Preparation of Magnetic Surface Ion-Imprinted Polymer Based on Functionalized Fe3O4 for Fast and Selective Adsorption of Cobalt Ions from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
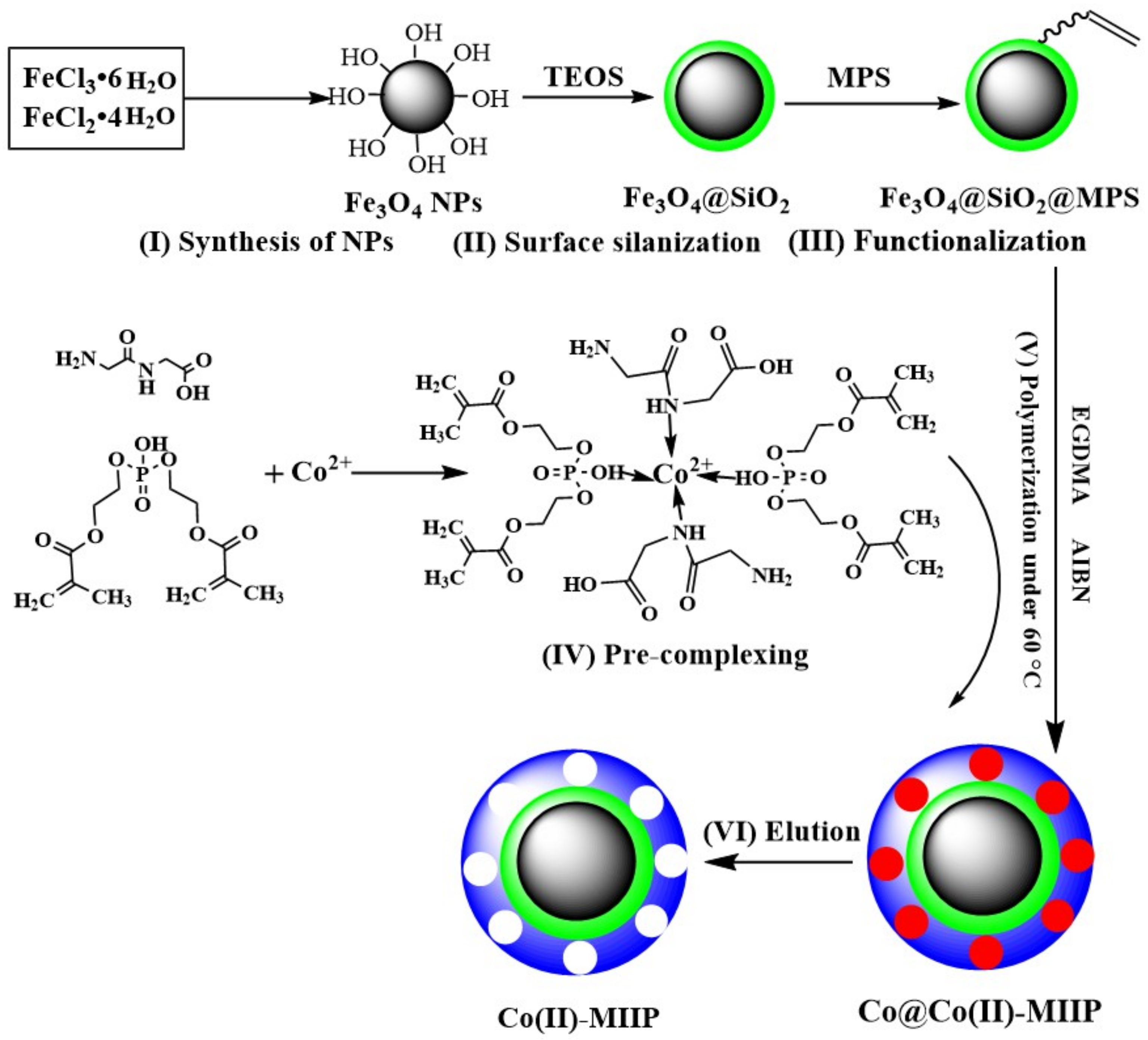
2.2. Synthesis of Fe3O4 NPs
2.3. Surface Silanization of Fe3O4 NPs
2.4. Functionalization of Fe3O4@SiO2
2.5. Synthesis of Co(II)-MIIP and Co(II)-NIP
2.6. Characterizations
2.7. Adsorption Experiments
2.8. Adsorption Selectivity
3. Results and Discussion
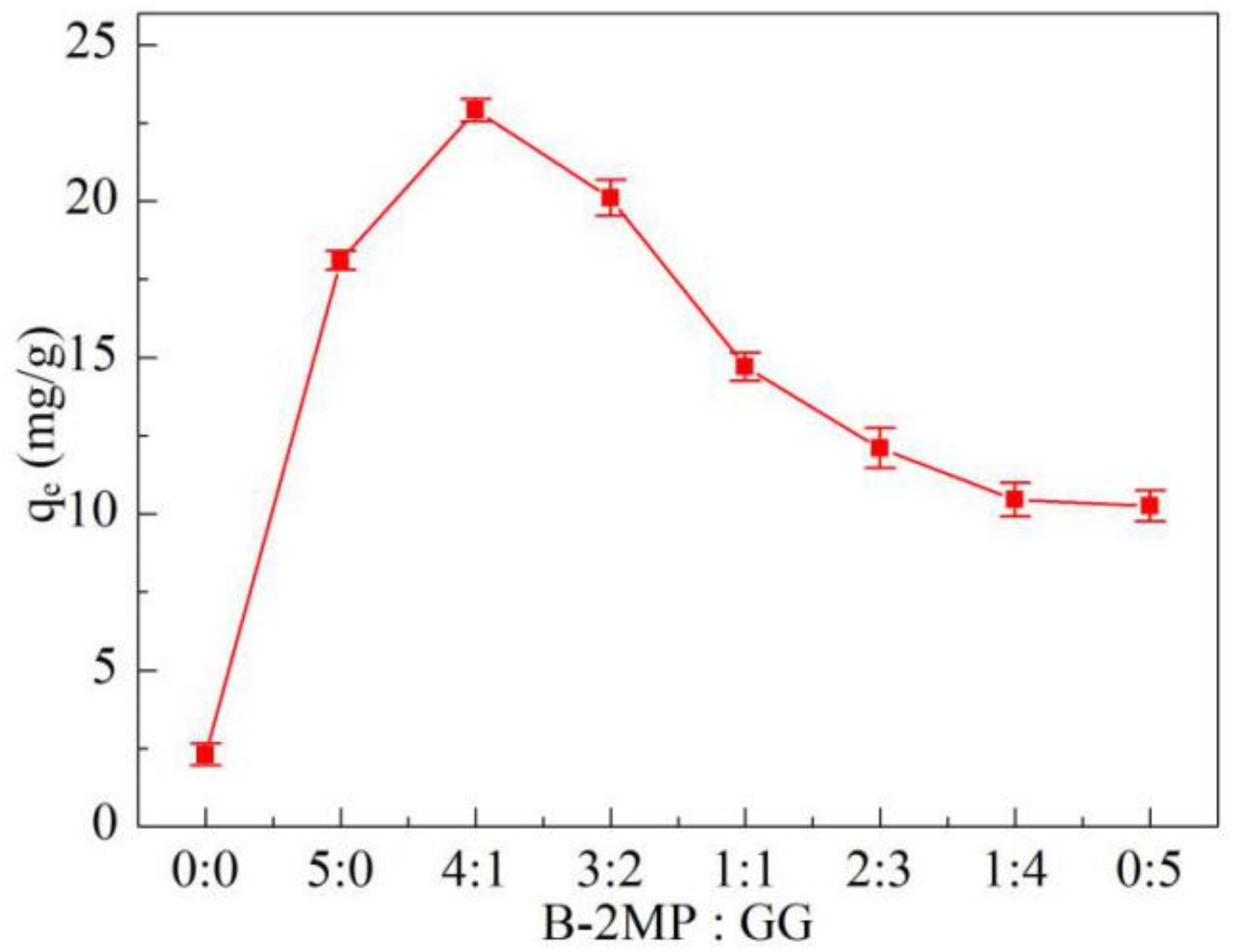
3.1. B-2MP and GG Dosage
3.2. The Amount of EGDMA
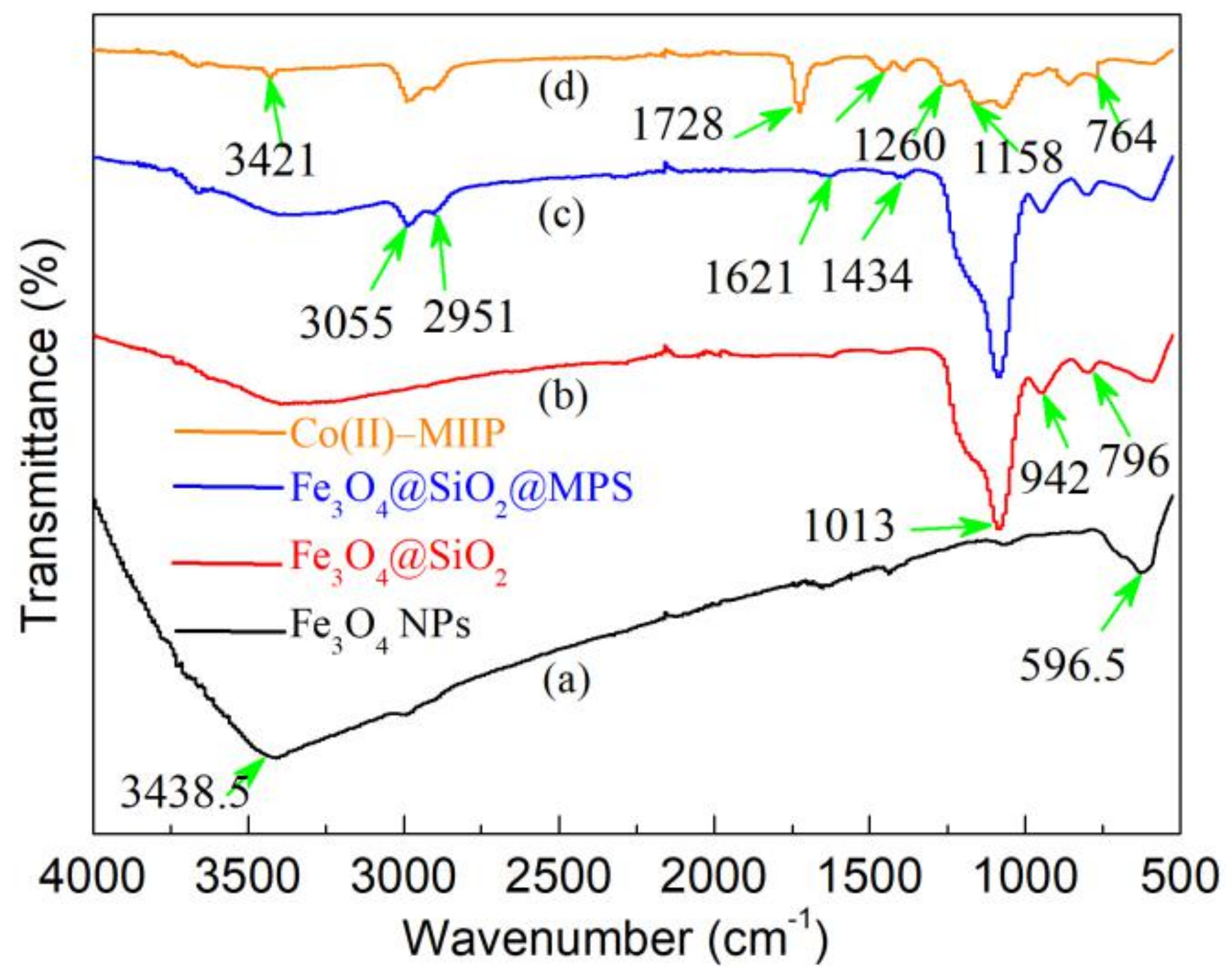
3.3. FT-IR
3.4. TGA
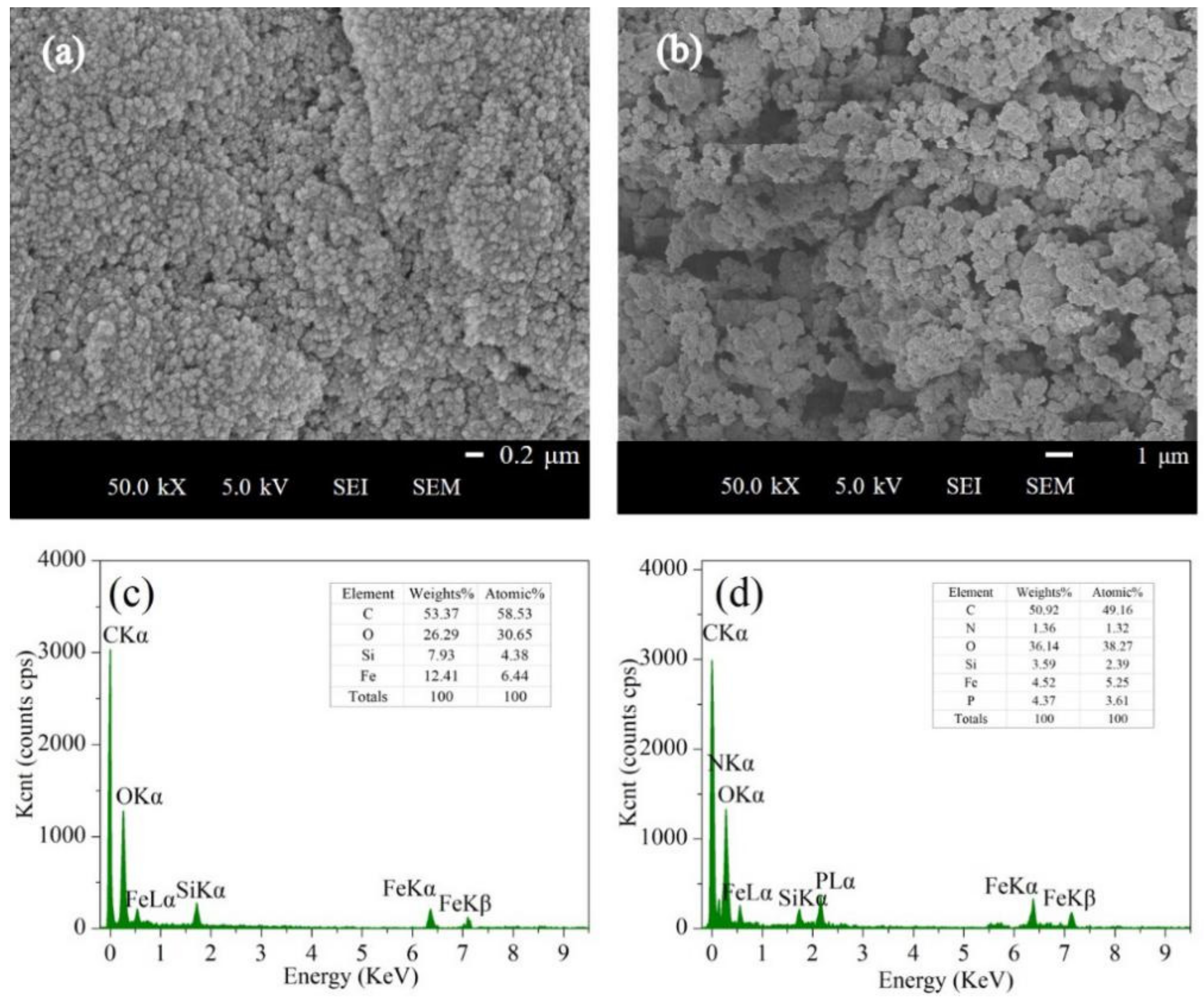
3.5. SEM and EDS
3.6. BET
3.7. VSM
3.8. XRD
3.9. Effect of Solution pH
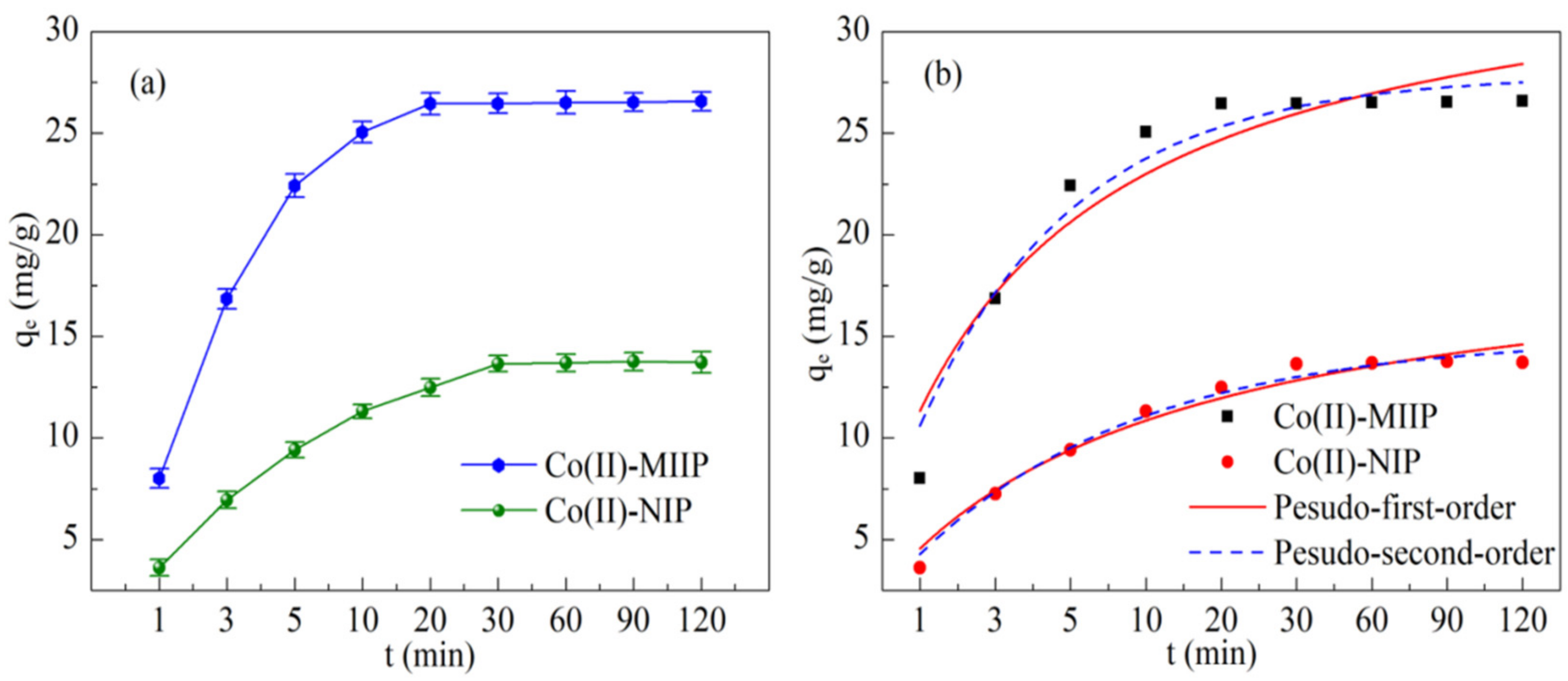
3.10. Adsorption Kinetics
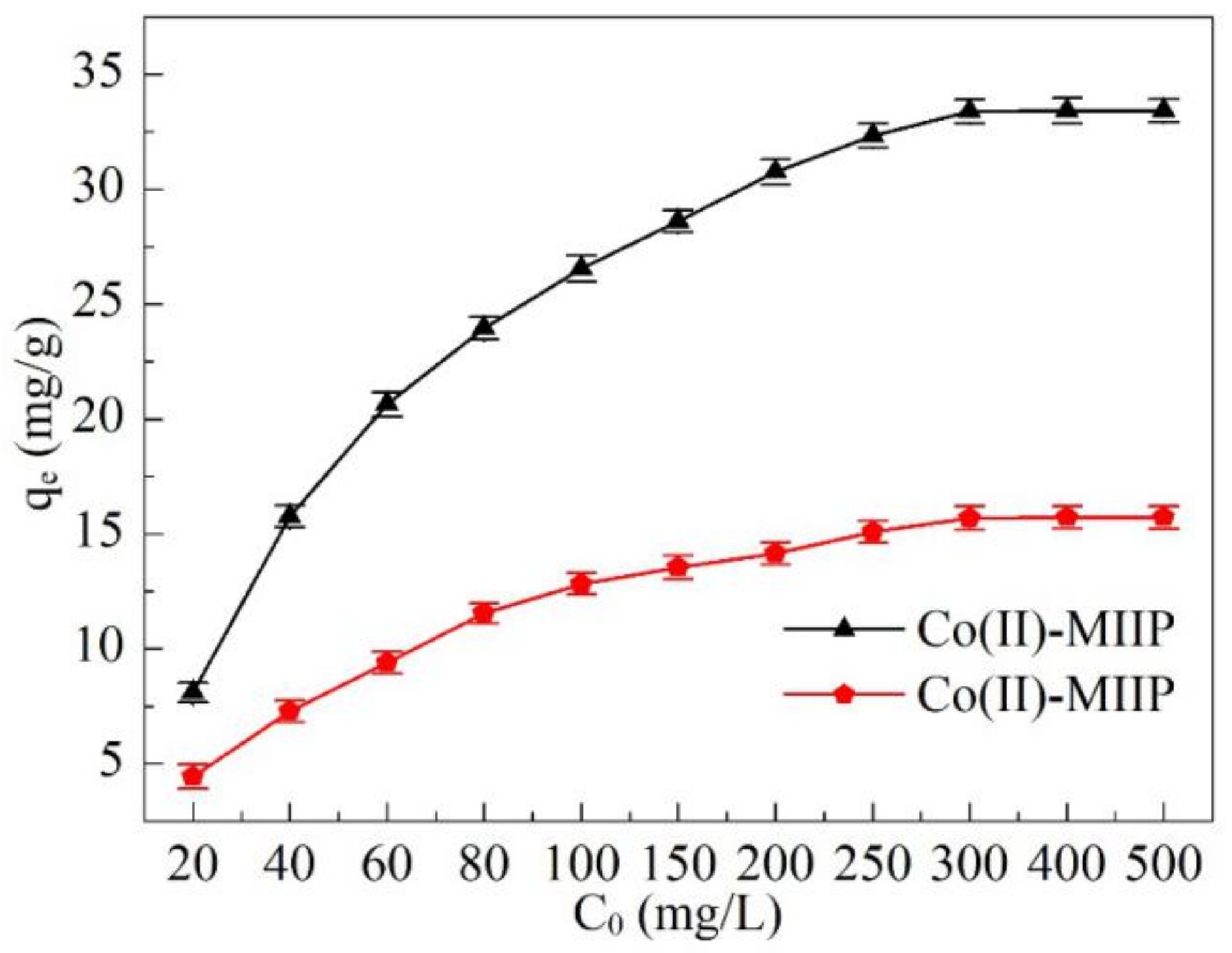
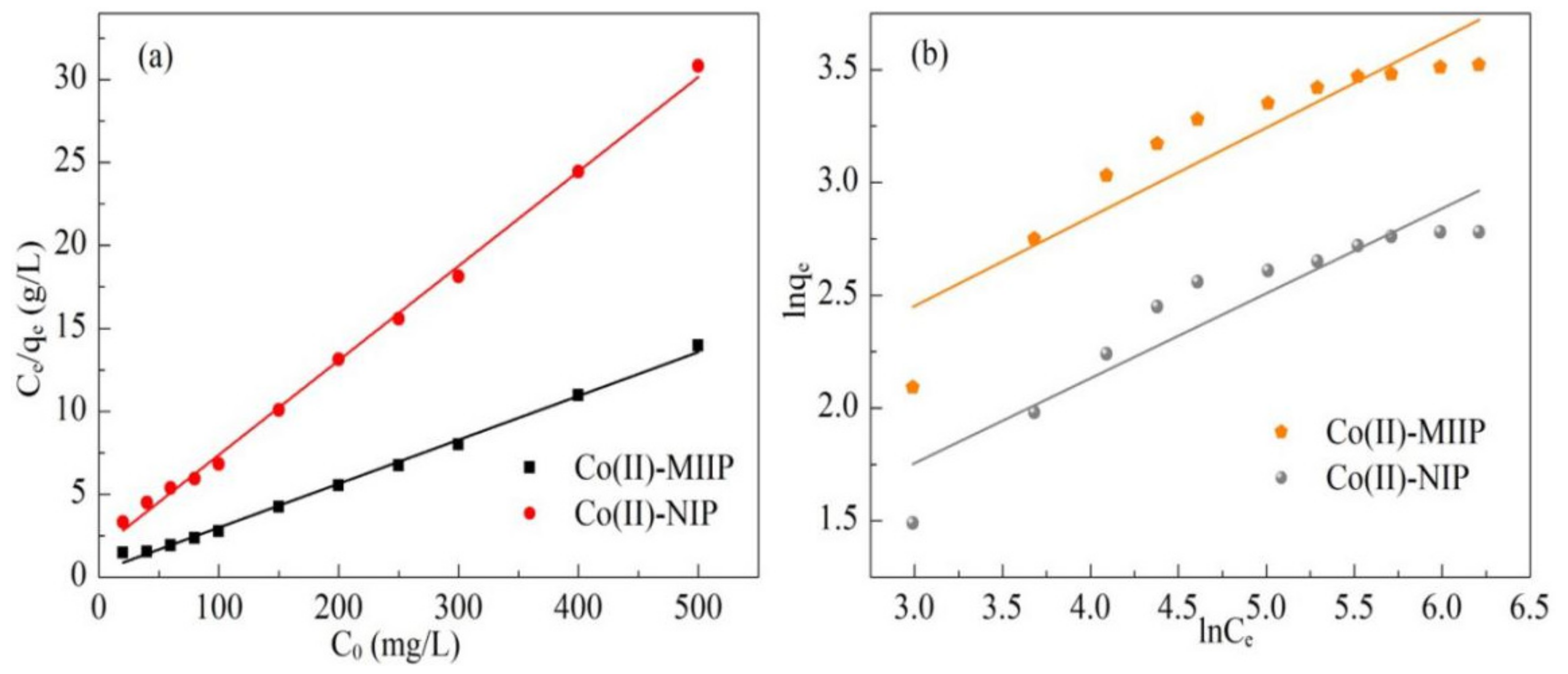
3.11. Adsorption Isotherm
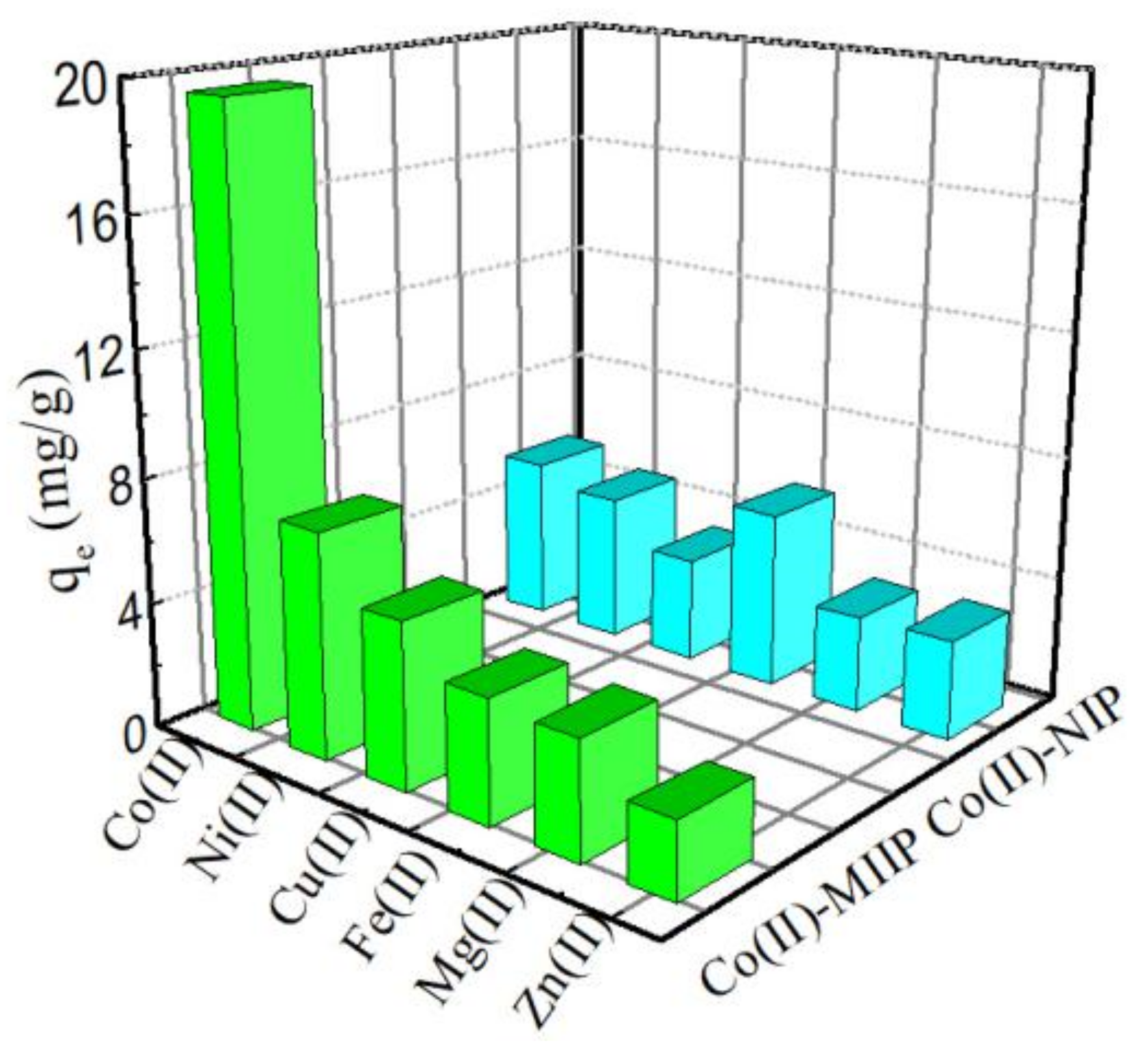
3.12. Adsorption Selectivity
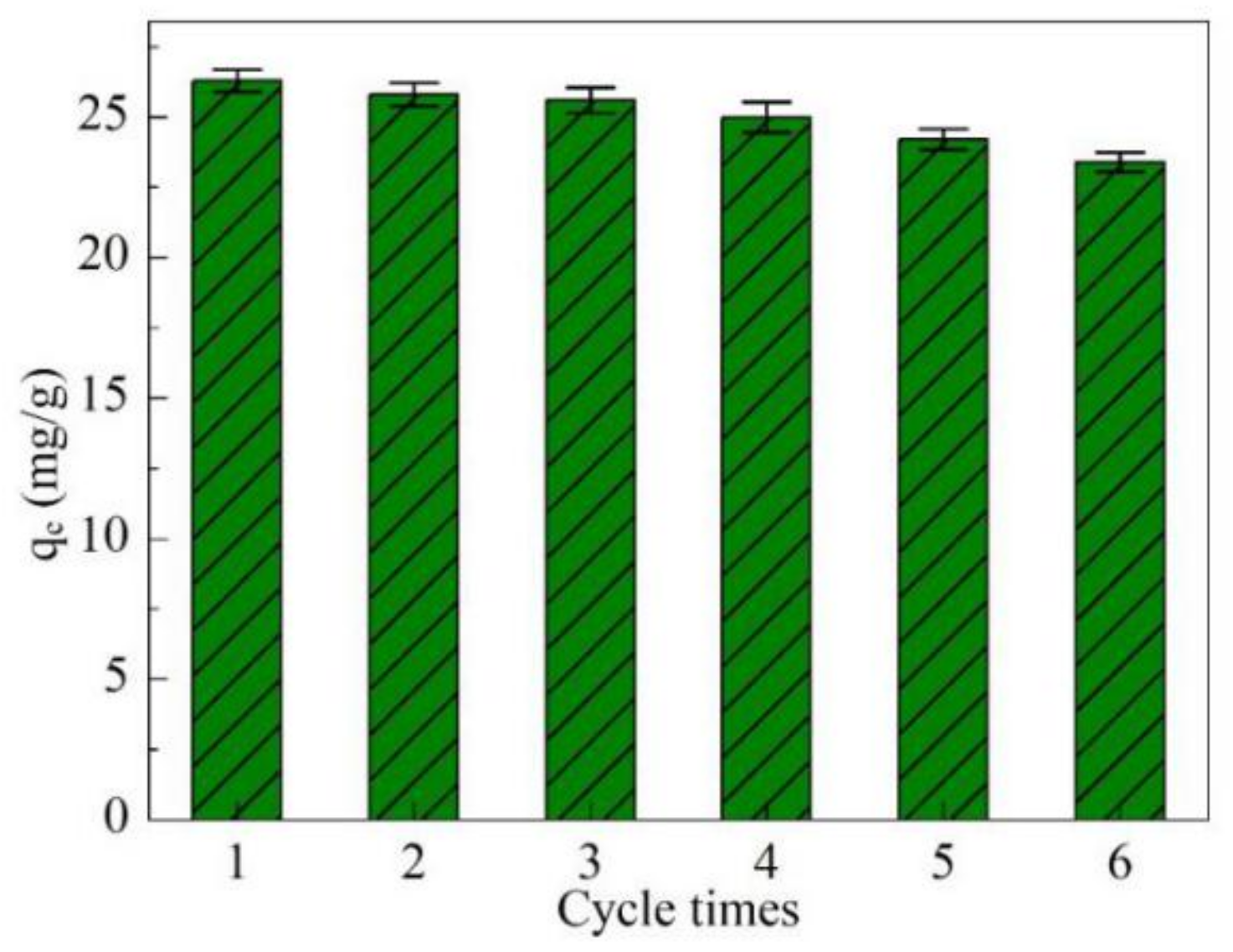
3.13. Reusability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dehaine, Q.; Tijsseling, L.T.; Glass, H.J.; Törmänen, T.; Butcher, A.R. Geometallurgy of cobalt ores: A review. Miner. Eng. 2021, 160, 106656. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, G.; Liu, Z.; Meng, M.; Jiang, Y.; Ni, L.; Guo, W.; Liu, F. Preparation of core-shell ion imprinted nanoparticles via photoinitiated polymerization at ambient temperature for dynamic removal of cobalt in aqueous solution. RSC Adv. 2015, 5, 85691–85704. [Google Scholar] [CrossRef]
- Khoddami, N.; Shemirani, F. A new magnetic ion-imprinted polymer as a highly selective sorbent for determination of cobalt in biological and environmental samples. Talanta 2016, 146, 244–252. [Google Scholar] [CrossRef]
- Torkashvand, M.; Gholivand, M.B.; Azizi, R. Synthesis, characterization and application of a novel ion-imprinted polymer based voltammetric sensor for selective extraction and trace determination of cobalt (II) ions. Sens. Actuat. B-Chem. 2017, 243, 283–291. [Google Scholar] [CrossRef]
- Biswal, B.K.; Jadhav, U.U.; Madhaiyan, M.; Ji, L.; Yang, E.; Cao, B. Biological leaching and chemical precipitation methods for recovery of Co and Li from spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 12343–12352. [Google Scholar] [CrossRef]
- Ren, J.; Li, R.; Liu, Y.; Cheng, Y.; Mu, D.; Zheng, R.; Liu, J.; Dai, C. The impact of aluminum impurity on the regenerated lithium nickel cobalt manganese oxide cathode materials from spent LIBs. New J. Chem. 2017, 41, 10959–10965. [Google Scholar] [CrossRef]
- Alkhadra, M.A.; Conforti, K.M.; Gao, T.; Tian, H.; Bazant, M.Z. Continuous separation of radionuclides from contaminated water by shock electrodialysis. Environ. Sci. Technol. 2020, 54, 527–536. [Google Scholar] [CrossRef]
- Tortora, F.; Innocenzi, V.; De Michelis, I.; Vegliò, F.; di Celso, G.M.; Prisciandaro, M. Recovery of anionic surfactant through acidification/ultrafiltration in a micellar-enhanced ultrafiltration process for Cobalt removal. Environ. Eng. Sci. 2018, 35, 493–500. [Google Scholar] [CrossRef]
- Vivas, E.L.; Cho, K. Efficient adsorptive removal of Cobalt(II) ions from water by dicalcium phosphate dihydrate. J. Environ. Manag. 2021, 283, 111990. [Google Scholar] [CrossRef]
- Saleh, T.A.; Musa, A.M.; Tawabini, B.; Ali, S.A. Aminomethylphosphonate chelating ligand and octadecyl alkyl chain in a resin for simultaneous removal of Co(II) ions and organic contaminants. J. Chem. Eng. Data 2016, 61, 3377–3385. [Google Scholar] [CrossRef]
- Hossein Beyki, M.; Shemirani, F.; Shirkhodaie, M. Aqueous Co(II) adsorption using 8-hydroxyquinoline anchored γ-Fe2O3@chitosan with Co(II) as imprinted ions. Int. J. Biol. Macromol. 2016, 87, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.F.; Mehamod, F.S.; Mohd Suah, F.B. Fabrication and binding characterization of ion imprinted polymers for highly selective Co2+ ions in an aqueous medium. J. Environ. Chem. Eng. 2019, 7, 103007. [Google Scholar] [CrossRef]
- Şarkaya, K.; Demir, A. The comparative investigation on synthesis, characterizations of silver ion-imprinting and non-imprinting cryogels, their impedance spectroscopies and relaxation mechanisms. Polym. Bull. 2019, 76, 5701–5716. [Google Scholar] [CrossRef]
- Chaipuang, A.; Phungpanya, C.; Thongpoon, C.; Watla-Iad, K.; Inkaew, P.; Machan, T.; Suwantong, O. Synthesis of copper(II) ion-imprinted polymers via suspension polymerization. Polym. Adv. Technol. 2018, 29, 3134–3141. [Google Scholar] [CrossRef]
- Huang, R.; Ma, X.; Li, X.; Guo, L.; Xie, X.; Zhang, M.; Li, J. A novel ion-imprinted polymer based on graphene oxide-mesoporous silica nanosheet for fast and efficient removal of chromium (VI) from aqueous solution. J. Colloid Interf. Sci. 2018, 514, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, J.; Zhou, X.; Fei, X.; Wang, Y.; Zhou, Y.; Zhong, L.; Han, X. Adsorption of molybdate on molybdate-imprinted chitosan/triethanolamine gel beads. Carbohyd. Polym. 2014, 114, 514–520. [Google Scholar] [CrossRef]
- Lee, H.; Choi, J.; Choi, S. Magnetic ion-imprinted polymer based on mesoporous silica for selective removal of Co(II) from radioactive wastewater. Sep. Sci. Technol. 2021, 56, 1842–1852. [Google Scholar] [CrossRef]
- Wang, R.; Lin, J.; Huang, S.; Wang, Q.; Hu, Q.; Peng, S.; Wu, L.; Zhou, Q. Disulfide cross-linked poly(methacrylic acid) iron oxide nanoparticles for efficiently selective adsorption of Pb(II) from aqueous solutions. ACS Omega 2021, 6, 976–987. [Google Scholar] [CrossRef]
- Dahaghin, Z.; Mousavi, H.Z.; Boutorabi, L. Application of magnetic ion-imprinted polymer as a new environmentally-friendly nonocomposite for a selective adsorption of the trace level of Cu(II) from aqueous solution and different samples. J. Mol. Liq. 2017, 243, 380–386. [Google Scholar] [CrossRef]
- Wu, L.; Luo, Z.; Jiang, H.; Zhao, Z.; Geng, W. Selective and rapid removal of Mo(VI) from water using functionalized Fe3O4-based Mo(VI) ion-imprinted polymer. Water Sci. Technol. 2021, 83, 435–448. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, S.; Zhou, Y.; Dong, P.; Hua, D. Synthesis of surface ion-imprinted magnetic microspheres by locating polymerization for rapid and selective separation of uranium (VI). RSC Adv. 2015, 5, 4153–4161. [Google Scholar] [CrossRef]
- Zhao, B.; He, M.; Chen, B.; Hu, B. Fe3O4 nanoparticles coated with double imprinted polymers for magnetic solid phase extraction of lead(II) from biological and environmental samples. Microchim. Acta 2019, 186, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Liu, L.; Deng, F.; Luo, S. Novel ion-imprinted polymer using crown ether as a functional monomer for selective removal of Pb(II) ions in real environmental water samples. J. Mater. Chem. A 2013, 1, 8280–8286. [Google Scholar] [CrossRef]
- Wang, H.; Shang, H.; Sun, X.; Hou, L.; Wen, M.; Qiao, Y. Preparation of thermo-sensitive surface ion-imprinted polymers based on multi-walled carbon nanotube composites for selective adsorption of lead(II) ion. Colloid Surface A 2020, 585, 124139. [Google Scholar] [CrossRef]
- Yuan, G.; Tu, H.; Liu, J.; Zhao, C.; Liao, J.; Yang, Y.; Yang, J.; Liu, N. A novel ion-imprinted polymer induced by the glycylglycine modified metal-organic framework for the selective removal of Co(II) from aqueous solutions. Chem. Eng. J. 2018, 333, 280–288. [Google Scholar] [CrossRef]
- Kong, D.; Wang, N.; Qiao, N.; Wang, Q.; Wang, Z.; Zhou, Z.; Ren, Z. Facile preparation of ion-imprinted chitosan microspheres enwrapping Fe3O4 and graphene oxide by inverse suspension cross-linking for highly selective removal of copper(II). ACS Sustain. Chem. Eng. 2017, 5, 7401–7409. [Google Scholar] [CrossRef]
- Xie, C.; Huang, X.; Wei, S.; Xiao, C.; Cao, J.; Wang, Z. Novel dual-template magnetic ion imprinted polymer for separation and analysis of Cd2+ and Pb2+ in soil and food. J. Clean. Prod. 2020, 262, 121387. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Zhang, M.; Jiao, J.; Zhang, H.; Du, J.; Zhang, B.; Ren, Z. Preparation of highly efficient ion-imprinted polymers with Fe3O4 nanoparticles as carrier for removal of Cr(VI) from aqueous solution. Sci. Total Environ. 2020, 699, 134334. [Google Scholar] [CrossRef]
- Guo, H.; Tang, Y.; Yu, Y.; Xue, L.; Qian, J. Covalent immobilization of α-amylase on magnetic particles as catalyst for hydrolysis of high-amylose starch. Int. J. Biol. Macromol. 2016, 87, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, H.; Yang, X.; Li, L. Synthesis of tri-layer hybrid microspheres with magnetic core and functional polymer shell. Eur. Polym. J. 2009, 45, 2023–2032. [Google Scholar] [CrossRef]
- Zhu, G.; Tang, H.; Qing, P.; Zhang, H.; Cheng, X.; Cai, Z.; Xu, H.; Zhang, Y. A monophosphonic group-functionalized ion-imprinted polymer for a removal of Fe3+ from highly concentrated basic chromium sulfate solution. Korean J. Chem. Eng. 2020, 37, 911–920. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Dib, S.S. Utilization of nano-olive stones in environmental remediation of methylene blue from water. J. Environ. Health Sci. 2020, 18, 63–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, B.; Zhu, Y.; Ao, H.; Qi, C.; Chen, F. Synthesis and characterization of magnetic iron oxide/calcium silicate mesoporous nanocomposites as a promising vehicle for drug delivery. ACS Appl. Mater. Inter. 2012, 4, 6969–6974. [Google Scholar] [CrossRef]
- Akbarnejad, S.; Amooey, A.A.; Ghasemi, S. High effective adsorption of acid fuchsin dye using magnetic biodegradable polymer-based nanocomposite from aqueous solutions. Microchem. J. 2019, 149, 103966. [Google Scholar] [CrossRef]
- Hassan, S.H.; Kamel, A.; Hassan, A.A.; Amr, A.; Abd El-Naby, H.; Al-Omar, M.; Sayed, A. CuFe2O4/polyaniline (PANI) nanocomposite for the hazard mercuric ion removal: Synthesis, characterization, and adsorption properties study. Molecules 2020, 25, 2721. [Google Scholar] [CrossRef]
- Tan, P.; Jiang, Y.; Liu, X.; Sun, L. Magnetically responsive porous materials for efficient adsorption and desorption processes. Chin. J. Chem. Eng. 2019, 27, 1324–1338. [Google Scholar] [CrossRef]
- Fayazi, M.; Taher, M.A.; Afzali, D.; Mostafavi, A.; Ghanei-Motlagh, M. Synthesis and application of novel ion-imprinted polymer coated magnetic multi-walled carbon nanotubes for selective solid phase extraction of lead(II) ions. Mater. Sci. Eng. C 2016, 60, 365–373. [Google Scholar] [CrossRef]
- Shafizadeh, F.; Taghizadeh, M.; Hassanpour, S. Preparation of a novel magnetic Pd(II) ion-imprinted polymer for the fast and selective adsorption of palladium ions from aqueous solutions. Environ. Sci. Pollut. R 2019, 26, 18493–18508. [Google Scholar] [CrossRef] [PubMed]
- Adibmehr, Z.; Faghihian, H. Preparation of highly selective magnetic cobalt ion-imprinted polymer based on functionalized SBA-15 for removal Co2+ from aqueous solutions. J. Environ. Health Sci. 2019, 17, 1213–1225. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Wang, J.; Guo, X.; Wang, X.; Choo, J.; Chen, L. Green multi-functional monomer based ion imprinted polymers for selective removal of copper ions from aqueous solution. J. Colloid Interf. Sci. 2019, 541, 376–386. [Google Scholar] [CrossRef]
- Velempini, T.; Pillay, K.; Mbianda, X.Y.; Arotiba, O.A. Carboxymethyl cellulose thiol-imprinted polymers: Synthesis, characterization and selective Hg(II) adsorption. J. Environ. Sci. China 2019, 79, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Moussa, S.I.; Ali, M.M.S.; Sheha, R.R. The performance of activated carbon/NiFe2O4 magnetic composite to retain heavy metal ions from aqueous solution. Chin. J. Chem. Eng. 2021, 29, 135–145. [Google Scholar] [CrossRef]
- Neolaka, Y.A.B.; Lawa, Y.; Naat, J.N.; Pau Riwu, A.A.; Darmokoesoemo, H.; Supriyanto, G.; Holdsworth, C.I.; Amenaghawon, A.N.; Kusuma, H.S. A Cr(VI)-imprinted-poly(4-VP-co-EGDMA) sorbent prepared using precipitation polymerization and its application for selective adsorptive removal and solid phase extraction of Cr(VI) ions from electroplating industrial wastewater. Reat. Funct. Polym. 2020, 147, 104451. [Google Scholar] [CrossRef]
- Li, M.; Feng, J.; Huang, K.; Tang, S.; Liu, R.; Li, H.; Ma, F.; Meng, X. Amino group functionalized SiO2@graphene oxide for efficient removal of Cu(II) from aqueous solutions. Chem. Eng. Res. Des. 2019, 145, 235–244. [Google Scholar] [CrossRef]
- Kenawy, I.M.; Ismail, M.A.; Hafez, M.A.H.; Hashem, M.A. Synthesis and characterization of novel ion-imprinted guanyl-modified cellulose for selective extraction of copper ions from geological and municipality sample. Int. J. Biol. Macromol. 2018, 115, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Al Abri, A.M.; Mohamad, S.; Abdul Halim, S.N.; Abu Bakar, N.K. Development of magnetic porous coordination polymer adsorbent for the removal and preconcentration of Pb(II) from environmental water samples. Environ. Sci. Pollut. R 2019, 26, 11410–11426. [Google Scholar] [CrossRef]
- Nchoe, O.B.; Klink, M.J.; Mtunzi, F.M.; Pakade, V.E. Synthesis, characterization, and application of β-cyclodextrin-based ion-imprinted polymer for selective sequestration of Cr(VI) ions from aqueous media: Kinetics and isotherm studies. J. Mol. Liq. 2020, 298, 111991. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Tapaswi, P.K.; Park, S.S.; Mathew, A.; Cho, H.; Ha, C. Ion-imprinted mesoporous silica hybrids for selective recognition of target metal ions. Micropor. Mesopor. Mat. 2013, 180, 162–171. [Google Scholar] [CrossRef]
- Le, Q.T.N.; Vivas, E.L.; Cho, K. Oxalated blast-furnace slag for the removal of Cobalt(II) ions from aqueous solutions. J. Ind. Eng. Chem. 2021, 95, 57–65. [Google Scholar] [CrossRef]


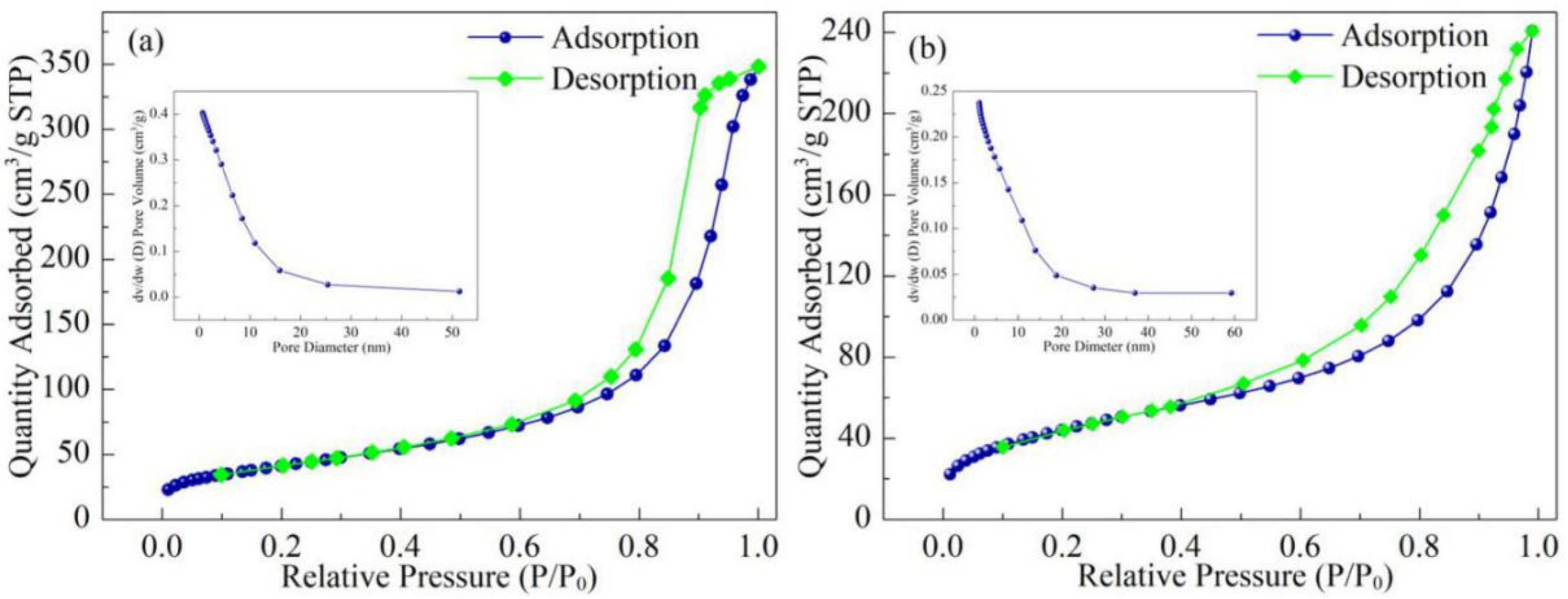
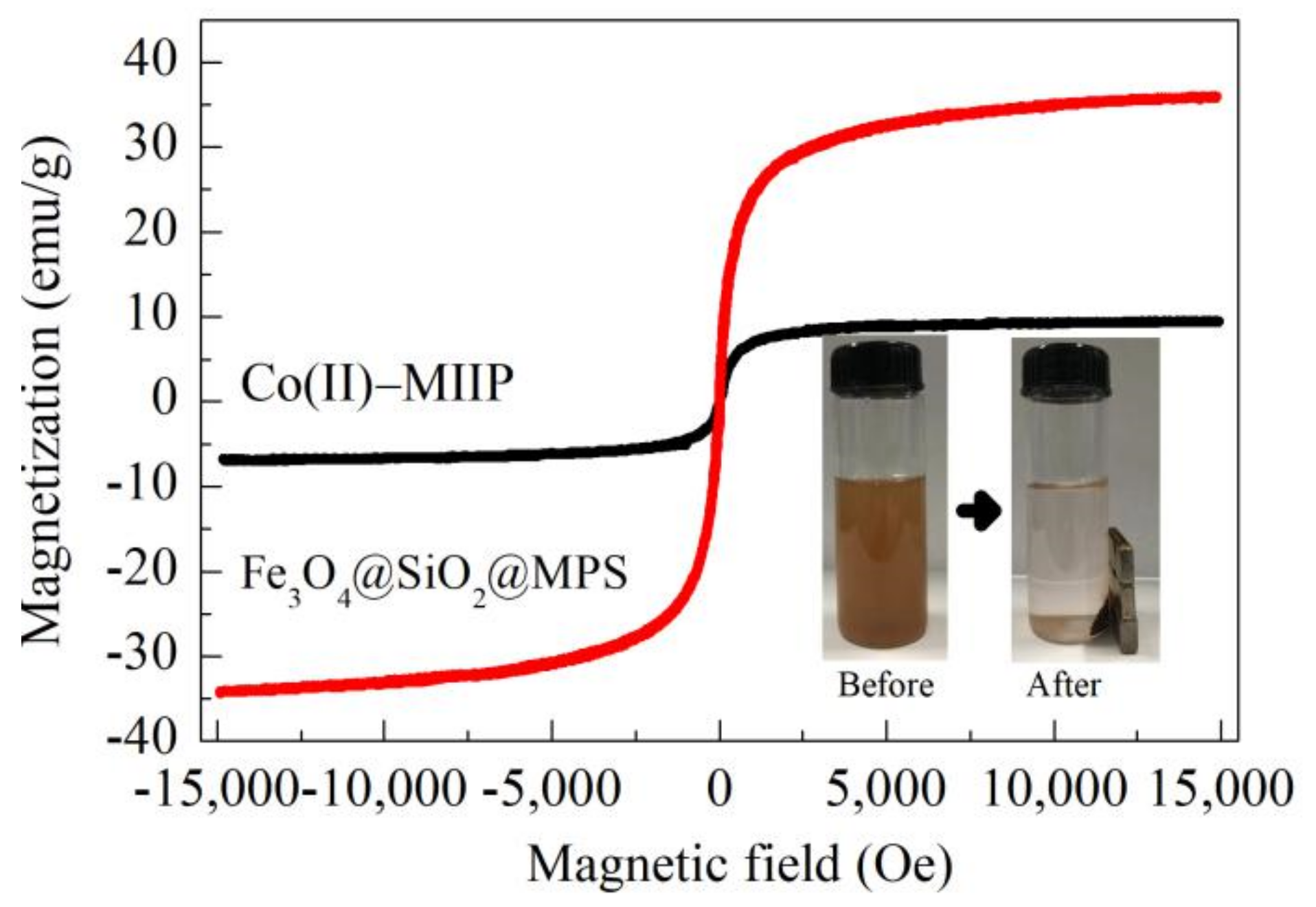
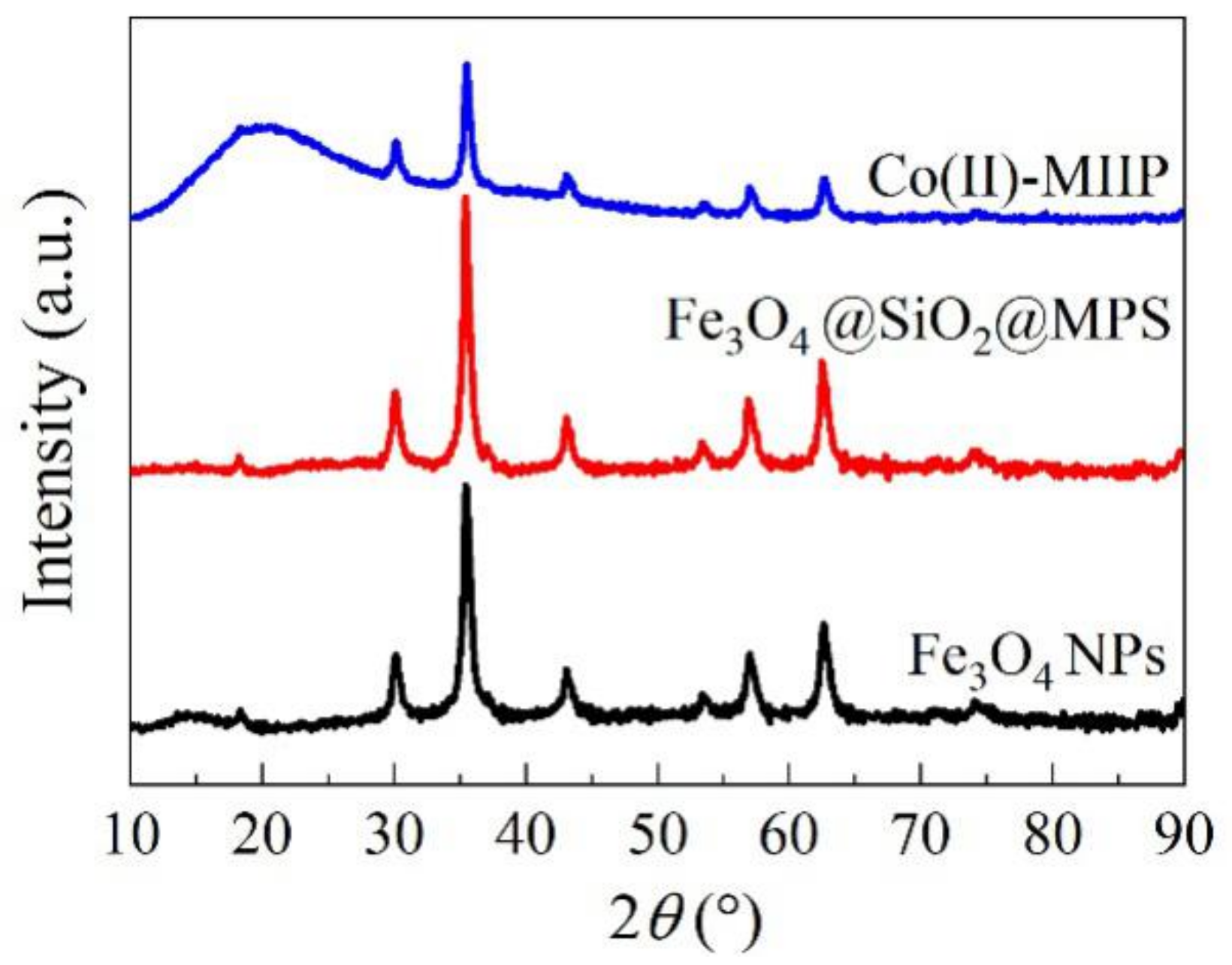
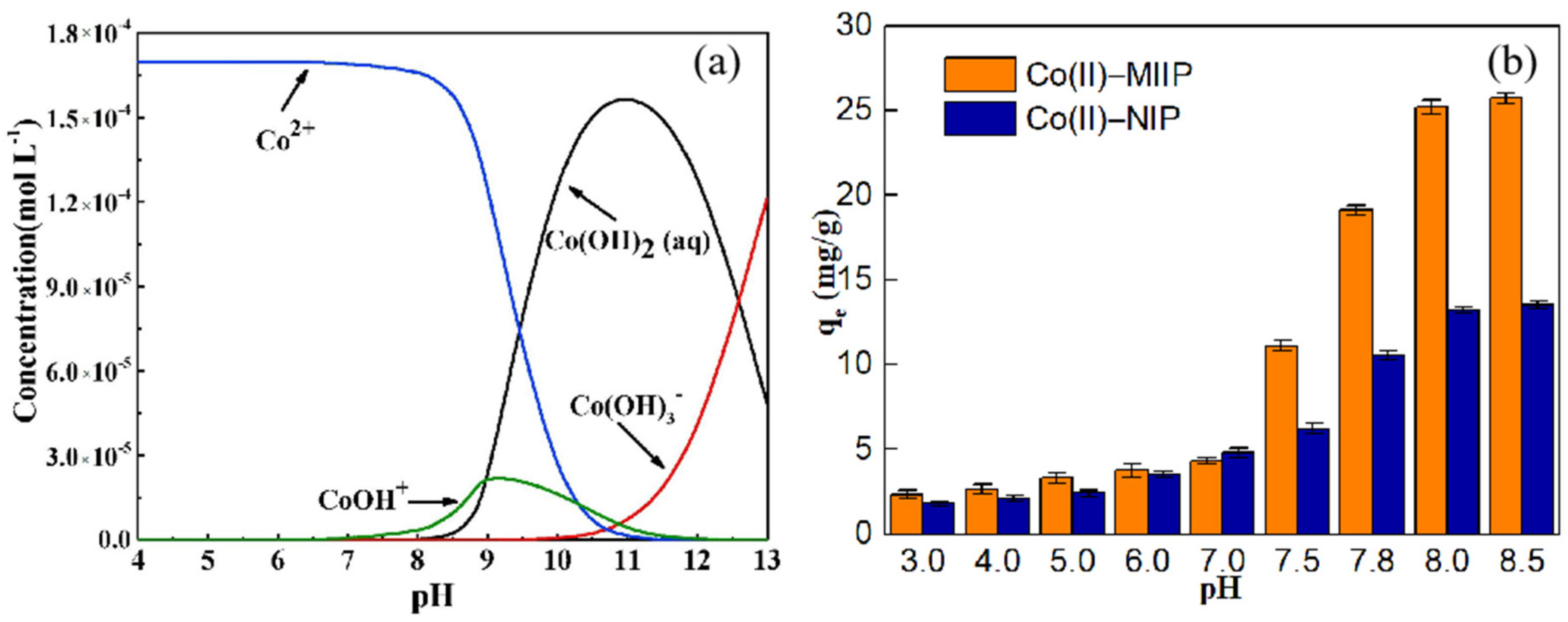
| Samples | Average Pore Diameter (nm) | Total Pore Volume (cm3·g−1) | Specific Surface Area (m2·g−1) |
|---|---|---|---|
| Fe3O4@SiO2@MPS | 10.37 | 0.4028 | 126.82 |
| Co(II)-MIIP | 9.31 | 0.2375 | 74.94 |
| qe, experimemt (mg·g−1) | Pseudo-First-Order | Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|---|
| k1 (1·min−1) | qe (mg·g−1) | R2 | k2 (g·mg−1·min−1) | qe (mg·g−1) | h0 | R2 | ||
| Co(II)-MIIP | 26.53 | 0.0119 | 35.43 | 0.906 | 0.433 | 27.85 | 1.78 | 0.955 |
| Co(II)-NIP | 13.72 | 0.0142 | 20.39 | 0.969 | 0.332 | 15.03 | 1.17 | 0.986 |
| Adsorbents | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm, experiment (mg·g−1) | KL (L·mg−1) | R2 | n | KF (mg·g−1) | R2 | |
| Co(II)-MIIP | 37.81 | 0.076 | 0.995 | 1.26 | 14.93 | 0.839 |
| Co(II)-NIP | 17.54 | 0.034 | 0.997 | 1.59 | 12.68 | 0.865 |
| Ions | Co(II)-MIIP | Co(II)-NIP | K′ | ||||
|---|---|---|---|---|---|---|---|
| q (mg·g−1) | Kd | K | q (mg·g−1) | Kd | K | ||
| Co(II) | 20.8 | 0.263 | 6.41 | 7.7 | 0.083 | 1.22 | 5.25 |
| Fe(II) | 3.9 | 0.041 | - | 6.4 | 0.068 | - | - |
| Co(II) | 22.4 | 0.29 | 5.15 | 9.2 | 0.101 | 1.27 | 4.05 |
| Cu(II) | 5.3 | 0.056 | - | 7.4 | 0.08 | - | - |
| Co(II) | 21.6 | 0.285 | 7.37 | 8.4 | 0.092 | 1.21 | 6.09 |
| Mg(II) | 3.7 | 0.038 | - | 7.1 | 0.076 | - | - |
| Co(II) | 24.5 | 0.263 | 10.42 | 7.3 | 0.079 | 0.87 | 11.82 |
| Zn(II) | 2.4 | 0.025 | - | 8.2 | 0.088 | - | - |
| Co(II) | 19.3 | 0.248 | 3.64 | 6.5 | 0.069 | 0.81 | 4.48 |
| Ni(II) | 6.2 | 0.066 | - | 7.8 | 0.085 | - | - |
| Adsorbents | Capacity (mg·g−1) | Equilibrium Time (min) | Cycles | Selectivity | Ref. |
|---|---|---|---|---|---|
| DCPD | 441 | 1440 | - | No | [9] |
| IIP-MAA | 15.38 | 20 | 5 | Poor | [12] |
| UiO-66-NH2 | 175.0 | 1200 | 5 | Yes | [25] |
| P-IIPs | 96.65 | 60 | 3 | Yes | [2] |
| IIPMO | 109.6098 | 30 | 5 | Yes | [48] |
| Slag-Ox | 576 | 120 | 3 | No | [49] |
| Co(II)-MIIP | 33.4 | 20 | 6 | Yes | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Jiang, H.; Wu, L.; Yu, N.; Luo, Z.; Geng, W. Preparation of Magnetic Surface Ion-Imprinted Polymer Based on Functionalized Fe3O4 for Fast and Selective Adsorption of Cobalt Ions from Water. Water 2022, 14, 261. https://doi.org/10.3390/w14020261
Zhao Z, Jiang H, Wu L, Yu N, Luo Z, Geng W. Preparation of Magnetic Surface Ion-Imprinted Polymer Based on Functionalized Fe3O4 for Fast and Selective Adsorption of Cobalt Ions from Water. Water. 2022; 14(2):261. https://doi.org/10.3390/w14020261
Chicago/Turabian StyleZhao, Zijian, Hui Jiang, Lang Wu, Ning Yu, Zhengwei Luo, and Wenhua Geng. 2022. "Preparation of Magnetic Surface Ion-Imprinted Polymer Based on Functionalized Fe3O4 for Fast and Selective Adsorption of Cobalt Ions from Water" Water 14, no. 2: 261. https://doi.org/10.3390/w14020261
APA StyleZhao, Z., Jiang, H., Wu, L., Yu, N., Luo, Z., & Geng, W. (2022). Preparation of Magnetic Surface Ion-Imprinted Polymer Based on Functionalized Fe3O4 for Fast and Selective Adsorption of Cobalt Ions from Water. Water, 14(2), 261. https://doi.org/10.3390/w14020261





