Key Factors for Activated Carbon Adsorption of Pharmaceutical Compounds from Wastewaters: A Multivariate Modelling Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Selection of the Target Pharmaceutical Compounds (PhCs)
2.3. Analytical Methods
2.3.1. Quantification of Pharmaceutical Compounds (PhCs)
2.3.2. Wastewaters Characterisation
2.3.3. PAC Characterisation
2.4. Batch Adsorption Tests
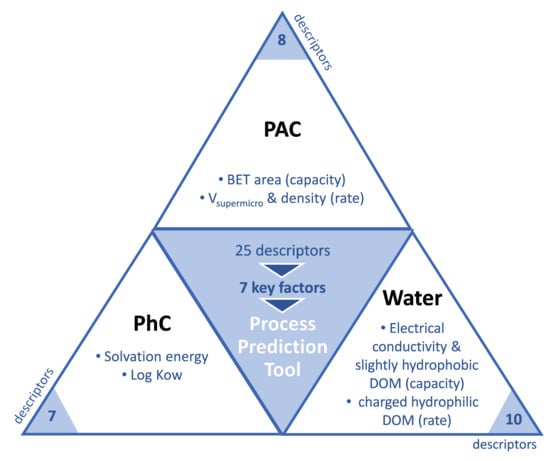
2.5. Parameters Used as Model Inputs
2.5.1. PhCs Parameters
Molecular Modeling
Molecular Data
2.5.2. Wastewater Parameters
2.5.3. PACs Parameters
2.6. Development of PLS Regression
2.7. PLS Regression Optimisation and Selection
3. Results and Discussion
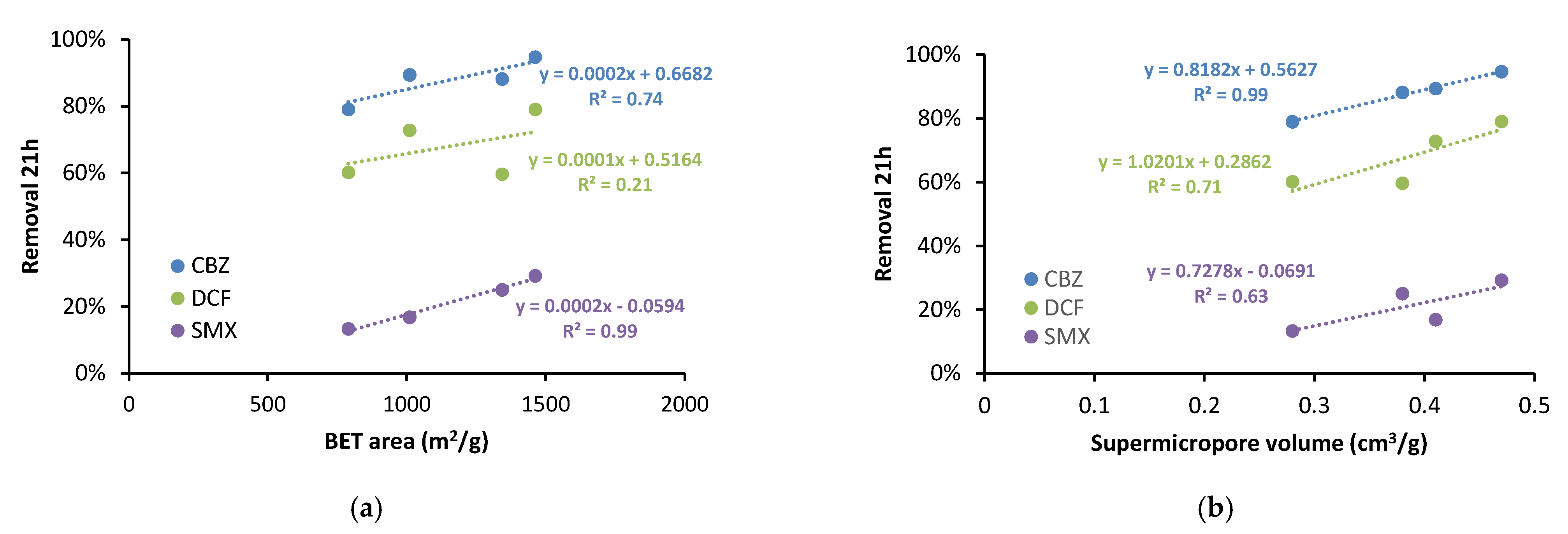
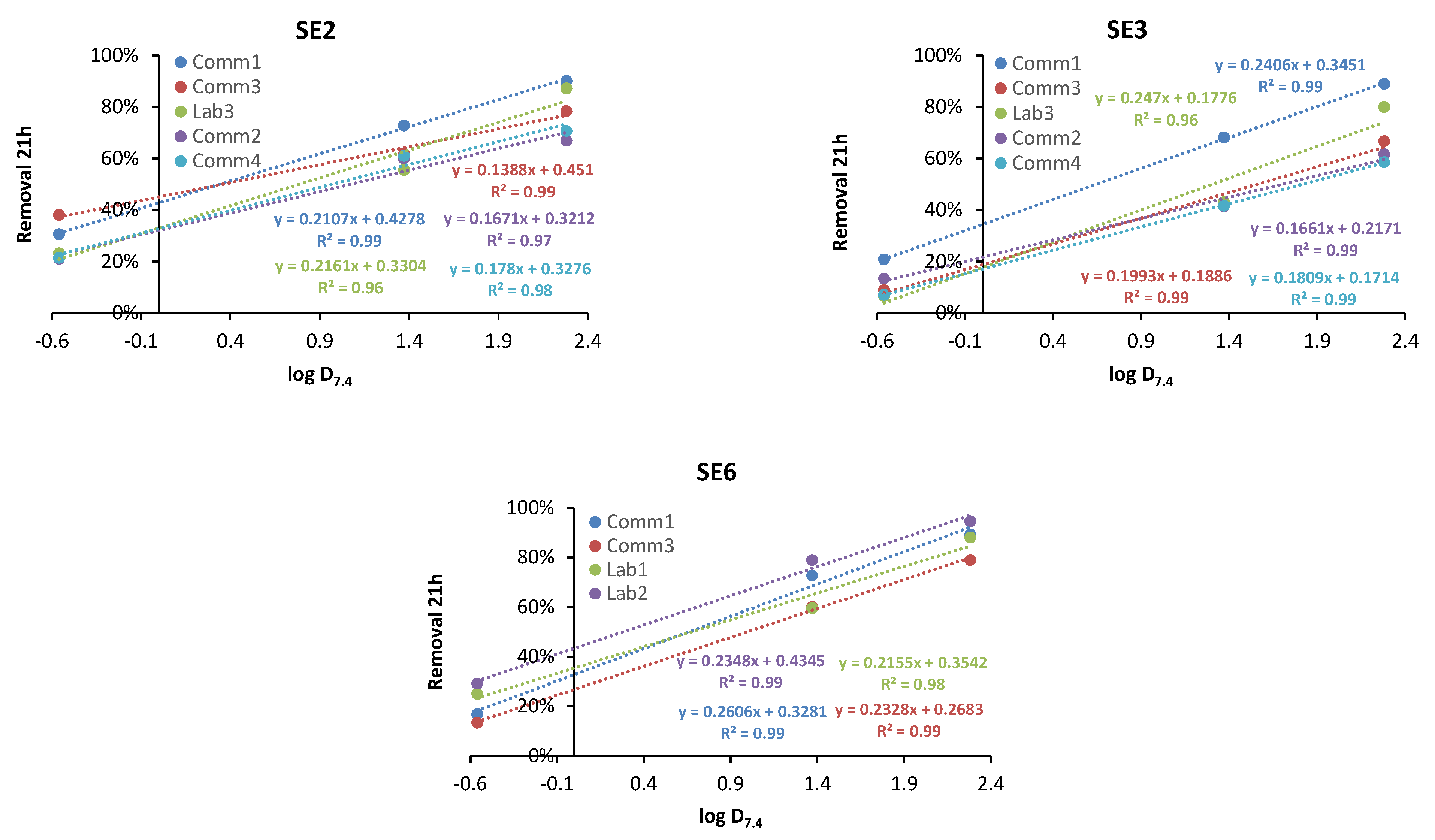
3.1. Univariate Analysis
3.2. Multivariate Analysis
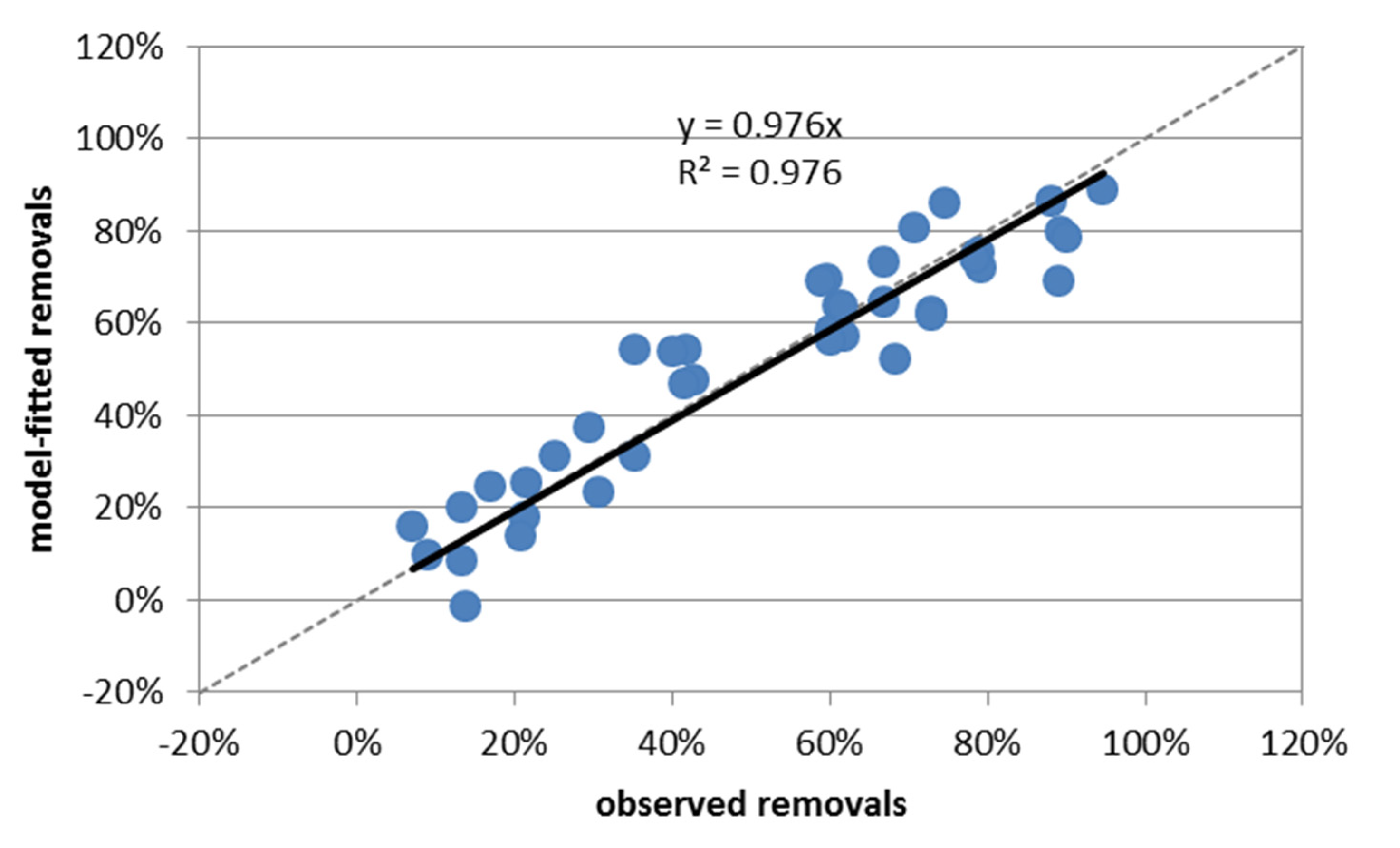
3.2.1. Fitting of Adsorption at 21 h
3.2.2. Fitting of Adsorption at 1 h
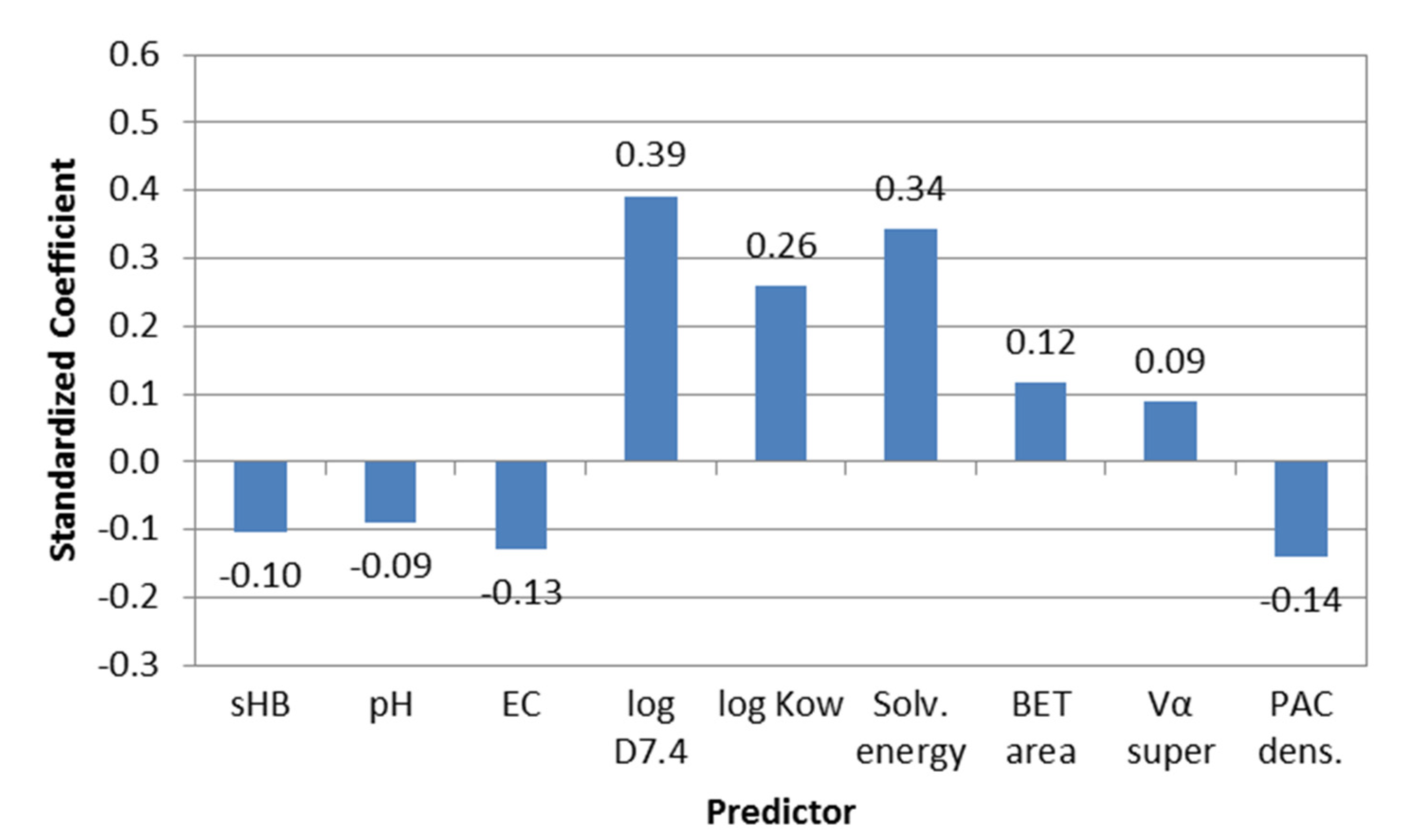
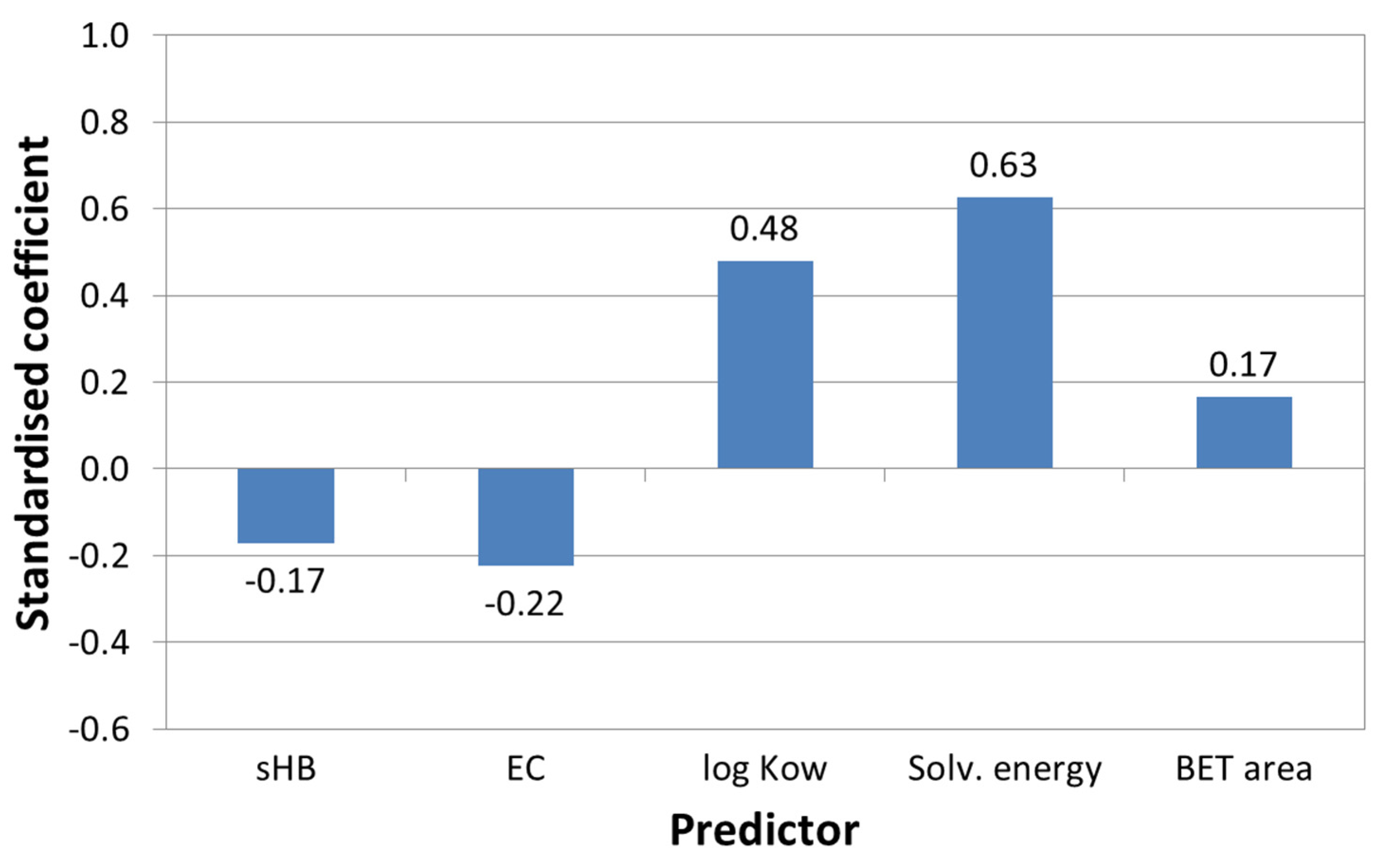
3.2.3. Assessing the Main Descriptors for Adsorption Capacity and Short-Term Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mestre, A.S.; Campinas, M.; Viegas, R.M.C.; Mesquita, E.; Carvalho, A.P.; Rosa, M.J. Activated carbons in full-scale advanced wastewater treatment (in press). In Advanced Materials for Sustainable Environmental Remediation: Terrestrial and Aquatic Environments (in Press); Giannakoudakis, D.A., Meili, L., Anastopoulos, I., Eds.; Elsevier: Boston, MA, USA, 2022; ISBN 0323904858. [Google Scholar]
- Ania, C.O.; Armstrong, P.A.; Bandosz, T.J.; Beguin, F.; Carvalho, A.P.; Celzard, A.; Frackowiak, E.; Gilarranz, M.A.; László, K.; Matos, J.; et al. Pereira, Engaging nanoporous carbons in “beyond adsorption” applications: Characterization, challenges and performance. Carbon 2020, 164, 69–84. [Google Scholar] [CrossRef]
- Zietzschmann, F.; Altmann, J.; Ruhl, A.S.; Dünnbier, U.; Dommisch, I.; Sperlich, A.; Meinel, F.; Jekel, M. Estimating organic micro-pollutant removal potential of activated carbons using UV absorption and carbon characteristics. Water Res. 2014, 56, 48–55. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Derome, C.; Buleté, A.; Vulliet, E.; Bressy, A.; Varrault, G.; Chebbo, G.; Rocher, V. Removal of emerging micropollutants from wastewater by activated carbon adsorption: Experimental study of different activated carbons and factors influencing the adsorption of micropollutants in wastewater. J. Environ. Chem. Eng. 2016, 4, 1102–1109. [Google Scholar] [CrossRef] [Green Version]
- Benstoem, F.; Pinnekamp, J. Characteristic numbers of granular activated carbon for the elimination of micropollutants from effluents of municipal wastewater treatment plants. Water Sci. Technol. 2017, 76, 279–285. [Google Scholar] [CrossRef] [PubMed]
- de Ridder, D.; Villacorte, L.; Verliefde, A.; Verberk, J.; Heijman, S.; Amy, G.; van Dijk, J. Modeling equilibrium adsorption of organic micropollutants onto activated carbon. Water Res. 2010, 44, 3077–3086. [Google Scholar] [CrossRef] [PubMed]
- Alves, T.C.; Cabrera-Codony, A.; Barceló, D.; Rodriguez-Mozaz, S.; Pinheiro, A.; Gonzalez-Olmos, R. Influencing factors on the removal of pharmaceuticals from water with micro-grain activated carbon. Water Res. 2018, 144, 402–412. [Google Scholar] [CrossRef]
- Guillossou, R.; Le Roux, J.; Mailler, R.; Morlay, C.; Vulliet, E.; Nauleau, F.; Rocher, V.; Gasperi, J. Influence of the properties of 7 micro-grain activated carbons on organic micropollutants removal from wastewater effluent. Chemosphere 2020, 243, 125306. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Mehmood, T.; Liland, K.H.; Snipen, L.; Sæbø, S. A review of variable selection methods in Partial Least Squares Regression. Chemom. Intell. Lab. Syst. 2012, 118, 62–69. [Google Scholar] [CrossRef]
- Sanches, S.; Galinha, C.; Crespo, M.B.; Pereira, V.; Crespo, J. Assessment of phenomena underlying the removal of micropollutants during water treatment by nanofiltration using multivariate statistical analysis. Sep. Purif. Technol. 2013, 118, 377–386. [Google Scholar] [CrossRef]
- Shahmansouri, A.; Bellona, C. Application of quantitative structure-property relationships (QSPRs) to predict the rejection of organic solutes by nanofiltration. Sep. Purif. Technol. 2013, 118, 627–638. [Google Scholar] [CrossRef]
- Brito, R.S.; Pinheiro, H.M.; Ferreira, F.; Matos, J.S.; Lourenço, N.D. In situ UV-Vis spectroscopy to estimate COD and TSS in wastewater drainage systems. Urban Water J. 2014, 11, 261–273. [Google Scholar] [CrossRef]
- Flyborg, L.; Björlenius, B.; Ullner, M.; Persson, K.M. A PLS model for predicting rejection of trace organic compounds by nanofiltration using treated wastewater as feed. Sep. Purif. Technol. 2017, 174, 212–221. [Google Scholar] [CrossRef]
- Jones, S.M.; Watts, M.J.; Wickramasinghe, S.R. Wickramasinghe, A Nanofiltration Decision Tool for Potable Reuse: A New Rejection Model for Recalcitrant CECs. Water Environ. Res. 2017, 89, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Doig, A.L.; Sun-Kou, M.D.R.; Doig, M.E.; Comina, G. Use of mathematical algorithms to evaluate the influence of physicochemical parameters affecting the adsorption of aromatic compounds on activated carbon. Rev. Colomb. Quim. 2015, 44, 25–29. [Google Scholar]
- Meinel, F.; Sperlich, A.; Jekel, M. Pilot-scale study of powdered activated carbon recirculation for micropollutant removal. Water Sci. Technol. 2016, 74, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Rosa, M.J.; Campinas, M.; Viegas, R.M.C.; Silva, C. Life Impetus Technical Guidelines for improved control of pharmaceutical compounds in urban activated sludge WWTPs. In Deliverable of LIFE IMPETUS Action D1 (Communication and Dissemination); LNEC: Lisbon, Portugal, 2019. [Google Scholar]
- Viegas, R.M.; Mestre, A.S.; Mesquita, E.; Campinas, M.; Andrade, M.A.; Carvalho, A.P.; Rosa, M.J. Assessing the applicability of a new carob waste-derived powdered activated carbon to control pharmaceutical compounds in wastewater treatment. Sci. Total Environ. 2020, 743, 140791. [Google Scholar] [CrossRef] [PubMed]
- Campinas, M.; Silva, C.; Viegas, R.M.; Coelho, R.; Lucas, H.; Rosa, M.J. To what extent may pharmaceuticals and pesticides be removed by PAC conventional addition to low-turbidity surface waters and what are the potential bottlenecks? J. Water Process Eng. 2020, 40, 101833. [Google Scholar] [CrossRef]
- Gaffney, V.D.J.; Cardoso, V.V.; Rodrigues, A.; Ferreira, E.; Benoliel, M.J.; Almeida, C.M. Analysis of pharmaceutical compounds in water by SPE-UPLC-ESI-MS/MS. Quim. Nova 2014, 37, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Clesceri, L.; Greenberg, A.; Eaton, A. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF): Washington, DC, USA, 1998. [Google Scholar]
- EN1484. Water analysis—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC); CEN: Brussels, Belgium, 1997. [Google Scholar]
- Chow, C.W.K.; Fabris, R.; Drikas, M. A rapid fractionation technique to characterise natural organic matter for the optimisation of water treatment processes. J. Water Supply Res. Technol. Aqua 2004, 53, 85–92. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Rouquerol, J.; Llewellyn, P. Is the bet equation applicable to microporous adsorbents? In Studies in Surface Science and Catalysis; Llewellyn, P.L., Rodriquez-Reinoso, F., Rouquerol, J., Seaton, N., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2007; pp. 49–56. [Google Scholar]
- ISO9277. Determination of Specific Surface Area of Solids by Gas Adsorption—BET Method, 2nd ed.; ISO: Geneva, Switzerland, 2010. [Google Scholar]
- Rodríguez-Reinoso, F.; Martin-Martinez, J.M.; Prado-Burguete, C.; McEnaney, B. A standard adsorption isotherm for the characterization of activated carbons. J. Phys. Chem. 1987, 91, 515–516. [Google Scholar] [CrossRef]
- Mestre, A.S.; Hesse, F.; Freire, C.; Ania, C.O.; Carvalho, A. Chemically activated high grade nanoporous carbons from low density renewable biomass (Agave sisalana) for the removal of pharmaceuticals. J. Colloid Interface Sci. 2019, 536, 681–693. [Google Scholar] [CrossRef]
- Mestre, A.; Pires, J.; Nogueira, J.M.; Parra, J.B.; Carvalho, A.; Ania, C.O. Waste-derived activated carbons for removal of ibuprofen from solution: Role of surface chemistry and pore structure. Bioresour. Technol. 2009, 100, 1720–1726. [Google Scholar] [CrossRef] [Green Version]
- Rosin, P.; Rammler, E. The Laws Governing the Fineness of powdered coal. J. Inst. Fuel 1933, 7, 29–36. [Google Scholar]
- Frisch, M.J.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06 functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Ho, J. Are thermodynamic cycles necessary for continuum solvent calculation of pKas and reduction potentials? Phys. Chem. Chem. Phys. 2015, 17, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Krasner, S.W.; Westerhoff, P.; Chen, B.; Rittmann, B.E.; Nam, S.-N.; Amy, G. Impact of Wastewater Treatment Processes on Organic Carbon, Organic Nitrogen, and DBP Precursors in Effluent Organic Matter. Environ. Sci. Technol. 2009, 43, 2911–2918. [Google Scholar] [CrossRef]
- Edzwald, J.K.; Tobiason, J.E. Enhanced coagulation: US requirements and a broader view. Water Sci. Technol. 1999, 40, 63–70. [Google Scholar] [CrossRef]
- Przepiórski, J. Chapter 9 Activated carbon filters and their industrial applications. In Interface Science and Technology; Bandosz, T.J., Ed.; Elsevier Ltd.: Oxford, UK, 2006; pp. 421–474. [Google Scholar]
- Ebie, K.; Li, F.; Azuma, Y.; Yuasa, A.; Hagishita, T. Pore distribution effect of activated carbon in adsorbing organic micropollutants from natural water. Water Res. 2001, 35, 167–179. [Google Scholar] [CrossRef]
- Kamakura, W. Kamakura’s Analytic Tools for Excel. Available online: www.katexcel.com/home (accessed on 11 October 2021).
- Diamantidis, N.; Karlis, D.; Giakoumakis, E. Unsupervised stratification of cross-validation for accuracy estimation. Artif. Intell. 2000, 116, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Abu-Mostafa, Y.S.; Magdon-Ismail, M.; Lin, H.-T. Learning from Data: A Short Course; AMLBook: USA, 2012. [Google Scholar]
- Pierna, J.A.F.; Abbas, O.; Baeten, V.; Dardenne, P. A Backward Variable Selection method for PLS regression (BVSPLS). Anal. Chim. Acta 2009, 642, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Malash, G.F.; El-Khaiary, M.I. Piecewise linear regression: A statistical method for the analysis of experimental adsorption data by the intraparticle-diffusion models. Chem. Eng. J. 2010, 163, 256–263. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Buleté, A.; Vulliet, E.; Deshayes, S.; Zedek, S.; Mirande-Bret, C.; Eudes, V.; Bressy, A.; et al. Removal of a wide range of emerging pollutants from wastewater treatment plant discharges by micro-grain activated carbon in fluidized bed as tertiary treatment at large pilot scale. Sci. Total Environ. 2016, 542, 983–996. [Google Scholar] [CrossRef] [Green Version]
- Kårelid, V.; Larsson, G.; Björlenius, B. Pilot-scale removal of pharmaceuticals in municipal wastewater: Comparison of granular and powdered activated carbon treatment at three wastewater treatment plants. J. Environ. Manag. 2017, 193, 491–502. [Google Scholar] [CrossRef]
- Zietzschmann, F.; Aschermann, G.; Jekel, M. Comparing and modeling organic micro-pollutant adsorption onto powdered activated carbon in different drinking waters and WWTP effluents. Water Res. 2016, 102, 190–201. [Google Scholar] [CrossRef]
- Campinas, M.; Rosa, M.J. The ionic strength effect on microcystin and natural organic matter surrogate adsorption onto PAC. J. Colloid Interface Sci. 2006, 299, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Campinas, M.; Viegas, R.M.; Rosa, M.J. Modelling and understanding the competitive adsorption of microcystins and tannic acid. Water Res. 2013, 47, 5690–5699. [Google Scholar] [CrossRef]
- Altmann, J.; Ruhl, A.S.; Zietzschmann, F.; Jekel, M. Direct comparison of ozonation and adsorption onto powdered activated carbon for micropollutant removal in advanced wastewater treatment. Water Res. 2014, 55, 185–193. [Google Scholar] [CrossRef]
- Matsui, Y.; Ando, N.; Yoshida, T.; Kurotobi, R.; Matsushita, T.; Ohno, K. Modeling high adsorption capacity and kinetics of organic macromolecules on super-powdered activated carbon. Water Res. 2011, 45, 1720–1728. [Google Scholar] [CrossRef] [PubMed]

| PhC Molecular Structure Therapeutic Class | Optimized Geometries and Dimensions |
|---|---|
Carbamazepine/CBZ Anti-epileptic & psychiatric drug | 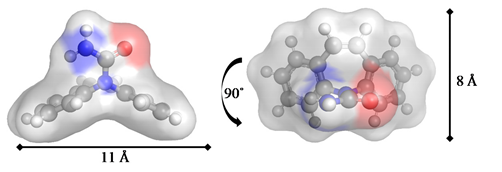 |
Diclofenac/DCF Non-steroidal analgesic & anti-inflammatory drug | 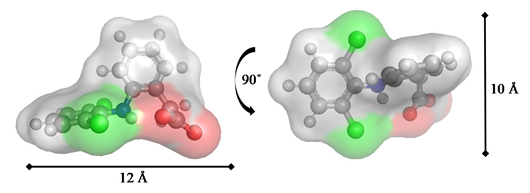 |
Sulfamethoxazole/SMX Antibiotic | 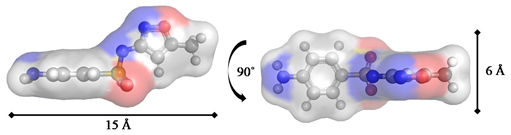 |
| Characteristics | CBZ | DCF | SMX |
|---|---|---|---|
| log Kow (-) | 2.67 | 4.06 | 0.89 |
| log D7.4 (-) | 2.28 | 1.37 | −0.56 |
| Polarizability (Å3) | 27.6 | 30.3 | 24.8 |
| Charge at pH 7.4 (-) | 0 | −1 | −1 |
| pKa (-) | 13.9 | 4.0 | 5.6 |
| Critical dimension 1 (Å) | 8 | 10 | 6 |
| Solvation energy (kJ/mol) | −15 | −60 | −71 |
| Characteristics | SE1 | SE2 | SE3 | SE4 | SE5 | SE6 |
|---|---|---|---|---|---|---|
| A254 (au cm−1) | 0.163 | 0.195 | 0.154 | 0.236 | 0.216 | 0.147 |
| A436 (au cm−1) | 0.013 | 0.020 | 0.012 | 0.021 | 0.020 | 0.014 |
| DOC (mg C/L) | 5.5 | 6.4 | 5.3 | 7.9 | 4.7 | 5.3 |
| SUVA (L/(mg×m)) | 3.0 | 3.1 | 2.9 | 3.0 | 4.6 | 2.8 |
| vHB (mg C/L) | 2.5 | 3.4 | 2.7 | 3.7 | 2.5 | 2.4 |
| sHB (mg C/L) | 0.6 | 1.1 | 1.3 | 2.0 | 0.9 | 1.2 |
| nHL (mg C/L) | 1.0 | 0.5 | 0.6 | 0.4 | 0.3 | 0.4 |
| cHL (mg C/L) | 1.4 | 1.4 | 0.7 | 1.9 | 1.0 | 1.3 |
| pH (-) | 7.2 | 7.3 | 7.8 | 7.6 | 7.3 | 7.4 |
| EC (µS/cm) | 1014 | 880 | 1330 | 1306 | 2450 | 704 |
| Comm1 | Comm2 | Comm3 | Comm4 | Lab1 | Lab2 | Lab3 | |
|---|---|---|---|---|---|---|---|
| Origin | Coal | BituminousCoal | Vegetable | Vegetable | Vegetable | Vegetable | Vegetable |
| Activation | Steam | NA | Steam | NA | Steam | Steam | Steam |
| Textural properties | |||||||
| Apparent surface (BET) area—ABET (m2/g) | 1010 | 746 | 790 | 1111 | 1343 | 1463 | 762 |
| BET constant—CBET (-) | 772 | 662 | 726 | 816 | 1387 | 983 | 1247 |
| Total pore volume—VTotal (cm3/g) | 0.66 | 0.52 | 0.53 | 0.83 | 0.72 | 0.90 | 0.56 |
| Mesopore volume—VMeso (cm3/g) 2 nm < φ < 50 nm | 0.25 | 0.24 | 0.23 | 0.44 | 0.25 | 0.43 | 0.28 |
| Total micropore volume—Vα total (cm3/g) φ < 2 nm | 0.41 | 0.28 | 0.30 | 0.39 | 0.47 | 0.47 | 0.28 |
| Supermicropore volume—Vα super (cm3/g) 0.7 nm < φ < 2 nm | 0.41 | 0.28 | 0.28 | 0.39 | 0.38 | 0.47 | 0.21 |
| Ultramicropore volume—Vα ultra (cm3/g) φ < 0.7 nm | 0.00 | 0.00 | 0.02 | 0.00 | 0.09 | 0.00 | 0.07 |
| Surface properties | |||||||
| pHPZC (-) | 7.4 | 8.2 | 8.0 | 7.9 | 10.1 | 9.6 | 8.0 |
| Overall surface charge at pH 7.4 ± 0.2 * (-) | 0 | positive | |||||
| Physical properties | |||||||
| Particle size D50 (µm) | 20 | 20 | 15 | 20 | 55 | 55 | 100 |
| Apparent density (kg/m3) | 385 | 417 | 400 | 370 | 430 | 372 | 550 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viegas, R.M.C.; Mestre, A.S.; Mesquita, E.; Machuqueiro, M.; Andrade, M.A.; Carvalho, A.P.; Rosa, M.J. Key Factors for Activated Carbon Adsorption of Pharmaceutical Compounds from Wastewaters: A Multivariate Modelling Approach. Water 2022, 14, 166. https://doi.org/10.3390/w14020166
Viegas RMC, Mestre AS, Mesquita E, Machuqueiro M, Andrade MA, Carvalho AP, Rosa MJ. Key Factors for Activated Carbon Adsorption of Pharmaceutical Compounds from Wastewaters: A Multivariate Modelling Approach. Water. 2022; 14(2):166. https://doi.org/10.3390/w14020166
Chicago/Turabian StyleViegas, Rui M. C., Ana S. Mestre, Elsa Mesquita, Miguel Machuqueiro, Marta A. Andrade, Ana P. Carvalho, and Maria João Rosa. 2022. "Key Factors for Activated Carbon Adsorption of Pharmaceutical Compounds from Wastewaters: A Multivariate Modelling Approach" Water 14, no. 2: 166. https://doi.org/10.3390/w14020166
APA StyleViegas, R. M. C., Mestre, A. S., Mesquita, E., Machuqueiro, M., Andrade, M. A., Carvalho, A. P., & Rosa, M. J. (2022). Key Factors for Activated Carbon Adsorption of Pharmaceutical Compounds from Wastewaters: A Multivariate Modelling Approach. Water, 14(2), 166. https://doi.org/10.3390/w14020166