Low-Cost Adsorbents for the Removal of Phenol/Phenolics, Pesticides, and Dyes from Wastewater Systems: A Review
Abstract
:1. Introduction
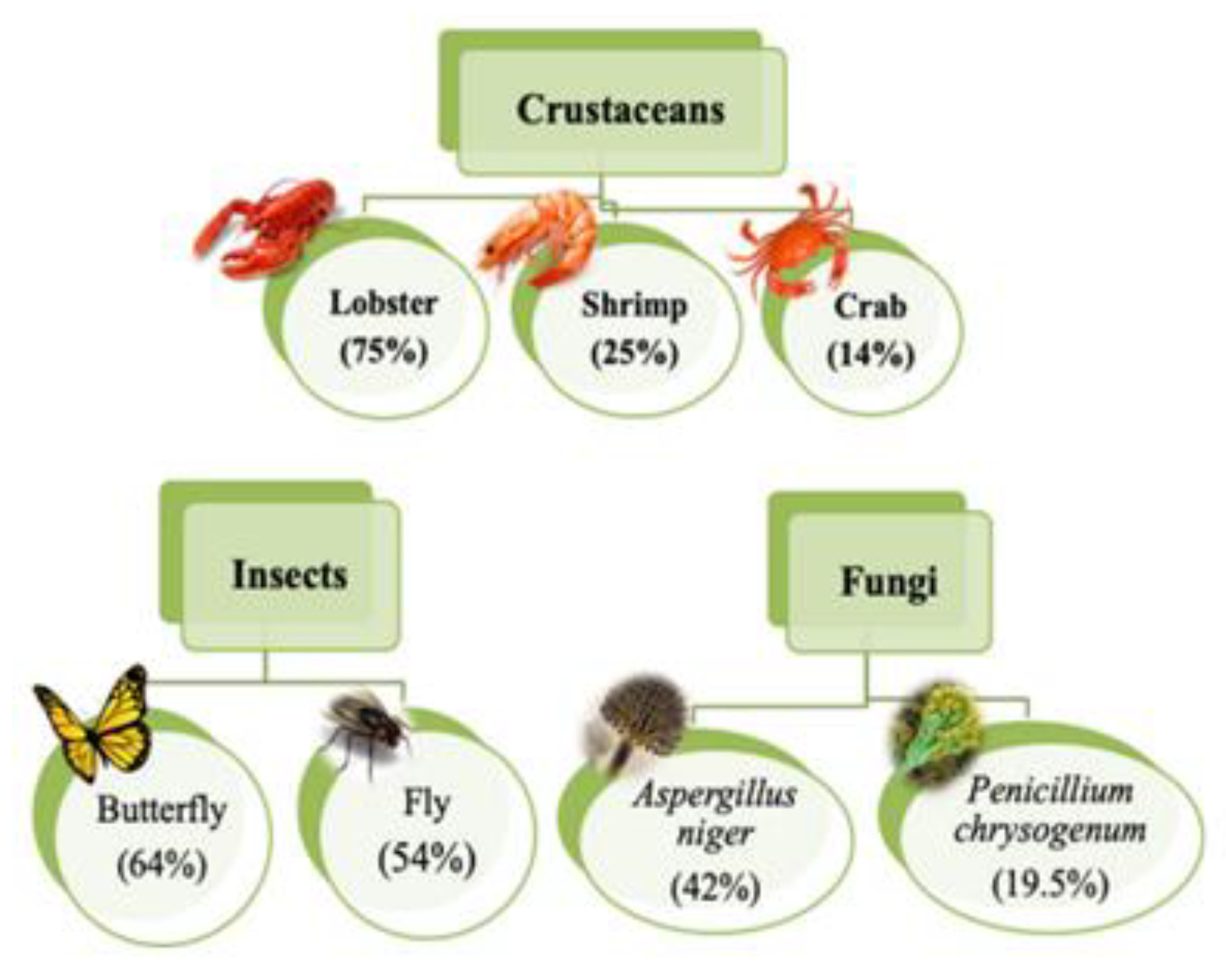
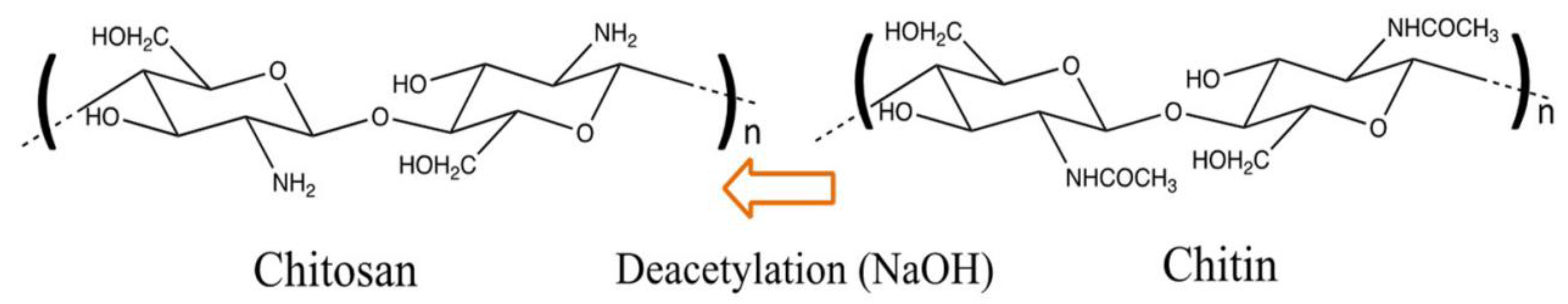
2. Types of Adsorbents
3. Adsorption of Phenol and Phenolic Compounds Using Different Adsorbents
4. Adsorption of Pesticides by Using Different Adsorbents
5. Adsorption of Dye Compounds Using Different Adsorbents
6. Conclusions and Future Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panagopoulos, A.; Giannika, V. Comparative techno-economic and environmental analysis of minimal liquid discharge (MLD) and zero liquid discharge (ZLD) desalination systems for seawater brine treatment and valorization. Sustain. Energy Technol. Assess. 2022, 53, 102477. [Google Scholar] [CrossRef]
- Panagopoulos, A. Brine management (saline water & wastewater effluents): Sustainable utilization and resource recovery strategy through Minimal and Zero Liquid Discharge (MLD & ZLD) desalination systems. Chem. Eng. Process. 2022, 176, 108944. [Google Scholar] [CrossRef]
- Panagopoulos, A. Techno-economic assessment and feasibility study of a zero liquid discharge (ZLD) desalination hybrid system in the Eastern Mediterranean. Chem. Eng. Process. 2022, 178, 109029. [Google Scholar] [CrossRef]
- Amine, M.; Dora, T.; Ben, S.; Bechir, H. Phenol removal from water by AG reverse osmosis membrane. Environ. Prog. Sustain. Energy. 2015, 34, 982–989. [Google Scholar]
- Mukherjee, A.; Mehta, R.; Saha, S. Removal of multiple pesticide residues from water by low-pressure thin-film composite membrane. Appl. Water Sci. 2020, 10, 244. [Google Scholar] [CrossRef]
- Nathan, G.; Nishil, M.; Wei, S.; Berry, M.; Tam, C. Dye Removal Using Sustainable Membrane Adsorbents Produced from Melamine Formaldehyde−Cellulose Nanocrystals and Hard Wood Pulp. Ind. Eng. Chem. Res. 2020, 59, 20854–20865. [Google Scholar]
- Vesna, P.; Sanja, S.; Nesic, A.; Sava, V. Adsorption of azo dyes on polymer materials. Hem. Ind. 2013, 67, 881–900. [Google Scholar]
- Moosavi, S.; Li, W.; Gan, S.; Zamiri, G.; Omid, A. Application of Efficient Magnetic Particles and Activated Carbon for Dye Removal from Wastewater. ACS Omega 2020, 5, 2684–20697. [Google Scholar] [CrossRef]
- Siyal, A.; Rashid, S.; Low, A.; Nurul, E. A review on recent developments in the adsorption of surfactants from wastewater. J. Environ. Manag. 2020, 254, 109797. [Google Scholar] [CrossRef]
- Mustafa, T.; Sen, T.; Ang, H.; Afrze, S. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Asep, B. Isotherm Adsorption of Carbon Microparticles Prepared from Pumpkin (Cucurbita maxima) Seeds Using Two-Parameter Monolayer Adsorption Models and Equations. Mor. J. Chem. 2020, 8, 745–761. [Google Scholar]
- Izabela, M.; Dariusz, W.; Aleksandra, S. Experimental investigation into CO2 capture from the cement plant by VPSA technology using zeolite 13X and activated carbon. J. CO2 Util. 2022, 61, 102027. [Google Scholar] [CrossRef]
- Kumar, P.; Ramesh, D.; Karthikeyan, S.; Subramanian, P. Activated carbon production from coconut leaflets through chemical activation: Process optimization using Taguchi approach. Bioresour. Technol. Rep. 2022, 19, 101155. [Google Scholar] [CrossRef]
- John, P.; Santos, M.; Noemi, Z. Synthesis, characterization and application of cross-linked chitosan/oxalic acid hydrogels to improve azo dye (Reactive Red 195) adsorption. React. Funct. Polym. 2020, 155, 104699. [Google Scholar] [CrossRef]
- Silva, A.; Healy, B.; Pinto, L.; Cadaval, T.; Breslin, C. Recent Developments in Chitosan-Based Adsorbents for the Removal of Pollutants from Aqueous Environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef]
- Paula, M.; Natalia, G.; Taina, K.; Franco, P. Adsorptive removal of basic dye onto sustainable chitosan beads: Equilibrium, kinetics, stability, continuous-mode adsorption and mechanism. Sustain. Chem. Pharm. 2020, 18, 100318. [Google Scholar] [CrossRef]
- Chitosan Market. Available online: https://www.alliedmarketresearch.com/chitosan-market (accessed on 2 September 2022).
- Zhang, X.; Yu, J.; Huang, Y.; Wang, Z. Experimental research on the gaseous PbCl2 adsorption by thermal alkali modified coal fly ash. J. Environ. Chem. Eng. 2022, 10, 107912. [Google Scholar] [CrossRef]
- Bing, Y.; Hu, H.; Biao, F.; Huang, Y.; Liu, H. Condensation and adsorption characteristics of gaseous selenium on coal-fired fly ash at low temperatures. Chemosphere 2022, 287, 132127. [Google Scholar] [CrossRef]
- Xin, Z.; Zhao, H.; Ji, P.; Wang, L.; Hu, X. Effect and mechanisms of synthesis conditions on the cadmium adsorption capacity of modified fly ash. Ecotoxicol. Environ. Safety 2021, 223, 112550. [Google Scholar] [CrossRef]
- Agus, T.; Hidayat, P.; Arif, H. Modified coal fly ash as low cost adsorbent for removal reactive dyes from batik industry. MATEC Web Conf. 2018, 154, 01037. [Google Scholar] [CrossRef] [Green Version]
- Sanjuan, A.; Argiz, C. Fineness of coal fly ash for use in cement and concrete. Fuels 2021, 2, 471–486. [Google Scholar] [CrossRef]
- Jiang, Z.; Kim, J.; John, L.; Barrera, V.; Valery, N. Improving the Dispersion and Integration of Single-Walled Carbon Nanotubes in Epoxy Composites through Functionalization. Nano Lett. 2003, 3, 1107–1113. [Google Scholar]
- Devi, R.; Gill, S. A squared bossed diaphragm piezoresistive pressure sensor based on CNTs for low pressure range with enhanced sensitivity. Microsyst. Technol. 2021, 27, 3225–3233. [Google Scholar] [CrossRef]
- Danikiewicz, A.; Wolany, W.; Cichocki, D. Carbon nanotubes manufacturing using the CVD equipment against the background of other methods. Arch. Mater. Sci. Eng. 2021, 64, 103–109. [Google Scholar]
- Rajabi, M.; Moradi, O.; Mahanpoor, K. Removal of dye molecules from aqueous solution by carbon nanotubes and carbon nanotube functional groups: Critical review. RSC Adv. 2017, 7, 47083–47090. [Google Scholar] [CrossRef] [Green Version]
- Carbon Nanotube Market. Available online: https://www.alliedmarketresearch.com/carbon-nanotube-market (accessed on 2 September 2022).
- Nour, T.; Ghadir, A.; Farag, S. Individual and competitive adsorption of phenol and nickel onto multi walled carbon nanotubes. J. Adv. Res. 2015, 6, 405–415. [Google Scholar]
- Pinero, E.; Amoros, C.; Szostak, K.; Beguin, F.; Delpeux, S. High surface area carbon nanotubes prepared by chemical activation. Carbon 2002, 40, 1597–1617. [Google Scholar]
- Sang, L.; Lee, S.; Jung, J.; Kim, H. Pore characterization of multi-walled carbon nanotubes modified by KOH. Chem. Phys. Lett. 2005, 416, 251–255. [Google Scholar]
- Sadeghpour, N.; Vadi, M.; Bagheri, N. Utilizing carbon nanotubes as efficient nanoadsorbent for pantprazole removal from aqueous solution samples: Kinetics, isotherm and thermodynamic studies. J. Chil. Chem. Soc. 2021, 66, 5324–5331. [Google Scholar] [CrossRef]
- Soma, C.; Jayanta, C.; Peng, H.; Chen, Z.; Arnab, M.; Rolf, S. Surface Area Measurement of Functionalized Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 24812–24815. [Google Scholar]
- Fernando, J.; Isabel, A.; Sandep, A.; Jose, B. Adsorption Equilibria of light organics on single walled carbon nanotube heterogeneous bundles: Thermodynamically aspects. J. Phys. Chem. 2011, 115, 2622–2629. [Google Scholar]
- Oleg, B.; Liu, J.; Joh, T. Characterization of single wall carbon nanotubes by nonane pre-adsorption. Carbon 2006, 44, 2039–2044. [Google Scholar]
- Jalil, A.; Karim, A.; Nordin, K.; Hassim, H. Grape-like mesostructured silica nanoparticle decorated single-walled carbon nanotubes: Silica growth and dye adsorptivity. RSC Adv. 2015, 5, 71796–71804. [Google Scholar] [CrossRef]
- Ho, S.M. Removal of dye by adsorption onto activated carbons: Review. Eurasian J. Anal. Chem. 2018, 13, 332–338. [Google Scholar]
- Ho, S.M. Removal of Dyes from Wastewater by Adsorption onto Activated Carbon: Mini Review. J. Geosci. Environ. Prot. 2020, 8, 120–131. [Google Scholar] [CrossRef]
- Sircar, S.; Rao, M. Activated carbon for gas separation and storage. Carbon 1996, 34, 1–12. [Google Scholar] [CrossRef]
- Reza, M.; Saidur, R.; Yun, C.; Bakar, A. Shammya, Preparation of activated carbon from biomass and its’ applications in water and gas purification, a review. Arab J. Basic Appl. Sci. 2020, 27, 208–238. [Google Scholar] [CrossRef]
- Octolia, T.; Mumfaijah, M.; Allo, Y.K.; Dahlan, K.; Ansanay, Y.O. The Effect of Chemical Activating Agent on the Properties of Activated Carbon from Sago Waste. Appl. Sci. 2021, 11, 11640. [Google Scholar]
- Mistar, M.; Ahmad, S.; Muslim, A.; Supardan, M. Preparation and characterization of a high surface area of activated carbon from Bambusa vulgaris—Effect of NaOH activation and pyrolysis temperature. IOP Conf. Ser. Mater. Sci. Eng. 2018, 334, 012051. [Google Scholar] [CrossRef]
- Toni, V.; Bergna, D.; Riikk, L.; Romar, H.; Hu, T. Activated Carbon Production from Peat Using ZnCl2: Characterization and Applications. BioResources 2014, 12, 8078–8092. [Google Scholar]
- Jiang, Z.; Liang, C.; Liu, Y.; You, W.; Han, C.; Li, C. Activated Carbons Chemically Modified by Concentrated H2SO4 for the Adsorption of the Pollutants from Wastewater and the Dibenzothiophene from Fuel Oils. Langmuir 2003, 19, 731–736. [Google Scholar] [CrossRef]
- Global Market Volume Activated Carbon. Available online: https://www.statista.com/statistics/963555/global-market-volume-activated-carbon/ (accessed on 2 September 2022).
- Available online: https://ihsmarkit.com/products/activated-carbon-chemical-economics-handbook.html (accessed on 2 September 2022).
- Pei, Y.; Mo, S.; Xie, Q.; Ma, L.; Lili, H. Stellerite-seeded facile synthesis of zeolite X with excellent aqueous Cd2+ and Ni2+ adsorption performance. Chin. J. Chem. Eng. 2022, in press. [Google Scholar] [CrossRef]
- Chung, K.; Park, D.; Kim, K.; Lee, C. Adsorption equilibria and kinetics of ethane and ethylene on zeolite 13X pellets. Micropor. Mesopor. Mater. 2022, 343, 112199. [Google Scholar] [CrossRef]
- Emami, S.; Razif, H.; Mohd, N.; Zakaria, R. Potential of zeolite and algae in biomass immobilization. BioMed Res. Int. 2018, 6563196. [Google Scholar] [CrossRef] [Green Version]
- Shruti, A.; Kosankar, P. Review on Zeolite Synthesis. Zeichen J. 2020, 6, 395–399. [Google Scholar]
- Bo, L.; Dong, B.; Fang, C.; Chen, Z.; Zhao, Z. Effective adsorption of methylene blue from aqueous solution by coal gangue-based zeolite granules in a fluidized bed: Fluidization characteristics and continuous adsorption. Powder Technol. 2022, 408, 117764. [Google Scholar] [CrossRef]
- Zeolites: Chemical Economics Handbook. Available online: https://ihsmarkit.com/products/zeolites-chemical-economics-handbook.html#:~:text=Mainland%20China%20is%20the%20largest,world%20production%20is%20highly%20decentralized (accessed on 2 September 2022).
- Villegas, L.; Mashhadi, N.; Chen, M. A Short Review of Techniques for Phenol Removal from Wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Global Market Value Phenol. Available online: https://www.statista.com/statistics/1244237/global-market-value-phenol/ (accessed on 2 September 2022).
- Raza, W.; Lee, J.; Raza, N.; Kim, K. Removal of phenolic compounds from industrial waste water based on membrane-based technologies. J. Ind. Eng. Chem. 2019, 71, 1–18. [Google Scholar] [CrossRef]
- Lehlogonolo, T.; Shepherd, T.; Frederick, L.; Evans, C. Adsorption of Phenol from Wastewater Using Calcined Magnesium-Zinc-Aluminium Layered Double Hydroxide Clay. Sustainability 2020, 12, 4273. [Google Scholar] [CrossRef]
- Masuma, S.; Rafat, M.; Sabrina, K.; Easir, A. Adsorption of Phenol from Aqueous Solution Using Activated Carbon prepared from Coconut Shell. J. Chem. Eng. 2017, 29, 9–13. [Google Scholar]
- Abdel-Ghani, N.; El-Chaghaby, G.; Helal, F. Preparation, characterization and phenol adsorption capacity of activated carbons from African beech wood sawdust. Glob. J. Environ. Sci. Manag. 2016, 2, 209–222. [Google Scholar] [CrossRef]
- Namasivayam, C.; Kavitha, D. Removal of phenol and chlorophenols from water by coir pith carbon: Equilibrium and rate studies. J. Environ. Sci. Eng. 2004, 46, 217–232. [Google Scholar] [PubMed]
- Potgieter, J.; Bada, S.; Vermaak, S. Adsorptive removal of various phenols from water by South African coal fly ash. Water SA 2009, 35, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Rahel, C.; Mridula, B. Study on the removal characteristics of phenol from aqueous solution by coal fly ash. Int. J. Adv. Technol. Eng. Sci. 2014, 2, 52–66. [Google Scholar]
- Neeru, C.; Vidya, S.; Agrawal, B.; Chandrajit, B. Removal of Phenol Using Fly Ash and Impregnated Fly Ash: An Approach to Equilibrium, Kinetic, and Thermodynamic Study. Sep. Sci. Technol. 2015, 50, 690–699. [Google Scholar] [CrossRef]
- Ruiz, J.; Gallegos, S.; Juarez, G.; Miranda, G.; Rivas, L.; Regil, O. Influence of the Textural Parameters of LDH-TiO2 Composites on Phenol Adsorption and Photodegradation Capacities. Int. J. Photoenergy 2019, 2019, 5783507. [Google Scholar] [CrossRef] [Green Version]
- Nourrdine, C.; Ahcene, S.; Chater, M. Adsorption of phenol from aqueous solution onto zeolites Y modified by silylation. Comptes. Rendus Chim. 2013, 16, 222–228. [Google Scholar]
- Saravanakumar, K.; Kumar, A. Removal of phenol from aqueous solution by adsorption using zeolite. Afr. J. Agric. Res. 2013, 8, 2965–2969. [Google Scholar]
- Kragulj, M.; Tričković, J.; Kukovecz, Á.; Jović, B.; Molnar, J.; Rončević, S.; Kónya, Z.; Dalmacija, B. Adsorption of chlorinated phenols on multiwalled carbon nanotubes. RSC Adv. 2015, 5, 24920–24929. [Google Scholar] [CrossRef]
- Hadi, M.; Sahu, J.; Behzad, H.; Ahmad, B.; McKay, G.; Mubarak, M. High-performance removal of toxic phenol by single-walled and multi-walled carbon nanotubes: Kinetics, adsorption, mechanism and optimization studies. J. Ind. Eng. Chem. 2016, 35, 63–74. [Google Scholar]
- Meng, X.; Li, J.; Hu, C.; Du, J. Adsorption of phenol, p-chloro phenol and p-nitrophenol onto functional chitosan. Bioresour. Technol. 2009, 100, 1168–1173. [Google Scholar]
- Shane, L.; Frederick, J.; Shepherd, M. The Effect of Metallic Composition of Layered Double Hydroxide Clay on the Removal of Phenol from Aqueous Solution. Chem. Eng. Trans. 2020, 81, 193–198. [Google Scholar]
- Xiaoman, H.; Wang, B.; Zhang, Q. Phenols removal from water by precursor preparation for Mg-Al layered double hydroxide: Isotherm, kinetic and mechanism. Mater. Chem. Phys. 2019, 221, 108–117. [Google Scholar] [CrossRef]
- Shazryenna, D.; Piakong, M. Removal of phenol by zeolite. Trans. Sci. Technol. 2016, 3, 107–113. [Google Scholar]
- Khalid, M.; Guy, J.; Renaud, A.; Patrick, M. Removal of Phenol from Water by Adsorption Using Zeolites. Ind. Eng. Chem. Res. 2004, 43, 5275–5280. [Google Scholar] [CrossRef]
- Ba, B.; Yamni, K.; Lgaz, H.; Ramola, S.; Dehmani, Y. Enhanced removal efficiency of NaY zeolite toward phenol from aqueous solution by modification with nickel (Ni-NaY). J. Saudi Chem. Soc. 2021, 25, 101224. [Google Scholar] [CrossRef]
- Syaifullah, M.; Saputra, E.; Sun, H. Removal of Phenol Using Sulphate Radicals Activated by Natural Zeolite-Supported Cobalt Catalysts. Water Air Soil Pollut. 2013, 224, 1721. [Google Scholar] [CrossRef]
- Asgari, G.; Mohammadi, A.; Ebrahimi, A. Adsorption of phenol from aqueous solution by modified zeolite with FeCl3. Int. J. Environ. Health Eng. 2013, 2, 6. [Google Scholar] [CrossRef]
- Nan, J.; Ran, S.; Sebastiaan, G.; Luuk, C. Adsorption of triclosan, trichlorophenol and phenol by high-silica zeolites: Adsorption efficiencies and mechanisms. Sep. Purif. Technol. 2020, 235, 116152. [Google Scholar] [CrossRef]
- Hasan, B.; Duygu, O.; Ali, G.; Duran, C.; Soylak, M. Removal of phenol from aqueous solutions by adsorption onto organomodified Tirebolu bentonite: Equilibrium, kinetic and thermodynamic study. J. Hazard Mater. 2009, 172, 353–362. [Google Scholar] [CrossRef]
- Feryal, A. Sorption of phenol and 4-chlorophenol onto pumice treated with cationic surfactant. J. Environ. Manag. 2005, 74, 239–244. [Google Scholar] [CrossRef]
- Srihari, V.; Das, A. Comparative studies on adsorptive removal of phenol by three agro-based carbons: Equilibrium and isotherm studies. Ecotoxicol. Environ. Saf. 2008, 71, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Gnanasundaram, N.; Rambabu, K.; Murugesan, T.; Show, P. Adsorptive removal of phenol using banyan root activated carbon. Chem. Eng. Commun. 2019, 208, 831–842. [Google Scholar] [CrossRef]
- Radhika, M.; Palanivelu, K. Adsorptive removal of chlorophenols from aqueous solution by low cost adsorbent--Kinetics and isotherm analysis. J. Hazard. Mater. 2006, 138, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, M.; Binupriya, A.; Yun, S.; Kavitha, D. Kinetic and isothermal studies on liquid-phase adsorption of 2,4-dichlorophenol by palm pith carbon. Bioresour. Technol. 2007, 98, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Sathishkumar, M.; Yun, E.; Choi, J.; Binupriya, A. Porogen effect on characteristics of banana pith carbon and the sorption of dichlorophenols. J. Colloid Interface Sci. 2008, 320, 22–29. [Google Scholar] [CrossRef]
- Yan, M.; Gao, N.; Chu, W.; Cong, L. Removal of phenol by powdered activated carbon adsorption. Front. Environ. Sci. Eng. 2013, 7, 158–165. [Google Scholar]
- Jun, T.; Huo, P.; Fu, Z.; Zhang, J.; Yang, Z.; Zhang, D. Characterization and phenol adsorption performance of activated carbon prepared from tea residue by NaOH activation. Environ. Technol. 2017, 40, 171–181. [Google Scholar] [CrossRef]
- Riaz, Q.; Abdul, R. A Study of the Adsorption of Phenol by Activated Carbon from Aqueous Solutions. Turk. J. Chem. 2002, 26, 357–361. [Google Scholar]
- Vimal, C.; Swamy, M.; Indra, M.; Prasad, B.; Mishra, M. Adsorptive removal of phenol by bagasse fly ash and activated carbon: Equilibrium, kinetics and thermodynamics. Colloids Surf. A Phys. Chem. Eng. Asp. 2006, 272, 89–104. [Google Scholar]
- Mitali, S.; Kumar, A. Use of fly ash for the removal of phenol and its analogues from contaminated water. Waste Manag. 2006, 26, 559–570. [Google Scholar] [CrossRef]
- Ugurlu, M.; Gurses, A.; Yalcin, M. Removal of Phenolic and Lignin Compounds from Bleached Kraft Mill Effluent by Fly Ash and Sepiolite. Adsorption 2005, 11, 87–97. [Google Scholar] [CrossRef]
- Shaokui, Z.; Yang, Z.; Do, J.; Yun, P. Removal of chlorophenols from groundwater by chitosan sorption. Water Res. 2004, 38, 2315–2322. [Google Scholar]
- Santos, D.; Rainer, H.; Rennio, F.; Gilson, B. Pesticides removal from industrial wastewater by a membrane bioreactor and post-treatment with either activated carbon, reverse osmosis or ozonation. J. Environ. Chem. Eng. 2020, 8, 104538. [Google Scholar] [CrossRef]
- Goh, P.; Ahmad, N.; Ismail, A.; Wong, W. Membrane technology for pesticide removal from aquatic environment: Status quo and way forward. Chemosphere 2022, 307, 136018. [Google Scholar] [CrossRef]
- Hanif, H.; Hashimi, R.; Ryan, Q. Toxic Effects of Pesticides on Humans, Plants, Animals, Pollinators and Beneficial Organisms. Asian Plant Res. J. 2020, 5, 37–47. [Google Scholar] [CrossRef]
- Vinay, K.; Ravi, S.; Prem, N.; Jai, K.; Fethiye, G.; Yogesh, C. Removal of Malathion from Aqueous Solutions and Waste Water Using Fly Ash. J. Water Resour. Prot. 2010, 2, 322–330. [Google Scholar]
- Jude, C.; Felix, C.; Augustine, A. Kinetics and Equilibrium Isotherms of Pesticides Adsorption onto Boiler Fly Ash. Terr. Aquat. Environ. Toxicol. 2012, 6, 21–29. [Google Scholar]
- Smedt, C.; Ferrer, F.; Leus, K.; Spanoghe, P. Removal of Pesticides from Aqueous Solutions by Adsorption on Zeolites as Solid Adsorbents. Adsorp. Sci. Technol. 2015, 33, 457–485. [Google Scholar] [CrossRef] [Green Version]
- Shattar, S.; Zakaria, N.; Foo, K. Utilization of montmorillonite as a refining solution for the treatment of ametryn, a second generation of pesticide. J. Environ. Chem. Eng. 2017, 5, 3235–3242. [Google Scholar] [CrossRef]
- Salman, M.; Hameed, H. Adsorption of 2,4-dichlorophenoxyacetic acid and carbofuran pesticides onto granular activated carbon. Desalination 2010, 256, 129–135. [Google Scholar] [CrossRef]
- Ignatowicz, K. A mass transfer model for the adsorption of pesticide on coconut shell based activated carbon. Int. J. Heat Mass Transfer. 2011, 54, 4931–4938. [Google Scholar] [CrossRef]
- Salman, M.; Nnoku, V.; Hameed, H. Bentazon and carbofuran adsorption onto date seed activated carbon: Kinetics and equilibrium. Chem. Eng. J. 2011, 173, 361–368. [Google Scholar] [CrossRef]
- Salman, M. Optimization of preparation conditions for activated carbon from palm oil fronds using response surface methodology on removal of pesticides from aqueous solution. Arab. J. Chem. 2014, 7, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Mandal, A.; Kumar, A.; Singh, N. Sorption mechanisms of pesticides removal from effluent matrix using biochar: Conclusions from molecular modelling studies validated by single-, binary and ternary solute experiments. J. Environ. Manag. 2021, 295, 113104. [Google Scholar] [CrossRef] [PubMed]
- Ourania, A.; Albanis, T.; Islam, M.; George, S. Preparation of activated carbons from agricultural residues for pesticide adsorption. Chemosphere 2010, 80, 1328–1336. [Google Scholar]
- Gholamreza, M.; Hiwa, H.; Ahmad, A. The investigation of diazinon pesticide removal from contaminated water by adsorption onto NH4Cl-induced activated carbon. Chem. Eng. J. 2013, 214, 172–179. [Google Scholar]
- Elcin, D.; Elif, C. Adsorption of 2,4-dichlorophenoxyacetic acid on peanut shells: Effect of initial concentration. Environ. Res. Technol. 2018, 1, 23–26. [Google Scholar]
- Mira, P.; Stojic, N.; Igor, K. Removal of pesticides from water using zeolites. Kuwait J. Sci. 2017, 44, 99–105. [Google Scholar]
- Hartini, W.; Ahmad, J.; Ali, N.; Endut, A. Study on the removal of pesticide in agricultural run off by granular activated carbon. Bioresour. Technol. 2011, 102, 5312–5318. [Google Scholar]
- Ajoy, S.; Shabeer, A.; Suman, G.; Kumar, R. Removal of mixed pesticides from aqueous solutions using organoclays: Evaluation of equilibrium and kinetic model. Bull. Environ. Contam. Toxicol. 2013, 91, 111–116. [Google Scholar] [CrossRef]
- Roya, M.; Azam, K.; Rad, M. Highly efficient removal of paraquat pesticide from aqueous solutions using a novel nano Kaolin modified with sulfuric acid via host–guest interactions. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 307–313. [Google Scholar]
- Ouardi, E.; Said, A.; Ali, A.; Jamaa, D. Removal of Carbaryl Pesticide from Aqueous Solution by Adsorption on Local Clay in Agadir. Am. J. Anal. Chem. 2013, 4, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Sergio, S.; Pero, J.; Ruiz, A.; Nuno, R.; Alves, A. Organochlorine pesticides removal from wastewater by pine bark adsorption after activated sludge treatment. Environ. Technol. 2011, 32, 673–683. [Google Scholar] [CrossRef]
- Oyarce, E.; Rivas, L.; Sotelo, S.; Plinio, C. Removal of Dyes by Polymer-Enhanced Ultrafiltration: An Overview. Polymers 2021, 13, 3450. [Google Scholar] [CrossRef]
- Chen, Y.; Ooi, W.; Hoe, B.; Chai, W.; Chiu, C. Removal of Ionic Dyes by Nanofiber Membrane Functionalized with Chitosan and Egg White Proteins: Membrane Preparation and Adsorption Efficiency. Membranes 2022, 12, 63. [Google Scholar] [CrossRef]
- Available online: https://www.globenewswire.com/news-release/2020/02/24/1989226/0/en/global-synthetic-dyes-market-outlook-2020-2030.html (accessed on 2 September 2022).
- Rana, K.; Faris, H.; Muayad, A.; Alberto, F. Removal of Dyes Using Graphene Oxide (GO) Mixed Matrix Membranes. Membranes 2020, 10, 366. [Google Scholar] [CrossRef]
- Available online: https://www.businesswire.com/news/home/20190219005492/en/Global-Dyes-Market-by-Type-Application-Region-and-Country---Forecast-to-2022---ResearchAndMarkets.com (accessed on 2 September 2022).
- Marmagne, O.; Coste, C. Color removal from textile plant effluents. Am. Dyest. Rep. 1996, 85, 15–21. [Google Scholar]
- Average Price of Kaolin. Available online: https://www.statista.com/statistics/248194/average-price-of-kaolin/#:~:text=The%20average%20kaolin%20price%20in,are%20found%20in%20central%20Georgia.&text=Kaolinite%20is%20a%20clay%20mineral,%2C%20orange%2C%20and%20yellow%20tints (accessed on 18 September 2022).
- Muthulakshmi, N.; Thangamani, K. Removal of Direct Dye using Activated Carbon Prepared from Prosopis juliflora Bark: Isothermal, Thermodynamic and Kinetic Studies. Chem. Sci. Rev. Lett. 2016, 5, 182–191. [Google Scholar]
- Ghani, N.; Lima, C.; Rawash, A.; Ghadir, A. Adsorption of coomassie brilliant blue R-250 dye onto novel activated carbon prepared from nigella sativa L waste: Equilibrium, kinetics and thermodynamics. J. Chil. Chem. Soc. 2017, 62, 3505–3511. [Google Scholar] [CrossRef]
- Pinedo, S.; Mendieta, S.; Segura, E.; Rios, S. Efficient removal of brilliant blue by clinoptilolite tuff modified with Fe3+ and Fe–Cu nanoparticles. Desalin. Water Treat. 2019, 144, 300–310. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.; Stitou, M. Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef] [Green Version]
- Bazrafshan, E.; Mostafapour, K.; Mahvi, H. Decolorization of reactive red 198 by adsorption onto ZnCl2 activated pistachio hull wastes. Int. J. Environ. Health Eng. 2014, 3, 7. [Google Scholar] [CrossRef]
- Xavier, A.; Pandi, M. Adsorption of Reactive Green and Reactive Red Dyes from the Aqueous Solution using Activated Carbons. Int. J. Sci. Res. 2015, 4, 454–459. [Google Scholar]
- Edris, B.; Amir, M.; Morteza, A. Reactive red-120 removal by activated carbon obtained from cumin herb wastes. Freseniu Environ. Bull. 2013, 22, 584–590. [Google Scholar]
- Adaobi, I.; Joseph, T.; Dominic, O. Adsorptive Removal of Vat Yellow 4 on Activated Mucuna pruriens (Velvet Bean) Seed Shells Carbon. Asian J. Chem. Sci. 2016, 1, 1–16. [Google Scholar]
- Ahmad, A.; Hameed, H.; Ahmad, L. Removal of disperse dye from aqueous solution using waste-derived activated carbon: Optimization study. J. Hazard. Mater. 2009, 170, 62–619. [Google Scholar] [CrossRef]
- Markandeya, S.; Sheo, P.; Lal, A. Removal of Disperse Orange and Disperse Blue dyes present in textile mill effluent using zeolite synthesized from cenospheres. Water Sci. Technol. 2021, 84, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Ahmad, A.; Khan, A. Removal of azo dye from aqueous solution by a low-cost activated carbon prepared from coal: Adsorption kinetics, isotherms study, and DFT simulation. Environ. Sci. Pollut. Res. 2021, 28, 10234–10247. [Google Scholar] [CrossRef]
- Abbasi, M.; Habibi, M. Optimization and characterization of Direct Blue 71 removal using nanocomposite of Chitosan-MWCNTs: Central composite design modeling. J. Taiwan Inst. Chem. Eng. 2016, 62, 112–121. [Google Scholar] [CrossRef]
- Ainoa, S.; Jose, A.; Maria, I.; Estrella, N.; Jose, G. Egg By-Products as a Tool to Remove Direct Blue 78 Dye from Wastewater: Kinetic, Equilibrium Modeling, Thermodynamics and Desorption Properties. Materials 2020, 13, 1262. [Google Scholar] [CrossRef]
- Setareh, M.; Omid, M. The study of thermodynamics and kinetics methyl orange and malachite green by SWCNTs, SWCNT-COOH and SWCNT-NH2 as adsorbents from aqueous solution. J. Ind. Eng. Chem. 2014, 20, 3186–3194. [Google Scholar] [CrossRef]
- Ramazan, B.; Bingu, l.; Nas, M.; Mehmet, H.; Sen, F. Synthesis and application of AuNi@AC nano adsorbents for the removal of Maxilon Blue 5G azo dye from aquatic mediums. Food Chem. Toxicol. 2022, 167, 113303. [Google Scholar] [CrossRef]
- Alkaim, A.; Sadik, Z.; Mahdi, D. Preparation, structure and adsorption properties of synthesized multiwall carbon nanotubes for highly effective removal of maxilon blue dye. Korean J. Chem. Eng. 2015, 32, 2456–2462. [Google Scholar] [CrossRef]
- Junaidi, H.; Shahri, M.; Ashrul, A.; Nurul, A.; Eny, K. Adsorption of Acid Blue 25 on Agricultural Wastes: Efficiency, Kinetics, Mechanism, and Regeneration. Air Soil Water Res. 2021, 14, 11786221211057496. [Google Scholar] [CrossRef]
- Mark, J.; Wan, Z. Adsorption of Acid Orange 33 Dye by Bentonite and Surfactant Modified Bentonite. Asian J. Chem. 2018, 30, 2383–2388. [Google Scholar]
- Malik, P. Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: A case study of Acid Yellow 36. Dyes Pigm. 2003, 56, 239–249. [Google Scholar] [CrossRef]
- Kaustubha, M.; Naidu, J.; Meikap, B.; Biswas, M. Removal of Crystal Violet from Wastewater by Activated Carbons Prepared from Rice Husk. Ind. Eng. Chem. Res. 2006, 45, 14, 5165–5171. [Google Scholar]
- Shirmardi, M.; Mahvi, A.; Hashemzadeh, B. The adsorption of malachite green (MG) as a cationic dye onto functionalized multi walled carbon nanotubes. Korean J. Chem. Eng. 2013, 30, 1603–1608. [Google Scholar] [CrossRef]
- Shirani, M.; Semnani, A.; Haddadi, H. Optimization of Simultaneous Removal of Methylene Blue, Crystal Violet, and Fuchsine from Aqueous Solutions by Magnetic NaY Zeolite Composite. Water Air Soil Pollut. 2014, 225, 2054. [Google Scholar] [CrossRef]
- Unal, G.; Ozcan, G.; Gizem, C. Removal of Methylene Blue from Aqueous Solution by Activated Carbon Prepared from Pea Shells (Pisum sativum). J. Chem. 2013, 2013, 614083. [Google Scholar] [CrossRef] [Green Version]
- Lunhong, A.; Zhang, C.; Wang, Y.; Li, M.; Meng, L. Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar]
- Belachew, N.; Hinsene, H. Preparation of Zeolite 4A for Adsorptive Removal of Methylene Blue: Optimization, Kinetics, Isotherm, and Mechanism Study. Silicon 2022, 14, 1629–1641. [Google Scholar] [CrossRef]
- Mansour, A.; Abeer, E.; Attia, A.; Beheary, S. Brilliant Green Dye Biosorption Using Activated Carbon Derived from Guava Tree Wood. Int. J. Chem. Eng. 2020, 2020, 8053828. [Google Scholar] [CrossRef]
- Raziq, M.; Lim, C.; Roshan, T.; Chau, Y.; Abdul, H. Machine learning approaches to predict adsorption capacity of Azolla pinnata in the removal of methylene blue. J. Taiwan Inst. Chem. Eng. 2022, 132, 104134. [Google Scholar] [CrossRef]
- Lu, Y.; Raziq, M.; Linda, L.; Namal, P. Effective and Simple NaOH-Modification Method to Remove Methyl Violet Dye via Ipomoea aquatica Roots. Adsorp. Sci. Technol. 2021, 2021, 5932222. [Google Scholar] [CrossRef]
- Kooh, M.; Dahri, M.; Lim, L. Removal of methyl violet 2B dye from aqueous solution using Nepenthes rafflesiana pitcher and leaves. Appl. Water Sci. 2017, 7, 3859–3868. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.; Kamarehie, B.; Almasi, A. Removal of Rhodamine B from aqueous solution by stalk corn activated carbon: Adsorption and kinetic study. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Cheng, W.; Li, N.; Pan, Y.; Jin, L. The Adsorption of Rhodamine B in Water by Modified Zeolites. Mod. Appl. Sci. 2016, 10, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Gaurav, B.; Kavita, J.; Neeraj, D. Utilization of carbon nanotubes for the removal of rhodamine B dye from aqueous solutions. J. Nanosci. Nanotechnol. 2014, 14, 4331–4336. [Google Scholar] [CrossRef]
- Bahram, M.; Talebi, R.; Naseri, A.; Nouri, S. Modeling and Optimization of removal of rhodamine B from wastewaters by adsorption on modified clay. Chiang Mai J. Sci. 2014, 41, 1230–1240. [Google Scholar]
- Marcelo, L.; Silva, J.; Samuel, C. Design, Cost Estimation and Sensitivity Analysis for a Production Process of Activated Carbon from Waste Nutshells by Physical Activation. Processes 2020, 8, 945. [Google Scholar] [CrossRef]
- Chilton, N.; Wayne, E.; Rao, M.; Losso, N. Activated carbon from pecan shell: Process description and economic analysis. Ind. Crops Prod. 2003, 17, 209–217. [Google Scholar]
- Keith, C.; John, P.; Gordon, M. Production of activated carbon from bamboo scaffolding waste-process design, evaluation and sensitivity analysis. Chem. Eng. J. 2005, 109, 147–165. [Google Scholar]
- Jia, L.; Lock, H. The production cost analysis of oil palm waste activated carbon: A pilot-scale evaluation. Greenh. Gases. 2020, 10, 999–1026. [Google Scholar] [CrossRef]
- Ng, C.; Ramu, M.; Wayne, M. Granular Activated Carbons from Agricultural By-Products: Process Description and Estimated Cost of Production. Bulletin Number 881, 2003, LSU AgCenter Bulletins, Louisiana State University. Available online: https://digitalcommons.lsu.edu/cgi/viewcontent.cgi?article=1034&context=agcenter_bulletins (accessed on 18 September 2022).
- Zahra, A.; Mohsen, M.; Habibollah, Y. The feasibility of cost-effective manufacturing activated carbon derived from walnut shells for large-scale CO2 capture. Environ. Sci. Pollut. Res. 2019, 26, 26542–26552. [Google Scholar] [CrossRef]
- Ozsoy, H.; Leeuwen, J. Removal of color from fruit candy waste by activated carbon adsorption. J. Food Eng. 2010, 101, 106–112. [Google Scholar] [CrossRef]
- Thomas, K.; Lynda, H.; Isabel, M. Activated carbons from flax shive and cotton gin waste as environmental adsorbents for the chlorinated hydrocarbon trichloroethylene. Bioresour. Technol. 2009, 100, 5045–5050. [Google Scholar]
- Tinnabhop, S.; Wanwisa, S. Economic comparative evaluation of combination of activated carbon generation and spent activated carbon regeneration plants. J. Eng. Sci. Technol. 2017, 12, 3329–3343. [Google Scholar]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Sanchez, R. Sustainable Asphalt Pavement: Application of Slaughterhouse Waste Oil and Fly Ash in Asphalt Binder. Master’s Thesis, Texas A&M University, Kingsville, TX, USA, 2014; ProQuest Dissertations Publishing, Ann Arbor, MI, USA; p. 1573599. [Google Scholar]
- Jaime, L.; Shin, K.; Kawi, S.; Wang, C. Conversion of Coal Fly Ash into Zeolite Materials: Synthesis and Characterizations, Process Design, and Its Cost-Benefit Analysis. Ind. Eng. Chem. Res. 2017, 56, 11565–11574. [Google Scholar]
- Biniwale, R.; Sadhana, R.; Hasan, M. Cost estimates for production of fly ash based zeolite—A. J. Sci. Ind. Res. 2001, 60, 574–579. [Google Scholar]
- Ihab, H.; Jian, Z. Simulation of Synthetic Zeolites-4A and 5A Manufacturing for Green Processing. Eng. Sci. Technol. Int. J. 2012, 2, 188–195. [Google Scholar]
- Keka, O.; Narayan, C.; Amar, S. Zeolite from fly ash: Synthesis and characterization. Bull. Mater. Sci. 2004, 27, 555–564. [Google Scholar]
- Sapawe, N.; Jalil, A.; Karim, A.; Jusoh, R. Cost-effective microwave rapid synthesis of zeolite NaA for removal of methylene blue. Chem. Eng. J. 2013, 229, 388–398. [Google Scholar] [CrossRef]
- CNT Composites: Cost and Production. Available online: https://sites.google.com/site/cntcomposites/cost-and-production#:~:text=The%20cost%20of%20carbon%20nanotubes,%24%2020%2D2000%2Fg (accessed on 18 September 2022).
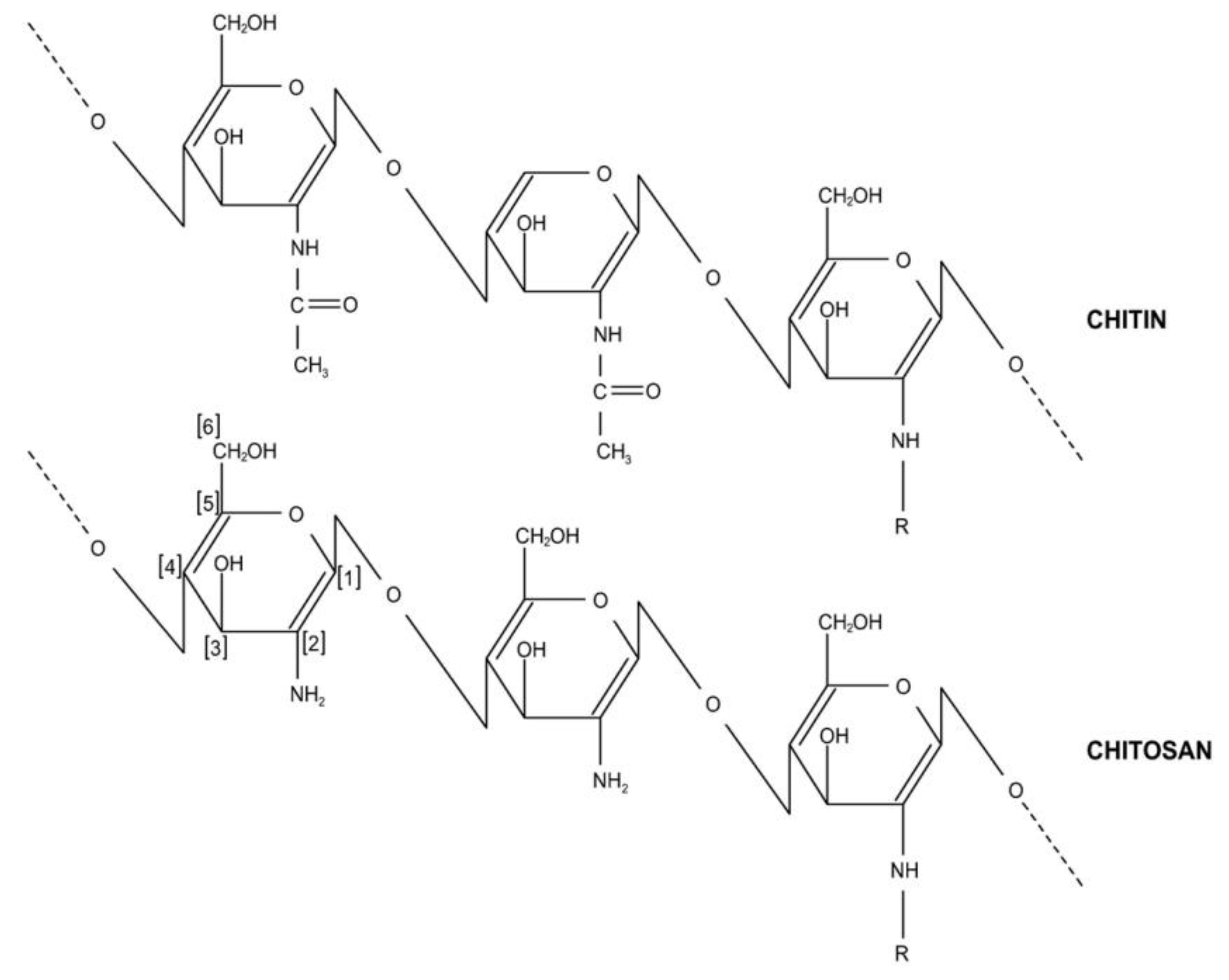
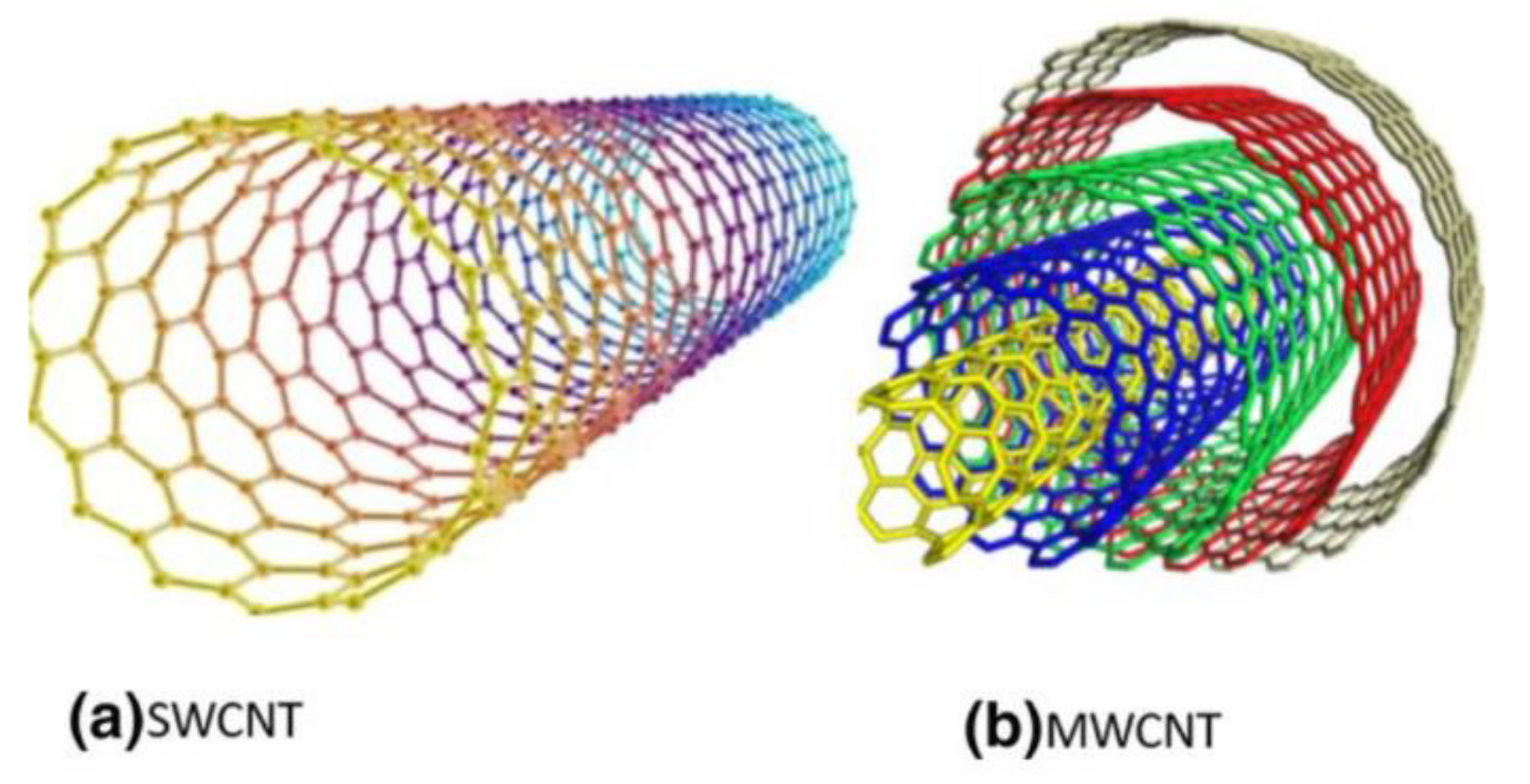
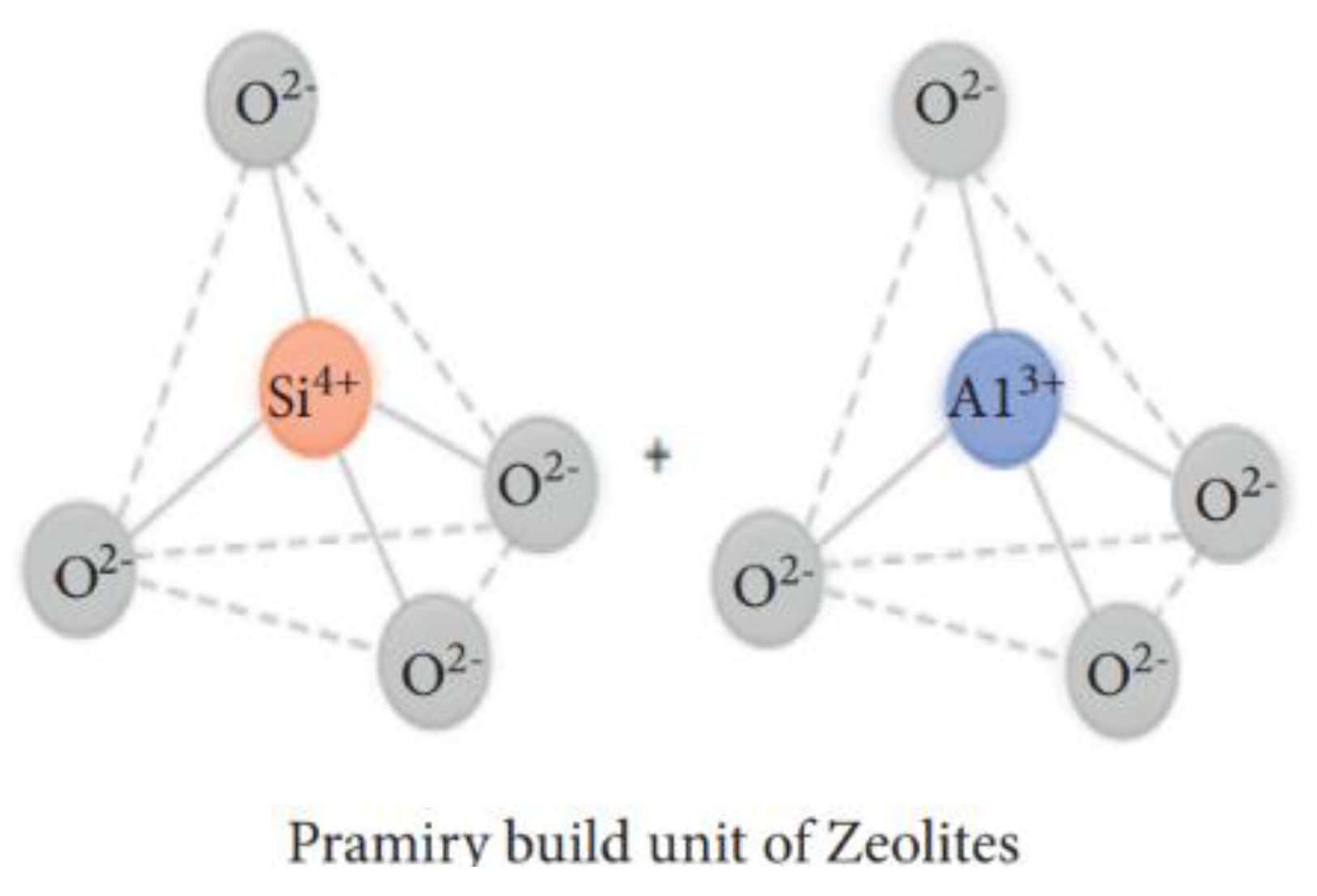
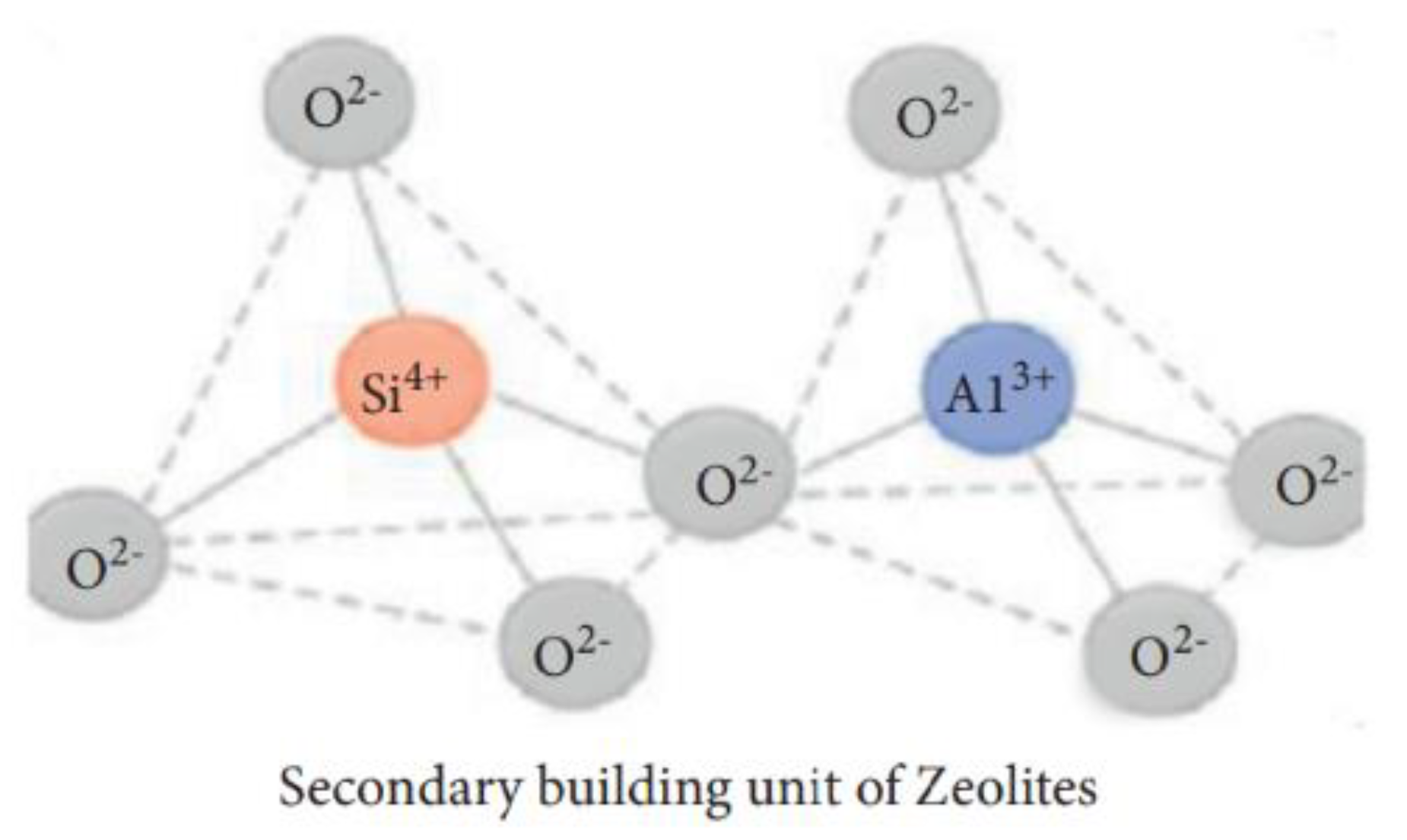
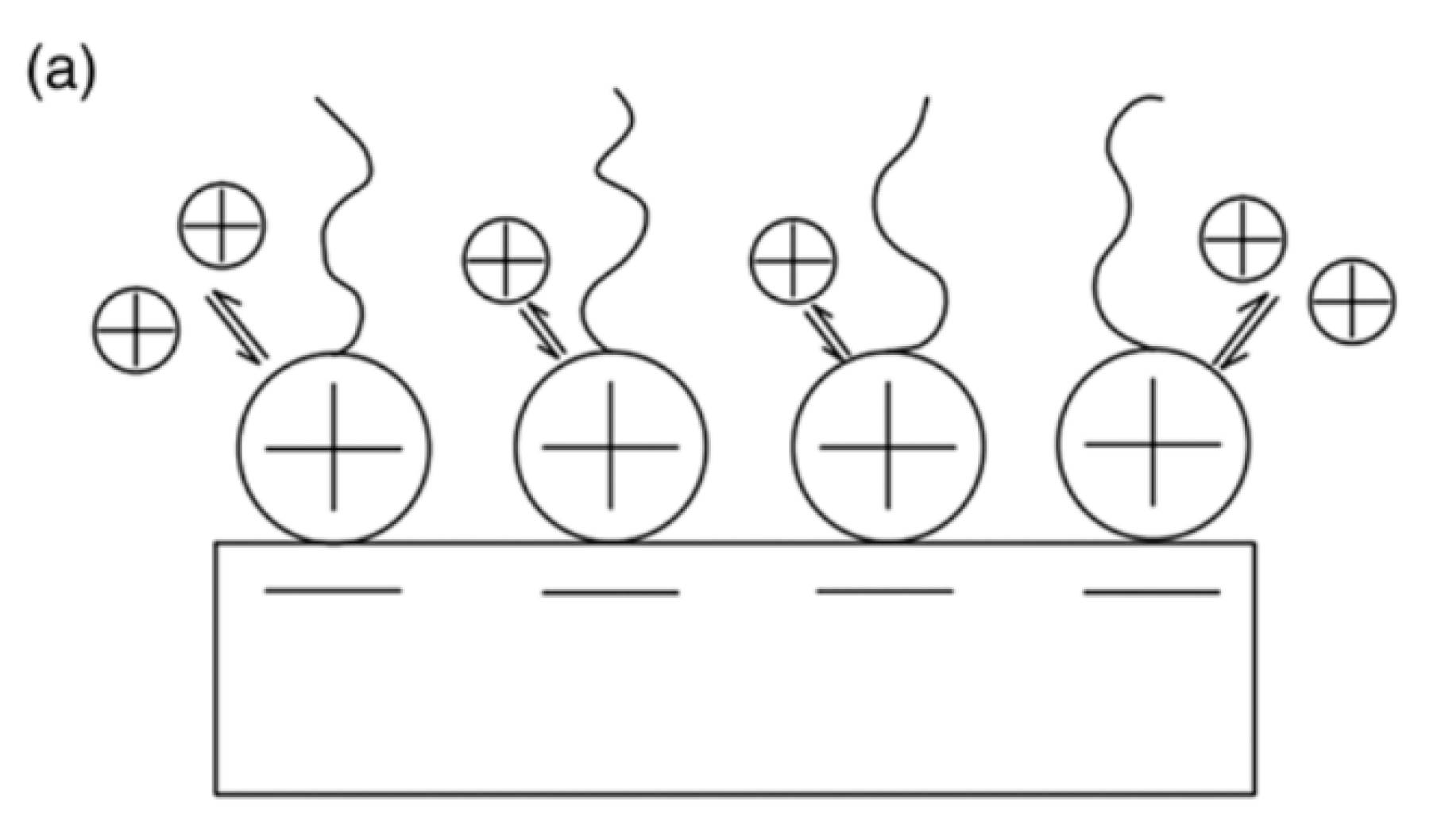
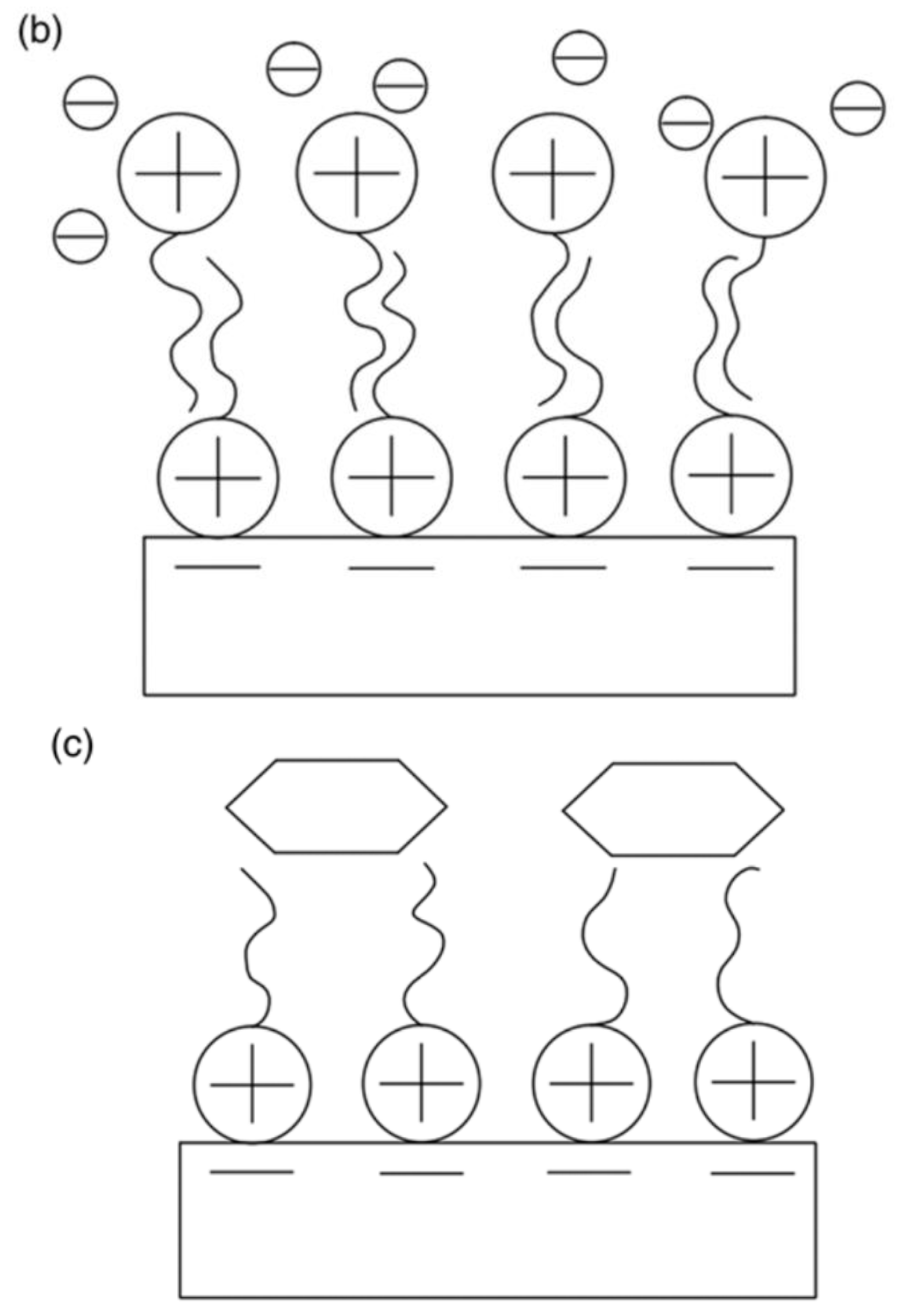
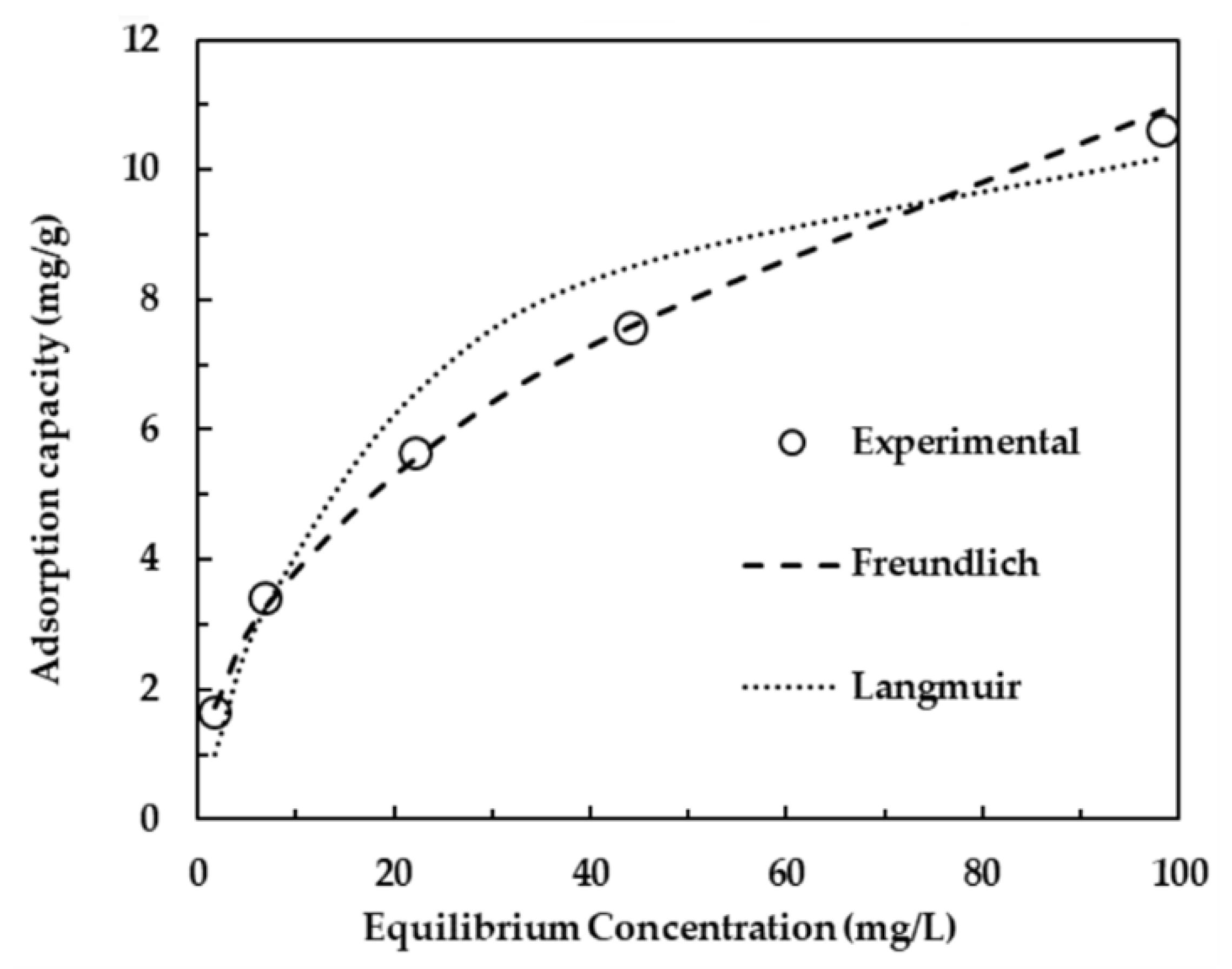
| Physical Adsorption | Chemical Adsorption |
|---|---|
| Van der Waal force of attraction is involved | Chemical bond is involved |
| The adsorption is considered a reversible process | The adsorption is considered an irreversible process |
| It does not require activation energy | Activation energy is needed |
| Lower value in heat of adsorption | Higher value in heat of adsorption |
| The adsorption occurs at lower temperatures | The adsorption occurs at higher temperature |
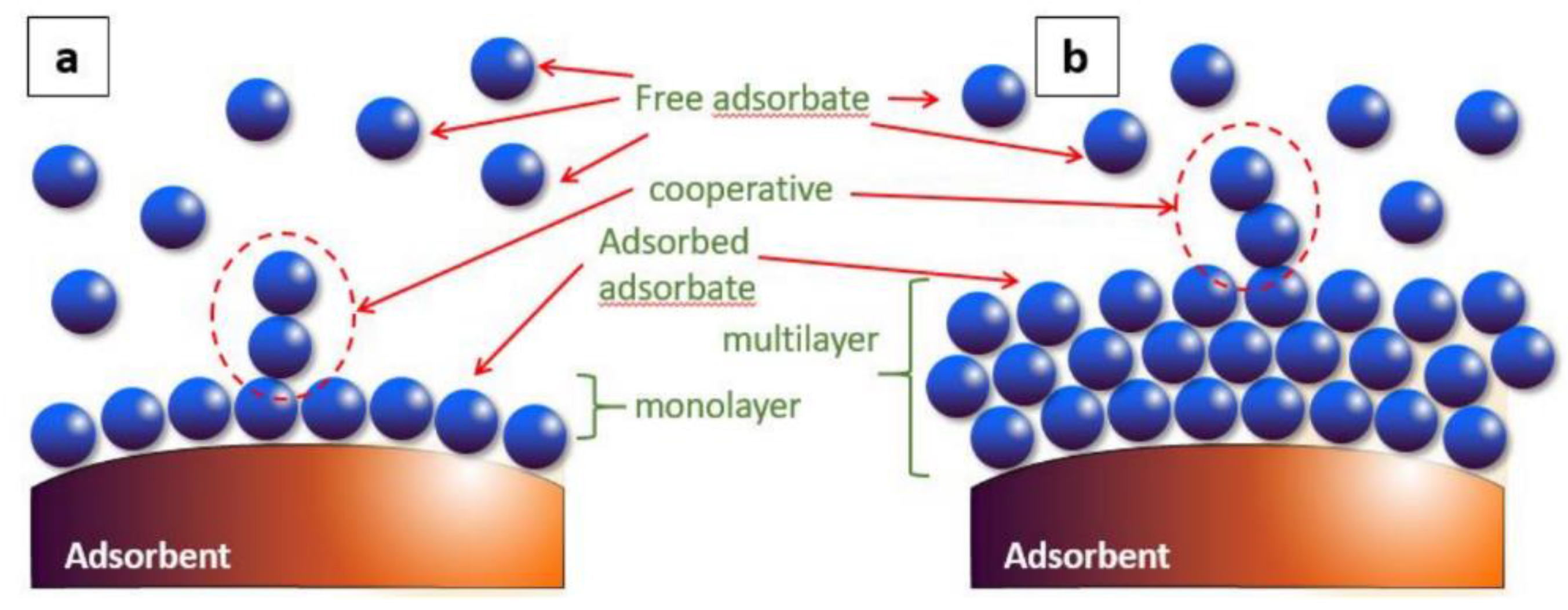
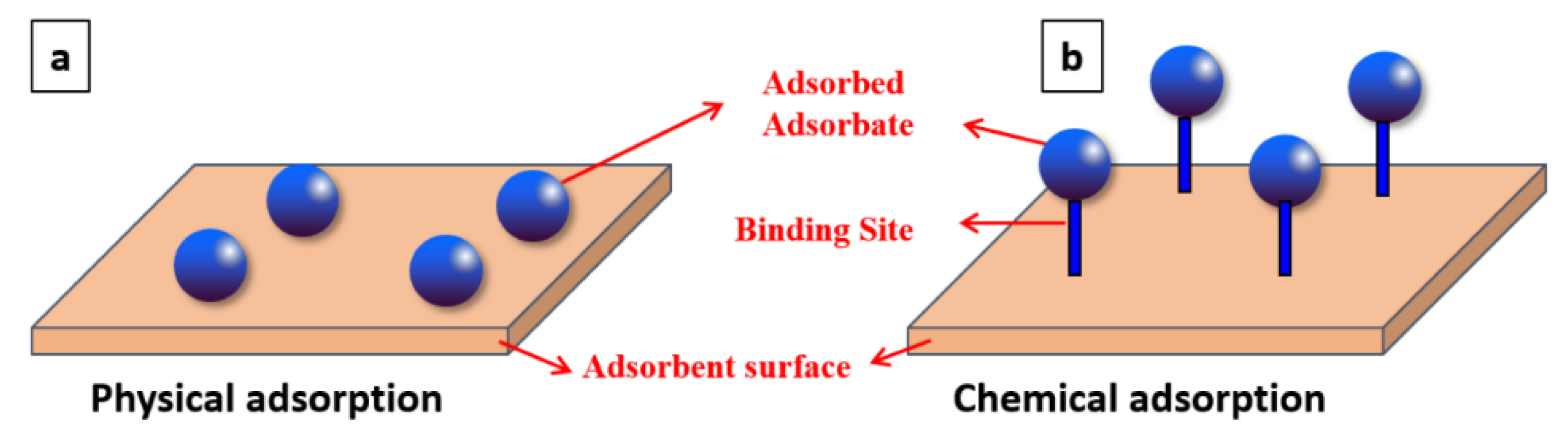
| It produces a multilayer (Figure 1) | It produces a monolayer (Figure 1) |
| Type | Physical Properties |
|---|---|
| Multi-walled carbon nanotube | Pore volume = 0.8 cm3/g |
| Specific surface area = 40–600 m2/g | |
| Outside diameter = 40–60 nm | |
| Core diameter = 5–10 nm [28] | |
| Length = 5–15 µm | |
| Surface area: 1000 m2/g | |
| Microspore volume = 0.5 cm3/g [29] | |
| Surface area = 407–650 m2/g | |
| Average pore width = 2.77 to 2.13 nm [30] | |
| Surface area = 98.7 m2/g | |
| Average pore diameter = 30.9 nm [31] | |
| Pore volume = 0.764 cm3/g | |
| Single-walled carbon nanotube | Surface area = 381 to 1068 m2/g |
| Average bundle size = 10–30 nm [32] | |
| Surface area = 302 m2/g [33] | |
| Pore volume of the bundles = 0.14 cm3/g | |
| Skeletal density = 0.806 g/cm3 | |
| Pore volume = 0.1 cm3/g | |
| Surface area = 911 m2/g | |
| Diameter = 9–25 Å [34] | |
| Surface area = 398 m2/g [35] | |
| Average pore size = 10.8 nm | |
| Pore volume = 0.92 cm3/g |
| Activating Agent | Microporous Volume (cm3/g) | Mesoporous Volume (cm3/g) | Total Pore Volume (cm3/g) | Surface Area (m2/g) | Average Pore Diameter (nm) | Yield (%) | Fixed Carbon (%) | Ash (%) |
|---|---|---|---|---|---|---|---|---|
| KOH | 0.101 | 0.085 | 0.186 | 374.03 | 5.12 | 30.5 | 43.78 | 10.9 |
| H3PO4 | 0.194 | 0.097 | 0.291 | 480.23 | 4.21 | 22.39 | 2.56 | 29.6 |
| Zinc chloride (ZnCl2) | 0.225 | 0.076 | 0.301 | 546.61 | 3.32 | 23.34 | 40.9 | 23.8 |
| Potassium Permanganate (KMnO4) | 0.152 | 0.092 | 0.244 | 274.92 | 4.3 | 29.98 | 14.02 | 26.02 |
| Phenol and Phenolic Compound | Unhealthy Effect | Sources | Structure |
|---|---|---|---|
| Chlorophenol | Mouth and throat burning | herbicides and disinfectants | 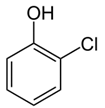 |
| Hydroquinone | Damage to chromosomes | Cosmetic industries |  |
| 2,4-dimethyl phenol | Nausea and headache | Insecticides and fungicides | 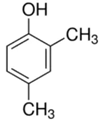 |
| Para-cresol | Renal and digestive system disorder | Photographic sources and paint | 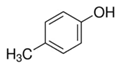 |
| Bisphenol A | Increase blood pressure and cardiovascular disease | Polycarbonate plastics | 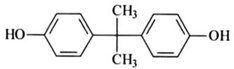 |
| Alkylphenol | Testicular damage | Surfactants and detergents | 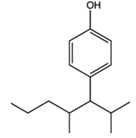 |
| Aminophenol | Skin allergies | Film and cosmetic products | 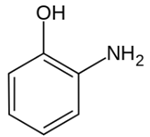 |
| Chlorocatechol | Eye irritation | Photography, dyeing fur | 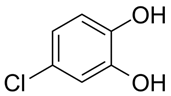 |
| Nitrophenol | Bluish skin and headache | Fungicides and insecticides | 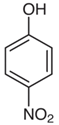 |
| Adsorbent | Adsorption Studies |
|---|---|
| Layered double hydroxide (LDH) clays | Neat clay has low phenol removal efficiency because of poor mixing of the clay with phenolic water [68]. Amorphous mixed metal oxide enhanced the surface area (8.7 to 29.73 m2/g) when heated at 500 °C. The highest percentage removal of phenol was 85% (contact time = 24 h, pH = 7, in the calcined clays). |
| Mg-Al LDH | The highest adsorption capacities for phenol and p-nitrophenol were 82.6 mg/g and 356.4 mg/g, respectively [69]. Adsorption kinetics were well-matched with pseudo-second-order kinetic model. |
| Natural Zeolite (clinoptilolite) | The adsorption efficiency of phenol increased (32% to 49%) when increasing the adsorbent dosage (5 g to 25 g). More phenol can be adsorbed with the increase in initial concentration due to the increase in the mass transfer driving force [70]. The highest adsorptive capacity was 0.7 mg/g, as indicated in the Langmuir model (R2 = 0.993). |
| Siliceous BEA zeolite | Zeolite has high Si/Al ratio [71]. High removal efficiency of phenol was obtained (the phenol concentration was less than 1.6 g/L). |
| NaY and nickel-modified NaY zeolites | The adsorption process achieved equilibrium within 120 min and at pH = 4. Ni-modified NaY zeolite showed the highest adsorption capacity (88.79%) compared to NaY (77.2%) due to low coordination number of nickel cations in the zeolite [72]. Adsorption kinetics followed pseudo-second-order model kinetic model and Freundlich model. |
| Zeolite | Natural zeolite-supported cobalt oxide catalysts were synthesized [73]. The adsorption efficiency of phenol was found to be 100% and 70% in cobalt-supported zeolite from Indonesia and Australia, respectively (0.2 g = catalyst, 25 ppm = phenol, 1 g = oxone, temperature = 25 °C). Higher activation energies were obtained in Australian sample (61.3 kJ/mol) compared to Indonesian sample (52.4 kJ/mol). |
| Modified zeolite with FeCl3 | Adsorption of phenol reached equilibrium within 100 min and at pH 3. Adsorption data were well-described by the Langmuir model (R2 = 0.98). Removal efficiency increased [74] from 65% to 95% (increase in surface area) with the adsorbent dose (up to 1 g). |
| High silicate zeolite | Removal of 2,4,6-trichlorophenol was more favorable when using FAU zeolite (lateral interaction) and showed S-shaped isotherm [75]. MFI zeolite has the highest adsorpion capacity due to close-fit phenomenon (interaction between adsorbate and adsorbent would be stronger). The zeolite pore structure was studied based on the opening sizes (5.1Å × 5.5 Å and 5.3 Å × 5.6 Å) and phenol size (4.34 Å × 0.87 Å × 5.55 Å). |
| Modified bentonite (cetyl trimethylammonium bromide) | The time to reach equilibrium was about 60 min at pH 9. The monolayer adsorption capacity was found to be 333 mg/g based on the Langmuir model [76]. Adsorption process was spontaneous and exothermic according to the thermodynamic studies. |
| Pumice | The pumice was treated with cationic surfactants (benzyldimethyl tetradecylammonium chloride and hexadecyltrimethyl ammonium bromide). The adsorption capacity increased with the increase in initial concentration [77]. The adsorption data fitted well with Freundlich model. |
| Rice husk, black gram husk, and green-gram-husk-based activated carbon | The optimum adsorption conditions obtained from the study were pH = 5.1, adsorbent dosage = 0.5 g/L, equilibrium time = 6 h). Adsorption process can best be described by the Redlich–Peterson isotherm [78]. |
| Banyan-root-based activated carbon | The maximum percentage removal was 89.2% in KOH-treated activated carbon [79]. The surface area and average pore radius were observed to be 988 m2/g and 24 nm, respectively. Adsorption process was spontaneous, exothermic, and obeyed Langmuir isotherm (maximum adsorption capacity = 26.95 mg/g). |
| Coconut-shell-based activated carbon | The percentage removal of parachlorophenol and 2,4,6-trichlorophenol was observed to be 99.9% and 99.8%, respectively [80]. The adsorption data supported Freundlich model and pseudo-second-order kinetic. |
| Palm-pith-based activated carbon | The small particle size (250–500 microns) and acidic conditions displayed a favorable behavior (adsorption capacity of 2,4-dichlorophenol = 19.16 mg/g). The entropy (Δ = 30.7 J/mol.K) and enthalpy value (7.16 kJ/mol) were reported [81]. |
| Banana-pith-based activated carbon | Based on the Langmuir model, the highest adsorption capacity of 2,4-dichlorophenol (pH = 2) was 129.4 mg/g in zinc-chloride-treated activated carbon [82]. Kinetic studies followed pseudo-first-order kinetic model. |
| Coal-, coconut-, and bamboo-based activated carbon | Adsorption capacities of phenol were found in the range of 13.16–24.96 mg/g, 10.86–21.22 mg/g, and 10.29–20.14 mg/g in bamboo-, coconut-, and coal-based activated carbon, respectively [83]. Free energy (−9.34 to −9.94 kJ/mol), enthalpy (−18.59 kJ/mol), and entropy (−30.05 J/K.mol) were reported. The adsorption data were well-described by the pseudo-second-order kinetic model and Freundlich model. |
| Tea-residue-based activated carbon | The kinetics of adsorption of phenol followed pseudo-second-order kinetic model. The highest adsorption capacity was 320 mg/g in NaOH-treated activated carbon. Surface area and total pore volume were found to be 819 m2/g and 0.443 cm3/g, respectively [84]. |
| Commercial activated carbon (Riedel-de Haen) | Removal of phenol was observed to be more than 99% when the shaking time was 10 min at room temperature [85]. Adsorption data can best be described by the Langmuir model (R2 = 0.9947). |
| Bagasse fly ash | The optimum conditions were determined (equilibrium time = 5 h, pH = 6.5, fly ash dose = 10 g/L) [86]. Adsorption process followed the Redlich–Peterson and pseudo-second-order kinetic model. The entropy change (1.8 J/kg.K) and heat of adsorption (0.5 MJ/kg) were highlighted. |
| Fly ash | Removal of phenol was strongly dependent on the initial adsorbate concentration and the molecular size [87]. According to thermodynamic studies, adsorption process was spontaneous (negative value of free energy). |
| Fly ash | The equilibrium time was found to be 60 min. Adsorption kinetics followed pseudo-second-order kinetic model [88]. External diffusion and pore diffusion can be observed during the experiment as indicated in intraparticle model. Removal of phenol increased when reducing the particle size but reduced when the temperature was increased. |
| Chitosan (D-glucosamine) | The adsorption efficiency from Freundlich model (0.17 to 0.4) was reported [89]. The highest adsorption capacity (Langmuir model) increased (0.1 to 0.18 mmol/kg) when the temperature was reduced (25 °C to 5 °C) in flake-type chitosan. The percentage removal reduced as pH increased due to the hydroxyl group (in the adsorbate) combined via hydrogen bond with chitosan (in hydroxyl group). Based on the Langmuir model, the highest adsorption capacities of 2,4,6-trichlorophenol, 3,4-dichlorophenol, 2,3-dichlorophenol, and 2,6 dichlorophenol were 0.7, 0.63, 0.6, and 1.08 mmol/kg, respectively (agitation speed = 150 rpm, equilibrium time = 4 days, natural pH). |
| Chemical Formula | Name | Molar Mass (g/mol) | Structure |
|---|---|---|---|
| C16H15Cl3O2 | methoxychlr | 345.65 | 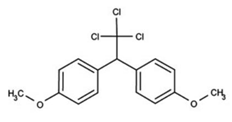 |
| C8H14ClN5 | atrazine | 215.69 | 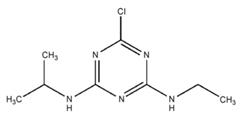 |
| (CH3O)2P(S)OC6H4NO2 | methyl parathion | 263.2 | 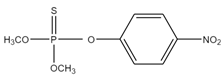 |
| C10H19O6PS2 | malathion | 330.35 | 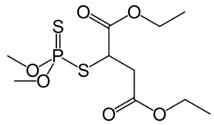 |
| C12H14Cl2N2 | paraquat dichloride | 257.16 | 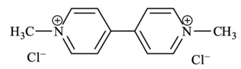 |
| C3H8NO5P | glyphosate | 169.07 | 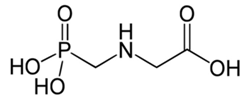 |
| C10H12N2O3S | bentazone | 240.28 | 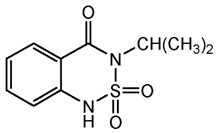 |
| C6H3Cl2NO2 | clopyralid | 192 | 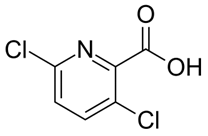 |
| C9H10ClN5O2 | imidacloprid | 255.661 | 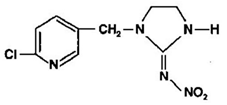 |
| C12H18N2O | isoproturon | 206.28 | 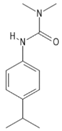 |
| C15H21NO4 | metalaxyl-M | 279.33 | 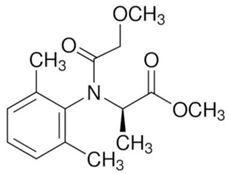 |
| WHO Class | LD50* for the Rat (mg/kg Body Weight) | Example in Terms of Active Ingredients | ||
|---|---|---|---|---|
| Oral | Dermal | |||
| Ia | Extremely hazardous | <5 | <50 | Aldicarb, Parathion, Mercuric chloride |
| Ib | Highly hazardous | 5–50 | 50–200 | Acrolein, Cadusafos, Ca-arsenate |
| II | Moderately hazardous | 50–2000 | 200–2000 | Alachlor, Bentazone, Copper sulfate |
| III | Slightly hazardous | Over 2000 | Over 2000 | Hexaconazole, Atrazine, Butachlor |
| U | Unlikely to present acute hazard | 5000 or higher | 5000 or higher | Mancozeb, Captan, Bifenox. |
| Pesticides | Adsorbent | Adsorption Studies |
|---|---|---|
| Ametryn Formula: C9H17N5S Molecular mass: 227.3 g/mol | Natural montmorillonite | Langmuir model: Maximum monolayer adsorption capacity was 188.8 mg/g. Thermodynamic studies: spontaneous and endothermic [96]. |
| Carbofuran Formula: C12H15NO3 Molecular mass: 221.256 g/mol 2,4-Dichlorophenoxyacetic acid Formula: C8H6Cl2O3 Molecular mass: 221.04 g/mol | Commercial granular-type activated carbon (Filtersorb 300) | Langmuir model: maximum adsorption capacities were 181.82 mg/ and 96.15 mg/g for 2,4-Dichlorophenoxyacetic acid and carbofuran, respectively. Adsorption kinetics followed pseudo-second-order kinetic isotherm [97]. |
| Hexachlorocyclohexane Formula: C6H6Cl6 Molecular mass: 290.8 g/mol | Coconut-shell-based activated carbon | Maximum monolayer adsorption capacity can be determined based on the Langmuir model [98]. |
| Bentazon Formula: C10H12N2O3S Molecular mass: 240.28 g/mol | Date seed activated carbon | Adsorption data were well-described by the Freundlich model and pseudo-second-order kinetic model [99]. The adsorption percentage of carbofuran was found to be higher compared to bentazon. |
| Bentazon, carbofuran, 2,4-Dichlorophenoxyacetic acid (2,4-D) | Palm-oil-frond-based activated carbon | The percentage removal of bentazon (48% to 95%), carbofuran (57% to 96%) and 2,4-D (71% to 98%) was studied with various activation temperatures (716 to 884 °C) and activation times (0.32 to 3.68 h) [100]. |
| Atrazine Formula: C8H14ClN5 Molecular mass: 215.69 g/mol Imidacloprid Formula: C9H10ClN5O2 Molecular mass: 255.661 g/mol Azoxystrobin Formula: C22H17N3O5 Molecular mass: 403.388 g/mol | Rice-straw-activated carbon | Experimental findings revealed pore filling and partitioning for imidacloprid, atrazine, and azoxystrobin, respectively [101]. |
| Bromopropylate Formula: C17H16Br2O3 Molecular mass: 428.120 g/mol | Corn-cob-based activated carbon | The adsorption data can best be described by the pseudo-second-order kinetic model and Langmuir isotherm [102]. |
| Diazinon Formula: C12H21N2O3PS Molecular mass: 304.34 g/mol | NH4Cl-induced activated carbon | Langmuir model: maximum adsorption capacity was 250 mg/g. Thermodynamic studies: The enthalpy (+77.5 kJ/mol), entropy (+296 J/K.mol), and free energy (−10.76 to −14.9 kJ/mol) were reported [103]. |
| 2,4-dichlorophenoxy acetic acid | Peanut-shell-based activated carbon | The adsorption data followed Freundlich model (R2 = 0.9347) and pseudo-first-order kinetic model R2 = 0.99). The percentage removal of 2,4-D reduced (78.6% to 47.2%) when the initial concentration was increased (10 to 50 mg/L) [104]. |
| atrazine | Zeolite (Fruška Gora) | The highest percentage removal was 73% (pH = 10, particle size = 1–2 mm, zeolite dos = 15 g) [105]. |
| Malathion | Coconut shell- and palm-shell-based activated carbon | Langmuir model: maximum adsorption capacity was 909 mg/g [106]. |
| Alachlor Formula: C14H20ClNO2 Molar mass: 269.7 g/mol Metolachlor Formula: C15H22ClNO2 molar mass: 283.8 g/mol Fipronil Formula: C12H4Cl2F6N4OS Molar mass: 437.14 g/mol Chlorpyriphos Formula: C9H11Cl3NO3PS Molar mass: 350.57 g/mol endosulfan sulfate formula: C9H6Cl6O4S molas mass: 422.9 g/mol | Organo-modified montmorillonite clays modified with octadecylamine, dimethyl- dialkylamine, octadecylamine, and aminopropyltriethoxysilane | The adsorption data followed Freundlich model (R2 = 0.951 to 0.992) and pseudo-second-order kinetic model [107]. Higher value of Freundlich constant (0.17 to 0.52 mg/g) represented higher affinity of these pesticides toward organo clays due to the hydrophobic interaction. The percentage removal of chlorpyriphos was 70% within 90 min due to high octanol–water partition coefficient and lower water solubility value. Based on the Freundlich model, adsorption of alachlor (1/n = 0.14), metolachlor (1/n = 0.25), fipronil (1/n = 0.09), and endosulfan sulfate (1/n = 0.21) was found to be non-linear and followed the L-type isotherms. |
| Paraquat Formula: C12H14Cl2N2 Molar mass: 257.16 g/mol | Kaolin modified with sulfuric acid | Equilibrium was achieved in 60 min. Adsorption data were fit well with Freundlich model [108]. |
| Carbaryl Formula: C12H11NO2 Malar mass: 201.225 g/mol | Clay (Agadir city) | Equilibrium was reached [109] in 120 min (initial concentration = 10 mg/L, mass of clay = 1 g/L, temperature = 20 °C). Adsorption process takes place in two stages, namely, transfer of external mass and diffusion phenomena. It was clear that the isoelectric point was pH 5.1. When the pH was more than pH 5.1, electrostatic repulsion happened and reduced the adsorption process. We can observe the high electronic density in carbaryl due to the Kekule forms of two nuclei. Adsorbed amount reduced (2.211, 2.001, 1.897, and 1.343 to 1.117 mg/g) when the temperature was 20 °C, 30 °C, 40 °C, 50 °C, and 60 °C due to releasing activation energy during the adsorption process. |
| Lindane Formula: C6H6Cl6 Molar mass: 290.81 g/mol Heptachlor Formula: C10H5Cl7 molar mass: 373.32 g/mol | Pine-bark-based activated carbon | The highest removal efficiencies of Lindane (76.6%) and heptachlor (77.7%) were reported [110]. |
| Classification of Dyes | Example of Dyes | Adsorbent | Adsorption Studies and Thermodynamic Investigations |
|---|---|---|---|
| Direct dye | Direct Brown MR | Prosopis juliflora bark-based activated carbon | The highest percentage removal was 90.2% (contact time = 60 min, initial concentration = 400 mg/L, adsorbent dosage = 100 mg, pH = 2, temperature = 30 °C). Thermodynamic studies: spontaneous (ΔG = −0.289 to −0.33 kJ/mol) and endothermic reaction (ΔH = 0.22 kJ/mol). Adsorption data were well-fitted with Langmuir model (R2 = 0.992, maximum adsorption capacity = 500 mg/) and Freundlich model (R2 = 0.996) [118]. |
| Direct dye | Brilliant blue | Nigella Sativa L waste-based activated carbon | Thermodynamic studies: exothermic (ΔH = −3.264 kJ/mol), spontaneous (ΔG = −4.37 kJ/mol), and the disorder reduces at the solution/solid interface (ΔS = −24.89 J/mol.K) [119]. The Freundlich model (R2 = 0.994) described adsorption on heterogeneous surfaces with different adsorption energies. Langmuir model: The highest adsorption capacity was 14.49 mg/g. |
| Direct dye | Brilliant blue | Zeolite | The highest percentage of removal was 87.02% in Zeolite-Fe(Fe-Cu) zeolite [120], and the adsorption process was endothermic (126.29 kJ/mol). Removal efficiency was 75.29% in zeolite-Fe, and the adsorption process was endothermic (52.6 kJ/mol). The optimum pH was in the range of 3 to 5. The kinetics of adsorption followed pseudo-second-order kinetic model in both samples. |
| Direct dye | Congo red | Pinus pinaster bark-based activated carbon | The maximum adsorption capacity for Congo Red was 0.47 mg/g (pH = 2, temperature = 25 °C, initial dye concentration = 5 mg/L, activated carbon dose = 10 g/L), but dropped to 0.3 mg/g at pH 9 [121]. The highest correlation coefficient (R2 = 0.98) can be observed in the pseudo-second-order kinetic model, indicating chemical sorption process. Thermodynamic studies: exothermic process. The adsorption capacity was reduced when the temperature was increased (25 °C to 60 °C). |
| Reactive dye | Reactive red 198 | Pistachio hull-waste-based activated carbon | The highest adsorption capacity was 253.67 mg/g (pH = 2, initial concentration = 80 mg/L, temperature = 25 °C) [122]. The Freundlich model (R2 = 0.9289) shows the best correlation coefficient compared to Langmuir model (R2 = 0.8839). |
| Reactive dye | Reactive red 2, reactive green 19 | Ground nut shell, baby-corn-based activated carbon | Percentage removal of reactive green dye (71.6% to 79.5%) and reactive red dye (58.7% to 73.3%) by using ground-nut-shell-based activated carbon increased when the initial concentration of dye was reduced (65 ppm to 40 ppm). [123]. Percentage removal of reactive red dye (61.5% to 78%) and reactive green dye (72% to 80.5%) using baby-corn-based activated carbon increased when the initial concentration of dye was reduced (65 ppm to 40 ppm) [123]. |
| Reactive dye | Reactive Red-120 | Cumin-herb-wastes-based activated carbon | The adsorption capacity was reduced with the increase in pH (pH 2 to 12) due to binding sites (activated carbon) being associated with hydrogen ions (such as bridging ligands between activated carbon surface and reactive red-120) at lower pH values [124]. The highest percentage removal was 95.8% (pH = 2, initial concentration = 50 mg/L, temperature = 22 °C). The Langmuir model (R2 = 0.976) shows the best correlation coefficient compared to Freundlich model (R2 = 0.9308), which represented monolayer adsorption. |
| Vat dyes | Vat yellow 4 | Mucuna pruriens seed-shells-based activated carbon | The optimum pH adsorption was at pH 2 because electrostatic attraction occurred (negative charge in anionic and positively charged in the adsorbent surface). Adsorption kinetics were well described by the pseudo-second-order model (R2 = 0.99) [125]. Adsorption data fit well with Langmuir model (R2 = 0.989 to 0.997, maximum adsorption capacity = 34.48 to 52.63 mg/g), indicating a monolayer adsorption process on the homogeneous surface. |
| Disperse dyes | Disperse Orange 30 | Rattan-sawdust-based activated carbon | The highest adsorption capacities were observed in specific conditions (temperature = 470 °C, activation time = 134 min, impregnation ratio of phosphoric acid:precursors = 1:4.5) [126]. |
| Disperse dyes | Disperse Orange 25, dispese Blue 7 | Zeolite | The maximum adsorption capacities were 125 mg/g and 109.8 mg/g for disperse orange and disperse blue, respectively. The removal of dye increased when the initial concentration was increased (10 to 40 mg/L) but dropped (from 60 to 40 mg/L) in higher concentrations (due to limited adsorption sites) [127]. |
| Azo dyes | Azo dyes | Coal-based activated carbon | The highest adsorption capacity was 333 mg/g in acidic conditions [128]. Thermodynamic studies: spontaneous (ΔG = −12.4 kJ/mol), endothermic (ΔH = 39.66 kJ/mol), and feasible (ΔS = 174.55 J/mol.K). Adsorption data were well-fitted with Langmuir model, pseudo-second-order kinetic isotherm, and D-R model. |
| Azo dyes | Direct Blue 71 | Chitosan-multi walled carbon nanotubes | The optimized experimental conditions were reported (pH = 6.25, concentration of dye = 3 mg/L, adsorbent dose = 0.1 g) [129]. Langmuir model: maximum adsorption capacity was 29.3 mg/g. The highest correlation coefficient can be seen in Langmuir model (R2 = 0.9985) and pseudo-second order model (R2 = 0.999). |
| Azo dyes | Direct Blue 78 | Egg-shell-based activated carbon | The maximum adsorption capacity was 13 mg/g (adsorbent dose = 0.5 g, pH = 5). Adsorption process can be described by the Freundlich model and pseudo-second-order kinetic model. Thermodynamic studies: spontaneous and endothermic process [130]. |
| Azo dyes | Methyl orange | Single-walled carbon nanotube (SWCNT), carboxylate functionalized single-walled carbon nanotube (SWCNT-COOH), amide functionalized single-walled carbon nanotube (SWCNT-NH2) | Equilibrium was achieved at various times [131] using different adsorbents, such as SWCNT-NH2, (15 min) and SWCNT-COOH (20 min), and single-walled carbon nanotube (20 min). |
| Azo dye | Maxilon Blue 5G | Gold–nickel supported activated carbon | Equilibrium was reached after 30 min. The adsorption kinetics followed the pseudo-second-order kinetic model. Maximum adsorption capacity was 542.9 mg/g [132]. |
| Azo dye | Maxilon Blue 5G | Multi-walled carbon nanotube | The highest adsorption capacity was 260.7 mg/g [133]. The homogeneity of the carbon nanotube surface site can be observed based on the Freundlich model. Thermodynamic studies: endothermic and spontaneous process |
| Acid dyes | Acid blue 25 | Banana peel, Durian-peel-based activated carbon | Langmuir model (R2 = 0.99): monolayer adsorption with uniform binding energy [134]. The highest adsorption capacities were found to be 70 mg/g and 89.7 mg/g in banana and durian-peel-based activated carbon, respectively, in the optimized conditions. The rate constant values were found to be 0.163 g/mg.min and 0.092 g/mg.min in banana and durian peel activated carbon, respectively, based on the pseudo-second-order kinetic model (R2 = 0.999). Thermodynamic studies: spontaneous (ΔG = −13.6 to −14.7 kJ/mol) and exothermic process (ΔH = −12.1 to −13.4 kJ/mol). |
| Acid dyes | Acid Orange 3 | Sodium-bentonite, CTAB modified bentonite | The maximum adsorption capacity was 50 mg/g (dye concentration = 50 mg/L, adsorbent dosage = 80 mg, time = 90 min, pH = 2) in the cetyltrimethylammonium bromide (CTAB) modified bentonite [135]. Thermodynamic studies: spontaneous and endothermic. The correlation coefficient in Langmuir model (R2 = 0.959) was high compared to Freundlich model (R2 = 0.957). |
| Acid dyes | Acid Yellow 36 | Saw dust, rice-husk-based activated carbon | The maximum adsorption capacities were 183.8 and 86.9 mg/g for saw dust and rice-husk-based activated carbon, respectively [136]. Kinetic studies revealed that intraparticle diffusion was a rate-limiting step. |
| Basic dyes | Crystal violet | Rice husk | The maximum adsorption of crystal violet onto sulfuric acid treated activated carbon and zinc chloride treated activated carbon was found to be 64.875 mg/g and 61.575 mg/g, respectively [137]. The adsorption data were described well by the Langmuir model and Freundlich model. The adsorption process was fully controlled by intra-particle diffusion. |
| Basic dye | Malachite green | Multi-walled carbon nanotube | The maximum adsorption capacity was 142.85 mg/g based on the Langmuir model (R2 = 0.997) [138]. |
| Basic dyes | Crystal violet | Magnetic NaY zeolite | The highest dye-removal percentage was 98.1% (pH = 10.3, temperature = 50 °C, time = 45 min, initial dye concentration = 10 mg/L, adsorbent mass = 46.2 mg) [139]. |
| Basic dyes | Methylene blue | Pea-shells-based activated carbon | The adsorption of methylene blue increased with an increase in the temperature (25 °C to 55 °C) due to an increase in diffusion rate (via external boundary layer and the internal pores of adsorbent) [140]. According to Langmuir model (R2 more than 0.99), the maximum adsorption capacities were increased from 246.91, 261.1, and 269.54 to 270 mg/g when the temperature was 25 °C, 35 °C, 45 °C, and 55 °C, respectively. Thermodynamic studies: free energy (−28.4 to −33.73 kJ/mol), enthalpy (1457 kJ/mol), and entropy (0.147 kJ/mol.K) values were reported. |
| Basic dyes | Methylene blue | Magnetite-loaded Multi-walled carbon nanotube | The highest adsorption capacity was 48.06 mg/g due to the electrostatic attraction and π-π stacking interaction [141]. Adsorption data can best be described by the Langmuir model and pseudo-second-order kinetic model. |
| Basic dyes | Methylene blue | Zeolite 4A (Ethiopia kaolin) | The highest percentage removal was 99.37% (time = 179.82 min, concentration of dyes = 10 mg/L, adsorbent dose = 39.05 mg). Langmuir model: maximum adsorption capacity was 44.35 mg/g [142]. |
| Basic dye | Brilliant green | Guava-tree-wood-based activated carbon | Removal of dye can be considered a physical process based on the Freundlich model (R2 = 0.975) [143]. Thermodynamic studies: free energy (−86.188 kJ/mol), enthalpy (43.025 kJ/mol), and entropy (128 J/mol.K) values were reported. It is clear that the percentage removal increased (83% to 99%) with increase in adsorbent dose (0.1 g to 0.8 g). |
| Basic dyes | Methylene blue | Azolla pinnata aquatic plant | The adsorption of dye can be predicted via machine-learning algorithms [144]. The support vector regression was considered the best machine-learning algorithm due to providing the highest correlation coefficient value (R2 = 0.994) compared to other algorithms. |
| Basic dyes | Methyl violet | Ipomoea aquatica root | The highest adsorption capacity was 551.5 mg/g in NaOH-treated Ipomoea aquatica root based on the Sips model [145]. SEM images demonstrated that different morphologies could be observed in the untreated (flat surface) and NaOH-treated Ipomoea aquatica root (irregular and rough surface). |
| Basic dyes | Methyl violet | Nepenthes rafflesiana pitcher and Nepenthes rafflesiana leaves | The adsorption kinetics were best represented by pseudo-second-order kinetic model in both samples [146]. The highest adsorption capacities were found to be 288.7 mg/g and 194 mg/g in Nepenthes rafflesiana pitcher and Nepenthes rafflesiana leaves, respectively. Thermodynamic studies: spontaneous and endothermic reaction. |
| Amphoteric dye | Rhodamine B | Corn-stalk-activated carbon | Adsorption data were described well by the Freundlich model and pseudo-second-order kinetic model [147]. The highest adsorption capacity was 5.6 mg/g (pH = 3, adsorbent dose = 2.5 g/L). |
| Amphoteric dye | Rhodamine B | Zeolite | The modified zeolites (HCl and cetyl trimethyl ammonium bromide) had a higher adsorption capacity than the unmodified zeolite [148]. The adsorption data followed Freundlich model and pseudo-second-order kinetic model. The highest adsorption capacity was 7.955 mg/g at 303 K based on the Langmuir model. |
| Amphoteric dye | Rhodamine B | Multi-walled carbon nanotubes | The adsorption data followed pseudo-second-order kinetic model, Langmuir model, and Temkin isotherm [149]. The percentage removal of dye increased when the contact time was increased but dropped when increasing the dye concentration. |
| Amphoteric dye | Rhodamine B | Modified clay | The highest removal efficiency of dye from dyeing factory (in Tabriz, Iran) reached 70% [150]. |
| Description | References |
|---|---|
| Activated carbon was produced from industrial waste nutshell via physical activation technique (water vapor). Cost of production was $2.15/kg. | Marcelo and co-workers (2020) [151] |
| Pecan-shell-based activated carbon was synthesized through steam activation and phosphoric acid activation. The estimated production cost was $2.72/kg for steam-activated carbon (based on 1400 kg/day output and 320 days per year production). For the phosphoric acid activation process, the production cost was estimated at $2.89/kg. (based on 3000 kg/day output and 320 days per year production). | Chilton and co-workers (2003) [152] |
| Bamboo was used to produce activated carbon. The cost of production was $1.93/kg. | Keith and co-workers (2005) [153] |
| Oil palm waste was used to produce activated carbon. For the zinc chloride activation process, the production cost was estimated at $3.24/kg. For the steam activation process, the production cost was $2.72/kg. The highest product cost was $8.60/kg AC through modification by magnesium oxide. | Jia and Lock, 2020 [154] |
| Sugarcane bagasse was used to prepare activated carbon. Cost of production was $3.12/kg (according to 1940 kg per day and 320 days per year production) | Ng and co-workers (2003) [155] |
| Walnut shell was used to synthesis activated carbon. The estimated cost of production was $1.83/kg (300 days/year of production and 2 labor forces per shift (two shifts) for 24 h/day) Production of activated carbon through carbonization (600 °C) and chemical activation process (KOH and phosphoric acid). | Zahra and co-workers (2019) [156] |
| The removal of color from fruit candy waste by using commercial activated carbon (Norit Darco 12 × 40, a lignite-based activated carbon). Estimated cost of decolorization process was reported as USD 19/ton for red colorant, USD 70/ton waste for yellow colorant, and USD 245/ton waste for blue fruit candy waste. | Ozsoy and co-workers (2010) [157] |
| Synthesis of flax-shive-based activated carbon through steam activation or phosphoric acid activation process. The percentage of yield of the activated carbon produced by chemical activation with phosphoric acid was 32%. The production cost was $7.10–8.90/kg. The percentage of yield of the activated carbon prepared by steam activation was 16%. The production cost was $1.50–4.30/kg. | Thomas and co-workers (2009) [158] |
| The sales prices for the generation (coconut shell as raw material), regeneration, and integrated plants were found to be 674.31, 514.66, and 536.66 USD/ton of product, respectively. Experimental findings showed that regeneration process of spent activated carbon obtained from petrochemical industries yielded more profits. | Tinnabhop and Wanwisa (2017) [159] |
| Description | References |
|---|---|
| The cost of waste baggase fly ash was $2 /ton (included transportation cost and rolling cost). The cost of finished product was $15 /ton (included transportation costs, chemicals, electrical energy, and labor fee). | Ahmaruzzaman, (2010) [160] |
| The cost of fly ash ranges from $15/ton to $40/ ton (majority of the cost was for transportation) | Sanchez (2014) [161] |
| Description | References |
|---|---|
| Total costs were recorded to be $1.3/kg based on the experimental results. The zeolite was produced from coal fly ash via the fusion method. For a 5000 kg/h raw material feed, a payback period of 7.1 years was feasible over 20-year operation time. Total equipment costs (fired heater, fusion reactor, cyclone, fluidized bed heat exchange tower, intermediate product storage, aging tank, hydrothermal reactor, pump, screw feeders, belt conveyor, multi-stage solids washers, rotary vacuum drum filter, rotary dryer, product storage, heat exchanger, forced draft fan, air compressor, stack) were $36,092,200. Overall results indicated that the utility prices for wastewater treatment were $83.79/1000 m3. | Jaime and co-workers (2017) [162] |
| This study was conducted in India. The Indian currency is called the Indian Rupee (Rs). Total project cost was Rs3.9crore with a plant capacity of 7500 kg/d. The profit margin was Rs5/kg and annual profits were Rs110.25lakh when the annual production was 2250 tons/year. The investment payback period was estimated to be 42 months. Overall results showed the cost of production for zeolite-A was Rs 18/kg, cheaper than commercial zeolite (Rs 23/kg). | Biniwale and co-workers (2001) [163] |
| Kaolin was used to produce zeolite. The zeolite 5A cost will be reduced (42 cents to 34 cents per pound) without heat integration. The product cost of the 67,000 metric tons/year plant was reduced to 22 cents/pound when the heat integration was included. | Ihab and Jian (2012) [164] |
| Zeolite of X-type was produced by using coal fly ash through alkali fusion and hydrothermal treatment. The cost was estimated to be cheaper than commercial 13X zeolite (almost one-fifth in the market). | Keka and co-workers (2004) [165] |
| Total cost for the production of NaA zeolite was found to be $0.003 and $1.16 by using microwave method and hydrothermal method, respectively. | Sapawe and co-workers (2013) [166] |
| Description | References |
|---|---|
| The cost of carbon nanotube is dependent on purity and supplier. The cost of multi-wall carbon nanotube is $0.5–100/g. The cost of single-wall carbon nanotube is $20–2000/g. | [167] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, S. Low-Cost Adsorbents for the Removal of Phenol/Phenolics, Pesticides, and Dyes from Wastewater Systems: A Review. Water 2022, 14, 3203. https://doi.org/10.3390/w14203203
Ho S. Low-Cost Adsorbents for the Removal of Phenol/Phenolics, Pesticides, and Dyes from Wastewater Systems: A Review. Water. 2022; 14(20):3203. https://doi.org/10.3390/w14203203
Chicago/Turabian StyleHo, Soonmin. 2022. "Low-Cost Adsorbents for the Removal of Phenol/Phenolics, Pesticides, and Dyes from Wastewater Systems: A Review" Water 14, no. 20: 3203. https://doi.org/10.3390/w14203203
APA StyleHo, S. (2022). Low-Cost Adsorbents for the Removal of Phenol/Phenolics, Pesticides, and Dyes from Wastewater Systems: A Review. Water, 14(20), 3203. https://doi.org/10.3390/w14203203






