Enhanced Catalytic Activity of a Coal-Based Powdered Activated Carbon by Thermal Treatment
Abstract
:1. Introduction
2. Results
2.1. OTC Removal by PACs Treated at Different Temperatures
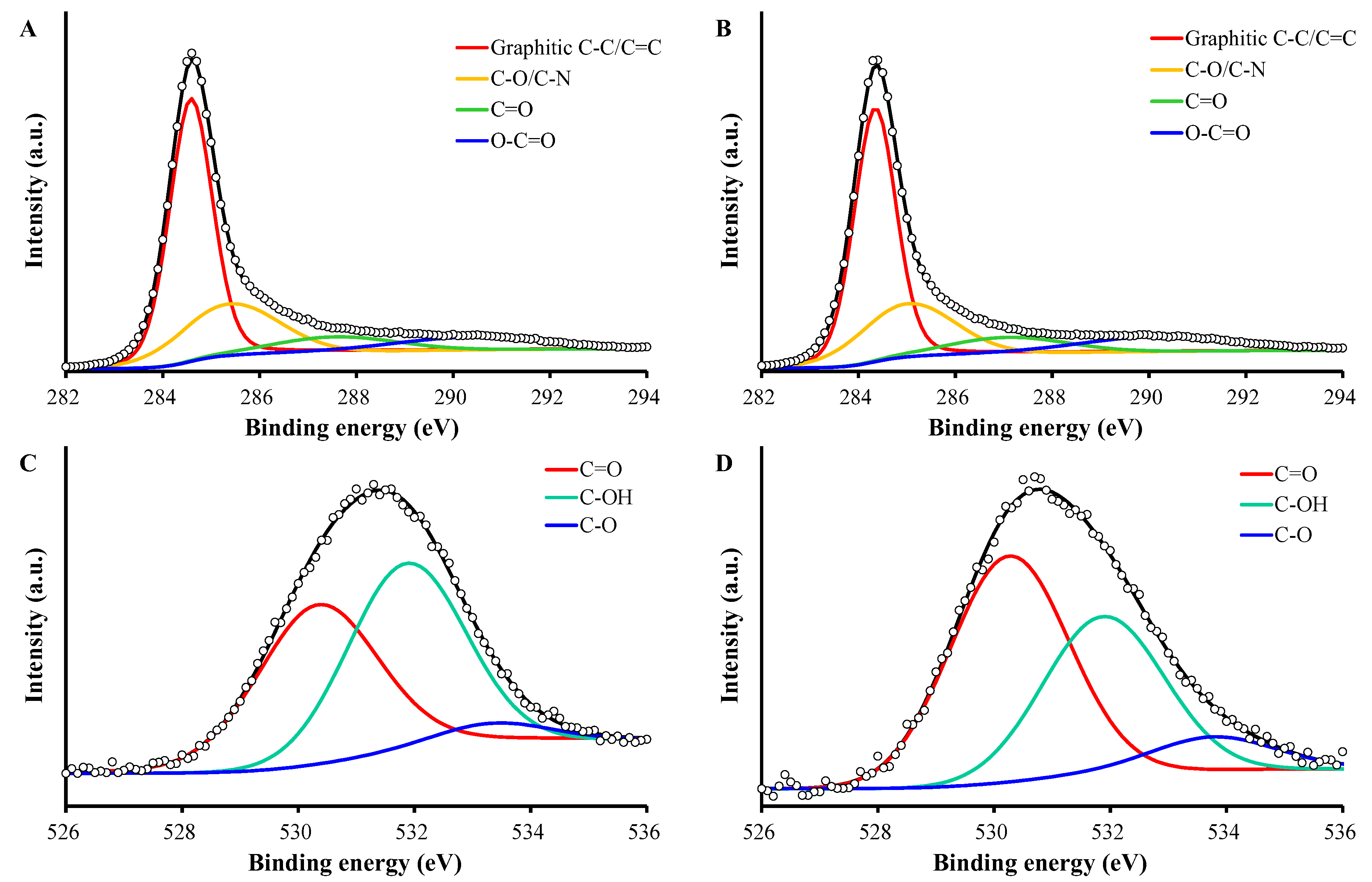
2.2. Characterization of PAC and PAC900
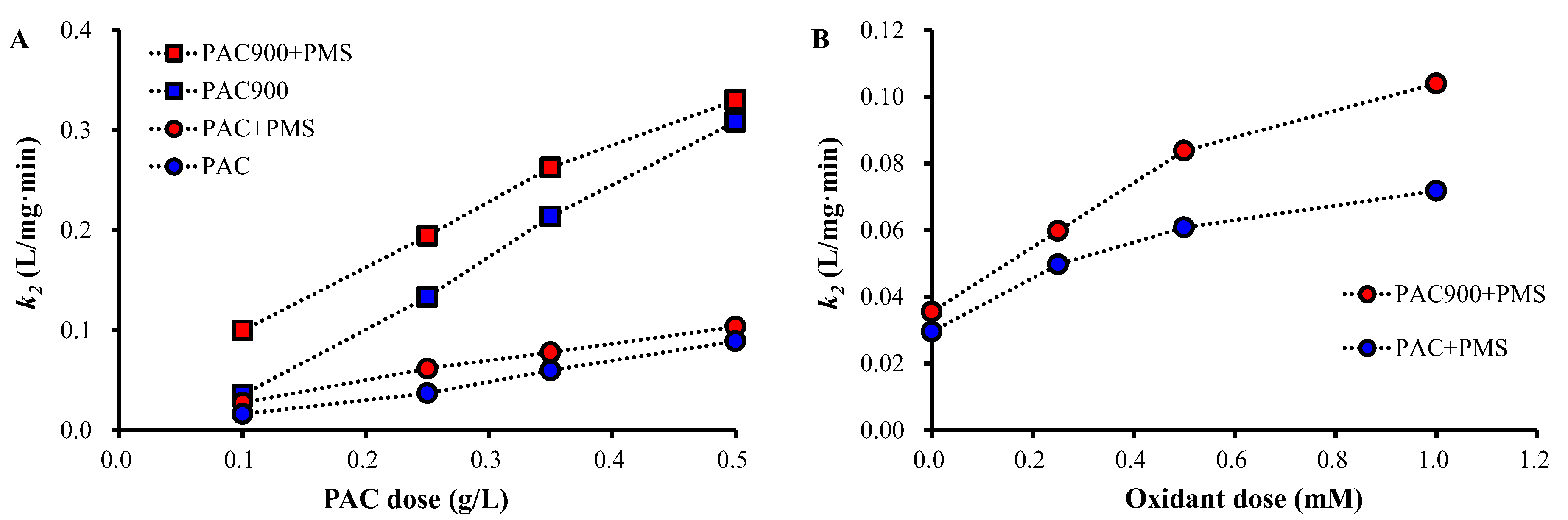
2.3. OTC Removal by PAC and PAC900 under Different Conditions
2.4. Radical Identification and the Possible Mechanisms of OTC Removal by Thermally Treated PAC
3. Materials and Methods
3.1. Materials
3.2. Batch Experiments
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gogate, P.R.; Pandit, A.B. A Review of Imperative Technologies for Wastewater Treatment I: Oxidation Technologies at Ambient Conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Zhao, Q.; Mao, Q.; Zhou, Y.; Wei, J.; Liu, X.; Yang, J.; Luo, L.; Zhang, J.; Chen, H.; Chen, H.; et al. Metal-Free Carbon Materials-Catalyzed Sulfate Radical-Based Advanced Oxidation Processes: A Review on Heterogeneous Catalysts and Applications. Chemosphere 2017, 189, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Tang, L.; Tang, J.; Zeng, G.; Huang, B.; Dong, H.; Zhang, Y.; Zhou, Y.; Deng, Y.; Ma, L.; et al. Catalytic Reduction–Adsorption for Removal of p-Nitrophenol and Its Conversion p-Aminophenol from Water by Gold Nanoparticles Supported on Oxidized Mesoporous Carbon. J. Colloid Interface Sci. 2016, 469, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, Y.-P.; Su, M.; Yuan, Z.-Y. Metal-Free Carbonaceous Materials as Promising Heterogeneous Catalysts. ChemCatChem 2015, 7, 2765–2787. [Google Scholar] [CrossRef]
- Sun, Y.; Cho, D.-W.; Graham, N.J.D.; Hou, D.; Yip, A.C.K.; Khan, E.; Song, H.; Li, Y.; Tsang, D.C.W. Degradation of Antibiotics by Modified Vacuum-UV Based Processes: Mechanistic Consequences of H2O2 and K2S2O8 in the Presence of Halide Ions. Sci. Total Environ. 2019, 664, 312–321. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, X.; Liu, S.; Liu, Y.; Zeng, G.; Ye, S.; Yin, Z.; Hu, X.; Liu, N. Catalytic Degradation of Estrogen by Persulfate Activated with Iron-Doped Graphitic Biochar: Process Variables Effects and Matrix Effects. Chem. Eng. J. 2019, 378, 122141. [Google Scholar] [CrossRef]
- Zhu, K.; Shen, Y.; Hou, J.; Gao, J.; He, D.; Huang, J.; He, H.; Lei, L.; Chen, W. One-Step Synthesis of Nitrogen and Sulfur Co-Doped Mesoporous Graphite-like Carbon Nanosheets as a Bifunctional Material for Tetracycline Removal via Adsorption and Catalytic Degradation Processes: Performance and Mechanism. Chem. Eng. J. 2021, 412, 128521. [Google Scholar] [CrossRef]
- Pham, V.L.; Kim, D.-G.; Ko, S.-O. Advanced Oxidative Degradation of Acetaminophen by Carbon Catalysts: Radical vs Non-Radical Pathways. Environ. Res. 2020, 188, 109767. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.; Wang, S. Activated Carbons as Green and Effective Catalysts for Generation of Reactive Radicals in Degradation of Aqueous Phenol. RSC Adv. 2013, 3, 21905. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, F.; Moradi, M. Application of Peroxymonosulfate and Its Activation Methods for Degradation of Environmental Organic Pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Yang, S.; Yang, X.; Shao, X.; Niu, R.; Wang, L. Activated Carbon Catalyzed Persulfate Oxidation of Azo Dye Acid Orange 7 at Ambient Temperature. J. Hazard. Mater. 2011, 186, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-G.; Ko, S.-O. Effects of Thermal Modification of a Biochar on Persulfate Activation and Mechanisms of Catalytic Degradation of a Pharmaceutical. Chem. Eng. J. 2020, 399, 125377. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-laton, I.; Gurmen, S.; Gafarli, I.; Khoei, S.; Safaltin, S.; Ozcelik, D.Y. Oxidative degradation of Bisphenol A by carbocatalytic activation of persulfate and peroxymonosulfate with reduced graphene oxide. J. Hazard. Mater. 2018, 360, 141–149. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.-D.; Lim, T.-T. Graphene and CNTs-Based Carbocatalysts in Persulfates Activation: Material Design and Catalytic Mechanisms. Chem. Eng. J. 2018, 354, 941–976. [Google Scholar] [CrossRef]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; NurAlam, S.M. A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Wu, T.; Jin, B.; Yang, L.; Qiu, J. Synthesis, modification strategies and applications of coal-based carbon materials. Fuel Process. Technol. 2022, 230, 107203. [Google Scholar] [CrossRef]
- Chowdhury, A.; Kumari, S.; Khan, A.A.; Chandra, M.R.; Hussain, S. Activated carbon loaded with Ni-Co-S nanoparticle for superior adsorption capacity of antibiotics and dye from wastewater: Kinetics and isotherms. Colloids Surf. A 2021, 611, 125868. [Google Scholar] [CrossRef]
- Poudel, M.B.; Shin, M.; Kim, H.J. Interface engineering of MIL-88 derived MnFe-LDH and MnFe2O3 on three-dimensional carbon nanofibers for the efficient adsorption of Cr(VI), Pb(II), and As(III) ions. Sep. Purif. Technol. 2022, 287, 120463. [Google Scholar] [CrossRef]
- Poudel, M.B.; Awasthi, G.P.; Kim, H.J. Novel insight into the adsorption of Cr(VI) and Pb(II) ions by MOF derived Co-Al layered double hydroxide @hematite nanorods on 3D porous carbon nanofiber network. Chem. Eng. J. 2021, 417, 129312. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, Y.; Zhang, Y.; Zhang, B.-T.; Yin, M.; Chen, L. Heterogeneous activation of persulfate by activated carbon supported iron for efficient amoxicillin degradation. Environ. Technol. Innov. 2021, 21, 101259. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Wen, H.; Cai, Z.; He, C.; Wang, Z.; Yan, W. Microwave assisted modification of activated carbons by organic acid ammoniums activation for enhanced adsorption of acid red 18. Powder Technol. 2018, 323, 230–237. [Google Scholar] [CrossRef]
- Ji, Q.; Luo, G.; Shi, M.; Zou, R.; Fang, C.; Xu, Y.; Li, X.; Yao, H. Acceleration of the reaction of H2S and SO2 by non-thermal plasma to improve the mercury adsorption performance of activated carbon. Chem. Eng. J. 2021, 423, 130144. [Google Scholar] [CrossRef]
- Chen, K.; He, Z.-J.; Liu, Z.H.; Ragauskas, A.J.; Li, B.-Z.; Yuan, Y.J. Emerging Modification Technologies of Lignin-based Activated Carbon toward Advanced Applications. ChemSusChem 2022, e202201284. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Pereira, R.; Pereira, M.F.R.; van der Zee, F.P.; Cervantes, F.J.; Alves, M.M. Thermal modification of activated carbon surface chemistry improves its capacity as redox mediator for azo dye reduction. J. Hazard. Mater. 2010, 83, 931–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nwamba, O.C.; Echeverria, E.; McIlroy, D.N.; Austin, A.; Shreeve, J.M.; Aston, D.E. Thermal Modification of Graphite for Fast Electron Transport and Increased Capacitance. ACS Appl. Nano Mater. 2019, 2, 228–240. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Świątkowski, A.; Skrzypczyńska, K.; Błażewicz, S.; Hryniewicz, J. The Effects of the Thermal Treatment of Activated Carbon on the Phenols Adsorption. Korean J. Chem. Eng. 2017, 34, 1081–1090. [Google Scholar] [CrossRef]
- Miyazato, T.; Nuryono, N.; Kobune, M.; Rusdiarso, B.; Otomo, R.; Kamiya, Y. Phosphate Recovery from an Aqueous Solution through Adsorption-Desorption Cycle over Thermally Treated Activated Carbon. J. Water Process Eng. 2020, 36, 101302. [Google Scholar] [CrossRef]
- Azam, K.; Shezad, N.; Shafiq, I.; Akhter, P.; Akhtar, F.; Jamil, F.; Shafique, S.; Park, Y.-K.; Hussain, M. A review on activated carbon modifications for the treatment of wastewater containing anionic dyes. Chemosphere 2022, 306, 135566. [Google Scholar] [CrossRef]
- Macías-García, A.; Corzo, M.G.; Domínguez, M.A.; Franco, M.A.; Naharro, J.M. Study of the adsorption and electroadsorption process of Cu (II) ions within thermally and chemically modified activated carbon. J. Hazard. Mater. 2017, 328, 46–55. [Google Scholar] [CrossRef]
- Menz, J.; Olsson, O.; Kümmerer, K. Antibiotic Residues in Livestock Manure: Does the EU Risk Assessment Sufficiently Protect against Microbial Toxicity and Selection of Resistant Bacteria in the Environment? J. Hazard. Mater. 2019, 379, 120807. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, Fate, and Risk Assessment of Typical Tetracycline Antibiotics in the Aquatic Environment: A Review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.J.; Murby, E.J.; Costanzo, S.D. Removal of Antibiotics in Conventional and Advanced Wastewater Treatment: Implications for Environmental Discharge and Wastewater Recycling. Water Res. 2007, 41, 4164–4176. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Wei, Q.; Wei, D. A Critical Review on Antibiotics and Hormones in Swine Wastewater: Water Pollution Problems and Control Approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of Persulfate (PS) and Peroxymonosulfate (PMS) and Application for the Degradation of Emerging Contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Ren, W.; Nie, G.; Zhou, P.; Zhang, H.; Duan, X.; Wang, S. The Intrinsic Nature of Persulfate Activation and N-Doping in Carbocatalysis. Environ. Sci. Technol. 2020, 54, 6438–6447. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Li, N.; Xing, X.; Sun, Y.; Zhang, Z.; Hao, Z. Gaseous Adsorption of Hexamethyldisiloxane on Carbons: Isotherms, Isosteric Heats and Kinetics. Chemosphere 2020, 247, 125862. [Google Scholar] [CrossRef]
- Rezma, S.; Birot, M.; Hafiane, A.; Deleuze, H. Physically Activated Microporous Carbon from a New Biomass Source: Date Palm Petioles. Comptes Rendus Chim. 2017, 20, 881–887. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A Review on Surface Modification of Activated Carbon for Carbon Dioxide Adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Lazim, Z.M.; Hadibarata, T.; Puteh, M.H.; Yusop, Z. Adsorption Characteristics of Bisphenol A onto Low-Cost Modified Phyto-Waste Material in Aqueous Solution. Water Air Soil Pollut. 2015, 226, 34. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, L.; Liang, Y.; Chen, Q.; Li, Z.; Li, C.; Wang, Z.; Li, W. Coconut-Based Activated Carbon Fibers for Efficient Adsorption of Various Organic Dyes. RSC Adv. 2018, 8, 42280–42291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashhadimoslem, H.; Safarzadeh Khosrowshahi, M.; Jafari, M.; Ghaemi, A.; Maleki, A. Adsorption Equilibrium, Thermodynamic, and Kinetic Study of O2/N2/CO2 on Functionalized Granular Activated Carbon. ACS Omega 2022, 7, 18409–18426. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhao, B.; Zhou, Q.; He, Y.; Wang, Z.; Radacsi, N. Fe-Doped ZnO/Reduced Graphene Oxide Nanocomposite with Synergic Enhanced Gas Sensing Performance for the Effective Detection of Formaldehyde. ACS Omega 2019, 4, 10252–10262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaworski, S.; Wierzbicki, M.; Sawosz, E.; Jung, A.; Gielerak, G.; Biernat, J.; Jaremek, H.; Łojkowski, W.; Woźniak, B.; Wojnarowicz, J.; et al. Graphene Oxide-Based Nanocomposites Decorated with Silver Nanoparticles as an Antibacterial Agent. Nanoscale Res. Lett. 2018, 13, 116. [Google Scholar] [CrossRef] [Green Version]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [Green Version]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [Green Version]
- Kordek, K.; Jiang, L.; Fan, K.; Zhu, Z.; Xu, L.; Al-Mamun, M.; Dou, Y.; Chen, S.; Liu, P.; Yin, H.; et al. Two-Step Activated Carbon Cloth with Oxygen-Rich Functional Groups as a High-Performance Additive-Free Air Electrode for Flexible Zinc–Air Batteries. Adv. Energy Mater. 2019, 9, 1802936. [Google Scholar] [CrossRef]
- Tangsir, S.; Hafshejani, L.D.; Lähde, A.; Maljanen, M.; Hooshmand, A.; Naseri, A.A.; Moazed, H.; Jokiniemi, J.; Bhatnagar, A. Water Defluoridation Using Al2O3 Nanoparticles Synthesized by Flame Spray Pyrolysis (FSP) Method. Chem. Eng. J. 2016, 288, 198–206. [Google Scholar] [CrossRef]
- Scaria, J.; Anupama, K.V.; Nidheesh, P.V. Tetracyclines in the Environment: An Overview on the Occurrence, Fate, Toxicity, Detection, Removal Methods, and Sludge Management. Sci. Total Environ. 2021, 771, 145291. [Google Scholar] [CrossRef]
- Ren, W.; Xiong, L.; Yuan, X.; Yu, Z.; Zhang, H.; Duan, X.; Wang, S. Activation of Peroxydisulfate on Carbon Nanotubes: Electron-Transfer Mechanism. Environ. Sci. Technol. 2019, 53, 14595–14603. [Google Scholar] [CrossRef]
- Tian, L.; Chen, P.; Jiang, X.-H.; Chen, L.-S.; Tong, L.-L.; Yang, H.-Y.; Fan, J.-P.; Wu, D.-S.; Zou, J.-P.; Luo, S.-L. Mineralization of Cyanides via a Novel Electro-Fenton System Generating OH and O2−. Water Res. 2022, 209, 117890. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Liu, Y.; Kim, S.H.; Dionysiou, D.D. Facile Preparation of Porous Mn/Fe3O4 Cubes as Peroxymonosulfate Activating Catalyst for Effective Bisphenol A Degradation. Chem. Eng. J. 2019, 376, 119193. [Google Scholar] [CrossRef]
- Xie, M.; Tang, J.; Kong, L.; Lu, W.; Natarajan, V.; Zhu, F.; Zhan, J. Cobalt Doped G-C3N4 Activation of Peroxymonosulfate for Monochlorophenols Degradation. Chem. Eng. J. 2019, 360, 1213–1222. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Y.; Fan, Z.; Liu, F.; Li, A. Carbonate-Enhanced Catalytic Activity and Stability of Co3O4 Nanowires for 1O2-Driven Bisphenol A Degradation via Peroxymonosulfate Activation: Critical Roles of Electron and Proton Acceptors. J. Hazard. Mater. 2020, 393, 122395. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhu, Y.; Lyu, L.; Zeng, Q.; Xing, X.; Hu, C. Electronic Structure Modulation of Graphitic Carbon Nitride by Oxygen Doping for Enhanced Catalytic Degradation of Organic Pollutants through Peroxymonosulfate Activation. Environ. Sci. Technol. 2018, 52, 14371–14380. [Google Scholar] [CrossRef]
- Yang, Y.; Banerjee, G.; Brudvig, G.W.; Kim, J.-H.; Pignatello, J.J. Oxidation of Organic Compounds in Water by Unactivated Peroxymonosulfate. Environ. Sci. Technol. 2018, 52, 5911–5919. [Google Scholar] [CrossRef]
- Komeily-Nia, Z.; Chen, J.-Y.; Nasri-Nasrabadi, B.; Lei, W.-W.; Yuan, B.; Zhang, J.; Qu, L.-T.; Gupta, A.; Li, J.-L. The Key Structural Features Governing the Free Radicals and Catalytic Activity of Graphite/Graphene Oxide. Phys. Chem. Chem. Phys. 2020, 22, 3112–3121. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.; Sun, H.; Ao, Z.; Wang, S.; Liu, S. Understanding of the Oxidation Behavior of Benzyl Alcohol by Peroxymonosulfate via Carbon Nanotubes Activation. ACS Catal. 2020, 10, 3516–3525. [Google Scholar] [CrossRef]
- Luo, H.; Liu, Y.; Lu, H.; Fang, Q.; Rong, H. Efficient Adsorption of Tetracycline from Aqueous Solutions by Modified Alginate Beads after the Removal of Cu(II) Ions. ACS Omega 2021, 6, 6240–6251. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Hossaini, Z.; Pourakbar, M. High-Rate Adsorption of Acetaminophen from the Contaminated Water onto Double-Oxidized Graphene Oxide. Chem. Eng. J. 2016, 287, 665–673. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Lou, Z.; Wang, Y.; Zhang, Y.; Qian, G. Method To Characterize Acid–Base Behavior of Biochar: Site Modeling and Theoretical Simulation. ACS Sustain. Chem. Eng. 2014, 2, 2501–2509. [Google Scholar] [CrossRef]

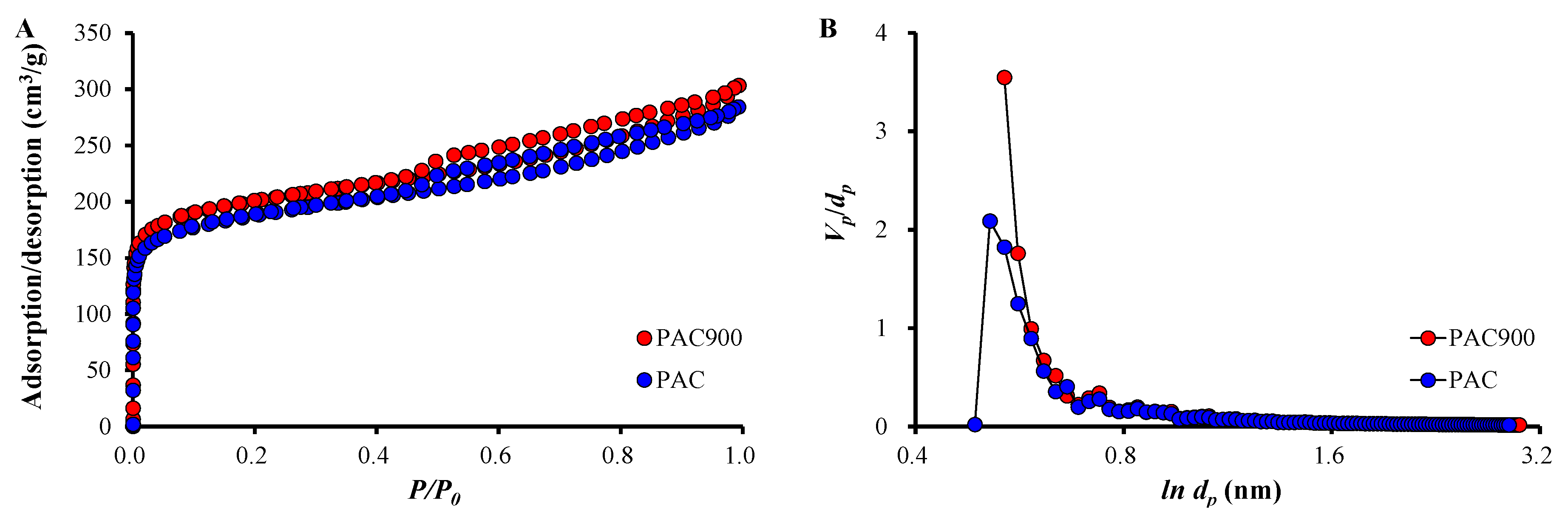



| SBET (m2/g) | Vp (m3/g) | dp (nm) | |
|---|---|---|---|
| PAC | 685.76 | 0.4375 | 2.552 |
| PAC900 | 734.53 | 0.4665 | 2.541 |
| D4 | D | D3 | G | D2 | D+G | 2D | ID/IG | ID/ID2 | I2D/IG | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PAC | Center (cm−1) | 1210 | 1344 | 1540 | 1588 | 1610 | 2679 | 2906 | 3.69 | 19.23 | 0.51 |
| Fraction (%) | 10.2 | 41.6 | 15.3 | 11.3 | 2.2 | 13.9 | 5.7 | ||||
| PAC900 | Center (cm−1) | 1200 | 1344 | 1550 | 1588 | 1610 | 2678 | 2904 | 3.80 | 33.13 | 0.58 |
| Fraction (%) | 7.4 | 46.1 | 13.8 | 12.1 | 1.4 | 12.2 | 7.0 |
| C1s | O1s | |||||||
|---|---|---|---|---|---|---|---|---|
| Graphitic C-C/C=C | C-O/C-N | C=O | O-C=O | C=O | C-OH | C-O | ||
| PAC | Position (eV) | 284.3 | 285.0 | 287.1 | 290.3 | 530.3 | 531.9 | 533.4 |
| Fraction (%) | 56 | 24 | 9 | 11 | 46 | 49 | 5 | |
| PAC900 | Position (eV) | 284.6 | 285.4 | 287.6 | 290.4 | 530.3 | 531.9 | 533.8 |
| Fraction (%) | 58 | 24 | 9 | 9 | 53 | 38 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-G.; Kim, T.-H.; Ko, S.-O. Enhanced Catalytic Activity of a Coal-Based Powdered Activated Carbon by Thermal Treatment. Water 2022, 14, 3308. https://doi.org/10.3390/w14203308
Kim D-G, Kim T-H, Ko S-O. Enhanced Catalytic Activity of a Coal-Based Powdered Activated Carbon by Thermal Treatment. Water. 2022; 14(20):3308. https://doi.org/10.3390/w14203308
Chicago/Turabian StyleKim, Do-Gun, Tae-Hoon Kim, and Seok-Oh Ko. 2022. "Enhanced Catalytic Activity of a Coal-Based Powdered Activated Carbon by Thermal Treatment" Water 14, no. 20: 3308. https://doi.org/10.3390/w14203308
APA StyleKim, D.-G., Kim, T.-H., & Ko, S.-O. (2022). Enhanced Catalytic Activity of a Coal-Based Powdered Activated Carbon by Thermal Treatment. Water, 14(20), 3308. https://doi.org/10.3390/w14203308












