Comparison of Four Plant-Based Bio-Coagulants Performances against Alum and Ferric Chloride in the Turbidity Improvement of Bentonite Synthetic Water
Abstract
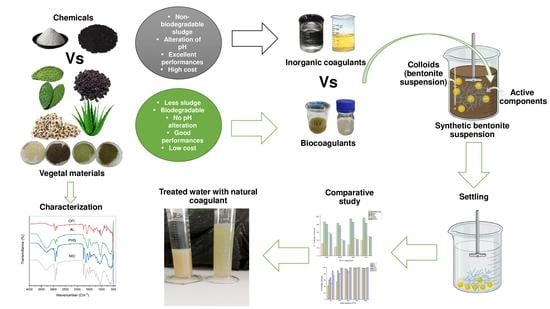
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Apparatus and Measurements
2.3. Preparation of the Bentonite Synthetic Wastewater
2.4. Preparation, Extraction, and Characterization of the Bio-Coagulants
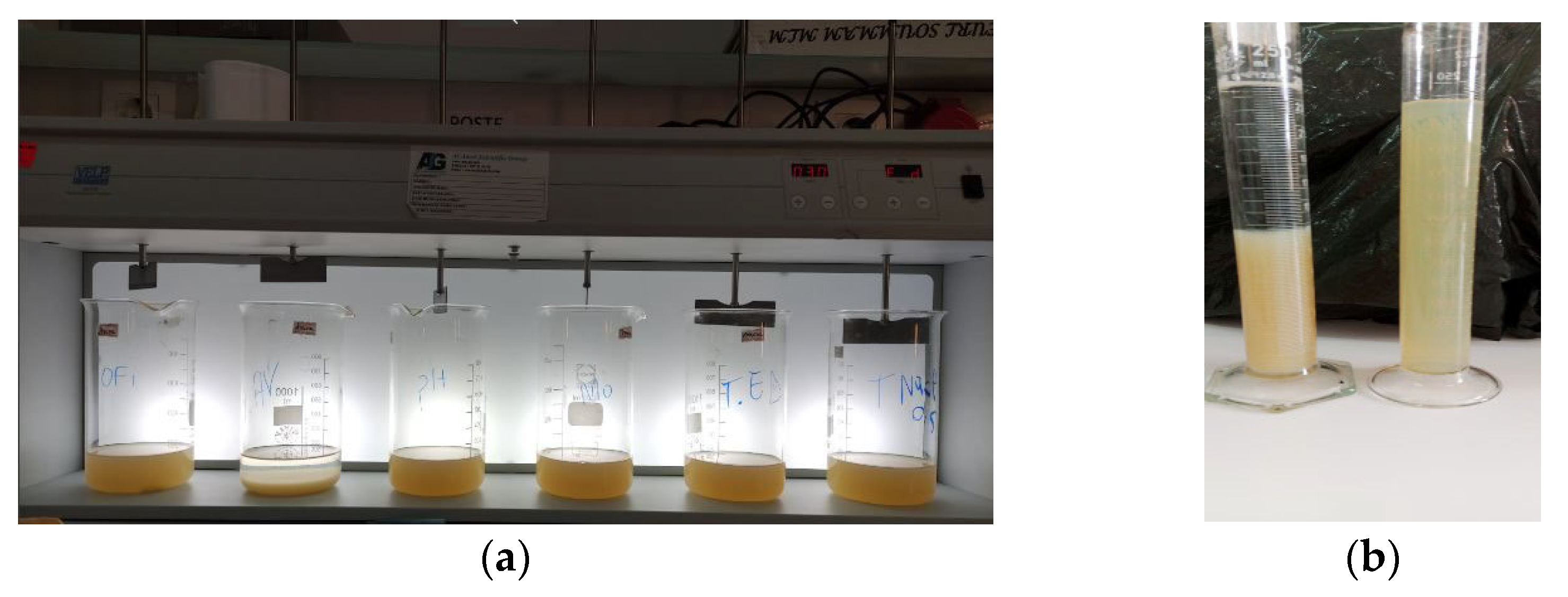
2.5. Coagulation-Flocculation Experiments
2.6. Comparison of the Sludge Production
3. Results and Discussion
3.1. FTIR Bio-Coagulants Characterization
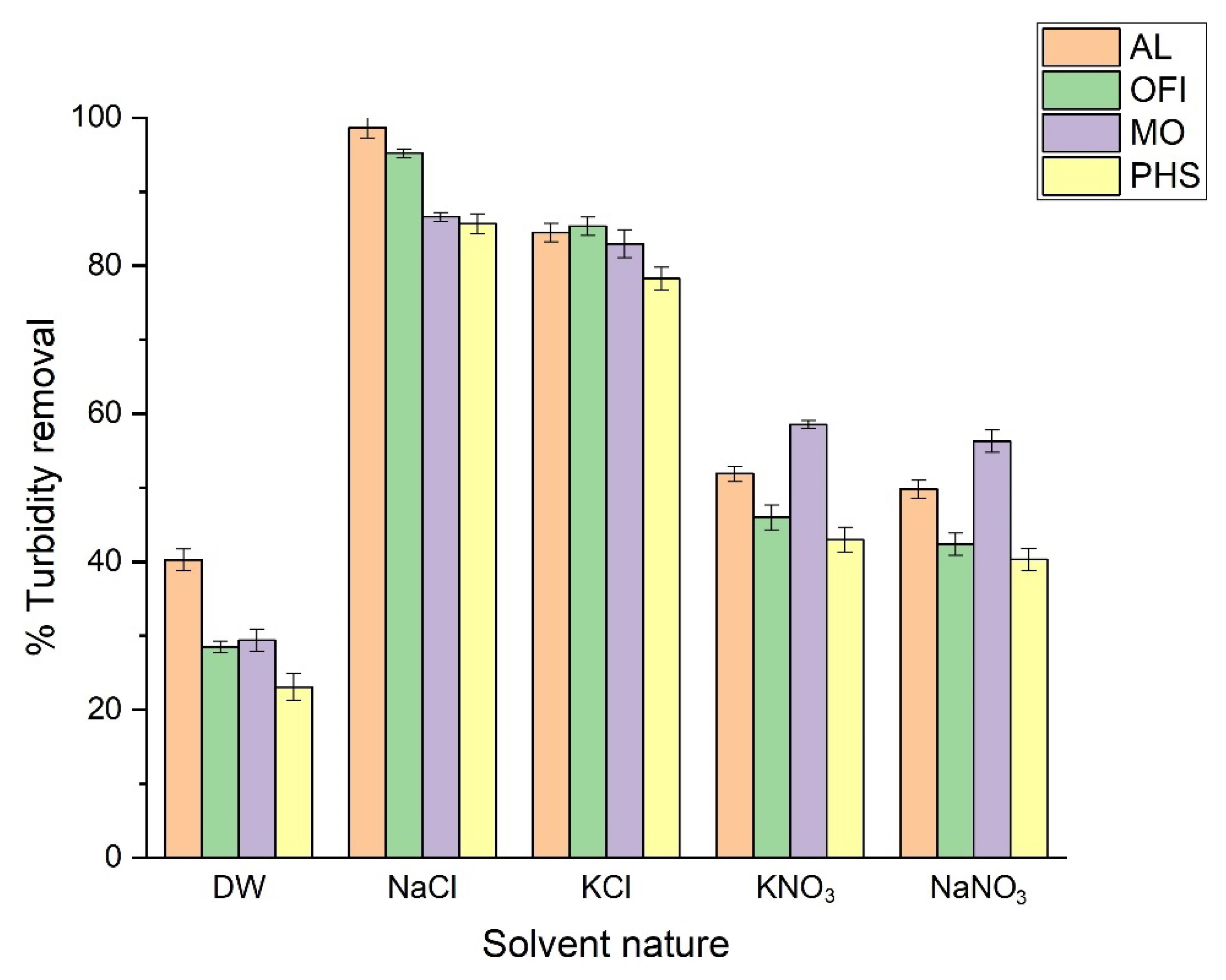
3.2. Effect of Solvent Nature on Turbidity Removal
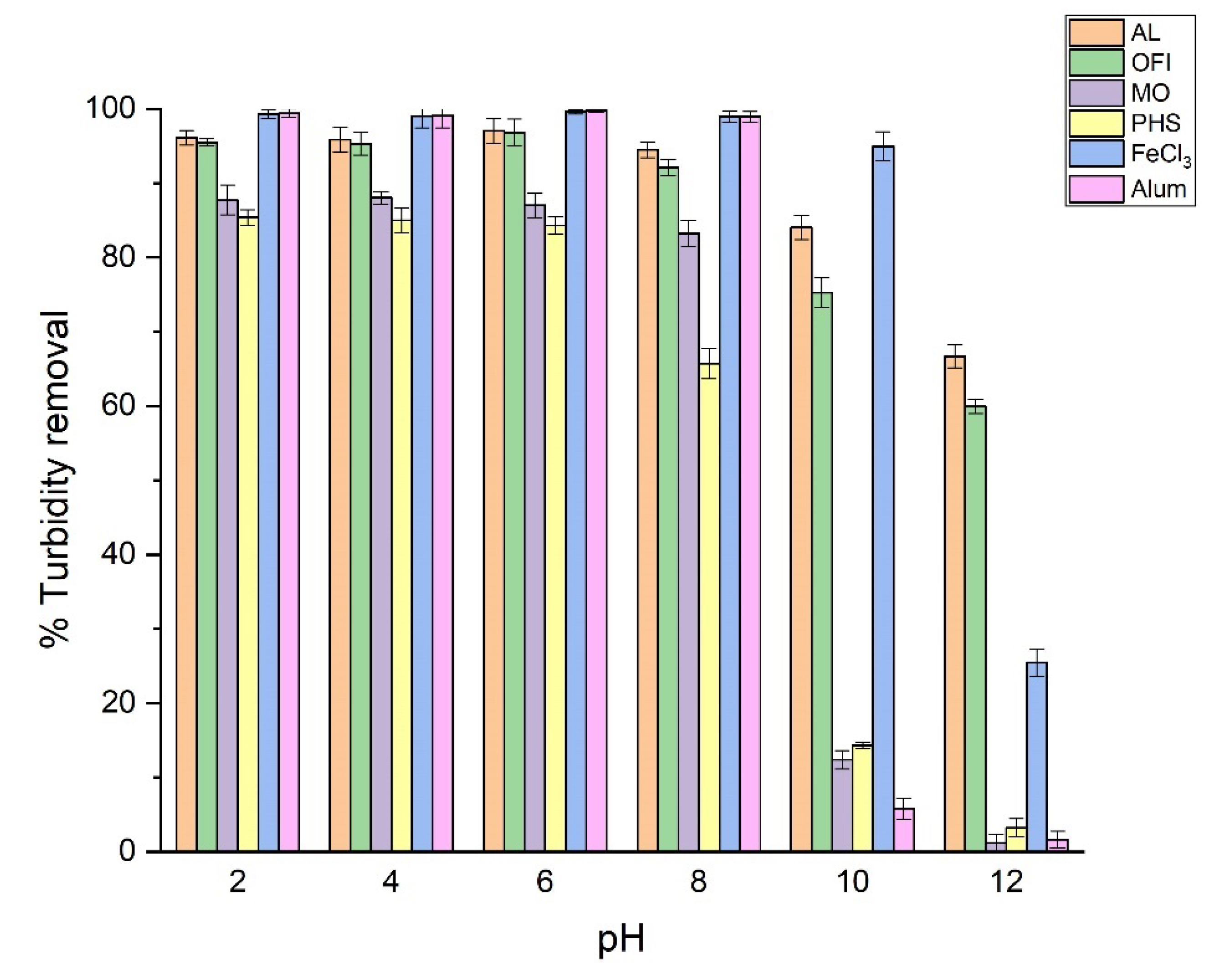
3.3. Effect of pH on Turbidity Removal
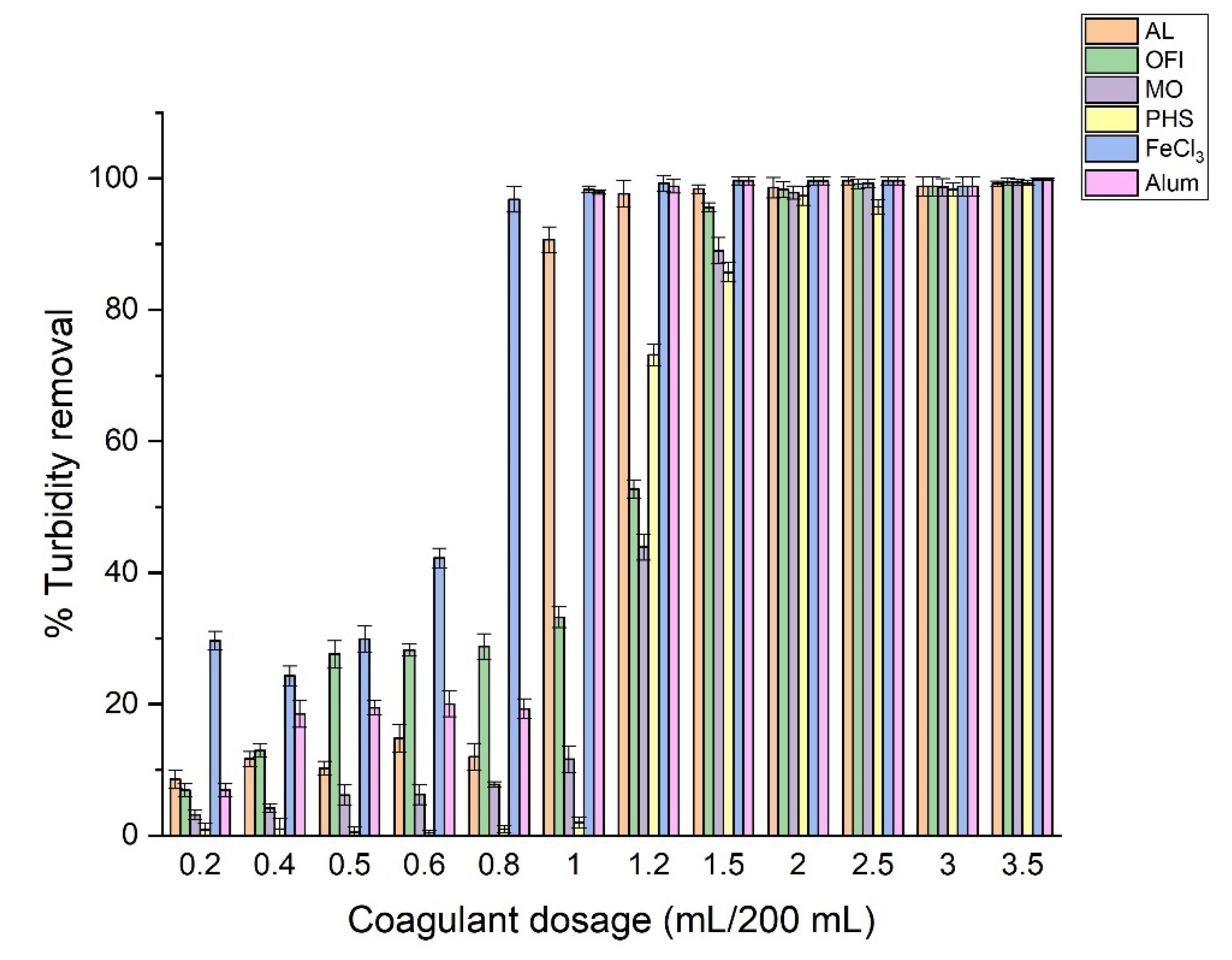
3.4. Effect of Bio-Coagulant Dosage on Turbidity Removal
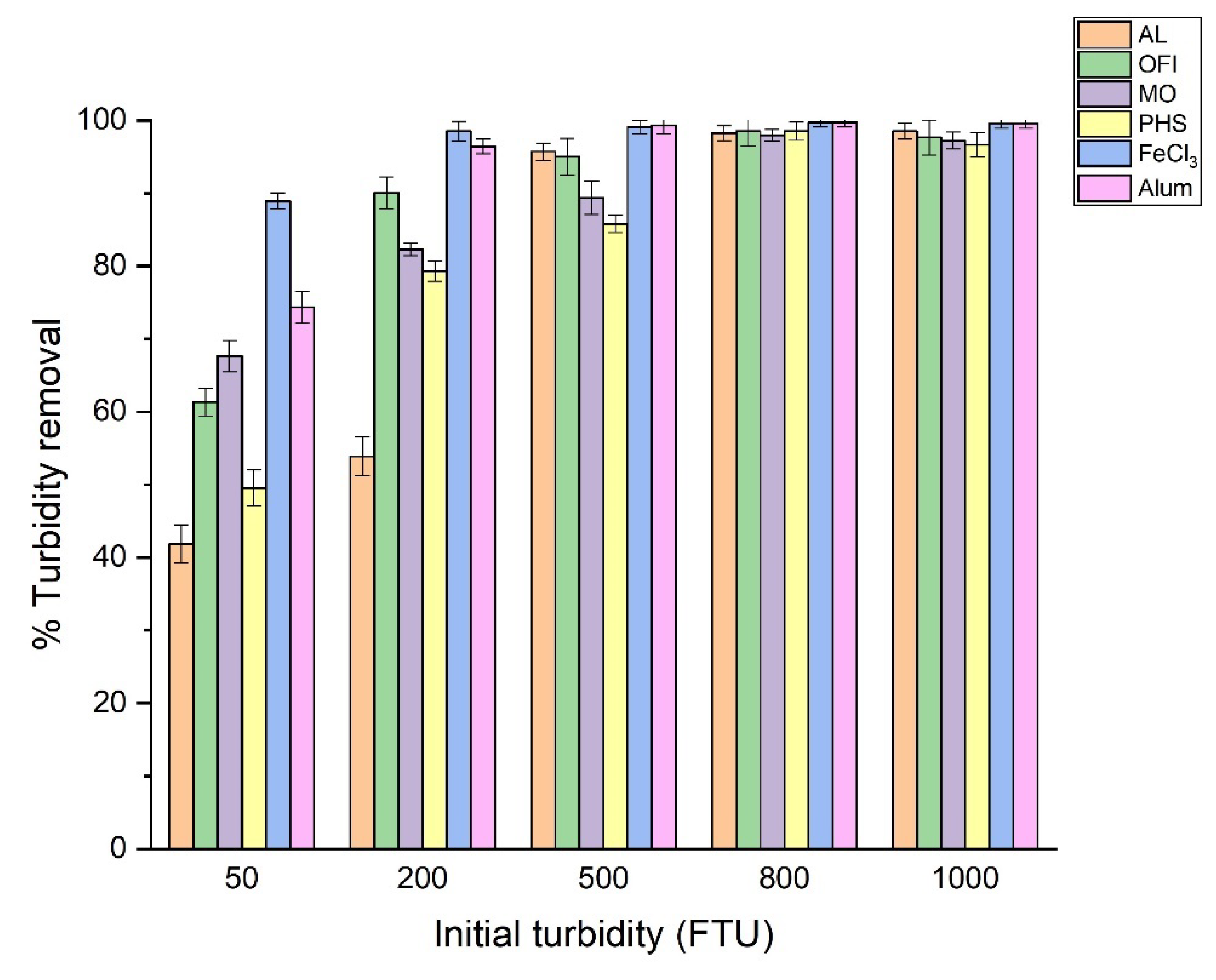
3.5. Effect of Initial Turbidity
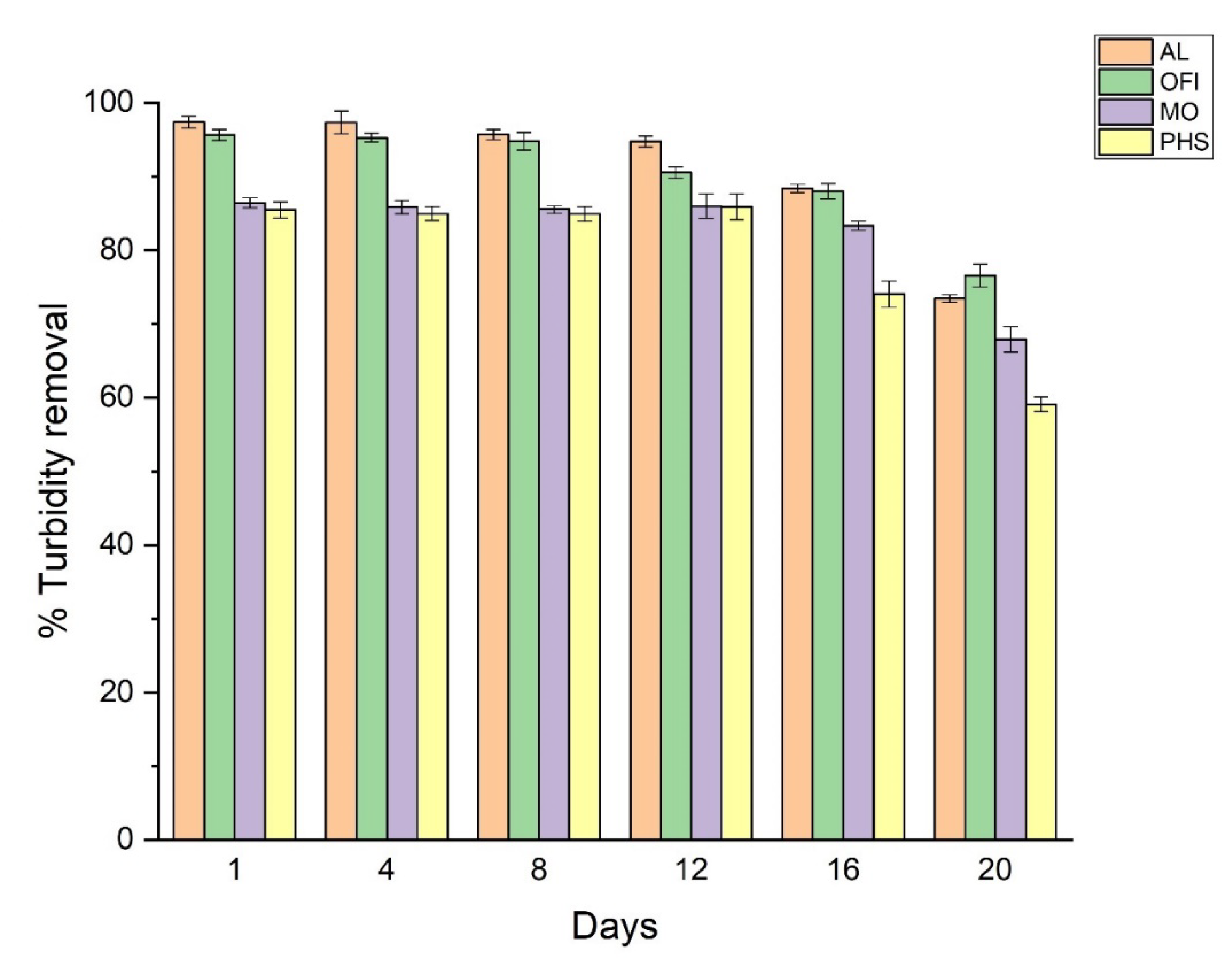
3.6. Effect of Bio-Coagulants Aging on Turbidity Removal
3.7. Comparison of Sludge Production
3.8. Variation of pH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AV | Aloe vera |
| OFI | Opuntia ficus indica |
| MO | Moringa oleifera |
| PHS | Pinus halepensis |
| FeCl3 | Ferric chloride |
| Alum | Aluminum sulfate |
| HCl | Hydrochloric acid |
| NaOH | Sodium hydroxide |
| NaCl | Sodium chloride |
| KCl | Potassium chloride |
| KNO3 | Potassium nitrate |
| NaNO3 | Sodium nitrate |
References
- Larsen, T.A.; Hoffmann, S.; Lüthi, C.; Truffer, B.; Maurer, M. Emerging solutions to the water challenges of an urbanizing world. Science 2016, 352, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H.; Wang, X.; Huppes, G.; Heijungs, R.; Ren, N.-Q. Environmental implications of increasingly stringent sewage discharge standards in municipal wastewater treatment plants: Case study of a cool area of China. J. Clean. Prod. 2015, 94, 278–283. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chang, K.-Y.; Pan, T.-M. Monascus purpureus NTU 568 fermented product improves memory and learning ability in rats with aluminium-induced alzheimer’s disease. J. Funct. Foods 2016, 21, 167–177. [Google Scholar] [CrossRef]
- Lugo-Arias, J.; Lugo-Arias, E.; Ovallos-Gazabon, D.; Arango, J.; de la Puente, M.; Silva, J. Effectiveness of the mixture of nopal and cassava starch as clarifying substances in water purification: A case study in Colombia. Heliyon 2020, 6, e04296. [Google Scholar] [CrossRef]
- Kurniawan, S.; Abdullah, S.; Imron, M.; Said, N.; Ismail, N.; Hasan, H.; Othman, A.; Purwanti, I. Challenges and opportunities of Biocoagulant/Bioflocculant application for drinking water and wastewater treatment and its potential for sludge recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef]
- Adebisi, G.A.; Chowdhury, Z.Z.; Alaba, P.A. Equilibrium, kinetic, and thermodynamic studies of lead ion and zinc ion adsorption from aqueous solution onto activated carbon prepared from palm oil mill effluent. J. Clean. Prod. 2017, 148, 958–968. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Imran, M.; Crowley, D.E.; Khalid, A.; Hussain, S.; Mumtaz, M.W.; Arshad, M. Microbial Biotechnology for decolorization of Textile Wastewaters. Rev. Environ. Sci. Bio/Technol. 2014, 14, 73–92. [Google Scholar] [CrossRef]
- Dasgupta, J.; Sikder, J.; Chakraborty, S.; Curcio, S.; Drioli, E. Remediation of textile effluents by membrane based treatment techniques: A state of the art review. J. Environ. Manag. 2015, 147, 55–72. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2004; Volume 1. [Google Scholar]
- Soros, A.; Amburgey, J.E.; Stauber, C.E.; Sobsey, M.D.; Casanova, L.M. Turbidity reduction in drinking water by coagulation-flocculation with chitosan polymers. J. Water Health 2019, 17, 204–218. [Google Scholar] [CrossRef]
- Vijayaraghavan, G.; Sivakumar, T.; Kumar, A.V. Application of plant based coagulants for waste water treatment. Int. J. Adv. Eng. Res. Stud. 2011, 1, 88–92. [Google Scholar]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.; Wu, T.Y. Recent advancement of coagulation–flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Rivera Cañón, A.F.; Hernández Carrillo, C.G.; Gómez Cuaspud, J.A. Evaluation of physicochemical properties of Nopal (Opuntia Ficus-Indica) as bio coagulant-flocculant for water treatment. J. Phys. Conf. Ser. 2021, 2046, 012057. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W. State of the art and sustainability of natural coagulants in water and wastewater treatment. J. Clean. Prod. 2020, 262, 121267. [Google Scholar] [CrossRef]
- Saleem, M.; Bachmann, R.T. A contemporary review on plant-based coagulants for applications in water treatment. J. Ind. Eng. Chem. 2019, 72, 281–297. [Google Scholar] [CrossRef]
- Bratby, J. Coagulation and Flocculation; Uplands: Croydon, UK, 1980. [Google Scholar]
- Williams, R.B.; Culp, G.L. Handbook of Public Water Systems; Van Nostrand Reinhold Co: New York, NY, USA, 1986; p. 379. [Google Scholar]
- Dick, M.; Dal Magro, L.; Rodrigues, R.C.; de Oliveira Rios, A.; Flôres, S.H. Valorization of Opuntia monacantha (Willd.) haw. Cladodes to obtain a mucilage with hydrocolloid features: Physicochemical and functional performance. Int. J. Biol. Macromol. 2019, 123, 900–909. [Google Scholar] [CrossRef]
- Albano, C.; Negro, C.; Tommasi, N.; Gerardi, C.; Mita, G.; Miceli, A.; De Bellis, L.; Blando, F. Betalains, phenols and antioxidant capacity in cactus pear [Opuntia ficus-indica (L.) mill.] fruits from Apulia (South Italy) genotypes. Antioxidants 2015, 4, 269–280. [Google Scholar] [CrossRef]
- Gassenschmidt, U.; Jany, K.D.; Bernhard, T.; Niebergall, H. Isolation and characterization of a flocculating protein from Moringa oleifera Lam. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1995, 1243, 477–481. [Google Scholar] [CrossRef]
- Mandloi, M.; Chaudhari, S.; Folkard, G.K. Evaluation of natural coagulants for direct filtration. Environ. Technol. 2004, 25, 481–489. [Google Scholar] [CrossRef]
- Hadadi, A.; Imessaoudene, A.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.A.; Mouni, L. An eco-friendly approach to dye wastewater treatment using a novel biocoagulant: Aleppo Pine Seeds (Pinus halepensis Mill.) Parametrical Study and Response Surface Methodology. 2022; Under examination for publication. [Google Scholar]
- Ihaddaden, S.; Aberkane, D.; Boukerroui, A.; Robert, D. Removal of methylene blue (basic dye) by coagulation-flocculation with biomaterials (bentonite and Opuntia Ficus indica). J. Water Process Eng. 2022, 49, 102952. [Google Scholar] [CrossRef]
- Vargas-Solano, S.V.; Rodríguez-González, F.; Martínez-Velarde, R.; Morales-García, S.S.; Jonathan, M.P. Removal of heavy metals present in water from the Yautepec River Morelos México, using Opuntia ficus-indica mucilage. Environ. Adv. 2021, 7, 100160. [Google Scholar] [CrossRef]
- Putra, R.S.; Putri, C.I.; Tyagustin, N.S. The combination of electroflotation-biocoagulation process using aloe vera for river water treatment. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1087, 012047. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K.; Khalfaoui, A.; Bouchareb, R.; Panico, A.; Gisonni, C.; Crispino, G.; Pirozzi, F.; Pizzi, A. Use of Aloe Vera as an organic coagulant for improving drinking water quality. Water 2021, 13, 2024. [Google Scholar] [CrossRef]
- Bouaouine, O.; Bourven, I.; Khalil, F.; Bressollier, P.; Baudu, M. Identification and role of Opuntia Ficus indica constituents in the flocculation mechanism of Colloidal Solutions. Sep. Purif. Technol. 2019, 209, 892–899. [Google Scholar] [CrossRef]
- Zurina, A.Z.; Mohd Fadzli, M.; Abdul Ghani, L.A. Preliminary study of rambutan (Nephelium lappaceum) seed as potential biocoagulant for turbidity removal. Adv. Mater. Res. 2014, 917, 96–105. [Google Scholar] [CrossRef]
- ELsayed, E.M.; El-Den, A.N.; Elkady, M.F.; Zaatout, A.A. Comparison of coagulation performance using natural coagulants against traditional ones. Sep. Sci. Technol. 2020, 56, 1779–1787. [Google Scholar] [CrossRef]
- Ghebremichael, K.A.; Gunaratna, K.R.; Henriksson, H.; Brumer, H.; Dalhammar, G. A simple purification and activity assay of the coagulant protein from Moringa oleifera seed. Water Res. 2005, 39, 2338–2344. [Google Scholar] [CrossRef]
- Aboulhassan, M.A.; Souabi, S.; Yaacoubi, A.; Baudu, M. Improvement of paint effluents coagulation using natural and synthetic coagulant aids. J. Hazard. Mater. 2006, 138, 40–45. [Google Scholar] [CrossRef]
- Yang, W.; Shang, J.; Sharma, P.; Li, B.; Liu, K.; Flury, M. Colloidal stability and aggregation kinetics of biochar colloids: Effects of pyrolysis temperature, cation type, and humic acid concentrations. Sci. Total Environ. 2019, 658, 1306–1315. [Google Scholar] [CrossRef]
- Marques, T.L.; Alves, V.N.; Coelho, L.M.; Coelho, N.M. Removal of ni(ii) from aqueous solution using Moringa oleifera seeds as a bioadsorbent. Water Sci. Technol. 2012, 65, 1435–1440. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K.; Khalfaoui, A.; Pizzi, A.; Medjahdi, G. The use of as natural coagulant in algerian drinking water treatment plant. J. Renew. Mater. 2022, 10, 625–637. [Google Scholar] [CrossRef]
- Kandasamy, K.; Venkatesh, M.; Khadar, Y.S.; Rajasingh, P. One-pot green synthesis of cds quantum dots using Opuntia ficus-indica fruit sap. Mater. Today Proc. 2020, 26, 3503–3506. [Google Scholar] [CrossRef]
- León-Martínez, F.M.; Cano-Barrita, P.F.; Castellanos, F.; Luna-Vicente, K.B.; Ramírez-Arellanes, S.; Gómez-Yáñez, C. Carbonation of high-calcium lime mortars containing cactus mucilage as additive: A spectroscopic approach. J. Mater. Sci. 2020, 56, 3778–3789. [Google Scholar] [CrossRef]
- De Souza, M.T.; Ambrosio, E.; de Almeida, C.A.; de Souza Freitas, T.K.; Santos, L.B.; de Cinque Almeida, V.; Garcia, J.C. The use of a natural coagulant (Opuntia ficus-indica) in the removal for organic materials of textile effluents. Environ. Monit. Assess. 2014, 186, 5261–5271. [Google Scholar] [CrossRef]
- Okuda, T.; Baes, A.U.; Nishijima, W.; Okada, M. Improvement of extraction method of coagulation active components from Moringa oleifera seed. Water Res. 1999, 33, 3373–3378. [Google Scholar] [CrossRef] [Green Version]
- Madrona, G.S.; Serpelloni, G.B.; Vieira, A.M.S.; Nishi, L.; Cardoso, K.C.; Bergamasco, R. Study of the effect of saline solution on the extraction of the Moringa oleifera seed’s active component for water treatment. Water Air Soil Pollut. 2010, 211, 409–415. [Google Scholar] [CrossRef]
- Baquerizo-Crespo, R.J.; Nuñez, Y.; Albite, J.; Macías-Alcívar, J.A.; Cedeño-Zambrano, N.; Dueñas-Rivadeneira, A.A.; Gomez-Salcedo, Y.; Rodríguez-Díaz, J.M. Biocoagulants as an Alternative for Water Treatment. In Advances in the Domain of Environmental Biotechnology; Springer: Singapore, 2021; pp. 313–334. [Google Scholar] [CrossRef]
- Dalvand, A.; Gholibegloo, E.; Ganjali, M.R.; Golchinpoor, N.; Khazaei, M.; Kamani, H.; Hosseini, S.S.; Mahvi, A.H. Comparison of moringa stenopetala seed extract as a clean coagulant with alum and Moringa stenopetala-Alum hybrid coagulant to remove direct dye from textile wastewater. Environ. Sci. Pollut. Res. 2016, 23, 16396–16405. [Google Scholar] [CrossRef]
- Oladoja, N.A. Headway on natural polymeric coagulants in water and wastewater treatment operations. J. Water Process Eng. 2015, 6, 174–192. [Google Scholar] [CrossRef]
- Kristianto, H. The potency of Indonesia native plants as natural coagulant: A Mini Review. Water Conserv. Sci. Eng. 2017, 2, 51–60. [Google Scholar] [CrossRef]
- Beyene, H.D.; Hailegebrial, T.D.; Dirersa, W.B. Investigation of coagulation activity of Cactus Powder in water treatment. J. Appl. Chem. 2016, 2016, 7815903. [Google Scholar] [CrossRef]
- Choudhary, M.; Ray, M.B.; Neogi, S. Evaluation of the potential application of cactus (Opuntia ficus-indica) as a bio-coagulant for pre-treatment of oil sands process-affected water. Sep. Purif. Technol. 2019, 209, 714–724. [Google Scholar] [CrossRef]
- Adjeroud-Abdellatif, N.; Hammoui, Y.; Boudria, A.; Agab, S.; Choulak, F.; Leclerc, J.-P.; Merzouk, B.; Madani, K. Effect of a natural coagulant extract from Opuntia ficus-indica cladode on electrocoagulation-electroflotation water treatment process. Int. J. Environ. Anal. Chem. 2020, 100, 1–25. [Google Scholar] [CrossRef]
- Othmani, B.; Gamelas, J.A.; Rasteiro, M.G.; Khadhraoui, M. Characterization of two cactus formulation-based flocculants and investigation on their flocculating ability for cationic and anionic dyes removal. Polymers 2020, 12, 1964. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.M.; Fugate, E.J.; Craver, V.O.; Smith, J.A.; Zimmerman, J.B. Toward understanding the efficacy and mechanism of Opuntia spp. as a natural coagulant for potential application in water treatment. Environ. Sci. Technol. 2008, 42, 4274–4279. [Google Scholar] [CrossRef] [PubMed]
- Nasim, T.; Pal, A.; Giri, A.; Goswami, L.; Bandyopadhyay, A. Exploring polyelectrolytic features of the exudate from native acacia nilotica for flocculating aqueous kaolin suspension. Sep. Purif. Technol. 2014, 131, 50–59. [Google Scholar] [CrossRef]
- Tie, J.; Li, P.; Xu, Z.; Zhou, Y.; Li, C.; Zhang, X. Removal of congo red from aqueous solution using Moringa oleifera seed cake as natural coagulant. Desalination Water Treat. 2014, 54, 2817–2824. [Google Scholar] [CrossRef]
- Chethana, M.; Sorokhaibam, L.G.; Bhandari, V.M.; Raja, S.; Ranade, V.V. Green approach to dye wastewater treatment using biocoagulants. ACS Sustain. Chem. Eng. 2016, 4, 2495–2507. [Google Scholar] [CrossRef]
- Aboulhassan, M.A.; Harif, S.; Souabi, S.; Yaacoubi, A. Efficient and sustainable treatment of industrial wastewater using a tannin-based polymer. Int. J. Sustain. Eng. 2021, 14, 1943–1949. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Nwodo, U.U.; Okoli, S.A.; Mabinya, L.V.; Okoh, A.I. Implications for public health demands alternatives to inorganic and synthetic flocculants: Bioflocculants as important candidates. MicrobiologyOpen 2016, 5, 177–211. [Google Scholar] [CrossRef] [Green Version]
- Ramli, S.F.; Aziz, H.A. Use of ferric chloride and chitosan as coagulant to remove turbidity and color from landfill leachate. Appl. Mech. Mater. 2015, 773–774, 1163–1167. [Google Scholar] [CrossRef]
- Barbot, E.; Dussouillez, P.; Bottero, J.Y.; Moulin, P. Coagulation of bentonite suspension by polyelectrolytes or ferric chloride: Floc Breakage and Reformation. Chem. Eng. J. 2010, 156, 83–91. [Google Scholar] [CrossRef]
- Al-Husseini, T.R.; Ghawi, A.H.; Ali, A.H. Performance of hydraulic jump rapid mixing for enhancement of turbidity removal from synthetic wastewater: A comparative study. J. Water Process Eng. 2019, 30, 100590. [Google Scholar] [CrossRef]
- Baghvand, A.; Zand, A.D.; Mehrdadi, N.; Karbassi, A. Optimizing coagulation process for low to high turbidity waters using aluminum and iron salts. Am. J. Environ. Sci. 2010, 6, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Bolto, B.; Gregory, J. Organic polyelectrolytes in water treatment. Water Res. 2007, 41, 2301–2324. [Google Scholar] [CrossRef]
- Tripathy, T.; De, B.R. Flocculation: A new way to treat the waste water. J. Phys. Sci. 2006, 10, 93–127. [Google Scholar]
- Zhou, Z.; Yang, Y.; Li, X.; Gao, W.; Liang, H.; Li, G. Coagulation efficiency and flocs characteristics of recycling sludge during treatment of low temperature and micro-polluted water. J. Environ. Sci 2012, 24, 1014–1020. [Google Scholar] [CrossRef]
- Ramavandi, B. Treatment of water turbidity and bacteria by using a coagulant extracted from plantago ovata. Water Resour. Ind. 2014, 6, 36–50. [Google Scholar] [CrossRef] [Green Version]
- Gaikwad, V.T.; Munavalli, G.R. Turbidity removal by conventional and ballasted coagulation with natural coagulants. Appl. Water Sci. 2019, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Asrafuzzaman, M.; Fakhruddin, A.N.; Hossain, M.A. Reduction of turbidity of water using locally available natural coagulants. ISRN Microbiol. 2011, 2011, 632189. [Google Scholar] [CrossRef] [Green Version]
- Kurniawan, S.B.; Abdullah, S.R.; Othman, A.R.; Purwanti, I.F.; Imron, M.F.; Ismail, N.I.; Ahmad, A.; Hasan, H.A. Isolation and characterisation of bioflocculant-producing bacteria from aquaculture effluent and its performance in treating high turbid water. J. Water Process Eng. 2021, 42, 102194. [Google Scholar] [CrossRef]
- Bahrodin, M.B.; Zaidi, N.S.; Hussein, N.; Sillanpää, M.; Prasetyo, D.D.; Syafiuddin, A. Recent advances on coagulation-based treatment of wastewater: Transition from chemical to natural coagulant. Curr. Pollut. Rep. 2021, 7, 379–391. [Google Scholar] [CrossRef]
- Anastasakis, K.; Kalderis, D.; Diamadopoulos, E. Flocculation behavior of Mallow and okra mucilage in treating wastewater. Desalination 2009, 249, 786–791. [Google Scholar] [CrossRef]
- Sowmeyan, R.; Santhosh, J.; Latha, R. Effectiveness of herbs in community water treatment. Int. Res. J. Biochem. Bioinform. 2011, 1, 297–303. [Google Scholar]
- Fatombi, J.K.; Lartiges, B.; Aminou, T.; Barres, O.; Caillet, C. A natural coagulant protein from copra (Cocos nucifera): Isolation, characterization, and potential for water purification. Sep. Purif. Technol. 2013, 116, 35–40. [Google Scholar] [CrossRef]
- Shak, K.P.; Wu, T.Y. Optimized use of alum together with unmodified Cassia obtusifolia seed gum as a coagulant aid in treatment of palm oil mill effluent under natural ph of wastewater. Ind. Crop. Prod. 2015, 76, 1169–1178. [Google Scholar] [CrossRef]
- Babayemi, K.A.; Onukwuli, O.D. Phosphate removal from phosphorus containing waste water by coagulation/flocculation process using gossypium spp. (GS) as coagulant. Curr. Adv. Environ. Sci. 2015, 3, 1–5. [Google Scholar] [CrossRef]
- Shamsnejati, S.; Chaibakhsh, N.; Pendashteh, A.R.; Hayeripour, S. Mucilaginous seed of ocimum basilicum as a natural coagulant for textile wastewater treatment. Ind. Crop. Prod. 2015, 69, 40–47. [Google Scholar] [CrossRef]
- Tie, J.; Jiang, M.; Li, H.; Zhang, S.; Zhang, X. A comparison between Moringa oleifera seed presscake extract and polyaluminum chloride in the removal of direct black 19 from synthetic wastewater. Ind. Crop. Prod. 2015, 74, 530–534. [Google Scholar] [CrossRef]
- Abidin, Z.Z.; Ismail, N.; Yunus, R.; Ahamad, I.S.; Idris, A. A preliminary study on Jatropha curcas as coagulant in wastewater treatment. Environ. Technol. 2011, 32, 971–977. [Google Scholar] [CrossRef]
- Kukić, D.V.; Šćiban, M.B.; Prodanović, J.M.; Tepić, A.N.; Vasić, M.A. Extracts of fava bean (Vicia faba L.) seeds as natural coagulants. Ecol. Eng. 2015, 84, 229–232. [Google Scholar] [CrossRef]
- Muthuraman, G.; Sasikala, S. Removal of turbidity from drinking water using natural coagulants. J. Ind. Eng. Chem. 2014, 20, 1727–1731. [Google Scholar] [CrossRef]
- Ward, O.P.; Moo-young, M.; Venkat, K. Enzymatic degradation of cell wall and related plant polysaccharides. Crit. Rev. Biotechnol. 1989, 8, 237–274. [Google Scholar] [CrossRef] [PubMed]
- Byong, H.L. Fundamentals of Food Biotechnology; Wiley-VCH Publishers Inc.: New York, NY, USA, 1996. [Google Scholar]
- Kristianto, H.; Rahman, H.; Prasetyo, S.; Sugih, A.K. Removal of congo red aqueous solution using Leucaena leucocephala seed’s extract as natural coagulant. Appl. Water Sci. 2019, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Ndabigengesere, A.; Narasiah, K.S. Quality of water treated by coagulation using Moringa oleifera seeds. Water Res. 1998, 32, 781–791. [Google Scholar] [CrossRef]
- Adnan, O.; Abidin, Z.Z.; Idris, A.; Kamarudin, S.; Al-Qubaisi, M.S. A novel biocoagulant agent from Mushroom Chitosan as water and wastewater therapy. Environ. Sci. Pollut. Res. 2017, 24, 20104–20112. [Google Scholar] [CrossRef]
- Amagloh, F.K.; Benang, A. Effectiveness of Moringa oleifera seed as coagulant for water purification. Afr. J. Agric. Res. 2009, 4, 119–123. [Google Scholar]
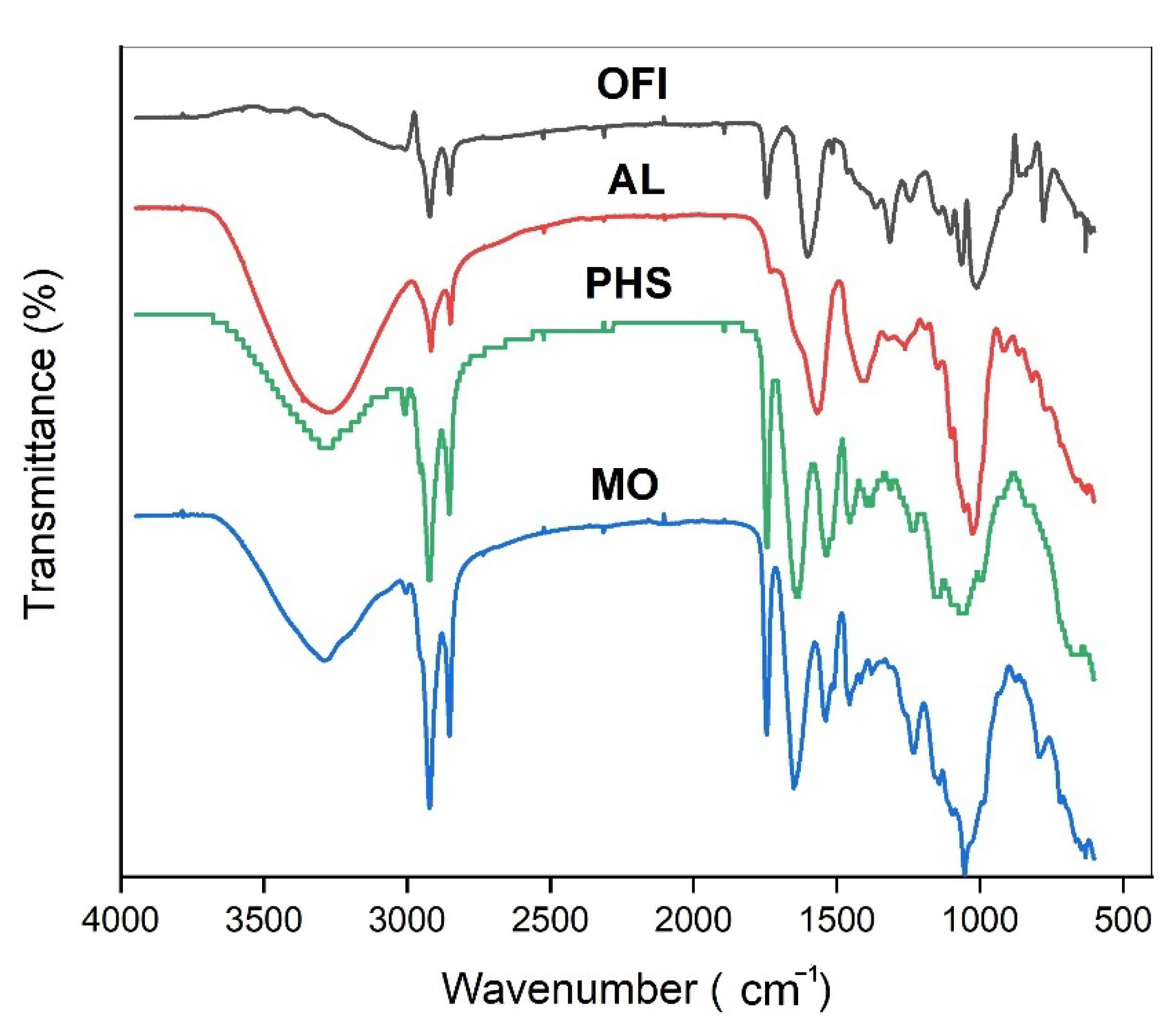
| Wave Number (cm−1) | Functional Group | References |
|---|---|---|
| 3278.85 2916.66 | Hydroxyl group OH/N-H bonds C-H asymmetric stretching in CH2 | [34,35,36,37] |
| 2848.6 | C-H symmetric stretching in CH2 | |
| 1567.72 | Carbonyl function C=O (primary amides) | |
| 1398.82 | CH3 primary aromatic amines | |
| 1537 | NH2 amine group | |
| 1025.83 | CO group/C-N stretching vibration of amine groups |
| Bio-Coagulant | Preparation | Effluent Type | Optimum pH | Optimum Dosage | Turbidity Removal | References |
|---|---|---|---|---|---|---|
| AV | NaCl extraction 0.5 M | Bentonite synthetic water | 2–8 | 2 mL/200 mL | 100% | This study |
| OFI | NaCl extraction 0.5 M | Bentonite synthetic water | 2–8 | 2.5 mL/200 mL | 100% | This study |
| MO | NaCl extraction 0.5 M | Bentonite synthetic water | 2–8 | 3 mL/200 mL | 99.71% | This study |
| PHS | NaCl extraction 0.5 M | Bentonite synthetic water | 2–8 | 3.5 mL/200 mL | 99.43% | This study |
| Malva sylvestris | Mucilage | Synthetic | 7 | 12 mg/L | 96.3–97.4% | [67] |
| Luffa cylindrica | Powder | Surface water | 9.4 | 8000 mg/L | 85% | [68] |
| Cicer arietinum | Water extract | Clay | / | 100 mg/L | 95.9% | [67] |
| Cocos nucifera | Water extract | / | 8 | 250 mg/L | 25% | [69] |
| Cassia obtusifolia | Powder | Palm oil | acidic | 2470 mg/L + 1115 mg/L aluminum sulfate | 82% | [70] |
| Gosspium spp | Water extract | Phosphate | 6 | 500 mg/L | >70% | [71] |
| Ocimum basilicum | Water extract at 50 °C + 0.9% NaCl/1 h | Textile (cationic dye) | 6.5 | 9.6 mg/L + 20 mg of aluminum sulfate | 68.5% | [72] |
| Moringa oleifra | 0.5 M (NH4)2SO4 extract | Synthetic | 5 | 5 mL | 94% | [73] |
| Jatropha curcas | 5% water extract | Synthetic | 3 | 80 mg/L | 90% | [74] |
| Vicia faba | Textile wastewater | Textile wastewater | 7 | 6.75 mg/L | 60–70% | [75] |
| Phaseolus vulgaris | NaCl extraction | Kaolinturbid water | 7 | 1 mL/L | 95% | [76] |
| Coagulant | Coagulant Dosage (mL/200 mL) | % Turbidity Removal | Sludge Volume (mL/L) |
|---|---|---|---|
| MO | 1.5 | 87 ± 2.5 | 25 ± 1 |
| PHS | 1.5 | 84.2 ± 3.7 | 30 ± 1 |
| OFI | 1.5 | 94.81 ± 2.5 | 26 ± 1 |
| AV | 1.5 | 87.1 ± 3.0 | 22 ± 1 |
| FeCl3 | 1.5 | 99.12 ± 3.7 | 52 ± 1 |
| Alum | 1.5 | 98.84 ± 3.7 | 62 ± 1 |
| Coagulant | Initial pH of Water (before Addition of Coagulant) | Final pH |
|---|---|---|
| AV | 7.23 ± 0.32 | 7.28 ± 0.42 |
| OFI | 7.25 ± 0.15 | |
| MO | 7.80 ± 0.28 | |
| PHS | 7.68 ± 0.31 | |
| FeCl3 | 3.28 ± 0.46 | |
| Alum | 4.12 ± 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadadi, A.; Imessaoudene, A.; Bollinger, J.-C.; Assadi, A.A.; Amrane, A.; Mouni, L. Comparison of Four Plant-Based Bio-Coagulants Performances against Alum and Ferric Chloride in the Turbidity Improvement of Bentonite Synthetic Water. Water 2022, 14, 3324. https://doi.org/10.3390/w14203324
Hadadi A, Imessaoudene A, Bollinger J-C, Assadi AA, Amrane A, Mouni L. Comparison of Four Plant-Based Bio-Coagulants Performances against Alum and Ferric Chloride in the Turbidity Improvement of Bentonite Synthetic Water. Water. 2022; 14(20):3324. https://doi.org/10.3390/w14203324
Chicago/Turabian StyleHadadi, Amina, Ali Imessaoudene, Jean-Claude Bollinger, Aymen Amine Assadi, Abdeltif Amrane, and Lotfi Mouni. 2022. "Comparison of Four Plant-Based Bio-Coagulants Performances against Alum and Ferric Chloride in the Turbidity Improvement of Bentonite Synthetic Water" Water 14, no. 20: 3324. https://doi.org/10.3390/w14203324