On the Presence and Ubiquity of the Exotic Batophora (J. Agardh) in the Mar Menor Lagoon (SE Spain)
Abstract
:1. Introduction
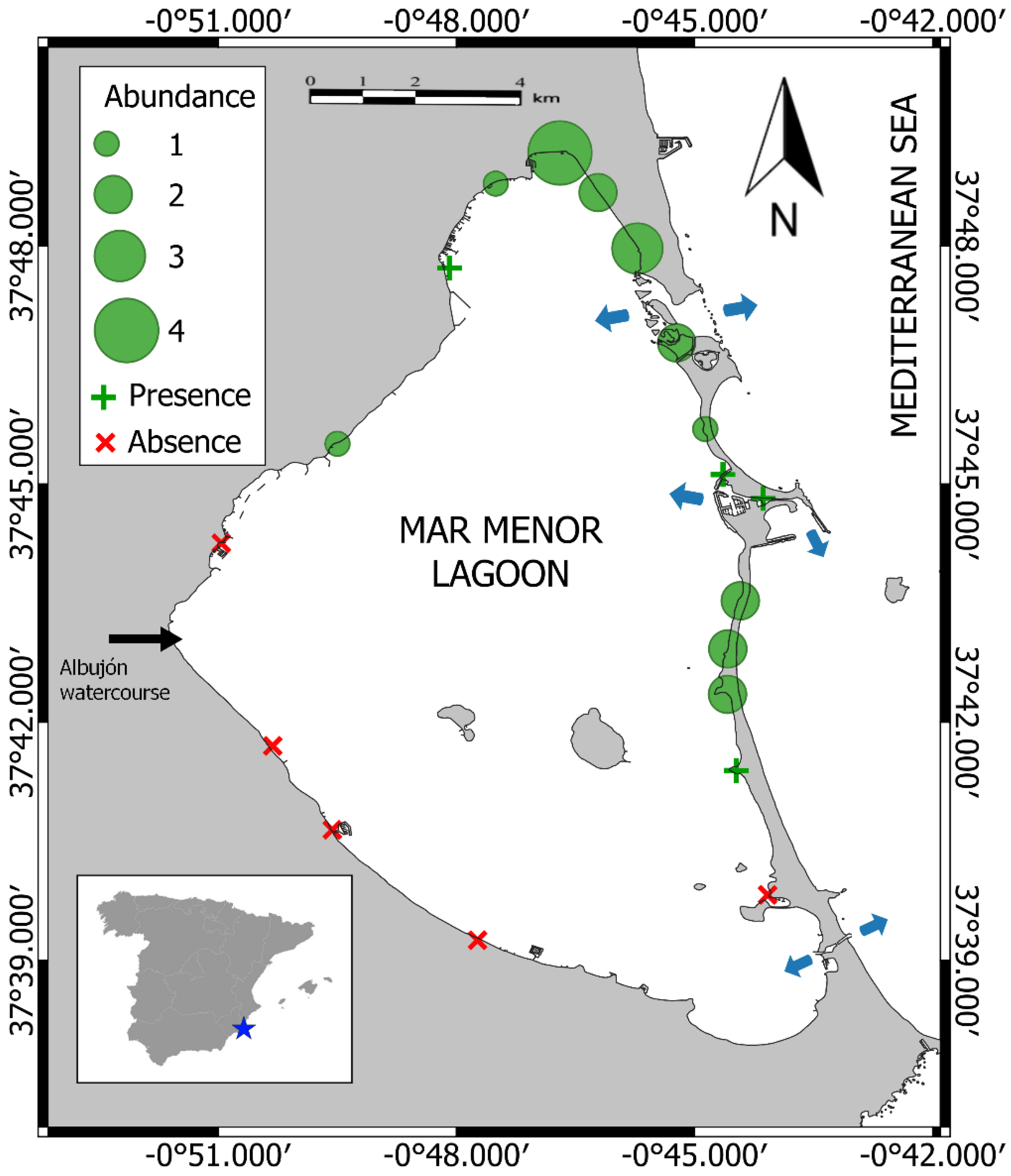
2. Materials and Methods
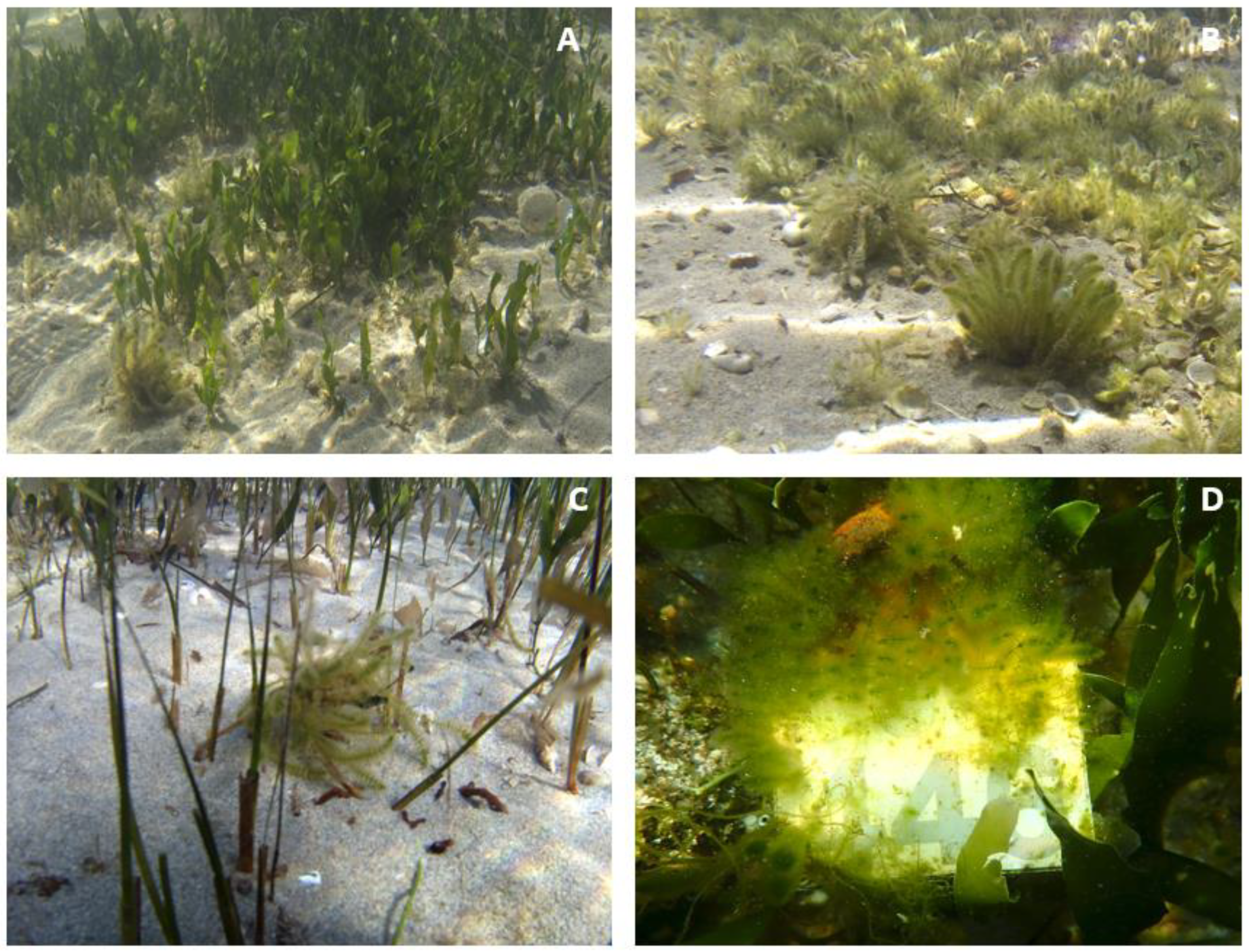
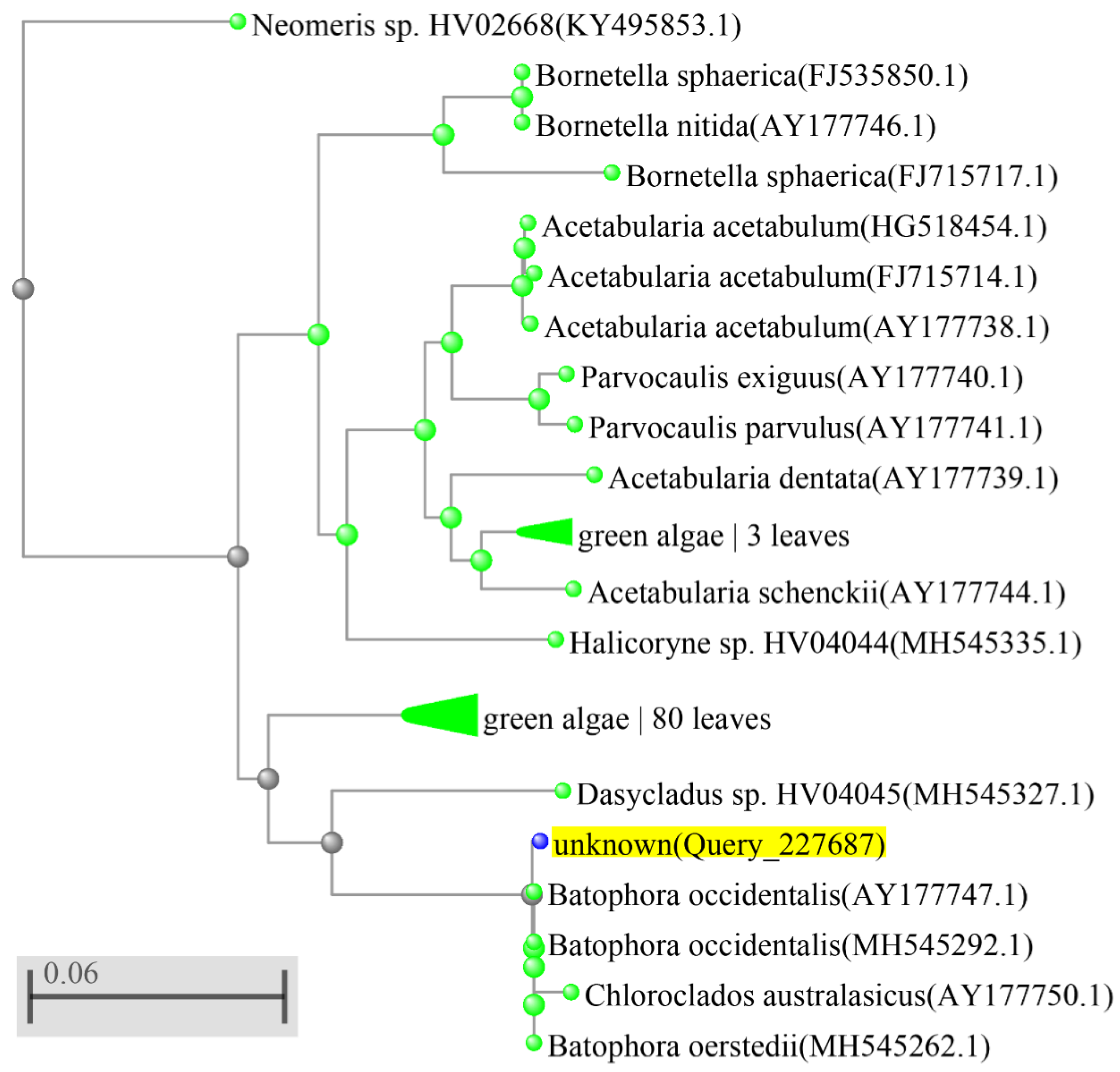
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Deposition of Sequences and of Expression Data
Acknowledgments
Conflicts of Interest
References
- Pérez-Ruzafa, A.; Marcos, C.; Bernal, C.M.; Quintino, V.; Freitas, R.; Rodrigues, A.M.; García-Sánchez, M.; Pérez-Ruzafa, I.M. Cymodocea nodosa vs. Caulerpa prolifera: Causes and consequences of a long-term history of interaction in macrophyte meadows in the Mar Menor coastal lagoon (Spain, southwestern Mediterranean). Estuar. Coast. Shelf Sci. 2012, 110, 101–115. [Google Scholar] [CrossRef]
- Álvarez-Rogel, J.; Barberá, G.G.; Maxwell, B.; Guerrero-Brotons, M.; Díaz-García, C.; Martínez-Sánchez, J.J.; Sallent, A.; Martínez-Ródenas, J.; González-Alcaraz, M.N.; Jiménez-Cárceles, F.J.; et al. The case of Mar Menor eutrophication: State of the art and description of tested Nature-Based Solutions. Ecol. Eng. 2020, 158, 106086. [Google Scholar] [CrossRef]
- Terrados, J.; Ros, J.D. Production dynamics in a macrophyte-dominated ecosystem: The Mar Menor coastal lagoon. Oecol. Aquat. 1991, 10, 255–270. [Google Scholar]
- Belando, M.D.; Bernardeau-Esteller, J.; Paradinas, I.; Ramos-Segura, A.; García-Muñoz, R.; García-Moreno, P.; Marín-Guirao, L.; Ruiz, J.M. Long-term coexistence between the macroalga Caulerpa prolifera and the seagrass Cymodocea nodosa in a Mediterranean lagoon. Aquat. Bot. 2021, 173, 103415. [Google Scholar] [CrossRef]
- Conesa, H.M.; Jiménez-Cárceles, F.J. The Mar Menor lagoon (SE Spain): A singular natural ecosystem threatened by human activities. Mar. Poll. Bull. 2007, 54, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Marcos, C.; Torres, I.; López-Capel, A.; Pérez-Ruzafa, A. Long term evolution of fisheries in a coastal lagoon related to changes in lagoon ecology and human pressures. Rev. Fish Biol. Fish. 2015, 25, 689–713. [Google Scholar] [CrossRef]
- García-Oliva, M.; Pérez-Ruzafa, Á.; Umgiesser, G.; McKiver, W.; Ghezzo, M.; De Pascalis, F.; Marcos, C. Assessing the hydrodynamic response of the Mar Menor lagoon to dredging inlets interventions through numerical modelling. Water 2018, 10, 959. [Google Scholar] [CrossRef]
- Giménez-Casalduero, F.; Marcos-Diego, C.; Oliva-Paterna, F.J.; Pérez-Ruzafa, A.; Robledano-Aymerich, F.; Torralva-Forero, M.M. Ecología lagunar. In Informe Integral Sobre el Estado Ecológico del Mar Menor; Comité de Asesoramiento Científico del Mar Menor: Murcia, Spain, 2017; Available online: https://www.miteco.gob.es/es/agua/temas/concesiones-y-autorizaciones/apendice14a19_tcm30-451146.pdf (accessed on 15 April 2022).
- Giménez-Casalduero, F.; Gomariz-Castillo, F.; Alonso-Sarría, F.; Cortés, E.; Izquierdo-Muñoz, A.; Ramos-Esplá, A.A. Pinna nobilis in the Mar Menor coastal lagoon: A story of colonization and uncertainty. Mar. Ecol. Prog. Ser. 2020, 652, 77–94. [Google Scholar] [CrossRef]
- Marín-Guirao, L.; Marín-Atucha, A.; Lloret-Barba, J.; Martínez-López, E.; García-Fernández, A.J. Effects of mining wastes on a seagrass ecosystem: Metal accumulation and bioavailability, seagrass dynamics and associated community structure. Mar. Environ. Res. 2005, 60, 317–337. [Google Scholar] [CrossRef]
- Lloret, J.; Marin, A.; Marin-Guirao, L.; Velasco, J. Changes in macrophytes distribution in a hypersaline coastal lagoon associated with the development of intensively irrigated agriculture. Ocean Coast. Manag. 2005, 48, 828–842. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Clemente-Navarro, P.; Mercado, J.M.; Fraile-Nuez, E.; Albentosa, M.; Marín-Guirao, L.; Santos, J. Nuevo Evento de Mortalidad Masiva de Organismos Marinos en el Mar Menor: Contexto y Factores; Informe de Asesoramiento Técnico del Instituto Español de Oceanografía (IEO): Murcia, Spain, 2021; 24p, Available online: http://www.ieo.es/documents/10640/38594/informe+IEO_MarMenor_60921.pdf/36c4e2fc-7ab3-420d-b73d-8cf33478885e (accessed on 15 April 2022).
- Giménez-Casalduero, F.; Ramos Esplá, A.A.; Izquierdo Muñoz, A.; Gomaríz Castillo, F.; Martínez Hernández, F.J.; González-Carrión, F. Mar Menor: Una Laguna Singular y Sensible. Evaluación Científica de su Estado; Alloctonous Marine Invertebrates in the Mar Menor Lagoon; Temas de Oceanografía, 9; Leon, V.M., Bellido, J.M., Eds.; Instituto Español de Oceanografía (IEO), Ministerio de Economía y Competitividad: Spain, 2016; pp. 157–178. Available online: http://www.ieo.es/documents/10640/31690/Temas+de+Oceanografía+09+-.pdf/72df5dbb-7de0-4286-89c2-cc3c7dc7c169 (accessed on 15 April 2022).
- Roman, S.; Perez-Ruzafa, A.; Lopez, E. First record in the Western Mediterranean Sea of Branchiomma boholense (Grube, 1878) (Polychaeta: Sabellidae), an alien species of Indo-Pacific origin. Cah. Biol. Mar. 2009, 50, 241–250. [Google Scholar]
- Serio, D.; Cormaci, M.; Furnari, G.; Boisset, F. First record of Palisada maris-rubri (Ceramiales, Rhodophyta) from the Mediterranean Sea along with three proposed transfers to the genus Palisada. Phycol. Res. 2010, 58, 9–16. [Google Scholar] [CrossRef]
- Marambio, M.; Franco, I.; Purcell, J.E.; Canepa, A.; Guerrero, E.; Fuentes, V. Aggregations of the invasive ctenophore Mnemiopsis leidyi in a hypersaline environment, the Mar Menor lagoon (NW Mediterranean). Aquat. Invasions 2013, 8, 243–248. [Google Scholar] [CrossRef]
- González-Wangüemert, M.; Domínguez-Godino, J.; Giménez-Casalduero, F.; Serrão, E.A. Genetic signature of a recent invasion: The ragged sea hare Bursatella leachii in Mar Menor (SE Spain). Biochem. Syst. Ecol. 2014, 54, 123–129. [Google Scholar] [CrossRef]
- López Soriano, J.; Quiñonero Salgado, S.; Verdejo Guirao, J.F.; Pla Ventura, M. Primeras citas de Cerithium scabridum Philippi, 1848 (Gastropoda: Cerithiidae) para la península ibérica. NEMUS 2018, 8, 133–136. [Google Scholar]
- Ballesteros, E.; Torras, X.; Pinedo, S.; García, M.; Mangialajo, L.; De Torres, M. A new methodology based on littoral community cartography dominated by macroalgae for the implementation of the European Water Framework Directive. Mar. Poll. Bull. 2007, 55, 172–180. [Google Scholar] [CrossRef]
- Coat, G.; Dion, P.; Noailles, M.C.; De Reviers, B.; Fontaine, J.M.; Berger-Perrot, Y.; Loiseaux-De Goër, S. Ulva armoricana (Ulvales, Chlorophyta) from the coasts of Brittany (France). II. Nuclear rDNA ITS sequence analysis. Eur. J. Phycol. 1998, 33, 81–86. [Google Scholar] [CrossRef]
- Zechman, F.W. Phylogeny of the Dasycladales (Chlorophyta, Ulvophyceae) based on analyses of rubisco large subunit (rbcL) gene sequences 1. J. Phycol. 2003, 39, 819–827. [Google Scholar] [CrossRef]
- Lefort, V.; Desper, R.; Gascuel, O. FastME 2.0: A Comprehensive, Accurate, and Fast Distance-Based Phylogeny Inference Program. Mol. Biol. Evol. 2015, 32, 2798–2800. [Google Scholar] [CrossRef]
- Dawes, C.J.; Mathieson, A.C. The Seaweeds of Florida; University Press of Florida: Gainesville, FL, USA, 2008; 591p. [Google Scholar]
- Suárez, A.M.; Martínez-Daranas, B.; Yusimí, A. Macroalgas Marinas de Cuba; Editorial UH: Habana, Cuba, 2015; 264p. [Google Scholar]
- Wynne, M.J. A checklist of benthic marine algae of the tropical and subtropical western Atlantic: Fourth revision. Nova Hedwigia Beiheft 2017, 145, 7–202. [Google Scholar]
- Reyes, J.; Sansón, M.; Afonso-Carrillo, J. Notes on some interesting marine algae new from the Canary Islands. Cryptogamic. Bot. 1993, 4, 50–59. [Google Scholar]
- Pedroche, F.F.; Silva, P.C.; Aguilar-Rosas, L.E.; Dreckmann, K.M.; Aguilar-Rosas, R. Catálogo de las Algas Marinas Bentónicas del Pacífico de México. I. Chlorophycota; Universidad Autónoma de Baja California: Mexicali, Mexico, 2005; pp. 17–146. [Google Scholar]
- Rodríguez-Reyes, J.C.; Marcano, A.M.; Figueroa, G.; Velásquez-Boadas, A.J.; Fernández, Y.; Martínez, E.F.; Duque Aguilera, M.G. Características morfológicas y reproductivas de tres especies de Batophora (Chlorophyta, Dasycladaceae) de la isla Margarita, Venezuela. Acta Biol. Venez. 2018, 38, 71–84. [Google Scholar]
- Prince, J.S.; Baker, S. Batophora largoensis new species (Chlorophyta, Dasycladaceae) from South Florida: Genetic confirmation. Bull. Mar. Sci. 1984, 3, 330–334. [Google Scholar]
- Prince, J.S.; Baker, S. Batophora largoensis new species (Chlorophyta, Dasycladaceae) from South Florida: Morphological and ultrastructural evidence. Bull. Mar. Sci. 1984, 34, 321–329. [Google Scholar]
- Berger, S.; Kaever, M.J. Dasycladales: An Illustrated Monograph of a Fascinating Algal Order; Oxford University Press: New York, NY, USA, 1992; 247p. [Google Scholar]
- Gómez-Poot, J.M.; Espinoza-Avalos, J.; Jiménez-Flores, S.G. Vegetative and reproductive characteristics of two species of Batophora (Chlorophyta, Dasycladaceae) from Chetumal Bay, Quintana Roo, Mexico. Bot. Mar. 2005, 45, 189–195. [Google Scholar] [CrossRef]
- Quan-Young, L.I.; Jiménez-Flores, S.G.; Espinoza-Ávalos, J. Flora béntica y reproducción de las algas Batophora spp. (Chlorophyta: Dasycladaceae) de una laguna costera contaminada (Bahía de Chetumal, México). Rev. Biol. Trop. 2006, 54, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Landry, B. Changes in the Distribution and Density of Florida Bay Macrophytes: 1995–2004. Master’s Thesis, University of North Carolina, Wilmington, NC, USA, 2005. Available online: https://libres.uncg.edu/ir/listing.aspx?id=1587 (accessed on 23 April 2022).
- Ruiz, J.M.; Marín-Guirao, L.; Bernardeau-Esteller, J.; Ramos-Segura, A.; García-Muñoz, R.; Sandoval-Gil, J.M. Spread of the invasive alga Caulerpa racemosa var. cylindracea (Caulerpales, Chlorophyta) along the Mediterranean Coast of the Murcia region (SE Spain). Anim. Biodivers. Conserv. 2011, 34, 73–82. [Google Scholar] [CrossRef]
- Iveša, L.; Djakovac, T.; Devescovi, M. Spreading patterns of the invasive Caulerpa cylindracea Sonder along the west Istrian Coast (northern Adriatic Sea, Croatia). Mar. Environ. Res. 2015, 107, 1–7. [Google Scholar] [CrossRef]
- Terradas-Fernández, M.; Valverde-Urrea, M.; Casado-Coy, N.; Sanz-Lazaro, C. The ecological condition of vermetid platforms affects the cover of the alien seaweed Caulerpa cylindracea. Sci. Mar. 2020, 84, 181–191. [Google Scholar] [CrossRef]
- Garcia-Pintado, J.; Martínez-Mena, M.; Barberá, G.G.; Albaladejo, J.; Castillo, V.M. Anthropogenic nutrient sources and loads from a Mediterranean catchment into a coastal lagoon: Mar Menor, Spain. Sci. Total Environ. 2007, 373, 220–239. [Google Scholar] [CrossRef]
- Ballesteros, E. On the presence of a species of Batophora J. Agardh, 1854 (Chlorophyta: Dasycladales) in Formentera, Balearic Islands. Boll. Soc. Hist. Nat. Bal. 2020, 63, 109–118. [Google Scholar]
- Pérez-Ruzafa, Á.; Marcos, C. La situación de Mar Menor: Seis mil años de historia, 50 años de resistencia y un ejemplo de ecosistema complejo. In Una Mirada Global Sobre el Mar Menor; Navarro Caballero, T.M., Ed.; Cátedra del Agua y la Sostenibilidad, Universidad de Murcia: Murcia, Spain, 2018; pp. 81–114. [Google Scholar]
- Gil-Rodriguez, M.C.; Afonso-Carrillo, J. Distribution of the family Dasycladaceae (Chlorophyta) in the Canary Islands [Acetabularia, Cymopolia, Dasycladus, Polyphysa, Algae]. Collect. Bot. 1982, 13, 831–839. [Google Scholar]
- Littler, D.S.; Littler, M.M. Caribbean reef plants. In An Identification Guide to the Reef Plants of the Caribbean, Bahamas, Florida and Gulf of Mexico; Offshore Graphics: Washington, DC, USA, 2000; 542p. [Google Scholar]
- Cormaci, M.; Furnari, G.; Alongi, G. Flora marina bentonica del Mediterraneo: Chlorophyta. Boll. Accad. Gioenia Sci. Nat. 2014, 47, 11–436. [Google Scholar]
- Bottalico, A.; Delle Foglie, C.I.; Perrone, C. Batophora J Agardh (Chlorophyta, Dasycladales): Un genere tropicale nuovo per il Mediterraneo. In Proceedings of the 98th Congresso Nazionale della Società Botanica Italiana, Catania, Italy, 24–26 September 2003. [Google Scholar]
- Morrison, D. Seasonality of Batophora oerstedi (Chlorophyta), a tropical macroalga. Mar. Ecol. Prog. Ser. 1984, 14, 235–244. [Google Scholar] [CrossRef]
- Thayer, G.W.; Murphey, P.L.; LaCroix, M.W. Responses of plant communities in western Florida Bay to the die-off of seagrasses. Bull. Mar. Sci. 1994, 54, 718–726. [Google Scholar]


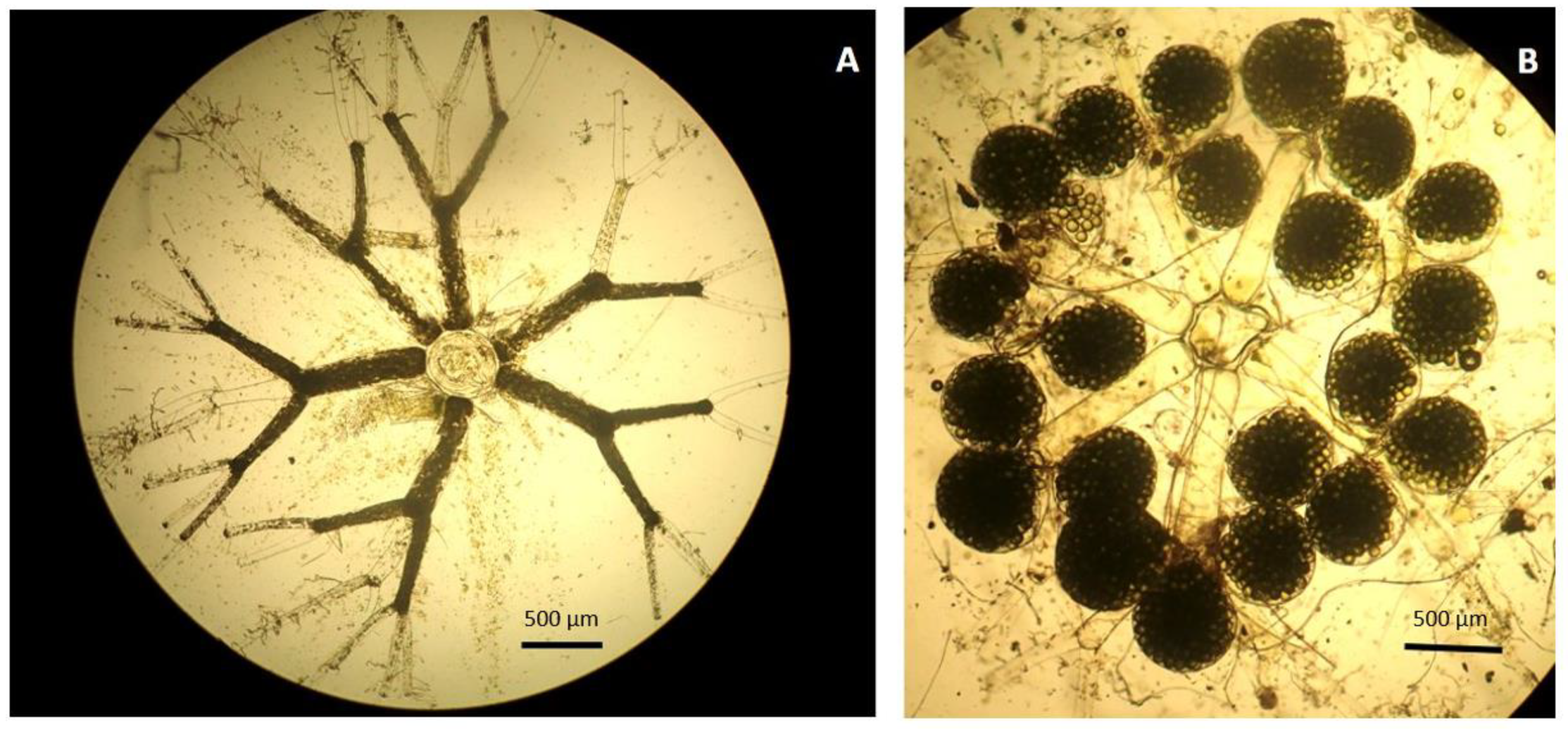
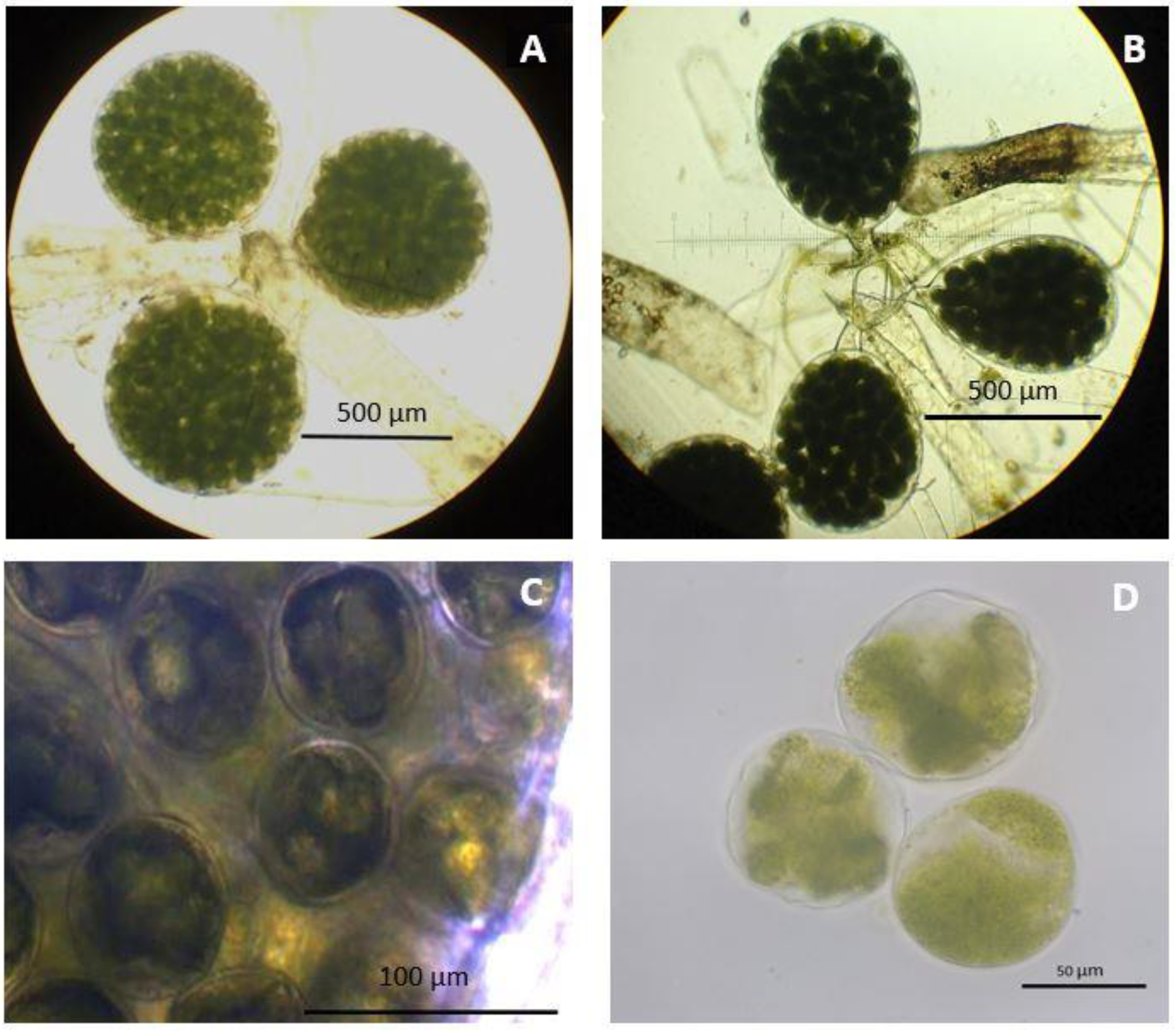
| Main Observed Characters | This Study | Ballesteros, 2020 |
|---|---|---|
| No. of branchlets shaping a whorl | 6–9 | 6–10 |
| Main axis diameter | 400–500 µm | 450 to 600 µm |
| First internode diameter | 175–250 µm | 140–180 µm |
| Gametophores location | 1st internode | 1st (2nd) internode |
| Gametophores shape | Spherical (oval) | Spherical |
| Gametophores diameter | 350 to 650 µm | 440 to 650 µm |
| Gametangia shape | Spherical | Spherical |
| Gametangia diameter | 59–68 µm | 60–87 µm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terradas-Fernández, M.; Valverde-Urrea, M.; López-Moya, F.; Fernández-Torquemada, Y. On the Presence and Ubiquity of the Exotic Batophora (J. Agardh) in the Mar Menor Lagoon (SE Spain). Water 2022, 14, 2909. https://doi.org/10.3390/w14182909
Terradas-Fernández M, Valverde-Urrea M, López-Moya F, Fernández-Torquemada Y. On the Presence and Ubiquity of the Exotic Batophora (J. Agardh) in the Mar Menor Lagoon (SE Spain). Water. 2022; 14(18):2909. https://doi.org/10.3390/w14182909
Chicago/Turabian StyleTerradas-Fernández, Marc, Miguel Valverde-Urrea, Federico López-Moya, and Yolanda Fernández-Torquemada. 2022. "On the Presence and Ubiquity of the Exotic Batophora (J. Agardh) in the Mar Menor Lagoon (SE Spain)" Water 14, no. 18: 2909. https://doi.org/10.3390/w14182909





