Study on the Migration Law of Dissolved Organic Matter in Mine Water Treatment Station
Abstract
:1. Introduction
2. Materials and Methods
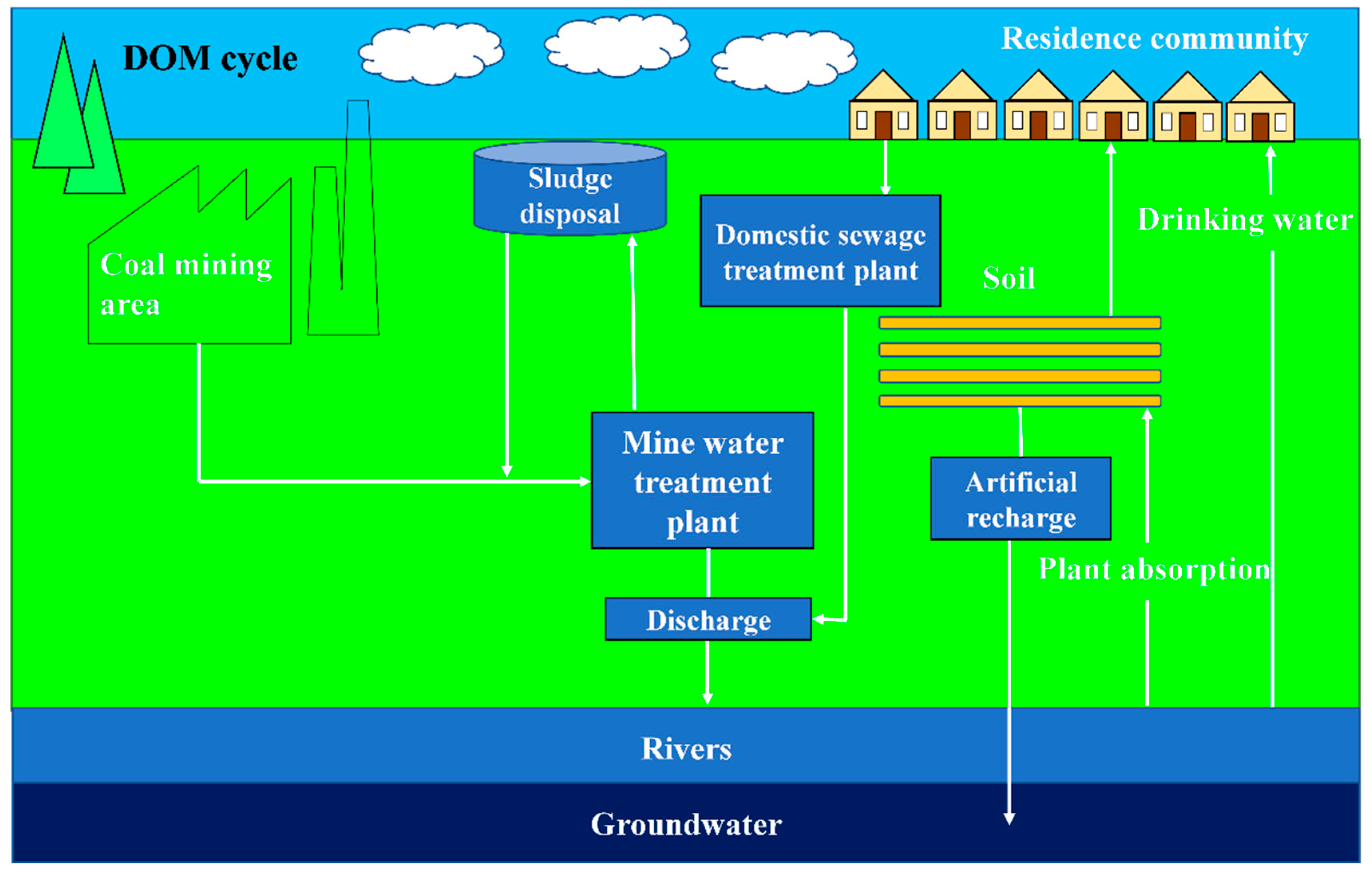
2.1. Study Area
2.2. Field Sampling
2.3. Parameters Measuring
2.3.1. Physicochemical Properties
2.3.2. TOC/UV254
2.3.3. EEM
2.4. Statistical Analysis
3. Results
3.1. Comparison of Biogeochemical Characteristics
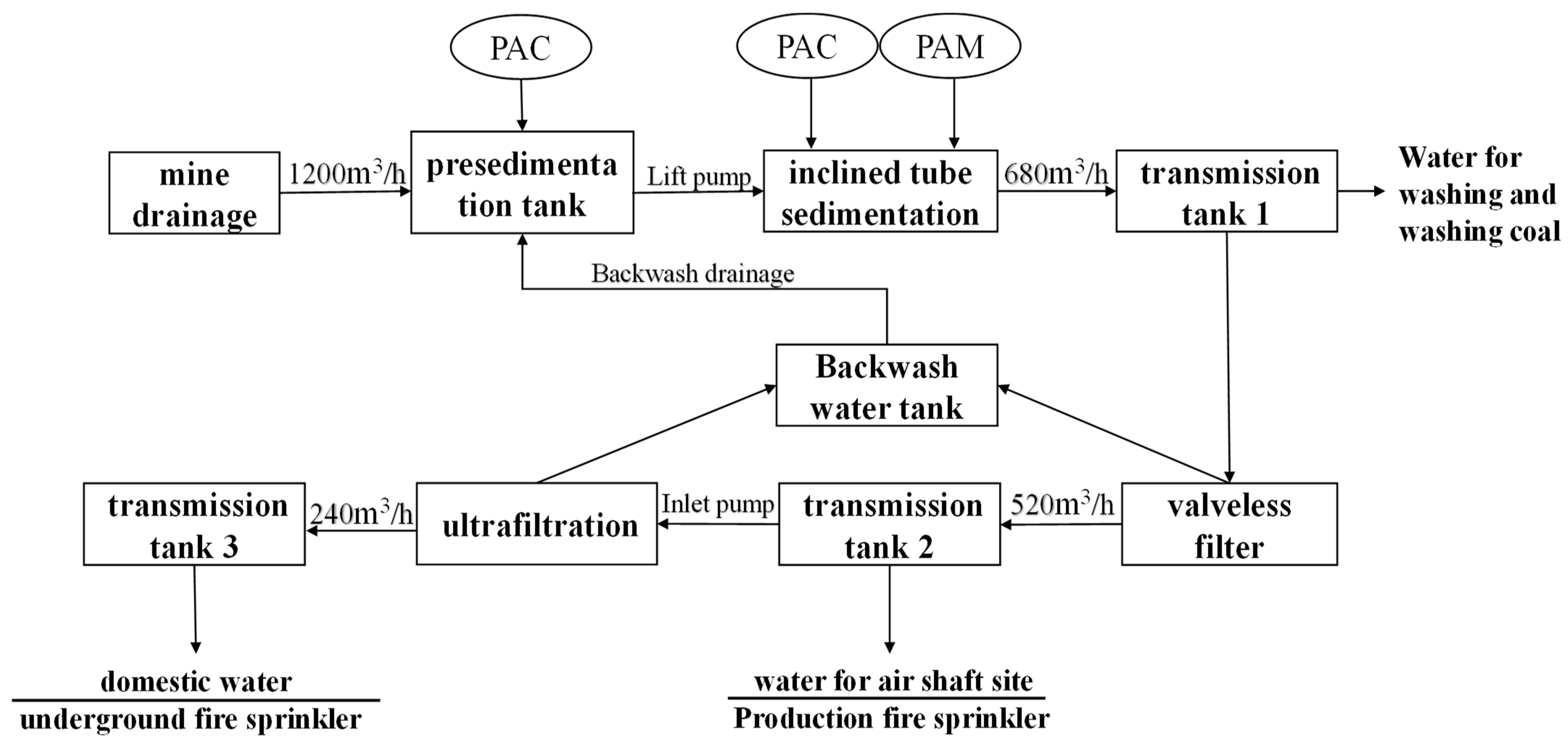
3.2. Water Quality and DOM Characteristics of the Influent and Effluent Water in the Caojiatan Coal Mine Water Treatment Station
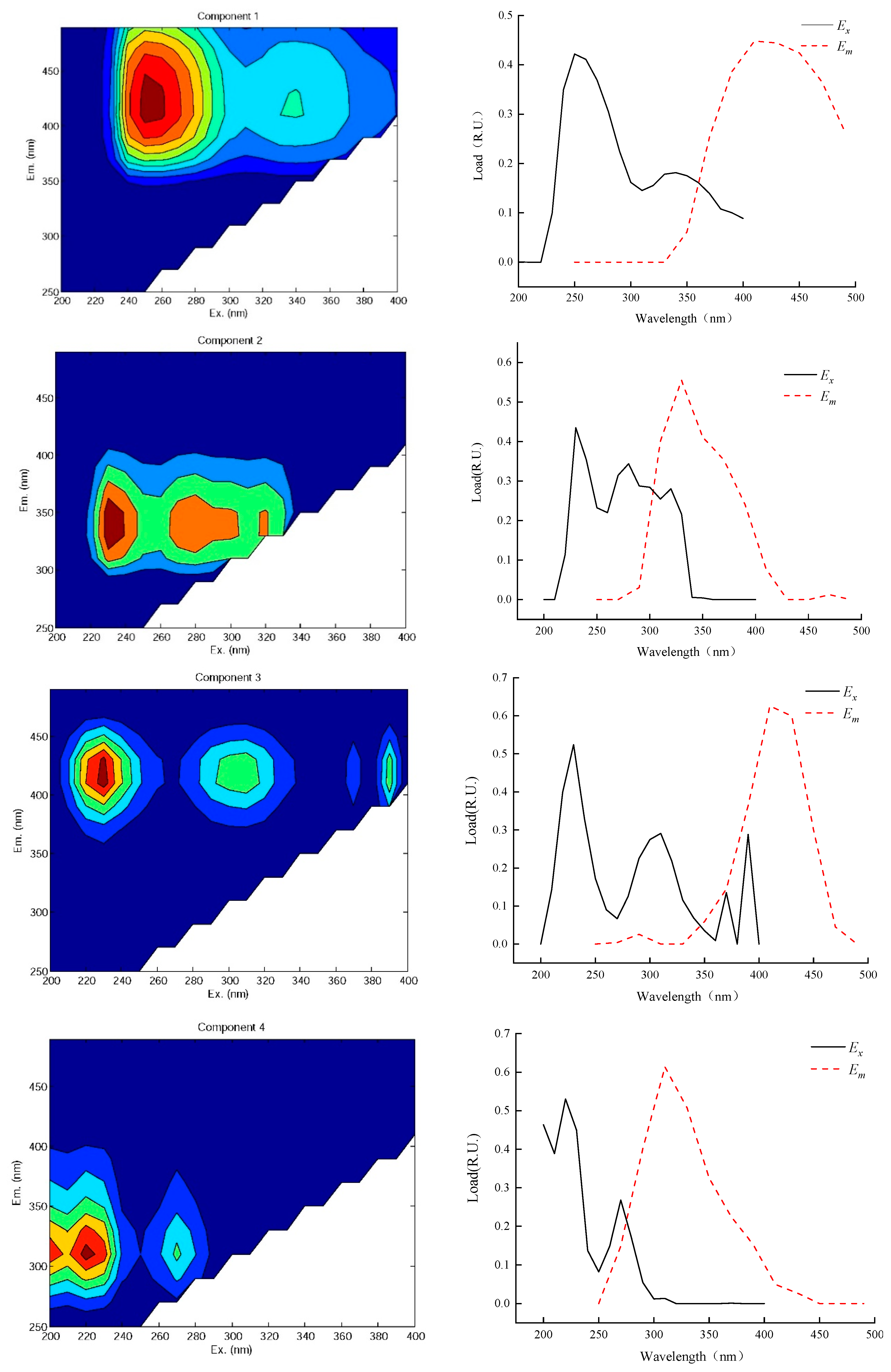
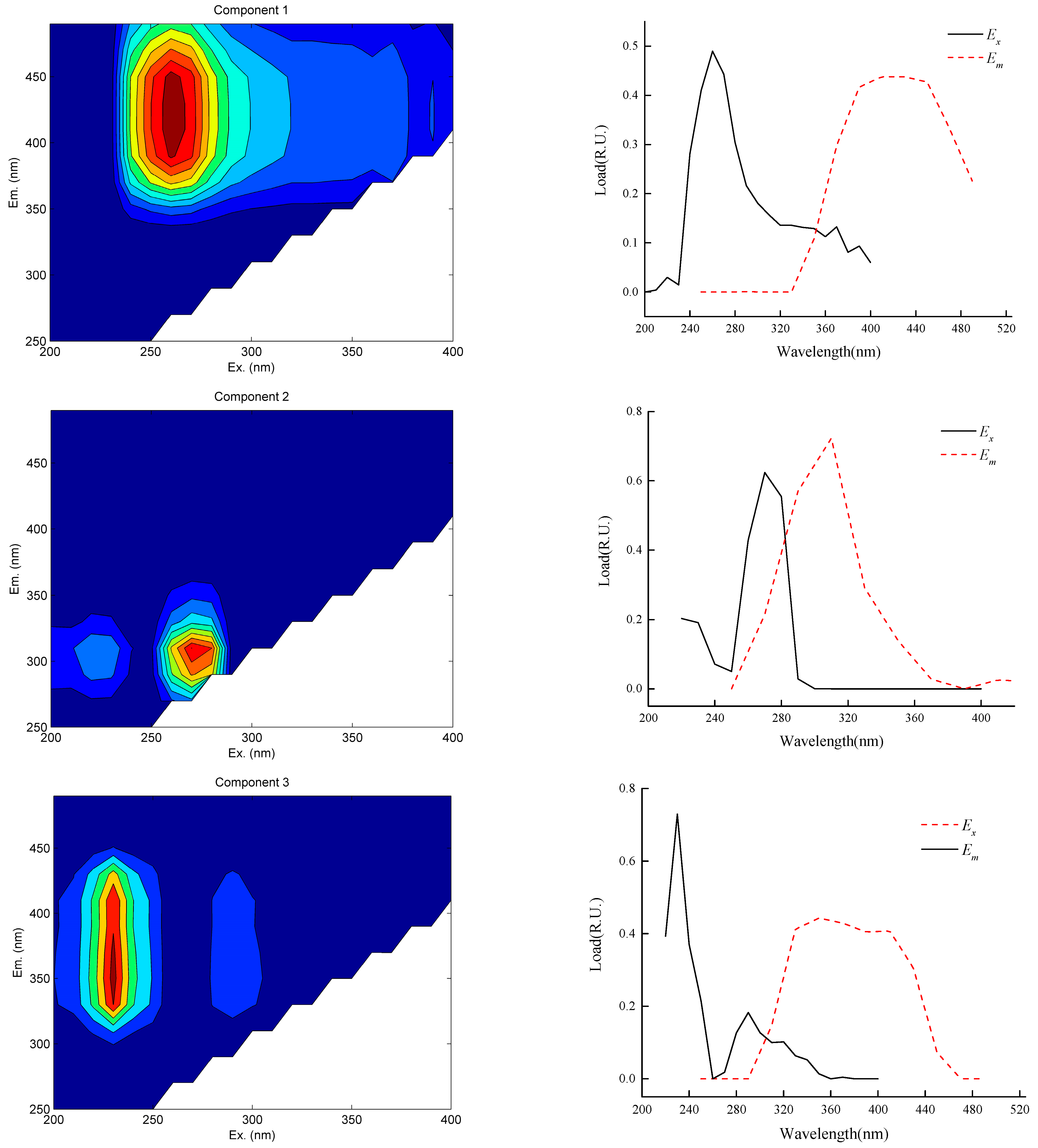
3.3. DOM Characteristics of the Water Influent and Effluent of Caojiatan Mine Water Treatment Station
3.4. Discussion on Removal Technology of Petroleum Pollutants Based on Tyrosine Components
4. Conclusions
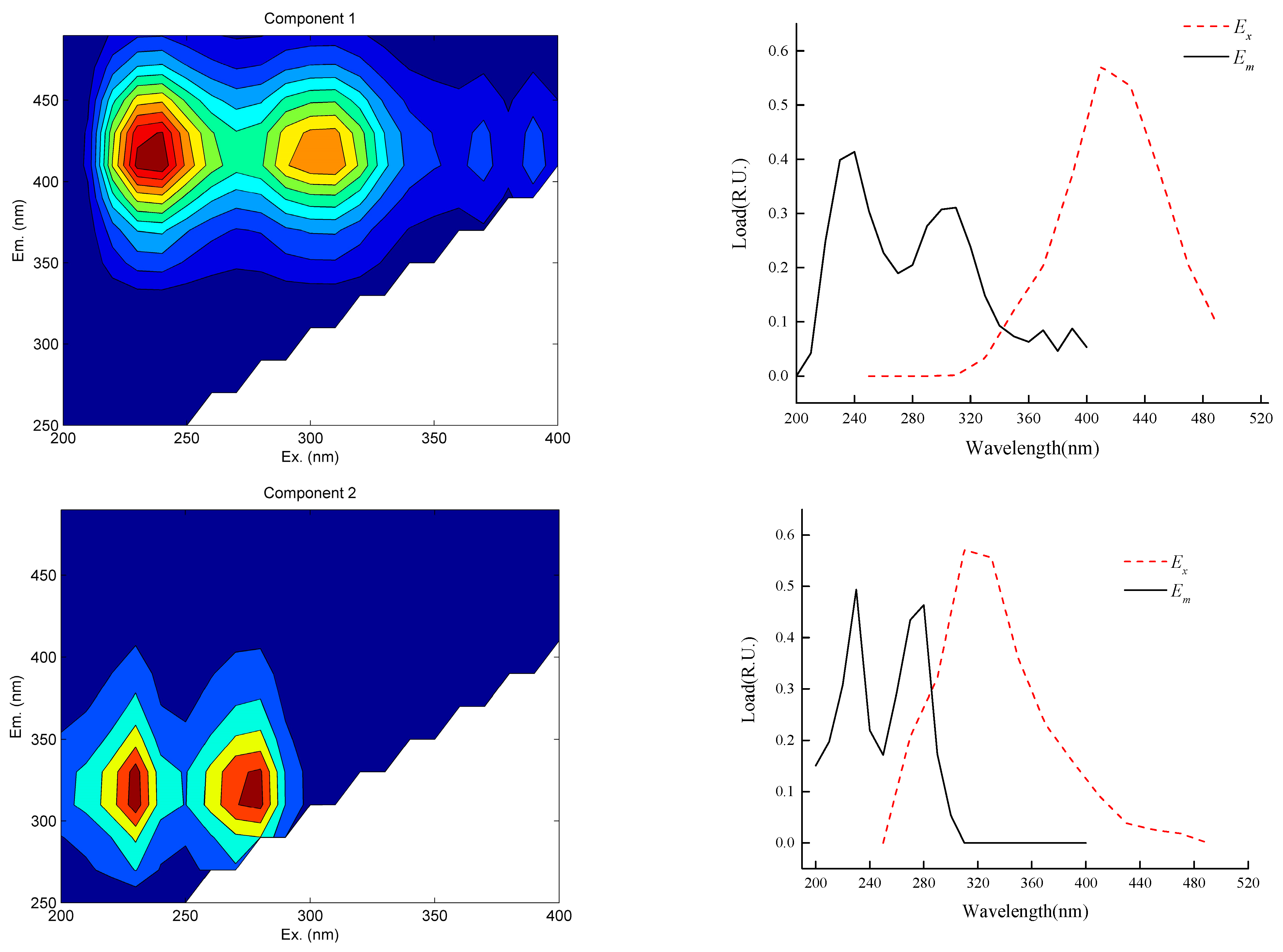
- The effluent from the mine water treatment station mainly contains four components. There is fulvic-acid-like acid in the C1/C3 ultraviolet region; the fulvic-acid-like acid generally exists in sandy soil and moves to the groundwater through rainfall and other processes. The C2 component is derived from tryptophan-like acids. In addition to the emulsified oil produced by a large number of microbial activities and underground shearer leakage, the C4 component is mainly derived from point source pollution formed by large-scale human activities.
- The treatment effect of the coagulation–filtration–disinfection process on COD and nitrite can reach more than 90% removal. The removal efficiency of TOC can reach 50%. After treatment, some of the TOC that cannot be removed remains. At the same time, the system has a poor treatment capacity for several organic ions, including EC, TDS, NH4+, SO42−, Cl−, and Mn, and has a certain removal capacity for Cu2+ and F−.
- Through the coagulation–filtration–disinfection process, protein-like tryptophan substances in the water are completely removed. The fluorescence intensity of the fulvic-acid-like components increases from 334.7272 a.U to 440.3296 a.U, whereas the fluorescence intensity of the protein tyrosine-like components decreases from 330.1814 a.U to 295.7762 a.U. This is because the alkanes, cycloalkanes, and other fatty hydrocarbons of the oil pollutants in the mine water are removed during the treatment process. On the basis of this, an ozone air-flotation combination-removal process is proposed to be suitable for the removal of emulsified oil in mine water.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuß, T.; Behounek, B.; Ulseth, A.J.; Singer, G.A. Land use controls stream ecosystem metabolism by shifting dissolved organic matter and nutrient regimes. Freshw. Biol. 2017, 62, 582–599. [Google Scholar] [CrossRef]
- Monteith, D.T.; Stoddard, J.L.; Evans, C.D.; de Wit, H.A.; Forsius, M.; Høgåsen, T.; Wilander, A.; Skjelkvåle, B.L.; Jeffries, D.S.; Vuorenmaa, J.; et al. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 2007, 450, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Parr, T.B.; Cronan, C.S.; Ohno, T.; Findlay, S.E.G.; Smith, S.M.C.; Simon, K.S. Urbanization changes the composition and bioavailability of dissolved organic matter in headwater streams. Limnol. Oceanogr. 2015, 60, 885–900. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yang, J.; Wang, H. Study on characteristics and sources of DOM in mine water in the middle of the Yellow River Basin Based on parallel factor method. J. China Coal Soc. 2021, 1–14. [Google Scholar] [CrossRef]
- Zou, Y.; Lv, R.; Yang, J. Three-dimensional excitation emiss-ion matrix fluorescence spectroscopic characterization of dissolved organic matt-er in coal mine water. J. China Coal Soc. 2012, 37, 1396–1400. [Google Scholar]
- Weber, J.H.; Wilson, S.A. The isolation and characterization of fulvic acid and humic acid from river water. Water Res. 1975, 9, 1079–1084. [Google Scholar] [CrossRef]
- Demars, B.O.L.; Friberg, N.; Thornton, B. Pulse of dissolved organic matter alters reciprocal carbon subsidies between autotrophs and bacteria in stream food webs. Ecol. Monogr. 2020, 90, e01399. [Google Scholar] [CrossRef] [Green Version]
- Awad, J.; Fisk, C.A.; Cox, J.W.; Anderson, S.J.; van Leeuwen, J. Modelling of THM formation potential and DOM removal based on drinking water catchment characteristics. Sci. Total Environ. 2018, 635, 761–768. [Google Scholar] [CrossRef]
- Bodmer, P. Linking Carbon Dynamics in Stream Ecosystems to Dissolved Organic Matter Quality. Ph.D. Dissertation, Freie Universität Berlin, Berlin, Germany, 2016. [Google Scholar]
- Bodmer, P.; Heinz, M.; Pusch, M.; Singer, G.; Premke, K. Carbon dynamics and their link to dissolved organic matter quality across contrasting stream ecosystems. Sci. Total Environ. 2016, 553, 574–586. [Google Scholar] [CrossRef]
- Glatzel, S.; Kalbitz, K.; Dalva, M.; Moore, T. Dissolved organic matter properties and their relationship to carbon dioxide efflux from restored peat bogs. Geoderma 2003, 113, 397–411. [Google Scholar] [CrossRef]
- Barros, N.; Cole, J.J.; Tranvik, L.J.; Prairie, Y.T.; Bastviken, D.; Huszar, V.L.M.; del Giorgio, P.; Roland, F. Carbon emission from hydroelectric reservoirs linked to reservoir age and latitude. Nat. Geosci. 2011, 4, 593–596. [Google Scholar] [CrossRef]
- Duan, H.; Feng, L.; Ma, R.; Zhang, Y.; Loiselle, S.A. Variability of particulate organic carbon in inland waters observed from MODIS Aqua imagery. Environ. Res. Lett. 2014, 9, 084011. [Google Scholar] [CrossRef]
- Prairie, Y.T.; Alm, J.; Beaulieu, J.; Barros, N.; Battin, T.; Cole, J.; del Giorgio, P.; DelSontro, T.; Guérin, F.; Harby, A.; et al. Greenhouse Gas Emissions from Freshwater Reservoirs: What Does the Atmosphere See? Ecosystems 2018, 21, 1058–1071. [Google Scholar] [CrossRef] [PubMed]
- Hertkorn, N.; Harir, M.; Koch, B.P. High field NMR Spectroscopy and FTICR Mass Spectrometry: Powerful Discovery Tools for the Characterization of Marine Dissolved Organic Matter. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 22–27 April 2012; p. 12595. [Google Scholar]
- Mosher, J.J.; Kaplan, L.A.; Podgorski, D.C.; McKenna, A.M.; Marshall, A.G. Longitudinal shifts in dissolved organic matter chemogeography and chemodiversity within headwater streams: A river continuum reprise. Biogeochemistry 2015, 124, 371–385. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Z.; Guo, L. Chemical evolution of Macondo crude oil during laboratory degradation as characterized by fluorescence EEMs and hydrocarbon composition. Mar. Pollut. Bull. 2013, 66, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, T.S.; Osburn, C.; Shields, M.R.; Yvon Lewis, S.; Young, J.; Guo, L.; Zhou, Z. Deepwater Horizon Oil in Gulf of Mexico Waters after 2 Years: Transformation into the Dissolved Organic Matter Pool. Environ. Sci. Technol. 2014, 48, 9288–9297. [Google Scholar] [CrossRef]
- He, D.; Wang, K.; Pang, Y.; He, C.; Li, P.; Li, Y.; Xiao, S.; Shi, Q.; Sun, Y. Hydrological management constraints on the chemistry of dissolved organic matter in the Three Gorges Reservoir. Water Res. 2020, 187, 116413. [Google Scholar] [CrossRef]
- Wen, Z.; Song, K.; Shang, Y.; Zhao, Y.; Fang, C.; Lyu, L. Differences in the distribution and optical properties of DOM between fresh and saline lakes in a semi-arid area of Northern China. Aquat. Sci. 2018, 80, 1–12. [Google Scholar] [CrossRef]
- Lyu, L.; Liu, G.; Shang, Y.; Wen, Z.; Hou, J.; Song, K. Characterization of dissolved organic matter (DOM) in an urbanized watershed using spectroscopic analysis. Chemosphere 2021, 277, 130210. [Google Scholar] [CrossRef] [PubMed]
- Li, W.T.; Chen, S.Y.; Xu, Z.X.; Li, Y.; Shuang, C.D.; Li, A.M. Characterization of Dissolved Organic Matter in Municipal Wastewater Using Fluorescence PARAFAC Analysis and Chromatography Multi-Excitation/Emission Scan: A Comparative Study. Environ. Sci. Technol. 2014, 48, 2603–2609. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Huang, T.L.; Wen, C.C.; Liang, W.G.; Lin, Z.S.; Yang, S.Y.; Li, K.; Cai, X.C. Influence of Storm Runoff on the Spectral Characteristics of Dissolved Organic Matter (DOM) in a Drinking Water Reservoir During the Flood Season. Huanjing kexue 2021, 42, 1391–1402. [Google Scholar] [CrossRef]
- Feng, W.; Zhu, Y.; Wu, F. The fluorescent characteristics and sources of dissolved organic matter in water of Tai Lake. Acta Sci Circumst 2016, 36, 475–482. [Google Scholar] [CrossRef]
- Khan, S.; Shahnaz, M.; Jehan, N.; Rehman, S.; Shah, M.T.; Din, I. Drinking water quality and human health risk in Charsadda district, Pakistan. J. Clean. Prod. 2013, 60, 93–101. [Google Scholar] [CrossRef]
- Li, Z.; Peng, H.; Xie, B.; Liu, C.; Nie, X.; Wang, D.; Huang, M.; Xiao, H.; Shi, L.; Zhang, X.; et al. Dissolved organic matter in surface runoff in the Loess Plateau of China: The role of rainfall events and land-use. Hydrol. Process. 2020, 34, 1446–1459. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Q.; Wang, T.; Zhang, X. Variation characteristics of mine water quality during maintenance of underground fully mechanized mi-ning equipment in Shenfu mining area. J. China Coal Soc. 2019, 44, 3710–3718. [Google Scholar] [CrossRef]
- Fu, Y.; He, K.; Zhou, C.; Mao, Y. Improvement of Saline-Alkaline Soil via Flue Gas Desulfurization Gypsum and Safety Analysis of the Associated Heavy Metals. J. Phys. Conf. Ser. 2021, 1838, 012051. [Google Scholar] [CrossRef]
- Zhou, Y.; Davidson, T.A.; Yao, X.; Zhang, Y.; Jeppesen, E.; de Souza, J.G.; Wu, H.; Shi, K.; Qin, B. How autochthonous dissolved organic matter responds to eutrophication and climate warming: Evidence from a cross-continental data analysis and experiments. Earth Sci. Rev. 2018, 185, 928–937. [Google Scholar] [CrossRef]
- Andersson, C.A.; Bro, R. The N-way Toolbox for MATLAB. Chemom. Intell. Lab. Syst. 2000, 52, 1–4. [Google Scholar] [CrossRef]
- Bahram, M.; Bro, R.; Stedmon, C.; Afkhami, A. Handling of Rayleigh and Raman scatter for PARAFAC modeling of fluorescence data using interpolation. J. Chemom. 2006, 20, 99–105. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, J.; Men, D.; Zhao, X.; Wu, S. Insight into the heavy metal binding properties of dissolved organic matter in mine water affected by water-rock interaction of coal seam goaf. Chemosphere 2021, 265, 129134. [Google Scholar] [CrossRef]
- Del Castillo, C.E.; Coble, P.G.; Conmy, R.N.; Müller Karger, F.E.; Vanderbloemen, L.; Vargo, G.A. Multispectral in situ measurements of organic matter and chlorophyll fluorescence in seawater: Documenting the intrusion of the Mississippi River plume in the West Florida Shelf. Limnol. Oceanogr. 2001, 46, 1836–1843. [Google Scholar] [CrossRef]
- Del Castillo, C.E.; Coble, P.G.; Morell, J.M.; López, J.M.; Corredor, J.E. Analysis of the optical properties of the Orinoco River plume by absorption and fluorescence spectroscopy. Mar. Chem. 1999, 66, 35–51. [Google Scholar] [CrossRef]
- Cory, R.M.; McKnight, D.M. Fluorescence Spectroscopy Reveals Ubiquitous Presence of Oxidized and Reduced Quinones in Dissolved Organic Matter. Environ. Sci. Technol. 2005, 39, 8142–8149. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.R.; Ruiz, G.M.; Dunsmuir, W.T.M.; Waite, T.D. Optimized Parameters for Fluorescence-Based Verification of Ballast Water Exchange by Ships. Environ. Sci. Technol. 2006, 40, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S. Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnol. Oceanogr. 2005, 50, 686–697. [Google Scholar] [CrossRef]
- Leenheer, J.A.; Croué, J.P. Peer reviewed: Characterizing aquatic dissolved organic matter. Environ. Sci. Technol. 2003, 37, 18A–26A. [Google Scholar] [CrossRef] [Green Version]
- Coble, P.G.; Del Castillo, C.E.; Avril, B. Distribution and optical properties of CDOM in the Arabian Sea during the 1995 Southwest Monsoon. Deep Sea Res. Part II Top. Stud. Oceanogr. 1998, 45, 2195–2223. [Google Scholar] [CrossRef]
- Dosskey, M.G.; Bertsch, P.M. Transport of Dissolved Organic Matter through a Sandy Forest Soil. Soil Sci. Soc. Am. J. 1997, 61, 920–927. [Google Scholar] [CrossRef]
- Fu, Q.L.; He, J.Z.; Blaney, L.; Zhou, D.M. Roxarsone binding to soil-derived dissolved organic matter: Insights from multi-spectroscopic techniques. Chemosphere 2016, 155, 225–233. [Google Scholar] [CrossRef]
- Dandajeh, H.A.; Ladommatos, N.; Hellier, P.; Eveleigh, A. Effects of unsaturation of C2 and C3 hydrocarbons on the formation of PAHs and on the toxicity of soot particles. Fuel 2017, 194, 306–320. [Google Scholar] [CrossRef]
- Ye, H.; Liu, B.; Wang, Q.; How, Z.T.; Zhan, Y.; Chelme-Ayala, P.; Guo, S.; Gamal El-Din, M.; Chen, C. Comprehensive chemical analysis and characterization of heavy oil electric desalting wastewaters in petroleum refineries. Sci. Total Environ. 2020, 724, 138117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.Q.; Su, R.G.; Bai, Y.; Zhang, C.S.; Shi, X.Y. Characterization of Chromophoric dissolved organic matter (CDOM) in Zhoushan fishery using excitation-emission matrix spectroscopy (EEMs) and parallel factor analysis (PARAFAC). Estuar. Coast. 2017, 40, 1325–1345. [Google Scholar] [CrossRef]
- Bao, Y.; Niu, J.; Xu, Z.; Gao, D.; Shi, J.; Sun, X.; Huang, Q. Removal of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) from water by coagulation: Mechanisms and influencing factors. J. Colloid Interface Sci. 2014, 434, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.Z.; Sun, Y.H.; He, C. Research on coagulation/sedimentation process for simulation of fluorine-containing wastewater treatment. Appl Mech Mater 2013, 361, 755–759. [Google Scholar] [CrossRef]
- Pan, Z. The Study of oil Pollution Recognition Measurement Method and Experiment Based on Fluorescence Spectrum Analysis. Ph.D. Dissertation, Yanshan University, Qinhuangdao, China, 2012. [Google Scholar]
- Sulzberger, B.; Durisch Kaiser, E. Chemical characterization of dissolved organic matter (DOM): A prerequisite for understanding UV-induced changes of DOM absorption properties and bioavailability. Aquat. Sci. 2009, 71, 104–126. [Google Scholar] [CrossRef] [Green Version]
- Balch, J.; Guéguen, C. Effects of molecular weight on the diffusion coefficient of aquatic dissolved organic matter and humic substances. Chemosphere 2015, 119, 498–503. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, E.J.; Jeon, Y.J.; Hur, J.; Oh, N.H. Hydrological changes of DOM composition and biodegradability of rivers in temperate monsoon climates. J. Hydrol. 2016, 540, 538–548. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Liu, L.; He, Z.; Giesy, J.P.; Bai, Y.; Sun, F.; Wu, F. Cation-induced coagulation of aquatic plant-derived dissolved organic matter: Investigation by EEM-PARAFAC and FT-IR spectroscopy. Environ. Pollut. 2018, 234, 726–734. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, Y.; Wang, M.; Zou, F.; Zhang, C.; Guan, Z.; Yan, M. In-situ characterization of dissolved organic matter removal by coagulation using differential UV–Visible absorbance spectroscopy. Chemosphere 2020, 242, 125062. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Bian, Y.; Hursthouse, A.S.; Xu, S.; Xiong, N.; Wan, P. The role of magnetic MOFs nanoparticles in enhanced iron coagulation of aquatic dissolved organic matter. Chemosphere 2020, 247, 125921. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Song, Q.; Xing, X.; Jian, W.J.; Liu, Y.; Zhao, Z.L. Analysis of fluoresce-nce spectrum characteristics of petroleum polluted water. Spectrosc. Spectr. Anal. 2014, 34, 2466–2471. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, N.; Shi, Y.; Wang, F.; Chen, D. Three di-mensional fluorescence spectrum characteristics of dissolved organic matter in coal mine area. Spectrosc. Spectr. Anal. 2008, 28, 174–177. [Google Scholar]
- Yang, S.; Wang, F.; Tang, Q.; Wang, P.; Xu, Z.; Liang, J. Utilization of ultra-light carbon foams for the purification of emulsified oil wastewater and their adsorption kinetics. Chem. Phys. 2019, 516, 139–146. [Google Scholar] [CrossRef]
- Dotson, A.; Westerhoff, P. Occurrence and removal of amino acids during drinking water treatment. J. Am. WATER Work. Assoc. 2009, 101, 101–115. [Google Scholar] [CrossRef]
- Zhou, Y.; Jeppesen, E.; Zhang, Y.; Shi, K.; Liu, X.; Zhu, G. Dissolved organic matter fluorescence at wavelength 275/342 nm as a key indicator for detection of point-source contamination in a large Chinese drinking water lake. Chemosphere 2016, 144, 503–509. [Google Scholar] [CrossRef]
- Fang, H. Study on biological stability of drinking water and removing organic matter by water purification processes. Ph.D. Dissertation, Southeast University, Nanjing, China, 2006. [Google Scholar]
- Lou, J.C.; Chen, B.H.; Chang, T.W.; Yang, H.W.; Han, J.Y. Variation and removal efficiency of assimilable organic carbon (AOC) in an advanced water treatment system. Environ. Monit. Assess. 2011, 178, 73–83. [Google Scholar] [CrossRef]
- Soonglerdsongpha, S.; Kasuga, I.; Kurisu, F.; Furumai, H. Comparison of assimilable organic carbon removal and bacterial community structures in biological activated carbon process for advanced drinking water treatment plants. Sustain. Environ. Res. 2011, 21, 59–64. [Google Scholar]
- Zhao, C.; Lin, D.; Wang, Z. Researching Progress of Micro-electrolysis Technology. Envir. Protec. Oil Gas Fields 2013, 23, 59–61. [Google Scholar] [CrossRef]
- Huang, J.G.; Zhong, L. Experimental study on pretreatment of oil refining wastewater by waste ferric/active carbon micro-electrolysis process. Sci. Technol. Chem. Indus. 2010, 5, 10–14. [Google Scholar] [CrossRef]
- Ma, S.; Wu, N.; Ma, Q.; Li, F.; Yang, J.; Li, D.; Li, J. Treatment of Oily Wastewater with Iron-Carbon Internal Electrolysis Process Enhanced by Ultrasonic. Adv. Sci. Lett. 2013, 19, 1869–1872. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Mozia, S. Removal of organic matter from water by PAC/UF system. Water Res. 2002, 36, 4137–4143. [Google Scholar] [CrossRef]
- Jin, P.; Jin, X.; Bjerkelund, V.A.; Østerhus, S.W.; Wang, X.C.; Yang, L. A study on the reactivity characteristics of dissolved effluent organic matter (EfOM) from municipal wastewater treatment plant during ozonation. Water Res. 2016, 88, 643–652. [Google Scholar] [CrossRef] [PubMed]




| Project | Analytical Method |
|---|---|
| pH | Glass electrode method |
| COD | Potassium permanganate method |
| copper | Atomic absorption spectrophotometry |
| Fluoride | Ion chromatography |
| Sulfate | Flame atomic absorption spectrophotometry |
| chloride | Ion chromatography |
| NO3− | UV spectrophotometry |
| Mn | Flame atomic absorption spectrophotometry |
| TDS | 105 °C dry weight method |
| Ec | Glass electrode method |
| NO2− | Spectrophotometry |
| NH4+ | Spectrophotometry |
| TOC | Combustion oxidation—non-dispersive infrared absorption method |
| UV254 | spectrophotometric method |
| EEMs | Three-dimensional fluorescence spectrometric |
| Grade | Pollutant | Removal Rate |
|---|---|---|
| A | COD | 94.3% |
| NO2− | 90% | |
| B | BIX | 51.12% |
| Cu2+ | 50% | |
| HIX | 45.82% | |
| TOC | 47.79% | |
| F− | 24.11% | |
| C | FI | 13.37% |
| Ec | 1.64% | |
| TDS | 2.5% | |
| NH4+ | 0% | |
| Mn | 0% | |
| D | NO3− | −85.47% |
| UV254 | −300% |
| Mining Area | Before Treatment (mg/L) | Treatment Process | After Treatment (mg/L) | Removal Rate (%) |
|---|---|---|---|---|
| Yanzhou | 6 | Coagulation sedimentation + oil scraper | 0.6 | 90% |
| Changzhi | 0.8 | Ozone oxidation + activated carbon adsorption | 0.019 | 97% |
| Huainan | 3.388 | Coagulation + filtration + adsorption | 0.136 | 96% |
| Huainan | 3.85 | Coagulation + sedimentation | 3.3 | 14% |
| Datong | 0.29 | Electrolytic air flotation + sand filter | 0.11 | 62% |
| Datong | 0.72 | Add C-F-O adsorbent | 0.05 | 93% |
| Huaibei | 4.2 | TiO2 supported activated carbon adsorption | 0.84 | 80% |
| Fushun | 17.3 | Lime softening and degreasing | 4.3 | 75% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Dong, S.; Jin, P.; Liang, J.; Yang, J.; Huang, Y. Study on the Migration Law of Dissolved Organic Matter in Mine Water Treatment Station. Water 2022, 14, 3339. https://doi.org/10.3390/w14203339
Zhang X, Dong S, Jin P, Liang J, Yang J, Huang Y. Study on the Migration Law of Dissolved Organic Matter in Mine Water Treatment Station. Water. 2022; 14(20):3339. https://doi.org/10.3390/w14203339
Chicago/Turabian StyleZhang, Xiyu, Shuning Dong, Pengkang Jin, Jidong Liang, Jian Yang, and Yongan Huang. 2022. "Study on the Migration Law of Dissolved Organic Matter in Mine Water Treatment Station" Water 14, no. 20: 3339. https://doi.org/10.3390/w14203339




