Efficient Removal of Methyl Red Dye by Using Bark of Hopbush
Abstract
:Highlights
- Analysis of low-cost, bio-adsorbent bark of the D. viscosa plant, proved to be an efficient ad-sorbent for the removal of methyl red dye from aqueous solution.
- The pseudo-second-order kinetic model and Freundlich adsorption isotherm appeared to be the best fit for describing the adsorption of methyl red onto D. viscosa plant bark.
- D. viscosa plant bark as an adsorbent showed good adsorption capability in tap water and river water.
Abstract
1. Introduction
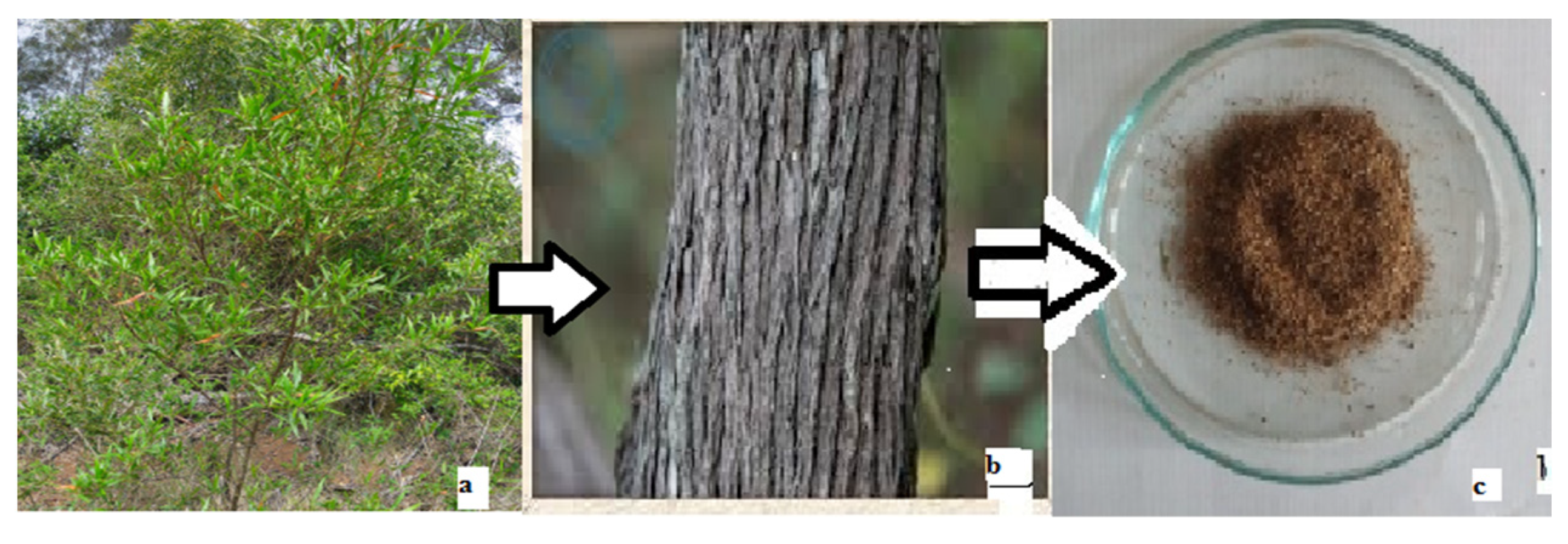
2. Materials and Methods
Characterizations
3. Results and Discussion
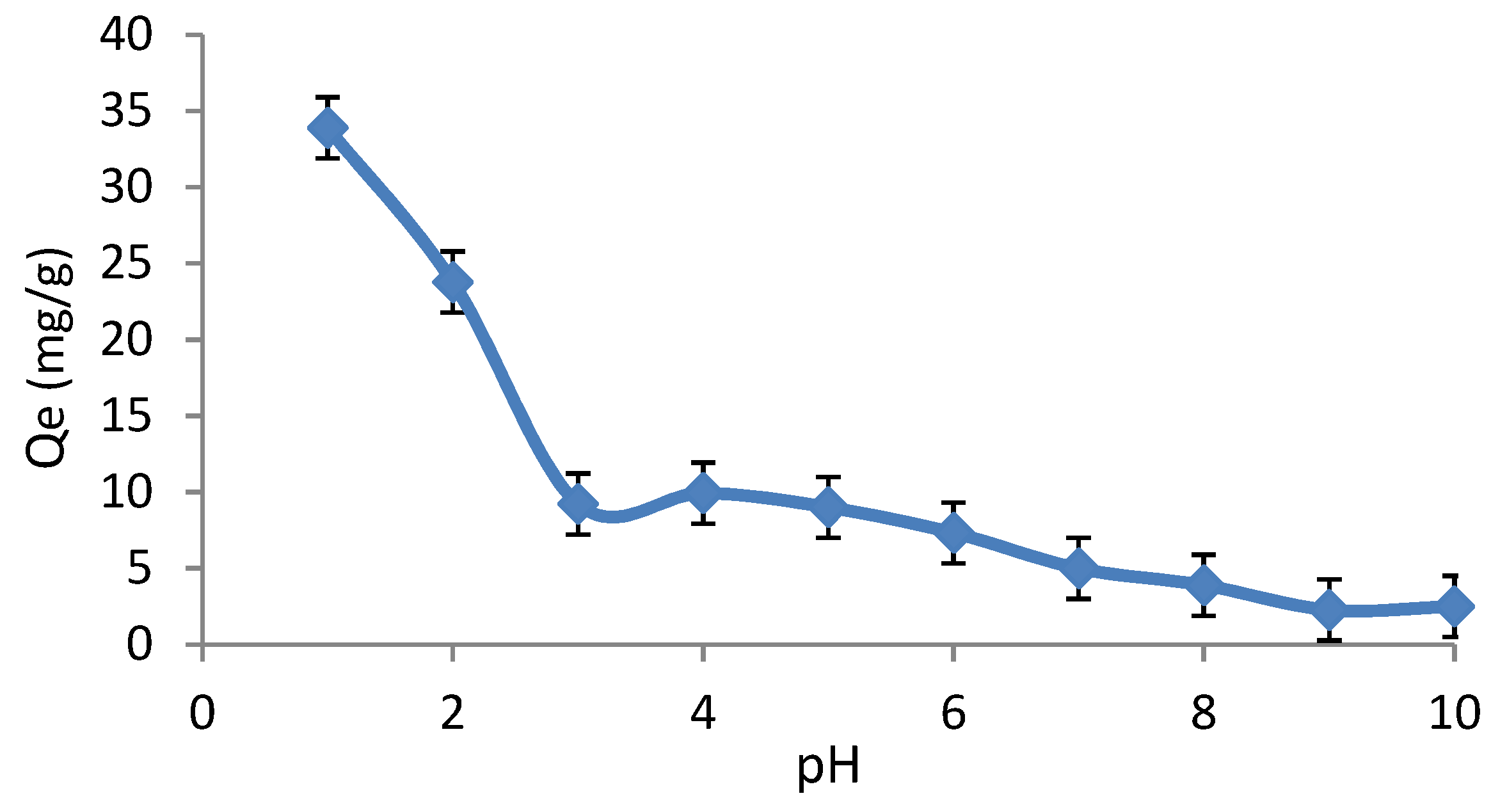
3.1. Effect of pH on Adsorption
3.2. Effect of Concentration of MR Dye on Adsorption
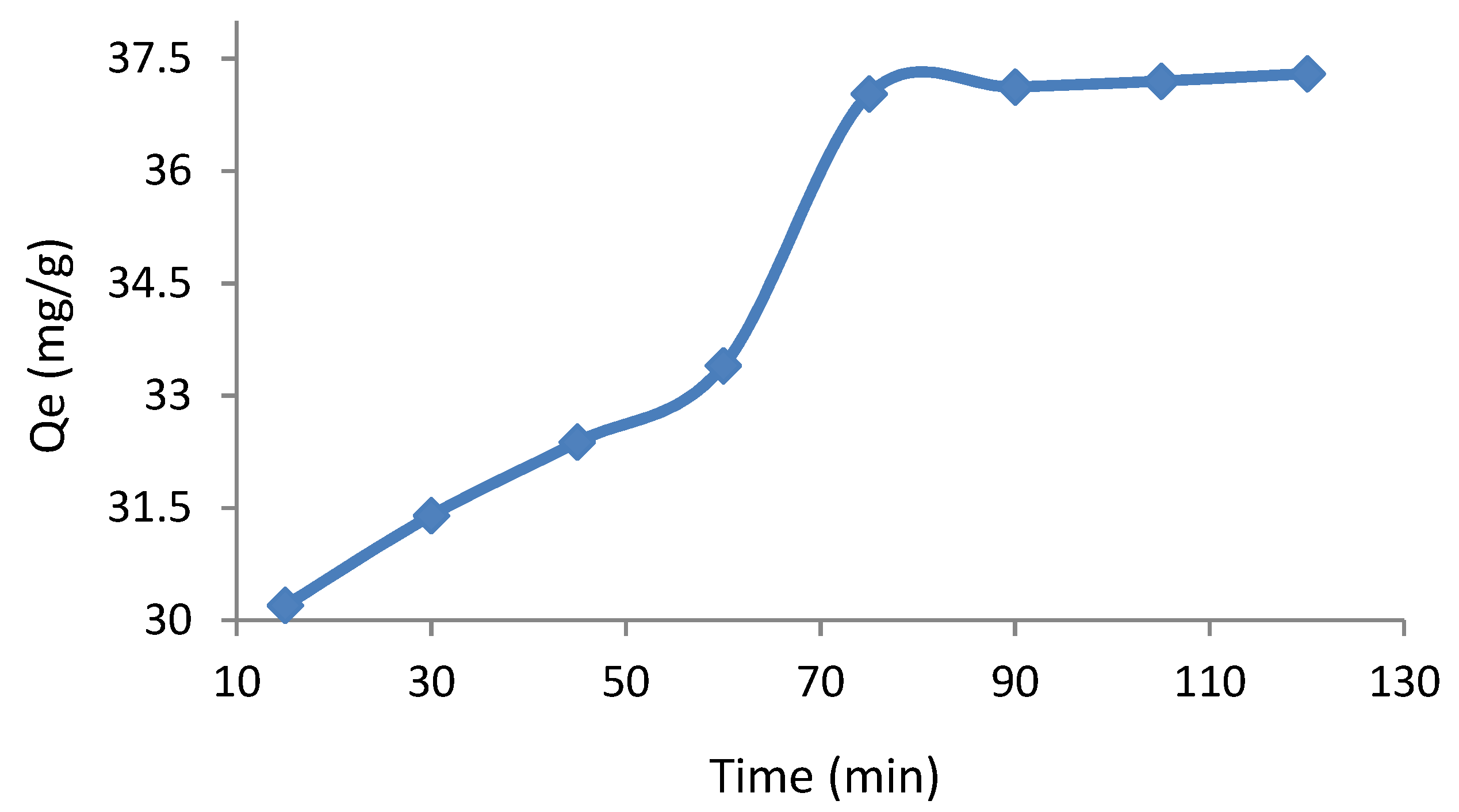
3.3. Effect of Contact Time on Adsorption
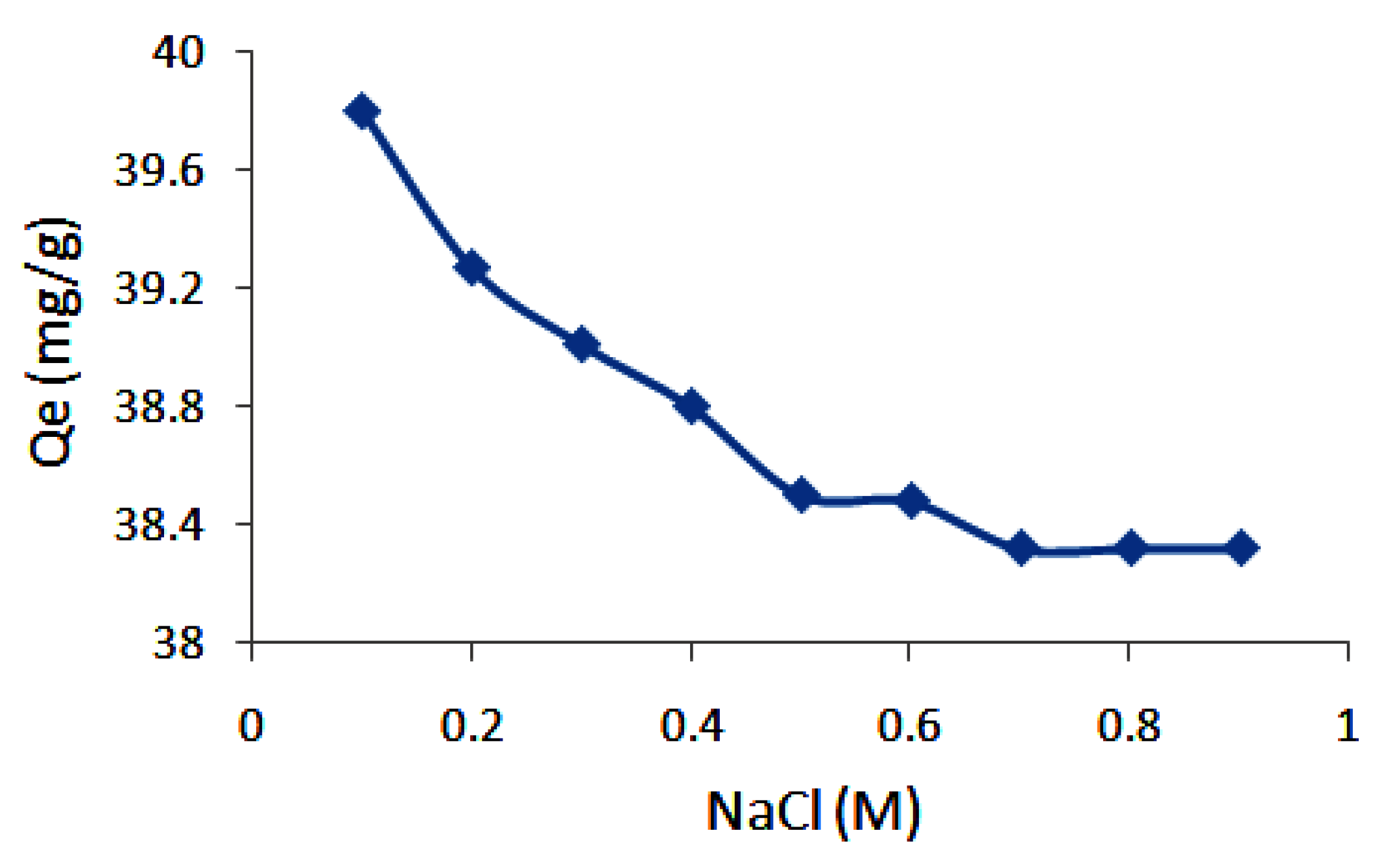
3.4. Effect of Ionic Strength on Adsorption
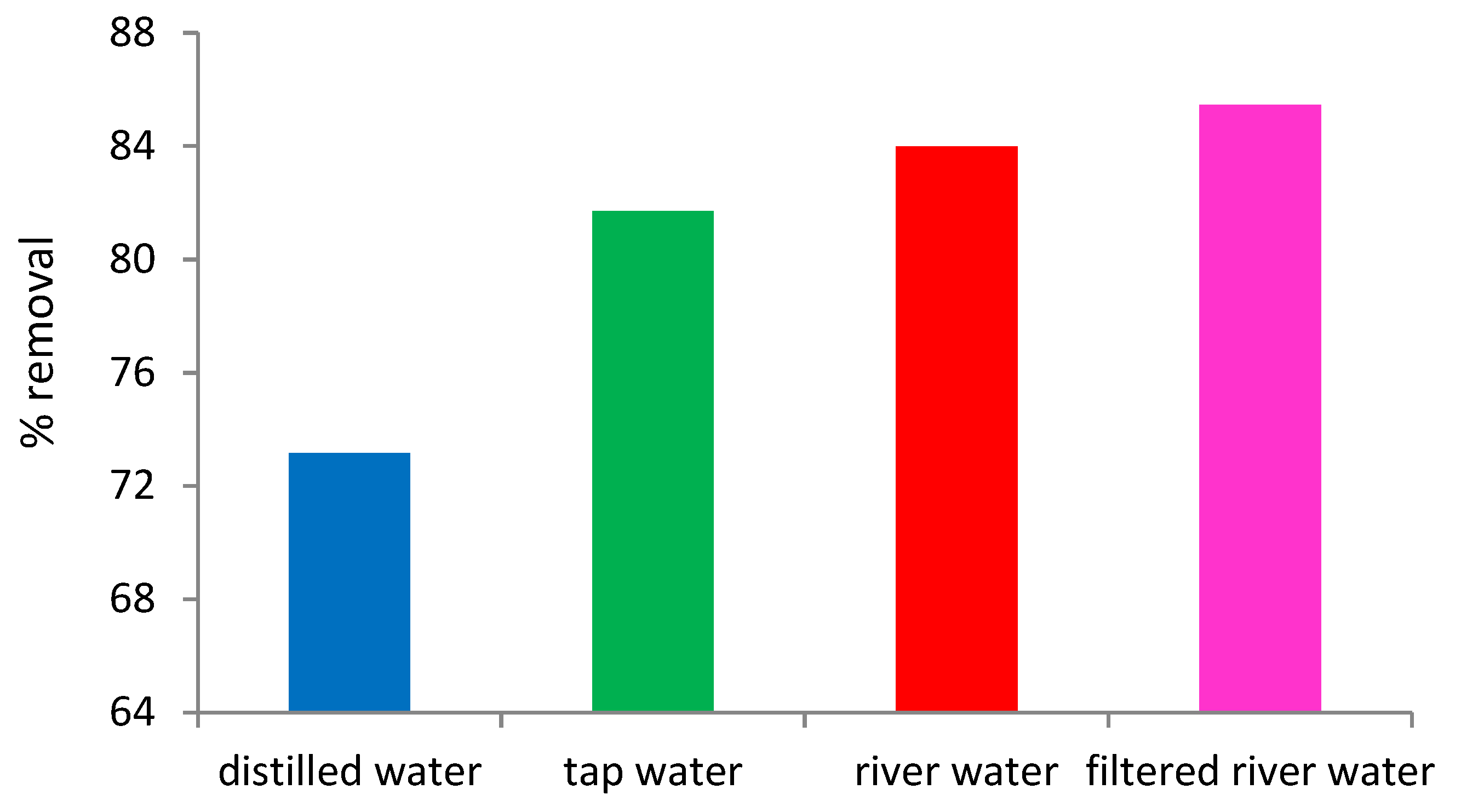
3.5. Effect of Water Obtained from Different Resources on the Adsoprption of MR Dye
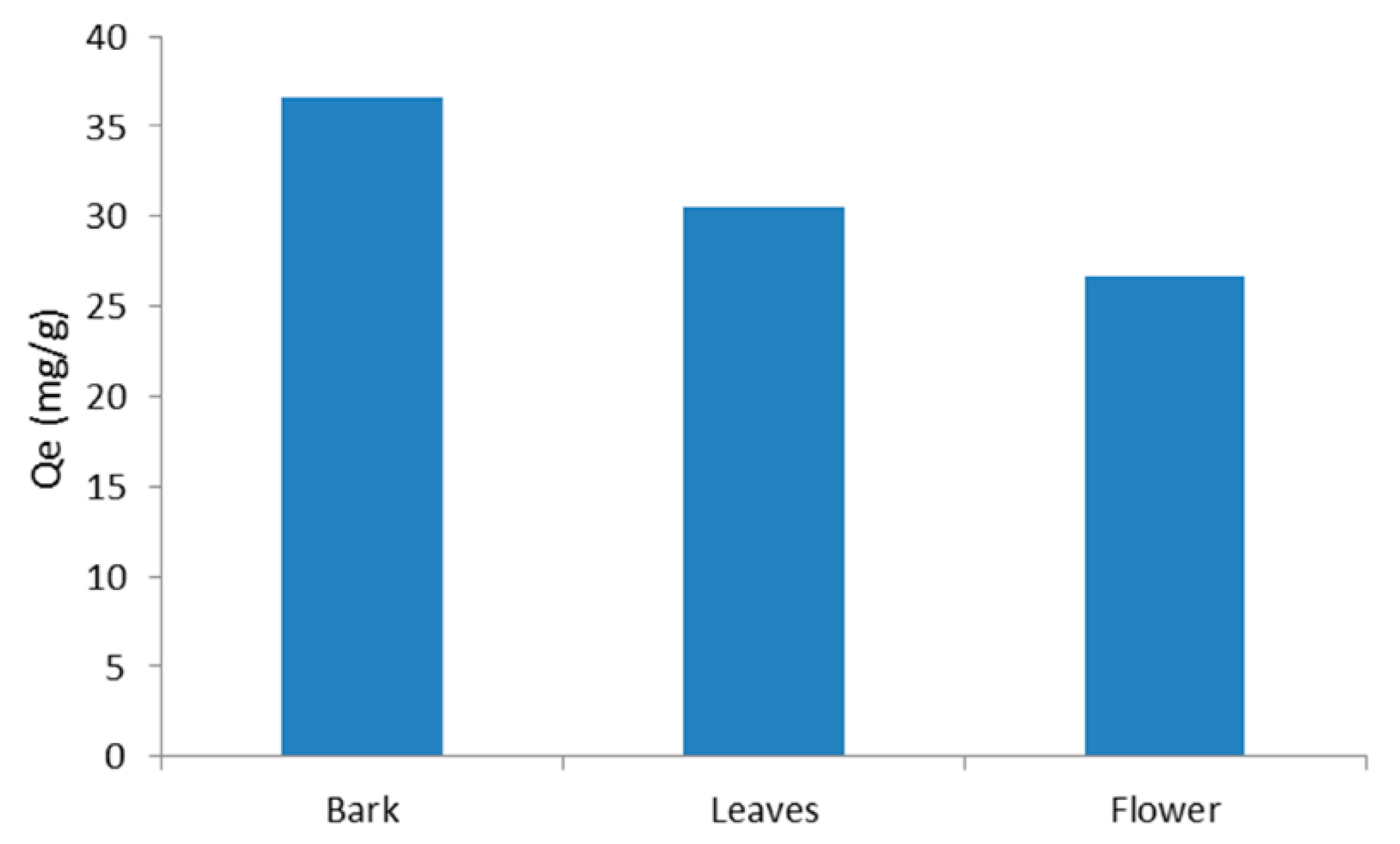
3.6. Comparison between Leaves, Flowers, and Bark Powder of D. viscosa Plant
3.7. Comparison of Adsorbents: Bark Powder of D. viscosa Plant, Animal Charcoal and Silica Gel
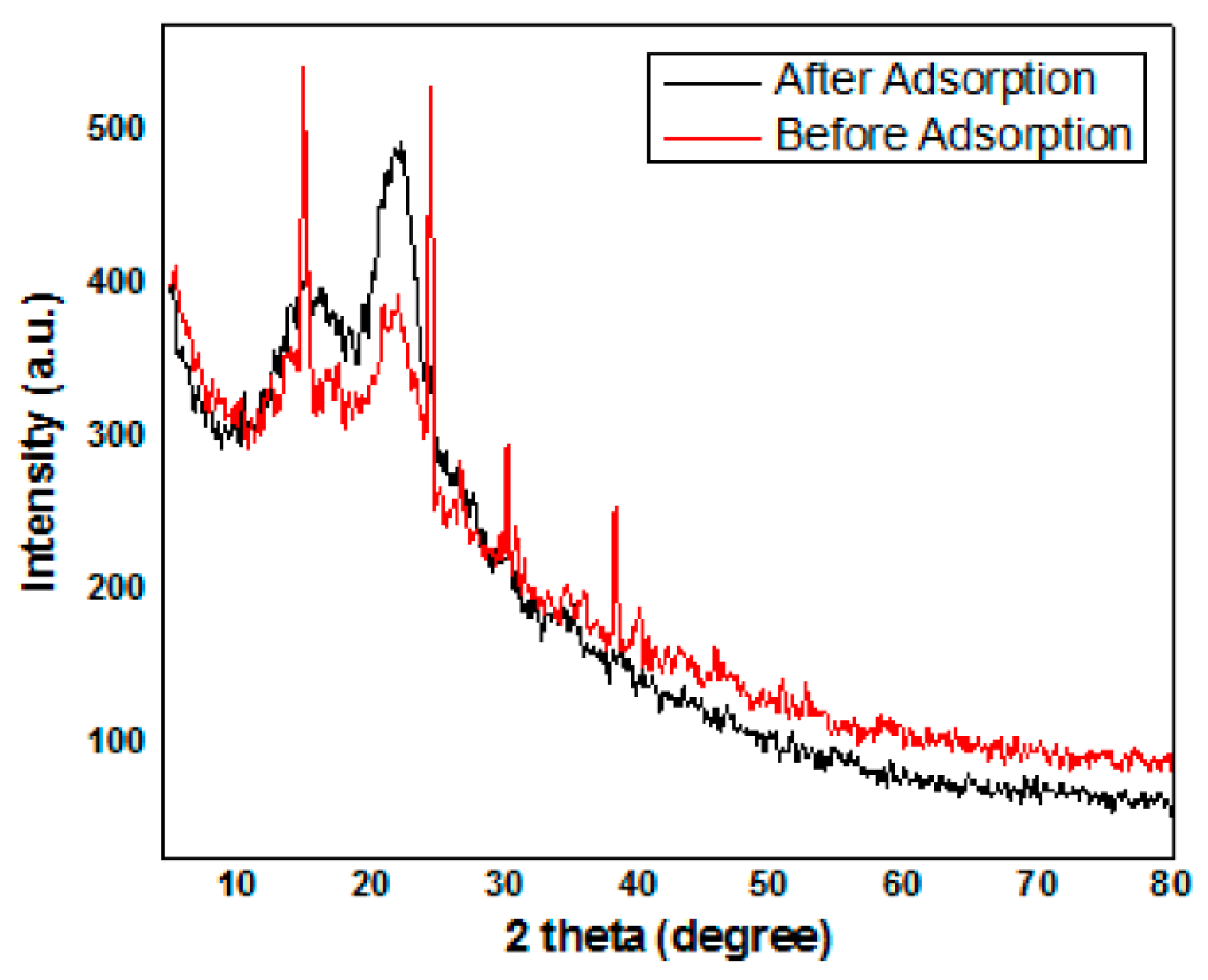
3.8. X-ray Diffraction Analysis
3.9. Order of Adsorpton Kinetics
3.10. Adsorption Isotherms
3.11. Comparison of Adsorption Capacity of D. viscosa Plant Bark with Previous Low-Cost Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azizullah, A.; Khattak, M.N.K.; Richter, P.; Häder, D.-P. Water pollution in pakistan and its impact on public health—A review. Environ. Int. 2011, 37, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Hinrichsen, D.; Tacio, H. The coming freshwater crisis is already here. In The Linkages between Population and Water; Woodrow Wilson International Center for Scholars: Washington, DC, USA, 2002; pp. 1–26. [Google Scholar]
- Gupta, V. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manage. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Sudha, M.; Saranya, A.; Selvakumar, G.; Sivakumar, N. Microbial degradation of azo dyes: A review. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 670–690. [Google Scholar]
- Hunge, Y.; Mohite, V.; Kumbhar, S.; Rajpure, K.; Moholkar, A.; Bhosale, C. Photoelectrocatalytic degradation of methyl red using sprayed WO3 thin films under visible light irradiation. J. Mater. Sci. Mater. Electron. 2015, 26, 8404–8412. [Google Scholar] [CrossRef]
- Anjaneyulu, Y.; Sreedhara Chary, N.; Samuel Suman Raj, D. Decolourization of industrial effluents—Available methods and emerging technologies—A review. Rev. Environ. Sci. Biotechnol. 2005, 4, 245–273. [Google Scholar] [CrossRef]
- Kumar, P.S.; Joshiba, G.J.; Femina, C.C.; Varshini, P.; Priyadharshini, S.; Karthick, M.; Jothirani, R. A critical review on recent developments in the low-cost adsorption of dyes from wastewater. Desalin. Water Treat. 2019, 172, 395–416. [Google Scholar] [CrossRef]
- Takkar, S.; Tyagi, B.; Kumar, N.; Kumari, T.; Iqbal, K.; Varma, A.; Thakur, I.S.; Mishra, A. Biodegradation of methyl red dye by a novel actinobacterium zhihengliuella sp. Istpl4: Kinetic studies, isotherm and biodegradation pathway. Environ. Technol. Innov. 2022, 26, 102348. [Google Scholar] [CrossRef]
- Patil, N.P.; Bholay, A.D.; Kapadnis, B.P.; Gaikwad, V.B. Biodegradation of model azo dye methyl red and other textile dyes by isolate bacillus circulans npp1. J. Pure Appl. Microbiol. 2016, 10, 2793–2800. [Google Scholar] [CrossRef]
- Vinoda, B.; Vinuth, M.; Bodke, Y.; Manjanna, J. Photocatalytic degradation of toxic methyl red dye using silica nanoparticles synthesized from rice husk ash. J Environ. Anal. Toxicol. 2015, 5. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.-S.; Lee, D.-J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Ahmad, N.; Bello, O.S. Modified durian seed as adsorbent for the removal of methyl red dye from aqueous solutions. Appl. Water Sci. 2015, 5, 407–423. [Google Scholar] [CrossRef] [Green Version]
- Rajoriya, S.; Saharan, V.K.; Pundir, A.S.; Nigam, M.; Roy, K. Adsorption of methyl red dye from aqueous solution onto eggshell waste material: Kinetics, isotherms and thermodynamic studies. Curr. Res. Green Sustain. Chem. 2021, 4, 100180. [Google Scholar] [CrossRef]
- Xiang, P.; Deng, C.; Liu, L.; Huang, Y. Study on the Adsorption of Methyl Red by Bentonite/Chitosan Composites. InIOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; p. 022079. [Google Scholar]
- Dawadi, K.B.; Bhattarai, M.; Homagai, P.L. Adsorptive removal of methyl red from aqueous solution using charred and xanthated sal (Shorea robusta) sawdust. Amrit Res. J. 2020, 1, 37–44. [Google Scholar] [CrossRef]
- Santhi, T.; Manonmani, S.; Smitha, T. Removal of malachite green from aqueous solution by activated carbon prepared from the epicarp of ricinus communis by adsorption. J. Hazard. Mater. 2010, 179, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.A.; Nassef, E.; Husain, M. Use of spent oil shale to remove methyl red dye from aqueous solutions. AIMS Mater. Sci. 2020, 7, 338–353. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O. Biosorption of pollutants by plant leaves: An empirical review. J. Environ. Chem. Eng. 2019, 7, 103100. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Robalds, A.; Tran, H.N.; Mitrogiannis, D.; Giannakoudakis, D.A.; Hosseini-Bandegharaei, A.; Dotto, G.L. Removal of heavy metals by leaves-derived biosorbents. Environ. Chem. Lett. 2019, 17, 755–766. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Bikiaris, D.N.; Kyzas, G.Z. Chitin adsorbents for toxic metals: A review. Int. J. Mol. Sci. 2017, 18, 114. [Google Scholar] [CrossRef]
- Babatunde, E.; Akolo, S.; Ighalo, J.; Kovo, A. Response Surface Optimisation of the Adsorption of Cu (ii) from Aqueous Solution by Crab Shell Chitosan. In Proceedings of the 3rd International Engineering Conference, Minna, Nigeria, 24–26 September 2019. [Google Scholar]
- An, H.; Park, B.; Kim, D. Crab shell for the removal of heavy metals from aqueous solution. Water Res. 2001, 35, 3551–3556. [Google Scholar] [CrossRef]
- Babarinde, A.; Onyiaocha, G.O. Equilibrium sorption of divalent metal ions onto groundnut (Arachis hypogaea) shell: Kinetics, isotherm and thermodynamics. Chem. Int. 2016, 2, 37–46. [Google Scholar]
- Olakunle, M.O.; Inyinbor, A.A.; Dada, A.O.; Bello, O.S. Combating dye pollution using cocoa pod husks: A sustainable approach. Int. J. Sustain. Eng. 2018, 11, 4–15. [Google Scholar] [CrossRef]
- Series, I.; Science, M. Utilization of cacao pod husk (Theobroma cacao L.) as activated carbon and catalyst in biodiesel production process from waste cooking oil. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; p. 012093. [Google Scholar]
- Mutavdžić Pavlović, D.; Ćurković, L.; Macan, J.; Žižek, K. Eggshell as a new biosorbent for the removal of pharmaceuticals from aqueous solutions. CLEAN–Soil Air Water 2017, 45, 1700082. [Google Scholar] [CrossRef]
- Bamukyaye, S.; Wanasolo, W. Performance of egg-shell and fish-scale as adsorbent materials for chromium(VI) removal from effluents of tannery industries in eastern uganda. Open Access Lib. J. 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Hevira, L.; Munaf, E.; Zein, R. The use of Terminalia catappa L. Fruit shell as biosorbent for the removal of Pb(II), Cd(II) and Cu(II) ion in liquid waste. J. Chem. Pharm. Res. 2015, 7, 79–89. [Google Scholar]
- Sud, D.; Mahajan, G.; Kaur, M. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.; Šoštarić, T.; Stojanović, M.; Milojković, J.; Mihajlović, M.; Stanojević, M.; Stanković, S. Removal of Pb2+ ions by raw corn silk (Zea mays L.) as a novel biosorbent. J. Taiwan Inst. Chem. Eng. 2016, 58, 407–416. [Google Scholar] [CrossRef]
- Petrović, M.; Šoštarić, T.; Stojanović, M.; Petrović, J.; Mihajlović, M.; Ćosović, A.; Stanković, S. Mechanism of adsorption of Cu2+ and Zn2+ on the corn silk (Zea mays L.). Ecol. Eng. 2017, 99, 83–90. [Google Scholar] [CrossRef]
- Eletta, O.A.; Ighalo, J.O. A review of fish scales as a source of biosorbent for the removal of pollutants from industrial effluents. J. Res. Inf. Civ. Eng. 2019, 16, 2479–2510. [Google Scholar]
- Ahmadifar, Z.; Dadvand Koohi, A. Characterization, preparation, and uses of nanomagnetic fe3o4 impregnated onto fish scale as more efficient adsorbent for Cu2+ ion adsorption. Environ. Sci. Pollut. Res. 2018, 25, 19687–19700. [Google Scholar] [CrossRef]
- Ribeiro, C.; Scheufele, F.; Alves, H.; Kroumov, A.; Espinoza-Quiñones, F.; Módenes, A.; Borba, C. Evaluation of hybrid neutralization/biosorption process for zinc ions removal from automotive battery effluent by dolomite and fish scales. Environ. Technol. 2019, 40, 2373–2388. [Google Scholar] [CrossRef]
- Olasehinde, E.F.; Okoronkwo, A.E.; Aiyesanmi, A.F. Biosorption of nickel from aqueous solution by tithonia diversifolia. Desalination Water Treat. 2009, 12, 352–359. [Google Scholar]
- Sekar, M.; Sakthi, V.; Rengaraj, S. Kinetics and equilibrium adsorption study of lead(II) onto activated carbon prepared from coconut shell. J. Colloid Interface Sci. 2004, 279, 307–313. [Google Scholar] [CrossRef]
- Al-Othman, Z.A.; Ali, R.; Naushad, M. Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: Adsorption kinetics, equilibrium and thermodynamic studies. Chem. Eng. J. 2012, 184, 238–247. [Google Scholar] [CrossRef]
- Romzi, A.A.; Kooh, M.R.R.; Lim, L.B.L.; Priyantha, N.; Chan, C.M. Environmentally friendly adsorbent derived from rock melon skin for effective removal of toxic brilliant green dye: Linear versus non-linear analyses. Int. J. Environ. Anal. Chem. 2021, 1–20. [Google Scholar] [CrossRef]
- Petrović, J.T.; Stojanović, M.D.; Milojković, J.V.; Petrović, M.S.; Šoštarić, T.D.; Laušević, M.D.; Mihajlović, M.L. Alkali modified hydrochar of grape pomace as a perspective adsorbent of pb2+ from aqueous solution. J. Environ. Manag. 2016, 182, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Şen, A.; Pereira, H.; Olivella, M.; Villaescusa, I. Heavy metals removal in aqueous environments using bark as a biosorbent. Int. J. Environ. Sci. Tech. 2015, 12, 391–404. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K.; Ang, H. Adsorption performance of continuous fixed bed column for the removal of methylene blue (MB) dye using eucalyptus sheathiana bark biomass. Res. Chem. Intermed. 2016, 42, 2343–2364. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K.; Ang, H.M. Adsorption removal of zinc(II) from aqueous phase by raw and base modified eucalyptus sheathiana bark: Kinetics, mechanism and equilibrium study. Process Saf. Environ. Prot. 2016, 102, 336–352. [Google Scholar] [CrossRef]
- AREMU, M.O.; Alade, A.; Bello, A.; Salam, K. Kinetics and thermodynamics of 2, 4, 6–trichlorophenol adsorption onto activated carbon derived from flamboyant pod bark. J. Int. Environ. Appl. Sci. 2018, 13, 158–166. [Google Scholar]
- Ajaelu, C.J.; Nwosu, V.; Ibironke, L.; Adeleye, A. Adsorptive removal of cationic dye from aqueous solution using chemically modified african border tree (Newbouldia laevis) bark. J. Appl. Sci. Environ. Manag. 2017, 21, 1323–1329. [Google Scholar] [CrossRef]
- Cutillas-Barreiro, L.; Ansias-Manso, L.; Fernández-Calviño, D.; Arias-Estévez, M.; Nóvoa-Muñoz, J.; Fernández-Sanjurjo, M.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Pine bark as bio-adsorbent for cd, cu, ni, pb and zn: Batch-type and stirred flow chamber experiments. J. Environ. Manag. 2014, 144, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Feng, L.; Wei, X.; Jin, J.; Wu, K. Study on the adsorption characteristics of congo red by sycamore bark activated carbon. In environment. technologies. resources. In Proceedings of the International Scientific and Practical Conference, Kharkov, Ukraine, 10–13 October 2017; pp. 64–69. [Google Scholar]
- Kooh, M.R.R.; Thotagamuge, R.; Chau, Y.-F.C.; Mahadi, A.H.; Lim, C.M. Machine learning approaches to predict adsorption capacity of azolla pinnata in the removal of methylene blue. J. Taiwan Inst. Chem. Eng. 2022, 132, 104134. [Google Scholar] [CrossRef]
- Eletta, O.; Ayandele, F.; Adeniyi, A.; Ighalo, J. Valorisation of sunflower (Tithonia diversifolia) stalks for the adsorption of Pb(II) and Fe(II) from aqueous media. NSChE J. 2020, 35, 107. [Google Scholar]
- Ighalo, J.O.; Adeniyi, A.G. Adsorption of pollutants by plant bark derived adsorbents: An empirical review. J. Water Process Eng. 2020, 35, 101228. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Traditional uses of iraqi medicinal plants. IOSR J. Pharm. 2018, 8, 32–96. [Google Scholar]
- Santhi, T.; Manonmani, S.; Smitha, T. Removal of methyl red from aqueous solution by activated carbon prepared from the annona squmosa seed by adsorption. Chem. Eng. Res. Bull. 2010, 14, 11–18. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, I.A. Interaction of dis-azo dyes with quaternized poly (dimethylaminoethyl methacrylate) as a function of the dye structure and polycation charge density. J. Appl. Polymer Sci. 2009, 112, 728–735. [Google Scholar] [CrossRef]
- Elabed, A.; Saoiabi, A.P. Réactivité Thermique et Cinétique de Dégradation du Bois d’Arganier: Application à l’Élaboration du Charbon Actif par Activation Chimique à l’Acide Phosphorique. Ph.D. Thesis, Université Mohammed V-AGDAL, Rabat, Morocco, 2007. [Google Scholar]
- Essaadaoui, Y.; Lebkiri, A.; Rifi, E.H.; Kadiri, L.; Ouass, A. Adsorption of cobalt from aqueous solutions onto bark of eucalyptus. Mediterr. J. Chem. 2018, 7, 145–155. [Google Scholar] [CrossRef]
- Murtaza, G.; Zia, M.H. Wastewater production, treatment and use in pakistan. In Proceedings of the Second Regional Workshop of the Project ‘Safe Use of Wastewater in Agriculture, New Delhi, India, 16–18 May 2012; pp. 16–18. [Google Scholar]
- Naeem, H.; Ajmal, M.; Muntha, S.; Ambreen, J.; Siddiq, M. Synthesis and characterization of graphene oxide sheets integrated with gold nanoparticles and their applications to adsorptive removal and catalytic reduction of water contaminants. RSC Adv. 2018, 8, 3599–3610. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Kelly, S.J.; Huang, X.; Liu, J. Biomimetic snowflake-shaped magnetic micro-/nanostructures for highly efficient adsorption of heavy metal ions and organic pollutants from aqueous solution. J. Mater. Chem. A 2014, 2, 11759–11767. [Google Scholar] [CrossRef]
- Mohamad, R.; Alias, M.Z.M.; Ghazi, R.M. Removal of methyl red using chemical impregnated activated carbon prepared from parkia speciosa pod (petai) as a potential adsorbent. J. Trop. Resour. Sustain. Sci. (JTRSS) 2017, 5, 62–65. [Google Scholar] [CrossRef]
- Enenebeaku, C.K.; Okorocha, N.J.; Uchechi, E.E.; Ukaga, I.C. Adsorption and equilibrium studies on the removal of methyl red from aqueous solution using white potato peel powder. Int. Lett. Chem. Phys. Astron. 2017, 72, 52. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Ahmed, N.A.B.; Adegoke, K.A.; Bello, O.S. Sorption studies of methyl red dye removal using lemon grass (Cymbopogon citratus). Chem. Data Collect. 2019, 22, 100249. [Google Scholar] [CrossRef]
- Tanzim, K.; Abedin, M.Z. A novel bioadsorbent for the removal of methyl red from aqueous solutions. IOSR J. Environ. Sci. Toxicol. Food Technol. 2015, 9, 87–91. [Google Scholar]
- Fosso-Kankeu, E.; Webster, A.; Ntwampe, I.; Waanders, F. Coagulation/flocculation potential of polyaluminium chloride and bentonite clay tested in the removal of methyl red and crystal violet. Arab. J. Sci. Eng. 2017, 42, 1389–1397. [Google Scholar] [CrossRef]
- Abdulhussein, H.A.; Hassan, A.A. Methyl red dye removal from aqueous solution by adsorption on rice hulls. Babylon Univ. Sci. 2015, 23, 627–637. [Google Scholar]

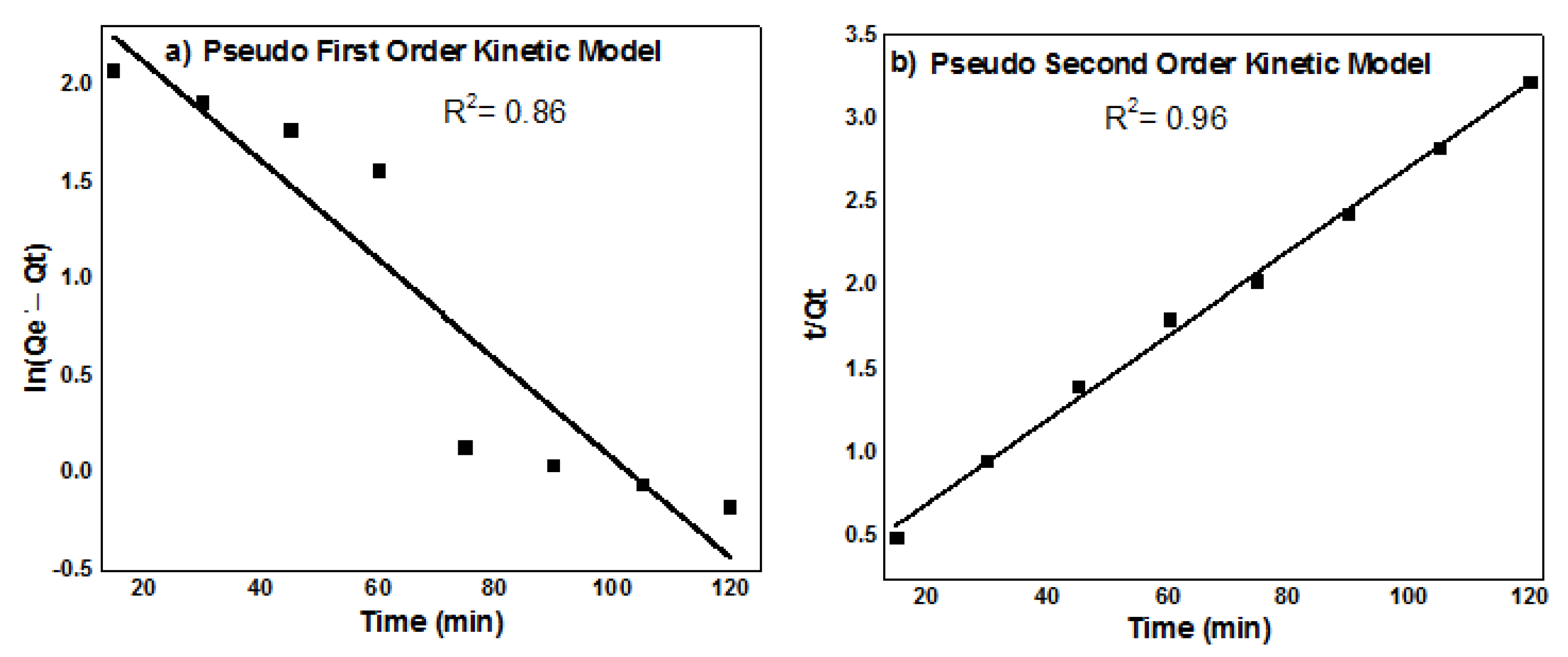
| Pseudo First Order Kinetic Model | Pseudo Second Order Kinetic Model | ||||
|---|---|---|---|---|---|
| k1 | Qe | R2 | k2 | Qe | R2 |
| −0.000213 | 13.9 | 0.86341 | 0.00328 | 39.75 | 0.96 |
| Adsorbent D. viscosa Plant Bark (Powder) | Langmuir Isotherms | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| Qm mg g−1 | KL (Lg−1) | R2 | KF | n | R2 | |
| - | 2.53614 | −0.00688 | 0.694 | 0.00707 | 0.33664 | 0.983 |
| Adsorbents | Adsorbent Dose (mg) | λmax (nm) | pH | Ci (ppm) | Contact Time (min) | Qe (mg/g) | % Removal | Reference |
|---|---|---|---|---|---|---|---|---|
| Bark of D. viscosa | 100 | 433 | 1 | 500 | 75 | 36.64 | 74 | Present work |
| Parkia speciosa Pod | 5000 | 410 | - | 10 | 30 | - | 100 | [58] |
| Pomelo peels | 1000 | 410 | 6.5 | 100 | 80 | - | 95 | [61] |
| White Potato Peel Powder | 1000 | - | 2 | 25 | 80 | 4.5 | 90.5 | [59] |
| Bentonite clay | 1600 | 536 | 2 | 100 | 25 | - | 98.4 | [62] |
| Rice hulls | 10,000 | - | 3 | 500 | 100 | 3.6 | 65 | [63] |
| Activated carbon prepared from Annona squmosa seeds | 200 | 540 | 4 | 200 | 100 | 27.7 | 50 | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul, S.; Kanwal, M.; Qazi, R.A.; Gul, H.; Khattak, R.; Khan, M.S.; Khitab, F.; Krauklis, A.E. Efficient Removal of Methyl Red Dye by Using Bark of Hopbush. Water 2022, 14, 2831. https://doi.org/10.3390/w14182831
Gul S, Kanwal M, Qazi RA, Gul H, Khattak R, Khan MS, Khitab F, Krauklis AE. Efficient Removal of Methyl Red Dye by Using Bark of Hopbush. Water. 2022; 14(18):2831. https://doi.org/10.3390/w14182831
Chicago/Turabian StyleGul, Salma, Mansha Kanwal, Raina Aman Qazi, Hajera Gul, Rozina Khattak, Muhammad Sufaid Khan, Fatima Khitab, and Andrey E. Krauklis. 2022. "Efficient Removal of Methyl Red Dye by Using Bark of Hopbush" Water 14, no. 18: 2831. https://doi.org/10.3390/w14182831







