Achieving Partial Nitritation in Anammox Start-Up Environment
Abstract
:1. Introduction
2. Materials and Methods
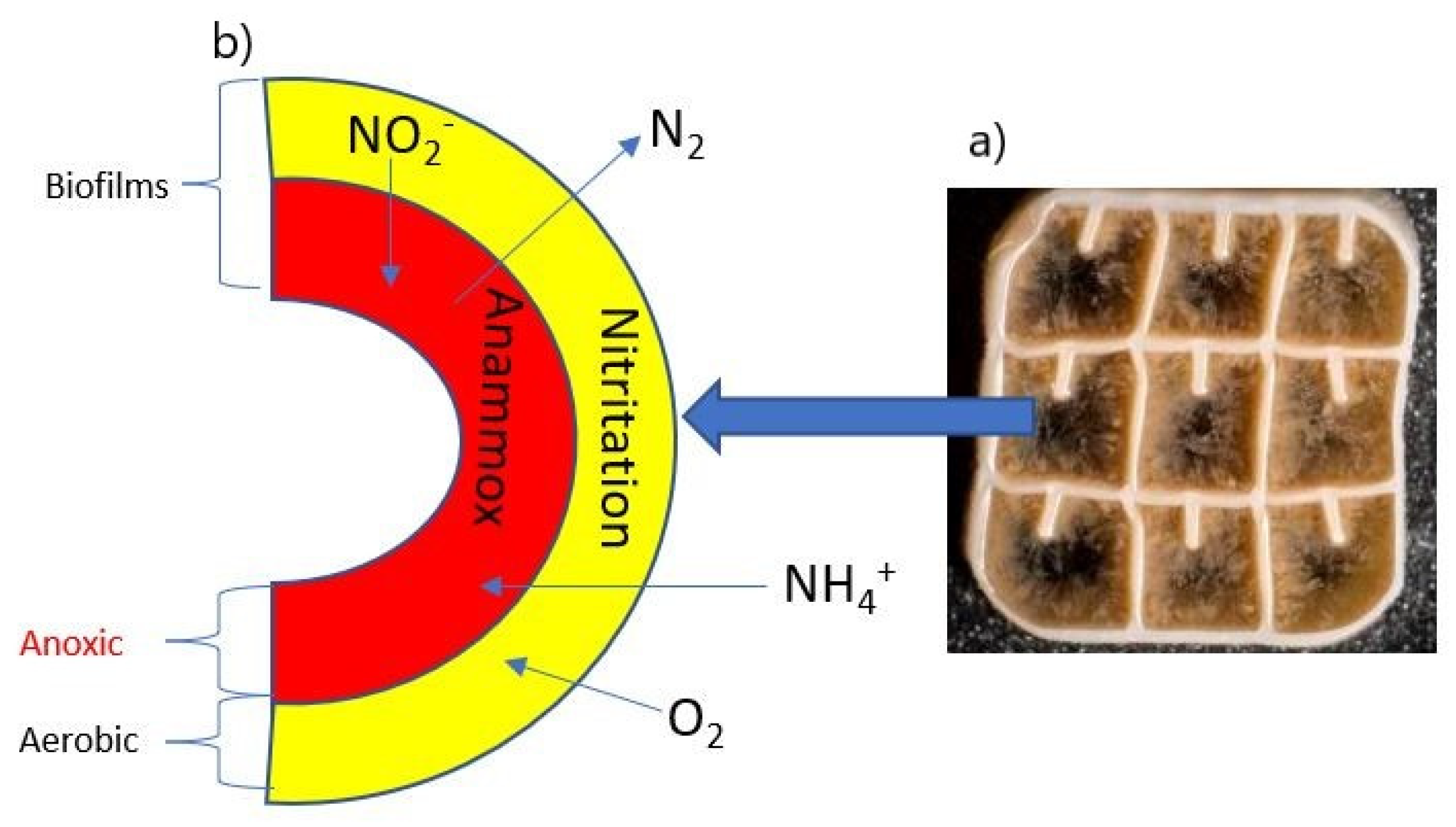
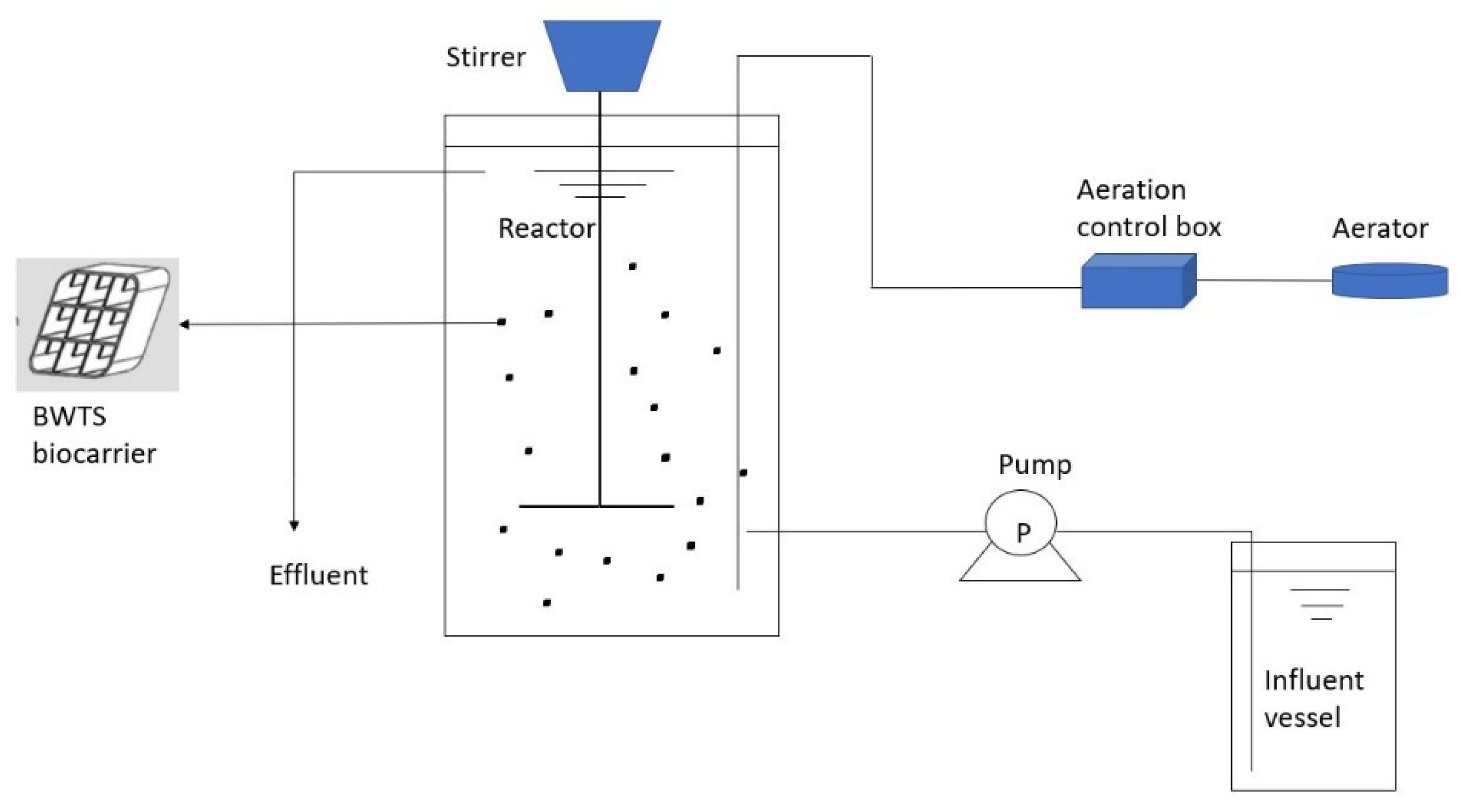
2.1. Experimental Setup of Moving Bed Biofilm Reactor
2.2. Operating Conditions
2.3. Synthetic Wastewater
2.4. Wastewater Constituients Analysis
2.5. Data Analysis
3. Results and Discussion
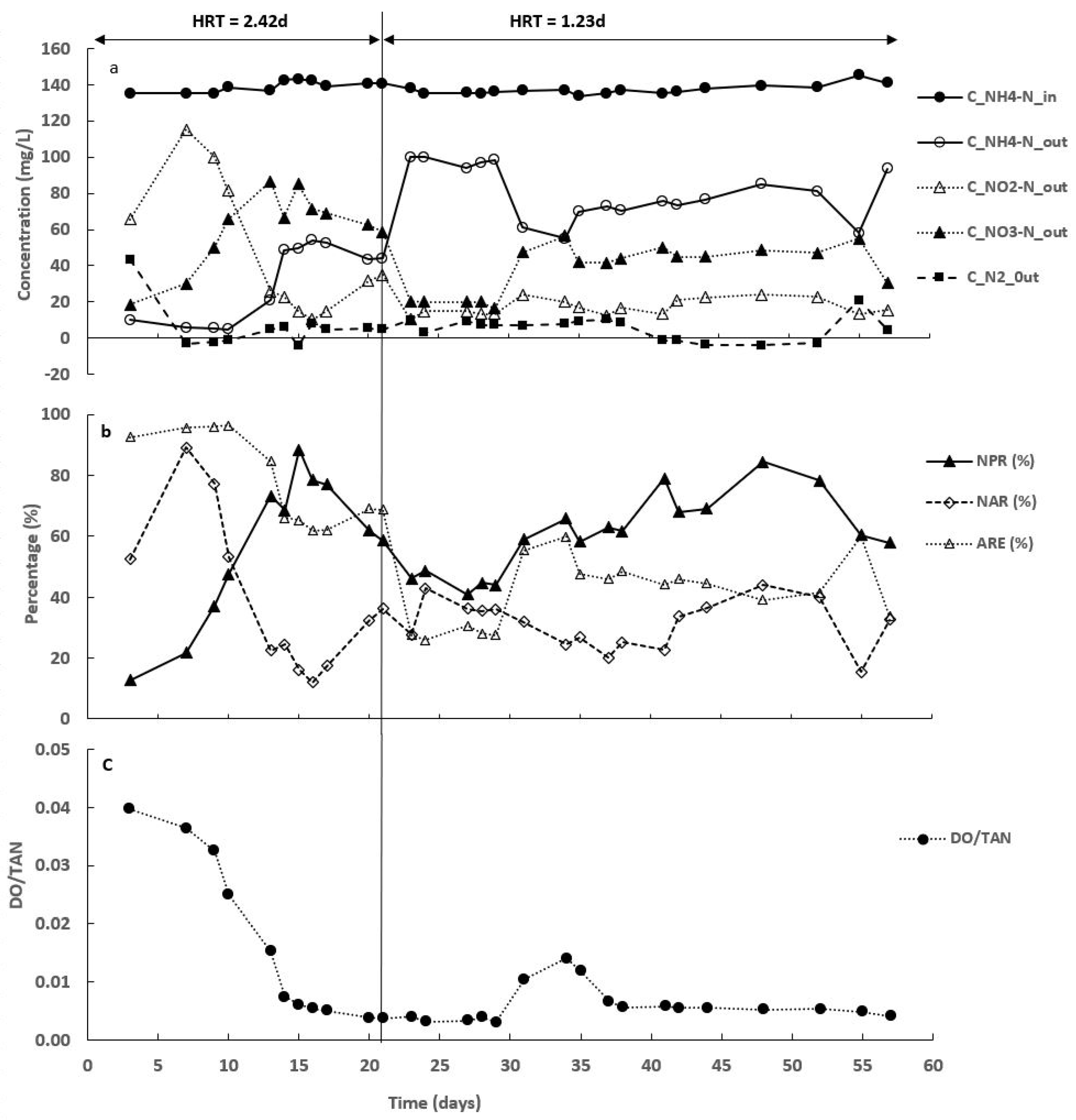
3.1. Synthetic Medium Concentration and Operating Conditions
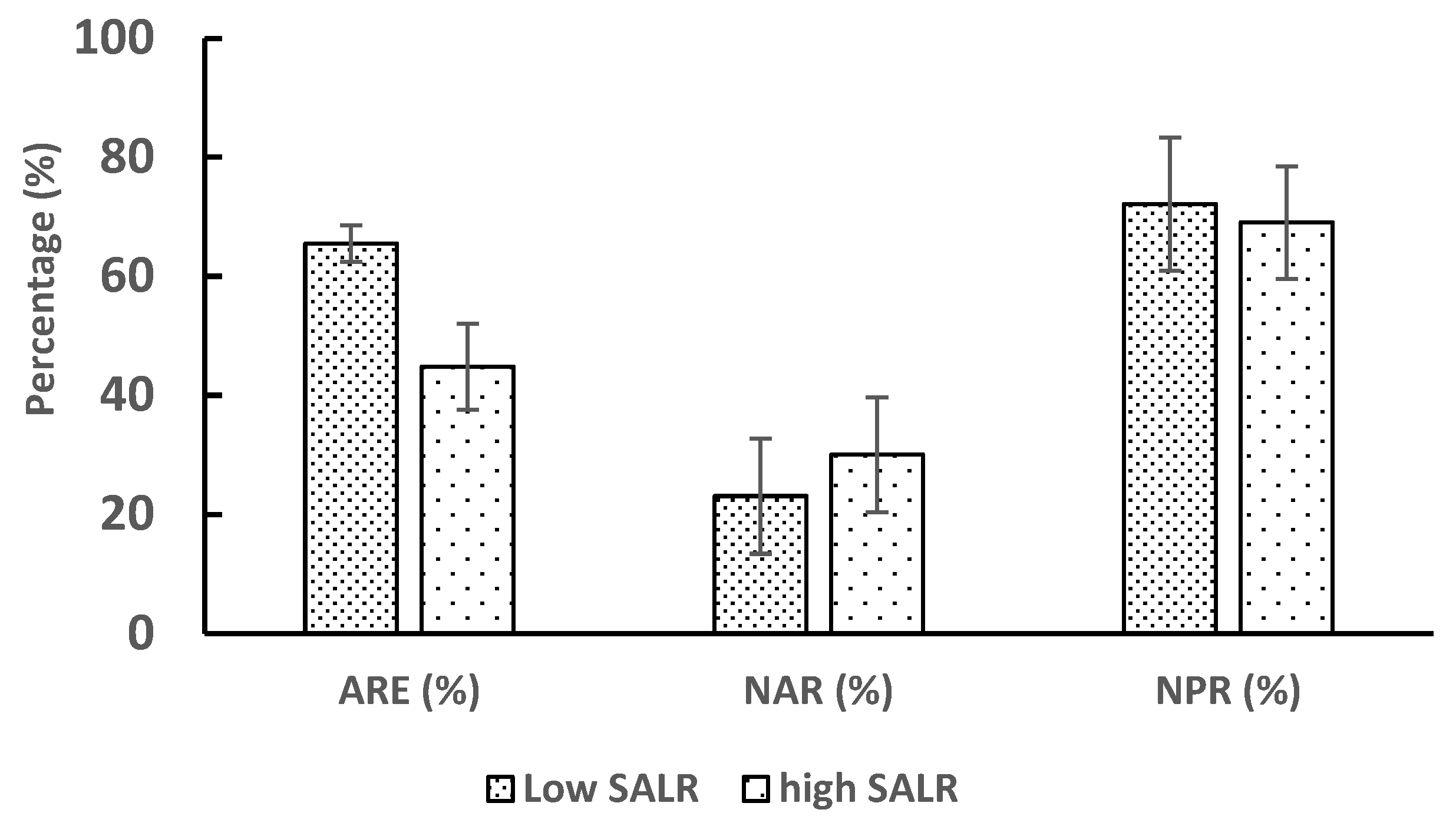
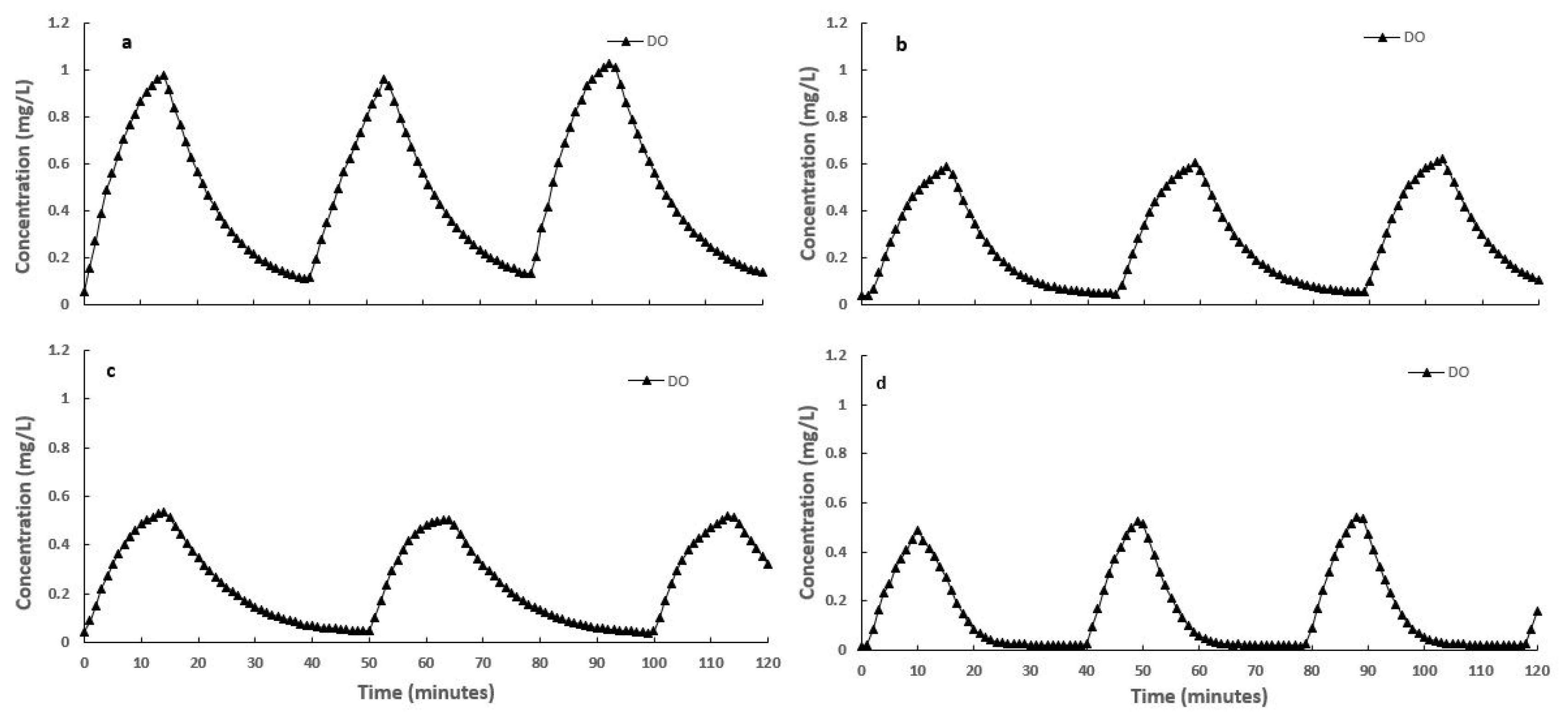
3.2. Nitrogen Compounds Transformations in Different SALR under Continuous Aeration
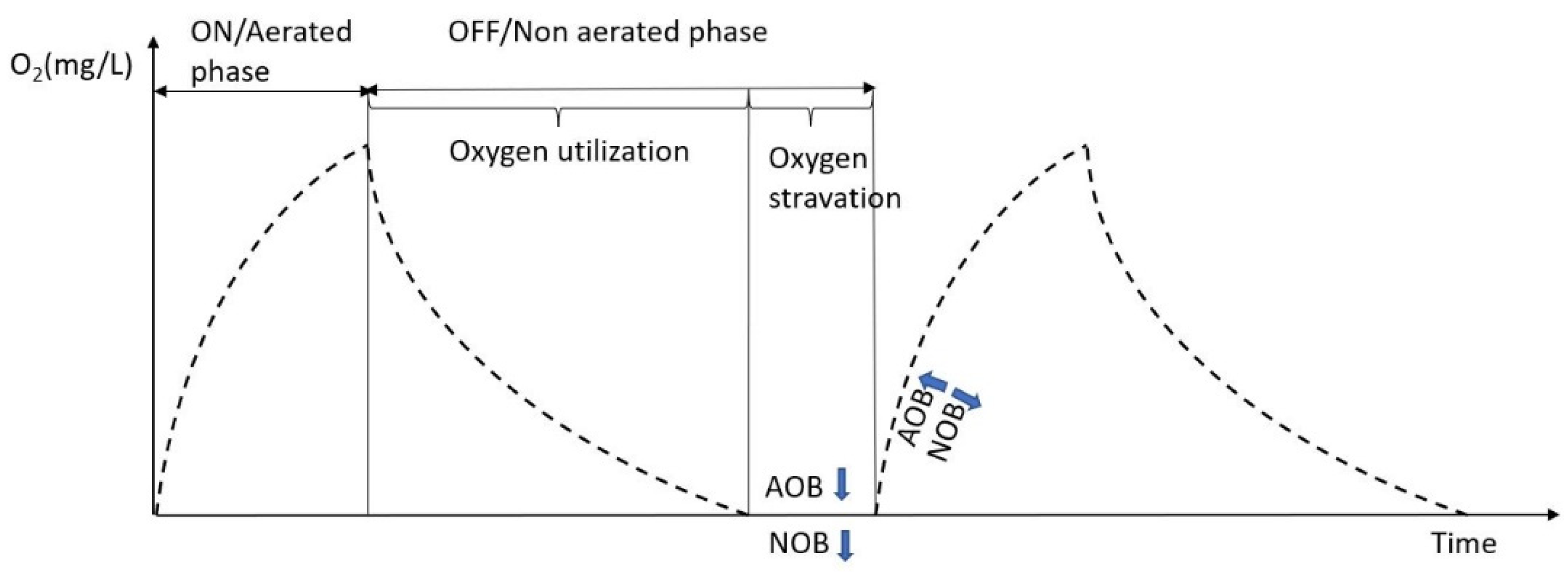
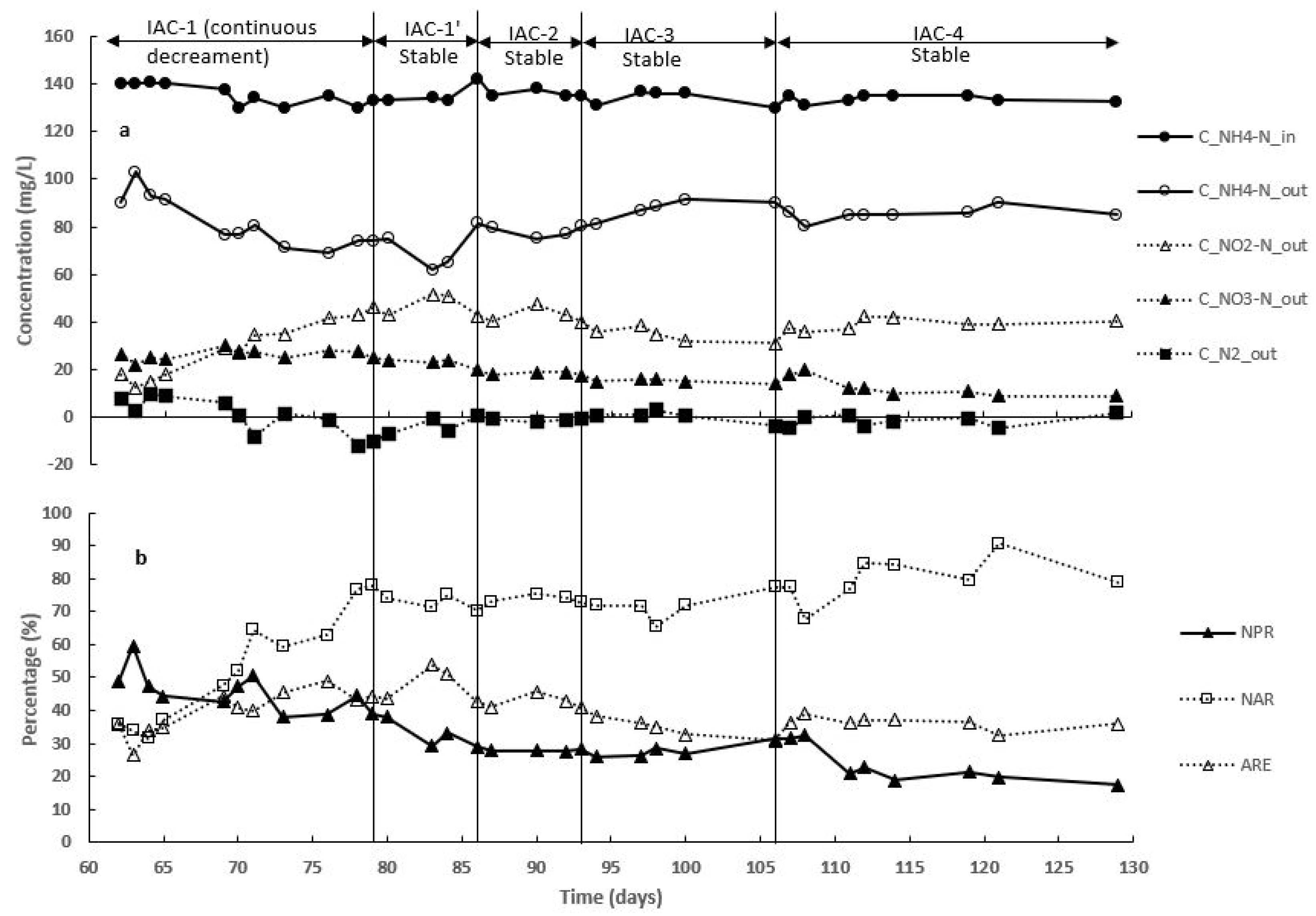
3.3. Effect of Intermittent Aeration Cycle on Nitrogen Compounds Transformation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahn, Y.-H. Sustainable nitrogen elimination biotechnologies: A review. Process Biochem. 2006, 41, 1709–1721. [Google Scholar] [CrossRef]
- Van Hulle, S.W.; Vandeweyer, H.J.; Meesschaert, B.D.; Vanrolleghem, P.A.; Dejans, P.; Dumoulin, A. Engineering aspect and practical application of autotrophic nitrogen removal from nitrogen rich stream. Chem. Eng. J. 2010, 162, 1–20. [Google Scholar] [CrossRef]
- Qiu, S.; Li, Z.; Hu, Y.; Shi, L.; Liu, R.; Shi, L.; Chen, L.; Zhan, X. What’s the best way to achieve successful mainstream partial nitritation-anammox application? Crit. Rev. Environ. Sci. Technol. 2020, 51, 1–33. [Google Scholar] [CrossRef]
- Cao, Y.; van Loosdrecht, M.C.M.; Daigger, G.T. Mainstream partial nitritation–anammox in municipal wastewater treatment: Status, bottlenecks, and further studies. Appl. Microbiol. Biotechnol. 2017, 101, 1365–1383. [Google Scholar] [CrossRef]
- Jetten, M.S.M.; Horn, S.J.; van Loosdrecht, M.C.M. Towards a more sustainable municipal wastewater treatment system. Sustain. Sanit. 1997, 35, 171–180. [Google Scholar] [CrossRef]
- Qian, W.; Peng, Y.; Li, X.; Zhang, Q.; Ma, B. The inhibitory effects of free ammonia on ammonia oxidizing bacteria and nitrite oxidizing bacteria under anaerobic condition. Bioresour. Technol. 2017, 243, 1247–1250. [Google Scholar] [CrossRef]
- Wiesmann, U. Biological nitrogen removal from wastewater. Adv. Biochem. Eng. Biotechnol. 1994, 51, 113–154. [Google Scholar]
- Zhou, Z.; Qi, M.; Wang, H. Achieving Partial Nitrification via Intermittent Aeration in SBR and Short-Term Effects of Different C/N Ratios on Reactor Performance and Microbial Community Structure. Water 2020, 12, 3485. [Google Scholar] [CrossRef]
- Xu, G. Partial nitrification adjusted by hydroxylamine in aerobic granules under high DO and ambient temperature and subsequent Anammox for low C/N wastewater treatment. Chem. Eng. J. 2012, 8, 338–345. [Google Scholar] [CrossRef]
- Schopf, A.; Delatolla, R.; Kirkwood, K.M. Partial nitritation at elevated loading rates: Design curves and biofilm characteristics. Bioprocess Biosyst. Eng. 2019, 42, 1809–1818. [Google Scholar] [CrossRef]
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.; Srinath, E.G. Inhibition of Nitrification by Ammonia and Nitrous Acid. J. Water Pollut. Control Fed. 1976, 48, 835–852. [Google Scholar]
- Ma, B.; Wang, S.; Cao, S.; Miao, Y.; Jia, F.; Du, R.; Peng, Y. Biological nitrogen removal from sewage via anammox: Recent advances. Bioresour. Technol. 2016, 200, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Elliott, D.; Nielsen, M.; Healy, M.G.; Zhan, X. Long-term partial nitrification in an intermittently aerated sequencing batch reactor (SBR) treating ammonium-rich wastewater under controlled oxygen-limited conditions. Biochem. Eng. J. 2011, 55, 215–222. [Google Scholar] [CrossRef]
- Kostera, J.; Youngblut, M.D.; Slosarczyk, J.M.; Pacheco, A.A. Kinetic and product distribution analysis of NO* reductase activity in Nitrosomonas europaea hydroxylamine oxidoreductase. J. Biol. Inorg. Chem. 2008, 13, 1073–1083. [Google Scholar] [CrossRef]
- Kampschreur, M.J.; Tan, N.C.G.; Kleerebezem, R.; Picioreanu, C.; Jetten, M.S.M.; Loosdrecht, M.C.M. van Effect of Dynamic Process Conditions on Nitrogen Oxides Emission from a Nitrifying Culture. Environ. Sci. Technol. 2008, 42, 429–435. [Google Scholar] [CrossRef]
- Bakke, R. High Tech Process in Combination with Natural Systems. Msc Lecture Presentation; University of South-Eastern Norway: Notodden, Norway, 2018. [Google Scholar]
- Eilersen, A.M.; Henze, M.; Kløft, L. Effect of volatile fatty acids and trimethylamine on nitrification in activated sludge. Water Res. 1994, 28, 1329–1336. [Google Scholar] [CrossRef]
- Peng, Y.; Song, X.; Peng, C.; Li, J.; Chen, Y. Biological nitrogen removal in SBR bypassing nitrate generation accomplished by chlorination and aeration time control. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2004, 49, 295–300. [Google Scholar] [CrossRef]
- Dinamarca, C.; Bakke, R. Apparent hydrogen consumption in acid reactors: Observations and implications. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2009, 59, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association; Eaton, A.D.; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; APHA-AWWA-WEF: Washington, DC, USA, 2005; ISBN 978-0-87553-047-5. [Google Scholar]
- Dimitrova, I.; Dabrowska, A.; Ekström, S. Start-up of a full-scale partial nitritation-anammox MBBR without inoculum at Klagshamn WWTP. Water Sci. Technol. 2020, 81, 2033–2042. [Google Scholar] [CrossRef]
- Kanders, L.; Ling, D.; Nehrenheim, E. Rapid start-up of one-stage deammonification MBBR without addition of external inoculum. Water Sci. Technol. 2016, 74, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zuo, J.; Shen, P.; Gu, X. Influence of temperature, pH value and organic substance on activity of ANAMMOX sludge. Huan Jing Ke Xue Huanjing Kexue 2006, 27, 691–695. [Google Scholar]
- Yumpu.com. Nitrification & Denitrification—The Water Planet Company. Available online: https://www.yumpu.com/en/document/view/11509528/nitrification-denitrification-the-water-planet-company (accessed on 15 September 2021).
- Bartrolí, A.; Pérez, J.; Carrera, J. Applying Ratio Control in a Continuous Granular Reactor to Achieve Full Nitritation under Stable Operating Conditions. Environ. Sci. Technol. 2010, 44, 8930–8935. [Google Scholar] [CrossRef]
- Choi, M.; Cho, K.; Jeong, D.; Chung, Y.-C.; Park, J.; Lee, S.; Bae, H. Effects of the ammonium loading rate on nitrite-oxidizing activity during nitrification at a high dose of inorganic carbon. J. Environ. Sci. Health Part A 2018, 53, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; He, C.; van Loosdrecht, M.C.M.; Pérez, J. Selection of ammonium oxidizing bacteria (AOB) over nitrite oxidizing bacteria (NOB) based on conversion rates. Chem. Eng. J. 2016, 304, 953–961. [Google Scholar] [CrossRef]
- Duan, H.; Ye, L.; Lu, X.; Yuan, Z. Overcoming Nitrite Oxidizing Bacteria Adaptation through Alternating Sludge Treatment with Free Nitrous Acid and Free Ammonia. Environ. Sci. Technol. 2019, 53, 1937–1946. [Google Scholar] [CrossRef]
- Klaus, S.; Baumler, R.; Rutherford, B.; Thesing, G.; Zhao, H.; Bott, C. Startup of a Partial Nitritation-Anammox MBBR and the Implementation of pH-Based Aeration Control. Water Environ. Res. 2017, 89, 500–508. [Google Scholar] [CrossRef]
- Jia, F.; Yang, Q.; Liu, X.; Li, X.; Li, B.; Zhang, L.; Peng, Y. Stratification of Extracellular Polymeric Substances (EPS) for Aggregated Anammox Microorganisms. Environ. Sci. Technol. 2017, 51, 3260–3268. [Google Scholar] [CrossRef]
- Kanders, L.; Areskoug, T.; Schneider, Y.; Ling, D.; Punzi, M.; Beier, M. Impact of seeding on the start-up of one-stage deammonification MBBRs. Environ. Technol. 2014, 35, 2767–2773. [Google Scholar] [CrossRef]
| Continuous Aeration | |||||
|---|---|---|---|---|---|
| S.N. | Flow Rate (L/d) | HRT (d) | SALR (gN/m2·d) | Duration | |
| 1 | 3.3 | 2.4 | 0.14 ± 0.1 | Day1–day 21 | |
| 2 | 6.5 | 1.2 | 0.27 ± 0.005 | Day 21–day 59 | |
| Intermittent Aeration | |||||
| Test | Cycle Length (min) | ON/OFF or Aerated/Non-Aerated Time (min/min) | Aerated/Non aerated DO (mg/L) | HRT (d) | Duration |
| IAC-1 | 40 | 15/25 | 1/0.1 | 1.2 | Day 59–day 79 |
| IAC-2 | 45 | 15/30 | 0.6/0.05 | 1.2 | Day 86–day 93 |
| IAC-3 | 50 | 15/35 | 0.5/0.05 | 1.2 | Day 93–day 106 |
| IAC-4 | 40 | 10/30 | 0.5/0.025 | 1.2 | Day 106–day 129 |
| Vitamin Composition | (g/L) | Mineral Composition | (g/L) |
|---|---|---|---|
| Thioctic acid p-aminobenzoic acid Pantothentic acid Vitamin B12 Thiamine Nicotinic acid Riboflavin Pyridoxine hydrochloride Folic acid Biotin | 0.05 0.05 0.05 0.001 0.05 0.05 0.05 0.1 0.02 0.02 | CuSO4·5H2O MnSO4·H2O FeSO4·7H2O NiCl2·6H2O ZnSO4·7H2O CoCl2·6H2O H3BO3 | 0.055 0.04 2.7 0.1 0.088 0.05 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathak, S.; Wang, S.; Janka, E. Achieving Partial Nitritation in Anammox Start-Up Environment. Water 2022, 14, 229. https://doi.org/10.3390/w14020229
Pathak S, Wang S, Janka E. Achieving Partial Nitritation in Anammox Start-Up Environment. Water. 2022; 14(2):229. https://doi.org/10.3390/w14020229
Chicago/Turabian StylePathak, Sabin, Shuai Wang, and Eshetu Janka. 2022. "Achieving Partial Nitritation in Anammox Start-Up Environment" Water 14, no. 2: 229. https://doi.org/10.3390/w14020229
APA StylePathak, S., Wang, S., & Janka, E. (2022). Achieving Partial Nitritation in Anammox Start-Up Environment. Water, 14(2), 229. https://doi.org/10.3390/w14020229






