Fenton Process for Treating Acrylic Manufacturing Wastewater: Parameter Optimization, Performance Evaluation, Degradation Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater
2.2. Chemicals
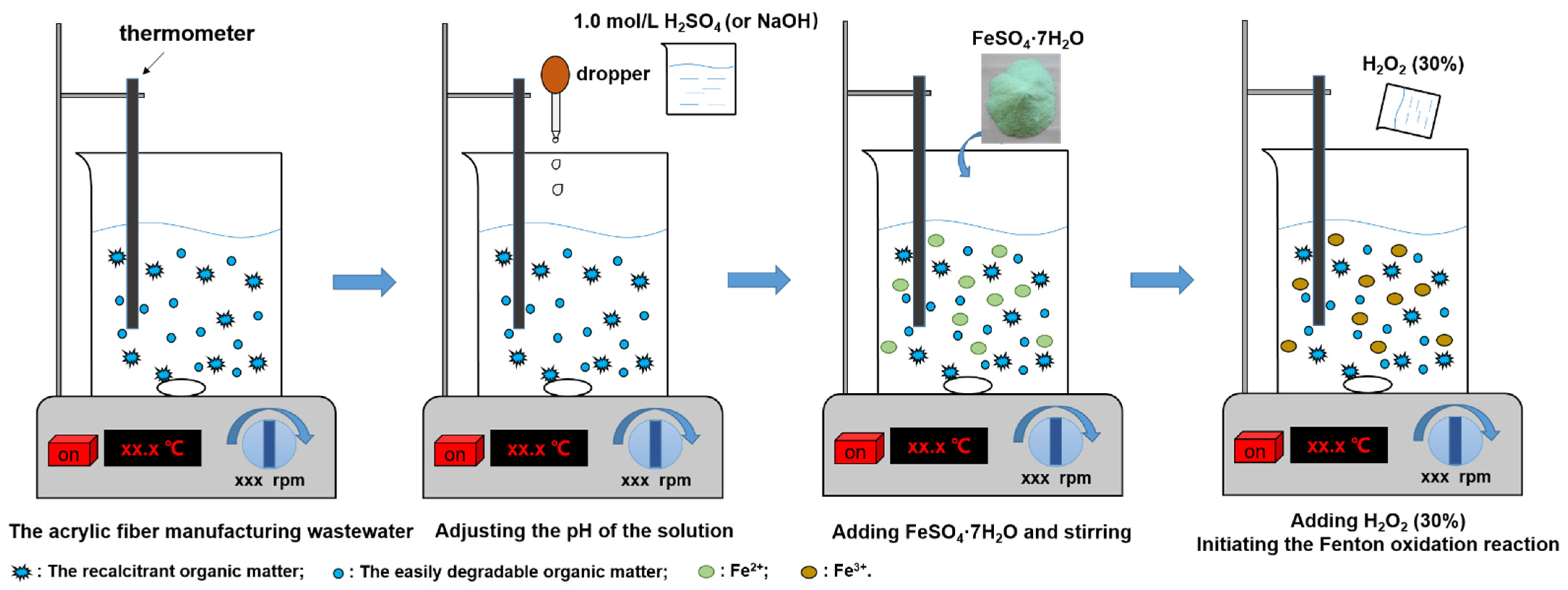
2.3. Experimental Method
2.4. Analytical Methods
3. Results and Discussion
3.1. Examination of Main Factors That Affect the Removal of COD
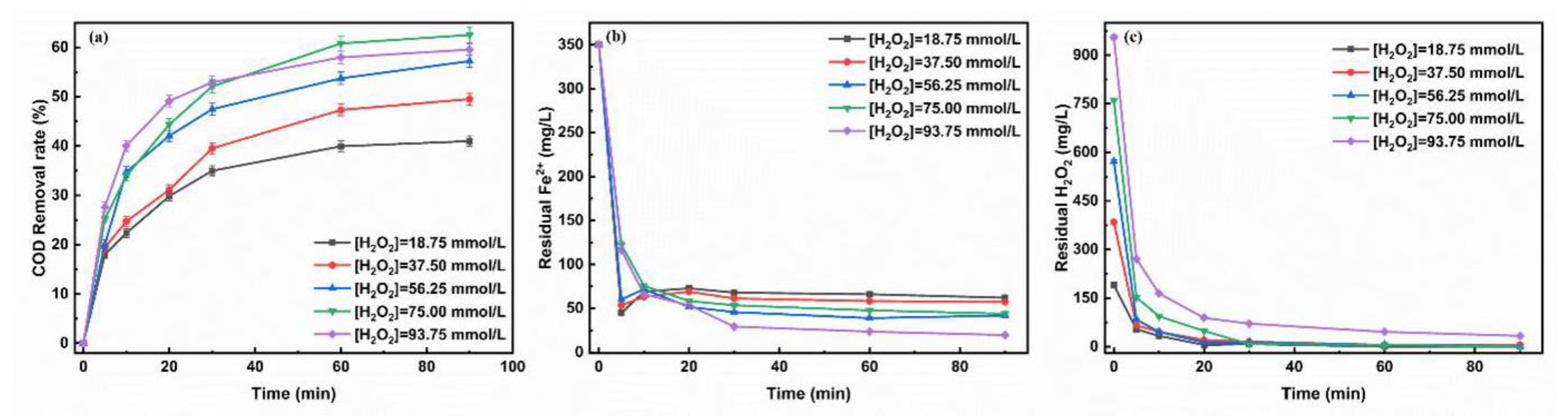
3.1.1. Initial Concentration of H2O2
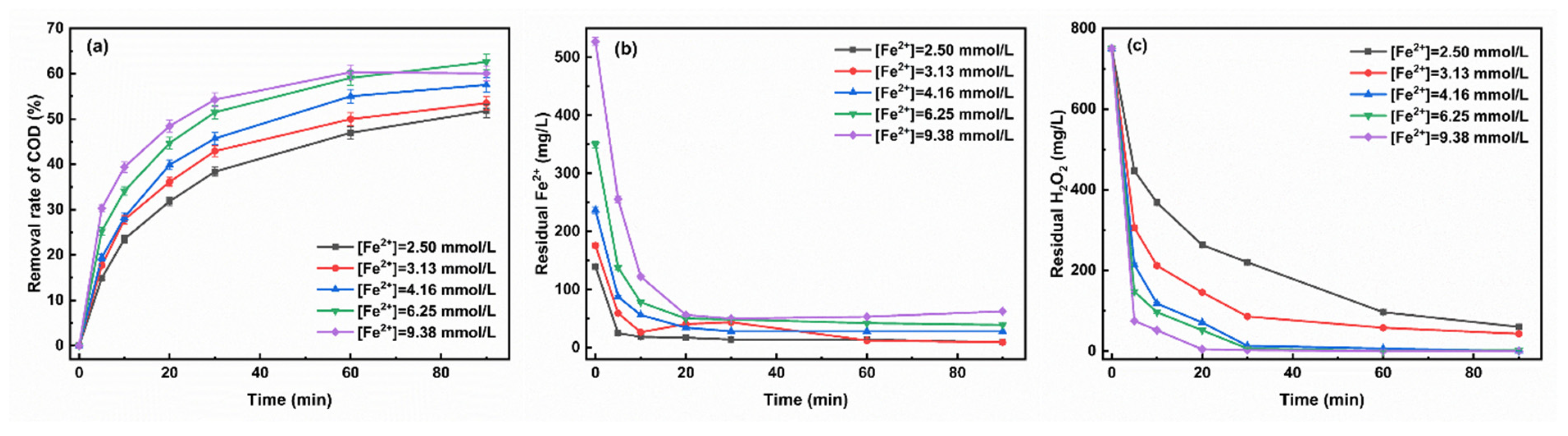
3.1.2. The Initial Concentration of Fe2+
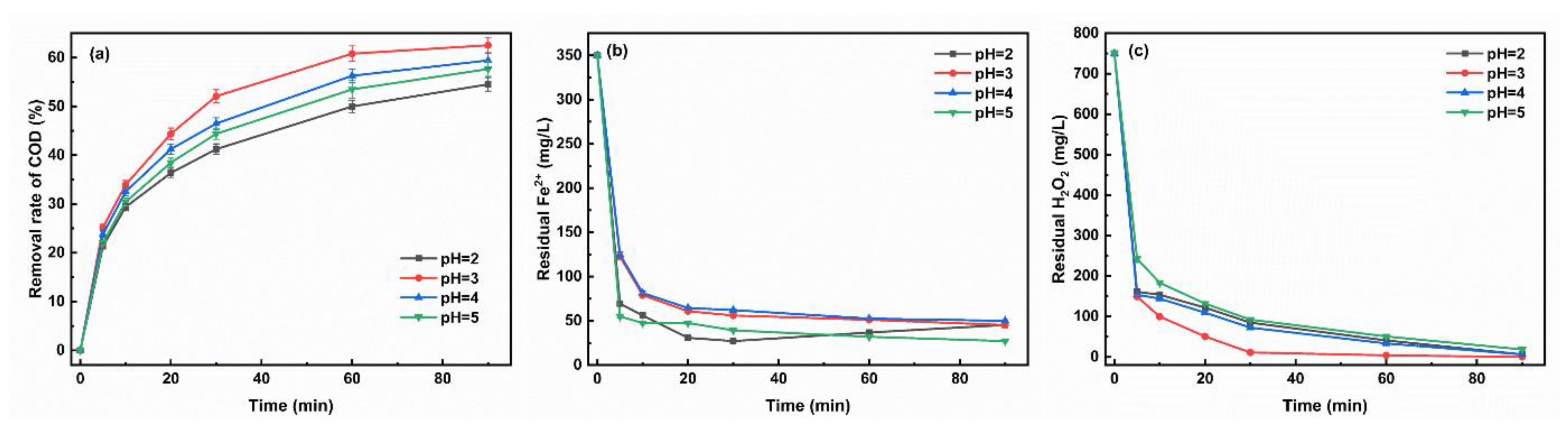
3.1.3. Initial pH
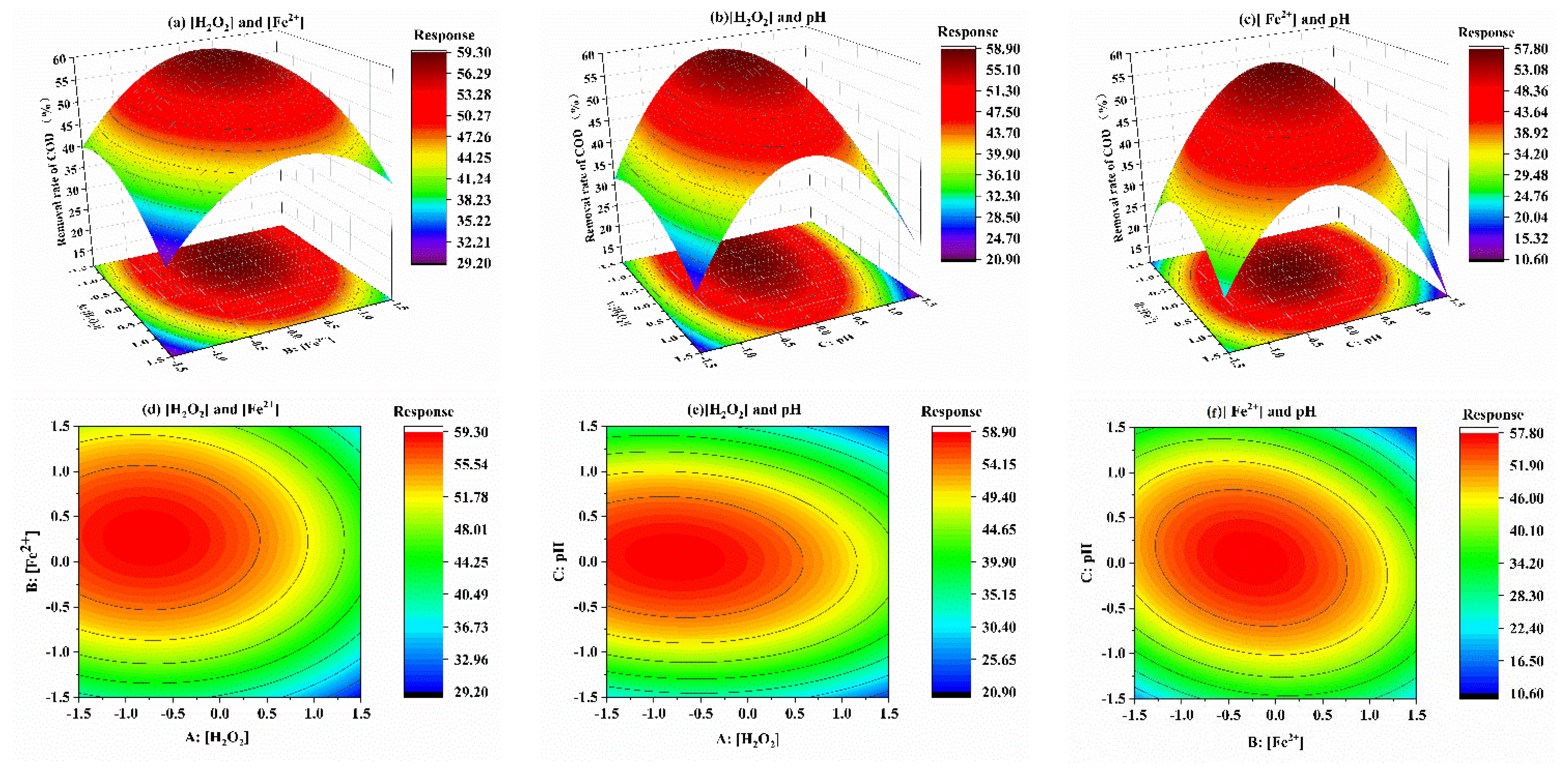
3.2. Response Surface Analysis
3.2.1. Regression Model and Analysis of Variance
3.2.2. Response Surface Optimization
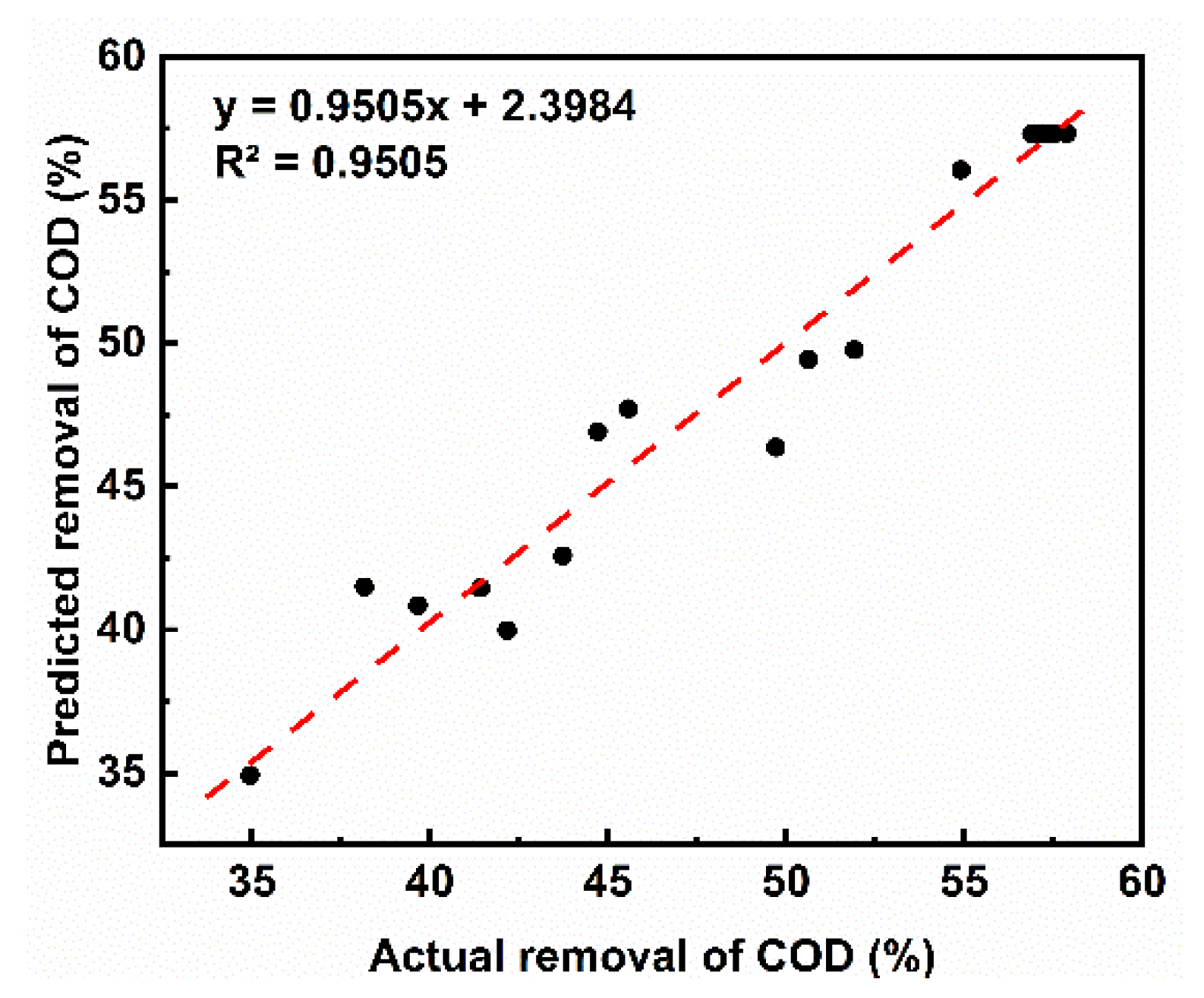
3.2.3. Verification of Optimal COD Degradation Conditions
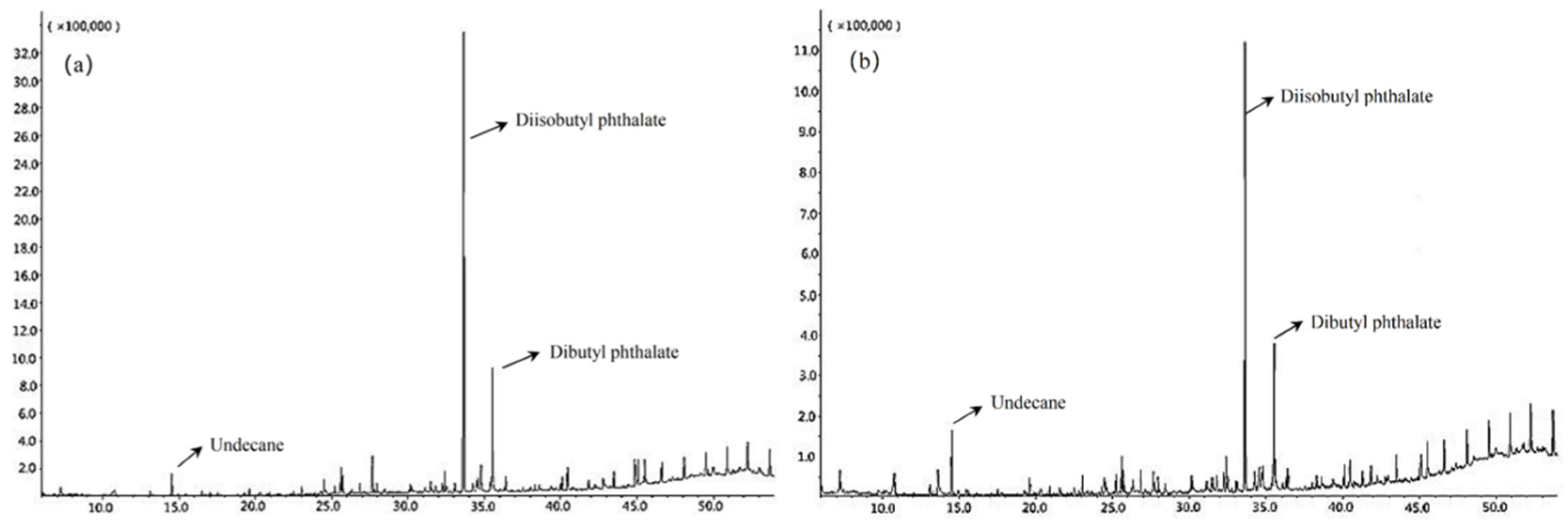
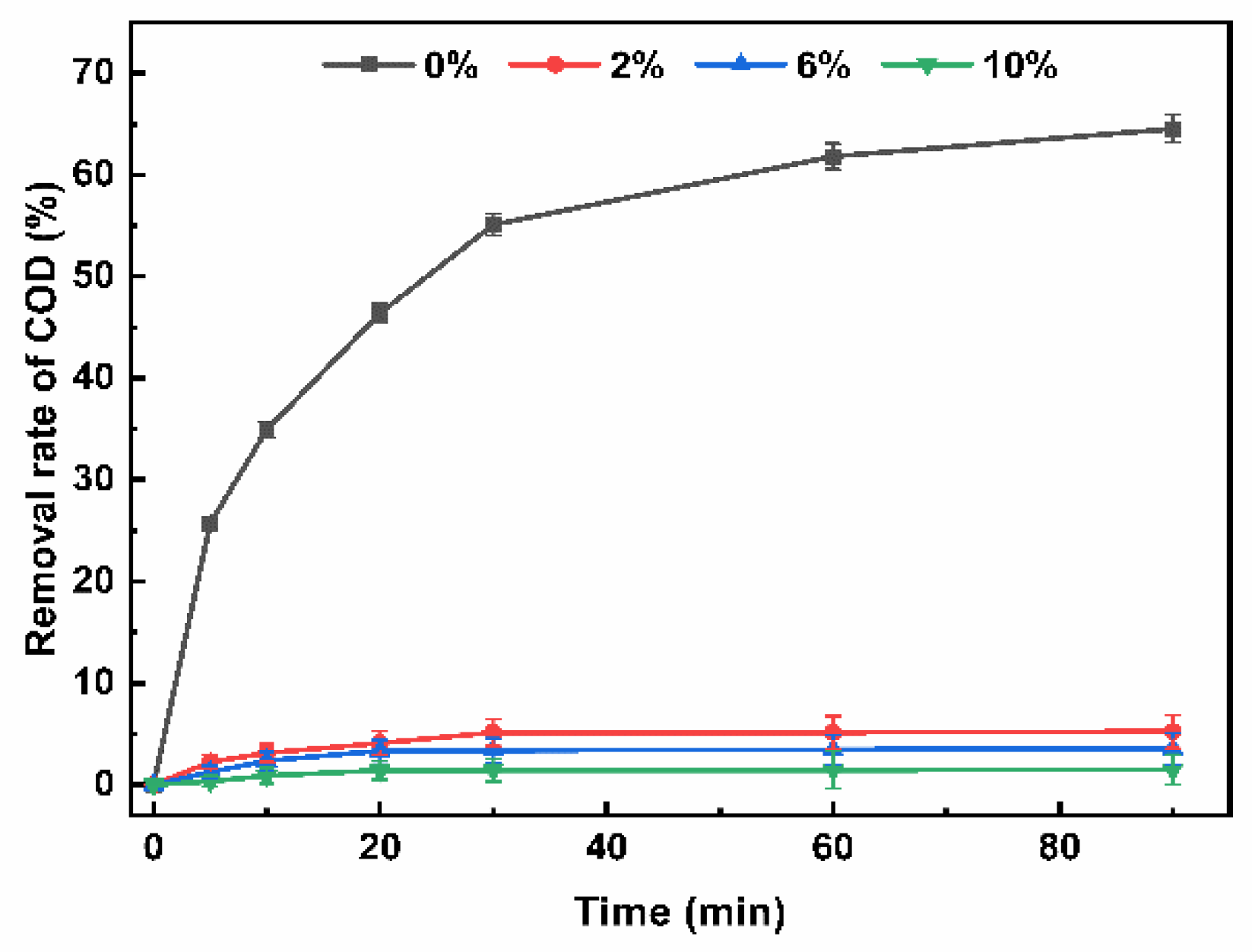
3.3. Removal Effect of Refractory Organic Matter
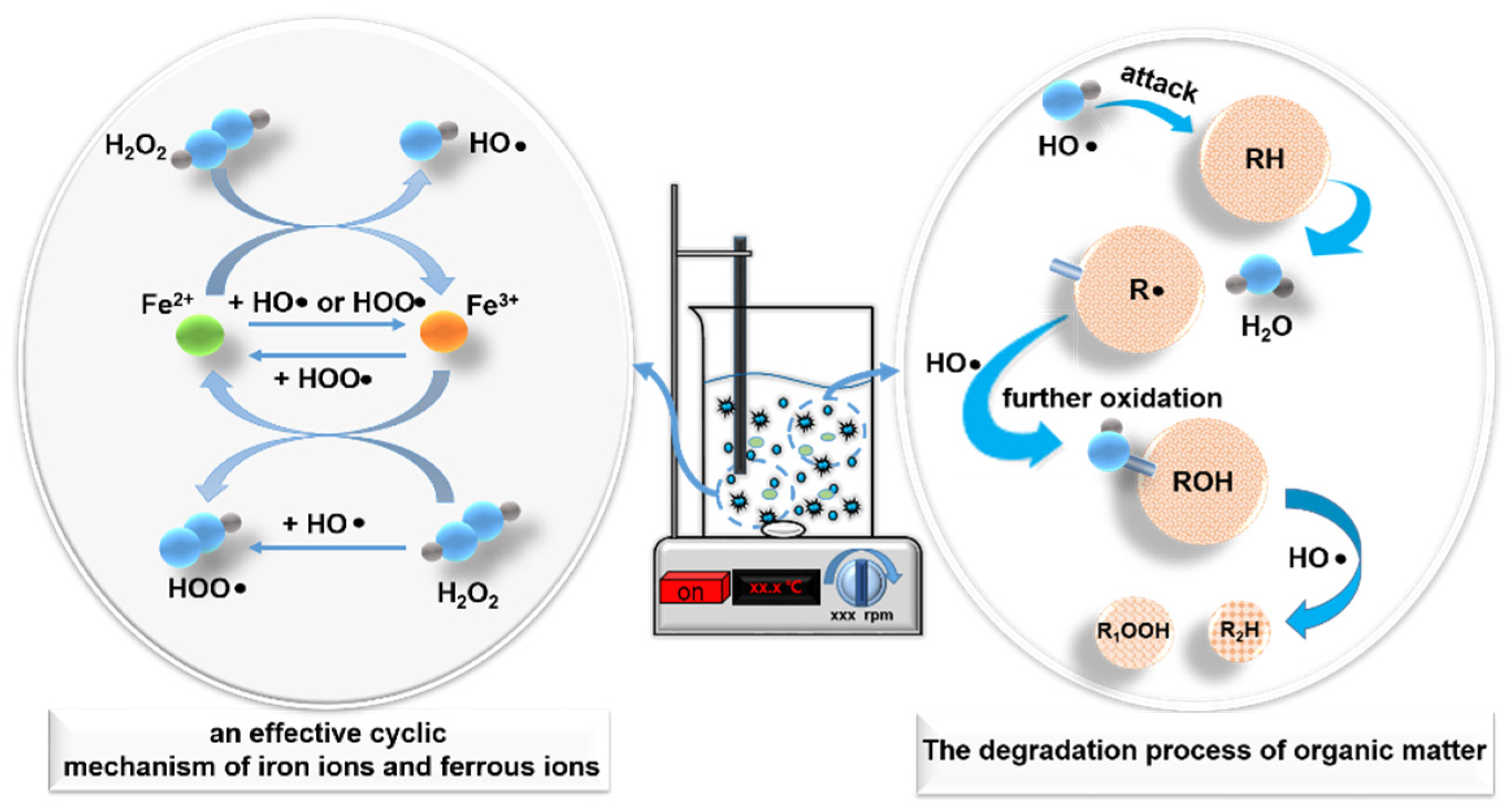
3.4. Mechanism Analysis
4. Conclusions
- (1)
- The optimization analysis combined with the response surface method showed that the optimal degradation conditions for acrylic fiber wastewater using the Fenton method are: (i) initial H2O2 concentration of 60.90 mmol/L; (ii) initial Fe2+ concentration of 7.44 mmol/L; (iii) pH of 3. The predicted degradation efficiency of the model equation was 59.8%, and the actual COD degradation rate was 63.2%.
- (2)
- The deviation between the actual and fitted model values was less than 5%, indicating that the model equation had a high degree of credibility. According to the analysis of the influence of factors and variables, it can be seen that the influence order of the three factors was [H2O2] > [Fe2+] > pH. In addition, the interaction between [Fe2+] and pH had the most significant impact on the degradation of COD.
- (3)
- For the actual acrylic fiber wastewater treatment, the removal rate of COD, TOC, NH4+-N, TN is 61.45%~66.51%, 67.82%~70.99%, 55.67%~60.97%, 56.45%~61.03%, respectively. The effluent met the textile dyeing and finishing industry water pollutant discharge standard “GB4287-2012”. The COD, TOC, NH4+-N, and TN were decreased to 93.3 ± 3.5 mg/L, 28.2 ± 0.7 mg/L, 26.4 ± 0.6 mg/L, 34.5 ± 0.6 mg/L, respectively.
- (4)
- HO• generated during electron transfer between H2O2 and Fe2+ effectively decompose organic pollutants in acrylic production wastewater. In this case, 13 kinds of aromatic hydrocarbons and long-chain alkanes in acrylic fiber wastewater had been effectively removed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pattnaik, P.; Dangayach, G.S.; Bhardwaj, A.K. A review on the sustainability of textile industries wastewater with and without treatment methodologies. Rev. Environ. Health 2018, 33, 163–203. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luan, Z.; Yu, L.; Ji, Z. Pretreatment of acrylic fiber manufacturing wastewater by the Fenton process. Desalination 2012, 284, 62–65. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Yamamoto, K.; Nakajima, F.; Fukushi, K. Bioaugmented membrane bioreactor (MBR) with a GAC-packed zone for high rate textile wastewater treatment. Water Res. 2011, 45, 2199–2206. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, G.; Meng, Q.; Xu, L.; Lv, B. Enhanced MBR by internal micro-electrolysis for degradation of anthraquinone dye wastewater. Chem. Eng. J. 2012, 210, 575–584. [Google Scholar] [CrossRef]
- Cinperi, N.C.; Ozturk, E.; Yigit, N.O.; Kitis, M. Treatment of woolen textile wastewater using membrane bioreactor, nanofiltration and reverse osmosis for reuse in production processes. J. Clean. Prod. 2019, 223, 837–848. [Google Scholar] [CrossRef]
- Deowan, S.A.; Galiano, F.; Hoinkis, J.; Johnson, D.; Altinkaya, S.A.; Gabriele, B.; Hilal, N.; Drioli, E.; Figoli, A. Novel low-fouling membrane bioreactor (MBR) for industrial wastewater treatment. J. Membr. Sci. 2016, 510, 524–532. [Google Scholar] [CrossRef]
- GilPavas, E.; Dobrosz-Gómez, I.; Gómez-García, M.Á. Coagulation-flocculation sequential with Fenton or Photo-Fenton processes as an alternative for the industrial textile wastewater treatment. J. Environ. Manag. 2017, 191, 189–197. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- Gong, C.; Zhang, Z.; Li, H.; Li, D.; Wu, B.; Sun, Y.; Cheng, Y. Electrocoagulation pretreatment of wet-spun acrylic fibers manufacturing wastewater to improve its biodegradability. J. Hazard. Mater. 2014, 274, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Song, Y.; Meng, X.; Pic, J.-S. Combination of Fenton oxidation and sequencing batch membrane bioreactor for treatment of dry-spun acrylic fiber wastewater. Environ. Earth Sci. 2015, 73, 4911–4921. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, Q.; Zhang, T.; Shi, Z.; Tian, Y.; Shi, S.; Smale, N.; Wang, J. Microbubble enhanced ozonation process for advanced treatment of wastewater produced in acrylic fiber manufacturing industry. J. Hazard. Mater. 2015, 287, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Song, Y.; Meng, X.; Tu, X.; Pic, J.-S. Transformation characteristics of organic pollutants in Fered-Fenton process for dry-spun acrylic fiber wastewater treatment. Water Sci. Technol. 2014, 70, 1976–1982. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, Q.; Shi, Z.; Fang, Y.; Shi, S.; Wang, J.; Wu, C. Advanced treatment of wet-spun acrylic fiber manufacturing wastewater using three-dimensional electrochemical oxidation. J. Environ. Sci. 2016, 50, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Han, X.; Lu, H.; Gao, Y.; Chen, X.; Yang, M. The role of in situ Fenton coagulation on the removal of benzoic acid. Chemosphere 2020, 238, 124632. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 37, 273–275. [Google Scholar] [CrossRef]
- He, D.-Q.; Zhang, Y.-J.; Pei, D.-N.; Huang, G.-X.; Liu, C.; Li, J.; Yu, H.-Q. Degradation of benzoic acid in an advanced oxidation process: The effects of reducing agents. J. Hazard. Mater. 2020, 382, 121090. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton Process and Related Electrochemical Technologies Based on Fenton’s Reaction Chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef] [PubMed]
- Walha, K.; Amar, R.B.; Quemeneur, F.; Jaouen, P. Treatment by nanofiltration and reverse osmosis of high salinity drilling water for seafood washing and processing Abstract. Desalination 2008, 219, 231–239. [Google Scholar] [CrossRef]
- Wei, J.; Song, Y.; Tu, X.; Zhao, L.; Zhi, E. Pretreatment of dry-spun acrylic fiber manufacturing wastewater by Fenton process: Optimization, kinetics and mechanisms. Chem. Eng. J. 2013, 218, 319–326. [Google Scholar] [CrossRef]
- Pérez, M.; Torrades, F.; Domènech, X.; Peral, J. Fenton and photo-Fenton oxidation of textile effluents. Water Res. 2002, 36, 2703–2710. [Google Scholar] [CrossRef]
- NEPA. Water Quality-Determination of the Chemical Oxygen Demand-Dichromate Method. China National Standard HJ828-2017; China National Environmental Protection Administration(NEPA), 2017. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201704/W020170606398873416325.pdf (accessed on 1 May 2017).
- NEPA. Water Quality-Determination of Ammonia Nitrogen-Nessler’s Reagent Spectrophotometry. China National Standard HJ535-2009; China National Environmental Protection Administration(NEPA), 2009. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201001/W020180319535685015821.pdf (accessed on 1 April 2010).
- NEPA. Water Quality—Determination of Iron—Phenanthroline Spectrophotometry. China National Standard HJ/T 345—2007; China National Environmental Protection Administration(NEPA), 2007. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/200703/W020120104561152753262.pdf (accessed on 1 May 2007).
- Zhao, H.; Dong, M.; Wang, Z.; Wang, H.; Qi, H. Roles of free radicals in NO oxidation by Fenton system and the enhancement on NO oxidation and H2O2 utilization efficiency. Environ. Technol. 2020, 41, 109–116. [Google Scholar] [CrossRef]
- LU, P. Spectrophotometric determination of hydrogen peroxide in Fenton advanced oxidation systems by potassium titanyl oxalate. Archit. Eng. Technol. Des. 2014, 582–582, 517. [Google Scholar] [CrossRef]
- Tekbaş, M.; Yatmaz, H.C.; Bektaş, N. Heterogeneous photo-Fenton oxidation of reactive azo dye solutions using iron exchanged zeolite as a catalyst. Microporous Mesoporous Mater. 2008, 115, 594–602. [Google Scholar] [CrossRef]
- Teel, A.L.; Warberg, C.R.; Atkinson, D.A.; Watts, R.J. Comparison of mineral and soluble iron Fenton’s catalysts for the treatment of trichloroethylene. Water Res. 2001, 35, 977–984. [Google Scholar] [CrossRef]
- Verma, M.; Haritash, A.K. Degradation of amoxicillin by Fenton and Fenton-integrated hybrid oxidation processes. J. Environ. Chem. Eng. 2019, 7, 102886. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Yousefi, Z.; Eslami, A.; Ardebilian, M.B. Municipal solid waste landfill leachate treatment by fenton, photo-fenton and fenton-like processes: Effect of some variables. Iran. J. Environ. Health Sci. Eng. 2012, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B: Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Rott, E.; Minke, R.; Bali, U.; Steinmetz, H. Removal of phosphonates from industrial wastewater with UV/FeII, Fenton and UV/Fenton treatment. Water Res. 2017, 122, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.L.; Gan, S.; Ng, H.K. Fenton based remediation of polycyclic aromatic hydrocarbons-contaminated soils. Chemosphere 2011, 83, 1414–1430. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Cannon, F.S.; Nieto-Delgado, C.; Brown, N.R.; Gu, X. Effect of preparation protocol on anchoring quaternary ammonium/epoxide-forming compound into granular activated carbon for perchlorate adsorption: Enhancement by Response Surface Methodology. Chem. Eng. J. 2013, 223, 309–317. [Google Scholar] [CrossRef]
- Luo, D. Optimization of total polysaccharide extraction from Dioscorea nipponica Makino using response surface methodology and uniform design. Carbohydr. Polym. 2012, 90, 284–288. [Google Scholar] [CrossRef]
- Behera, S.K.; Meena, H.; Chakraborty, S.; Meikap, B.C. Application of response surface methodology (RSM) for optimization of leaching parameters for ash reduction from low-grade coal. Int. J. Min. Sci. Technol. 2018, 28, 621–629. [Google Scholar] [CrossRef]
- NEPA. Discharge Standards of Water Pollutants for Dyeing and Finishing of Textile Industry. China National Standard GB4287-2012. China National Environmental Protection Administration(NEPA), 2012. Available online: https://www.doc88.com/p-998284323481.html?r=1 (accessed on 1 January 2013).
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef]
- Cai, Q.Q.; Lee, B.C.Y.; Ong, S.L.; Hu, J.Y. Fluidized-bed Fenton technologies for recalcitrant industrial wastewater treatment–Recent advances, challenges and perspective. Water Res. 2021, 190, 116692. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. Advanced oxidation of phenol: A comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem. Eng. J. 2012, 183, 1–9. [Google Scholar] [CrossRef]
- Gu, X.; Qin, N.; Zhang, P.; Hu, Y.; Zhang, Y.-N.; Zhao, G. In-situ synthesis of {111}TiO2/Ti photoelectrode to boost efficient removal of dimethyl phthalate based on a bi-functional interface. Chem. Eng. J. 2021, 422, 129980. [Google Scholar] [CrossRef]
- Liang, D.; Li, N.; An, J.; Ma, J.; Wu, Y.; Liu, H. Fenton-based technologies as efficient advanced oxidation processes for microcystin-LR degradation. Sci. Total Environ. 2021, 753, 141809. [Google Scholar] [CrossRef] [PubMed]
| Parameter | COD mg·L−1 | BOD5 mg·L−1 | TOC mg·L−1 | NH3-N mg·L−1 | TN mg·L−1 | BOD5/COD | pH |
|---|---|---|---|---|---|---|---|
| Amount | 249–270 | 8–10 | 90–100 | 60–66 | 78–95 | 0.030–0.040 | 5.4–5.8 |
| Run | Factor | Response, R (%) | |||||
|---|---|---|---|---|---|---|---|
| A: [H2O2] | B: [Fe2+] | C: pH | |||||
| Coded Level | Corresponding Value (mmol·L−1) | Coded Level | Corresponding Value (mmol·L−1) | Coded Level | - | ||
| S1 | −1 | 56.25 | −1 | 4.16 | 0 | 3 | 51.92 ± 1.25 |
| S2 | 1 | 93.75 | −1 | 4.16 | 0 | 3 | 43.73 ± 1.02 |
| S3 | −1 | 56.25 | 1 | 9.38 | 0 | 3 | 54.91 ± 1.28 |
| S4 | 1 | 93.75 | 1 | 9.38 | 0 | 3 | 45.57 ± 1.06 |
| S5 | −1 | 56.25 | 0 | 6.25 | −1 | 2 | 44.72 ± 0.98 |
| S6 | 1 | 93.75 | 0 | 6.25 | −1 | 2 | 39.66 ± 0.89 |
| S7 | −1 | 56.25 | 0 | 6.25 | 1 | 4 | 50.63 ± 1.05 |
| S8 | 1 | 93.75 | 0 | 6.25 | 1 | 4 | 42.17 ± 1.07 |
| S9 | 0 | 75.00 | −1 | 4.16 | −1 | 2 | 34.95 ± 0.67 |
| S10 | 0 | 75.00 | 1 | 9.38 | −1 | 2 | 49.71 ± 1.09 |
| S11 | 0 | 75.00 | −1 | 4.16 | 1 | 4 | 38.16 ± 0.88 |
| S12 | 0 | 75.00 | 1 | 9.38 | 1 | 4 | 41.42 ± 0.87 |
| S13 | 0 | 75.00 | 0 | 6.25 | 0 | 3 | 57.04 ± 1.35 |
| S14 | 0 | 75.00 | 0 | 6.25 | 0 | 3 | 57.88 ± 1.39 |
| S15 | 0 | 75.00 | 0 | 6.25 | 0 | 3 | 56.89 ± 1.38 |
| S16 | 0 | 75.00 | 0 | 6.25 | 0 | 3 | 57.28 ± 1.42 |
| S17 | 0 | 75.00 | 0 | 6.25 | 0 | 3 | 57.54 ± 1.36 |
| Source | Sum of Squares (SS) | Degree of Freedom (df) | Mean Square (MS) | F-Value (F) | p-Value (p) |
|---|---|---|---|---|---|
| Model | 906.22 | 9 | 100.69 | 14.94 | 0.0009 |
| A-[H2O2] | 120.51 | 1 | 120.51 | 17.89 | 0.0039 |
| B-[Fe2+] | 65.27 | 1 | 65.27 | 9.69 | 0.0170 |
| C-pH | 1.39 | 1 | 1.39 | 0.21 | 0.6629 |
| A × B | 0.33 | 1 | 0.33 | 0.049 | 0.8310 |
| A × C | 2.89 | 1 | 2.89 | 0.43 | 0.5334 |
| B × C | 33.06 | 1 | 33.06 | 4.91 | 0.0623 |
| A2 | 26.94 | 1 | 26.94 | 4.00 | 0.0857 |
| B2 | 139.90 | 1 | 139.90 | 20.76 | 0.0026 |
| C2 | 464.37 | 1 | 464.37 | 68.92 | <0.0001 |
| Residual | 47.16 | 7 | 6.74 | ||
| Lack of Fit | 46.54 | 3 | 15.51 | 99.01 | 0.0003 |
| Pure Error | 0.63 | 4 | 0.16 | ||
| Cor Total | 953.38 | 16 | |||
| R2 | 0.95 | ||||
| Raj2 | 0.89 |
| Water Quality Index | Influent | Effluent | Emission Limit |
|---|---|---|---|
| pH | 5.5 ± 0.2 | 6.4 ± 0.2 | 6~9 |
| COD/(mg·L−1) | 259.6 ± 8.5 | 93.3 ± 3.5 | 200 |
| TOC/(mg·L−1) | 92.3 ± 2.5 | 28.2 ± 0.7 | 30 |
| NH4+-N/(mg·L−1) | 63.5 ± 2.6 | 26.4 ± 0.6 | 25 |
| TN/(mg·L−1) | 83.8 ± 3.2 | 34.5 ± 0.6 | 50 |
| Number | Peak Time (min) | Organic Matter | Structural Formula | Molecular Formula | The Degree of Match (%) | Occasion | |
|---|---|---|---|---|---|---|---|
| Influent | Effluent | ||||||
| 1 | 14.532 | Undecane |  | C11H24 | 94 | √ | √ |
| 2 | 24.509 | 1,4,5-Trimethylnaphthalene | 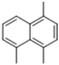 | C13H14 | 62 | √ | |
| 3 | 25.643 | 2,6-Di-tert-butyl-4-methylphenol | 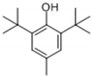 | C15H24O | 96 | √ | |
| 4 | 25.732 | 2,4-di- tert -butylphenol | 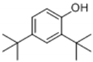 | C14H22O | 94 | √ | |
| 5 | 27.657 | 2,2,4-trimethyl-1,3-pentanediol diisobutyrate |  | C16H30O4 | 59 | √ | |
| 6 | 32.404 | Octadecane |  | C18H38 | 99 | √ | |
| 7 | 33.656 | Diisobutyl phthalate |  | C16H22O4 | 90 | √ | √ |
| 8 | 35.535 | Dibutyl phthalate | 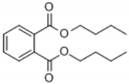 | C16H22O4 | 94 | √ | √ |
| 9 | 40.446 | 2,6-diphenylpyridine | 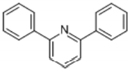 | C17H13N | 97 | √ | |
| 10 | 43.488 | Tetracosane |  | C24H50 | 98 | √ | |
| 11 | 44.882 | Benzo(H)quinoline | 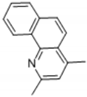 | C15H13N | 50 | √ | |
| 12 | 45.073 | Heneicosane |  | C21H44 | 93 | √ | |
| 13 | 45.51 | DEHP, di-(2-ethylhexyl) phthalate. |  | C16H22O4 | 90 | √ | |
| 14 | 46.617 48.100 49.528 | Hexacosane |  | C26H54 | 98 | √ | |
| 15 | 50.907 53.703 | Octacosane | 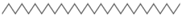 | C28H58 | 98 | √ | |
| 16 | 52.240 | Eicosane |  | C20H42 | 98 | √ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Zhang, C.; Su, P.; Lu, W.; Zhang, Z.; Wang, X.; Hu, W. Fenton Process for Treating Acrylic Manufacturing Wastewater: Parameter Optimization, Performance Evaluation, Degradation Mechanism. Water 2022, 14, 2913. https://doi.org/10.3390/w14182913
Lin Z, Zhang C, Su P, Lu W, Zhang Z, Wang X, Hu W. Fenton Process for Treating Acrylic Manufacturing Wastewater: Parameter Optimization, Performance Evaluation, Degradation Mechanism. Water. 2022; 14(18):2913. https://doi.org/10.3390/w14182913
Chicago/Turabian StyleLin, Zhiwei, Chunhui Zhang, Peidong Su, Wenjing Lu, Zhao Zhang, Xinling Wang, and Wanyue Hu. 2022. "Fenton Process for Treating Acrylic Manufacturing Wastewater: Parameter Optimization, Performance Evaluation, Degradation Mechanism" Water 14, no. 18: 2913. https://doi.org/10.3390/w14182913
APA StyleLin, Z., Zhang, C., Su, P., Lu, W., Zhang, Z., Wang, X., & Hu, W. (2022). Fenton Process for Treating Acrylic Manufacturing Wastewater: Parameter Optimization, Performance Evaluation, Degradation Mechanism. Water, 14(18), 2913. https://doi.org/10.3390/w14182913







