Selective and Competitive Adsorption of Anions in Solution on Porous Adsorbent from Zea mays Steams: Kinetic and Equilibrium Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bioadsorbents
2.3. Adsorption Kinetics
2.4. Adsorption Isotherms
2.5. Multicomponent Adsorption
3. Results
3.1. Adsorption Kinetics
3.2. Adsorption Equilibrium
3.3. Multicomponent Adsorption Test Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Li, B.; Huang, H.; Zhao, N.; Zhang, M.; Cao, L. Investigation into lanthanum-coated biochar obtained from urban dewatered sewage sludge for enhanced phosphate adsorption. Sci. Total Environ. 2020, 714, 136839. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Zhang, B.; Wang, R.; Zhao, Z. Biochar as an adsorbent for inorganic nitrogen and phosphorus removal from water: A review. Environ. Sci. Pollut. Res. 2017, 24, 26297–26309. [Google Scholar] [CrossRef]
- Lazaratou, C.; Vayenas, D.; Papoulis, D. The role of clays, clay minerals and clay-based materials for nitrate removal from water systems: A review. Appl. Clay Sci. 2020, 185, 105377. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, X.; Wang, X.; Gao, B.; Yue, Q.; Song, W.; Zhang, L.; Wang, H. FTIR, Raman, and XPS analysis during phosphate, nitrate and Cr(VI) removal by amine cross-linking biosorbent. J. Colloid Interface Sci. 2016, 468, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, A.; Ghasemi, F.; Derakhshani, E.; Shahabi, H. Thermodynamic, kinetic and isotherm studies of sulfate removal from aqueous solutions by graphene and graphite nanoparticles. Desalin. Water Treat. 2017, 80, 247–254. [Google Scholar] [CrossRef]
- US-EPA; U.S.E.P.A. 2018. Edition of the Drinking Water Standards and Health Advisories Tables. 2018. Available online: https://www.epa.gov/system/files/documents/2022-01/dwtable2018.pdf (accessed on 7 September 2022).
- WHO. Nitrate and Nitrite in Drinking-Water. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/75380/WHO_SDE_WSH_04.03_56_eng.pdf (accessed on 7 September 2022).
- Belkada, F.D.; Kitous, O.; Drouiche, N.; Aoudj, S.; Bouchelaghem, O.; Abdi, N.; Grib, H.; Mameri, N. Electrodialysis for fluoride and nitrate removal from synthesized photovoltaic industry wastewater. Sep. Purif. Technol. 2018, 204, 108–115. [Google Scholar] [CrossRef]
- Liu, T.; Hu, S.; Yuan, Z.; Guo, J. High-level nitrogen removal by simultaneous partial nitritation, anammox and nitrite/nitrate-dependent anaerobic methane oxidation. Water Res. 2019, 166, 115057. [Google Scholar] [CrossRef]
- Qin, P.; Lu, S.; Liu, X.; Wang, G.; Zhang, Y.; Li, D.; Wan, Z. Removal of tri-(2-chloroisopropyl) phosphate (TCPP) by three types of constructed wetlands. Sci. Total Environ. 2020, 749, 141668. [Google Scholar] [CrossRef]
- Nur, H.M.; Yüzer, B.; Aydin, M.I.; Aydin, S.; Öngen, A.; Selçuk, H. Desalination and fate of nutrient transport in domestic wastewater using electrodialysis membrane process. Desalin. Water Treat. 2019, 172, 323–329. [Google Scholar] [CrossRef]
- Liu, R.; Sui, Y.; Wang, X. Metal–organic framework-based ultrafiltration membrane separation with capacitive-type for enhanced phosphate removal. Chem. Eng. J. 2019, 371, 903–913. [Google Scholar] [CrossRef]
- Boeykens, S.P.; Piol, M.N.; Legal, L.S.; Saralegui, A.B.; Vázquez, C. Eutrophication decrease: Phosphate adsorption processes in presence of nitrates. J. Environ. Manag. 2017, 203, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Li, Y.; Liu, T.; Huang, Q.; Yang, S.; Wang, W.; Jin, P. The simultaneous adsorption of nitrate and phosphate by an organic-modified aluminum-manganese bimetal oxide: Adsorption properties and mechanisms. Appl. Surf. Sci. 2019, 478, 539–551. [Google Scholar] [CrossRef]
- Cao, W.; Dang, Z.; Zhou, X.-Q.; Yi, X.-Y.; Wu, P.; Zhu, N.-W.; Lu, G.-N. Removal of sulphate from aqueous solution using modified rice straw: Preparation, characterization and adsorption performance. Carbohydr. Polym. 2011, 85, 571–577. [Google Scholar] [CrossRef]
- Alagha, O.; Manzar, M.S.; Zubair, M.; Anil, I.; Mu’Azu, N.D.; Qureshi, A. Comparative Adsorptive Removal of Phosphate and Nitrate from Wastewater Using Biochar-MgAl LDH Nanocomposites: Coexisting Anions Effect and Mechanistic Studies. Nanomaterials 2020, 10, 336. [Google Scholar] [CrossRef]
- Li, J.-H.; Lv, G.-H.; Bai, W.-B.; Liu, Q.; Zhang, Y.-C.; Song, J.-Q. Modification and use of biochar from wheat straw (Triticum aestivumL.) for nitrate and phosphate removal from water. Desalin. Water Treat. 2016, 57, 4681–4693. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, B.; Gao, Q.; Gao, Y.; Liu, S. Adsorption of phosphorus by different biochars. Spectrosc. Lett. 2017, 50, 73–80. [Google Scholar] [CrossRef]
- Shukla, N.; Sahoo, D.; Remya, N. Biochar from microwave pyrolysis of rice husk for tertiary wastewater treatment and soil nourishment. J. Clean. Prod. 2019, 235, 1073–1079. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Shen, F.; Li, T. Phosphate adsorption on lanthanum loaded biochar. Chemosphere 2016, 150, 1–7. [Google Scholar] [CrossRef]
- Zare, L.; Ghasemi-Fasaei, R. Investigation of equilibrium isotherm and kinetic modeling to asses sorption characteristics of nitrate onto palm leaf biochar. Iran. J. Chem. Chem. Eng. 2018, 38, 143–153. [Google Scholar]
- Manjunath, S.V.; Kumar, M. Evaluation of single-component and multi-component adsorption of metronidazole, phosphate and nitrate on activated carbon from Prosopıs julıflora. Chem. Eng. J. 2018, 346, 525–534. [Google Scholar] [CrossRef]
- Villabona-Ortiz, Á.; Tejada-Tovar, C.; Ortega-Toro, R. Effect of temperature in removing of anions in solution on biochar using Zea mays stalks as a precursor. J. Water L. Dev. 2021, 50, 64–68. [Google Scholar] [CrossRef]
- ASTM. ASTM D 4130-15; Standard Test Method for Sulfate in Brackish Water, Seawater, and Brines. ASTM: West Conshohocken, PA, USA, 2018; pp. 1–5. [Google Scholar]
- ASTM. ASTM D7781-14; Standard Test Method for Nitrite-Nitrate in Water by Nitrate Reductase. ASTM: West Conshohocken, PA, USA, 2018; pp. 1–8. [Google Scholar]
- ASTM. ASTM D 515-60; Standard Test Method for Phosphate Ion in Water. ASTM: West Conshohocken, PA, USA, 2018; pp. 1–4. [Google Scholar]
- Lonappan, L.; Rouissi, T.; Brar, S.K.; Verma, M.; Surampalli, R.Y. An insight into the adsorption of diclofenac on different biochars: Mechanisms, surface chemistry, and thermodynamics. Bioresour. Technol. 2018, 249, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]
- Riahi, K.; Chaabane, S.; Ben Thayer, B. A kinetic modeling study of phosphate adsorption onto Phoenix dactylifera L. date palm fibers in batch mode. J. Saudi Chem. Soc. 2017, 21, S143–S152. [Google Scholar] [CrossRef]
- Naushad, M.; Sharma, G.; Kumar, A.; Sharma, S.; Ghfar, A.A.; Bhatnagar, A.; Stadler, F.J.; Khan, M.R. Efficient removal of toxic phosphate anions from aqueous environment using pectin based quaternary amino anion exchanger. Int. J. Biol. Macromol. 2018, 106, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rasmey, A.-H.M.; Aboseidah, A.A.; Youssef, A.K. Application of langmuir and freundlich isotherm models on biosorption of Pb2+ by freez-dried biomass of Pseudomonas aeruginosa. Egypt. J. Microbiol. 2018, 53, 37–48. [Google Scholar] [CrossRef]
- Xi, Y.; Huang, M.; Luo, X. Enhanced phosphate adsorption performance by innovative anion imprinted polymers with dual interaction. Appl. Surf. Sci. 2019, 467, 135–142. [Google Scholar] [CrossRef]
- Rodiguez, M.H.; Yperman, J.; Carleer, R.; Maggen, J.; Dadi, D.; Gryglewicz, G.; Van der Bruggen, B.; Hernández, J.F.; Otero-Calvis, A. Adsorption of Ni(II) on spent coffee and coffee husk based activated carbon. J. Environ. Chem. Eng. 2018, 6, 1161–1170. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Z.; Fu, Y.; Chen, Y.; Pan, Z.; Wang, R.; Tan, Z. Comparative analysis on adsorption properties and mechanisms of nitrate and phosphate by modified corn stalks. RSC Adv. 2018, 8, 36468–36476. [Google Scholar] [CrossRef]
- Bashir, M.T.; Ali, S.; Idris, A.; Haroon, R. Kinetic and thermodynamic study of nitrate adsorption from aqueous solution by lignocellulose-based anion resins. Desalination Water Treat. 2017, 62, 449–456. [Google Scholar] [CrossRef]
- Balarak, D.; Zafariyan, M.; Igwegbe, C.A.; Onyechi, K.K.; Ighalo, J.O. Adsorption of acid blue 92 dye from aqueous solutions by single-walled carbon nanotubes: Isothermal, kinetic, and thermodynamic studies. Environ. Process. 2021, 8, 869–888. [Google Scholar] [CrossRef]
- Golie, W.M.; Upadhyayula, S. An investigation on biosorption of nitrate from water by chitosan based organic-inorganic hybrid biocomposites. Int. J. Biol. Macromol. 2017, 97, 489–502. [Google Scholar] [CrossRef]
- William Kajjumba, G.; Emik, S.; Öngen, A.; Kurtulus Özcan, H.; Aydın, S. Modelling of adsorption kinetic processes—Errors, theory and application. In Advanced Sorption Process Applications; Intechopen: London, UK, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Meenakshi, S. Synthesis and characterization of Zn–Al LDHs/activated carbon composite and its adsorption properties for phosphate and nitrate ions in aqueous medium. J. Mol. Liq. 2019, 296, 111766. [Google Scholar] [CrossRef]
- Halajnia, A.; Oustan, S.; Najafi, N.; Khataee, A.; Lakzian, A. Adsorption–desorption characteristics of nitrate, phosphate and sulfate on Mg–Al layered double hydroxide. Appl. Clay Sci. 2013, 80, 305–312. [Google Scholar] [CrossRef]
- Matusik, J. Arsenate, orthophosphate, sulfate, and nitrate sorption equilibria and kinetics for halloysite and kaolinites with an induced positive charge. Chem. Eng. J. 2014, 246, 244–253. [Google Scholar] [CrossRef]
- Qiao, H.; Mei, L.; Chen, G.; Liu, H.; Peng, C.; Ke, F.; Hou, R.; Wan, X.; Cai, H. Adsorption of nitrate and phosphate from aqueous solution using amine cross-linked tea wastes. Appl. Surf. Sci. 2019, 483, 114–122. [Google Scholar] [CrossRef]
- Barroso-Solares, S.; Merillas, B.; Cimavilla-Román, P.; Rodriguez-Perez, M.; Pinto, J. Enhanced nitrates-polluted water remediation by polyurethane/sepiolite cellular nanocomposites. J. Clean. Prod. 2020, 254, 120038. [Google Scholar] [CrossRef]
- Banu, H.A.T.; Karthikeyan, P.; Meenakshi, S. Comparative studies on revival of nitrate and phosphate ions using quaternized corn husk and jackfruit peel. Bioresour. Technol. Rep. 2019, 8, 100331. [Google Scholar] [CrossRef]
- Xia, F.; Yang, H.; Li, L.; Ren, Y.; Shi, D.; Chai, H.; Ai, H.; He, Q.; Gu, L. Enhanced nitrate adsorption by using cetyltrimethylammonium chloride pre-loaded activated carbon. Environ. Technol. 2019, 41, 3562–3572. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, H.; Ma, F.; Zhang, T.; Nan, X. Effects of dairy manure biochar on adsorption of sulfate onto light sierozem and its mechanisms. RSC Adv. 2019, 9, 5218–5223. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.; Burton, G.R.; Rousseau, S.; Qian, J. Kinetics and thermodynamics of sulfate adsorption on magnetite at elevated temperatures. J. Solut. Chem. 2019, 48, 1488–1502. [Google Scholar] [CrossRef]
- Salami, A.H.; Bonakdari, H.; Akhbari, A.; Shamshiri, A.; Mousavi, S.F.; Farzin, S.; Hassanvand, M.R.; Noori, A. Performance assessment of modified clinoptilolite and magnetic nanotubes on sulfate removal and potential application in natural river samples. J. Incl. Phenom. Macrocycl. Chem. 2020, 97, 51–63. [Google Scholar] [CrossRef]
- Ao, H.; Cao, W.; Hong, Y.; Wu, J.; Wei, L. Adsorption of sulfate ion from water by zirconium oxide-modified biochar derived from pomelo peel. Sci. Total Environ. 2020, 708, 135092. [Google Scholar] [CrossRef]
- Runtti, H.; Tynjälä, P.; Tuomikoski, S.; Kangas, T.; Hu, T.; Rämö, J.; Lassi, U. Utilisation of barium-modified analcime in sulphate removal: Isotherms, kinetics and thermodynamics studies. J. Water Process. Eng. 2017, 16, 319–328. [Google Scholar] [CrossRef]
- Sereshti, H.; Afsharian, E.Z.; Bidhendi, M.E.; Nodeh, H.R.; Kamboh, M.A.; Yilmaz, M. Removal of phosphate and nitrate ions aqueous using strontium magnetic graphene oxide nanocomposite: Isotherms, kinetics, and thermodynamics studies. Environ. Prog. Sustain. Energy 2020, 39, e13332. [Google Scholar] [CrossRef]
- Dong, S.; Ji, Q.; Wang, Y.; Liu, H.; Qu, J. Enhanced phosphate removal using zirconium hydroxide encapsulated in quaternized cellulose. J. Environ. Sci. 2020, 89, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lee, X.; Grattieri, M.; Yuan, M.; Cai, R.; Macazo, F.C.; Minteer, S.D. Modified biochar for phosphate adsorption in environmentally relevant conditions. Chem. Eng. J. 2020, 380, 122375. [Google Scholar] [CrossRef]
- Iftekhar, S.; Küçük, M.E.; Srivastava, V.; Repo, E.; Sillanpää, M. Application of zinc-aluminium layered double hydroxides for adsorptive removal of phosphate and sulfate: Equilibrium, kinetic and thermodynamic. Chemosphere 2018, 209, 470–479. [Google Scholar] [CrossRef]
- Constantino, L.V.; Quirino, J.N.; Monteiro, A.M.; Abrão, T.; Parreira, P.S.; Urbano, A.; Santos, M.J. Sorption-desorption of selenite and selenate on Mg-Al layered double hydroxide in competition with nitrate, sulfate and phosphate. Chemosphere 2017, 181, 627–634. [Google Scholar] [CrossRef]
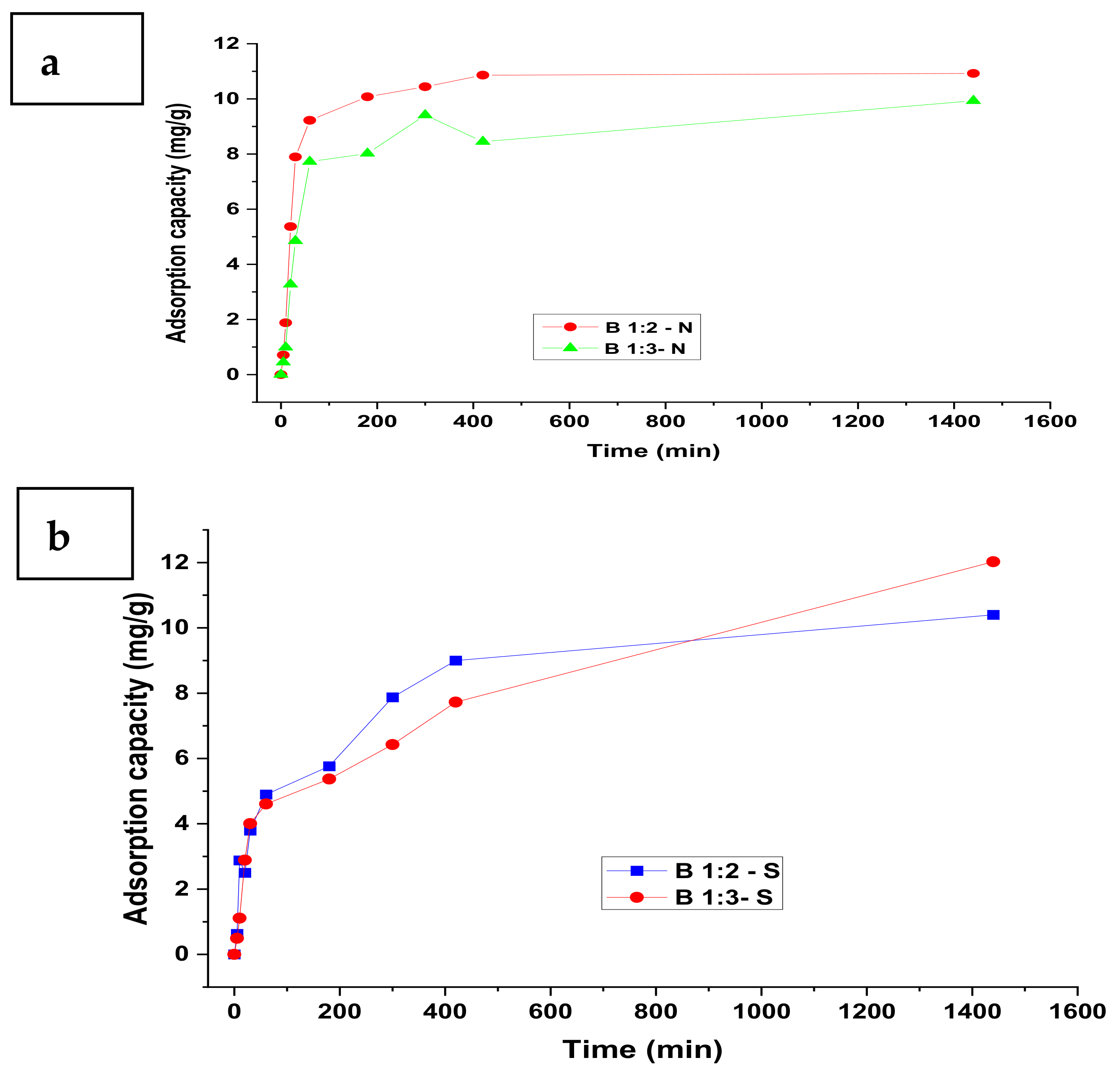
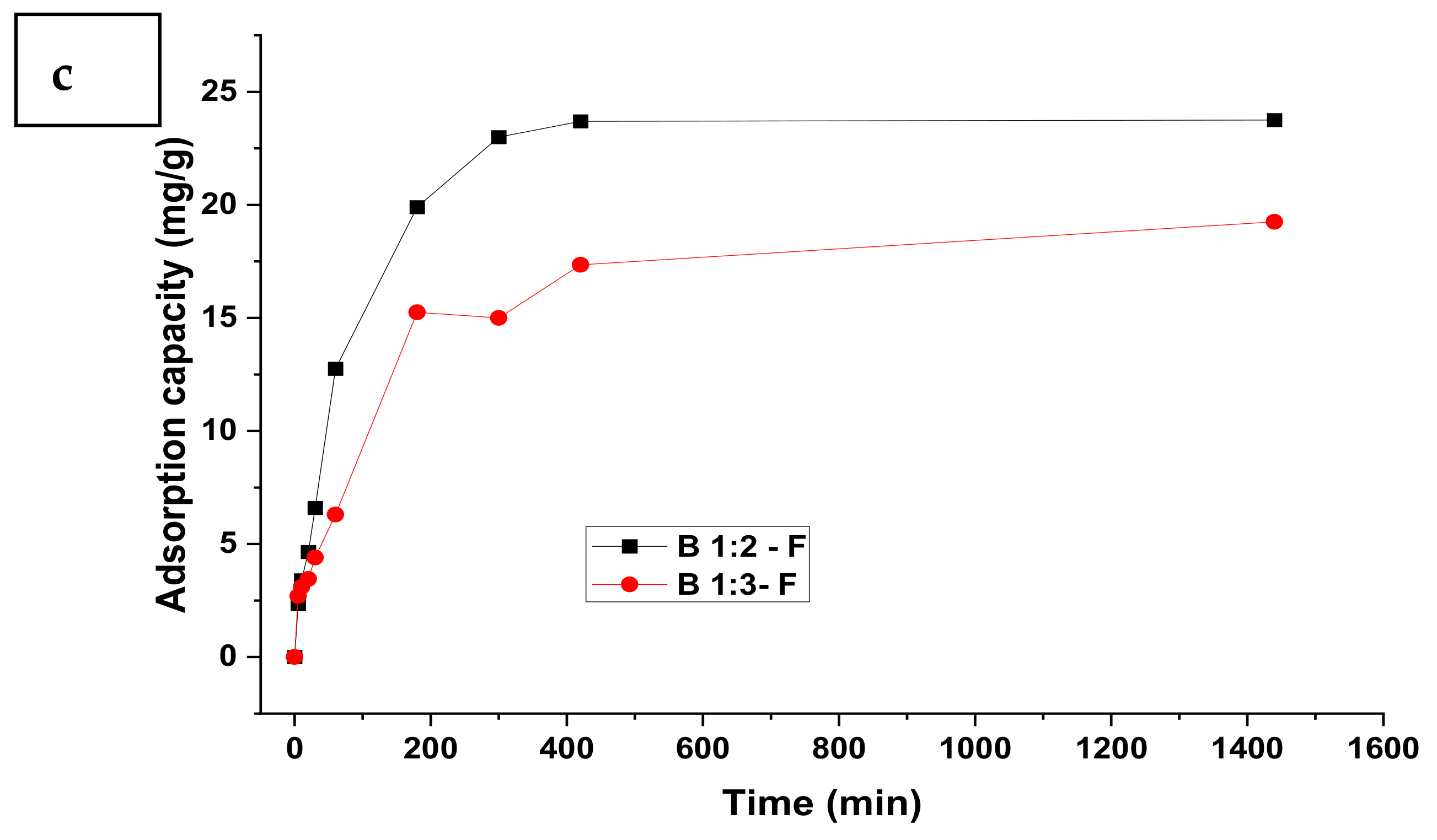
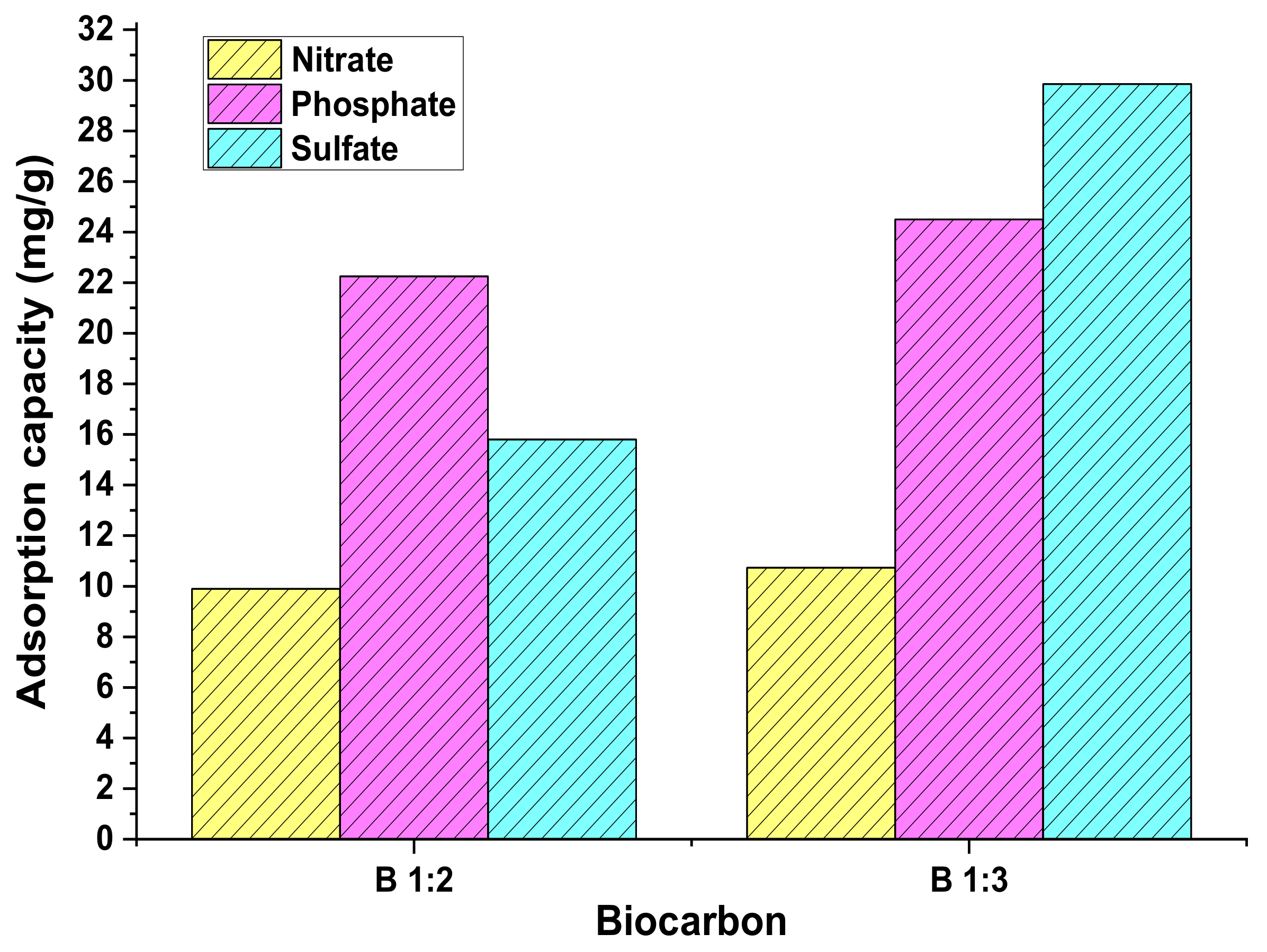
| Model | Parameter | Nitrate | Sulphate | Phosphate | |||
|---|---|---|---|---|---|---|---|
| B 1:2 | B 1:3 | B 1:2 | B 1:3 | B 1:2 | B 1:3 | ||
| Pseudo-first order | qe | 10.64 | 9.09 | 8.62 | 11.08 | 23.59 | 18.12 |
| k1 | 0.03 | 0.02 | 0.02 | 0.00039 | 0.01 | 0.008 | |
| R2 | 0.96 | 0.96 | 0.87 | 0.79 | 0.99 | 0.97 | |
| Pseudo-second order | k2 | 11.53 | 10.05 | 9.90 | 10.83 | 26.83 | 20.95 |
| qe | 0.004 | 0.003 | 0.001 | 8.78 × 10−4 | 5.26 × 10−4 | 4.82 × 10−4 | |
| R2 | 0.93 | 0.94 | 0.94 | 0.87 | 0.99 | 0.97 | |
| Elovich | β | 0.53 | 0.55 | 0.59 | 0.55 | 0.21 | 0.29 |
| α | 1.33 | 0.64 | 0.53 | 0.38 | 1.08 | 0.75 | |
| R2 | 0.81 | 0.88 | 0.97 | 0.94 | 0.94 | 0.93 | |
| Isotherm Model | Parameters | Nitrate | Sulphate | Phosphate | |||
|---|---|---|---|---|---|---|---|
| B 1:2 | B 1:3 | B 1:2 | B 1:3 | B 1:2 | B 1:3 | ||
| Langmuir | qmax (mg/g) | 9788.17 | 487.93 | 28.68 | 7625.07 | 8400.49 | 73834.8 |
| KL (L/mg) | 3.68 × 10−5 | 2.73 × 10−4 | 0.01 | 2.28 × 10−5 | 3.77 × 10−6 | 2.99 × 10−6 | |
| R2 | 0.99 | 0.95 | 0.76 | 0.89 | 0.59 | 0.61 | |
| Freundlich | kf (mg/g (L/mg)1/n) | 0.13 | 0.12 | 0.45 | 0.11 | 7.21 | 1.4 × 10−4 |
| n | 0.98 | 0.98 | 1.33 | 0.90 | 0.39 | 0.35 | |
| R2 | 0.99 | 0.95 | 0.71 | 0.90 | 0.85 | 0.89 | |
| Dubinin–Radushkevich | qDR (mg/g) | 11.93 | 12.03 | 12.64 | 15.03 | 44.558 | 47.75 |
| KDR (mol2/kJ2) | 1.46 × 10−4 | 1.71 × 10−4 | 9.02 × 10−5 | 1.65 × 10−4 | 3.13 × 10−4 | 5.41 | |
| E (KJ/mol) | 58.54 | 54.01 | 74.45 | 55.01 | 39.99 | 30.39 | |
| R2 | 0.82 | 0.89 | 0.86 | 0.99 | 0.79 | 0.79 | |
| Pollutant | Adsorbent | qmax (mg/g) | Reference |
|---|---|---|---|
| Nitrate | Zn–Al LDHs/activated carbon composite | 73.742 | [39] |
| Amine crosslinked tea waste | 136.43 | [42] | |
| Polyurethane/sepiolite cellular nanocomposites | 23.30 | [43] | |
| Corn husk quaternized with N,N-dimethylformamide, ethylenediamine, and triethylamine | 79.09 | [44] | |
| Jackfruit peel quaternized with N,N-dimethylformamide, ethylenediamine, and triethylamine | 62.91 | ||
| Cetylpyridinium bromide modified zeolite | 28.06 | [16] | |
| Unmodified activated carbon | 3.86 | [45] | |
| 25% CTAC-modified activated carbon | 7.1 | ||
| Activated carbon modified with CTAC at 50%. | 10.5 | ||
| 100% CTAC-modified activated carbon | 14.3 | ||
| Sulphate | Manure biochar | 78.4 | [46] |
| Mangenite | 16.4 | [47] | |
| Clinoptilolite | 74.63 | [48] | |
| Magnetic nanotubes | 94 | ||
| Zirconium oxide-modified biochar derived from pomelo peel | 35.21 | [49] | |
| Pomelo peel | 1.02 | ||
| Barium-modified analcime | 2.3 | [50] | |
| Barium-modified acid-washed analcime | 13.7 | ||
| Barium-modified zeolite | 3.8 | ||
| Phosphate | Strontium magnetic graphene oxide nanocomposite | 238.09 | [51] |
| Zirconium hydroxide encapsulated in quaternized cellulose | 83.6 | [52] | |
| Lanthanum-coated biochar from urban dewatered sewage sludge | 93.91 | [1] | |
| Biochar from wheat straw modified with chitosan | 77.61 | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villabona-Ortíz, A.; Ortega-Toro, R.; Tejada-Tovar, C. Selective and Competitive Adsorption of Anions in Solution on Porous Adsorbent from Zea mays Steams: Kinetic and Equilibrium Study. Water 2022, 14, 2906. https://doi.org/10.3390/w14182906
Villabona-Ortíz A, Ortega-Toro R, Tejada-Tovar C. Selective and Competitive Adsorption of Anions in Solution on Porous Adsorbent from Zea mays Steams: Kinetic and Equilibrium Study. Water. 2022; 14(18):2906. https://doi.org/10.3390/w14182906
Chicago/Turabian StyleVillabona-Ortíz, Angel, Rodrigo Ortega-Toro, and Candelaria Tejada-Tovar. 2022. "Selective and Competitive Adsorption of Anions in Solution on Porous Adsorbent from Zea mays Steams: Kinetic and Equilibrium Study" Water 14, no. 18: 2906. https://doi.org/10.3390/w14182906








