Coordination among Water Transport, Photosynthesis and Nutrition under Climate Change: Stronger Responses of a Native than an Invasive Herb
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Leaf Gas Exchange and Hydraulic Conductance Measurements
2.3. Leaf Nutrient Concentrations, Chlorophyll Concentration, δ13C
2.4. Leaf Structure and Anatomy
2.5. Statistical Analyses
3. Results
3.1. Temperature and [CO2] Effects on Leaf Structure
3.2. Temperature and [CO2] Effects on Leaf Hydraulic and Photosynthetic Characteristics
3.3. Temperature and [CO2] Effects on Leaf Nutrient Properties and Leaf Construction Cost
3.4. Coordination among Physiological Processes
4. Discussion
4.1. Coordinated Changes in Water Transport and Photosynthesis, and Water Relations
4.2. Changes in Photosynthetic Capacity in Relation to N
4.3. Coordinated Changes in Leaf Structure and Function
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability. In Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Medlyn, B.E.; Barton, C.V.M.; Broadmeadow, M.S.J.; Ceulemans, R.; De Angelis, P.; Forstreuter, M.; Freeman, M.; Jackson, S.B.; Kellomaki, S.; Laitat, E.; et al. Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: A synthesis. New Phytol. 2001, 149, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Lammertsma, E.I.; de Boer, H.J.; Dekker, S.C.; Dilcher, D.L.; Lotter, A.F.; Wagner-Cremer, F. Global CO2 rise leads to reduced maximum stomatal conductance in Florida vegetation. Proc. Natl. Acad. Sci. USA 2011, 108, 4035–4040. [Google Scholar] [CrossRef]
- Guerrieri, R.; Belmecheri, S.; Ollinger, S.V.; Asbjornsen, H.; Jennings, K.; Xiao, J.; Stocker, B.D.; Martin, M.; Hollinger, D.Y.; Bracho-Garrillo, R.; et al. Disentangling the role of photosynthesis and stomatal conductance on rising forest water-use efficiency. Proc. Natl. Acad. Sci. USA 2019, 116, 16909–16914. [Google Scholar] [CrossRef] [PubMed]
- Mathias, J.M.; Thomas, R.B. Global tree intrinsic water use efficiency is enhanced by increased atmospheric CO2 and modulated by climate and plant functional types. Proc. Natl. Acad. Sci. USA 2021, 118, e2014286118. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.; Humphries, S.W. A mechanistic evaluation of photosynthetic acclimation at elevated CO2. Glob. Change Biol. 2000, 6, 1005–1011. [Google Scholar] [CrossRef]
- Luomala, E.M.; Laitinen, K.; Kellomaki, S.; Vapaavuori, E. Variable photosynthetic acclimation in consecutive cohorts of Scots pine needles during 3 years of growth at elevated CO2 and elevated temperature. Plant Cell Environ. 2003, 26, 645–660. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–371. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Taylor, J.M. Effects of elevated CO2 on stem growth, vessel area and hydraulic conductivity of oak and cherry seedlings. New Phytol. 1996, 133, 617–626. [Google Scholar] [CrossRef]
- Heath, J.; Kerstiens, G.; Tyree, M.T. Stem hydraulic conductance of European beech (Fagus sylvatica L.) and pedunculate oak (Quercus robur L.) grown in elevated CO2. J. Exp. Bot. 1997, 48, 1487–1489. [Google Scholar] [CrossRef]
- Bunce, J.A.; Ziska, L.H. Decreased hydraulic conductance in plants at elevated carbon dioxide. Plant Cell Environ. 1998, 21, 121–126. [Google Scholar] [CrossRef]
- Tognetti, R.; Longobucco, A.; Miglietta, F.; Raschi, A. Water relations, stomatal response and transpiration of Quercus pubescens trees during summer in a Mediterranean carbon dioxide spring. Tree Physiol. 1999, 19, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, R.; Longobucco, A.; Raschi, A.; Miglietta, F.; Fumagalli, I. Responses of two Populus clones to elevated atmospheric CO2 concentration in the field. Ann. For. Sci. 1999, 56, 493–500. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Tschaplinski, T.J.; Norby, R.J. Plant water relations at elevated CO2—Implications for water-limited environments. Plant Cell Environ. 2002, 25, 319–331. [Google Scholar] [CrossRef]
- Eguchi, N.; Morii, N.; Ueda, T.; Funada, R.; Takagi, K.; Hiura, T.; Sasa, K.; Koike, T. Changes in petiole hydraulic properties and leaf water flow in birch and oak saplings in a CO2-enriched atmosphere. Tree Physiol. 2008, 28, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Domec, J.C.; Palmroth, S.; Ward, E.; Maier, C.A.; Therezien, M.; Oren, R. Acclimation of leaf hydraulic conductance and stomatal conductance of Pinus taeda (loblolly pine) to long-term growth in elevated CO2 (free-air CO2 enrichment) and N-fertilization. Plant Cell Environ. 2009, 32, 1500–1512. [Google Scholar] [CrossRef]
- Sperry, J.S.; Venturas, M.D.; Todd, H.N.; Trugman, A.T.; Anderegg, W.R.; Wang, Y.; Tai, X. The impact of rising CO2 and acclimation on the response of US forests to global warming. Proc. Natl. Acad. Sci. USA 2019, 116, 25734–25744. [Google Scholar] [CrossRef]
- Hao, G.Y.; Holbrook, N.M.; Zwieniecki, M.A.; Gutschick, V.P.; BassiriRad, H. Coordinated responses of plant hydraulic architecture with the reduction of stomatal conductance under elevated CO2 concentration. Tree Physiol. 2018, 38, 1041–1052. [Google Scholar] [CrossRef]
- Medeiros, J.S.; Ward, J.K. Increasing atmospheric [CO2] from glacial to future concentrations affects drought tolerance via impacts on leaves, xylem and their integrated function. New Phytol. 2013, 199, 738–748. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.S.; Montagu, K.D.; Conroy, J.P. Changes in wood density of Eucalyptus camaldulensis due to temperature: The physiological link between water viscosity and wood anatomy. For. Ecol. Manag. 2004, 193, 157–165. [Google Scholar] [CrossRef]
- Phillips, N.G.; Attard, R.D.; Ghannoum, O.; Lewis, J.D.; Logan, B.A.; Tissue, D.T. Impact of variable [CO2] and temperature on water transport structure-function relationships in Eucalyptus. Tree Physiol. 2011, 31, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.M.; Sack, L.; Bernacchi, C.J.; Ort, D.R. Soybean leaf hydraulic conductance does not acclimate to growth at elevated [CO2] or temperature in growth chambers or in the field. Ann. Bot. 2013, 112, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Way, D.A.; Domec, J.C.; Jackson, R.B. Elevated growth temperatures alter hydraulic characteristics in trembling aspen (Populus tremuloides) seedlings: Implications for tree drought tolerance. Plant Cell Environ. 2013, 36, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Navas, M.L. Plant growth and competition at elevated CO2: On winners, losers and functional groups. New Phytol. 2003, 157, 175–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Cristiano, P.M.; Zhang, Y.F.; Campanello, P.I.; Tan, Z.H.; Zhang, Y.P.; Cao, K.; Goldstein, G. Carbon economy of subtropical forests. In Tropical Tree Physiology; Goldstein, G., Santiago, L.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 337–355. [Google Scholar]
- Santiago, L.S.; Goldstein, G.; Meinzer, F.C.; Fisher, J.B.; Machado, K.; Woodruff, D.; Jones, T. Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia 2004, 140, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Feild, T.S.; Jordan, G.J. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 2007, 144, 1890–1898. [Google Scholar] [CrossRef]
- Zhang, J.L.; Cao, K.F. Stem hydraulics mediates leaf water status, carbon gain, nutrient use efficiencies and plant growth rates across dipterocarp species. Funct. Ecol. 2009, 23, 658–667. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Meinzer, F.C.; Qi, J.H.; Goldstein, G.; Cao, K.F. Midday stomatal conductance is more related to stem rather than leaf water status in subtropical deciduous and evergreen broadleaf trees. Plant Cell Environ. 2013, 36, 149–158. [Google Scholar] [CrossRef]
- Siddiq, Z.; Zhang, Y.-J. Cool-dry season depression in gas exchange of canopy leaves and water flux of tropical trees at the northern limit of Asian tropics. Plant Ecol. 2022, 223, 171–183. [Google Scholar] [CrossRef]
- Sack, L.; Tyree, M.T.; Holbrook, N.M. Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytol. 2005, 167, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Field, C.; Mooney, H.A. The photosynthesis-nitrogen relationship in wild plants. In On the Economy of Plant Form and Function; Givnish, T., Ed.; Cambridge University Press: London, UK, 1986; pp. 25–55. [Google Scholar]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Cao, K.F.; Sack, L.; Li, N.; Wei, X.M.; Goldstein, G. Extending the generality of leaf economic design principles in the cycads, an ancient lineage. New Phytol. 2015, 206, 817–829. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Yang, D.; Zhang, K.-Y.; Bai, X.-L.; Wang, Y.-S.-D.; Wu, H.-D.; Ding, L.-Z.; Zhang, Y.-J.; Zhang, J.-L. Higher water and nutrient use efficiencies in savanna than in rainforest lianas result in no difference in photosynthesis. Tree Physiol. 2021, 42, 145–159. [Google Scholar] [CrossRef]
- Niu, S.L.; Sherry, R.A.; Zhou, X.H.; Wan, S.Q.; Luo, Y.Q. Nitrogen regulation of the climate-carbon feedback: Evidence from a long-term global change experiment. Ecology 2010, 91, 3261–3273. [Google Scholar] [CrossRef]
- Barber, S.A. A diffusion and mass-flow concept of soil nutrient availability. Soil Sci. 1962, 93, 39–49. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Sack, L.; Goldstein, G.; Cao, K.-F. Hydraulic determination of leaf nutrient concentrations in cycads. Mem. NY Bot. Gard 2018, 117, 179–192. [Google Scholar]
- Yin, Y.; Zhou, Y.-B.; Li, H.; Zhang, S.-Z.; Fang, Y.-T.; Zhang, Y.-J.; Zou, X. Linking tree water use efficiency with calcium and precipitation. Tree Physiol. 2022; in press. [Google Scholar] [CrossRef]
- Tyree, M.T.; Alexander, J.D. Plant water relations and the effects of elevated CO2—A review and suggestions for future research. Vegetatio 1993, 104, 47–62. [Google Scholar] [CrossRef]
- McDowell, N.G.; Sapes, G.; Pivovaroff, A.; Adams, H.D.; Allen, C.D.; Anderegg, W.R.; Arend, M.; Breshears, D.D.; Brodribb, T.; Choat, B.; et al. Mechanisms of woody-plant mortality under rising drought, CO2 and vapour pressure deficit. Nat. Rev. Earth Environ. 2022, 3, 294–308. [Google Scholar] [CrossRef]
- Smith, S.D.; Huxman, T.E.; Zitzer, S.F.; Charlet, T.N.; Housman, D.C.; Coleman, J.S.; Fenstermaker, L.K.; Seemann, J.R.; Nowak, R.S. Elevated CO2 increases productivity and invasive species success in an arid ecosystem. Nature 2000, 408, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Raizada, P.; Singh, A.; Raghubanshi, A.S. Comparative response of seedlings of selected native dry tropical and alien invasive species to CO2 enrichment. J. Plant Ecol. 2009, 2, 69–75. [Google Scholar] [CrossRef]
- Song, L.Y.; Wu, J.R.; Li, C.H.; Li, F.R.; Peng, S.L.; Chen, B.M. Different responses of invasive and native species to elevated CO2 concentration. Acta Oecologica.-Int. J. Ecol. 2009, 35, 128–135. [Google Scholar] [CrossRef]
- Stephens, K.L.; Dantzler-Kyer, M.E.; Patten, M.A.; Souza, L. Differential responses to global change of aquatic and terrestrial invasive species: Evidences from a meta-analysis. Ecosphere 2019, 10, e02680. [Google Scholar] [CrossRef]
- Bradford, M.A.; Schumacher, H.B.; Catovsky, S.; Eggers, T.; Newingtion, J.E.; Tordoff, G.M. Impacts of invasive plant species on riparian plant assemblages: Interactions with elevated atmospheric carbon dioxide and nitrogen deposition. Oecologia 2007, 152, 791–803. [Google Scholar] [CrossRef]
- Qing, H.; Cai, Y.; Xiao, Y.; Yao, Y.; An, S. Leaf nitrogen partition between photosynthesis and structural defense in invasive and native tall form Spartina alterniflora populations: Effects of nitrogen treatments. Biol. Invasions 2012, 14, 2039–2048. [Google Scholar] [CrossRef]
- Manea, A.; Leishman, M.R. Competitive interactions between native and invasive exotic plant species are altered under elevated carbon dioxide. Oecologia 2011, 165, 735–744. [Google Scholar] [CrossRef]
- He, L.; Kong, J.; Li, G.; Meng, G.; Chen, K. Similar responses in morphology, growth, biomass allocation, and photosynthesis in invasive Wedelia trilobata and native congeners to CO2 enrichment. Plant Ecol. 2018, 219, 145–157. [Google Scholar] [CrossRef]
- Pattison, R.R.; Goldstein, G.; Ares, A. Growth, biomass allocation and photosynthesis of invasive and native Hawaiian rainforest species. Oecologia 1998, 117, 449–459. [Google Scholar] [CrossRef]
- Baruch, Z.; Goldstein, G. Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia 1999, 121, 183–192. [Google Scholar] [CrossRef]
- Stratton, L.C.; Goldstein, G. Carbon uptake, growth and resource-use efficiency in one invasive and six native Hawaiian dry forest tree species. Tree Physiol. 2001, 21, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.L.; Auge, H.; Ebeling, S.K. Invasive Buddleja davidii allocates more nitrogen to its photosynthetic machinery than five native woody species. Oecologia 2007, 153, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.L.; Lei, Y.B.; Wang, R.F.; Callaway, R.M.; Valiente-Banuet, A.; Inderjit; Li, Y.P.; Zheng, Y.L. Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proc. Natl. Acad. Sci. USA 2009, 106, 1853–1856. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Olszyk, D.M.; Rygiewicz, P.T.; Tingey, D.T.; Johnson, M.G. Foliar nitrogen concentrations and natural abundance of N-15 suggest nitrogen allocation patterns of Douglas-fir and mycorrhizal fungi during development in elevated carbon dioxide concentration and temperature. Tree Physiol. 2001, 21, 1113–1122. [Google Scholar] [CrossRef]
- An, Y.A.; Wan, S.Q.; Zhou, X.H.; Subedar, A.A.; Wallace, L.L.; Luo, Y.Q. Plant nitrogen concentration, use efficiency, and contents in a tallgrass prairie ecosystem under experimental warming. Glob. Change Biol. 2005, 11, 1733–1744. [Google Scholar] [CrossRef]
- Takashima, T.; Hikosaka, K.; Hirose, T. Photosynthesis or persistence: Nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ. 2004, 27, 1047–1054. [Google Scholar] [CrossRef]
- Cavaleri, M.A.; Sack, L. Comparative water use of native and invasive plants at multiple scales: A global meta-analysis. Ecology 2010, 91, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Cronk, Q.C.B.; Fuller, J.L. Plant Invaders: The Threat to Natural Ecosystems; Chapman Hall: London, UK, 1995. [Google Scholar]
- Lin, R.; Chen, Y. Flora of China; Science Press: Beijing, China, 1985; p. 74. [Google Scholar]
- Sack, L.; Melcher, P.J.; Zwieniecki, M.A.; Holbrook, N.M. The hydraulic conductance of the angiosperm leaf lamina: A comparison of three measurement methods. J. Exp. Bot. 2002, 53, 2177–2184. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M. Declining hydraulic efficiency as transpiring leaves desiccate: Two types of response. Plant Cell Environ. 2006, 29, 2205–2215. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C. Measurement of leaf hydraulic conductance and stomatal conductance and their responses to irradiance and dehydration using the evaporative flux method (EFM). J. Vis. Exp. 2012, 70, e4179. [Google Scholar] [CrossRef] [Green Version]
- Melcher, P.J.; Michele Holbrook, N.; Burns, M.J.; Zwieniecki, M.A.; Cobb, A.R.; Brodribb, T.J.; Choat, B.; Sack, L. Measurements of stem xylem hydraulic conductivity in the laboratory and field. Methods Ecol. Evol. 2012, 3, 685–694. [Google Scholar] [CrossRef]
- McDowell, S.C. Photosynthetic characteristics of invasive and noninvasive species of Rubus (Rosaceae). Am. J. Bot. 2002, 89, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Marquard, R.; Tipton, J. Relationship between extractable chlorophyll and an in-situ method to estimate leaf greenness. Hortscience 1987, 22, 1327. [Google Scholar]
- Marenco, R.A.; Antezana-Vera, S.A.; Nascimento, H.C.S. Relationship between specific leaf area, leaf thickness, leaf water content and SPAD-502 readings in six Amazonian tree species. Photosynthetica 2009, 47, 184–190. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 4th ed.; W. H. Freeman and Co.: New York, NY, USA, 2011. [Google Scholar]
- Vu, J.C.V.; Allen Jr, L.H.; Bowes, G. Leaf ultrastructure, carbohydrates and protein of soybeans grown under CO2 enrichment. Environ. Exp. Bot. 1989, 29, 141–147. [Google Scholar] [CrossRef]
- Ellsworth, D.S. CO2 enrichment in a maturing pine forest: Are CO2 exchange and water status in the canopy affected? Plant Cell Environ. 1999, 22, 461–472. [Google Scholar] [CrossRef]
- Apple, M.E.; Olszyk, D.M.; Ormrod, D.P.; Lewis, J.; Southworth, D.; Tingey, D.T. Morphology and stomatal function of Douglas Fir needles exposed to climate change: Elevated CO2 and temperature. Int. J. Plant Sci. 2000, 161, 127–132. [Google Scholar] [CrossRef]
- Ghannoum, O.; Phillips, N.G.; Sears, M.A.; Logan, B.A.; Lewis, J.D.; Conroy, J.P.; Tissue, D.T. Photosynthetic responses of two eucalypts to industrial-age changes in atmospheric [CO2] and temperature. Plant Cell Environ. 2010, 33, 1671–1681. [Google Scholar] [CrossRef]
- Feng, Y.L. Photosynthesis, nitrogen allocation and specific leaf area in invasive Eupatorium adenophorum and native Eupatorium japonicum grown at different irradiances. Physiol. Plant. 2008, 133, 318–326. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Feild, T.S. Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol. Lett. 2010, 13, 175–183. [Google Scholar] [CrossRef]
- Eamus, D.; Berryman, C.A.; Duff, G.A. Assimilation, stomatal conductance, specific leaf area and chlorophyll responses to elevated CO2 of Maranthes Corymbosa, a tropical monsoon rain-forest species. Aust. J. Plant Physiol. 1993, 20, 741–755. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Jordan, G.J. Water supply and demand remain balanced during leaf acclimation of Nothofagus cunninghamii trees. New Phytol. 2011, 192, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhao, W.-l.; Cao, K.F. Global convergence in the balance between leaf water supply and demand across vascular land plants. Funct. Plant Biol. 2020, 47, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.; Nijs, I.; Behaeghe, T.; Impens, I. Elevated CO2 and temperature have different effects on leaf anatomy of perennial ryegrass in spring and summer. Ann. Bot. 1996, 78, 489–497. [Google Scholar] [CrossRef]
- Reddy, K.R.; Robana, R.R.; Hodges, H.F.; Liu, X.J.; McKinion, J.M. Interactions of CO2 enrichment and temperature on cotton growth and leaf characteristics. Environ. Exp. Bot. 1998, 39, 117–129. [Google Scholar] [CrossRef]
- Beerling, D.J.; Chaloner, W.G. The impact of atmospheric CO2 and temperature change on stomatal density: Observations from Quercus robur Lammas leaves. Ann. Bot. 1993, 71, 231–235. [Google Scholar] [CrossRef]
- Luomala, E.M.; Laitinen, K.; Sutinen, S.; Kellomaki, S.; Vapaavuori, E. Stomatal density, anatomy and nutrient concentrations of Scots pine needles are affected by elevated CO2 and temperature. Plant Cell Environ. 2005, 28, 733–749. [Google Scholar] [CrossRef]
- Woodward, F.I. Stomatal numbers are sensitive to increases in CO2 from preindustrial levels. Nature 1987, 327, 617–618. [Google Scholar] [CrossRef]
- Woodward, F.I.; Kelly, C.K. The influence of CO2 concentration on stomatal density. New Phytol. 1995, 131, 311–327. [Google Scholar] [CrossRef]
- Ferris, R.; Long, L.; Bunn, S.M.; Robinson, K.M.; Bradshaw, H.D.; Rae, A.M.; Taylor, G. Leaf stomatal and epidermal cell development: Identification of putative quantitative trait loci in relation to elevated carbon dioxide concentration in poplar. Tree Physiol. 2002, 22, 633–640. [Google Scholar] [CrossRef] [Green Version]

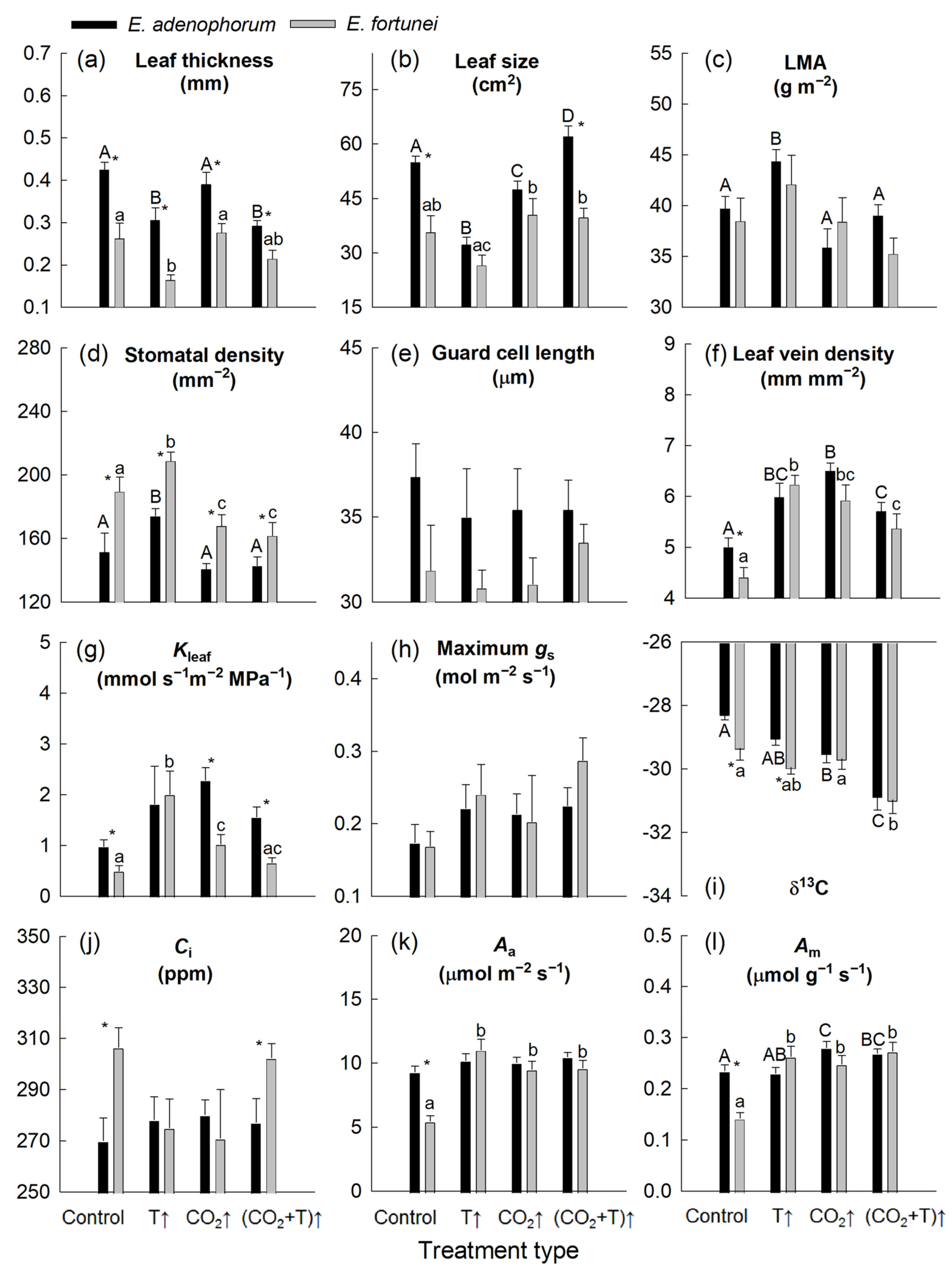
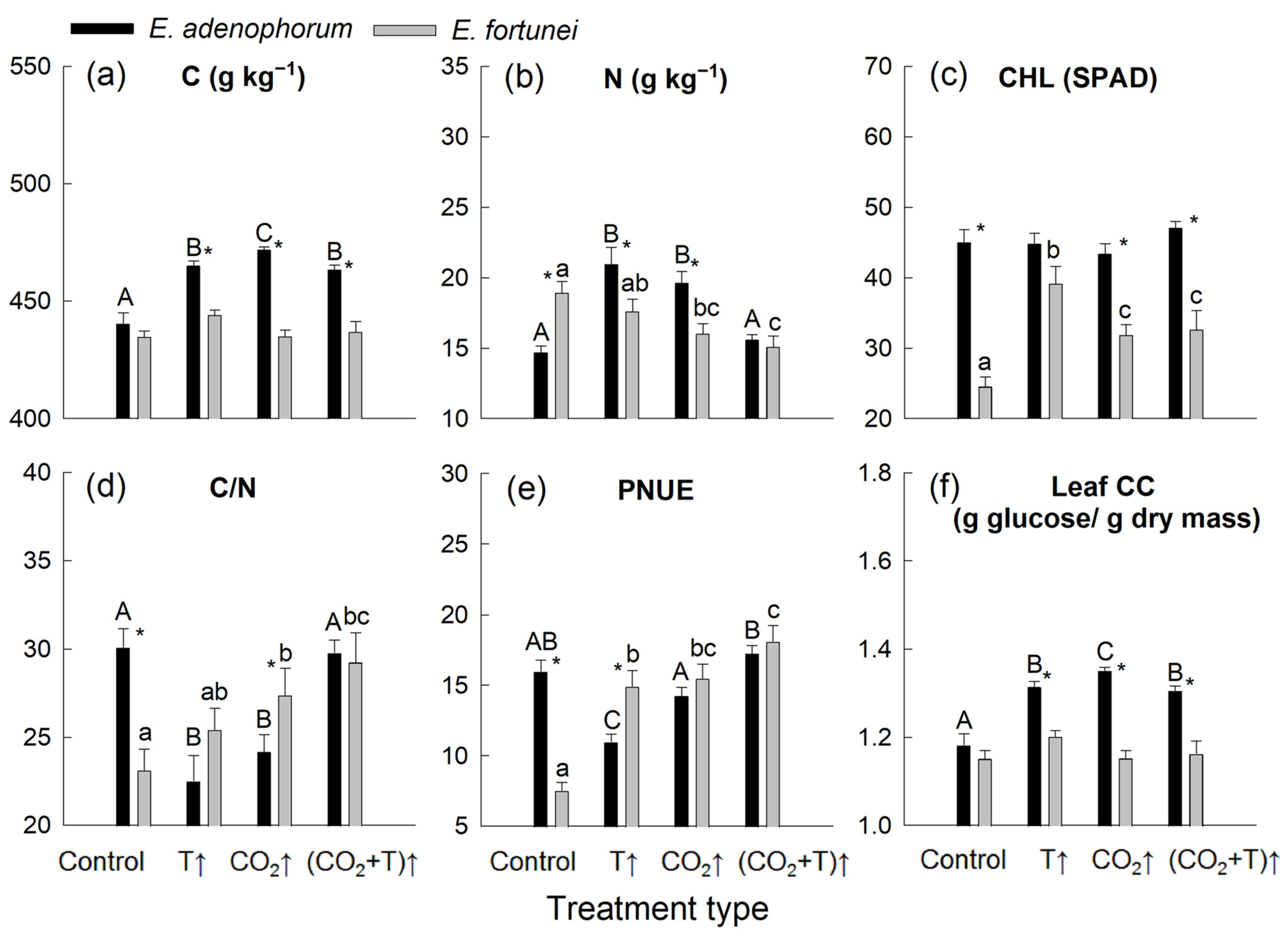

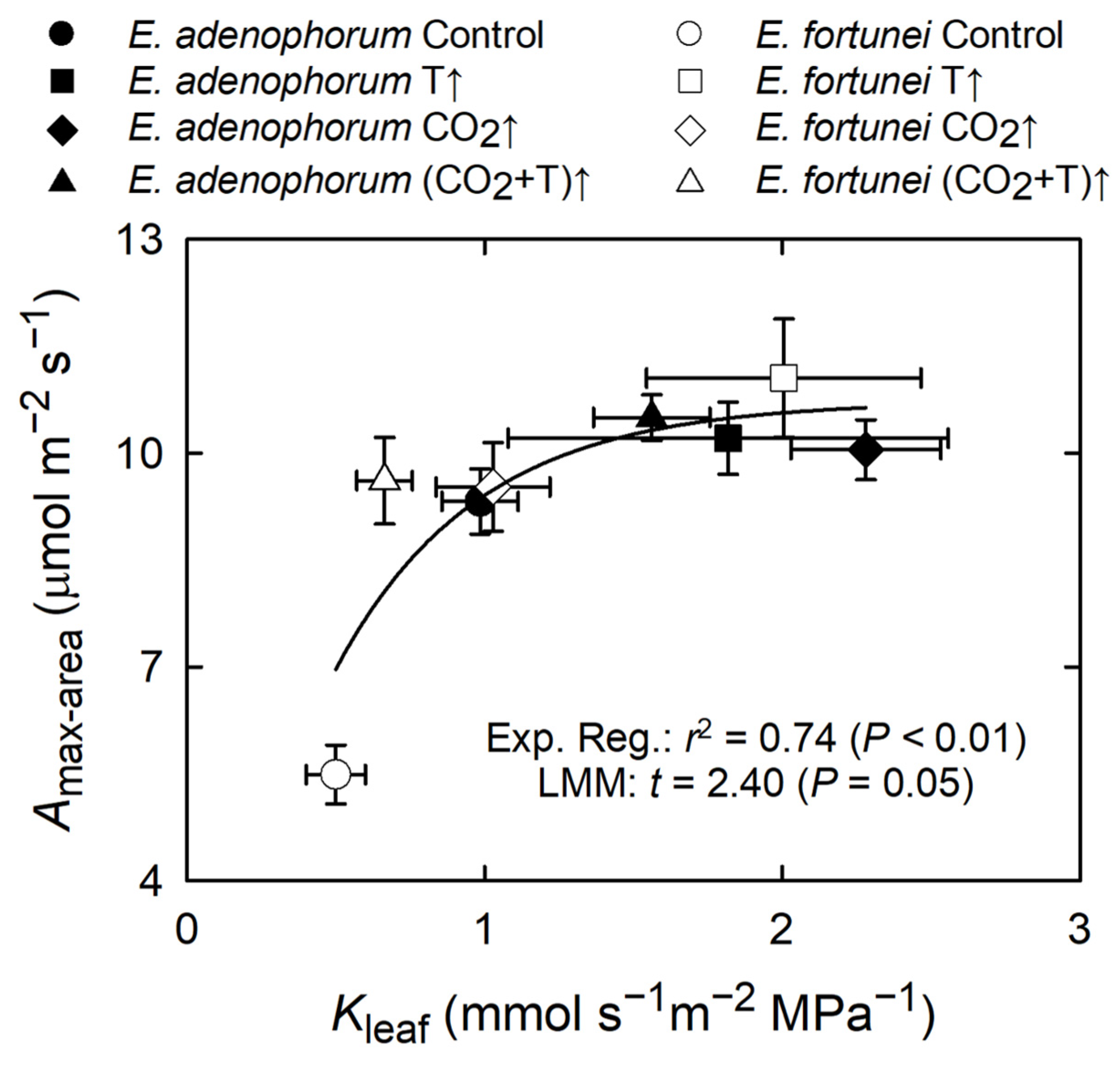



| T | CO2 | Species | T × CO2 | T × Species | CO2 × Species | |
|---|---|---|---|---|---|---|
| Structure | ||||||
| Leaf thickness | * | ns | * | ns | ns | ns |
| Leaf size | * | * | * | * | ns | ns |
| LMA | ns | * | ns | ns | ns | ns |
| Stomatal density | ns | * | * | * | ns | ns |
| Guard cell length | ns | ns | * | ns | ns | ns |
| Leaf vein density | * | * | ns | * | ns | ns |
| Physiology | ||||||
| Kleaf | * | ns | ns | * | ns | ns |
| Maximum gs | * | ns | ns | ns | ns | ns |
| δ13C | * | * | * | ns | ns | ns |
| Ci | ns | ns | * | ns | ns | ns |
| Aa | * | * | * | * | * | ns |
| Am | * | * | * | * | * | ns |
| Nutrition | ||||||
| C | * | * | * | * | ns | * |
| N | ns | * | ns | * | ns | * |
| CHL | * | ns | * | ns | * | ns |
| C/N | ns | * | * | * | ns | * |
| PNUE | * | * | ns | ns | * | * |
| Leaf CC | * | * | * | * | ns | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.-H.; Yan, Q.-S.; Tasnim, R.; Zhang, L.; Fu, P.-L.; Fan, Z.-X.; Zhang, Y.-J. Coordination among Water Transport, Photosynthesis and Nutrition under Climate Change: Stronger Responses of a Native than an Invasive Herb. Water 2022, 14, 2828. https://doi.org/10.3390/w14182828
Qi J-H, Yan Q-S, Tasnim R, Zhang L, Fu P-L, Fan Z-X, Zhang Y-J. Coordination among Water Transport, Photosynthesis and Nutrition under Climate Change: Stronger Responses of a Native than an Invasive Herb. Water. 2022; 14(18):2828. https://doi.org/10.3390/w14182828
Chicago/Turabian StyleQi, Jin-Hua, Qiao-Shun Yan, Rafa Tasnim, Lan Zhang, Pei-Li Fu, Ze-Xin Fan, and Yong-Jiang Zhang. 2022. "Coordination among Water Transport, Photosynthesis and Nutrition under Climate Change: Stronger Responses of a Native than an Invasive Herb" Water 14, no. 18: 2828. https://doi.org/10.3390/w14182828
APA StyleQi, J.-H., Yan, Q.-S., Tasnim, R., Zhang, L., Fu, P.-L., Fan, Z.-X., & Zhang, Y.-J. (2022). Coordination among Water Transport, Photosynthesis and Nutrition under Climate Change: Stronger Responses of a Native than an Invasive Herb. Water, 14(18), 2828. https://doi.org/10.3390/w14182828








