater treatment with high carbon source demand.
The results of this study showed that the carbon source released from sugarcane bagasse could be utilized by AD flora (Figure 5). The sugarcane-bagasse-loaded SBBR inoculated with AD flora operated stably for 60 cycles with approximately 20% NO3−–N removal (Figure 6), which compared poorly to the NO3−–N removal obtained from other sugarcane bagasse-constructed reactors in previous publications. For example, Sun et al. [35] constructed a biofiltration system using sugarcane bagasse (2.5% dosing ratio), sandy loam soil, and lake bottom sediment to enhance anoxic denitrification, and the NO3−–N removal rate could exceed 60%, while Sareh et al. [36] constructed a column reactor using sugarcane bagasse (30%) and soil (70%) to treat nitrate-containing synthetic wastewater, and this reactor operated stably for 98 d, with the highest NO3−–N removal efficiency and removal rate reaching up to 85% and 50 mg/L, respectively. The low nitrate–nitrogen removal performance of the SBBR in this experiment compared with the others mentioned above was related to the low bagasse dosing ratio (0.5%), the differences in reactor type, and the denitrification type. In general, sugarcane bagasse, as an additional carbon source, can enhance anoxic denitrification by denitrifying bacteria, and the results of this experiment suggest that the application of sugarcane bagasse to denitrification by AD bacteria is also feasible.
The practical application of sugarcane bagasse as a carbon source and filler to enhance denitrification is mainly based on the biofilm wastewater treatment process [35]. For example, Wang et al. [34] used sugarcane bagasse and iron as raw materials to fabricate a composite filter material to construct a denitrification filter reactor, and used sugarcane bagasse at an 8% dosing ratio to construct a tubular biological wastewater purification device, both of which achieved good denitrification performance [37]. The above reports and the results of this experiment indicate that the biofilm wastewater treatment process is an effective method for the practical application of AD with sugarcane bagasse. However, it is worth noting that in the study by Wang and Cui, the influent water was the tail water of WWTPs and rural domestic wastewater treatment facilities, with a low NO3−–N concentration when the total nitrogen removal rate of the reactors constructed with sugarcane bagasse exceeded 80% [34,37]. Accordingly, it is speculated that a high bagasse dosing ratio and low influent nitrogen load are favorable for long-term and stable NO3−–N removal in the practical application of the bagasse-enhanced AD process.
5. Conclusions
Sugarcane bagasse can serve as a carbon source that can easily be utilized by microorganisms over a short period. High-temperature and high-pressure pretreatments did not significantly affect the carbon release capacity of bagasse. Sugarcane bagasse as an additional carbon source performed remarkably well in enhancing the AD flora to treat low-C/N-nitrate-containing synthetic wastewater. In the SBBR constructed with sugarcane bagasse, bagasse as the carbon source and filler promoted the denitrification effect of the AD flora in low C/N wastewater. The nitrate removal efficiency was significantly higher than that of the control, and the effluent COD could stably reach the Class I and A standards of the Discharge Standard for Pollutants from Urban Wastewater Treatment Plants (GB 18918-2002). The results of this study provide a reference for the application of AD in low C/N wastewater treatment, and for biomass resource recovery and utilization.
Author Contributions
Conceptualization, M.C. and X.M.; methodology, Q.T. and J.Z.; investigation, M.C. and X.L.; resources, Y.D.; funding acquisition, S.M. and X.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Research Project of the Leshan Science and Technology Bureau (20SZD066) and the Key Laboratory of Development and Application of Rural Renewable Energy, through the Ministry of Agriculture and Rural Affairs in China (2019010).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, J.; Feng, L.; Pi, S.; Cui, D.; Ma, F.; Zhao, H.P.; Li, A. A critical review of aerobic denitrification: Insights into the intracellular electron transfer. Sci. Total Environ. 2022, 731, 139080. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.L.; Ali, A.; Ren, Y.; Su, J.F.; Wang, Z. A mechanistic review on aerobic denitrification for nitrogen removal in water treatment. Sci. Total Environ. 2022, 847, 157452. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, W.; Liang, D.; Zhang, J.; Li, J.; Li, P.; Wu, Y.; Bian, X.; Ding, F. Rapid start-up and advanced nutrient removal of simultaneous nitrification, endogenous denitrification and phosphorus removal aerobic granular sequence batch reactor for treating low C/N domestic wastewater. Environ. Res. 2022, 212, 113464. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Quan, J.; Huang, K.; Zhao, J.; Xing, G.; Wu, P.; Chen, Y.; Ding, X.; Hu, Y. Effects of C/N ratio and dissolved oxygen on aerobic denitrification process: A mathematical modeling study. Chemosphere 2021, 272, 129521. [Google Scholar] [CrossRef]
- Wang, X.J.; Wang, D.S.; Li, J.; Du, S.D.; Zhou, T.; Wu, G.D. Separation identification and the characteristics research of simultaneous removal of nitrogen and carbon about Marine heterotrophic nitrification and aerobic denitrification strain y6. China Environ. Sci. 2017, 37, 686–695. [Google Scholar] [CrossRef]
- Wiboonluk, P.; Cholticha, P.; Sorawit, P. Optimization and evaluation of a bottom substrate denitrification tank for nitrate removal from a recirculating aquaculture system. J. Environ. Sci. 2013, 25, 1557–1564. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Fan, Y.; Liu, Y.; Yu, M.; He, C.; Wu, J. Bioaugmentation of low C/N ratio wastewater: Effect of acetate and propionate on nutrient removal, substrate transformation, and microbial community behavior. Bioresour. Technol. 2019, 306, 122465. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biotechnol. Adv. 2016, 34, 1103–1112. [Google Scholar] [CrossRef]
- Fu, X.; Hou, R.; Yang, P.; Qian, S.; Feng, Z.; Chen, Z.; Wang, F.; Yuan, R.; Chen, H.; Zhou, B. Application of external carbon source in heterotrophic denitrification of domestic sewage: A review. Sci. Total Environ. 2022, 817, 153061. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Y.; Liang, D.; Wang, G.; Xie, J.; Zhu, X. Enhanced denitrification of sewage via bio-microcapsules embedding heterotrophic nitrification-aerobic denitrification bacteria Acinetobacter pittii SY9 and corn cob. Bioresour. Technol. 2022, 358, 127260. [Google Scholar] [CrossRef]
- Feng, L.; Pi, S.; Zhu, W.; Wang, X.; Xu, X. Nitrification and aerobic denitrification in solid phase denitrification systems with various biodegradable carriers for ammonium-contaminated water purification. J. Chem. Technol. Biotechnol. 2019, 94, 3569–3577. [Google Scholar] [CrossRef]
- Bilba, K.; Arsene, M.A. Silane treatment of bagasse fiber for reinforcement of cementitious composites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1488–1495. [Google Scholar] [CrossRef]
- Bao, G.Y.; Lan, Y.H. Sugarcane biorefinery—New development on utilization of bagasse. Sugarcane Canesugar. 2011, 5, 59–65. [Google Scholar] [CrossRef]
- Ajala, E.O.; Ighalo, J.O.; Ajala, M.A.; Adeniyi, A.G.; Ayanshola, A.M. Sugarcane bagasse: A biomass sufficiently applied for improving global energy, environment and economic sustainability. Bioresour. Bioprocess. 2021, 8, 87. [Google Scholar] [CrossRef]
- Hu, B.; Wang, T.; Ye, J.; Zhao, J.; Yang, L.; Wu, P.; Duan, J.; Ye, G. Effects of carbon sources and operation modes on the performances of aerobic denitrification process and its microbial community shifts. J. Environ. Manag. 2019, 239, 299–305. [Google Scholar] [CrossRef]
- Yang, X.R. Determination of COD by Doal-wavelength Equivalent Absorbance Spectrophotometry. J. Wuhan Univ. Technol. 2010, 32, 177–180. [Google Scholar] [CrossRef]
- Wang, J.; Rong, H.; Zhang, C. Evaluation of the impact of dissolved oxygen concentration on biofilm microbial community in sequencing batch biofilm reactor. J. Biosci. Bioeng. 2018, 125, 532–542. [Google Scholar] [CrossRef]
- Di, J.Z.; Li, T.D.; Zhao, W. Release law of sugarcane slag carbon source and orthogonal testing of sulfate-reducing bacteria. J. Agro-Environ. Sci. 2019, 38, 1151–1157. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; Jiang, X.; Zhou, P.; Zhang, J.; Zheng, Z. Treatment of artificial secondary effluent for effective nitrogen removal using a combination of corncob carbon source and bamboo charcoal filter. Int. Biodeterior. Biodegrad. 2016, 115, 164–170. [Google Scholar] [CrossRef]
- Pan, Z.; Zhou, J.; Lin, Z.; Wang, Y.; Zhao, P.; Zhou, J.; Liu, S.; He, X. Effects of COD/TN ratio on nitrogen removal efficiency, microbial community for high saline wastewater treatment based on heterotrophic nitrification-aerobic denitrification process. Bioresour. Technol. 2020, 301, 122726. [Google Scholar] [CrossRef]
- Wang, T.; Chen, M.; Liang, X.; Chen, F.; He, T.; Li, Z. Corrigendum: The alkali-tolerant bacterium of Bacillus thuringiensis EM-A1 can effectively perform heterotrophic nitrification and aerobic denitrification. Front. Environ. Sci. 2022, 19, 818316. [Google Scholar] [CrossRef]
- Su, J.F.; Ma, M.; Ma, F.; Lu, J.S. Kinetic analysis of heterotrophic nitrification -aerobic denitrification by an oligotrophic Acinetobacter sp. SYF26. Environ. Eng. Sci. 2017, 34, 844–851. [Google Scholar] [CrossRef]
- Prangya, R.R.; Puspendu, B.; Rajesh, R.D. Simultaneous removal of nitrogen and phosphorous from domestic wastewater using Bacillus cereus GS-5 strain exhibiting heterotrophic nitrification, aerobic denitrification and denitrifying phosphorous removal. Bioresour. Technol. 2017, 244, 484–495. [Google Scholar] [CrossRef]
- Zeng, M.; Zeng, Y.; Zhang, B.; Cheng, Y.; Long, B.; Wu, J.; Ren, S.; Liu, Y. Coupling of endogenous/exogenous nitrification and denitrification in an aerobic granular sequencing batch reactor. Environ. Technol. 2022, 20, 68380. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ma, B.; Li, X.; Zhang, Q.; Peng, Y. Advanced nitrogen removal without addition of external carbon source in an anaerobic/aerobic/anoxic sequencing batch reactor. Bioprocess Biosyst. Eng. 2019, 42, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Xiao, L.Y.; Hai, L.S.; Jia, J.W.; Jia, Y.X. Alkali-treated cellulose carrier enhancing denitrification in membrane bioreactor. Int. Biodeter. Biodegr. 2019, 145, 104813. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, B.; Xie, F.; Zhang, X.; Zhou, A.; Wang, S.; Yue, X. Study on the preparation and feasibility of a novel adding-type biological slow-release carbon source. J. Environ. Manag. 2022, 316, 115236. [Google Scholar] [CrossRef]
- Simon, A.; Luca, Q.; Bert, G.; Inez, D.; Simon, B.; Monica, A.; Tomas, A.; Fernando, M.; Anton, K.; Markus, H.; et al. Integration of biopolymer production with process water treatment at a sugar factory. New Biotechnol. 2014, 31, 308–323. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Liu, Z.; Yang, W.; Hu, J.; Jia, Z.; Hu, B. Biofilm: A strategy for the dominance of comammox Nitrospira. J. Clean. Prod. 2022, 363, 132316. [Google Scholar] [CrossRef]
- Chang, J.; Ma, L.; Zhou, Y.; Zhang, S.; Wang, W. Remediation of nitrate-contaminated wastewater using denitrification biofilters with straws of ornamental flowers added as carbon source. Water Sci. Technol. 2016, 74, 416–423. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, E.; Sun, S.; Tao, X.; Zhong, L.; Hu, K. Piggery wastewater treatment by Acinetobacter sp. TX5 immobilized with spent mushroom substrate in a fixed-bed reactor. Sci. Total Environ. 2018, 644, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. The Research and Application of Sustainable Long-release Carbon Material with Agricultural Waste. Ph.D. Thesis, Nanjing University, Nanjing, China, January 2017. [Google Scholar] [CrossRef]
- Zhong, S.Q.; Yang, Y.; Tao, R.; Li, L.; Zhang, M.; Zhao, J.C. Carbon releasing characteristics and denitrification effects of five plant materials. Chin. J. Environ. Eng. 2014, 8, 1817–1824. Available online: http://www.cjee.ac.cn/article/id/20140520?viewType=HTML (accessed on 1 September 2022).
- Wang, P.C. Performance of Nitrogen and Antibiotics Removal from the Effluent of Wastewater Treatment Plants by Biomass-iron Mixture Denitrification Biological Filter. Master’s Thesis, East China Normal University, Shanghai, China, May 2021. [Google Scholar] [CrossRef]
- Sun, F. The Study on the Improving Technology of Nitrogen and Phosphorus Removal Efficiency in Biofiltration System by Different’ Electron Donors. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, June 2021. [Google Scholar] [CrossRef]
- Sareh, T.; Hadi, M.; Abd, A.N.; Seyyed, E.H.G.; Saeed, B.; Amit, B. Investigation on the performance of sugarcane bagasse as a new carbon source in two hydraulic dimensions of denitrification beds. J. Clean. Prod. 2017, 140, 1176–1181. [Google Scholar] [CrossRef]
- Cui, H. A Tubular Bio-reactor Device Used for Enhancing Denitrification of the Effluent from Rural Wastewater Treatment Facilities and the Demonstration Project. Ph.D. Thesis, East China Normal University, Shanghai, China, May 2019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).