New Margin-Based Biochar for Removing Hydrogen Sulfide Generated during the Anaerobic Wastewater Treatment
Abstract
:1. Introduction
- i.
- Valorizing sludge from sewage treatment plants and margins (source of pollution).
- ii.
- Reduction in the costs of consumables by using the mixture of sludge and margins as raw material to produce biochar as adsorbent.
2. Materials and Methods
2.1. Biochar Production
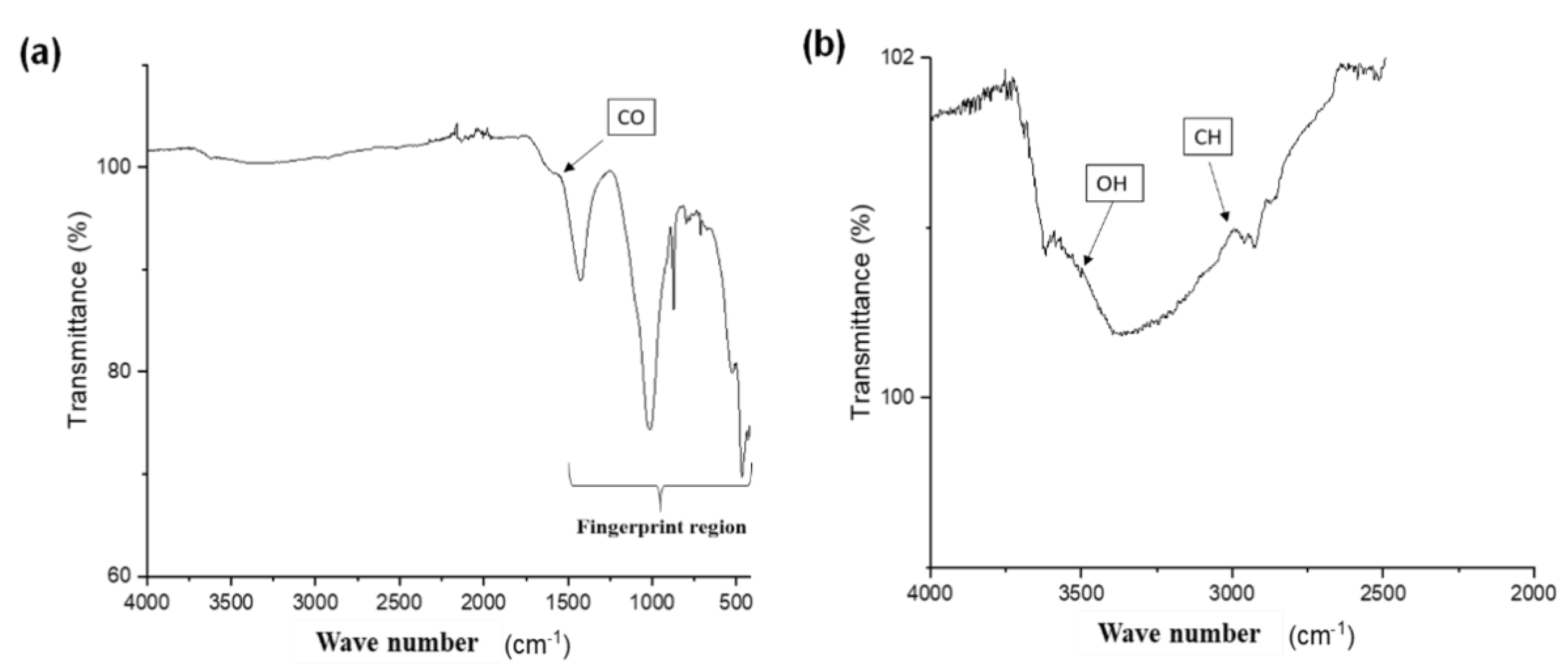
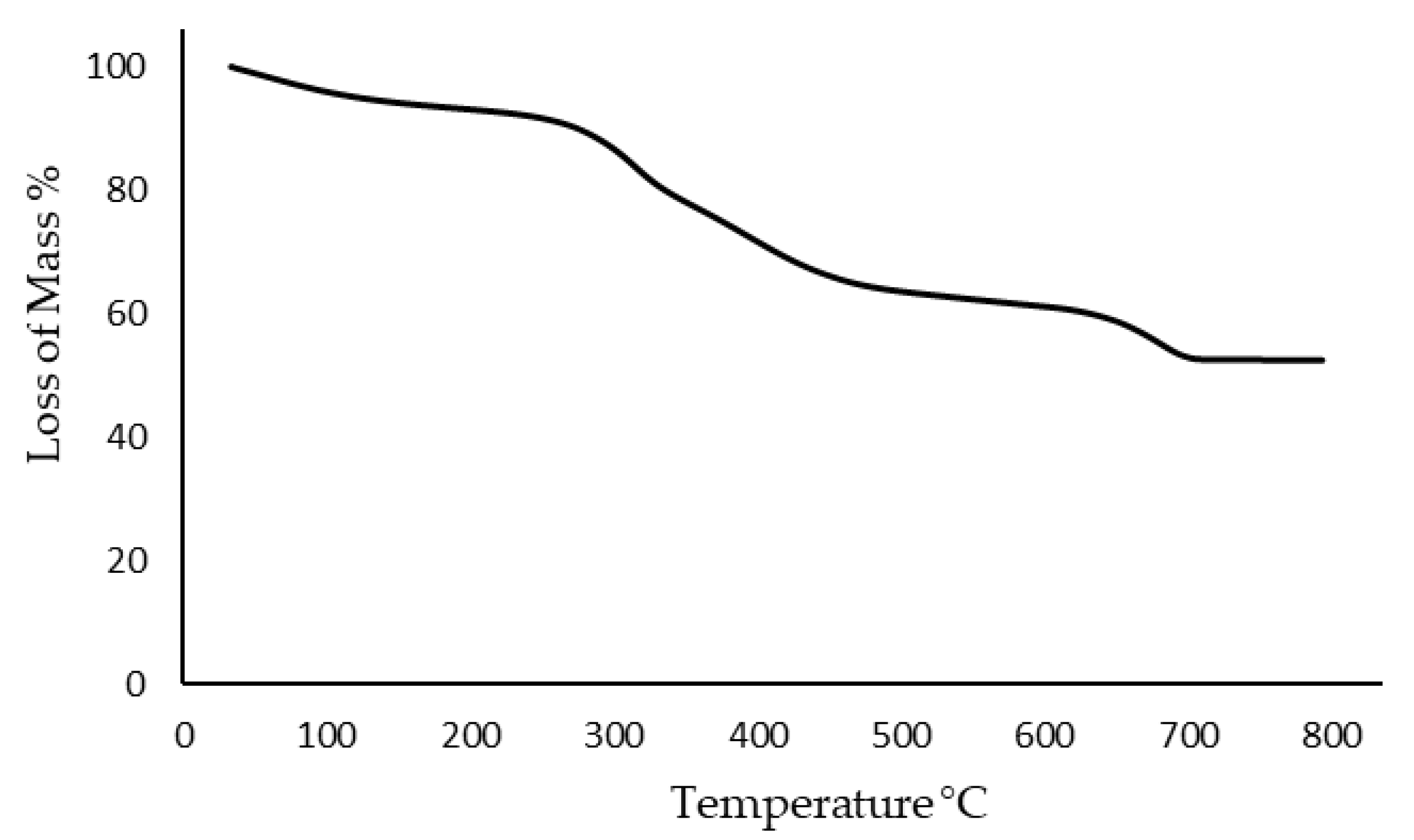
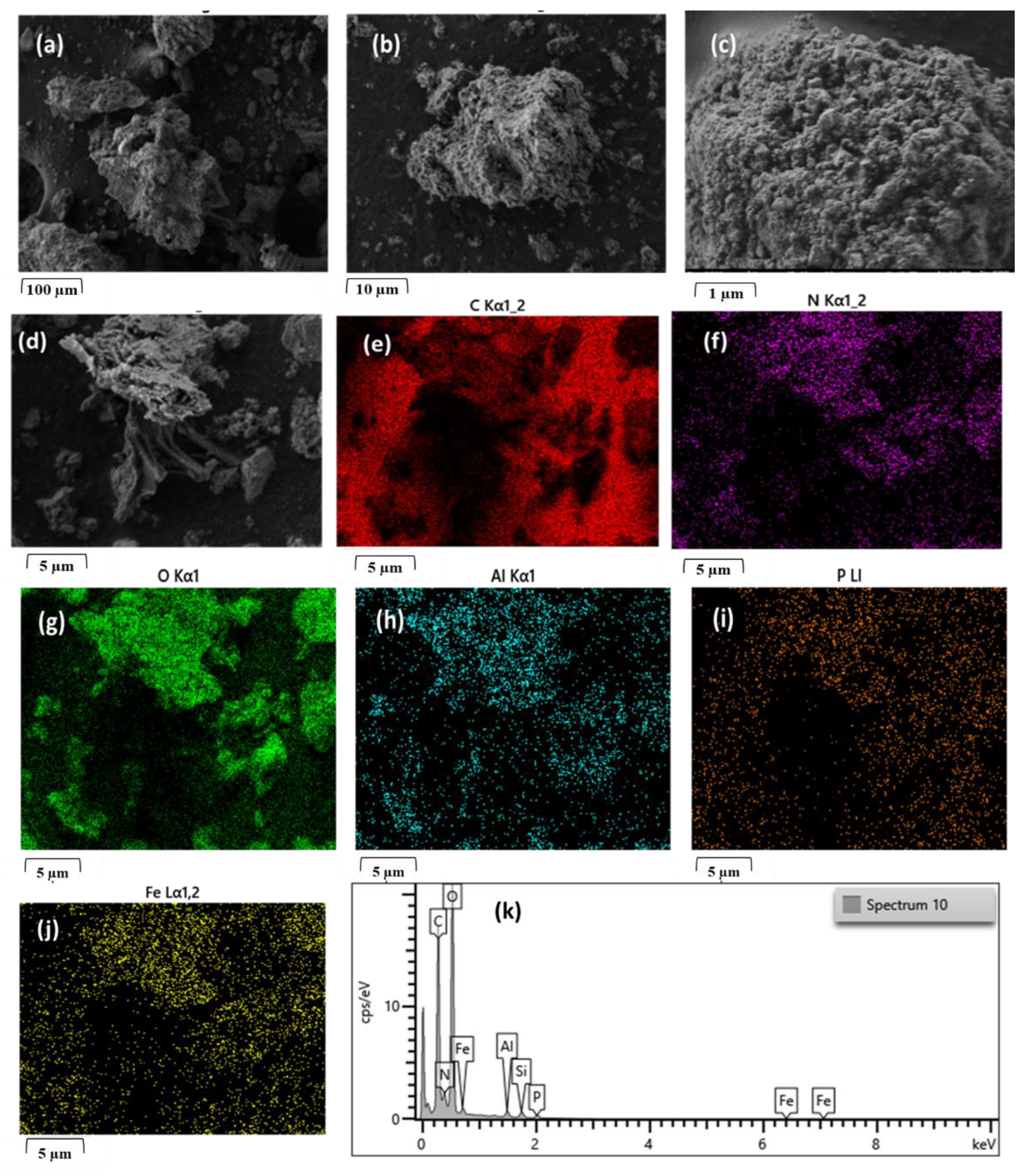
2.2. Physicochemical Characterization of Biochar
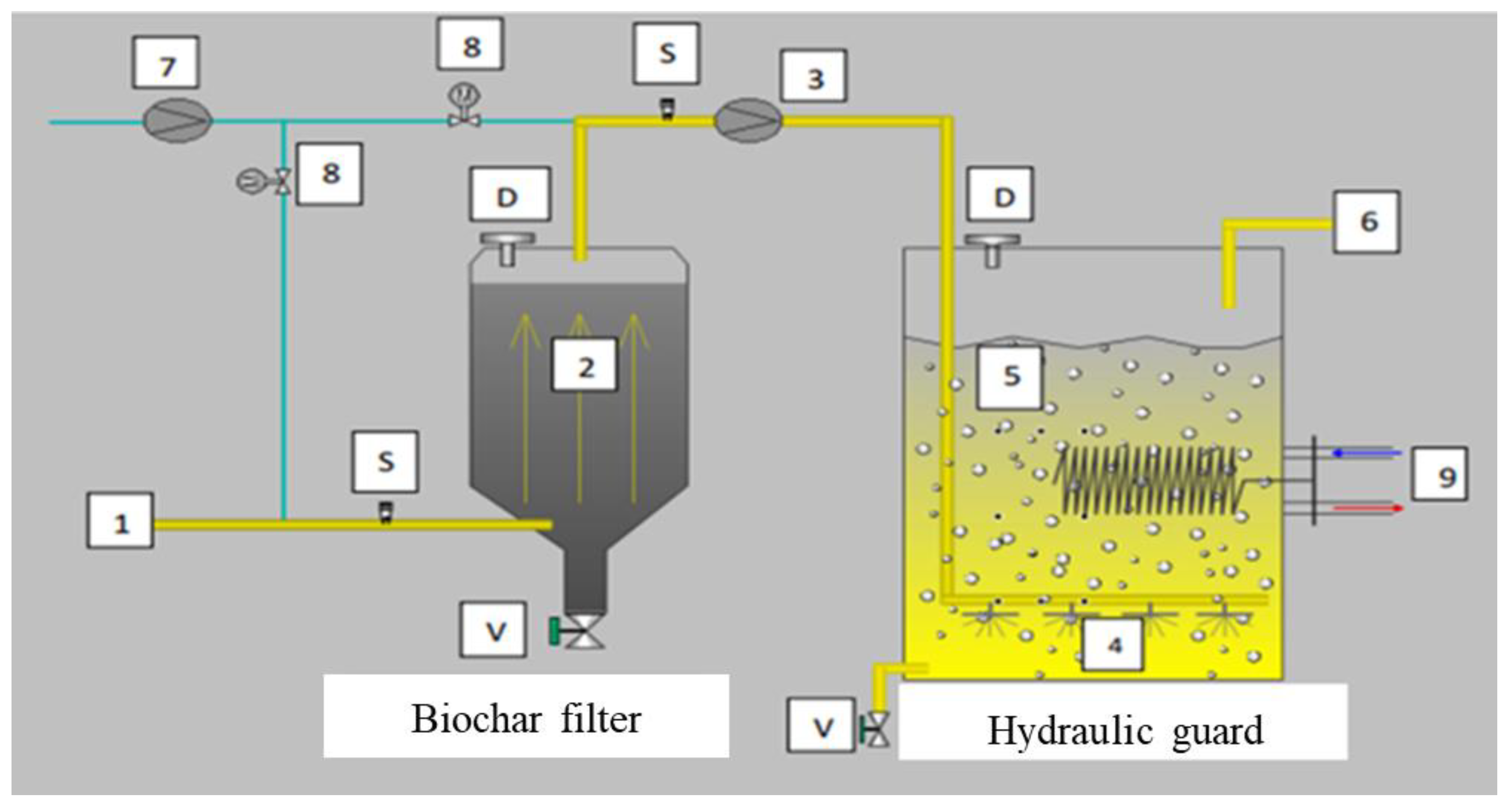
2.3. Description of the Pilot
- ✓
- A biochar filter (2) whose biochar is produced from a mixture of sewage plant sludge and margin used as an adsorbent for the pre-filtration of sulfur compounds in biogas and, more particularly, hydrogen sulfide (H2S).
- ✓
- Hydraulic guard (5) to ensure minimum hydraulic pressure through the biochar filter and can serve as an additional biological treatment reactor.
2.4. Statistical Analysis
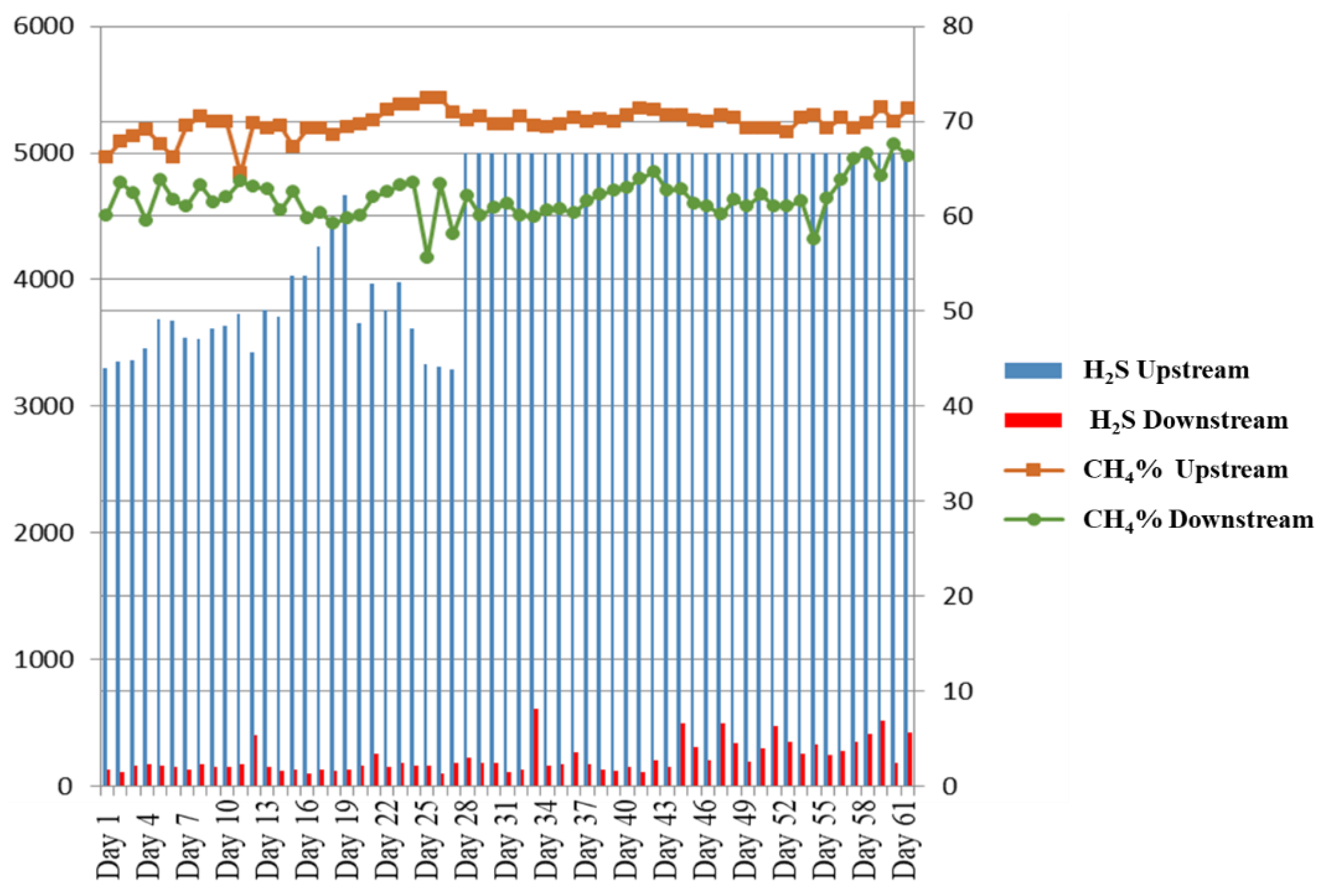
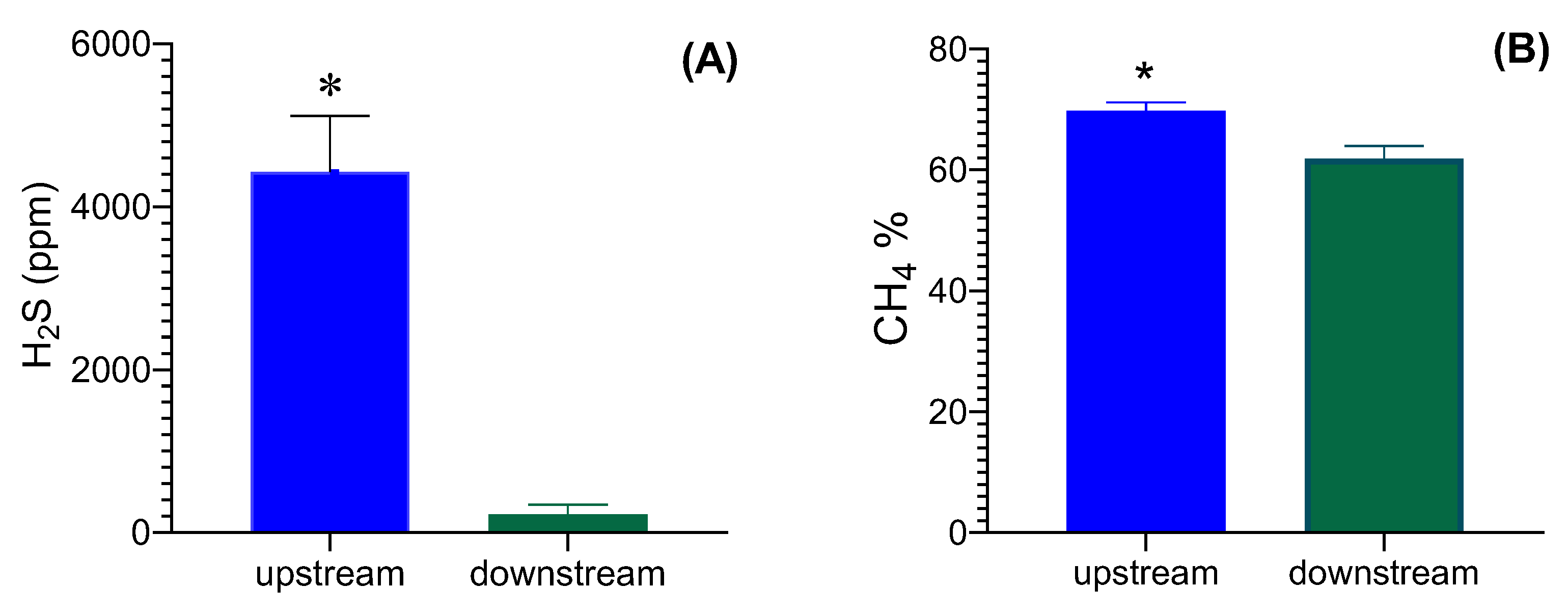
3. Results and Discussion
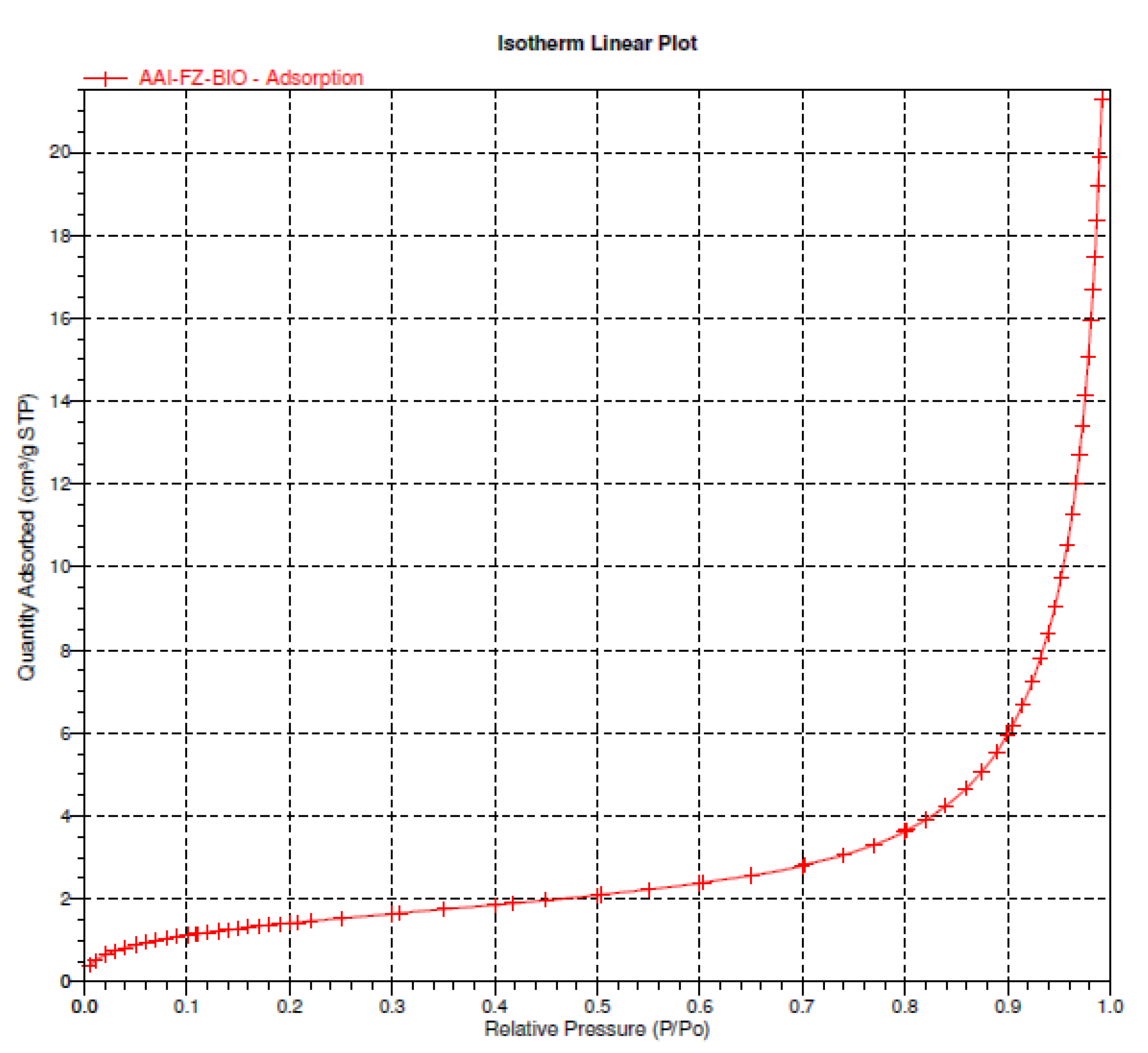
Physicochemical Characterization of Biochar
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasi, S.; Läntelä, J.; Rintala, J. Trace Compounds Affecting Biogas Energy Utilisation—A Review. Energy Convers. Manag. 2011, 52, 3369–3375. [Google Scholar] [CrossRef]
- Hong, J.; Hong, J.; Otaki, M.; Jolliet, O. Environmental and Economic Life Cycle Assessment for Sewage Sludge Treatment Processes in Japan. Waste Manag. 2009, 29, 696–703. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Boivin, S. A Review of Biogas Purification Processes. Biofuels Bioprod. Biorefining 2009, 3, 42–71. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; dos Santos Lins, P.V.; de Magalhães Oliveira, L.M.; Sepulveda, P.; Ighalo, J.O.; Rajapaksha, A.U.; Meili, L. Sewage Sludge-Derived Biochar for the Adsorptive Removal of Wastewater Pollutants: A Critical Review. Environ. Pollut. 2022, 293, 118581. [Google Scholar] [CrossRef]
- Ben Ayed, R.; Moreau, F.; Ben Hlima, H.; Rebai, A.; Ercisli, S.; Kadoo, N.; Hanana, M.; Assouguem, A.; Ullah, R.; Ali, E.A. SNP discovery and structural insights into OeFAD2 unravelling high oleic/linoleic ratio in olive oil. Comput. Struct. Biotechnol. J. 2022, 20, 1229–1243. [Google Scholar] [CrossRef]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar Prepared from Co-Pyrolysis of Municipal Sewage Sludge and Tea Waste for the Adsorption of Methylene Blue from Aqueous Solutions: Kinetics, Isotherm, Thermodynamic and Mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Assouguem, A.; Kara, M.; Mechchate, H.; Al-Mekhlafi, F.A.; Nasr, F.; Farah, A.; Lazraq, A. Evaluation of the Impact of Different Management Methods on Tetranychus urticae (Acari: Tetranychidae) and Their Predators in Citrus Orchards. Plants 2022, 11, 623. [Google Scholar] [CrossRef]
- Li, J.; Yu, G.; Pan, L.; Li, C.; You, F.; Wang, Y. Ciprofloxacin Adsorption by Biochar Derived from Co-Pyrolysis of Sewage Sludge and Bamboo Waste. Environ. Sci. Pollut. Res. 2020, 27, 22806–22817. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Bhatia, D.; Dhiman, J.; Samuel, J.; Prasad, R.; Singh, J. A Sustainable Paradigm of Sewage Sludge Biochar: Valorization, Opportunities, Challenges and Future Prospects. J. Clean. Prod. 2020, 269, 122259. [Google Scholar] [CrossRef]
- Gasquet, V. H2S Removal from Biogas Using Raw and Formulated Thermal Treatment Residues: Performance Comparison and Understanding of Adsorption Mechanisms 2020. Waste Biomass Valorization 2020, 11, 5363–5373. [Google Scholar] [CrossRef]
- Assouguem, A.; Kara, M.; Ramzi, A.; Annemer, S.; Kowalczyk, A.; Ali, E.A.; Moharram, B.A.; Lazraq, A.; Farah, A. Evaluation of the Effect of Four Bioactive Compounds in Combination with Chemical Product against Two Spider Mites Tetranychus urticae and Eutetranychus orientalis (Acari: Tetranychidae). Evid. Based Complement. Altern. Med. 2022, 2022, 2004623. [Google Scholar] [CrossRef] [PubMed]
- Kambo, H.S.; Dutta, A. A Comparative Review of Biochar and Hydrochar in Terms of Production, Physico-Chemical Properties and Applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal Responses to Biochar in Soil—Concepts and Mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Hale, S.; Hanley, K.; Lehmann, J.; Zimmerman, A.; Cornelissen, G. Effects of Chemical, Biological, and Physical Aging As Well As Soil Addition on the Sorption of Pyrene to Activated Carbon and Biochar. Environ. Sci. Technol. 2011, 45, 10445–10453. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Choi, Y.-K.; Kan, E. Effects of Dairy Manure-Derived Biochar on Psychrophilic, Mesophilic and Thermophilic Anaerobic Digestions of Dairy Manure. Bioresour. Technol. 2018, 250, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Kohlstock, D.; Haupt, T.; Heldt, E.; Heldt, N.; Kraft, E. Biochar as Additive in Biogas-Production from Bio-Waste. Energies 2016, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Linville, J.L.; Ignacio-de Leon, P.A.A.; Schoene, R.P.; Urgun-Demirtas, M. Towards a Sustainable Paradigm of Waste-to-Energy Process: Enhanced Anaerobic Digestion of Sludge with Woody Biochar. J. Clean. Prod. 2016, 135, 1054–1064. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Schoene, R.P.; Snyder, S.W. Producing Pipeline-Quality Biomethane via Anaerobic Digestion of Sludge Amended with Corn Stover Biochar with in-Situ CO2 Removal. Appl. Energy 2015, 158, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Ma, W.; Lu, T.; Jiang, Z.; Xiong, R.; Huang, C. Multifunctional Nanofibrous Membranes with Sunlight-Driven Self-Cleaning Performance for Complex Oily Wastewater Remediation. J. Colloid Interface Sci. 2022, 608, 164–174. [Google Scholar] [CrossRef]
- Ma, W.; Jiang, Z.; Lu, T.; Xiong, R.; Huang, C. Lightweight, Elastic and Superhydrophobic Multifunctional Nanofibrous Aerogel for Self-Cleaning, Oil/Water Separation and Pressure Sensing. Chem. Eng. J. 2022, 430, 132989. [Google Scholar] [CrossRef]
- Liu, C.; Jarochowska, E.; Du, Y.; Vachard, D.; Munnecke, A. Microfacies and Carbon Isotope Records of Mississippian Carbonates from the Isolated Bama Platform of Youjiang Basin, South China: Possible Responses to Climate-Driven Upwelling. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 438, 96–112. [Google Scholar] [CrossRef]
- Effect of Process Parameters on Production of Biochar from Biomass Waste through Pyrolysis: A Review—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S1364032115012010?casa_token=xRxhtKN9VeMAAAAA:STy7YFbCGeuTirFgHpZFw5uul9SiQpRvcGRrjLKHl9CODOiqvnCXgfCp5DAHby6ZCHn2wfpTzyk (accessed on 23 July 2022).
- Biochar: Production, Properties and Emerging Role as a Support for Enzyme Immobilization—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0959652620303140?casa_token=6_KadULQi5kAAAAA:hsTQ-M5a1MCd068PlsLPYnNQyYzATxkQ5jBE9r3sG9ZYusmwU7GTzemLlVq4yArEifwfCs0f4CI (accessed on 23 July 2022).
- Younis, U.; Rahi, A.A.; Danish, S.; Ali, M.A.; Ahmed, N.; Datta, R.; Fahad, S.; Holatko, J.; Hammerschmiedt, T.; Brtnicky, M.; et al. Fourier Transform Infrared Spectroscopy Vibrational Bands Study of Spinacia Oleracea and Trigonella Corniculata under Biochar Amendment in Naturally Contaminated Soil. PLoS ONE 2021, 16, e0253390. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Yan, Y.; Lamb, D.; Naidu, R.; Bolan, N.S.; Liu, Y.; Ok, Y.S.; Donne, S.W.; Semple, K.T. Thermal Stability of Biochar and Its Effects on Cadmium Sorption Capacity. Bioresour. Technol. 2017, 246, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downie, A.; Crosky, A.; Munroe, P. Physical Properties of Biochar. In Biochar for Environmental Management; Routledge: London, UK, 2009; ISBN 978-1-84977-055-2. [Google Scholar]
- Tan, Z.; Zou, J.; Zhang, L.; Huang, Q. Morphology, Pore Size Distribution, and Nutrient Characteristics in Biochars under Different Pyrolysis Temperatures and Atmospheres. J. Mater. Cycles Waste Manag. 2018, 20, 1036–1049. [Google Scholar] [CrossRef]
- Gondim, R.S.; Muniz, C.R.; Lima, C.E.P.; Santos, C.L.A.D. Explaining the water-holding capacity of biochar by scanning electron microscope images. Rev. Caatinga 2018, 31, 972–979. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Zhang, W.; Wang, S.; Zhuang, L.; Yang, Y.; Qiu, R. Characterization of Sewage Sludge-Derived Biochars from Different Feedstocks and Pyrolysis Temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 137–143. [Google Scholar] [CrossRef]
- Vikash Kumar, K.; Sivasankara Raju, R. Statistical Modeling and Optimization of Al-MMCs Reinforced with Coconut Shell Ash Particulates. In Innovative Product Design and Intelligent Manufacturing Systems; Deepak, B.B.V.L., Parhi, D., Jena, P.C., Eds.; Springer: Singapore, 2020; pp. 703–712. [Google Scholar]
- Saletnik, B.; Zaguła, G.; Bajcar, M.; Tarapatskyy, M.; Bobula, G.; Puchalski, C. Biochar as a Multifunctional Component of the Environment—A Review. Appl. Sci. 2019, 9, 1139. [Google Scholar] [CrossRef] [Green Version]
- Lassoued, N.; Bilal, E.; Rejeb, S.; Guénole-Bilal, I.; Khelil, N.; Rejeb, M.N.; Gallice, F. Behavior Canola (Brassica Napus) Following a Sewage Sludge Treatment. Carpathian J. Earth Environ. Sci. 2013, 8, 155–165. [Google Scholar]
- Adachi, A.; Ouadrhiri, F.E.; Kara, M.; El Manssouri, I.; Assouguem, A.; Almutairi, M.H.; Bayram, R.; Mohamed, H.R.H.; Peluso, I.; Eloutassi, N.; et al. Decolorization and Degradation of Methyl Orange Azo Dye in Aqueous Solution by the Electro Fenton Process: Application of Optimization. Catalysts 2022, 12, 665. [Google Scholar] [CrossRef]
- Zahoor, M.; Wahab, M.; Salman, S.M.; Sohail, A.; Ali, E.A.; Ullah, R. Removal of doxycycline from water using Dalbergia sissoo waste biomass based activated carbon and magnetic oxide/activated bioinorganic nanocomposite in batch adsorption and adsorption/membrane hybrid processes. Bioinorg. Chem. Appl. 2022. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Guillet-Nicolas, R.; García-Martínez, J.; Thommes, M. Recent Advances in the Textural Characterization of Hierarchically Structured Nanoporous Materials. Chem. Soc. Rev. 2017, 46, 389–414. [Google Scholar] [CrossRef] [PubMed]
- Ayiania, M.; Carbajal-Gamarra, F.M.; Garcia-Perez, T.; Frear, C.; Suliman, W.; Garcia-Perez, M. Production and Characterization of H2S and PO43− Carbonaceous Adsorbents from Anaerobic Digested Fibers. Biomass Bioenergy 2019, 120, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Huang, H.; Zhang, Y.; Xu, Z.; Cao, X. Biochar as Both Electron Donor and Electron Shuttle for the Reduction Transformation of Cr(VI) during Its Sorption. Environ. Pollut. 2019, 244, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.J.; Balaguer, M.D.; Rigola, M. Feasibility of Activated Carbon Production from Biological Sludge by Chemical Activation with ZnCl2 and H2SO4. Environ. Technol. 1996, 17, 667–671. [Google Scholar] [CrossRef]
- Ogasawara, S.; Kuroda, M.; Wakao, N. Preparation of Activated Carbon by Thermal Decomposition of Used Automotive Tires. Available online: https://pubs.acs.org/doi/pdf/10.1021/ie00072a030 (accessed on 22 June 2022).

| Element | Concentration Found for Synthesized Biochar (mg/kg) | Limit Value According to the Standard | ||
|---|---|---|---|---|
| IBI | BQM | EBC | ||
| B | 42 | - | - | - |
| Na | 890 | - | - | - |
| Mg | 16.506 | - | - | - |
| Al | 21.345 | - | - | - |
| Si | 658 | - | - | - |
| P | 21.408 | - | - | - |
| Ca | 144.651 | - | - | - |
| Mn | 346 | - | - | - |
| Fe | 16.464 | - | - | - |
| Co | 5 | - | - | - |
| Ni | 33 | 47–420 | 10 | 30 |
| Cu | 727 | 143–6000 | 40 | 100 |
| Zn | 1315 | 416–7400 | 150 | 400 |
| As | 0 | 13–100 | 10 | 13 |
| Cd | 4.9 | 1.4–39 | 3 | 1 |
| Pb | 117 | 121–300 | 60 | 120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaga, Y.; Benmessaoud, S.; Kara, M.; Assouguem, A.; Al-Ghamdi, A.A.; Al-Hemaid, F.M.; Elshikh, M.S.; Ullah, R.; Banach, A.; Bahhou, J. New Margin-Based Biochar for Removing Hydrogen Sulfide Generated during the Anaerobic Wastewater Treatment. Water 2022, 14, 3319. https://doi.org/10.3390/w14203319
Gaga Y, Benmessaoud S, Kara M, Assouguem A, Al-Ghamdi AA, Al-Hemaid FM, Elshikh MS, Ullah R, Banach A, Bahhou J. New Margin-Based Biochar for Removing Hydrogen Sulfide Generated during the Anaerobic Wastewater Treatment. Water. 2022; 14(20):3319. https://doi.org/10.3390/w14203319
Chicago/Turabian StyleGaga, Younes, Safaa Benmessaoud, Mohammed Kara, Amine Assouguem, Abdullah Ahmed Al-Ghamdi, Fahad M. Al-Hemaid, Mohamed S. Elshikh, Riaz Ullah, Artur Banach, and Jamila Bahhou. 2022. "New Margin-Based Biochar for Removing Hydrogen Sulfide Generated during the Anaerobic Wastewater Treatment" Water 14, no. 20: 3319. https://doi.org/10.3390/w14203319
APA StyleGaga, Y., Benmessaoud, S., Kara, M., Assouguem, A., Al-Ghamdi, A. A., Al-Hemaid, F. M., Elshikh, M. S., Ullah, R., Banach, A., & Bahhou, J. (2022). New Margin-Based Biochar for Removing Hydrogen Sulfide Generated during the Anaerobic Wastewater Treatment. Water, 14(20), 3319. https://doi.org/10.3390/w14203319









