Mobility of Potentially Toxic Elements (Pb, Zn, Cd, As, Sb) in Agricultural Carbonated Soils Contaminated by Mine Tailings (Northern Tunisia): A New Kinetic Leaching Approach with Organic Acids
Abstract
:1. Introduction
2. Sampling and Methods
2.1. Soil Sampling
2.2. Grain Size, Physico-Chemical Analysis of the Rhizospheric Soils
2.3. Mineralogical and Chemical Characterization of the Rhizospheric Soils
2.4. Kinetic Chemical Extractions
2.5. Statistical Analysis
3. Results
3.1. Soil Characteristics
3.1.1. Soil Granulometry
3.1.2. Physicochemical Parameters
3.1.3. Soil Mineralogy
3.1.4. PTE Total Concentrations
3.2. Kinetic Leaching Tests
4. Discussion
- ➢
- B1: Cd > Zn > Sb
- ➢
- H3: Zn > Cd > Sb
- ➢
- J2: Cd > Zn
- ➢
- G7: Cd > Zn
- ➢
- Z2: Zn > Cd
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Note
- De Kimpe, C.R.; Morel, J.L. Urban soil management: A growing concern. Soil Sci. 2000, 165, 31–40. [Google Scholar] [CrossRef]
- Morel, J.L.; Heinrich, A.B. SUITMA—Soils in urban, industrial, traffic, mining and military areas—An interdisciplinary working group of the ‘International Union of Soil Science’ (IUSS) dedicated to soils strongly modified by human activities. J. Soil Sediment 2008, 8, 206–207. [Google Scholar] [CrossRef]
- Reeves, D.; Brook, R. Hyperaccumulation of lead and zinc by two metallophytes from mining areas of central Europe. Environ. Pollut. Ser. A Ecol. Biol. 1983, 31, 277–285. [Google Scholar] [CrossRef]
- Cooke, J.A.; Andrewss, S.M.; Johnson, M.S. Lead-zinc, cadmium and fluoride in small mammals from contaminated grassland established on fluorspar tailings. Water Air Soil Pollut. 1990, 51, 43–54. [Google Scholar] [CrossRef]
- Jean, L. Mobilisation du Chrome et du Nickel à Partir de sols Contaminés, en Présence de Complexants: Transfert et Accumulation de ces Métaux chez Datura Innoxia. Thèse de Doctorat, Université de Limoges, Limoges, France, 2007; 221p. [Google Scholar]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Remediation technologies for metal-contaminated soils and groundwater an evaluation. Eng. Geol. 2001, 60, 193–207. [Google Scholar] [CrossRef]
- Ure, A.M.; Davidson, C.M. Chemical Speciation in the Environment; Blackie: Glasgow, UK, 2001; pp. 265–321. [Google Scholar]
- Peijnenburg, W.J.G.M.; Posthuma, L.; Eijsackers, H.J.P.; Allen, H.E. A conceptual framework for implementation of bioavailability of metals for environmental management purposes. Ecotoxicol. Environ. Saf. 1997, 37, 163–172. [Google Scholar] [CrossRef]
- Des Mines, D.G. Annuaire Statistique, Mines et Dérivés; Ministère de L’industrie (1997–2005): Tunis, Tunisia, 2005; 30p.
- Souissi, F.; Souissi, R.; Bouchardon, J.; Moutte, J.; Marguerite, M.; Chakroun, O.M.; Ghorbel, M. Mineralogical and Geochimical Characterization of Mine Tailing and the Effect of Pb, Zn, Cd and Cu Mobility on the Quality of Soils and Stream Sediments in Northern Tunisia; International Congress, Solid Waste Management and Sustainable Development: Hammamet, Tunisie, 2008; pp. 313–317. [Google Scholar]
- Souissi, R.; Souissi, F.; Chakroun, H.K.; Bouchardon, J.L. Mineralogical and Geochemical Characterization of Mine Tailings and Pb, Zn, and Cd Mobility in a Carbonate Setting (Northern Tunisia). Mine Water Environ. 2013, 32, 16–27. [Google Scholar] [CrossRef]
- Chakroun, H.D.; Souissi, F.; Souissi, R.; Bouchardon, J.L.; Moutte, J.; Abdeljaoued, S. Heavy metals distribution and mobility in flotation tailings and agricultural soils near the abandoned Pb-Zn district of Jebel Hallouf-Sidi Bouaouane (NW Tunisia). Carpathian J. Earth Environ. Sci. 2013, 8, 249–263. [Google Scholar]
- Othmani, M.A.; Souissi, F.; Bouzahzah, H.; Bussiere, B.; Ferreira da Silva, E.; Benzaazoua, M. The flotation tailings of the former Pb–Zn mine of Touiref (NW Tunisia): Mineralogy, mine drainage prediction, base-metal speciation assessment and geochemical modeling. Environ. Sci. Pollut. Res. 2014, 22, 2877–2890. [Google Scholar] [CrossRef]
- Souissi, R.; Souissi, F.; Ghorbel, M.; Munoz, M.; Courjault-Radé, P. Mobility of Pb, Zn, and Cd in a soil developed on a carbonated bedrock in a semi-arid climate and contaminated by Pb–Zn tailing, Jebel Ressas (NE Tunisia). Environ. Earth Sci. 2015, 73, 3501–3512. [Google Scholar] [CrossRef]
- Daldoul, G.; Souissi, R.; Souissi, F.; Jemmali, N.; Chakroun, H.K. Assessment and mobility of heavy metals in carbonated soils contaminated by old mine tailings in North Tunisia. J. Afr. Earth Sci. 2015, 110, 150–159. [Google Scholar] [CrossRef]
- Tlil, H.; Souissi, R.; Souissi, F.; Lattanzi, P.; Podda, F.; Concas, S.; Ardau, C.; Cidu, R. Environmental mineralogy and geochemistry of Pb–Zn mine wastes, Northern Tunisia. Rend. Lincei 2017, 28, 133–141. [Google Scholar] [CrossRef]
- Achour, Y.; Souissi, R.; Tlil, H.; Heino, M.M.; Souissi, F. Heavy Metals (Pb, Zn, Cd) and Metalloids (Sb, As) in Carbonated Soils Contaminated by Mine Tailings (North Tunisia). In Conference of the Arabian Journal of Geosciences; Springer: Cham, Switzerland, 2019; pp. 227–230. [Google Scholar] [CrossRef]
- Community bureau of reference, European Commission.
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate traces metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Maiz, I.; Arambarri, I.; Garcia, R.; Millán, E. Evaluation of heavy metal availability in polluted soils by two sequential extraction procedures using factor analysis. Environ. Pollut. 2000, 110, 3–9. [Google Scholar] [CrossRef]
- Onireti, O.O.; Lin, C.; Qin, J. Combined effects of low-molecular-weight organic acids on mobilization of arsenic and lead from multi-contaminated soils. Chemosphere 2017, 170, 161–168. [Google Scholar] [CrossRef]
- Shan, X.; Wang, Z.; Wang, W.; Zhang, S.; Wen, B. Labile rhizosphere soil solution fraction for prediction of bioavailability of heavy metals and rare earth elements to plants. Anal. Bioanal. Chem. 2003, 375, 400–407. [Google Scholar] [CrossRef]
- Feng, M.H.; Shan, X.Q.; Zhang, S.Z.; Bei Wen, B. Comparison of a rhizosphere-based method with other one-step extraction methods for assessing the bioavailability of soil metals to wheat. Chemosphere 2005, 59, 939–949. [Google Scholar] [CrossRef]
- Ash, C.; Drabek, O.; Tejnecký, V.; Jehlicka, J.; Michon, N. Sustainable soil washing: Shredded card filtration of potentially toxic elements after leaching from soil using organic acid solutions. PLoS ONE 2016, 11, e0149882. [Google Scholar] [CrossRef]
- Ash, C.; Tejnecký, V.; Boruvka, L.; Abek, O. Different low-molecular-mass organic acids specifically control leaching of arsenic and lead from contaminated soil. J. Contam. Hydrol. 2016, 187, 18–30. [Google Scholar] [CrossRef]
- Van Hees, P.A.W.; Lundstrom, U.S.; Giesler, R. Low molecular weight organic € acids and their Al-complexes in soil solution-composition, distribution and seasonal variation in three podzolized soils. Geoderma 2000, 94, 173e200. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zeng, G.; Zhou, L.; Wang, X.; Wang, Y.; Wang, C.; Hu, X.; Xu, W. Enhanced efficiency of cadmium removal by Boehmeria nivea (L.) Gaud. in the presence of exogenous citric and oxalic acids. J. Environ. Sci. 2014, 26, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.C.S.; Almeida, C.M.R.; Basto, M.C.P.; Teresa, M.; Vasconcelos, S.D. Influence of season and salinity on the exudation of aliphatic low molecular weight organic acids (ALMWOAs) by Phragmites australis and Halimione portulacoides roots. J. Sea Res. 2015, 95, 180–187. [Google Scholar] [CrossRef]
- Garau, G.; Mele, E.; Castaldi, P.; Lauro, G.P.; Deiana, S. Role of polygalacturonic acid and the cooperative effect of caffeic and malic acids on the toxicity of Cu(II) towards triticale plants (×Triticosecale Wittm). Biol. Fertil. Soils 2015, 51, 535–544. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, T.; Zhong, S.; Zhou, F.; Zhang, Y.; Ma, Y.; Fu, Q. Long-term effects of low-molecular-weight organic acids on remobilization of Cd, Cr, Pb, and As in alkaline coastal wetland soil. Environ. Pollut. Bioavailab. 2021, 33, 266–277. [Google Scholar] [CrossRef]
- Soil Texture Unit Code 1067; Lamotte, IA, USA; Chestertown, MD, USA. Available online: https://lamotte.com/products/soil/individual-soil-plant-tissue-test-kits/soil-texture-test-1067 (accessed on 9 June 2022).
- Van Bladel, R.; Frankart, R.; Gheyi, H.R. A comparison of three methods of determining the cation exchange capacity of calcareous soils. Geoderma 1975, 13, 289–298. [Google Scholar] [CrossRef]
- Wei, W.; Cui, J.; Wei, Z. Effects of low molecular weight organic acids on the immobilization of aqueous Pb(II) using phosphate rock and different crystallized hydroxyapatite. Chemosphere 2014, 105, 14–23. [Google Scholar] [CrossRef]
- Najafi, S.; Jalali, M. Effects of organic acids on cadmium and copper sorption and desorption by two calcareous soils. Environ Monit. Assess. 2015, 187, 585. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Dresler, S.; Matraszek, R. Exogenous malic and acetic acids reduce cadmium phytotoxicity and enhance cadmium accumulation in roots of sunflower plants. Plant Physiol. Bioch. 2015, 94, 225–234. [Google Scholar] [CrossRef]
- Onireti, O.O.; Lin, C. Mobilization of soil-borne arsenic by three common organic acids: Dosage and time effects. Chemosphere 2016, 147, 352–360. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Shan, X.Q. Effects of low-molecular-weight organic acids on uptake of lanthanum by wheat roots. Plant Soil 2004, 261, 163–170. [Google Scholar] [CrossRef]
- Vítková, M.; Komárek, M.; Tejnecký, V.; Šillerová, H. Interactions of nano-oxides with low-molecular-weight organic acids in a contaminated soil. J. Hazard. Mater. 2015, 293, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Bermond, A.; Ghestem, J.-P.; Yousfi, I. Kinetic approach to the chemical speciation of trace metals in soils. Analyst 1998, 123, 785–789. [Google Scholar] [CrossRef]
- Kim, A.G. Leaching methods applied to CUB: Standard, regulatory, and other. In Proceedings of the 15th International Symposium on Management and Use of Coal Combustion Products, St. Petersburg, FL, USA, 27–30 January 2003. [Google Scholar]
- Assessment of Laboratory Leaching Tests for Predicting the Impacts of Fill Material on Ground Water and Surface Water Quality—A Report to the Legislature; Washington State Department of General Administration Olympia: Washington, DC, USA, 2003.
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martinez-Martinez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Arrêté du 8 Janvier 1998 Fixant les Prescriptions Techniques Applicables aux Epandages de Boues sur les sols Agricoles pris en Application du Décret n 97-1133 du 8 Décembre 1997 relatif à L’épandage des Boues Issues du Traitement des Eaux Usées. Available online: https://aida.ineris.fr/reglementation/arrete-080198-fixant-prescriptions-techniques-applicables-epandages-boues-sols (accessed on 9 June 2022).
- Lapakko, K. Metal mine rock and waste characterization tools: An overview. Min. Miner. Sustain. Dev. 2002, 67, 1–30. [Google Scholar]
- Plante, B.; Benzaazoua, M.; Bussière, B.; Kandji, E.B.; Chopard, A.; Bouzahzah, H. Use of EDTA in modified kinetic testing for contaminated drainage prediction from waste rocks: Case of the Lac Tio mine. Environ. Sci. Pollut. Res. 2015, 22, 7882–7896. [Google Scholar] [CrossRef]
- Souissi, F. Etude Gîtologique et Conditions de Formation des Gisements de Fluorine (Pb–Zn– Ba) du Jebel Zaghouan (J. Stah et Sidi Taya) et du Jebel Oust, Tunisie Nordorientale; The’se Universite’ Paul Sabatier, Toulouse III: Toulouse, France, 1987; p. 220. [Google Scholar]
- Souissi, F. Minéralogie et Géochimie des Gîtes Minéraux et leur Impact sur L’environnement. Mémoire d’Habilitation Universitaire; Département de Géologie, Faculté des Sciences de Tunis, Université de Tunis el Manar: Tunis, Tunisia, 2007; pp. 47–65. [Google Scholar]
- Ghorbel, M.; Marguerite, M.; Courjault-Rade, P.; Destrigneville, C.; Parseval, P.; Souissi, R.; Souissi, F.; Ben Mammou, A.; Abdeljaouad, S. Health risk assessment for human exposure by direct ingestion of Pb, Cd, Zn bearing dust in the former miners’ village of Jebel Ressas (NE Tunisia). Eur. J. Mineral. 2010, 22, 639–649. [Google Scholar] [CrossRef]
- Cornu, S.; Clozel, B. Extractions Séquentielles et Spéciation des Eléments Trace Métalliques Dans les Sols Naturels: Analyse Critique. Etudes et Gestion des Sols; Association Française Pour l’Etude des Sols: Ardon, France, 2000; Volume 7, pp. 179–189. [Google Scholar]
- Jin, C.W.; Zheng, S.J.; He, Y.F.; Zhou, G.D.; Zhou, Z.X. Lead contamination in tea garden soils and factors affecting its bioavailability. Chemosphere 2005, 59, 1151–1159. [Google Scholar] [CrossRef]
- Chen, C.; Amirbahman, A.; Fisher, N.; Harding, G.; Lamborg, C.; Nacci, D.; Taylor, D. Methyl mercury in Marine Ecosystems: Spatial Patterns and Processes of Production, Bioaccumulation, and Biomagnification. Ecohealth 2008, 5, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Ettler, V.; Mihaljevic, M.; Sebekb, O.; Grygar, T. Assessment of single extractions for the determination of mobile forms of metals in highly polluted soils and sediments—Analytical and thermodynamic approaches. Anal. Chim. Acta 2007, 602, 131–140. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Behavioural properties of trace metal in soils. Appl. Geochem. 1993, 8, 3–9. [Google Scholar] [CrossRef]
- Lions, J. Etude Hydrogéochimique de la Mobilité de Polluants Inorganiques Dans les Sédiments de Curage mis en Dépôts; Expérimentations, Etude in Situ et Modélisations. Thèse: Hydrogéologie et Hydrologie Quantitatives; Ecole Nationale Supérieure des Mines de Paris: Paris, France, 2004; 248p. [Google Scholar]
- Organization Mondiale de la Santé. 2006. Available online: http://www.lenntech.fr/applications/potable/normes/normes-oms-eau-potable.html (accessed on 9 June 2022).
- Garcia, G.; Zanuzzi, A.L.; Faz, A. Evaluation of heavy metal availability prior to an in situ soil phytoremediation program. Biodegradation 2005, 16, 187–194. [Google Scholar] [CrossRef]
- Gerritse, R.G.; De Willigen, P.; Raats, P.A.C. Transport and fixation of orthophosphate in acid, homogeneous soils. III. Experimental study of acid, sandy soil columns heavily treated with pig slurry. Agric. Environm. 1982, 7, 175–185. [Google Scholar] [CrossRef]
- Harter, R.D. Effect of Soil pH on Adsorption of Lead, Copper, Zinc, and Nickel1. Soil Sci. Soc. Am. J. 1983, 47, 47. [Google Scholar] [CrossRef]
- Hatira, A.; Gallal, T.; Rouiller, I.; Guillet, B. Stabilité et solubilité des complexes formés entre le cuivre, le plomb, le zinc et les acides fulviques. Sci. Sol 1990, 28, 123–135. [Google Scholar]
- Johnson, C.E.; Petras, R.J. Distribution of Zinc and Lead Fractions within a Forest Spodosol. Soil Sci. Soc. Am. J. 1998, 62, 782. [Google Scholar] [CrossRef]
- Morin, G.; Juillot, F.; Ildefonse, P.; Calas, G.; Samama, J.C.; Chevallier, P.; Brown, G.E. Mineralogy of lead in a soil developed on a Pb-mineralized sandstone (Largentiere, France). Am. Mineral. 2001, 86, 92–104. [Google Scholar] [CrossRef]
- Razo, I.; Carrizales, L.; Castro, J.; Diaz-Barriga, F.; Monroy, M. Arsenic and heavy metal pollution of soil, water and sediments in a semi-arid climate mining area in Mexico. Water Air Soil Pollut. 2004, 152, 129–152. [Google Scholar] [CrossRef]
- Ullrich, M.S.; Ramsey, H.M.; Helios-Rybicka, E. ‘Total and exchangeable concentrations of heavy metals in the soil near Bytom, an area of Pb/Zn mining and smelting in Upper Silesia, Poland’. Appl. Geochem. 1999, 14, 187–196. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals in Soils: Their Origins, Chemical Behavior et Bioavailability; Wiley, John & Sons: London, UK, 1990; 339p. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Behavior of Metals in Soils; EPA/540/S-92/018; Technology Innovative Office: Washington, DC, USA, 1992; 25p, Available online: http://www.irishstatutebook.ie/eli/1992/act/7/enacted/en/html# (accessed on 9 June 2022).
- Mckenzie, R.M. The adsorption of Lead and other heavy metals on oxides of Manganese and Iron. Aust. J. Soil Res. 1980, 18, 61–73. [Google Scholar] [CrossRef]
- Tipping, E.; Thompson, D.W.; Ohnstad, M.; Hetherington, N.B. Effects of pH on the release of metals from naturally-occurring oxides of Mn and Fe. Environ. Technol. Lett. 1986, 7, 109–114. [Google Scholar] [CrossRef]
- Li, X.; Thornton, I. Chemical partitioning of trace and major elements in soils contaminated by mining and smelting activities. Appl. Geochem. 2001, 16, 1693–1706. [Google Scholar] [CrossRef]
- Ramos-Arroyo, Y.R.; Siebe, C. Weathering of sulphide minerals and trace element speciation in tailings of various ages in the Guanajuato mining district, Mexico. Catena 2007, 71, 497–506. [Google Scholar] [CrossRef]
- Gee, J.R.; Masson, D.G.; Watts, A.B.; Mitchell, N.C. Offshore continuation of volcanic rift zones, El Hierro, Canary Islands. J. Volcanol. Geotherm. Res. 2001, 105, 107–119. [Google Scholar] [CrossRef]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley and Sons: New York, NY, USA, 1979. [Google Scholar]
- Chuan, M.C.; Shu, G.Y.; Liu, J.C. Solubility of heavy metals in a contaminated soil: Effects of redox potential and pH. Water Air Soil Pollut. 1996, 90, 543–556. [Google Scholar] [CrossRef]
- Quemeneur, M. Les Processus Biogéochimiques Impliqués Dans la Mobilité de L’arsenic: Recherche de Bios Indicateurs. Thèse de Doctorat en Géosciences. Laboratoire des Interactions Microorganismes-Minéraux-Matière Organiques dans les Sols, Nancy 1; Faculté des Sciences et Techniques STMP, Université Henri Poincaré: Nancy, France, 2008; 245p. [Google Scholar]
- Laperche, V.; Bodénan, F.; Dictor, M.C.; Baranger, P. Guide Méthodologique de l‘Arsenic, Appliqué à la Gestion des Sites et Sols Pollués; Rapport BRGM RP-52066-Fance: Orléans, France, 2003. [Google Scholar]
- Bhattacharya, P.; Welch, A.H.; Stollenwerk, K.G.; McLaughlin, M.J.; Bundschuh, J.; Panaullah, G. Arsenic in the environment: Biology and chemistry. Sci. Total Environ. 2007, 379, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.; Glaubig, R.A. Anion Sorption on a Calcareous, Montmorillonitic SoilArsenic. Soil Sci. Soc. Am. J. 1988, 52, 1297–1300. [Google Scholar] [CrossRef]
- Casiot, C.; Ujevic, M.; Munoz, M.; Seidel, J.L.; Elbaz-Poulichet, F. Antimony and arsenic mobility in a creek draining an antimony mine abandoned 85 years ago (upper Orb basin, France). Appl. Geochem. 2007, 22, 788–798. [Google Scholar] [CrossRef]
- Masson, M.; Schafer, J.; Blanc, G.; Dabrin, A.; Castelle, S.; Lavaux, G. Behavior of arsenic and antimony in the surface freshwater reaches of a highly turbid estuary, the Gironde Estuary, France. Appl. Geochem. 2009, 24, 1747–1756. [Google Scholar] [CrossRef]
- Milham, L.; Craw, D. Antimony mobilization through two contrasting gold ore processing systems, New Zealand. Mine Water Environ. 2009, 28, 136–145. [Google Scholar] [CrossRef]
- Ashley, P.M.; Craw, D.; Graham, B.P.; Chappell, D.A. Environmental mobility of antimony around mesothermal stibnite deposits, New South Wales, Australia and southern New Zealand. J. Geochem. Explor 2003, 77, 1–14. [Google Scholar] [CrossRef]
- Hiller, E.; Lalinská, B.; Chovan, M.; Jurkovič, Č.; Klimko, T.; Jankulár, M.; Hovorič, R.; Šottník, P.; Fľaková, R.; Ženišová, Z.; et al. Arsenic and antimony contamination of waters, stream sediments and soils in the vicinity of abandoned antimony mines in the Western Carpathians, Slovakia. Appl. Geochem. 2012, 27, 598–614. [Google Scholar] [CrossRef]
- Gebel, T. Arsenic and antimony: Comparative approach on mechanistic toxicology. Chem.-Biol. Interact. 1997, 107, 131–144. [Google Scholar] [CrossRef]
- Smith, E.; Naidu, R.; Alston, A.M. Arsenic in the soil environment; a review. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: San Diego, CA, USA, 1998; Volume 64, pp. 149–195. [Google Scholar]
- Filella, M.; Belzile, N.; Chen, Y. Antimony in the environment: A review focused on natural waters, I. Occurrence. Earth-Sci. Rev. 2002, 57, 125–176. [Google Scholar] [CrossRef]
- Klein, C.; Hurlbut, C.S. Manual of Mineralogy, 20th ed.; John Wiley & Sons: New York, NY, USA, 1985; 596p. [Google Scholar]
- Cathala, N.; Salsac, L. Absorption du cuivre par les racines de maïs (Zea mays, L.) et de tournesol (Helianthus annuus, L.). Plant Soil 1975, 42, 65–83. [Google Scholar] [CrossRef]
- Hinsinger, P.; Marschner, P. Rhizosphere-perspectives and challenges—A tribute to Lorenz Hiltner 12–17 September 2004—Munich, Germany. Plant Soil 2006, 283, vii–viii. [Google Scholar] [CrossRef]
- Khan, A.G. Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 355–364. [Google Scholar] [CrossRef]
- Crowley, D.E.; Rengel, Z. Biology and chemistry of nutrient availability in the rhizosphere. In Mineral Nutrition of Crops: Fundamental and Implications; Rengel, Z., Ed.; Haworth Press: New York, NY, USA, 1999; pp. 1–40. [Google Scholar]
- Cabala, J.; Teper, L. Metalliferous constituents of rhizosphere soils contaminated by Zn–Pb mining in Southern Poland. Water Air Soil Pollut. 2007, 178, 351–362. [Google Scholar] [CrossRef]
- Cieśliński, G.; Van Rees, K.C.J.; Szmigielska, A.M.; Krishnamurti, G.S.R.; Huang, P.M. Low-molecular-weight organic acids in rhizosphere soils of durum wheat and their effect on cadmium bioaccumulation. Plant Soil 1998, 203, 109–117. [Google Scholar] [CrossRef]
- Shan, X.Q.; Lian, J.; Wen, B. Effect of organic acids on adsorption and desorption of rare earth elements. Chemosphere 2002, 47, 701–710. [Google Scholar] [CrossRef]
- Gonzaga, M.I.S.; Ma, L.Q.; Santos, J.A.G.; Matias, M.I.S. Rhizosphere characteristics of two arsenic hyperaccumulating Pteris ferns. Sci. Total Environ. 2009, 407, 4711–4716. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.G.M.; Snow, E.T.; Tanaka, A. Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci. Total Environ. 2003, 308, 83–96. [Google Scholar] [CrossRef]
- Fitz, W.J.; Wenzel, W.W. Arsenic transformations in the soil–rhizosphere–plant system: Fundamentals and potential application to phytoremediation. J. Biotechnol. 2002, 99, 259–278. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking Water-Quality, 4th ed.; WHO: Switzerland, Geneva, 2011; p. 564. [Google Scholar]
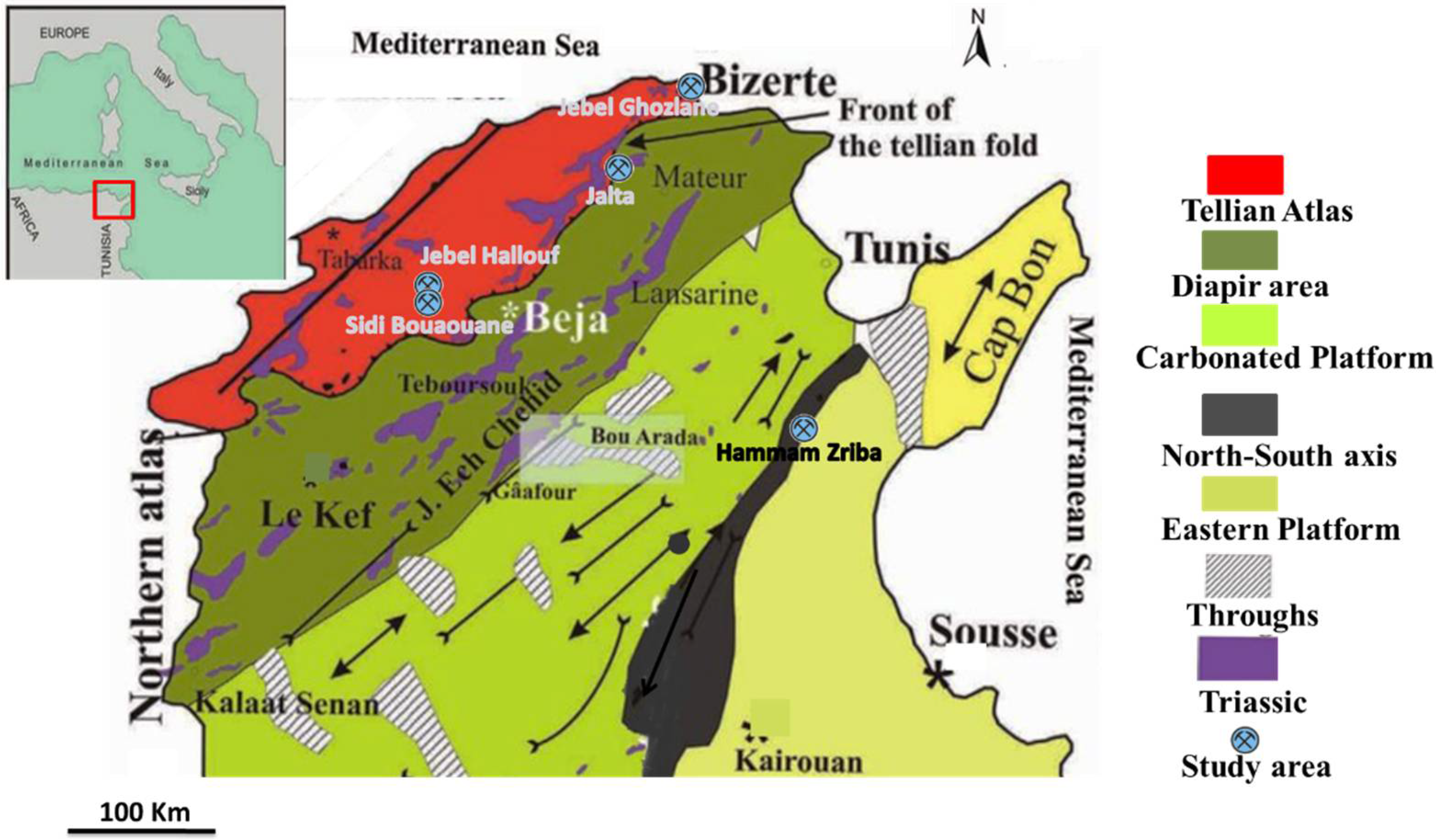





| Sample | Locality | Geographic Coordinates | |||
|---|---|---|---|---|---|
| Reference | Contaminated | Control | Latitude | Longitude | |
| H3 | x | Jebel Hallouf | 36°41′46.19″ N | 9°0′25.15″ E | |
| BT | x | Bouaouane | 36°42′41.87″ N | 9°01′15.94″ E | |
| B1 | x | Bouaouane | 36°41′33.67″ N | 9°0′58.97″ E | |
| J2 | x | Jalta | 37°04′27.10″ N | 9°31′47.76″ E | |
| Z2 | x | Zaghouan | 36°20′27.46″ N | 10°12′39.98″ E | |
| ZT | x | Zaghouan | 36°22′57.38″ N | 10°11′01.28″ E | |
| GT | x | Jebel Ghozlane | 37°18′43.75″ N | 9°46′46.61″ E | |
| G7 | x | Jebel Ghozlane | 37°19′27.87″ N | 9°46′16.18″ E | |
| Procedure | References | |||
|---|---|---|---|---|
| Reagent | Temperature | Extraction Ratio | Shaking Time | [22] [23] [37] modified |
| Low molecular weight organic acids (LMWOAs) solution 0.01 mol L−1, consisting of acetic, lactic, citric, malic and formic acids with a molar ratio of 4:2:1:1:1, pH 2.8 | Room temperature | 1/10 (4 g soil/40 mL LMWOAs) | 0 min 5 min 15 min 30 min 60 min 120 min 960 min | |
| Samples | EC (μS·cm−1) | pH | (% CaCO3) | CEC (meq/100 g) |
|---|---|---|---|---|
| H3 | 217 | 8.3 | 75 | 5.5 |
| BT | 237 | 7.8 | 40 | - |
| B1 | 499 | 7.8 | 46 | 18.4 |
| J2 | 367 | 7.1 | 2 | 23.1 |
| Z2 | 115 | 7.7 | 24 | 1.8 |
| ZT | 271 | 7.1 | 22 | - |
| GT | 233 | 8.0 | 50 | - |
| G7 | 865 | 7.8 | 33 | 3.2 |
| Sample | B1 | BT | H3 | J2 | GT | G7 | Z2 | |
|---|---|---|---|---|---|---|---|---|
| Mineralogy | ||||||||
| Quartz | + | + | + | + | + | + | + | |
| Calcite | + | + | + | + | + | + | + | |
| Barite | - | - | - | - | - | - | + | |
| Hemimorphite | - | - | - | - | - | - | + | |
| Fluorite | - | - | - | - | - | - | + | |
| Anglesite | - | - | - | + | - | - | + | |
| Montmorillonite | - | - | - | - | + | - | - | |
| Dolomite | - | - | - | + | - | + | - | |
| Illite | - | - | - | + | - | - | - | |
| Kaolinite | + | + | + | + | + | - | - | |
| Cerusite | + | - | + | - | - | - | - | |
| Pyrite | - | - | - | - | - | - | + | |
| Sphalerite | - | - | - | - | - | - | + | |
| Jordanite | - | - | - | + | - | - | - | |
| Gypsum | + | - | - | + | - | - | - | |
| Samples | As (mg·kg−1) | Sb (mg·kg−1) | Pb (mg·kg−1) | Zn (mg·kg−1) | Cd (mg·kg−1) | |
|---|---|---|---|---|---|---|
| B1 | 683 | 145 | 17,350 | 8620 | 64 | |
| BT | 44 | nd a | 391 | 418 | 7 | |
| H3 | 669 | 103 | 10,321 | 7951 | 55 | |
| J2 | 110 | nd a | 3061 | 1240 | 9 | |
| G7 | 348 | nd a | 7943 | 37,000 | 205 | |
| GT | Nd | nd a | 110 | 331 | 1 | |
| Z2 | Nd | nd a | 2235 | 12,674 | 35 | |
| ZT | Nd | 8 | 91 | 493 | Nd | |
| M.C (SRM type 2710) | Measured | 648 | 44 | 4905 | 6521 | 19 |
| Standard | 626 | 384 | 5532 | 6952 | 218 | |
| European standards for agricultural soils | - | - | 100 | 300 | 2 | |
| [As Leachate] | [Sb Leachate] | [Cd Leachate] | [Pb Leachate] | [Zn Leachate] | [Ca Leachate] | [mg·Leachate] | [As Total] | [Sb Total] | [Cd Total] | [Pb Total] | [Zn Total] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [As leachate] | 1.00 | |||||||||||
| [Sb leachate] | 0.97 | 1.00 | ||||||||||
| [Cd leachate] | 0.21 | 0.30 | 1.00 | |||||||||
| [Pb leachate] | 0.11 | −0.08 | −0.71 | 1.00 | ||||||||
| [Zn leachate] | −0.33 | −0.27 | 0.81 | −0.71 | 1.00 | |||||||
| [Ca leachate] | −0.51 | −0.62 | −0.86 | 0.76 | −0.55 | 1.00 | ||||||
| [mg·Leachate] | −0.11 | 0.01 | −0.27 | 0.14 | −0.48 | 0.32 | 1.00 | |||||
| [As Total] | 0.76 | 0.61 | 0.23 | 0.43 | −0.11 | −0.25 | −0.35 | 1.00 | ||||
| [Sb Total] | 0.92 * | 0.80 | 0.03 | 0.40 | −0.38 | −0.27 | −0.30 | 0.90 | 1.00 | |||
| [Cd Total] | −0,11 | −0.08 | 0.89 * | −0.49 | 0.89 | −0.55 | −0.31 | 0.21 | −0.16 | 1.00 | ||
| [Pb Total] | 0.92 | 0.86 | 0.41 | 0.14 | −0.08 | −0.54 | −0.24 | 0.92 * | 0.91 * | 0.21 | 1.00 | |
| [Zn Total] | −0.27 | −0.21 | 0.86 | −0.63 | 0.98 | −0.55 | −0.36 | −0.01 | −0.33 | 0.97 ** | 0.02 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achour, Y.; Souissi, R.; Tlil, H.; Souissi, F.; Motelica-Heino, M. Mobility of Potentially Toxic Elements (Pb, Zn, Cd, As, Sb) in Agricultural Carbonated Soils Contaminated by Mine Tailings (Northern Tunisia): A New Kinetic Leaching Approach with Organic Acids. Water 2022, 14, 3337. https://doi.org/10.3390/w14203337
Achour Y, Souissi R, Tlil H, Souissi F, Motelica-Heino M. Mobility of Potentially Toxic Elements (Pb, Zn, Cd, As, Sb) in Agricultural Carbonated Soils Contaminated by Mine Tailings (Northern Tunisia): A New Kinetic Leaching Approach with Organic Acids. Water. 2022; 14(20):3337. https://doi.org/10.3390/w14203337
Chicago/Turabian StyleAchour, Yosra, Radhia Souissi, Haifa Tlil, Fouad Souissi, and Mikael Motelica-Heino. 2022. "Mobility of Potentially Toxic Elements (Pb, Zn, Cd, As, Sb) in Agricultural Carbonated Soils Contaminated by Mine Tailings (Northern Tunisia): A New Kinetic Leaching Approach with Organic Acids" Water 14, no. 20: 3337. https://doi.org/10.3390/w14203337




