Silver Nanoparticles (Ag NPs) Boost Mitigation Powers of Chenopodium Quinoa (Q6 Line) Grown under In Vitro Salt-Stressing Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishment of Plant Materials
2.2. Synthesis, Characterization, and Preparation of Ag NPs
Characterization of Ag NPs
2.3. Chlorophyll Content
2.4. Proline Determination
2.5. Protein Content
2.6. Ion Analysis
2.7. Data Analysis
3. Results
3.1. Assessments of Plant Growth Parameters
3.2. Chlorophyll Content
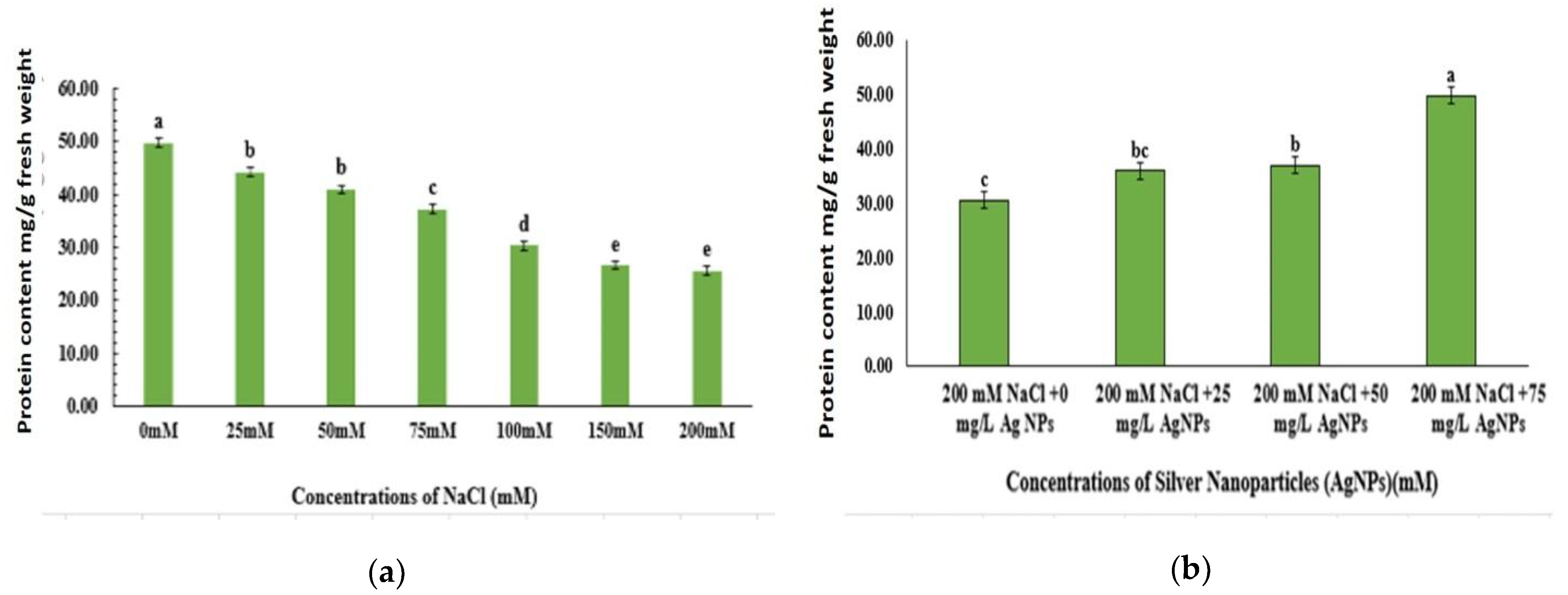
3.3. Protein Content
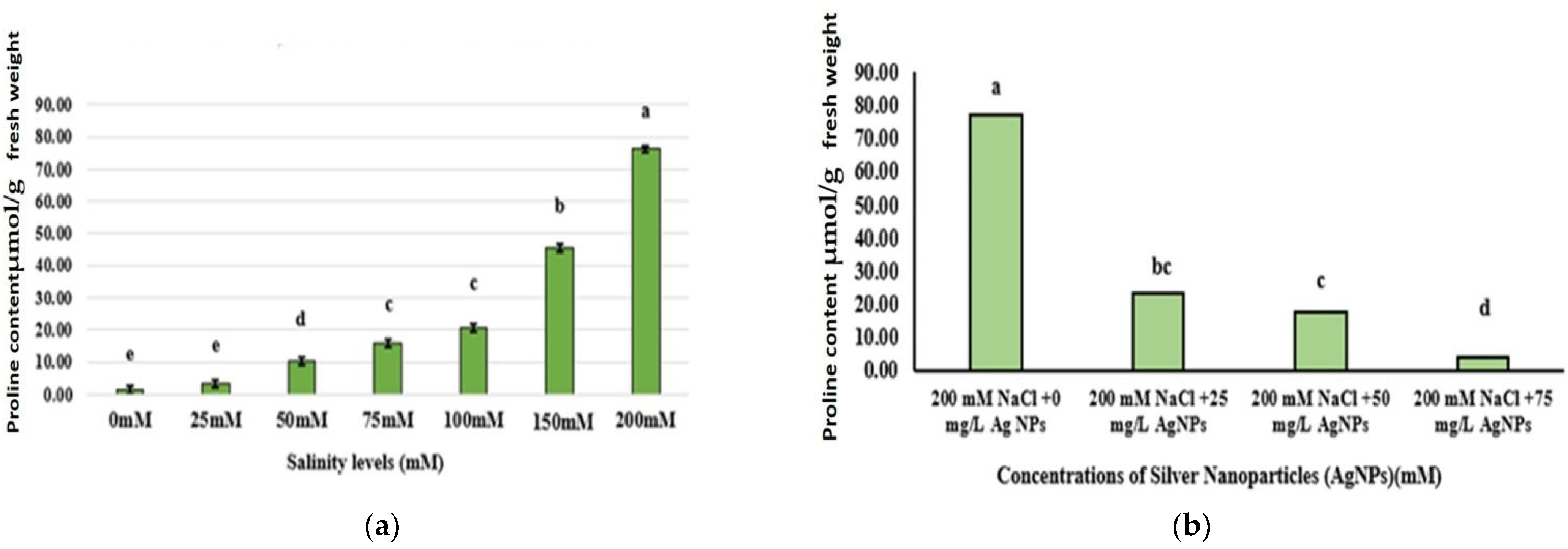
3.4. Proline Content
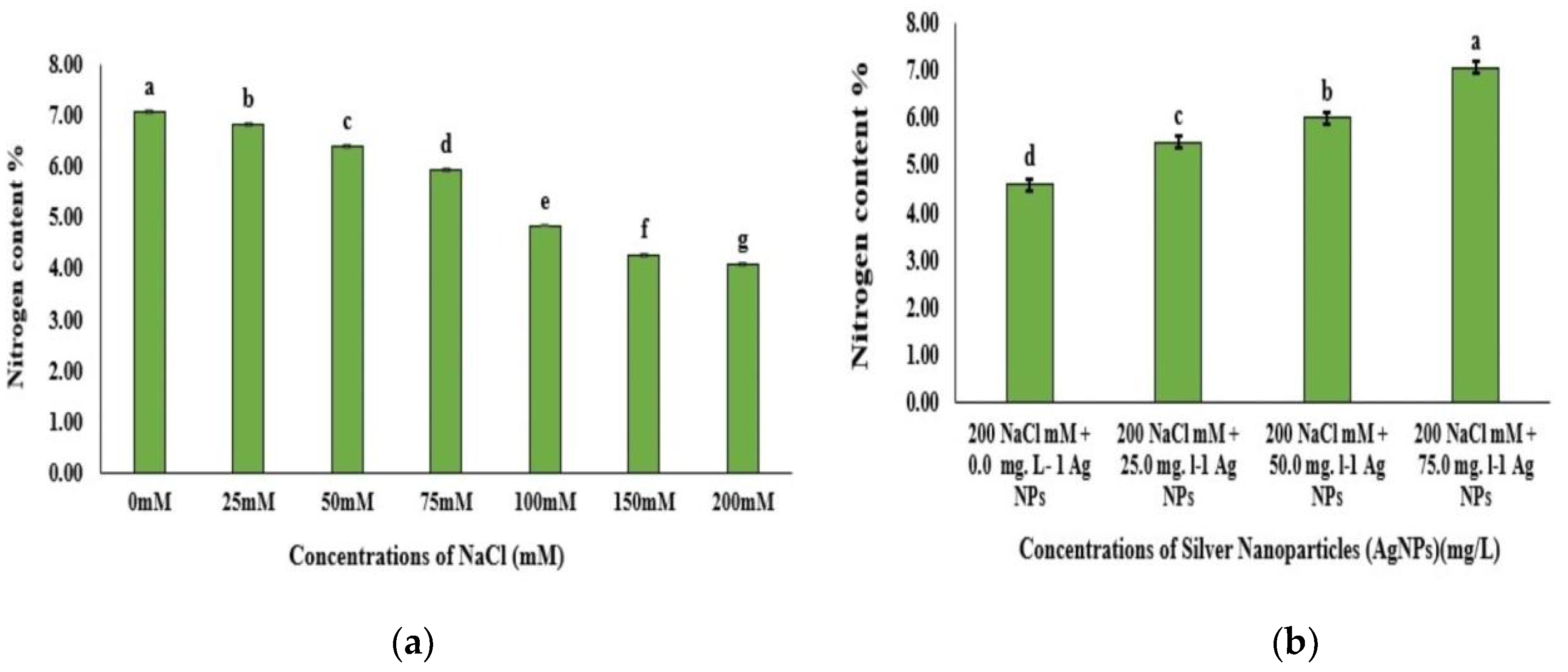
3.5. Nitrogen Content
3.6. Ion Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maughan, P.J.; Bonifacio, A.; Coleman, C.E.; Jellen, E.N.; Stevens, M.R.; Fairbanks, D.J. Quinoa (Chenopodium quinoa). In Pulses, Sugar and Tuber Crops; Springer: Berlin/Heidelberg, Germany, 2007; pp. 147–158. [Google Scholar]
- Adolf, V.I.; Shabala, S.; Andersen, M.N.; Razzaghi, F.; Jacobsen, S.E. Varietal differences of quinoa’s tolerance to saline conditions. Plant Soil 2012, 357, 117–129. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Martínez, E.A.; Orsini, F.; Antognoni, F.; Jacobsen, S.E. Quinoa–A model crop for understanding salt-tolerance mechanisms in halophytes. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2016, 150, 357–371. [Google Scholar] [CrossRef]
- Cocozza, C.; Pulvento, C.; Lavini, A.; Riccardi, M.; d’Andria, R.; Tognetti, R. Effects of Increasing Salinity Stress and Decreasing Water Availability on Ecophysiological Traits of Quinoa (Chenopodium quinoa Willd.) Grown in a Mediterranean-Type Agroecosystem. J. Agron. Crop Sci. 2013, 199, 229–240. [Google Scholar] [CrossRef]
- Bastidas, E.G.; Roura, R.; Rizzolo, D.A.D.; Massanes, T.; Gomis, R. Quinoa (Chenopodium quinoa Willd), from nutritional value to potential health benefits: An integrative review. J. Nutr. Food Sci. 2016, 6, 3. [Google Scholar]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and abiotic stresses in plants. In Abiotic and Biotic Stress in Plants; De Oliveira, A.B., Ed.; IntechOpen: London, UK, 2019; pp. 1–19. [Google Scholar]
- Ahmad, M.; Ali, Q.; Hafeez, M.M.; Malik, A. Improvement for biotic and abiotic stress tolerance in crop plants. Biol. Clin. Sci. Res. J. 2021, 2021, e004. [Google Scholar] [CrossRef]
- Scheben, A.; Yuan, Y.; Edwards, D. Advances in genomics for adapting crops to climate change. Curr. Plant Biol. 2016, 6, 2–10. [Google Scholar] [CrossRef]
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef]
- Saleem, M.A.; Ahmed Basra, S.M.; Afzal, I.; Iqbal, S.; Saddiq, M.S.; Naz, S. Exploring the Potential of Quinoa Accessions for Salt Tolerance in Soilless Culture. Int. J. Agric. Biol. 2017, 19, 233–240. [Google Scholar] [CrossRef]
- Stoleru, V.; Slabu, C.; Vitanescu, M.; Peres, C.; Cojocaru, A.; Covasa, M.; Mihalache, G. Tolerance of three Quinoa cultivars (Chenopodium quinoa Willd.) to salinity and alkalinity stress during germination stage. Agronomy 2019, 9, 287. [Google Scholar] [CrossRef]
- Cai, Z.Q.; Gao, Q. Comparative physiological and biochemical mechanisms of salt tolerance in five contrasting highland quinoa cultivars. BMC Plant Biol. 2020, 20, 70. [Google Scholar] [CrossRef]
- Shabala, S.; Hariadi, Y.; Jacobsen, S.-E. Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. J. Plant. Physiol. 2013, 170, 914. [Google Scholar] [CrossRef] [PubMed]
- Orsini, F.; Accorsi, M.; Gianquinto, G.; Dinelli, G.; Antognoni, F.; Carrasco, K.B.R.; Martinez, E.A.; Alnayef, M.; Marotti, I.; Bosi, S.; et al. Beyond the ionic and osmotic response to salinity in Chenopodium quinoa: Functional elements of successful halophytism. Funct. Plant Biol. 2011, 38, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Adolf, V.I.; Jacobsen, S.-E.; Shabala, S. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.). Environ. Exp. Bot. 2013, 92, 43–54. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Hussain, I.; Singh, H.; Singh, S.C. Plant-nanoparticle interaction: An approach to improve agricultural practices and plant productivity. Int. J. Pharm. Sci. Invent. 2015, 4, 25–40. [Google Scholar]
- Laware, S.L.; Raskar, S. Effect of titanium dioxide nanoparticles on hydrolytic and antioxidant enzymes during seed germination in onion. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 749–760. [Google Scholar]
- Hussein, M.M.; Abou-Baker, N.H. The contribution of nano zinc to alleviate salinity stress on cotton plants. R. Soc. Open Sci. 2018, 5, 171809. [Google Scholar] [CrossRef]
- Al Gethami, F.R.; El Sayed, H.E.S.A. Assessment Various Concentrations of ZnON anoparticles on Micropropagation for Chenopodium quinoa Willd. Plant. J. Adv. Biol. Biotechnol. 2020, 23, 33–42. [Google Scholar] [CrossRef]
- Mishra, V.; Mishra, R.K.; Dikshit, A.; Pandey, A.C. Interactions of nanoparticles with plants: An emerging prospective in the agriculture industry. In Emerging Technologies and Management of Crop Stress Tolerance; Academic Press: Cambridge, MA, USA, 2014; pp. 159–180. [Google Scholar]
- Michalek, S.; Swiecilo, A.; Molas, J. Effect of silver nanoparticles and ions on seeds epiphytic microorganisms’ activity and early stages of sweetcorn development. Przem. Chem. 2018, 97, 1654–1658. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Song, K.C.; Lee, S.M.; Park, T.S.; Lee, B.S. Preparation of colloidal silver nanoparticles by chemical reduction method. Korean J. Chem. Eng. 2009, 26, 153–155. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Jones, J.B., Jr. A Laboratory Guide of Exercises in Conducting Soil Test, and Plant Analyses; No. 631.42 J6; CRC: Horsham, PA, USA, 1984. [Google Scholar]
- Kirk, P.L. Kjeldahl method for total nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- SAS Institute, Inc. SAS/STAT 9.1 User’s Guide; SAS Institute, Inc.: Cary, NC, USA, 2004. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Ul Hussan, M.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar]
- Long, N.V. Effects of salinity stress on growth and yield of quinoa. Vietnam J. Agric. Sci. 2016, 14, 321–327. [Google Scholar]
- Jacobsen, S.E. The worldwide potential for quinoa (Chenopodium quinoa Willd.). Food Rev. Int. 2003, 19, 167–177. [Google Scholar] [CrossRef]
- Rahneshan, Z.; Nasibi, F.; Moghadam, A.A. Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. J. Plant Interact. 2018, 13, 73–82. [Google Scholar] [CrossRef]
- Husen, A.; Iqbal, M. Nanomaterials and plant potential: An overview. In Nanomaterials and Plant Potential; Springer: Berlin/Heidelberg, Germany, 2019; pp. 3–29. [Google Scholar]
- Mohamed, A.K.S.; Qayyum, M.F.; Abdel-Hadi, A.M.; Rehman, R.A.; Ali, S.; Rizwan, M. Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat. Arch. Agron. Soil Sci. 2017, 63, 1736–1747. [Google Scholar] [CrossRef]
- Aghdaei, M.; Salehi, H.; Sarmast, M.K. Effects of silver nanoparticles on Tecomella undulata (Roxb) Seem, micropropagation. Adv. Hortic. Sci. 2012, 26, 21–24. [Google Scholar]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.H.; Saradhi, P.P.; Khanna, P.K.; Arora, S. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 2012, 167, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Jasim, B.; Thomas, R.; Mathew, J.; Radhakrishnan, E.K. Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.). Saudi Pharm. J. 2012, 25, 443–447. [Google Scholar] [CrossRef]
- Wahid, I.; Kumari, S.; Ahmad, R.; Hussain, S.J.; Alamri, S.; Siddiqui, M.H.; Khan, M.I.R. Silver Nanoparticle Regulates Salt Tolerance in Wheat Through Changes in ABA Concentration, Ion Homeostasis, and Defense Systems. Biomolecules 2020, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Awan, S.A.; Raza, M.A.; Rizwan, M.; Tariq, R.; Ali, S.; Huang, L. Silver nanoparticles improved the plant growth and reduced the sodium and chlorine accumulation in pearl millet: A life cycle study. Environ. Sci. Pollut. Res. 2021, 28, 13712–13724. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, M.; Shabala, L.; Shabala, S. Physiological and molecular mechanisms mediating xylem Na+ loading in barley in the context of salinity stress tolerance. Plant Cell Environ. 2017, 40, 1009–1020. [Google Scholar] [CrossRef]
- Amjad, M.; Akhtar, S.S.; Yang, A.; Akhtar, J.; Jacobsen, S.E. Antioxidative response of quinoa exposed to iso-osmotic, ionic, and non-ionic salt stress. J. Agron. Crop Sci. 2015, 201, 452–460. [Google Scholar] [CrossRef]
- Rangani, J.; Panda, A.; Patel, M.; Parida, A.K. Regulation of ROS through proficient modulations of antioxidative defense system maintains the structural and functional integrity of photosynthetic apparatus and confers drought tolerance in the facultative halophyte Salvadora persica L. J. Photochem. Photobiol. B Biol. 2018, 189, 214–233. [Google Scholar] [CrossRef]
- Hatami, M.; Ghorbanpour, M. Defense enzyme activities and biochemical variations of Pelargonium zonale in response to nanosilver application and dark storage. Turk. J. Biol. 2014, 38, 130–139. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Faisal, M.; Al Sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 33, 2429–2437. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Alhmad, M.F.A.; Abdelfattah, K.E. The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J. Plant Growth Regul. 2017, 36, 60–70. [Google Scholar] [CrossRef]
- Salama, H.M. Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int. Res. J. Biotechnol. 2012, 3, 190–197. [Google Scholar]
- Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. The impact of cerium oxide nanoparticles on the salt stress responses of Brassica napus L. Environ. Pollut. 2016, 219, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Peterson, A.J.; Morris, C.F.; Murphy, K.M. Quinoa seed quality response to sodium chloride and sodium sulfate salinity. Front. Plant Sci. 2016, 7, 790. [Google Scholar] [CrossRef]
- Dawood, M.G.; Taie, H.A.A.; Nassar, R.M.A.; Abdelhamid, M.T.; Schmidhalter, U. The changes induced in the physiological, biochemical, and anatomical characteristics of Vicia faba by the exogenous application of proline under seawater stress. S. Afr. J. Bot. 2014, 93, 54–63. [Google Scholar] [CrossRef]
- Ramadan, A.A.; Abd Elhamid, E.M.; Sadak, M.S. Comparative study for the effect of arginine and sodium nitroprusside on sunflower plants grown under salinity stress conditions. Bull. Natl. Res. Cent. 2019, 43, 118. [Google Scholar] [CrossRef]

| Salinity Levels | Shoot Length (cm) | Leaf Number | Plant FW (mg) | Plant DW (mg) |
|---|---|---|---|---|
| 0 mM | 3.14 a,* | 6.90 a,b,c | 306.3 a | 17.14 a |
| 25 mM | 2.91 a | 6.10 a,b,c | 203.0 a,b,c | 13.05 a,b,c |
| 50 mM | 2.89 a | 7.50 a,b | 253.4 a,b | 15.27 a,b |
| 75 mM | 2.77 a | 8.50 a | 233.7 a,b | 14.20 a,b,c |
| 100 mM | 1.99 b | 8.00 a | 179.5 b,c | 11.81 a,b,c |
| 150 mM | 1.33 c | 4.80 bc | 140.7 b,c | 8.48 b,c |
| 200 mM | 1.04 c | 4.20 c | 103.00 c | 7.41 c |
| p-values | ||||
| TRT | <0.0001 | <0.0001 | <0.0001 | 0.0006 |
| Highest NaCl Level 200 mM NaCl + Ag NP Levels (mg/L) | Shoot Length (cm) | Leaf Number | Plant FW (mg) | Plant DW (mg) |
|---|---|---|---|---|
| 200 mM + 0.0 | 1.04 c,* | 4.20 c | 103.0 b | 7.41 c |
| 200 mM + 25.0 | 2.65 b | 5.10 b,c | 152.5 b | 10.14 b,c |
| 200 mM + 50.0 | 3.28 a,b | 6.40 a,b | 255.2 a | 13.70 a,b |
| 200 mM + 75.0 1 | 3.56 a | 7.90 a | 252.8 a | 14.55 a |
| p-values | ||||
| TRT | <0.0001 | <0.0001 | <0.0001 | 0.0002 |
| NaCl Levels | Chla (mg/g) | Chlb (mg/g) | Total Chl (mg/g) |
|---|---|---|---|
| 0 mM | 0.9314 a,* | 0.4591 a | 1.3879 a |
| 25 mM | 0.8278 b | 0.3730 b | 1.1986 b |
| 50 mM | 0.6712 c | 0.3245 b | 0.9939 c |
| 75 mM | 0.5825 d | 0.2494 c | 0.8304 d |
| 100 mM | 0.4300 e | 0.2345 c,d | 0.6632 e |
| 150 mM | 0.3430 f | 0.1728 d | 0.5148 f |
| 200 mM | 0.1832 g | 0.0732 e | 0.2559 g |
| p-values | |||
| TRT | <0.0001 | <0.0001 | <0.0001 |
| Highest Salinity Level 200 mM NaCl + Ag NP Levels (mg/L) | Chla (mg/g) | Chlb (mg/g) | Total Chl (mg/g) |
|---|---|---|---|
| 200 Mm + 0.0 | 0.9314 a,* | 0.4591 a | 1.3879 a |
| 200 Mm + 25.0 | 0.8278 b | 0.3730 b | 1.1986 b |
| 200 Mm + 50.0 | 0.6712 c | 0.3245 b | 0.9939 c |
| 200 Mm + 75.0 | 0.5825 d | 0.2494 c | 0.8304 d |
| p-values | |||
| TRT | <0.0001 | <0.0001 | <0.0001 |
| NaCl Levels | Ca (ppm) | P (ppm) | K (ppm) | Na (ppm) |
|---|---|---|---|---|
| 0 mM (control) | 13,507.00 a,* | 1375.65 a | 492.08 d | 624.17 e |
| 25 mM | 11,655.00 b | 1336.82 a.b | 525.83 d | 697.08 d,e |
| 50 mM | 10,245.00 c | 1270.45 b,c | 555.50 d | 755.50 d |
| 75 mM | 9395.77 d | 1213.19 c,d | 584.08 d | 784.08 d |
| 100 mM | 9152.63 e | 1147.97 d | 778.75 c | 949.58 c |
| 150 mM | 8342.50 f | 820.14 e | 1445.83 b | 1425.58 b |
| 200 mM | 6792.20 g | 735.97 f | 2567.00 a | 2734.50 a |
| p-values | ||||
| TRT | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Highest Salinity Level 200 mM NaCl + Ag NP Levels mg/L | Ca (ppm) | P (ppm) | K (ppm) | Na (ppm) |
|---|---|---|---|---|
| 200 Mm + 0.0 | 9300.58 d,* | 753.69 d | 2537.50 a | 2734.50 a |
| 200 Mm + 25.0 | 10,615.00 c | 1072.68 c | 1350.00 b | 1644.17 b |
| 200 Mm + 50.0 | 11,951.00 b | 1719.39 b | 778.75 c | 949.58 c |
| 200 Mm + 75.0 | 13,501.00 a | 2670.62 a | 450.92 d | 459.42 d |
| p-values | ||||
| TRT | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibli, R.; Mohusaien, R.; Abu-Zurayk, R.; Qudah, T.; Tahtamouni, R. Silver Nanoparticles (Ag NPs) Boost Mitigation Powers of Chenopodium Quinoa (Q6 Line) Grown under In Vitro Salt-Stressing Conditions. Water 2022, 14, 3099. https://doi.org/10.3390/w14193099
Shibli R, Mohusaien R, Abu-Zurayk R, Qudah T, Tahtamouni R. Silver Nanoparticles (Ag NPs) Boost Mitigation Powers of Chenopodium Quinoa (Q6 Line) Grown under In Vitro Salt-Stressing Conditions. Water. 2022; 14(19):3099. https://doi.org/10.3390/w14193099
Chicago/Turabian StyleShibli, Rida, Ruba Mohusaien, Rund Abu-Zurayk, Tamara Qudah, and Reham Tahtamouni. 2022. "Silver Nanoparticles (Ag NPs) Boost Mitigation Powers of Chenopodium Quinoa (Q6 Line) Grown under In Vitro Salt-Stressing Conditions" Water 14, no. 19: 3099. https://doi.org/10.3390/w14193099
APA StyleShibli, R., Mohusaien, R., Abu-Zurayk, R., Qudah, T., & Tahtamouni, R. (2022). Silver Nanoparticles (Ag NPs) Boost Mitigation Powers of Chenopodium Quinoa (Q6 Line) Grown under In Vitro Salt-Stressing Conditions. Water, 14(19), 3099. https://doi.org/10.3390/w14193099








